Abstract
Background
Asthma is a common pediatric chronic respiratory disease worldwide. Previous studies showed the prevalence of childhood asthma increased in developed countries as well as in Taiwan in the late 20th century. Recently, several reports from different parts of the world showed a reversed trend in this epidemic of childhood asthma prevalence. This study investigated the trend of childhood asthma through serial cross-section questionnaire surveys in the southern part of Taiwan, and identified associated factors related to this trend in elementary school children.
Methods
We used the Chinese version of the International Study of Asthma and Allergies in Childhood (ISAAC)29 questionnaire to assess the asthma status of elementary school students aged 6–12 years in Tainan city in 3 independent study periods, namely, 2008–2009, 2010–2012, and 2017–2018. We assessed the trend of “asthma” and “related respiratory symptoms” across 3 study periods.
Results
Of the 19,633 respondents, 17,545 (89.4%) completed the questionnaires. After adjustment for covariates, the prevalence of asthma and related respiratory symptoms was significantly lower in 2017–2018 than in the 2 earlier periods. Among the protective factors, the increasing rate of breastfeeding might be partly responsible for the observed reduced prevalence of current asthma and exercise-induced wheeze, but not physician-diagnosed asthma. The presence of pets in the house was the risk factor that correlated with the prevalence of nocturnal cough. Pearson correlation analysis showed a significant correlation of the prevalence of physician-diagnosed asthma, current asthma, and exercise-induced wheezing with the concentrations of air pollutant particles with aerodynamic diameter ≤10 μM (PM10) (r = 0.84, 0.77 and 0.81, respectively).
Conclusion
The prevalence of asthma and related respiratory symptoms has declined in elementary school-age children in southern Taiwan. The increased prevalence of breastfeeding, decreased rate of the presence of pets in the house, and improvement in outdoor air pollution seem to be related to this decreasing trend of asthma in school children. Our findings will provide the scientific base to empower prevention policy to reverse the trend of childhood asthma prevalence.
Trial registration
N/A
Keywords: Asthma prevalence, Children, Trend, Air pollution
Abbreviations: CO, carbon monoxide; EPA, Environmental Protection Administration; ISAAC, International Study of Asthma and Allergies in Childhood; NO2, nitrogen dioxide pollutant particles with aerodynamic diameter ≤10 μM; O3, ozone; OR, odds ratio; PM2.5, particles with aerodynamic diameter ≤2.5(PM10, μg/m3); PM10, particles with aerodynamic diameter ≤10 μm (PM10, μg/m3); ppb, part per billion; ppm, part per million; SO2, sulfur dioxide
Introduction
Asthma is a common chronic respiratory disease in children and is a significant burden for affected individuals, family caregivers, and societies.1 The worldwide prevalence of asthma and its severity have increased over time starting from the second half of the 20th century.2 After the 1990s, estimates of temporal trends in the prevalence of asthma in several countries were inconsistent. The trend of the prevalence of asthma seemed to level off in some countries, while it continued to increase in others.2,3 Recently, 2 large-scale nationwide population-based studies have found that the prevalence of asthma among children has plateaued or even declined in developed countries.4,5
Several studies have reported an association between a wide range of risk factors and childhood asthma. For example, indoor exposure to molds or fungi during postnatal life was associated with increased risks of asthma and wheezing.6,7 Pet keeping during infancy was a risk factor for subsequent development of asthma.8,9 One systemic review and meta-analysis suggested that breastfeeding was negatively associated with asthma.10 Nevertheless, the effect of some risk factors on the prevalence of asthma remains uncertain due to the wide range of ethnicities and environmental factors.
This study aimed to assess temporal trends in the prevalence of asthma and related respiratory symptoms in elementary school children living in Tainan city, Southern Taiwan, based on the standardized questionnaire of the International Study of Asthma and Allergies in Childhood (ISAAC) and identified factors associated with the trends of asthma and related respiratory symptoms using 3 independent questionnaire surveys over the past 10 years.
Methods
Study design and study population
This study consisted of 7 repeated cross-sectional surveys divided into 3 periods from 2008 to 2018, conducted in the same city and using the same methodology (Table 1). A total of 14 elementary schools in Tainan city were investigated. All school students aged 6–12 years were eligible for participation in this study. The Chinese version of the ISAAC questionnaire was answered by parents or guardians of the children in the study.
Table 1.
Questionnaire information according to the study periods (N = 17,545).
| Variable | Study period, years |
P value | ||
|---|---|---|---|---|
| 2008–2009 (n = 4188) | 2010–2012 (n = 8485) | 2017–2018 (n = 4872) | ||
| Age (years) | 9.81 ± 1.73 | 9.78 ± 1.69 | 9.17 ± 1.75ab | <0.001 |
| Male sex | 2165 (51.7) | 4218 (49.7) | 2429 (49.9) | 0.092 |
| Outcome | ||||
| Physician-diagnosed asthma | 459 (11.0) | 929 (10.9) | 440 (9.0)ab | 0.001 |
| Current asthma | 232 (5.5) | 455 (5.4) | 227 (4.7) | 0.116 |
| Exercise-induced wheeze | 240 (5.7) | 510 (6.0) | 180 (3.7)ab | <0.001 |
| Nocturnal cough | 418 (10.0) | 1072 (12.6)a | 420 (8.6)b | <0.001 |
| Breastfeeding | 753 (18.0) | 2143 (25.3)a | 2919 (59.9)ab | <0.001 |
| Allergy history | ||||
| Paternal asthma | 104 (2.5) | 168 (2.0) | 166 (3.4)ab | <0.001 |
| Paternal allergic rhinitis | 1008 (24.1) | 2076 (24.5) | 1291 (26.5)ab | 0.011 |
| Paternal atopic dermatitis | 168 (4.0) | 391 (4.6) | 313 (6.4)ab | <0.001 |
| Maternal asthma | 90 (2.1) | 229 (2.7) | 152 (3.1)a | 0.017 |
| Maternal allergic rhinitis | 806 (19.2) | 1816 (21.4)a | 1159 (23.8)ab | <0.001 |
| Maternal atopic dermatitis | 186 (4.4) | 426 (5.0) | 328 (6.7)ab | <0.001 |
| Indoor environment | ||||
| Pets | 752 (18.0) | 1548 (18.2) | 765 (15.7)ab | 0.001 |
| Visible mold | 362 (8.6) | 860 (10.1)a | 472 (9.7) | 0.028 |
| Cockroaches | 2937 (70.1) | 5543 (65.3)a | 3169 (65.0)a | <0.001 |
| Air pollution (city level) | ||||
| SO2, ppb | 4.6 ± 0.2 | 4.1 ± 0.4a | 2.8 ± 0.2ab | <0.001 |
| CO, ppm | 0.53 ± 0.04 | 0.46 ± 0.05a | 0.39 ± 0.03ab | <0.001 |
| O3, ppb | 34.7 ± 1.3 | 31.9 ± 1.6a | 32.0 ± 0.2ab | <0.001 |
| PM10, μg/m3 | 69.1 ± 0.6 | 69.2 ± 5.5 | 53.6 ± 3.4ab | <0.001 |
| PM2.5, μg/m3 | 45.0 ± 0.7 | 37.0 ± 4.0a | 24.7 ± 1.2ab | <0.001 |
| NO2, ppb | 18.4 ± 0.0 | 16.4 ± 0.0a | 13.0 ± 0.0ab | <0.001 |
SO2, sulfur dioxide; CO, carbon monoxide; O3, ozone; PM, particulate matter; NO2, nitrogen dioxide.
“a” and “b” indicate significant differences versus the “2008–2009 year” and “2010–2012 year” groups, respectively, in the Bonferroni multiple comparison.
Data are presented as frequencies (percentages) or means ± standard deviations
Data collection and definitions of study outcomes
Data regarding age, sex, breast-feeding status after birth, environmental living conditions, parental allergy histories, physician diagnosis of asthma, current asthma, and related respiratory symptoms were collected using standardized ISAAC questionnaires. The same questionnaire was used across study years.
Asthma
“Physician-diagnosed asthma” was defined according to the parental answer to the question, "Has your child ever been diagnosed with asthma by a physician?" “Current asthma” was defined according to affirmative responses to both of the following questions: "Has your child ever been diagnosed with asthma by a physician in the last 12 months?" and "Has your child's chest sounded wheezy or whistling in the last 12 months?"
Related respiratory symptoms
“Exercise-induced wheeze” was defined according to the parental answer to the question: "In the past 12 months, has your child's chest sounded wheezy during or after exercise?"
“Nocturnal cough” was defined according to the parental answer to the question: "In the past 12 months, has your child had a dry cough at night, apart from a cough associated with a cold or chest infection?"
Demographic and individual factors
We collected data on sex, age, breastfeeding status after birth (breastfeeding duration of more than 3 months was regarded as breastfeeding), parental allergy history, and indoor environmental factors, including the presence of pets, visible mold, and cockroaches at home.
Concentration of air pollutants
We obtained data on air pollutants from Taiwanese Environmental Protection Administration (EPA) air quality monitoring stations. The annual average concentrations of sulfur dioxide (SO2, ppb), carbon monoxide (CO, ppm), ozone (O3, ppb), particles with aerodynamic diameter ≤10 μm (PM10, μg/m3), particles with aerodynamic diameter ≤2.5 μm (PM2.5, μg/m3), and nitrogen dioxide (NO2, ppb) were calculated from the daily average values obtained at the monitoring stations in Tainan city where the 14 schools were located. The maximum distance between the schools and the monitoring stations was less than 10 km.
Statistical analysis
The crude prevalence of asthma-related outcomes and related respiratory symptoms among the subjects in different calendar years was calculated. We further standardized the prevalence according to the distribution of age (per year) and sex in 2018. The association between baseline characteristics and the risk of outcomes of interest was investigated using univariate and multivariable logistic regression analyses, separately. The adjusted covariates were sex, age, breastfeeding, parental allergy history, and indoor environmental factors, including the presence of pets, visible mold, and cockroaches at home. We divided the calendar year into 3 periods, namely, 2008–2009, 2010–2012, and 2017–2018, and performed logistic regression analysis to evaluate the temporal trend of asthma and related respiratory symptoms. The air pollution parameters were assessed at the city level for the analyzed cohort in this study; therefore, the valid sample size for the Pearson's correlation between air pollution parameters and outcomes (the prevalence each year) was 7 because the study was conducted for 7 study years. All tests were 2-tailed, and P < 0.05 was considered statistically significant. Data analyses were conducted using SPSS 25 (IBM SPSS Inc., Chicago, Illinois).
Results
Baseline characteristics
A total of 19 633 respondents out of 24 519 invited children were included in this study. Of these respondents, 17 545 (89.4%) had completed questionnaires and were eligible for analysis (Table 1). The mean ages were 9.81 years in 2008–2009, 9.78 years in 2010–2012, and 9.17 years in 2017–2018, with a decreasing trend over the study period (P < 0.001). There was no significant difference in the sex distribution across the 3 study periods. Notably, the prevalence of breastfeeding increased substantially from 18% in 2008–2009 and 25.3% in 2010–2012 to 59.9% in 2017–2018 (P < 0.001). On the other hand, the percentages of the presence of pets increased slightly in 2010–2012, and decreased in 2017–2018. The annual average concentrations of ambient air pollutants, including SO2, CO, PM2.5, and NO2, decreased over the study periods. The annual average concentration of PM10 was the lowest in 2017–2018 compared with the average concentration in previous periods.
Annual prevalence of asthma and related respiratory symptoms
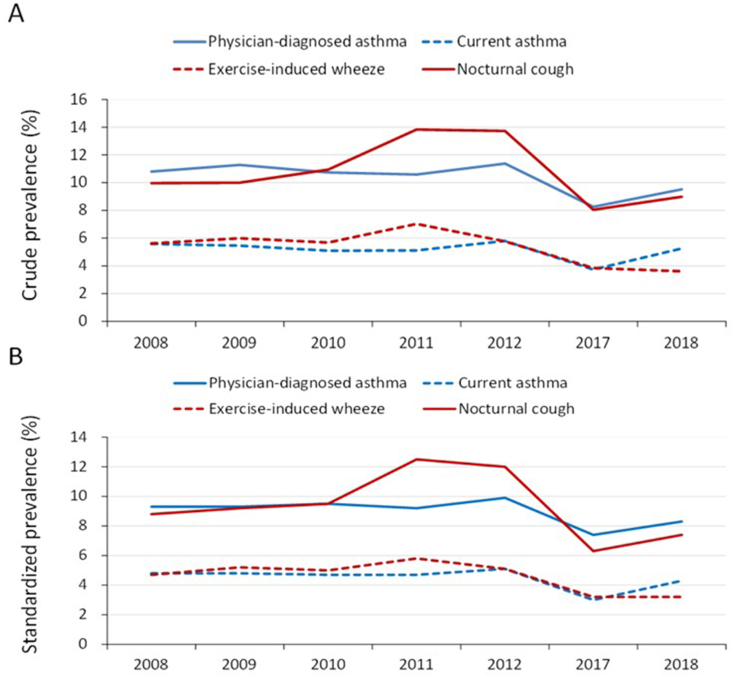
The crude and standardized (by age and sex) prevalence of physician-diagnosed asthma, current asthma, exercise-induced wheeze and nocturnal cough among the studied school children in different calendar years are shown in Fig. 1. There was a peak period between 2010 and 2012 for the prevalence of asthma (physician-diagnosed and current asthma) and related respiratory symptoms (exercise-induced wheeze and nocturnal cough), but these values decreased in 2017 and 2018, respectively.
Fig. 1.
The crude prevalence (A) and standardized prevalence (B) of physician-diagnosed asthma, current asthma, exercise-induced wheeze and nocturnal cough across the study years. Standardization was performed according to the sex and age distribution (per year) of the middle-year population in 2018
Associations between factors and the prevalence of asthma and related respiratory symptoms
Next, we explored associations between factors and the prevalence of physician-diagnosed asthma (Table 2). Using the period 2008–2009 as the reference period, the prevalence of physician-diagnosed asthma was significantly lower in the period 2017–2018 (OR = 0.77, p < 0.001). After covariate adjustment, we found that age, male sex, parental allergy history, and visible mold in the household were positively associated with the risk of physician-diagnosed asthma.
Table 2.
Association between baseline characteristics and the risk of physician-diagnosed asthma.
| Predictor | Univariate |
Multivariablea |
||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Study period, years | ||||
| 2008–2009 | Reference | Reference | ||
| 2010–2012 | 1.00 (0.89–1.12) | 0.985 | 0.99 (0.88–1.12) | 0.921 |
| 2017–2018 | 0.81 (0.70–0.93) | 0.002 | 0.77 (0.66–0.89) | 0.001 |
| Age | 1.03 (1.002–1.06) | 0.033 | 1.04 (1.01–1.08) | 0.004 |
| Male sex | 1.50 (1.36–1.65) | <0.001 | 1.51 (1.37–1.67) | <0.001 |
| Breastfeeding | 0.89 (0.80–0.99) | 0.028 | 0.92 (0.82–1.04) | 0.182 |
| Allergy history | ||||
| Paternal asthma | 3.57 (2.88–4.42) | <0.001 | 3.20 (2.56–4.00) | <0.001 |
| Paternal allergic rhinitis | 1.83 (1.65–2.03) | <0.001 | 1.67 (1.50–1.86) | <0.001 |
| Paternal atopic dermatitis | 1.86 (1.55–2.23) | <0.001 | 1.44 (1.19–1.75) | <0.001 |
| Maternal asthma | 4.24 (3.47–5.19) | <0.001 | 3.52 (2.85–4.34) | <0.001 |
| Maternal allergic rhinitis | 2.04 (1.84–2.26) | <0.001 | 1.78 (1.60–1.99) | <0.001 |
| Maternal atopic dermatitis | 2.07 (1.74–2.46) | <0.001 | 1.61 (1.34–1.93) | <0.001 |
| Indoor environment | ||||
| Pets | 0.92 (0.80–1.04) | 0.186 | 0.88 (0.77–1.01) | 0.066 |
| Visible mold | 1.30 (1.12–1.51) | 0.001 | 1.17 (1.002–1.37) | 0.047 |
| Cockroaches | 1.16 (1.04–1.28) | 0.006 | 1.10 (0.99–1.23) | 0.083 |
OR, odds ratio; CI, confidence interval.
Bold font indicates statistical significance (P-value < 0.05).
Adjusted for all shown measures
The results shown in Table 3 suggested that the period 2017–2018 (OR = 0.78, p = 0.019), age (OR = 0.95, p = 0.009), and breastfeeding (OR = 0.83, p = 0.022) were inversely associated with the prevalence of current asthma. In contrast, male sex, parental allergy history, and visible mold were positively associated with the prevalence of current asthma.
Table 3.
Association between baseline characteristics and the risk of current asthma.
| Predictor | Univariate |
Multivariablea |
||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Study period | ||||
| 2008–2009 | Reference | Reference | ||
| 2010–2012 | 0.97 (0.82–1.14) | 0.679 | 0.97 (0.82–1.15) | 0.730 |
| 2017–2018 | 0.83 (0.69–1.01) | 0.057 | 0.78 (0.64–0.96) | 0.019 |
| Age, per year | 0.94 (0.91–0.98) | 0.003 | 0.95 (0.91–0.99) | 0.009 |
| Male sex | 1.66 (1.45–1.91) | <0.001 | 1.68 (1.46–1.93) | <0.001 |
| Breastfeeding | 0.86 (0.74–0.99) | 0.037 | 0.83 (0.71–0.97) | 0.022 |
| Allergy history | ||||
| Paternal asthma | 3.87 (2.98–5.02) | <0.001 | 3.30 (2.51–4.33) | <0.001 |
| Paternal allergic rhinitis | 1.85 (1.61–2.12) | <0.001 | 1.64 (1.42–1.89) | <0.001 |
| Paternal atopic dermatitis | 2.31 (1.84–2.89) | <0.001 | 1.77 (1.40–2.24) | <0.001 |
| Maternal asthma | 4.25 (3.32–5.43) | <0.001 | 3.44 (2.65–4.45) | <0.001 |
| Maternal allergic rhinitis | 2.05 (1.78–2.36) | <0.001 | 1.72 (1.48–1.99) | <0.001 |
| Maternal atopic dermatitis | 2.03 (1.61–2.55) | <0.001 | 1.47 (1.15–1.87) | 0.002 |
| Indoor environment | ||||
| Pets | 1.01 (0.85–1.20) | 0.905 | 1.01 (0.84–1.20) | 0.943 |
| Visible mold | 1.40 (1.15–1.71) | 0.001 | 1.25 (1.02–1.54) | 0.032 |
| Cockroaches | 1.15 (0.99–1.33) | 0.060 | 1.09 (0.94–1.26) | 0.276 |
OR, odds ratio; CI, confidence interval.
Bold font indicates statistical significance (P-value < 0.05).
Adjusted for all shown measures
Table 4 shows that the period 2017–2018 (OR = 0.69; p < 0.001) and breastfeeding (OR = 0.79, p = 0.004) were inversely associated with the prevalence of exercise–induced wheeze. In contrast, age, male sex, and parental allergy history were positively associated with the prevalence of exercise-induced wheeze.
Table 4.
Association between baseline characteristics and the risk of exercise-induced wheeze.
| Predictor | Univariate |
Multivariablea |
||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Study period | ||||
| 2008–2009 | Reference | Reference | ||
| 2010–2012 | 1.05 (0.90–1.23) | 0.530 | 1.06 (0.90–1.24) | 0.487 |
| 2017–2018 | 0.63 (0.52–0.77) | <0.001 | 0.69 (0.56–0.85) | <0.001 |
| Age, per year | 1.13 (1.08–1.17) | <0.001 | 1.11 (1.07–1.16) | <0.001 |
| Male sex | 1.25 (1.09–1.43) | 0.001 | 1.25 (1.09–1.43) | 0.001 |
| Breastfeeding | 0.68 (0.59–0.80) | <0.001 | 0.79 (0.67–0.93) | 0.004 |
| Allergic history | ||||
| Paternal asthma | 2.21 (1.62–3.01) | <0.001 | 2.02 (1.47–2.78) | <0.001 |
| Paternal allergic rhinitis | 1.39 (1.20–1.60) | <0.001 | 1.32 (1.14–1.53) | <0.001 |
| Paternal atopic dermatitis | 1.94 (1.53–2.46) | <0.001 | 1.68 (1.31–2.15) | <0.001 |
| Maternal asthma | 3.12 (2.38–4.07) | <0.001 | 2.67 (2.02–3.53) | <0.001 |
| Maternal allergic rhinitis | 1.54 (1.33–1.78) | <0.001 | 1.40 (1.20–1.63) | <0.001 |
| Maternal atopic dermatitis | 1.88 (1.49–2.37) | <0.001 | 1.57 (1.23–2.00) | <0.001 |
| Indoor environment | ||||
| Pets | 1.18 (1.003–1.40) | 0.046 | 1.11 (0.94–1.31) | 0.229 |
| Visible mold | 1.25 (1.01–1.53) | 0.038 | 1.15 (0.93–1.42) | 0.194 |
| Cockroaches | 1.07 (0.93–1.23) | 0.372 | 1.02 (0.89–1.18) | 0.755 |
OR, odds ratio; CI, confidence interval.
Bold font indicates statistical significance (P-value < 0.05).
Adjusted for all shown measures
The prevalence of nocturnal cough significantly increased in the period 2010–2012 (OR = 1.3, p < 0.001) compared with that in the period 2008–2009; whereas it significantly decreased in 2017–2018 compared with that in 2008–2009 (OR = 0.77; p = 0.001) (Table 5). Older students have lower risk of nocturnal cough. In contrast, male sex, parental allergy history, the presence of pets, and visible mold in the household were significantly associated with the risk of nocturnal cough.
Table 5.
Association between baseline characteristics and the risk of nocturnal cough.
| Predictor | Univariate |
Multivariablea |
||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Study period | ||||
| 2008–2009 | Reference | Reference | ||
| 2010–2012 | 1.30 (1.16–1.47) | <0.001 | 1.30 (1.15–1.46) | <0.001 |
| 2017–2018 | 0.85 (0.74–0.98) | 0.026 | 0.77 (0.66–0.89) | 0.001 |
| Age, per year | 0.90 (0.87–0.92) | <0.001 | 0.89 (0.86–0.91) | <0.001 |
| Male sex | 1.22 (1.11–1.34) | <0.001 | 1.23 (1.12–1.36) | <0.001 |
| Breastfeeding | 0.91 (0.82–1.01) | 0.080 | 0.92 (0.82–1.02) | 0.123 |
| Allergic history | ||||
| Paternal asthma | 1.90 (1.48–2.42) | <0.001 | 1.72 (1.34–2.22) | <0.001 |
| Paternal allergic rhinitis | 1.52 (1.37–1.68) | <0.001 | 1.38 (1.24–1.53) | <0.001 |
| Paternal atopic dermatitis | 1.85 (1.55–2.22) | <0.001 | 1.53 (1.27–1.84) | <0.001 |
| Maternal asthma | 2.36 (1.89–2.96) | <0.001 | 1.94 (1.54–2.45) | <0.001 |
| Maternal allergic rhinitis | 1.78 (1.61–1.98) | <0.001 | 1.56 (1.40–1.74) | <0.001 |
| Maternal atopic dermatitis | 1.90 (1.60–2.26) | <0.001 | 1.52 (1.27–1.82) | <0.001 |
| Indoor environment | ||||
| Pets | 1.19 (1.05–1.34) | 0.005 | 1.21 (1.07–1.37) | 0.002 |
| Visible mold | 1.75 (1.53–2.01) | <0.001 | 1.59 (1.38–1.83) | <0.001 |
| Cockroaches | 1.17 (1.06–1.30) | 0.003 | 1.11 (0.99–1.23) | 0.062 |
OR, odds ratio; CI, confidence interval.
Bold font indicates statistical significance (P-value < 0.05).
Adjusted for all shown measures
The correlation of air pollutants and the prevalence of asthma and related respiratory symptoms
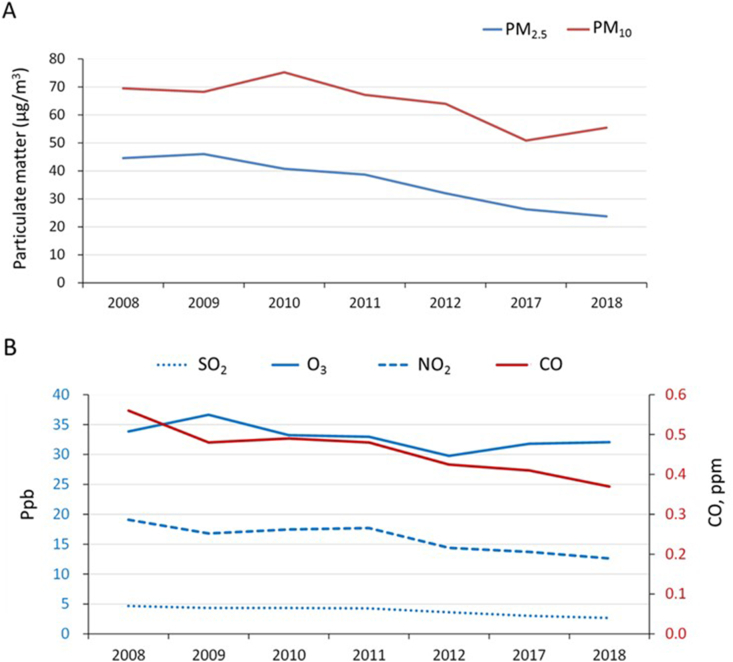
Decreasing trends of air particulate matter, namely, PM2.5 and PM10, and the other examined air pollutants, namely, SO2, O3, NO2, and CO, in the period of 2008–2018 were observed in this study (Fig. 2). We found significant correlations between PM10 and the prevalence of physician-diagnosed asthma (r = 0.840), current asthma (r = 0.771), and exercise-induced wheeze (r = 0.813) (Table 6). Moreover, the prevalence of exercise-induced wheeze was correlated with the levels of SO2, PM2.5, and PM10 (Table 6).
Fig. 2.
Air pollution data across the study years. Abbreviations: PM, particulate matter; SO2, sulfur dioxide; O3, ozone; NO2, nitrogen dioxide; CO, carbon monoxide
Table 6.
Correlations of different air pollution parameters with outcomes.
| Air pollution/outcomes | Physician-diagnosed asthma | Current asthma | Exercise-induced wheeze | Nocturnal cough |
|---|---|---|---|---|
| SO2, ppb | 0.682 | 0.595 | 0.815∗ | 0.492 |
| CO, ppm | 0.521 | 0.450 | 0.622 | 0.289 |
| O3, ppb | 0.103 | 0.161 | 0.287 | −0.146 |
| PM10, μg/m3 | 0.840∗ | 0.771∗ | 0.813∗ | 0.566 |
| PM2.5, μg/m3 | 0.648 | 0.591 | 0.763∗ | 0.387 |
| NO2, ppb | 0.558 | 0.493 | 0.725 | 0.405 |
∗ P-value <0.05
Discussion
In the present study, we found lowest prevalence of physician-diagnosed asthma and current asthma in the period 2017–2018; a leveling trend in the period 2010–2012, and a decline in the period 2017–2018 for the prevalence of related respiratory symptoms in school children in the southern part of Taiwan. The prevalence of breastfeeding rose significantly. The prevalence of pet keeping and concentration of PM10 increased slightly in the period 2010–2012 and decreased in period 2017–2018.
From the 1990s to the early 2000s, several epidemiological studies using ISAAC questionnaires evaluated the prevalence of childhood asthma among school-age children in Taiwan.11, 12, 13 The prevalence of asthma among children aged 7–15 years in Taipei city, Northern Taiwan, increased from 1.3% in 1974 to 5.1% in 1985.11 Subsequently, in the same city, Wu et al reported that the prevalence of asthma in children aged 6–7 years was 12.7% in 1994, 14.4% in 2002, and 13.0% in 2007.12 In a survey conducted among children aged 6–12 years in Tainan, the prevalence also increased from 6.46% in 1993 to 8.45% in 1997.13 The increase in asthma prevalence in Taipei city from 1974 to 1985 could not be explained by air pollution or exposure to new allergens. Other factors that may have influenced the prevalence of asthma indeed were needed to be explored.11 Wu et al found a significant increase in the prevalence of “wheeze ever” and “nocturnal cough in the past 12 months” on comparing 2007 with 1994 and 2002. But a significant decrease in the prevalence of “severe symptoms of wheeze in the past 12 months” was detected. There were no significant changes in the prevalence of current wheeze or physician-diagnosed asthma. The trends (increase in the prevalence of wheeze ever and nocturnal cough and decrease in symptom severity of wheeze) could be primarily explained by changes in diagnostic practice and increased awareness of asthma among physicians and parents as this would lead to a greater proportion of children with mild or transient wheeze ever being noticed. Wu et al believed that the reasons of steady trend in prevalence of asthma would be multifactorial and it needed further study to explore the real reason.12 Animal dander allergy is the likely factor that increased correspondingly with the prevalence of childhood asthma among primary school children in Tainan city in the previous study.13
Many risk factors might explain the epidemic rise of asthma among children worldwide from 1960 to 2000. The major explanation for the increase in asthma was the move of children indoors. The consequences of the indoor lifestyle included increased sensitization to indoor allergens, decreased physical activity, and the rise in obesity.14
In the United States, researchers investigated asthma prevalence rates during the period from 2001 to 2013 using National Health Interview Survey (NHIS) data and found that childhood asthma prevalence increased from 2001 to 2009 followed by a plateau then a decline in 2013. Although this analysis of NHIS data cannot answer the question of why trends change but does provide a comprehensive national picture and some insight into the complexity of asthma prevalence.4 The researchers suggested the trends of obesity that may have driven increasing prevalence of asthma may have also leveled off.15 In Lanzhou, China, the prevalence of asthma and that of asthma-related symptoms were lower in 2017 than in 1994–1995 in children of 6–12 years old from elementary schools. The worse indoor environmental conditions in households contributed to the higher prevalence of asthma and symptoms for the children in 1994–1995. Improvement of respiratory disease for the children was partly attributable to the protective effects of breastfeeding in 2017.16
In fact, documenting reductions in asthma prevalence is complicated, as parallel cohort studies with specific age windows are needed to establish patterns with the comparison group ideally from the same geographical region.2 These challenges might in part explain why previous studies from Australia and the United Kingdom have not consistently shown reductions in asthma prevalence and why temporal trends in European and Asian countries between the 1970s and mid-2000s have been conflicting.2
Our results suggested that breastfeeding might partially contribute to the observed decrease in the prevalence of current asthma. However, the influence of breastfeeding on the prevention of asthma and related respiratory symptoms remains controversial.10,17,18 According to the Tasmanian Longitudinal Health Study, in participants who were followed from childhood to middle age, the results showed that breastfeeding reduced the risk of childhood asthma and conversely increased the risk of adult asthma, but only in those participants with familial predisposition.19 A previous systematic review provided supportive evidence that breastfeeding might have a modest protective effect against asthma in late childhood-adolescence.17 The authors suggested that breastfeeding-related reductions in childhood wheezing might relate to the known beneficial immunological factors that could reduce childhood viral infections that predispose individuals to asthma.10,17
The roles of pet keeping on childhood asthma and respiration-symptoms also remained controversial.8,9,20,21 Luo et al found pet keeping early were positively associated with current wheezing and current dry cough; pet keeping currently were positively associated with current wheezing and diagnosed asthma.22 In this study, the presence of pets was a risk factor for nocturnal cough and might be responsible for the change of the prevalence of nocturnal cough. Due to the limitation of questionnaire, we did not have evidence to find there were any changes in the social status and lifestyle of the population in those study years.
Epidemiological studies have shown that outdoor pollution has effects on respiratory health, including an increased prevalence of asthma and allergic diseases.23 Exposure to PM2.5 and PM10 and nitrate was associated with an increased risk of asthma and persistent wheezing.24 Exposure to PM10 was associated with an increase in hospitalizations for asthma and, in asthmatic children, with the frequency of asthmatic symptoms (wheezing and cough).25 Garcia et al conducted a well-designed study in California and reported that decreases in ambient nitrogen dioxide and PM2.5 from 1993 to 2014 were significantly associated with lower asthma incidence among children.26 Recent decrease of asthma prevalence among children aged from 6 to 7 years old might be related to the decreasing tendency of air pollutant concentration in Fukuoka city, Japan.27 Similarly, the reduction in outdoor air pollution observed in this study might partially explain the observed decreasing trend. In addition, one of the traffic–related outdoor air pollutants, diesel particulate matter (DPM), has been linked to the negative impact on respiratory health. Douglas et al found that DPM was significantly associated with asthma emergency department visits in Los Angeles County. They suggested that reducing exposure to DPM might serve to improve asthma outcomes.28 In addition to air pollution, we also analyzed mean annual temperature and annual precipitation. The mean annual temperature increased slightly over study period whereas annual precipitation fluctuated during study period. We tried to find the association between the outcomes and mean annual temperature and annual precipitation by Pearson's correlation. However, there was no statistical significance (data not shown).
The main strengths of this study are the use of the standardized method in repeated surveys and the large sample size. Our study also has several limitations. First, data were not available during 2013–2016. We cannot determine the exact year responsible for the decreasing prevalence. Second, data were collected from 14 schools located in Tainan city. According to the National Health Insurance Research Database from the National Health Insurance Bureau, Taiwan, the yearly incidence of childhood asthma at the age of 6–12 years remained the same during 2008 and 2015 (data not shown). Therefore, the decreasing trend of childhood asthma prevalence found in Southern Taiwan may reflect changes in local environmental risk factors; for example, the southern part of Taiwan is more rural and agriculture based than the urban and industry-based areas in the other parts of Taiwan. Moreover, it was not able to classify the diseases severity using the questionnaire in the present study, so we are not able to find whether the clinical severity of asthma was also improved or getting worse in those study years. Other confounding risk factors, such as breastfeeding rates and conditions of living households, may also reflect the difference in prevalence rates of childhood asthma and related respiratory symptoms between the southern part and other parts of Taiwan. Therefore, an island-wide prospective survey of school children is needed to answer the question of whether the prevalence of primary school children is decreasing in Taiwan.
Conclusion
In summary, our data showed that the recent prevalence of asthma and related respiratory symptoms in Tainan city reached a peak in the period 2010–2012 and then declined in the 2017–2018 period. The increasing prevalence of breastfeeding, decreasing rates of pet keeping, and the improvement in air pollution might be associated with the reduced prevalence of asthma in school children. Further investigation is warranted to validate the findings in this study.
Author contributions
W.-Y.C and J.-Y.W conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted. W.-Y. C, C.-W.L, J.L, and P.-S. C conducted survey questionnaires and data collection and entry. W.-Y. C and H.-J. T performed statistical and data analyses. All authors have reviewed and revised the manuscript and approved the final manuscript as submitted.
Availability of data and materials
By request to corresponding author.
Ethics approval
This study was approved by National Cheng Kung University Human Research Ethics Committee (No. 107–364).
Declaration of competing interest
The authors have no competing interests to disclose.
Acknowledgments
The authors wish to thank the school teachers at the studied primary schools for their cooperation, the staff from the Tainan (Nan-Yin) Association of the Care of Children with Allergy and Asthma for their help, and the Public Health Bureau, Tainan City Government, Taiwan, for financial support. This study was, in part, supported by a research grant from the Chi-Mei Medical Center (CCFHR10902), and received funding in part from the Center of Allergy and Clinical Immunology Research (ACIR) and the Headquarters of University Advancement at the National Cheng Kung University, which is sponsored by the Ministry of Education in Taiwan.
Footnotes
Full list of author information is available at the end of the article.
References
- 1.Asher I., Pearce N. Global burden of asthma among children. Int J Tubercul Lung Dis. 2014;18(11):1269–1278. doi: 10.5588/ijtld.14.0170. [DOI] [PubMed] [Google Scholar]
- 2.Eder W., Ege M.J., von Mutius E. The asthma epidemic. N Engl J Med. 2006;355(21):2226–2235. doi: 10.1056/NEJMra054308. 23. [DOI] [PubMed] [Google Scholar]
- 3.Anandan C., Nurmatov U., van Schayck O.C., Sheikh A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy. 2010;65(2):152–167. doi: 10.1111/j.1398-9995.2009.02244.x. [DOI] [PubMed] [Google Scholar]
- 4.Akinbami L.J., Simon A.E., Rossen L.M. Changing trends in asthma prevalence among children. Pediatrics. 2016;137(1):1–7. doi: 10.1542/peds.2015-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim B.K., Kim J.Y., Kang M.K. Allergies are still on the rise? A 6-year nationwide population-based study in Korea. Allergol Int. 2016;65(2):186–191. doi: 10.1016/j.alit.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Beasley R., Semprini A., Mitchell E.A. Risk factors for asthma: is prevention possible? Lancet. 2015;386(9998):1075–1085. doi: 10.1016/S0140-6736(15)00156-7. [DOI] [PubMed] [Google Scholar]
- 7.Castro-Rodriguez J.A., Forno E., Rodriguez-Martinez C.E., Celedón J.C. Risk and protective factors for childhood asthma: what is the evidence? J Allergy Clin Immunol Pract. 2016;4(6):1111–1122. doi: 10.1016/j.jaip.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apelberg B.J., Aoki Y., Jaakkola J.J. Systematic review: exposure to pets and risk of asthma and asthma-like symptoms. J Allergy Clin Immunol. 2001;107(3):455–460. doi: 10.1067/mai.2001.113240. [DOI] [PubMed] [Google Scholar]
- 9.Lombardi E., Simoni M., La Grutta S. Effects of pet exposure in the first year of life on respiratory and allergic symptoms in 7-yr-old children. The SIDRIA-2 study. Pediatr Allergy Immunol. 2010;21(2 Pt 1):268–276. doi: 10.1111/j.1399-3038.2009.00910.x. [DOI] [PubMed] [Google Scholar]
- 10.Dogaru C.M., Nyffenegger D., Pescatore A.M., Spycher B.D., Kuehni C.E. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am J Epidemiol. 2014;179(10):1153–1167. doi: 10.1093/aje/kwu072. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh K.H., Shen J.J. Prevalence of childhood asthma in XXX, Taiwan, and other Asian Pacific countries. J Asthma. 1988;25(2):73–82. doi: 10.3109/02770908809071357. [DOI] [PubMed] [Google Scholar]
- 12.Wu W.F., Wan K.S., Wang S.J., Yang W., Liu W.L. Prevalence, severity, and time trends of allergic conditions in 6-to-7-year-old schoolchildren in XXX. J Investig Allergol Clin Immunol. 2011;21(7):556–562. [PubMed] [Google Scholar]
- 13.Tsuang H.C., Su H.J., Kao F.F., Shih H.C. Effects of changing risk factors on increasing asthma prevalence in southern Taiwan. Paediatr Perinat Epidemiol. 2003;17(1):3–9. doi: 10.1046/j.1365-3016.2003.00466.x. [DOI] [PubMed] [Google Scholar]
- 14.Platts-Mills T.A. The allergy epidemics: 1870-2010. J Allergy Clin Immunol. 2015;136(1):3–13. doi: 10.1016/j.jaci.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogden C.L., Carroll M.D., Kit B.K., Flegal K.M. Prevalence of childhood and adult obesity in the United States, 2011-2012. J Am Med Assoc. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao S., Wen D., Li S. Changes in children's asthma prevalence over two decades in Lanzhou: effects of socioeconomic, parental and household factors. J Thorac Dis. 2020;12(10):6365–6378. doi: 10.21037/jtd-19-crh-aq-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lodge C.J., Tan D.J., Lau M.X. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):38–53. doi: 10.1111/apa.13132. [DOI] [PubMed] [Google Scholar]
- 18.Brew B.K., Allen C.W., Toelle B.G., Marks G.B. Systematic review and meta-analysis investigating breast feeding and childhood wheezing illness. Paediatr Perinat Epidemiol. 2011;25(6):507–518. doi: 10.1111/j.1365-3016.2011.01233.x. [DOI] [PubMed] [Google Scholar]
- 19.Matheson M.C., Erbas B., Balasuriya A. Breast-feeding and atopic disease: a cohort study from childhood to middle age. J Allergy Clin Immunol. 2007;120(5):1051–1057. doi: 10.1016/j.jaci.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 20.Perzanowski M.S., Rönmark E., Platts-Mills T.A., Lundbäck B. Effect of cat and dog ownership on sensitization and development of asthma among preteenage children. Am J Respir Crit Care Med. 2002;166(5):696–702. doi: 10.1164/rccm.2201035. [DOI] [PubMed] [Google Scholar]
- 21.Ownby D.R., Johnson C.C., Peterson E.L. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. J Am Med Assoc. 2002;288(8):963–972. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 22.Luo S., Sun Y., Hou J. Pet keeping in childhood and asthma and allergy among children in Tianjin area, China. PloS One. 2018;13(5) doi: 10.1371/journal.pone.0197274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pawankar R., Wang J.Y., Wang I.J. Asia pacific association of allergy asthma and clinical immunology white paper 2020 on climate change, air pollution, and biodiversity in Asia-pacific and impact on allergic diseases. Asia Pac Allergy. 2020;10(1):e11. doi: 10.5415/apallergy.2020.10.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holst G.J., Pedersen C.B., Thygesen M. Air pollution and family related determinants of asthma onset and persistent wheezing in children: nationwide case-control study. BMJ. 2020;370:m2791. doi: 10.1136/bmj.m2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romeo E., De Sario M., Forastiere F. [PM 10 exposure and asthma exacerbations in pediatric age: a meta-analysis of panel and time-series studies] Epidemiol Prev. 2006;30(4-5):245–254. [PubMed] [Google Scholar]
- 26.Garcia E., Berhane K.T., Islam T. Association of changes in air quality with incident asthma in children in California, 1993-2014. J Am Med Assoc. 2019;321(19):1906–1915. doi: 10.1001/jama.2019.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odajima H., Kawano T., Wakatsuki M. Annual changes in the prevalence of asthma may be related to air pollution in Fukuoka: 29 years of observation. ERJ Open Res. 2020;6(2) doi: 10.1183/23120541.00166-2020. 00166-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douglas J.A., Archer R.S., Alexander S.E. Ecological determinants of respiratory health: examining associations between asthma emergency department visits, diesel particulate matter, and public parks and open space in Los Angeles, California. Prev Med Rep. 2019;14:100855. doi: 10.1016/j.pmedr.2019.100855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asher M.I., Keil U., Anderson H.R. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–489. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
By request to corresponding author.