Abstract
Objectives
Symptomatic local treatment of vaginal atrophy (VA) in menopausal women includes hormonal and nonhormonal preparations. Some women may be reluctant to use vaginal estradiol preparations because of the concern for developing breast cancer and endometrial hyperplasia. Therefore, it is necessary to compare the therapeutic effectiveness of alternative vaginal drugs, such as promestriene, an estrogen agonist, and sodium hyaluronate (NaH), a nonhormonal, water-based agent.
Methods
Ninety-one postmenopausal women diagnosed with symptomatic VA were divided into three groups and treated for 12 weeks; 30 women with vaginal estradiol (VE), 30 women with promestriene, and 31 women with NaH. Composite scoring, vaginal maturation index (VMI), pH, frequency of sexual activity, serum hormone levels, and endometrial thickness were evaluated VA before and after treatment.
Results
In the comparison of VA examination findings composite scoring, VMI, and vaginal pH values, three different drugs were found to be effective in the treatment (P < 0.05). The VMI following VE treatment was significantly higher than that after NaH treatment (P = 0.031), whereas the promestriene group had a more positive change than the others in terms of increase in after treatment composite scoring and sexual activity frequency (P = 0.031, P = 0.020). There were no differences between the groups in terms of pre and after treatment serum E2 levels and endometrial thickness.
Conclusions
Based on these findings, we can conclude that the use of promestriene or NaH can prove to be as effective and well tolerated as vaginal estradiol in the symptomatic treatment of vaginal atrophy.
Keywords: Estradiol, Menopause, Promestriene, Sodium hyaluronate, Vaginal atrophy
INTRODUCTION
Vulvovaginal atrophy (VVA) is a widely prevalent, progressive, and often untreated condition observed in postmenopausal women. The main symptoms of VVA include vaginal dryness, burning, itching, and bleeding which are included in the genitourinary syndrome of menopause (GSM) [1,2]. The new definition of GSM comprises genital symptoms of VVA mentioned before, sexual symptoms (dyspareunia and other sexual dysfunctions), and urinary symptoms (dysuria, frequency, urgency, recurrent urinary infections) [2]. These symptoms may be observed in > 50% of postmenopausal women [3]. In those studies which were conducted on a large number of post-menopausal patients for solving problems that stem from lack of estrogen and affect life quality negatively, it was found out that most of the patients did not know that these symptoms originated from the menopausal period and they felt embarrassed while talking about their complaints [4]. Moreover, only a few menopausal women consult a physician for symptoms of vaginal atrophy. Although the vasomotor symptoms typically improve over time, VVA is a chronic and progressive condition that does not resolve without treatment [5]. Estrogen-containing preparations are mainly used for the treatment of VVA [6,7]. Oral estrogen or the combination of estrogen and progesterone, which is used in hormone replacement therapy in menopausal women, may be useful for the treatment of urogenital atrophy with vasomotor symptoms.
The use of estrogen-containing oral preparations cannot be used in patients with breast cancer or those with a history of endometrial neoplasia because of the concern for continuing estrogenic stimulation of these sensitive tissues [8,9]. Systemic absorption of local vaginal estradiol (LVE) preparations used in the treatment of VVA is considered to be less than oral preparations [10]. However, it has been reported that the use of LVE cream may cause side effects such as breast tenderness, endometrial hyperplasia, and vaginal bleeding in some women [11]. Additionally, the effect of vaginal estrogen therapy in women with a history of thrombosis has not been studied yet. Therefore, the ideal estrogen-containing therapeutic agent for VVA should be absorbed minimally, reduce the symptoms with minimal side effects, and does not stimulate estrogen-sensitive tissues other than the vagina.
Nonhormonal strategies may be used in women of any age in which hormonal treatments are contraindicated. The prescription of vaginal moisturizers and lubricants and the maintenance of sexual activity may help improve VVA related symptoms. However, a few clinical trials have been performed to assess the efficacy of such products. Lubricants are short-acting substances (water-, silicone-, or oil-based) which are useful to reduce friction during sexual activity, whereas moisturizers are longer acting than lubricants and may exert a trophic effect [12].
Hyaluronic acid (HA) is a sulfur-free glycosaminoglycan (GAG), which is located in the extracellular matrix of all mammalian tissues. It provides proliferative differentiation of the vaginal epithelium, making vaginal cell attachment with each other more robust with desmosomes. HA also strengthens the collagen matrix and moisturizes the vagina by supporting the waterretaining ability of the vaginal mucosa [13].
This study aimed to compare the effectiveness of three drugs that could be applied to the vagina: estradiol vaginal tablets, promestriene, which is a less potent analog of estradiol with minimal absorption, and HA, a nonhormonal preparation used for the treatment of VVA. The effects of local hormonal and nonhormonal agents in the treatment of VVA has been studied in very few studies so far. Also, we did not find any other research comparing the effects of different types of estrogenic agents with a nonhormonal (HA) agent at the same time in VVA/GSM therapy. So we expect this study might contribute to the literature.
MATERIALS AND METHODS
This prospective and open-label study was carried out between January and August 2013 at Department of Obstetrics and Gynecology, Sakarya University School of Medicine, Sakarya, Turkey. Ethical approval was obtained from the Pharmaceutical and Medical Devices Agency of the Ministry of Health of Turkey (approval No. 988618). Written informed consent was obtained from all patients.
Study population
A total of 140 sexually active menopausal Caucasian women with VVA were enrolled in the study. The inclusion criteria were as follows: age > 40 years, menopause for ≥ 1 year, serum estradiol (E2) level < 20 pg/mL, follicle-stimulating hormone (FSH) level > 40 IU, endometrial thickness ≤ 5 mm at baseline transvaginal ultrasonographic examination. Exclusion criteria were as follows: identification of mass during breast examination, history of breast cancer, or pathologic findings in favor of malignancy in mammography, family history of breast cancer, history of endometrial hyperplasia. Women with a history of menopausal hormone therapy in the last 12 months and women who were using antihypertensive or anti-diabetic drugs or had a history of thromboembolism or previous myocardial infarction were also excluded. Also, women having any type of pelvic organ prolapse, cervical or pelvic mass, and those without vaginal intercourse, were also excluded from the study.
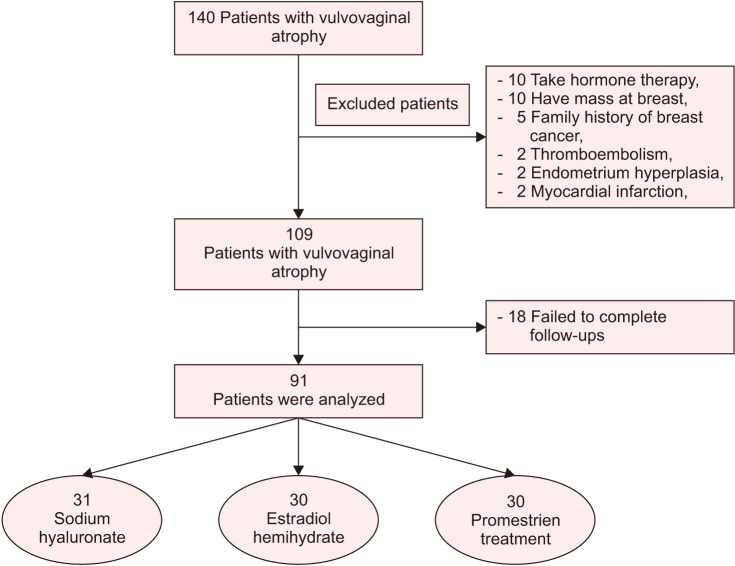
Forty-nine were women excluded from the study; 10 women had a mass at the breast examination, 5 women had a family history of breast cancer and 2 women were with endometrial hyperplasia, 10 women had a history of hormone therapy during the last 12 months, 2 women had a history of thromboembolism, 2 women had a history of myocardial infarction, and 18 women failed to complete the follow-ups. Thus, the study was completed with 91 patients (Fig. 1).
Fig. 1. Trial flow chart.
Patients were distributed to three different treatment groups for VVA. After giving women detailed counseling about the effects of drugs, the appointment to the drug groups was made according to their preferences. Thirty-one women were grouped as the sodium hyaluronate arm, having sodium hyaluronate (Cicatridina vaginal ovule, 2 g; Farma-Derma s.r.l., Bolognese, Italy) every day for the first 20 days, and then one ovule every other day. Thirty women were grouped as the estradiol treatment arm, having 1 estradiol hemihydrate vaginal tablet (Vagifem 10 µg vaginal tablet; Novo Nordisk, Plainsboro, NJ, USA) every day for 2 weeks, followed by 1 tablet twice a week. As the last group, 30 women were included in the promestriene treatment arm. They received one ovule (Colpotrophine vaginal soft capsule, 10 mg; Teva Pharmaceutical Industries Ltd., Cincinnati, OH, USA) every day for the first three weeks, and then one ovule every other day. The duration of treatments was determined to be 12 weeks. All data were reported in Figure 1.
At the end of the treatment, the groups were compared according to the efficacy of the drugs. Gynecological examinations of the women were performed by the same physician (G.I.) before, during, and after the treatment and the vaginal atrophy was graded by visual scoring; pale, thin, and dry changes in the vulvar and vaginal surfaces on examination shows VVA. In case of severe atrophy, petechial dots may be observed on the smooth and non-elastic vaginal mucosa without rugal folds. The grading of vaginal health indicated the degree of epithelial atrophy (0 = none, 1 = mild, 2 = moderate, 3 = severe) [13]. The composite scoring was performed by asking the patients about the severity of atrophy symptoms such as vaginal dryness, dyspareunia, and burning. Scores were calculated by the sum of the numbers which were given for each parameter; 0 = none, 1 = mild, 2 = moderate, and 3 = severe, as described previously [14,15]. The frequency (number/month) of women's sexual activities was noted before and after the treatment. To evaluate the vaginal maturation index (VMI), the vaginal swab was taken from the right side wall of the vagina with a smear brush. Vaginal swabs were evaluated by the same pathologist (M.Y.) without knowing whether the preparations were taken before or after the treatment. VMI was calculated according to the formula, maturation value: (0 × % of parabasal cells) × (0.5 × % of intermediate cells) × (1.0 × % of superficial cells) [16].
The pH was monitored from the vaginal side with the pH-indicator stent (Merck KGaA, Darmstadt, Germany) which was held over with the help of pens. Endometrial thickness was measured by transvaginal ultrasonography (TV USG). For ultrasonographic examination, a 6.5 MHz vaginal endoprobe (Voluson PRO 730; General Electrics Healthcare, Chicago, IL, USA) was used. Serum FSH and E2 levels were evaluated by Chemiluminescence Microparticle Immunoassay using the Architect i2000SR® kits (Abbott Diagnostics, Chicago, IL, USA). After the treatment; atrophy symptoms, VMI, endometrium thickness by TV USG, and serum FSH, E2 levels were re-evaluated. During the course of the treatment, the week in which the reduction in complaints was first noticed was recorded. Any side effects such as itching, discharge, burning, and abnormal uterine or vaginal bleeding were also recorded.
Statistical analysis
Power analysis was performed using G*Power (G*Power ver. 3.1.9.2; Franz Faul, Universitat, Kiel, Germany). The sample size was calculated as 28 for each group, assuming an effect size of 0.40, type I error of 0.05, and type II error of 0.10 (power = 0.90). However, we allocated a minimum of 30 subjects per group to cover the number of patients lost to follow-up. Continuous variables were assessed by the Kolmogorov–Smirnov normality test. To make the comparison between three groups one-way ANOVA was used for the variables with the normal distribution. Tukey's multiple comparison test was used in the post-hoc binary comparisons. Since there was a significant difference between the ages of groups, age-adjusted comparisons were evaluated by ANCOVA for normally distributed continuous variables. Kruskal–Wallis test was used for the variables without the normal distribution. Dunn test was used in post-hoc binary comparisons. For the comparison of the continuous variables between before treatment and after treatment measurements; a dependent two-sample t-test or Wilcoxon test was used. Continuous variables with normal distribution were shown with mean ± standard deviation, while continuous variables with no normal distribution were shown with median [interquartile range]. Categorical variables were analyzed by the chi-square test. Categorical variables were shown by number (n) and percentage (%) and P < 0.05 was considered statistically significant. Analyses were performed using commercial statistical software (IBM SPSS Statistics ver. 22.0; IBM, Armonk, NY, USA).
RESULTS
Mean age, menopausal age, and menopausal durations of women among groups were given in Table 1. Because of the age difference between groups, corrected P values for normally distributed continuous variables were calculated and represented in the last column of the Tables 2 and 3.
Table 1. Demographic features of groups.
| Variable | Sodium hyaluronate (n = 31) | Estradiol hemihydrate (n = 30) | Promestriene (n = 30) | P value |
|---|---|---|---|---|
| Age (y) | 52.45 ± 5.27a | 56.93 ± 7.78 | 52.77 ± 3.72a | 0.005* |
| Body mass index (kg/m2) | 29.03 ± 4.81 | 28.58 ± 3.56 | 30.15 ± 5.70 | 0.426 |
| Menopause age (y) | 46.65 ± 4.97 | 46.23 ± 5.35 | 46.30 ± 3.58 | 0.934 |
| Menopause duration (y) | 5.74 ± 3.61a | 10.70 ± 7.11 | 6.50 ± 5.64a | 0.002* |
Data are presented as mean ± standard deviation.
*P < 0.05.
aStatistically significant difference was found with the comparison of vaginal estradiol hemihydrate group.
Table 2. Comparison of symptom, examination, and cytologic findings of the groups before and after treatment.
| Sodium hyaluronate (n = 31) | Estradiol hemihydrate (n = 30) | Promestriene (n = 30) | P1 | P3 | |
|---|---|---|---|---|---|
| Composite score values | |||||
| Before | 5.03 ± 2.82 | 4.77 ± 2.92 | 4.47 ± 3.00 | 0.754 | 0.604 |
| After | 1.93 ± 2.00 | 1.90 ± 1.63 | 1.17 ± 1.37a | 0.143 | 0.031* |
| Pre-post treatment difference | 3.10 ± 2.01 | 2.87 ± 1.81 | 3.30 ± 2.35 | 0.720 | 0.874 |
| P2 | < 0.001* | < 0.001* | < 0.001* | ||
| Vaginal atrophy appearance | |||||
| Before | 29 (93.5) | 29 (96.7) | 29 (96.7) | 0.789 | - |
| After | 7 (22.6) | 9 (30.0) | 3 (10.0) | 0.156 | - |
| P2 | < 0.001* | < 0.001* | < 0.001* | ||
| Vaginal pH value | |||||
| Before | 6 [5.5–6.0] | 6 [5.5–6.0] | 5.75 [5.5–6.0] | 0.215 | - |
| After | 5.5 [5.0–6.0] | 5 [5.0–5.5] | 5 [5.0–5.5] | 0.423 | - |
| Pre-post treatment difference | 0.61 ± 0.53 | 0.68 ± 0.51 | 0.52 ± 0.55 | 0.484 | 0.682 |
| P2 | < 0.001* | < 0.001* | < 0.001* | ||
| Vaginal maturation index (VMI) | |||||
| Before | 55 [12.5–90] | 52.5 [8.7–82.1] | 60 [12.5–95] | 0.636 | - |
| After | 90 [25–95] | 95 [60–95] | 88.75 [31.8–95] | 0.399 | - |
| Pre-post treatment difference | 0 [0–25]a | 7 [4.38–41.25] | 6.25 [0–30] | 0.031* | - |
| P2 | 0.022* | < 0.001* | 0.003* | ||
| Superficial cell counts by groups | |||||
| Before | 30 [5–80] | 20 [0–73.75] | 30 [5–80] | 0.747 | - |
| After | 80 [10–90] | 90 [28.75–90] | 80 [8.75–90] | 0.418 | - |
| Pre-post treatment difference | 0 [0–30] | 10 [3–70] | 5 [0–35] | 0.064 | - |
| P2 | 0.009* | < 0.001* | 0.008* | ||
| Sexual activity (number/month) | |||||
| Before | 4 [2–4] | 2 [1–4] | 3.5 [2–4] | 0.384 | - |
| After | 4 [2–4] | 2 [1–4] | 4 [2–5.25] | 0.310 | - |
| Pre-post treatment difference | 0 [0–1] | 0 [0–0.25] | 0 [0–1] | 0.831 | - |
| P2 | 0.077 | 0.158 | 0.020* | ||
| The week when the treatment effect is felt | 3 [2–3] | 4 [2.75–4] | 3 [2–3] | 0.229 | - |
Data are presented as mean ± standard deviation, number (%), or median [interquartile range].
*P < 0.05.
aSignificantly different from the vaginal estradiol group. No significant difference was found in other binary comparisons.
P1: Comparison result among groups.
P2: Comparison result between pre-treatment and post-treatment in a group.
P3: Age-adjusted comparison result among groups.
Table 3. Comparison of serum follicle-stimulating hormone (FSH), E2 levels, and endometrium thickness before and after treatment in all groups.
| Sodium hyaluronate (n = 31) | Estradiol hemihydrate (n = 30) | Promestriene (n = 30) | P1 | P3 | |
|---|---|---|---|---|---|
| Serum FSH values (IU/mL) | |||||
| Before | 49.5 [36–70] | 56.9 [44.7–89.2] | 52.8 [41.2–66.4] | 0.416 | - |
| After | 53.6 [35–71.2] | 56.8 [43.5–82.5] | 54 [39.6–66.8] | 0.586 | - |
| Pre-post treatment difference | –1.00 ± 8.09 | 2.92 ± 9.33 | 2.32 ± 7.94 | 0.155 | 0.289 |
| P2 | 0.845 | 0.024* | 0.157 | ||
| Serum E2 values (pg/mL) | |||||
| Before | 11 [8–17] | 10.5 [8–16] | 12.5 [9.5–16.5] | 0.391 | - |
| After | 8 [8–15] | 8 [8–14.25] | 12 [8–15.25] | 0.218 | - |
| Pre-post treatment difference | 1.13 ± 6.90 | 0.67 ± 10.22 | 3.00 ± 14.88 | 0.691 | 0.666 |
| P2 | 0.228 | 0.585 | 0.074 | ||
| Endometrium thickness (mm) | |||||
| Before | 2.34 ± 1.47 | 2.19 ± 1.12 | 2.3 0± 1.55 | 0.905 | 0.915 |
| After | 2.24 ± 1.30 | 2.32±1.20 | 2.48 ± 1.81 | 0.807 | 0.878 |
| Pre-post treatment difference | 0 [–0.2 to 0.1] | 0 [0 to 0.2] | 0 [–0.03 to 0.1] | 0.489 | - |
| P2 | 0.384 | 0.112 | 0.203 |
Data are presented as median [interquartile range] or mean ± standard deviation.
*P < 0.05.
P1: Comparison result among groups.
P2: Comparison result between pre-treatment and post-treatment in a group.
P3: Age-adjusted comparison result among groups.
When composite scores that account for the constellation of symptoms such as vaginal dryness, vaginal pain, dyspareunia, and burning were evaluated and compared within all groups after treatment, the scores decreased significantly in all groups and VVA symptoms were improved (P < 0.001; Table 2). When the P values were adjusted according to the age difference, it was noted that the composite score of the group using promestriene has the highest reduction after treatment. Therefore it appears that promestriene is more effective in reducing VVA symptoms.
Before and after the treatment, the severity of the appearance of vaginal atrophy and vaginal pH was also assessed. The severity of vaginal atrophy and vaginal pH values were decreased in all groups after treatment (P < 0.001; Table 2).
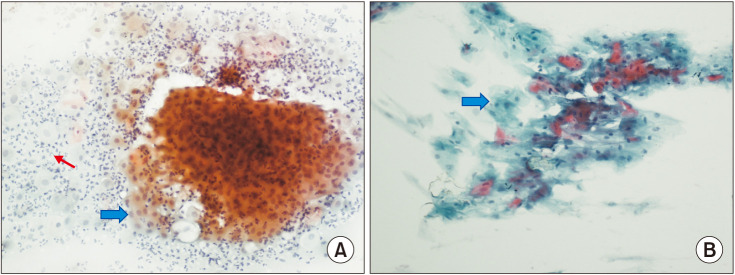
The VMI was assessed in vaginal swabs taken before and after treatment. The increased VMI values were noticed in all groups. Among the groups, the efficacy of VMI was significantly higher in the estradiol group than in the HA group (P = 0.031). No significant difference was found in other binary comparisons (Table 2). It was observed that vaginal superficial cells which is one of the parameters of VMI, increased significantly in all groups after treatment (Table 2, Fig. 2).
Fig. 2. Vaginal smear pictures showing changes in vaginal cells before and after treatment. (A) Atrophic vaginitis findings are observed in the before treatment smear material of the patient. Parabasal cells with the blue arrow, polymorphonuclear leukocytes with the red arrow (smear picture ×200). (B) A significant increase in the number of superficial cells (marked with a blue arrow) is seen in the patient's after treatment smear material (smear picture, ×200).
The frequency of monthly sexual activity was recorded before and after treatment (Table 2). There was a statistically significant increase in the number of sexual activities in the promestriene group after the treatment (P = 0.020). However, the changes in the other groups were not significant.
During the treatment period, the patients were asked about when their complaints started to decrease and the effect of the treatment was first noticed. There was no significant difference between the groups in terms of the week when the effect of treatment started. Symptoms such as vaginal dryness and dyspareunia during coital activity diminished or completely reversed after 3 weeks of treatment in vaginal HA and promestriene groups, and 4 weeks after in vaginal estradiol group.
Serum FSH and E2 levels and endometrium thickness of the women were evaluated before and after treatment (Table 3). There was no statistically significant difference between the groups in terms of serum FSH, E2 levels, and endometrium thickness before the treatment. At the end of the treatment, the serum E2 level and endometrium thickness were not altered significantly in all three groups. However, in the vaginal estradiol group, serum FSH level was found to be significantly decreased compared to other groups at the end of the treatment.
When the side effects originating from the treatment are asked, there were no side effects including itching, discharge, burning in all participants among three groups. No abnormal uterine bleeding was noted in any of the patients during the treatment.
DISCUSSION
Low-dose vaginal estradiol, low-potency-estrogen agonist promestriene, and nonhormonal vaginal HA applications were found to be similarly effective in the treatment of menopausal vaginal atrophy. The vaginal dryness symptoms of women regressed within 3–4 weeks following the administration of the drugs, and the proportion of superficial cells increased significantly in vaginal smears taken 3 months later in all groups.
Estrogen receptors play a role in the supportive mechanism of the pelvis by controlling the synthesis and breakdown of collagen. Therefore, vaginal estrogen application forms the basis of the treatment for vaginal atrophy [17,18,19]. Although the use of vaginal estrogen forms the basis of treatment, it is thought that vaginal estradiol may be risky in patients with a history of hormone-dependent cancers or undiagnosed vaginal bleeding or breast mass [8,9,20]. Therefore, alternative treatment methods have begun to be investigated. The treatment of VVA in women whom estradiol administration may pose a threat, the administration of a nonhormonal, water-based agent such as HA, or an estrogenic agent that exerts its estrogenic effects on vaginal mucosa with minimal systemic absorption property such as promestriene seem to be the plausible treatment options [21,22].
In this prospective open-label study, we investigated the efficacy of three different drugs used in the treatment of vaginal atrophy that could be alternative to each other; sodium hyaluronate, estradiol hemihydrate, and promestriene. All three drugs were found to be equally effective in reducing vaginal atrophy symptoms and lowering vaginal pH. In the comparison made in terms of VMI values, it was found that VMI increased at a statistically significant level in all three groups. However, it was found that the VMI increase in the vaginal estradiol group was statistically significantly higher than in the HA group (Table 2). Although we have observed similar effectiveness between these three agents in the treatment of vaginal atrophy, it will be more appropriate to administer according to the patient's need, if possible. This is important as the absorption of vaginal preparations and the residence time of the tissue varies from person to person. For example in a group of women with VVA and an increased risk of endometrial cancer, it may be more appropriate to start treatment with an estrogen-free preparation to reduce anxiety for the treatment of the patient. In this context, preparations containing primarily sodium hyaluronate followed by promestriene may be preferred. As a matter of fact, in the 2013 the North American Menopause Society (NAMS) guideline about the management of symptomatic VVA, it is recommended that first-line treatments should be performed with nonhormonal vaginal lubricants and moisturizers in such risky groups of patients [5]. The NAMS also stated that for symptomatic patients who do not respond to these initial preparations, low dose vaginal estrogens may be an option. This is why we preferred to investigate the effects of promestriene which is a less potent analog of estradiol with minimal systemic absorption property. Promestriene is a well-tolerated, effective vaginal antiatrophic agent in studies evaluating the efficacy and safety of promestriene use [22,23,24].
Del Pup et al. [24] stated that vaginal promestriene is less absorbable than estradiol hemihydrate and to pass into systemic circulation less. Serum estrone sulfate levels were increased in women using vaginal estradiol hemihydrate, whereas no significant change was seen in promestriene users. Del Pub [22] reported that promestriene could be used as the first choice especially in estrogen-associated cancer patients because of minimal absorption and lower systemic hormonal side effects. In our study, we did not find any difference between the groups using vaginal promestriene and vaginal estradiol and HA in terms of serum estradiol levels and endometrial thickness measurements before and after drug use (Table 3). Despite similar effectiveness with the other two drugs in the reversal of VVA symptoms, it was observed that there was a statistically significant increase in sexual frequency activity in the promestrien group (Table 2).
When we compared our patients in terms of vaginal atrophy symptoms, there was no difference in basal and post-therapy pelvic examination findings among the three groups. Pale, smooth, and non-elastic vaginal mucosa without rugal folds were reversed very well in all three groups. The microscopic examination findings studied in terms of evaluating the effectiveness of the drugs applied in the treatment of VVA were also compatible with the clinical findings. In the maturation index, which was examined to show the change in the rate of epithelial cells in the vaginal swab, it was found that all three drugs caused a statistically significant increase in superficial cells. However, the increase in the ratio of superficial cells and therefore VMI was highest in the vaginal estradiol group (Table 2). Nevertheless, the significant increase found in the VMI value of the HA group confirmed a positive effect of HA at the cellular level in the treatment of VA. Sodium hyaluronate is an extracellular matrix component that holds water by promoting collagen synthesis with other GAGs and this plays a fundamental role in tissue repair [25]. Based on our findings, it seems that HA may have proliferative and water retaining (moisturizer) effects on vaginal mucosal cells. Estradiol hemihydrate may be more effective in promoting the proliferation of healthy vaginal epithelial tissue and reduce the symptoms of atrophy. Due to different mechanisms of action in vaginal mucosal cells, the use of sodium hyaluronate in combination with estradiol hemihydrate on consecutive days may be considered in patients with insufficient response to low dose estrogen preparations.
The average age of women in the estradiol hemihydrate group, which we included in the study, is higher than the HA and promestriene groups. This finding may pose a bias in the evaluation of our study data. To eliminate the negative effect of the age difference between the study groups on the evaluation of the obtained data, the age-adjusted P values (P3) were calculated for the variables with normal distribution and were added to the last column of tables. There was no significant difference found in parameters that were used to evaluate the efficacy of VVA treatment (Table 2). The effects of drugs on serum hormone values and endometrial thickness were also not significant based upon age-adjusted P values (Table 3). Based on these results, we can say that the age difference between the study groups did not alter the conclusion of the data obtained.
Another point to be emphasized is that the dose of estradiol hemihydrate preparation used in our study is 10 µg. A lower dose of the drug; i.e., 10 µg is effective in the treatment of VVA [26]. The NAMS recommended the selection of the lowest possible estrogen preparation in the local treatment of atrophic vaginitis [5]. We did not see any unwanted effects neither on endometrial thickness nor levels of serum hormones (E2 and FSH) with 10 µg estradiol hemihydrate preparation. No drug-related side effects were reported in any of the women included in the study.
When the VVA parameters (composite score, VMI, pH) were evaluated after treatment, we observed that the efficacy of low potency estrogen promestriene in local treatment was comparable to the vaginal estradiol hemihydrate of 10 µg. Based on the effects on the endometrial thickness and serum E2 levels, it can be further concluded that promestriene is as safe as 10 µg vaginal estradiol tablets for the endometrium.
One of the possible limitations of the study was that the evaluation of the effects of the drugs ended in the 12th week. The basic information that guides us in this regard is based on the product use recommendations of the manufacturers and the NAMS 2013 position statement.
According to the NAMS 2013 position statement, women may need only a short course (1–3 months) of therapy to become symptom-free with first-line therapies which include nonhormonal, long-acting vaginal moisturizers and low-dose vaginal estrogen [5]. Simon et al. [27] also reported that in patients who underwent low-dose estrogen therapy, symptomatic relief developed in most of the cases within a short time after the start of treatment, in some stubborn cases the desired result could be achieved after the 12th week.
In Del Pup et al.'s promestriene review [24], the duration of drug administration in different studies varies between 1 month and 3 months. For the application of promestriene, it is recommended to apply once a day for the first 20–30 days, then every other day. For vaginal HA, it is recommended to apply one ovule a day for the first 20–30 days, and then one every other day [21]. The basic logic here is the information that the absorption of the drug from the mucosa will slow down after vaginal mucosal cornification developing within the first 2 weeks. In light of the above information, we think that 12 weeks of drug administration will be sufficient time to evaluate the symptomatic relief of the patients. The fact that symptomatic relief started in the groups in which we applied medication within 3–4 weeks supports this idea.
In conclusion, for the treatment of atrophic vaginitis, in addition to the local estradiol hemihydrate, the use of nonhormonal, water-based vaginal preparations such as sodium hyaluronate or an estrogen agonist with very low or negligible systemic absorption and effective local estrogenic action such as promestriene will provide additional the therapeutic interventions.
ACKNOWLEDGMENTS
This research was supported by the Sakarya University Scientific Research Project Commission (No. 2013-80-02-005).
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Nappi RE, Kokot-Kierepa M. Women's voices in the menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67:233–238. doi: 10.1016/j.maturitas.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Portman DJ, Gass ML Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. J Sex Med. 2014;11:2865–2872. doi: 10.1111/jsm.12686. [DOI] [PubMed] [Google Scholar]
- 3.Chin SN, Trinkaus M, Simmons C, Flynn C, Dranitsaris G, Bolivar R, et al. Prevalence and severity of urogenital symptoms in postmenopausal women receiving endocrine therapy for breast cancer. Clin Breast Cancer. 2009;9:108–117. doi: 10.3816/CBC.2009.n.020. [DOI] [PubMed] [Google Scholar]
- 4.Faubion SS, Sood R, Kapoor E. Genitourinary syndrome of menopause: management strategies for the clinician. Mayo Clin Proc. 2017;92:1842–1849. doi: 10.1016/j.mayocp.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20:888–902. doi: 10.1097/GME.0b013e3182a122c2. quiz 903-4. [DOI] [PubMed] [Google Scholar]
- 6.Kokot-Kierepa M, Bartuzi A, Kulik-Rechberger B, Rechberger T. [Local estrogen therapy--clinical implications--2012 update] Ginekol Pol. 2012;83:772–777. Polish. [PubMed] [Google Scholar]
- 7.Simunić V, Banović I, Ciglar S, Jeren L, Pavicić Baldani D, et al. Local estrogen treatment in patients with urogenital symptoms. Int J Gynaecol Obstet. 2003;82:187–197. doi: 10.1016/s0020-7292(03)00200-5. [DOI] [PubMed] [Google Scholar]
- 8.Crandall C, Petersen L, Ganz PA, Greendale GA. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519–530. doi: 10.1097/01.gme.0000117061.40493.ab. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SM, Partridge AH. Premature menopause in young breast cancer: effects on quality of life and treatment interventions. J Thorac Dis. 2013;5 Suppl 1:S55–S61. doi: 10.3978/j.issn.2072-1439.2013.06.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016;2016:CD001500. doi: 10.1002/14651858.CD001500.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rioux JE, Devlin MC, Gelfand MM, Steinberg WM, Hepburn DS. 17β-estradiol vaginal tablet versus conjugated equine estrogen vaginal cream to relieve menopausal atrophic vaginitis. Menopause. 2018;25:1208–1213. doi: 10.1097/GME.0000000000001220. [DOI] [PubMed] [Google Scholar]
- 12.Mehta A, Bachmann G. Vulvovaginal complaints. Clin Obstet Gynecol. 2008;51:549–555. doi: 10.1097/GRF.0b013e3181809a26. [DOI] [PubMed] [Google Scholar]
- 13.Bachmann GA, Nevadunsky NS. Diagnosis and treatment of atrophic vaginitis. Am Fam Physician. 2000;61:3090–3096. [PubMed] [Google Scholar]
- 14.Castelo-Branco C, Cancelo MJ, Villero J, Nohales F, Juliá MD. Management of post-menopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52 Suppl 1:S46–S52. doi: 10.1016/j.maturitas.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Bachmann G, Lobo RA, Gut R, Nachtigall L, Notelovitz M. Efficacy of low-dose estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Obstet Gynecol. 2008;111:67–76. doi: 10.1097/01.AOG.0000296714.12226.0f. [DOI] [PubMed] [Google Scholar]
- 16.Meisels A. The maturation value. Acta Cytol. 1967;11:249. [PubMed] [Google Scholar]
- 17.Jaisamrarn U, Triratanachat S, Chaikittisilpa S, Grob P, Prasauskas V, Taechakraichana N. Ultra-low-dose estriol and lactobacilli in the local treatment of postmenopausal vaginal atrophy. Climacteric. 2013;16:347–355. doi: 10.3109/13697137.2013.769097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iosif CS, Batra S, Ek A, Astedt B. Estrogen receptors in the human female lower uninary tract. Am J Obstet Gynecol. 1981;141:817–820. doi: 10.1016/0002-9378(81)90710-9. [DOI] [PubMed] [Google Scholar]
- 19.Chung da J, Bai SW. Roles of sex steroid receptors and cell cycle regulation in pathogenesis of pelvic organ prolapse. Curr Opin Obstet Gynecol. 2006;18:551–554. doi: 10.1097/01.gco.0000242959.63362.1e. [DOI] [PubMed] [Google Scholar]
- 20.Lee YK, Chung HH, Kim JW, Park NH, Song YS, Kang SB. Vaginal pH-balanced gel for the control of atrophic vaginitis among breast cancer survivors: a randomized controlled trial. Obstet Gynecol. 2011;117:922–927. doi: 10.1097/AOG.0b013e3182118790. [DOI] [PubMed] [Google Scholar]
- 21.Ekin M, Yaşar L, Savan K, Temur M, Uhri M, Gencer I, et al. The comparison of hyaluronic acid vaginal tablets with estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Arch Gynecol Obstet. 2011;283:539–543. doi: 10.1007/s00404-010-1382-8. [DOI] [PubMed] [Google Scholar]
- 22.Del Pup L. Management of vaginal dryness and dyspareunia in estrogen sensitive cancer patients. Gynecol Endocrinol. 2012;28:740–745. doi: 10.3109/09513590.2011.652717. [DOI] [PubMed] [Google Scholar]
- 23.Sun AJ, Lin SQ, Jing LH, Wang ZY, Ye JL, Zhang Y. [Safety of promestriene capsule used in postmenopausal atrophic vaginitis] Zhonghua Fu Chan Ke Za Zhi. 2009;44:593–596. Chinese. [PubMed] [Google Scholar]
- 24.Del Pup L, Postruznik D, Corona G. Effect of one-month treatment with vaginal promestriene on serum estrone sulfate levels in cancer patients: a pilot study. Maturitas. 2012;72:93–94. doi: 10.1016/j.maturitas.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Grimaldi EF, Restaino S, Inglese S, Foltran L, Sorz A, Di Lorenzo G, et al. Role of high molecular weight hyaluronic acid in postmenopausal vaginal discomfort. Minerva Ginecol. 2012;64:321–329. [PubMed] [Google Scholar]
- 26.Naumova I, Castelo-Branco C. Current treatment options for postmenopausal vaginal atrophy. Int J Womens Health. 2018;10:387–395. doi: 10.2147/IJWH.S158913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon J, Nachtigall L, Ulrich LG, Eugster-Hausmann M, Gut R. Endometrial safety of ultra-low-dose estradiol vaginal tablets. Obstet Gynecol. 2010;116:876–883. doi: 10.1097/AOG.0b013e3181f386bb. [DOI] [PubMed] [Google Scholar]