Abstract
Inflammation is an a physiological response instead an essential response of the organism to injury and its adequate resolution is essential to restore homeostasis. However, defective resolution can be the precursor of severe forms of chronic inflammation and fibrosis. Nowadays, it is known that an excessive inflammatory response underlies the most prevalent human pathologies worldwide. Therefore, great biomedical research efforts have been driven toward discovering new strategies to promote the resolution of inflammation with fewer side-effects and more specificity than the available anti-inflammatory treatments. In this line, the use of endogenous specialized pro-resolving mediators (SPMs) has gained a prominent interest. Among the different SPMs described, lipoxins stand out as one of the most studied and their deficiency has been widely associated with a wide range of pathologies. In this review, we examined the current knowledge on the therapeutic potential of lipoxins to treat diseases characterized by a severe inflammatory background affecting main physiological systems, paying special attention to the signaling pathways involved. Altogether, we provide an updated overview of the evidence suggesting that increasing endogenously generated lipoxins may emerge as a new therapeutic approach to prevent and treat many of the most prevalent diseases underpinned by an increased inflammatory response.
Keywords: lipoxin, inflammation, oxidative stress, lipid mediators, pathology
Introduction
Inflammation can be defined as the physiological response initiated by cells and tissues that aims to protect the organism against infections or injuries caused by exogenous or endogenous agents (1). Interestingly, it also plays an important role in processes like ovulation (2) or physiological interactions with microbiota (3). Acute inflammation entails two stages: an initial phase that comprises the onset of the inflammatory reaction to eliminate the danger signal, and a subsequent resolution phase wherein inflammation is blunted to restore homeostasis ( Figure 1 ). Different immune cell populations (i.e., macrophages, neutrophils, lymphocytes) and related mediators (cytokines, eicosanoids, immunoglobulins, among other) establish an orchestrated system that mediates these two phases as well as the transition between them (1, 4).
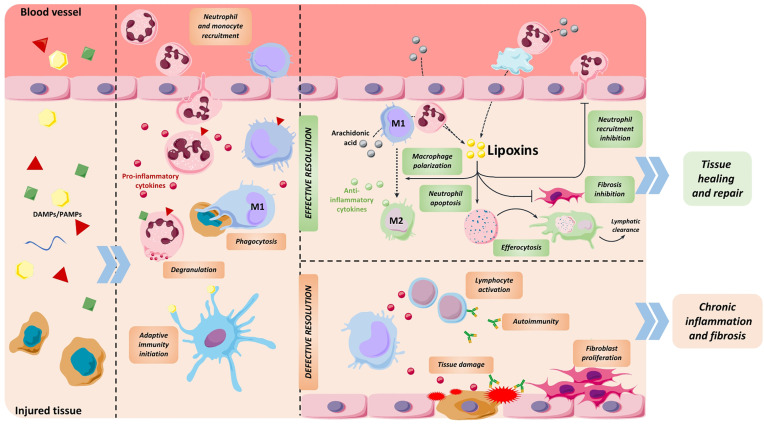
Figure 1.
Stages of inflammatory process. Release of pathogen and damage-associated molecular patterns (PAMPs and DAMPs) from injured tissue initiates inflammation by promoting the recruitment of neutrophils followed by monocytes. These immune cells blunt the source of damage by exerting different functions like degranulation to release toxic substances, phagocytosis, release of pro-inflammatory cytokines and initiating antigen recognition. At the end of this phase, immune cells shift to a resolutive phenotype, generating LXs and other specialized pro-resolving mediators that support the end of the inflammatory response. During resolution, these compounds prevent neutrophil recruitment and induce their apoptosis, polarize macrophages towards an anti-inflammatory phenotype, promote efferocytosis and inhibit fibroblast proliferation, among other functions. As a result, tissue is appropriately repaired. If the resolution phase is defective or insufficient, pro-inflammatory stimuli persist and end up damaging the tissue, causing chronic inflammation and subsequent fibrosis and organ dysfunction.
Within the molecules that actively participate in the resolution phase, special attention has been paid to specialized pro-resolving mediators (SPMs) (4), which promote resolution of inflammation by reducing the levels of pro-inflammatory cytokines and reactive oxygen species (ROS) and by modulating correct tissue repair (5–9), among other functions. So far, four types of SPMs have been classified according to the precursor molecule they originate from and the enzyme implicated in their metabolism ( Table 1 ). These include lipoxins (LXs), which derivate from omega 6 arachidonic acid and are the subject of this review, and other SPMs such as resolvins, maresins and protectins, that are structurally distinct and result from a different biosynthetic pathway derived from omega 3 fatty acids. Despite having similar pro-resolving actions, they can exert their function through different receptors driving to alternative signaling pathways [for review (10)].
Table 1.
Classification of principal specialized pro-resolving lipid mediators.
| SPM | Main enzymes | Main precursor molecule | References |
|---|---|---|---|
| Lipoxins | 5-, 12- and 15-lipoxygenases | Arachidonic Acid (AA) | (1) |
| Aspirin-Triggered Lipoxins | Acetylated COX-2 Cytochrome P450 enzymes |
||
| D-Series Resolvins | Acetylated COX-2 5- and 15-lipooxygenases |
Docosahexaenoic acid (DHA) | (2) |
| E-Series Resolvins | Acetylated COX-2 Cytochrome P450 enzymes 5- and 15-lipoxygenases |
Eicosapentaenoic Acid (EPA) | (2, 3) |
| Protectins | Acetylated COX-2 15-lipooxygenase |
Docosahexaenoic acid (DHA) | (4) |
| Maresins | 12-lipoxygenase | (5) |
LXs were the first SPM to be described (11) and the most studied to date since their role has been found to be essential in various inflammatory diseases (5, 12). Generation of native LXA4 and LXB4 results from the sequential lipoxygenation of the arachidonic acid present in the lipid membrane by the action of 5, 12 and/or 15-lipoxygenases (13) ( Figure 2 ). An alternative biosynthetic route requiring aspirin-mediated acetylation or statin-induced nitrosylation of COX-2 generates LX 15-R-epimers called 15-epi-lipoxins or aspirin-triggered lipoxins (ATLs) (14, 15). Both pathways of LX biosynthesis are driven by the coordinate interaction of distinct cell types such as neutrophils, eosinophils, macrophages, endothelial cells, epithelial cells, parenchymal cells or platelets in a process known as transcellular biosynthesis, which also occurs in the synthesis of pro-inflammatory eicosanoids (16). Transcellular biosynthesis allows cells to rapidly switch the production of pro-inflammatory mediators to anti-inflammatory based on the distinct cell types present in their environment, thus adapting eicosanoid synthesis to inflammatory or resolutive contexts (17).
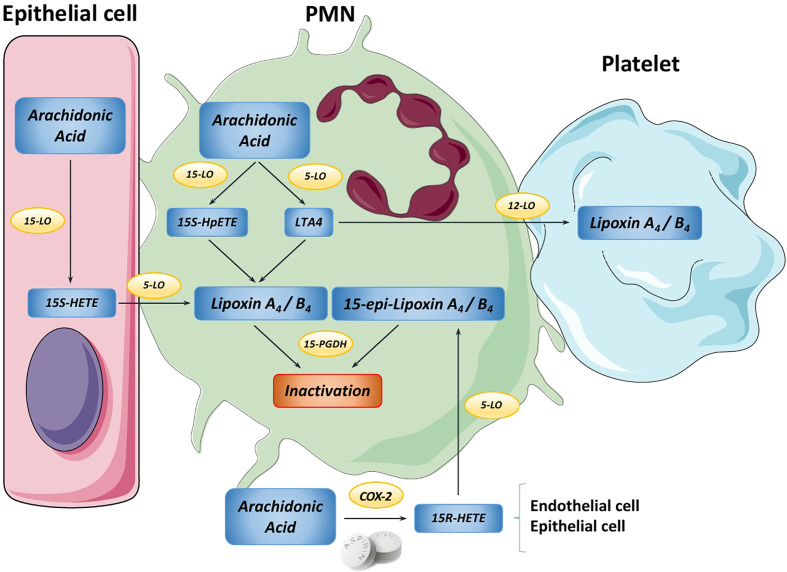
Figure 2.
Overview of the main lipoxin biosynthetic pathways. In the PMN cell, Arachidonic Acid (AA) can be converted into either 15S-HpETE or Leukotriene A4 (LTA4) by 15-LO and 5-LO, respectively. Then, these metabolites are transformed into native lipoxin A4 or B4. Moreover, platelets can use LTA4 from PMNs to produce LXA4/B4 in a process known as transcellular biosynthesis. Likewise, in epithelial cells AA is transformed by 15-LO into 15-HETE, which is transferred to PMNs to produce LXA4/B4. Endothelial cells can use AA to yield the intermediate 15R-HETE by COX-2, step that is promoted by aspirin. This transitional molecule is metabolized by PMNs to generate the “aspirin-triggered” 15-epi-lipoxins A4 and B4. Finally, both native and aspirin-triggered LXs can be rapidly inactivated by 15-PGDH, stopping the downstream signaling.
LXs mainly exert their functions by binding with high affinity to a G-protein coupled receptor named N-formyl peptide receptor 2 (FPR2), also called formyl peptide receptor-like 1 (FPRL1) or ALX receptor (ALXR) (18). ALXR is expressed in a wide array of tissues, including bone marrow, brain, lung, gastrointestinal tract and heart (18) and activates a plethora of cell type-specific pathways (12). Consequently, LXs are able to modulate a large variety of processes showing diverse actions depending on the cell type they act on (8). Among their functions, LXs are capable of blocking the arrival of excessive PMNs to the inflammatory site (19–22) and switching macrophage phenotype from pro-inflammatory (M1) to anti-inflammatory (M2), stimulating efferocytosis and repair-associated mechanisms (23–25). SPMs can also blunt the cytotoxicity of NK cells (26) and decrease antibody production and proliferation in memory B cells preventing maladaptive immunity and autoimmune reactions (27). They also affect non-immune cells; for example, they elicit anti-fibrotic responses by repressing metalloproteinases (MMP) and inducing tissue inhibitors of metalloproteinase (TIMPs) expression in fibroblasts (28). In cardiomyocytes, they have been described to induce the antioxidant NRF2 pathway, thus reducing damage from hypoxia/reoxygenation (29). The recently developed Atlas of Inflammation Resolution (AIR) is a web resource that gathers updated data on the various processes modulated by LXs and other SPMs with in-depth information about the underlying molecular pathways and their interactions (30).
The effect of LXs is destined to be local and transitory, therefore they are rapidly metabolized and inactivated by modifications at different carbons by distinct enzymes, primarily 15-hydroxyprostaglandin dehydrogenase (PGDH) (12, 31). Since this inactivation is stereospecific, ATL degradation occurs at approximately 50% of the conversion rate of native LXA4, resulting in an increased half-life (32). However, to further prolong LX action, synthetic LX analogs, such as LXA4-methyl ester (LXA4-ME), benzo-LXA4 and 15-epi-16-(p-fluoro)-phenoxy-lipoxin A4 (ATLa), have been designed by substitutions at different carbons (33). These analogs resisted rapid conversion while retaining their intrinsic properties and biological functions (31), resulting in a more potent bioactivity (18).
It is now widely accepted that a defective resolution phase may lead to chronic inflammation, a more persistent response that eventually causes tissue fibrosis, necrosis and irreversible damage, severely affecting appropriate functioning of organs (4). Impaired resolution may be enhanced by reduced dietary intake of fatty acids, by genetic polymorphisms affecting SPM biosynthesis and SPM receptors functionality and expression, and by abnormal downstream signaling upon receptor activation (34). In recent years, chronic inflammation has been demonstrated to underpin pathologies not previously thought to be inflammatory, like atherosclerosis, Alzheimer’s disease, cardiovascular diseases or even cancer (35, 36), which underscores the importance of an effective and tightly regulated resolution process. Indeed, current therapies for these diseases include treatment with generic corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs), however their main drawbacks are their potential side-effects, including hyperglycemia, hypertension, osteoporosis, increased bleeding or even neurological alterations and treatment resistance in certain patients (37, 38). As an alternative, LXs and other SPMs arise as an effective anti-inflammatory treatment with reduced side effects (39). Furthermore, reduced circulating levels of LXs which reach nanomolar concentrations under physiological conditions- have been related to worsened prognosis and disease progression, suggesting that LXs can serve as predictive biomarkers as observed in sputum from severe asthma patients (40), urine from lupus patients (41) or plasma from tuberculosis patients (42). In the following sections, we will provide an up-to-date analysis in relation to the current research on the role of LXs in pathologies affecting the main physiological organ systems.
Neurological Diseases
Alzheimer’s Disease
Alzheimer’s disease (AD) is a neurodegenerative disorder representing the most common cause of dementia in the elderly. It is characterized by the loss of cognitive functioning (thinking, remembering, and reasoning) and behavioral skills to such an extent that it interferes with a person’s life and daily activities. Although its causes are not fully understood, the deposition of toxic β-amyloid and P-Tau aggregates in the brain has been reported to interfere with neuronal circuits and activate pro-inflammatory signaling in microglia, thus initiating brain damage (43, 44).
Since both neurons and glia express ALXR (45), increasing LXs production in the brain may blunt inflammation and ameliorate the outcome of AD patients. Interestingly, LXA4 levels in brains from AD patients are reduced in contrast to healthy controls (n=10), suggesting that AD-associated neuroinflammation may be worsened by defective resolution mechanisms (45). In fact, treatment with LXs and their derivatives exerted beneficial effects in a human microglial cell line (46) and protected against the harmful accumulation of both β-amyloid and P-Tau aggregates by improving their phagocytic elimination via upregulation of IL-10 and TGF-β anti-inflammatory pathways in different mouse models of AD by either s.c. or intracerebroventricular (ICV) injection (47–50). These beneficial effect observed upon LX treatment can be partially explained by the decreased activity of the NF-κB/IL-1β pathway, which in turn downregulates p38, ERK, JNK and GSK3β kinases responsible for neuroinflammation and Tau phosphorylation (49, 51–53).
Stroke
Stroke is one of the leading causes of death worldwide and of disability in adults. Ischemic stroke is the most prevalent and occurs when a brain artery is totally or partially blocked by a clot or burst, prompting ischemia and preventing the brain from receiving oxygen and nutrients and triggering an inflammatory response that causes severe brain damage (54). Within the most common symptoms, patients exhibit trouble with speaking and understanding, paralysis in the face, arms or legs, partial blindness, loss of coordination and headache (54). Current therapies are limited, have side effects and their efficiency depends on a narrow time window, therefore research on alternative treatments is mandatory (54, 55). In addition, within the first five years after stroke, a high percentage of patients develop depressive symptoms, worsening their prognosis (56). Interestingly, it has been shown that circulating LX levels are lower in patients with ischemic stroke (n= 75 patients vs. 35 healthy) (57) and with depression compared to controls (n=143 patients vs. 44 healthy) (58). Concordantly, LXs and their analogs have been extensively demonstrated to play an important protective role in animal models of stroke (55). Thus, ICV inoculation of LXA4-ME immediately after occlusion reduced neurological dysfunction, infarct volume and histological damage in a rat model (59, 60). LXA4-ME treatment managed to decrease the number of apoptotic neurons (59) and to inhibit neutrophil infiltration and microglia activation, overall reducing pro-inflammatory cytokine levels mainly by downregulating NF-κB pathway (60). Both LXA4-ME (ICV) and BML-111 –ALXR analog- (i.v.) treatment allowed for the maintenance of blood-brain barrier, since MMP-9 and MMP-3 expression and activity were decreased whereas TIMP-1 was increased, further protecting against cerebral ischemia (55, 61, 62). Similar results were observed with ATL (i.v.), which also prevented leukocyte-platelet aggregations within cerebral microvasculature, therefore reducing the risk of cerebral atherothrombosis (63). Interestingly, treatment with rosiglitazone, a PPARγ agonist, promoted neuroprotection in a murine model of stroke in part by inducing 5-LO synthesis, thus increasing LXA4 (64). Other molecular pathways implicated in LX-associated neuroprotection are NRF2/HO-1 and autophagy, both involved in the antioxidant response (65, 66).
Generally, all these data support the idea that LX treatment emerge as a new therapeutic tool for brain diseases since these compounds can control increased inflammation and oxidative stress associated with brain damage. More clinical and experimental approaches are needed to elucidate the potential and the specific mechanism behind LX-mediated neuroprotection. Thus, additional data on the anti-apoptotic and pro-survival actions of LX in neurons or their role in the maintenance of the blood-brain barrier can reveal the mechanisms supporting LX-dependent neuroprotection.
Cardiovascular Diseases
Atherosclerosis
Atherosclerosis, the formation of fibro-fatty lesions in the artery wall, causes much morbidity and mortality worldwide as it is commonly associated with myocardial infarction or stroke, as well as peripheral artery disease. The main risk factors include hypercholesterolemia and blood lipid dysregulation but also hypertension, cigarette smoking and diabetes mellitus. Increasing evidence points to a role for the immune system, being increased pro-inflammatory mediators emerging factors for the prognosis of patients (36, 67).
Current research has revealed that deficient LX levels are tightly related to atheroma development. In fact, LX precursors profile was found reduced in unstable atherosclerotic plaques compared to stable plaques, suggesting that changes on pro-resolving lipids may promote plaque inflammation and rupture (68). Studies in rabbit atherosclerotic arteries showed reduced levels of LXs along with exacerbated levels of pro-inflammatory cytokines associated to a defective resolution process during atherogenesis (69, 70). Interestingly, statins, the most prevalent atherosclerosis medication, can upregulate the lipoxygenase pathway (71), and simvastatin, atorvastatin and lovastatin treatments were found to promote 15-epi-LXA4 formation independently of aspirin, indicating that synthesis of 15-epi-LXA4 could also be statin-triggered (15).
Although the molecular mechanism of LX-associated atheroprotection is not completely known, evidence suggests that LXs modulate the inflammatory process within atherosclerotic plaques (foam cells, ROS, cytokines) as well as elements interacting with them, such as vascular smooth muscle and immune cells, resulting in reduced necrosis and increased plaque stability (72). LXs treatment has been demonstrated to inhibit foam cell formation and apoptosis in macrophages mainly by blunting both the expression and signaling of CD36, the main receptor involved in oxLDL uptake (69). In addition, i.p. administration of LXA4 and benzo-LXA4 reduced aortic expression of pro-inflammatory cytokines (IL-1β and IL-6) and adhesion molecules (MCP-1, VCAM-1 and ICAM-1) in a mouse model of atherosclerosis (73). ATL was also found to inhibit vascular smooth muscle cell migration, preventing atherosclerotic lesions (74).
Therefore, the atheroprotective mechanisms elicited by LXs include reduced infiltration of inflammatory cells that can modulate the promotion of pro-resolution phenotypes inside the plaque. The evidence of statin-triggered LXs may explain some of the anti-inflammatory effects of statin therapy (75) and represents an interesting approach to increase LX levels and promote resolution in atherosclerotic patients especially by inducing plaque stabilization and preventing the appearance of acute cardiovascular events. However, additional studies are required to completely understand their synthesis mechanism as well as the resolution potential of statin treatment. Lastly, LXs role in plaque stabilization by preventing rupture and adverse thrombotic events is of paramount relevance in the context of atherosclerosis and it will undoubtedly be addressed in future research.
Myocardial Ischemia/Reperfusion Injury
Myocardial ischemia/reperfusion (I/R) injury occurs due to blood restoration after a critical period of coronary artery obstruction, which is associated with clinical interventions such as thrombolysis, angioplasty, and coronary bypass surgery. This reperfusion injury involves the activation of an inflammatory cascade and is manifested as functional impairment, arrhythmia, and accelerated progression of cell death in certain critically injured myocytes. Among the main mediators of reperfusion injury are oxygen radicals, calcium mishandling, and excessive inflammation (76). The primary therapeutic approach consists of improving the blood flow to the cardiac muscle and establishing a medical treatment for the main symptoms caused after damage. However, it is still necessary to find specific and more effective alternatives aimed at recovering lost cardiac functionality. Experimental evidence suggests that LX treatment may ameliorate myocardial injury outcome. Chen et al. were the first to describe a protective role for LXA4 in a rabbit model of myocardial I/R following cardiac arrest. I.v. administration of this SPM inhibited the expression of pro-inflammatory cytokines, reducing the apoptosis of cardiac cells (77). In addition, Zhao et al. demonstrated that LXA4 preconditioning and post-conditioning (i.v.) in myocardial I/R injury attenuated myocardial metabolic disturbance, inhibiting the inflammatory reaction and oxidative stress (78). This protective mechanism appears to occur by a downregulation of caspase 12 and GRP-78, both implicated in apoptosis (79).
Myocarditis
Myocarditis is a pathology caused by the inflammation of the cardiac muscle that can be associated to viral infections, toxic substances, or autoimmune processes. It is also considered one of the main precursors of dilated cardiomyopathy and one of the main causes of cardiac transplant in young adults (80).
LXs exhibited a protective effect in murine models of myocarditis. Part of this effect is achieved by cardiac inhibition of PI3K/Akt and NF-κB pro-inflammatory pathways (81), whose deleterious roles in cardiac pathology have been widely described (82, 83). Furthermore, the activation of NRF2 antioxidant response represents one of the main effects of LX-mediated protection as observed in a myocarditis model. We recently described that in cardiomyocytes, this activation occurs via CaMKK2-AMPKα pathway (84). Interestingly, patients with severe heart failure also exhibit decreased plasma levels of LXs, indicating deficient resolution of inflammation (n = 18 mild-to-moderate vs. 16 severe chronic heart failure) (85).
Altogether, current evidence demonstrates that LXs are able to efficiently coordinate the pro-resolutive response in the heart, suggesting that LX-based therapy could be a more effective alternative to treat cardiac diseases preventing cardiac inflammation, remodeling and dysfunction.
Respiratory Diseases
Acute Lung Injury
Acute lung injury (ALI), and its most severe form, acute respiratory distress syndrome (ARDS) are manifestations of the lung to an inflammatory response and have high morbidity and mortality in the present (86). They are characterized by severe hypoxemia, hypercapnia, diffuse infiltration in the chest X-ray and a substantial reduction in pulmonary compliance ultimately leading to respiratory failure (86).
Inflammation and activation of immune cells are critical for ALI and ARDS development, since the release of pro-inflammatory cytokines and proteases increases alveolar-capillary barrier permeability, disrupting the appropriate clearance of alveolar fluid and leading to pulmonary edema (87, 88). In this context, research in animal models of ALI revealed that i.v. LXA4 treatment diminished the production of pro-inflammatory cytokines and ROS, thus improving alveolar fluid clearance (89–91). BML-111 (i.p.) and ATL (i.v.) exhibited similar results including preventing neutrophil infiltration, promoting its clearance (92, 93) and inhibiting the formation of neutrophil-platelet aggregates (87).
LXs and analogs also modulate pulmonary cells by preventing apoptosis and promoting proliferation of alveolar type II cells (94), reducing inflammatory signaling in microvascular endothelial cells (95, 96) and promoting autophagy in alveolar macrophages (97). In addition, they diminish fibrosis and support appropriate alveolar epithelial repair by inhibiting collagen production and proliferation of lung fibroblasts (94). LXs regulate these processes by antagonizing TLR4/NF-κB and MAPK/AP-1 signaling (95, 96, 98) and by activating both the anti-inflammatory and anti-fibrotic ACE2-Ang- (1–7)-Mas axis, mainly through an upregulation of levels and activity of ACE2 (90, 99) and the antioxidant NRF2/HO-1 pathway (96, 100). Altogether, the ability of LXs to maintain the integrity of pulmonary epithelia and to prevent infiltration of immune cells accentuates their therapeutic potential in relation to ALI and ARDS, especially by blunting neutrophilia, the hallmark of these pathologies.
Asthma
Asthma is characterized by acute episodes of shortness of breath, coughing and chest tightness due to an underlying chronic inflammatory process sustained by a combined action of immune cells and bronchial epithelial cells (101). It affects 300 million people worldwide, being one of the most common chronic diseases in children and adults (102). Corticosteroid treatment is the most common medication for asthma, however, a small percentage of patients develop a more severe form that is unresponsive to this treatment, demanding more effective alternative therapies (103).
The potential of LXs to serve not only as a treatment but also as a biomarker for asthma has been extensively examined, as LXs concentration can be easily evaluated in sputum or exhaled breath. Decreased LXA4 levels have been correlated with increased asthma severity in both adults (n = 12 mild asthma, 15 moderate, 24 severe and 10 healthy) (40, 104) and children (n=36 mild asthma, 42 moderate, 28 severe vs. 40 healthy) (105–107). In fact, alveolar macrophages obtained from patients with severe asthma synthesize less LXs per se and in response to LPS treatment (n=11 severe asthma, 12 non-severe and 14 healthy) (103). In severe asthmatic children, alveolar macrophages were also found to be apoptotic and less functional (n=28 severe vs. 10 healthy) (108). This impaired LX synthesis explains in part the neutrophilia and eosinophilia observed in asthma, especially in severe cases (103). Indeed, LXs and analogs have been observed to attenuate eosinophil function as well as T lymphocyte and mast cell activity, both in vitro and in vivo (109–111). In relation to this, LXs managed to enhance NK cells functions in patients with asthma (112), including NK-induced apoptosis in eosinophils preventing enhanced inflammation (113).
Notably, 5-10% of asthmatic patients suffer from aspirin-intolerant asthma (AIA), developing exacerbated inflammation and asthmatic attacks upon aspirin treatment in contrast to aspirin-tolerant asthmatics (ATA) (114). LXs may play a major role in this syndrome since Sanak et al. proved that aspirin-treated blood from AIA patients produced less LXA4 and ATL than blood from ATA patients (n= 14 AIA vs. 11 ATA) (114). Similarly, urinary ATL levels were lower in AIA patients when compared to ATA patients (n=15 AIA vs. 16 ATA) (115). An interesting approach would be evaluating whether exogenous LX administration could ameliorate symptoms in AIA patients by balancing LX deficiency.
In light of the encouraging results obtained, recent advances in asthma treatment include a pilot application based on the inhalation of two LX analogs to treat children with acute episodes of asthma, which turned out to be a safer and more efficient alternative than some of the current asthma medications (116).
Idiopathic Pulmonary Fibrosis
Idiopathic pulmonary fibrosis (IPF) is a type of interstitial lung disease characterized by a progressive fibrotic process in the lungs that severely obstructs gas exchange, eventually causing respiratory failure and death, with mean survival ranging between 3-5 years (117, 118). The only treatment available is lung transplantation, with alternative therapies focusing on delaying fibrosis development and improving patients’ quality of life.
LXs have been reported to exert anti-fibrotic actions in the lung, mainly by inhibiting TGF-β signaling and collagen I production in fibroblasts (94, 119, 120), and by preventing fibroblasts proliferation and differentiation into myofibroblasts (94). These effects have also been observed in human lung myofibroblasts obtained from IPF patients, together with a reduction of α-smooth muscle actin (SMA) expression and actin stress fibers formation and contraction (121). Likewise, mice subjected to bleomycin-induced lung fibrosis and treated i.t. with ATL exhibited reduced inflammation and fibrosis, resulting in amelioration of pulmonary performance and mouse survival (122, 123).
Cystic Fibrosis
Cystic fibrosis (CF) is an autosomal recessive disorder caused by a mutation in the gene encoding for cystic fibrosis transmembrane conductance regulator (CFTR) (124). It is one of the most common genetic diseases, with a prevalence of 1:2500 in Caucasians and a median life expectancy under 50 years of age (124, 125). Defects on CFTR lead to mucus accumulation, bacterial infection and exacerbated inflammation, eventually causing respiratory failure (124). In this sense, LXA4 treatment in both in vitro and in vivo (i.v.) CF models was found to reduce bacterial and neutrophil counts (126), as well as prevent epithelial barrier disruption upon pathogen infection (127).
Interestingly, CFTR and LXs appear to be profoundly interrelated. Mattoscio et al. found that inhibition of CFTR in platelets decreased LXA4 synthesis (128). In fact, platelets from CF patients produce 40% less LXA4 than healthy controls (n = 6 homozygous, n = 8 heterozygous and n = 4 healthy). CFTR-mutated animals also exhibit reduced LXA4 levels, whereas restoring CFTR activity augmented them (129).
LXs were also found to affect the function of other pulmonary ion transport channels inducing Cl- secretion through calcium-dependent chloride channels, inhibiting Na+ absorption by epithelial sodium channels and activating KATP channel (130–132). As a result, LXs promote mucus clearance and airway epithelial repair, preserving airway surface liquid layer and protecting against bacterial infection and lung inflammation, which underscores their therapeutic potential to ameliorate respiratory diseases.
Renal Diseases
Renal Ischemia/Reperfusion Injury
Renal ischemia/reperfusion injury is caused by a sudden temporary impairment of kidney´s blood flow. It is usually associated with a robust inflammatory and oxidative stress response to hypoxia and reperfusion which impairs organ function (133). Although its pathophysiology is not completely understood, oxygen radicals generated at reperfusion phase initiates a cascade of deleterious cellular responses leading to inflammation, cell death, and acute kidney failure.
Treatment with ATLa (i.v.) was found to restore renal function and morphology and to diminish pro-inflammatory cytokine production and neutrophil infiltration in a mouse model of acute renal failure (134). Transcriptomic analysis performed in this model revealed that pre-treatment with this analog also managed to downregulate pro-fibrotic and pro-apoptotic genes, including collagen, transgelin and Fos-like proteins, while upregulating genes implicated in the antioxidant defense, cell growth and transport proteins, like glutathione, angiogenin and aquaporin (135). At the molecular level it has been proposed that this protective effect is mediated by MAPKs, mainly p38 and ERK, and PPAR/NRF2 pathways (136, 137).
Renal Fibrosis
Renal fibrosis is mainly mediated by excessive proliferation of mesangial cells, which plays an important role in glomerular inflammation. Both i.v. administration of LXA4 and benzo-LXA4 improved renal fibrosis in rats by diminishing renal apoptosis, TNFα and IFNγ expression, TGF-β and PAI-1 activation, and collagen deposition (138). Attenuation of MAPK, Akt and Smads signaling pathways are responsible for these effects. Interestingly, Brennan et al. observed that LX upregulates the expression of let-7c miRNA to promote the anti-fibrotic response, manifesting the existence of LX-activated miRNA pathways (139). Due to their ability to inhibit adverse remodeling in the kidney, LX treatment represents a great alternative to prevent the development of renal fibrosis and subsequent chronic kidney disease.
Diabetic Kidney Disease
Diabetic kidney disease occurs in >30% of patients with type 2 diabetes mellitus and is characterized by a maladaptive response of the renal parenchyma, which is intensified by the development of a chronic inflammatory response, finally leading to renal failure. Results from a clinical trial in chronic kidney disease patients revealed that aspirin treatment increased ATL levels in diabetic patients, however the effects of this increase were not evaluated (140). Studies in diabetic kidney disease animal models concluded that i.p. injection of LXA4 and benzo-LXA4 attenuated the development of the disease, including pro-inflammatory and pro-fibrotic signaling (141). Transcriptomic profile of this model found enrichment of classical pro-inflammatory pathways (TNFα, NF-κB, TGF-β) and identified activation of early growth response-1 (EGR-1) network to be involved in diabetic pathology as well (141). LXs managed to downregulate the transcriptional network of these mediators, thus representing an effective treatment to prevent renal inflammation and fibrosis developed in diabetic pathology.
Periodontal Diseases
Periodontal diseases (PD) represent a broad group of diseases characterized by chronic inflammation in the supporting structures of the teeth, in particular gingivae, bones and ligaments. It is typically initiated by bacterial infection, which induces an inflammatory reaction in the gingivae –termed gingivitis– that subsequently progresses into PD when untreated (142). PD can evolve into periodontitis, a much more severe form of the disease that can ultimately cause loss of teeth. The global prevalence of PD has been estimated to range between 10-15% of the population (142).
PD has been described to be initiated mainly by the inflammation of the periodontium, which consequently induces PMN recruitment since they represent the first line of defense against bacterial infection (143). An exacerbated recruitment of these immune cells to the periodontium weakens periodontal tissue leading to PD. LXs have been widely described to ameliorate PD outcome (144) showing that in animal models are able to reduce the release of pro-inflammatory mediators as well as PMN recruitment to the affected area (145). Studies in patients have proposed that reduced serum or salivary levels of LXA4 in patients can be useful as PD marker (n >65) (146–148).
The protective mechanism appears to lie in the inhibition of pro-inflammatory cytokines that modulate PMNs, hampering their recruitment and pro-inflammatory signaling thus preventing the onset of periodontal inflammation (149). In this sense, LXA4 treatment has also been found to abolish NF-κB and TNFα signaling in periodontal ligament cells, which play a pivotal role exacerbating the inflammatory response (150). Furthermore, increased circulating ROS levels and blood aggregation caused by P. gingivalis infection were successfully reduced upon LXA4 treatment, and this effect appears to be dependent on platelet-PMN interaction (151). In a different context, this SPM has also been shown to stimulate proliferation and migration of stem cells of the apical papilla -the main source of dentin-like structures- as well as inhibiting their pro-inflammatory activation, which may indicate LXs have also regenerative potential besides their anti-inflammatory properties (152). In fact, currently, targeting PD with LX-based treatment has reached clinical trial in the form of oral rinse (https://clinicaltrials.gov/ct2/show/NCT02342691).
Arthritis and Rheumatic Diseases
Rheumatoid arthritis (RA), an autoimmune disease causing severe destructive inflammation of the joints with associated systemic complications, is one of the most prevalent rheumatic disease to date (153). Current treatments are based on corticosteroids, potent anti-inflammatory drugs that are also well known to have adverse effects (154).
Studies in animal models of RA have reported that administration of either LXA4 (i.art.) or BML-111 (i.p.) attenuates arthritis in mice by diminishing joint erosion, pro-inflammatory cytokines release and immune cell infiltration (155, 156). In the same line, it was demonstrated that 12-LO/15-LO deficiency exacerbates the development of arthritis in mice partly due to a reduction in LXA4 levels (157). In this context, LXA4 treatment has been shown to prevent pro-inflammatory activation of fibroblast-like synoviocytes, the main cell type responsible for immune activation and inflammation in RA (158). Regarding the molecular pathways underlying this beneficial effect, it is known that LXA4 abrogates IL-6 expression (158), counteracts MMP/TIMPs imbalance in tissue degradation and fibrosis (159), and opposes IL-1β and TGF-β pro-inflammatory and pro-fibrotic actions in human fibroblast-like synoviocytes (158, 159). These effects occur in part by inhibition of p38 MAPK signaling pathway (PMID 33221976).
Besides RA, LXA4 treatment (i.p.) has been found to alleviate osteoarthritis in rodent models (160, 161) and its deficiency appears to be partly responsible for the onset of systemic lupus erythematosus (162). Indeed, it has been described that corticosteroids can inhibit LX production in the long term, which could explain the negative effects of the prolonged use of these drugs (163). Altogether, this evidence exhibits the crucial role that LXs and other SPMs play in rheumatic disorders as modulators of immunosuppressive and anti-inflammatory processes. Nonetheless, additional research should be conducted to strongly determine the specific effects of SPMs in rheumatic pathologies to appropriately evaluate their benefits.
Concluding Remarks
Persistent inflammation underpins many of the most prevalent pathologies at the present. Thus, a chronic inflammatory scenario is present in many cardiovascular, pulmonary, or neurological diseases as well as metabolic disorders and even cancer. LXs are endogenous pro-resolving lipid mediators whose levels are significantly reduced in a wide range of pathologies affecting the main systems we have reviewed. These mediators can play a decisive role in many of these pathologies, mainly due to their capacity to both halt the inflammatory signaling and reduce oxidative stress ( Figure 3 ). Exogenous administration of LXs or stimulation of their endogenous synthesis represent interesting alternative therapies that have provided successful results in animal models. LXs exert beneficial effects at different routes of administration, and synthetic analogs with more potent and prolonged actions are commercially available. Moreover, these SPMs have more reduced side-effects than most current treatments due to their endogenous nature. All these factors make them attractive targets from a pharmacological point of view. Nonetheless, additional evaluation of pharmacokinetics as well as potential side-effects is necessary to fully determine their safety and effective dose. Altogether, LX-based therapies emerge as a promising approach to kick off resolution pharmacology, which has exhibited great potential and could provide alternatives to treat current health problems with high incidence worldwide in the near future.
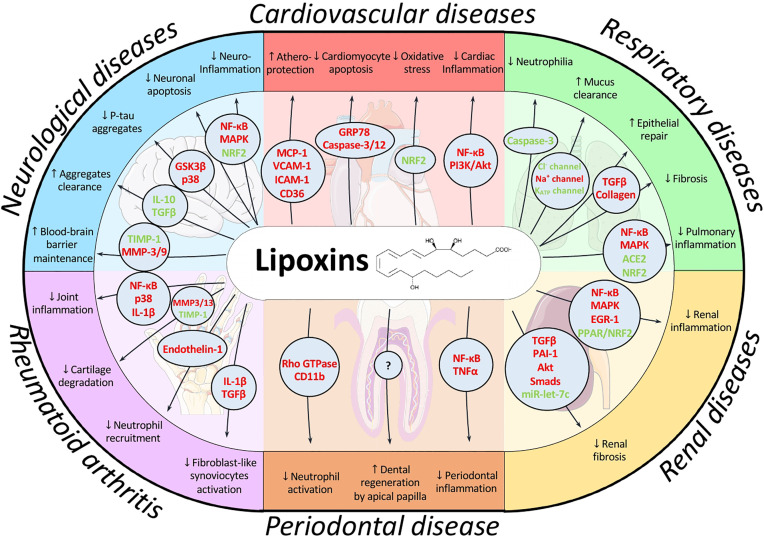
Figure 3.
Schematic overview of the main signaling pathways modulated by lipoxins and their effects in the pathologies addressed in this review. LXs have been described to coordinate a wide range of signaling pathways depending on cell context, allowing for a fine-tune regulation of the resolution process. For example, they induce apoptosis in neutrophils to promote their clearance while they prevent apoptosis in cardiomyocytes thus exhibiting cardioprotective actions. By modulating different pathways, LXs can exert protective effects on different cell types and environments, as observed by their effects on the brain, heart, lung, liver, periodontium and joints. Mediators highlighted in red indicate downregulation or inhibition by LX action whereas green indicates upregulation or activation.
Author Contributions
RJ, SS-G, and PP wrote the manuscript. MF-V and LB provided funding and corrected the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Ministerio de Economía, Industria y Competitividad, Ministerio de Ciencia, Investigación y Universidades, and Agencia Estatal de Investigación (SAF2017-82436R), Centro de Investigación Biomédica en Red en Enfermedades Cardiovasculares (CB16/11/00222), Consorcio de Investigación en Red de la Comunidad de Madrid, S2017/BMD-3686 and Fondo Europeo de Desarrollo Regional. RI holds a FPU PhD fellowship of the Ministerio de Ciencia, Investigación y Universidades (FPU16/00827).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ACE2, Angiotensin-converting enzyme 2; AD, Alzheimer disease; AIA, Aspirin-intolerant asthma; ALI, Acute lung injury; ALXR, Lipoxin receptor; AMPK, AMP-activated protein kinase; AP-1, Activator protein 1; ARDS, Acute respiratory distress syndrome; ATA, Aspirin-tolerant asthma; ATL, Aspirin triggered lipoxin; ATLa, 15-epi-16-(p-fluoro)-phenoxy-lipoxin A4; CaMKK2, Calcium/calmodulin dependent protein kinase kinase 2; CF, Cystic fibrosis; CFTR, Cystic fibrosis transmembrane conductance regulator; ERK, Extracellular signal-regulated kinases; GSK3β, Glycogen synthase kinase 3 beta; HO-1, Heme-oxygenase 1; i.art., intra-articular; ICV, Intracerebroventricular; IFNγ, Interferon gamma; IL, Interleukin; i.p., Intraperitoneal; IPF, Idiopathic pulmonary fibrosis; I/R, Ischemia/Reperfusion; i.t., Intratracheal; i.v., Intravenous; JNK, c-Jun N-terminal kinase; LO, Lipoxygenase; LX(s), Lipoxin(s); LXA4, Lipoxin A4; LXA4-ME, LXA4-methyl ester; MAPK, Mitogen-activated protein kinase; MMP, Matrix metalloproteinase; NF-κB, Nuclear factor κB; NK, Natural killer cells; NRF2, Nuclear factor erythroid 2-related factor 2; PD, Periodontal disease; PI3K, Phosphatidylinositol 3-kinase; PMN, Polymorphonuclear cell; PPAR, Peroxisome proliferator- activated receptors; s.c., Subcutaneous; SPM, Specialized pro-resolving mediators; TGF-β, Transforming growth factor beta; TIMP, Tissue inhibitor of metalloproteinase; TNFα, Tumor necrosis factor alpha; RA, Rheumatoid arthritis; ROS, Reactive oxygen species.
References
- 1. Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, et al. Resolution of Inflammation: State of the Art, Definitions and Terms. FASEB J (2007) 21:325–32. 10.1096/fj.06-7227rev [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Duffy DM, Ko C, Jo M, Brannstrom M, Curry TE. Ovulation: Parallels With Inflammatory Processes. Endocr Rev (2019) 40:369–416. 10.1210/er.2018-00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fiocchi C. What is “Physiological” Intestinal Inflammation and How Does it Differ From “Pathological” Inflammation? Inflamm Bowel Dis (2008) 14:S77–8. 10.1002/ibd.20618 [DOI] [PubMed] [Google Scholar]
- 4. Schett G, Neurath MF. Resolution of Chronic Inflammatory Disease: Universal and Tissue-Specific Concepts. Nat Commun (2018) 9(9):3261. 10.1038/s41467-018-05800-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basil MC, Levy BD. Specialized Pro-Resolving Mediators: Endogenous Regulators of Infection and Inflammation. Nat Rev Immunol (2016) 16:51–67. 10.1038/nri.2015.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lehmann C, Homann J, Ball AK, Blöcher R, Kleinschmidt TK, Basavarajappa D, et al. Lipoxin and Resolvin Biosynthesis is Dependent on 5-Lipoxygenase Activating Protein. FASEB J (2015) 29:5029–43. 10.1096/fj.15-275487 [DOI] [PubMed] [Google Scholar]
- 7. Jozsef L, Zouki C, Petasis NA, Serhan CN, Filep JG. Lipoxin A4 and Aspirin-Triggered 15-Epi-Lipoxin A4 Inhibit Peroxynitrite Formation, NF-Kappa B and AP-1 Activation, and IL-8 Gene Expression in Human Leukocytes. Proc Natl Acad Sci U S A (2002) 99:13266–71. 10.1073/pnas.202296999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McMahon B, Godson C. Lipoxins: Endogenous Regulators of Inflammation. Am J Physiol Renal Physiol (2004) 286:F189–201. 10.1152/ajprenal.00224.2003 [DOI] [PubMed] [Google Scholar]
- 9. El Kebir D, József L, Pan W, Wang L, Petasis NA, Serhan CN, et al. 15-Epi-Lipoxin A 4 Inhibits Myeloperoxidase Signaling and Enhances Resolution of Acute Lung Injury. Am J Respir Crit Care Med (2009) 180:311–9. 10.1164/rccm.200810-1601OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hansen TV, Vik A, Serhan CN. The Protectin Family of Specialized Pro-Resolving Mediators: Potent Immunoresolvents Enabling Innovative Approaches to Target Obesity and Diabetes. Front Pharmacol (2019) 9:1582. 10.3389/fphar.2018.01582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Serhan CN, Hamberg M, Samuelsson B. Lipoxins: Novel Series of Biologically Active Compounds Formed From Arachidonic Acid in Human Leukocytes. Proc Natl Acad Sci USA (1984) 81:5335–9. 10.1073/pnas.81.17.5335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chandrasekharan JA, Sharma-Walia N. Lipoxins: Nature’s Way to Resolve Inflammation. J Inflamm Res (2015) 8:181–92. 10.2147/JIR.S90380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Capra V, Rovati GE, Mangano P, Buccellati C, Murphy RC, Sala A. Transcellular Biosynthesis of Eicosanoid Lipid Mediators. Biochim Biophys Acta - Mol Cell Biol Lipids (2015) 1851:377–82. 10.1016/j.bbalip.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 14. Claria J, Serhan CN. Aspirin Triggers Previously Undescribed Bioactive Eicosanoids by Human Endothelial Cell-Leukocyte Interactions. Proc Natl Acad Sci U S A (1995) 92:9475–9. 10.1073/pnas.92.21.9475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levy BD. Myocardial 15-Epi-Lipoxin A4 Generation Provides a New Mechanism for the Immunomodulatory Effects of Statins and Thiazolidinediones. Circulation (2006) 114:873–5. 10.1161/CIRCULATIONAHA.106.647925 [DOI] [PubMed] [Google Scholar]
- 16. Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and Lipoxins: Structures, Biosynthesis, and Biological Effects. Science (80- ) (1987) 237:1171–6. 10.1126/science.2820055 [DOI] [PubMed] [Google Scholar]
- 17. Sala A, Folco G, Murphy RC. Transcellular Biosynthesis of Eicosanoids. Pharmacol Rep (2010) 62:503–10. 10.1016/S1734-1140(10)70306-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiang N, Serhan CN, Dahlén S-E, Drazen JM, Hay DWP, Rovati GE, et al. The Lipoxin Receptor ALX: Potent Ligand-Specific and Stereoselective Actions In Vivo. Pharmacol Rev (2006) 58:463–87. 10.1124/pr.58.3.4 [DOI] [PubMed] [Google Scholar]
- 19. Filep JG, Zouki C, Petasis NA, Hachicha M, Serhan CN. Anti-Inflammatory Actions of lipoxin-A4 Stable Analogs in Human Whole Blood: Modulation of Leukocyte Adhesion Molecule Expression and Inhibition of Neutrophil-Endothelial Interactions. Prostaglandins Other Lipid Mediat (1999) 59:3. 10.1016/S0090-6980(99)90238-1 [DOI] [PubMed] [Google Scholar]
- 20. Colgan SP, Serhan CN, Parkos CA, Delp-Archer C, Madara JL. Lipoxin A4 Modulates Transmigration of Human Neutrophils Across Intestinal Epithelial Monolayers. J Clin Invest (1993) 92:75–82. 10.1172/JCI116601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee TH, Horton CE, Kyan-Aung U, Haskard D, Crea AEG, Spur BW. Lipoxin A4 and Lipoxin B4 Inhibit Chemotactic Responses of Human Neutrophils Stimulated by Leukotriene B4 and N-Formyl-L-Methionyl-L-Leucyl-L-Phenylalanine. Clin Sci (1989) 77:195–203. 10.1042/cs0770195 [DOI] [PubMed] [Google Scholar]
- 22. Hedqvist P, Raud J, Palmertz U, Haeggström J, Nicolaou KC, Dahlen S-E. Lipoxin A 4 Inhibits Leukotriene B 4 ,-Induced Inflammation in the Hamster Cheek Pouch. Acta Physiol Scand (1989) 137:571–2. 10.1111/j.1748-1716.1989.tb08805.x [DOI] [PubMed] [Google Scholar]
- 23. Prieto P, Cuenca J, Través PG, Fernández-Velasco M, Martín-Sanz P, Boscá L. Lipoxin A4 Impairment of Apoptotic Signaling in Macrophages: Implication of the PI3K/Akt and the ERK/Nrf-2 Defense Pathways. Cell Death Differ (2010) 17:1179–88. 10.1038/cdd.2009.220 [DOI] [PubMed] [Google Scholar]
- 24. Wang Q, Parekh D, D’Souza V, Dancer R, Patel J, Bartis D, et al. S102 Lipoxin A4 Improves Efferocytosis Via Inhibition of the Hmgb1 in Human Alveolar Macrophages. Thorax (2014) 69:A54–5. 10.1136/thoraxjnl-2014-206260.108 [DOI] [Google Scholar]
- 25. Börgeson E, Johnson AMF, Lee YS, Till A, Syed GH, Ali-Shah ST, et al. Lipoxin A4 Attenuates Obesity-Induced Adipose Inflammation and Associated Liver and Kidney Disease. Cell Metab (2015) 22:125–37. 10.1016/j.cmet.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramstedt U, Serhan CN, Nicolaou KC, Webber SE, Wigzell H, Samuelsson B. Lipoxin A-Induced Inhibition of Human Natural Killer Cell Cytotoxicity: Studies on Stereospecificity of Inhibition and Mode of Action. J Immunol (1987) 138:266–70. [PubMed] [Google Scholar]
- 27. Ramon S, Bancos S, Serhan CN, Phipps RP. Lipoxin A 4 Modulates Adaptive Immunity by Decreasing Memory B -Cell Responses Via an ALX / FPR 2-Dependent Mechanism. Eur J Immunol (2014) 44:357–69. 10.1002/eji.201343316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sodin-Semrl S, Taddeo B, Tseng D, Varga J, Fiore S. Lipoxin A 4 Inhibits IL-1β-Induced IL-6, IL-8, and Matrix Metalloproteinase-3 Production in Human Synovial Fibroblasts and Enhances Synthesis of Tissue Inhibitors of Metalloproteinases. J Immunol (2000) 164:2660–6. 10.4049/jimmunol.164.5.2660 [DOI] [PubMed] [Google Scholar]
- 29. Chen X-Q, Wu S-H, Zhou Y, Tang Y-R. Lipoxin A4-Induced Heme Oxygenase-1 Protects Cardiomyocytes Against Hypoxia/Reoxygenation Injury Via P38 MAPK Activation and Nrf2/ARE Complex. PLoS One (2013) 8:e67120. 10.1371/journal.pone.0067120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Serhan CN, Gupta SK, Perretti M, Godson C, Brennan E, Li Y, et al. The Atlas of Inflammation Resolution (Air). Mol Aspects Med (2020) 74:100894. 10.1016/j.mam.2020.100894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parkinson JF. Lipoxin and Synthetic Lipoxin Analogs: An Overview of Anti-Inflammatory Functions and New Concepts in Immunomodulation. Inflamm Allergy Drug Targets (2006) 5:91–106. 10.2174/187152806776383125 [DOI] [PubMed] [Google Scholar]
- 32. Clish CB, Levy BD, Chiang N, Tai HH, Serhan CN. Oxidoreductases in Lipoxin A4 Metabolic Inactivation: A Novel Role for 15-Onoprostaglandin 13-Reductase/Leukotriene B4 12-Hydroxydehydrogenase in Inflammation. J Biol Chem (2000) 275:25372–80. 10.1074/jbc.M002863200 [DOI] [PubMed] [Google Scholar]
- 33. Serhan CN, Maddox JF, Petasis NA, Akritopoulou-Zanze I, Papayianni A, Brady HR, et al. Design of Lipoxin A4 Stable Analogs That Block Transmigration and Adhesion of Human Neutrophils. Biochemistry (1995) 34:14609–15. 10.1021/bi00044a041 [DOI] [PubMed] [Google Scholar]
- 34. Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LAJ, et al. Resolution of in Flammation: State of the Art, Definitions and Terms. FASEB J (2007) 21:325–32. 10.1096/fj.06-7227rev [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the Activation and Regulation of Innate and Adaptive Immunity. Nat Rev Immunol (2011) 11:519–31. 10.1038/nri3024 [DOI] [PubMed] [Google Scholar]
- 36. Golia E, Limongelli G, Natale F, Fimiani F, Maddaloni V, Pariggiano I, et al. Inflammation and Cardiovascular Disease: From Pathogenesis to Therapeutic Target. Curr Atheroscler Rep (2014) 16:435. 10.1007/s11883-014-0435-z [DOI] [PubMed] [Google Scholar]
- 37. Swartz SL, Dluhy RG. Corticosteroids: Clinical Pharmacology and Therapeutic Use. Drugs (1978) 16:238–55. 10.2165/00003495-197816030-00006 [DOI] [PubMed] [Google Scholar]
- 38. Vonkeman HE, van de Laar MAFJ. Nonsteroidal Anti-Inflammatory Drugs: Adverse Effects and Their Prevention. Semin Arthritis Rheum (2010) 39:294–312. 10.1016/j.semarthrit.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 39. Kieran NE, Maderna P, Godson C. Lipoxins: Potential Anti-Inflammatory, Proresolution, and Antifibrotic Mediators in Renal Disease. Kidney Int (2004) 65:1145–54. 10.1111/j.1523-1755.2004.00487.x [DOI] [PubMed] [Google Scholar]
- 40. Vachier I, Bonnans C, Chavis C, Farce M, Godard P, Bousquet J, et al. Severe Asthma is Associated With a Loss of LX4, an Endogenous Anti-Inflammatory Compound. J Allergy Clin Immunol (2005) 115:55–60. 10.1016/j.jaci.2004.09.038 [DOI] [PubMed] [Google Scholar]
- 41. Abd El Baky N, Abdul Salam M, Sedky Abdou M, Effat D, Mansour L. Urinary Lipoxin A4 as a Biomarker for Systemic Lupus Erythematosus. Egypt Rheumatol Rehabil (2015) 42:55. 10.4103/1110-161x.157861 [DOI] [Google Scholar]
- 42. Pavan Kumar N, Moideen K, Nancy A, Viswanathan V, Shruthi BS, Shanmugam S, et al. Plasma Eicosanoid Levels in Tuberculosis and Tuberculosis-Diabetes Co-Morbidity Are Associated With Lung Pathology and Bacterial Burden. Front Cell Infect Microbiol (2019) 9:335. 10.3389/fcimb.2019.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Palop JJ, Mucke L. Amyloid-Beta-Induced Neuronal Dysfunction in Alzheimer’s Disease: From Synapses Toward Neural Networks. Nat Neurosci (2010) 13:812–8. 10.1038/nn.2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lippens G, Sillen A, Landrieu I, Amniai L, Sibille N, Barbier P, et al. Tau Aggregation in Alzheimer’s Disease: What Role for Phosphorylation? Prion (2007) 1:21–5. 10.4161/pri.1.1.4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang X, Zhu M, Hjorth E, Cortés-Toro V, Eyjolfsdottir H, Graff C, et al. Resolution of Inflammation is Altered in Alzheimer’s Disease. Alzheimers Dement (2015) 11:40–50.e2. 10.1016/j.jalz.2013.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhu M, Wang X, Hjorth E, Colas RA, Schroeder L, Granholm A-C, et al. Pro-Resolving Lipid Mediators Improve Neuronal Survival and Increase Aβ42 Phagocytosis. Mol Neurobiol (2016) 53:2733–49. 10.1007/s12035-015-9544-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kantarci A, Aytan N, Palaska I, Stephens D, Crabtree L, Benincasa C, et al. Combined Administration of Resolvin E1 and Lipoxin A4 Resolves Inflammation in a Murine Model of Alzheimer’s Disease. Exp Neurol (2018) 300:111–20. 10.1016/j.expneurol.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 48. Medeiros R, Kitazawa M, Passos GF, Baglietto-Vargas D, Cheng D, Cribbs DH, et al. Aspirin-Triggered Lipoxin A4 Stimulates Alternative Activation of Microglia and Reduces Alzheimer Disease–Like Pathology in Mice. Am J Pathol (2013) 182:1780–9. 10.1016/j.ajpath.2013.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dunn HC, Ager RR, Baglietto-Vargas D, Cheng D, Kitazawa M, Cribbs DH, et al. Restoration of Lipoxin A4 Signaling Reduces Alzheimer’s Disease-Like Pathology in the 3xtg-AD Mouse Model. J Alzheimers Dis (2014) 43:893–903. 10.3233/JAD-141335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu J, Wang A, Min Z, Xiong Y, Yan Q, Zhang J, et al. Lipoxin A4 Inhibits the Production of Proinflammatory Cytokines Induced by β-Amyloid In Vitro and In Vivo. Biochem Biophys Res Commun (2011) 408:382–7. 10.1016/j.bbrc.2011.04.013 [DOI] [PubMed] [Google Scholar]
- 51. Luo C-L, Li Q-Q, Chen X-P, Zhang X-M, Li L-L, Li B-X, et al. Lipoxin A4 Attenuates Brain Damage and Downregulates the Production of Pro-Inflammatory Cytokines and Phosphorylated Mitogen-Activated Protein Kinases in a Mouse Model of Traumatic Brain Injury. Brain Res (2013) 1502:1–10. 10.1016/j.brainres.2013.01.037 [DOI] [PubMed] [Google Scholar]
- 52. Wu L, Miao S, Zou L-B, Wu P, Hao H, Tang K, et al. Lipoxin A4 Inhibits 5-Lipoxygenase Translocation and Leukotrienes Biosynthesis to Exert a Neuroprotective Effect in Cerebral Ischemia/Reperfusion Injury. J Mol Neurosci (2012) 48:185–200. 10.1007/s12031-012-9807-4 [DOI] [PubMed] [Google Scholar]
- 53. Wang Y-P, Wu Y, Li L-Y, Zheng J, Liu R-G, Zhou J-P, et al. Aspirin-Triggered Lipoxin A4 Attenuates LPS-Induced Pro-Inflammatory Responses by Inhibiting Activation of NF-κb and MAPKs in BV-2 Microglial Cells. J Neuroinflamm (2011) 8:95. 10.1186/1742-2094-8-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Beal CC. Gender and Stroke Symptoms. J Neurosci Nurs (2010) 42:80–7. 10.1097/JNN.0b013e3181ce5c70 [DOI] [PubMed] [Google Scholar]
- 55. Hawkins KE, DeMars KM, Alexander JC, de Leon LG, Pacheco SC, Graves C, et al. Targeting Resolution of Neuroinflammation After Ischemic Stroke With a Lipoxin A4 Analog: Protective Mechanisms and Long-Term Effects on Neurological Recovery. Brain Behav (2017) 7:e00688. 10.1002/brb3.688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ayerbe L, Ayis S, Wolfe CDA, Rudd AG. Natural History, Predictors and Outcomes of Depression After Stroke: Systematic Review and Meta-Analysis. Br J Psychiatry (2013) 202:14–21. 10.1192/bjp.bp.111.107664 [DOI] [PubMed] [Google Scholar]
- 57. Szczuko M, Kotlęga D, Palma J, Zembroń-Łacny A, Tylutka A, Gołąb-Janowska M, et al. Lipoxins, RevD1 and 9, 13 HODE as the Most Important Derivatives After an Early Incident of Ischemic Stroke. Sci Rep (2020) 10:12849. 10.1038/s41598-020-69831-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liang S, Li X, Huang W, Gong H. Change of Serum Myeloperoxidase and Lipoxin A4 Level in Coronary Heart Disease Patients With Anxiety and/or Depression. J Cent South Univ (Medical Sci) (2013) 38:370–5. 10.3969/j.issn.1672-7347.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 59. Wu Y, Ye XH, Guo PP, Xu SP, Wang J, Yuan SY, et al. Neuroprotective Effect of Lipoxin A4 Methyl Ester in a Rat Model of Permanent Focal Cerebral Ischemia. J Mol Neurosci (2010) 42:226–34. 10.1007/s12031-010-9355-8 [DOI] [PubMed] [Google Scholar]
- 60. Ye XH, Wu Y, Guo PP, Wang J, Yuan SY, Shang Y, et al. Lipoxin A4 Analogue Protects Brain and Reduces Inflammation in a Rat Model of Focal Cerebral Ischemia Reperfusion. Brain Res (2010) 1323:174–83. 10.1016/j.brainres.2010.01.079 [DOI] [PubMed] [Google Scholar]
- 61. Wu Y, Wang YP, Guo P, Ye XH, Wang J, Yuan SY, et al. A Lipoxin A 4 Analog Ameliorates Blood-Brain Barrier Dysfunction and Reduces MMP-9 Expression in a Rat Model of Focal Cerebral Ischemia-Reperfusion Injury. J Mol Neurosci (2012) 46:483–91. 10.1007/s12031-011-9620-5 [DOI] [PubMed] [Google Scholar]
- 62. Hawkins KE, DeMars KM, Singh J, Yang C, Cho HS, Frankowski JC, et al. Neurovascular Protection by Post-Ischemic Intravenous Injections of the Lipoxin A4 Receptor Agonist, BML-111, in a Rat Model of Ischemic Stroke. J Neurochem (2014) 129:130–42. 10.1111/jnc.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vital SA, Becker F, Holloway PM, Russell J, Perretti M, Granger DN, et al. Formyl-Peptide Receptor 2/3/Lipoxin A4 Receptor Regulates Neutrophil-Platelet Aggregation and Attenuates Cerebral Inflammation: Impact for Therapy in Cardiovascular Disease. Circulation (2016) 133:2169–79. 10.1161/CIRCULATIONAHA.115.020633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sobrado M, Pereira MP, Ballesteros I, Hurtado O, Fernandez-Lopez D, Pradillo JM, et al. Synthesis of Lipoxin A4 by 5-Lipoxygenase Mediates PPARgamma-Dependent, Neuroprotective Effects of Rosiglitazone in Experimental Stroke. J Neurosci (2009) 29:3875–84. 10.1523/JNEUROSCI.5529-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wu L, Liu ZJ, Miao S, Zou LB, Cai L, Wu P, et al. Lipoxin A 4 Ameliorates Cerebral Ischaemia/Reperfusion Injury Through Upregulation of Nuclear Factor Erythroid 2-Related Factor 2. Neurol Res (2013) 35:968–75. 10.1179/1743132813Y.0000000242 [DOI] [PubMed] [Google Scholar]
- 66. Wu L, Li H-H, Wu Q, Miao S, Liu Z-J, Wu P, et al. Lipoxin A4 Activates Nrf2 Pathway and Ameliorates Cell Damage in Cultured Cortical Astrocytes Exposed to Oxygen-Glucose Deprivation/Reperfusion Insults. J Mol Neurosci (2015) 56:848–57. 10.1007/s12031-015-0525-6 [DOI] [PubMed] [Google Scholar]
- 67. Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers (2019) 5:56. 10.1038/s41572-019-0106-z [DOI] [PubMed] [Google Scholar]
- 68. Stegemann C, Drozdov I, Shalhoub J, Humphries J, Ladroue C, Didangelos A, et al. Comparative Lipidomics Profiling of Human Atherosclerotic Plaques. Circ Cardiovasc Genet (2011) 4:232–42. 10.1161/CIRCGENETICS.110.959098 [DOI] [PubMed] [Google Scholar]
- 69. Mai J, Liu W, Fang Y, Zhang S, Qiu Q, Yang Y, et al. The Atheroprotective Role of Lipoxin A4 Prevents oxLDL-induced Apoptotic Signaling in Macrophages Via JNK Pathway. Atherosclerosis (2018) 278:259–68. 10.1016/j.atherosclerosis.2018.09.025 [DOI] [PubMed] [Google Scholar]
- 70. Petri MH, Laguna-Fernandez A, Arnardottir H, Wheelock CE, Perretti M, Hansson GK, et al. Aspirin-Triggered Lipoxin A4 Inhibits Atherosclerosis Progression in Apolipoprotein E –/– Mice. Br J Pharmacol (2017) 174:4043–54. 10.1111/bph.13707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhou G, Ge S, Liu D, Xu G, Zhang R, Yin Q, et al. Atorvastatin Reduces Plaque Vulnerability in an Atherosclerotic Rabbit Model by Altering the 5-Lipoxygenase Pathway. Cardiology (2010) 115:221–8. 10.1159/000296017 [DOI] [PubMed] [Google Scholar]
- 72. Fredman G, Hellmann J, Proto JD, Kuriakose G, Colas RA, Dorweiler B, et al. An Imbalance Between Specialized Pro-Resolving Lipid Mediators and Pro-Inflammatory Leukotrienes Promotes Instability of Atherosclerotic Plaques. Nat Commun (2016) 7:12859. 10.1038/ncomms12859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Brennan EP, Mohan M, McClelland A, de Gaetano M, Tikellis C, Marai M, et al. Lipoxins Protect Against Inflammation in Diabetes-Associated Atherosclerosis. Diabetes (2018) 67:2657–67. 10.2337/db17-1317 [DOI] [PubMed] [Google Scholar]
- 74. Ho KJ, Spite M, Owens CD, Lancero H, Kroemer AHK, Pande R, et al. Aspirin-Triggered Lipoxin and Resolvin E1 Modulate Vascular Smooth Muscle Phenotype and Correlate With Peripheral Atherosclerosis. Am J Pathol (2010) 177:2116–23. 10.2353/ajpath.2010.091082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Spite M, Serhan CN. Novel Lipid Mediators Promote Resolution of Acute Inflammation: Impact of Aspirin and Statins. Circ Res (2010) 107:1170–84. 10.1161/CIRCRESAHA.110.223883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wu MY, Yiang GT, Liao WT, Tsai APY, Cheng YL, Cheng PW, et al. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol Biochem (2018) 46:1650–67. 10.1159/000489241 [DOI] [PubMed] [Google Scholar]
- 77. Chen Z, Wu Z, Huang C, Zhao Y, Zhou Y, Zhou X, et al. Effect of Lipoxin A4 on Myocardial Ischemia Reperfusion Injury Following Cardiac Arrest in a Rabbit Model. Inflammation (2013) 36:468–75. 10.1007/s10753-012-9567-x [DOI] [PubMed] [Google Scholar]
- 78. Zhao Q, Hu X, Shao L, Wu G, Du J, Xia J. LipoxinA4 Attenuates Myocardial Ischemia Reperfusion Injury Via a Mechanism Related to Downregulation of GRP-78 and caspase-12 in Rats. Heart Vessels (2014) 29:667–78. 10.1007/s00380-013-0418-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bi X, Zhang G, Wang X, Nguyen C, May HI, Li X, et al. Endoplasmic Reticulum Chaperone GRP78 Protects Heart From Ischemia/Reperfusion Injury Through Akt Activation. Circ Res (2018) 122:1545–54. 10.1161/CIRCRESAHA.117.312641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, et al. Myocarditis and Inflammatory Cardiomyopathy: Current Evidence and Future Directions. Nat Rev Cardiol (2020) 18:169–93. 10.1038/s41569-020-00435-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shi Y, Pan H, Zhang H-Z, Zhao X-Y, Jin J, Wang H-Y. Lipoxin A4 Mitigates Experimental Autoimmune Myocarditis by Regulating Inflammatory Response, NF-κb and PI3K/Akt Signaling Pathway in Mice. Eur Rev Med Pharmacol Sci (2017) 21:1850–9. [PubMed] [Google Scholar]
- 82. Aoyagi T, Matsui T. Phosphoinositide-3 Kinase Signaling in Cardiac Hypertrophy and Heart Failure. Curr Pharm Des (2011) 17:1818–24. 10.2174/138161211796390976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gupta S, Young D, Maitra RK, Gupta A, Popovic ZB, Yong SL, et al. Prevention of Cardiac Hypertrophy and Heart Failure by Silencing of NF-κb. J Mol Biol (2008) 375:637–49. 10.1016/j.jmb.2007.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jaén RI, Fernández-Velasco M, Terrón V, Sánchez-García S, Zaragoza C, Canales-Bueno N, et al. BML-111 Treatment Prevents Cardiac Apoptosis and Oxidative Stress in a Mouse Model of Autoimmune Myocarditis. FASEB J (2020) 34:10531–46. 10.1096/fj.202000611R [DOI] [PubMed] [Google Scholar]
- 85. Reina-Couto M, Carvalho J, Valente MJ, Vale L, Afonso J, Carvalho F, et al. Impaired Resolution of Inflammation in Human Chronic Heart Failure. Eur J Clin Invest (2014) 44:527–38. 10.1111/eci.12265 [DOI] [PubMed] [Google Scholar]
- 86. Johnson ER, Matthay MA. Acute Lung Injury: Epidemiology, Pathogenesis, and Treatment. J Aerosol Med Pulm Drug Deliv (2010) 23:243–52. 10.1089/jamp.2009.0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ortiz-Muñoz G, Mallavia B, Bins A, Headley M, Krummel MF, Looney MR. Aspirin-Triggered 15-Epi-Lipoxin A4 Regulates Neutrophil-Platelet Aggregation and Attenuates Acute Lung Injury in Mice. Blood (2014) 124:2625–34. 10.1182/blood-2014-03-562876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Matthay MA. Alveolar Fluid Clearance in Patients With ARDS: Does it Make a Difference? Chest (American Coll Chest Physicians) 122(6 Suppl):340S–3S. 10.1378/chest.122.6_suppl.340S [DOI] [PubMed] [Google Scholar]
- 89. Yang Y, Cheng Y, Lian QQ, Yang L, Qi W, Wu DR, et al. Contribution of CFTR to Alveolar Fluid Clearance by Lipoxin A4 Via PI3K/Akt Pathway in LPS-Induced Acute Lung Injury. Mediators Inflamm (2013) 2013. 10.1155/2013/862628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chen QF, Kuang XD, Yuan QF, Hao H, Zhang T, Huang YH, et al. Lipoxin A4 Attenuates LPS-Induced Acute Lung Injury Via Activation of the ACE2-Ang-(1-7)-Mas Axis. Innate Immun (2018) 24:285–96. 10.1177/1753425918785008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jin SW, Zhang L, Lian QQ, Liu D, Wu P, Yao SL, et al. Posttreatment With Aspirin-Triggered Lipoxin A4 Analog Attenuates Lipopolysaccharide-Induced Acute Lung Injury in Mice: The Role of Heme Oxygenase-1. Anesth Analg (2007) 104:369–77. 10.1213/01.ane.0000252414.00363.c4 [DOI] [PubMed] [Google Scholar]
- 92. Tang M, Chen L, Li B, Wang Y, Li S, Wen A, et al. BML-111 Attenuates Acute Lung Injury in Endotoxemic Mice. J Surg Res (2016) 200:619–30. 10.1016/j.jss.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 93. El Kebir D, Jozsef L, Pan W, Wang L, Petasis NA, Serhan CN, et al. 15-Epi-Lipoxin A4 Inhibits Myeloperoxidase Signaling and Enhances Resolution of Acute Lung Injury. Am J Respir Crit Care Med (2009) 180(4):311–9. 10.1164/rccm.200810-1601OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zheng S, D’Souza VK, Bartis D, Dancer RCA, Parekh D, Naidu B, et al. Lipoxin A4 Promotes Lung Epithelial Repair Whilst Inhibiting Fibroblast Proliferation. ERJ Open Res (2016) 2:00079-2015. 10.1183/23120541.00079-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li H, Shi H, Ma N, Zi P, Liu Q, Sun R. BML-111 Alleviates Acute Lung Injury Through Regulating the Expression of lncRNA Malat1. Arch Biochem Biophys (2018) 649:15–21. 10.1016/j.abb.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 96. Lv W, Lv C, Yu S, Yang Y, Kong H, Xie J, et al. Lipoxin A4 Attenuation of Endothelial Inflammation Response Mimicking Pancreatitis-Induced Lung Injury. Exp Biol Med (2013) 238:1388–95. 10.1177/1535370213502611 [DOI] [PubMed] [Google Scholar]
- 97. Liu H, Zhou K, Liao L, Zhang T, Yang M, Sun C. Lipoxin A4 Receptor Agonist BML-111 Induces Autophagy in Alveolar Macrophages and Protects From Acute Lung Injury by Activating MAPK Signaling. Respir Res (2018) 19:243. 10.1186/s12931-018-0937-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Li HB, Wang GZ, Gong J, Wu ZY, Guo S, Li B, et al. BML-111 Attenuates Hemorrhagic Shock-Induced Acute Lung Injury Through Inhibiting Activation of Mitogen-Activated Protein Kinase Pathway in Rats. J Surg Res (2013) 183:710–9. 10.1016/j.jss.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 99. Hu Q, Hu Z, Chen Q, Huang Y, Mao Z, Xu F, et al. BML-111 Equilibrated ACE-AngII-AT1R and ACE2-Ang-(1-7)-Mas Axis to Protect Hepatic Fibrosis in Rats. Prostaglandins Other Lipid Mediat (2017) 131:75–82. 10.1016/j.prostaglandins.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 100. Wang YZ, Zhang YC, Cheng JS, Ni Q, Li PJ, Wang SW, et al. BML-111, a Lipoxin Receptor Agonist, Ameliorates ‘Two-Hit’-Induced Acute Pancreatitis-Associated Lung Injury in Mice by the Upregulation of Heme Oxygenase-1. Artif Cells Nanomed Biotechnol (2014) 42:110–20. 10.3109/21691401.2013.794355 [DOI] [PubMed] [Google Scholar]
- 101. Lambrecht BN, Hammad H. The Immunology of Asthma. Nat Immunol (2015) 16:45–56. 10.1038/ni.3049 [DOI] [PubMed] [Google Scholar]
- 102. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet (2018) 391:783–800. 10.1016/S0140-6736(17)33311-1 [DOI] [PubMed] [Google Scholar]
- 103. Bhavsar PK, Levy BD, Hew MJ, Pfeffer MA, Kazani S, Israel E, et al. Corticosteroid Suppression of Lipoxin A4and Leukotriene B4from Alveolar Macrophages in Severe Asthma. Respir Res (2010) 11:71. 10.1186/1465-9921-11-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gras D, Bourdin A, Vachier I, De Senneville L, Bonnans C, Chanez P. An Ex Vivo Model of Severe Asthma Using Reconstituted Human Bronchial Epithelium. J Allergy Clin Immunol (2012) 129:1259–66.e1. 10.1016/j.jaci.2012.01.073 [DOI] [PubMed] [Google Scholar]
- 105. Wu S-H, Yin P-L, Zhang Y-M, Tao H-X. Reversed Changes of Lipoxin A 4 and Leukotrienes in Children With Asthma in Different Severity Degree. Pediatr Pulmonol (2010) 45:333–40. 10.1002/ppul.21186 [DOI] [PubMed] [Google Scholar]
- 106. Eke Gungor H, Tahan F, Gokahmetoglu S, Saraymen B. Decreased Levels of Lipoxin A4 and Annexin A1 in Wheezy Infants. Int Arch Allergy Immunol (2014) 163:193–7. 10.1159/000358490 [DOI] [PubMed] [Google Scholar]
- 107. Hasan RA, O’Brien E, Mancuso P. Lipoxin A 4 and 8-Isoprostane in the Exhaled Breath Condensate of Children Hospitalized for Status Asthmaticus. Pediatr Crit Care Med (2012) 13:141–5. 10.1097/PCC.0b013e3182231644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Fitzpatrick AM, Holguin F, Teague WG, Brown LAS. Alveolar Macrophage Phagocytosis is Impaired in Children With Poorly Controlled Asthma. J Allergy Clin Immunol (2008) 121:1372–8.e3. 10.1016/j.jaci.2008.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, et al. Multi-Pronged Inhibition of Airway Hyper-Responsiveness and Inflammation by Lipoxin A4. Nat Med (2002) 8:1018–23. 10.1038/nm748 [DOI] [PubMed] [Google Scholar]
- 110. Levy BD, Lukacs NW, Berlin AA, Schmidt B, Guilford WJ, Serhan CN, et al. Lipoxin A 4 Stable Analogs Reduce Allergic Airway Responses Via Mechanisms Distinct From CysLT1 Receptor Antagonism. FASEB J (2007) 21:3877–84. 10.1096/fj.07-8653com [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Martin N, Ruddick A, Arthur GK, Wan H, Woodman L, Brightling CE, et al. Primary Human Airway Epithelial Cell-Dependent Inhibition of Human Lung Mast Cell Degranulation. PLoS One (2012) 7:e43545. 10.1371/journal.pone.0043545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Duvall MG, Barnig C, Cernadas M, Ricklefs I, Krishnamoorthy N, Grossman NL, et al. Natural Killer Cell–Mediated Inflammation Resolution is Disabled in Severe Asthma. Sci Immunol (2017) 2(10):eaam5446. 10.1126/sciimmunol.aam5446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, et al. Lipoxin A4 Regulates Natural Killer Cell and Type 2 Innate Lymphoid Cell Activation in Asthma. Sci Transl Med (2013) 5(174):74ra26. 10.1126/scitranslmed.3004812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sanak M, Levy BD, Clish CB, Chiang N, Gronert K, Mastalerz L, et al. Aspirin-Tolerant Asthmatics Generate More Lipoxins Than Aspirin-Intolerant Asthmatics. Eur Respir J (2000) 16:44–9. 10.1034/j.1399-3003.2000.16a08.x [DOI] [PubMed] [Google Scholar]
- 115. Yamaguchi H, Higashi N, Mita H, Ono E, Komase Y, Nakagawa T, et al. Urinary Concentrations of 15-Epimer of Lipoxin A4 are Lower in Patients With Aspirin-Intolerant Compared With Aspirin-Tolerant Asthma. Clin Exp Allergy (2011) 41:1711–8. 10.1111/j.1365-2222.2011.03839.x [DOI] [PubMed] [Google Scholar]
- 116. Kong X, Wu S-H, Zhang L, Chen X-Q. Pilot Application of Lipoxin A4 Analog and Lipoxin A4 Receptor Agonist in Asthmatic Children With Acute Episodes. Exp Ther Med (2017) 14:2284–90. 10.3892/etm.2017.4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Meyer KC. Pulmonary Fibrosis, Part I: Epidemiology, Pathogenesis, and Diagnosis. Expert Rev Respir Med (2017) 11:343–59. 10.1080/17476348.2017.1312346 [DOI] [PubMed] [Google Scholar]
- 118. Rajagopal K, Bryant AJ, Sahay S, Wareing N, Zhou Y, Pandit LM, et al. Idiopathic Pulmonary Fibrosis and Pulmonary Hypertension: Heracles Meets the Hydra. Br J Pharmacol (2020) 178(1):172–86. 10.1111/bph.15036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sato Y, Kitasato H, Murakami Y, Hashimoto A, Endo H, Kondo H, et al. Down-Regulation of Lipoxin A4 Receptor by Thromboxane A 2 Signaling in RAW246.7 Cells In Vitro and Bleomycin-Induced Lung Fibrosis In Vivo. BioMed Pharmacother (2004) 58:381–7. 10.1016/j.biopha.2004.05.006 [DOI] [PubMed] [Google Scholar]
- 120. Wu SH, Wu XH, Lu C, Dong L, Chen ZQ. Lipoxin A4 Inhibits Proliferation of Human Lung Fibroblasts Induced by Connective Tissue Growth Factor. Am J Respir Cell Mol Biol (2006) 34:65–72. 10.1165/rcmb.2005-0184OC [DOI] [PubMed] [Google Scholar]
- 121. Roach KM, Feghali-Bostwick CA, Amrani Y, Bradding P. Lipoxin A 4 Attenuates Constitutive and TGF-β1–Dependent Profibrotic Activity in Human Lung Myofibroblasts. J Immunol (2015) 195:2852–60. 10.4049/jimmunol.1500936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Martins V, Valenca SS, Farias-Filho FA, Molinaro R, Simoes RL, Ferreira TP, et al. ATLa, an Aspirin-Triggered Lipoxin A4 Synthetic Analog, Prevents the Inflammatory and Fibrotic Effects of Bleomycin-Induced Pulmonary Fibrosis. J Immunol (2009) 182:5374–81. 10.4049/jimmunol.0802259 [DOI] [PubMed] [Google Scholar]
- 123. Guilherme RF, Xisto DG, Kunkel SL, Freire-De-lima CG, Rocco PRM, Neves JS, et al. Pulmonary Antifibrotic Mechanisms Aspirin-Triggered Lipoxin A4 Synthetic Analog. Am J Respir Cell Mol Biol (2013) 49:1029–37. 10.1165/rcmb.2012-0462OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Turcios NL. Cystic Fibrosis Lung Disease: An Overview. Respir Care (2020) 65:233–51. 10.4187/respcare.06697 [DOI] [PubMed] [Google Scholar]
- 125. Rang C, Keating D, Wilson J, Kotsimbos T. Re-Imagining Cystic Fibrosis Care: Next Generation Thinking. Eur Respir J (2020) 318:1902443. 10.1183/13993003.02443-2019 [DOI] [PubMed] [Google Scholar]
- 126. Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, et al. Defective Lipoxin-Mediated Anti-Inflammatory Activity in the Cystic Fibrosis Airway. Nat Immunol (2004) 5:388–92. 10.1038/ni1056 [DOI] [PubMed] [Google Scholar]
- 127. Higgins G, Torre CF, Tyrrell J, McNally P, Harvey BJ, Urbach V. Lipoxin A4 Prevents Tight Junction Disruption and Delays the Colonization of Cystic Fibrosis Bronchial Epithelial Cells by Pseudomonas Aeruginosa. Am J Physiol - Lung Cell Mol Physiol (2016) 310:L1053–61. 10.1152/ajplung.00368.2015 [DOI] [PubMed] [Google Scholar]
- 128. Mattoscio D, Evangelista V, De Cristofaro R, Recchiuti A, Pandolfi A, Di Silvestre S, et al. Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Expression in Human Platelets: Impact on Mediators and Mechanisms of the Inflammatory Response. FASEB J (2010) 24:3970–80. 10.1096/fj.10-159921 [DOI] [PubMed] [Google Scholar]
- 129. Wu H, Yang J, Su EM, Li L, Zhao C, Yang X, et al. Lipoxin A4 and Platelet Activating Factor are Involved in E. Coli or LPS-Induced Lung Inflammation in CFTR-Deficient Mice. PLoS One (2014) 9(3):e93003. 10.1371/journal.pone.0093003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Buchanan PJ, McNally P, Harvey BJ, Urbach V. Lipoxin A4-Mediated KATP Potassium Channel Activation Results in Cystic Fibrosis Airway Epithelial Repair. Am J Physiol - Lung Cell Mol Physiol (2013) 305. 10.1152/ajplung.00058.2013 [DOI] [PubMed] [Google Scholar]
- 131. Al-Alawi M, Buchanan P, Verriere V, Higgins G, McCabe O, Costello RW, et al. Physiological Levels of Lipoxin A4 Inhibit ENaC and Restore Airway Surface Liquid Height in Cystic Fibrosis Bronchial Epithelium. Physiol Rep (2014) 2. 10.14814/phy2.12093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Verrière V, Higgins G, Al-Alawi M, Costello RW, McNally P, Chiron R, et al. Lipoxin A4 Stimulates Calcium-Activated Chloride Currents and Increases Airway Surface Liquid Height in Normal and Cystic Fibrosis Airway Epithelia. PLoS One (2012) 7(5):e37746. 10.1371/journal.pone.0037746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Malek M, Nematbakhsh M. Renal Ischemia/Reperfusion Injury; From Pathophysiology to Treatment. J Renal Inj Prev (2015) 4:20–7. 10.12861/jrip.2015.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Leonard MO, Hannan K, Burne MJ, Lappin DWP, Doran P, Coleman P, et al. 15-Epi-16-(Para-Fluorophenoxy)-Lipoxin A(4)-Methyl Ester, a Synthetic Analogue of 15-Epi-Lipoxin A(4), is Protective in Experimental Ischemic Acute Renal Failure. J Am Soc Nephrol (2002) 13:1657–62. 10.1097/01.asn.0000015795.74094.91 [DOI] [PubMed] [Google Scholar]
- 135. Kieran NE, Doran PP, Connolly SB, Greenan M-C, Higgins DF, Leonard M, et al. Modification of the Transcriptomic Response to Renal Ischemia/Reperfusion Injury by Lipoxin Analog. Kidney Int (2003) 64:480–92. 10.1046/j.1523-1755.2003.00106.x [DOI] [PubMed] [Google Scholar]
- 136. Wu S-H, Chen X-Q, Lü J, Wang M-J. BML-111 Attenuates Renal Ischemia/Reperfusion Injury Via Peroxisome Proliferator-Activated Receptor-α-Regulated Heme Oxygenase-1. Inflammation (2016) 39:611–24. 10.1007/s10753-015-0286-y [DOI] [PubMed] [Google Scholar]
- 137. Wu S-H, Wang M-J, Lü J, Chen X-Q. Signal Transduction Involved in Lipoxin A4-induced Protection of Tubular Epithelial Cells Against Hypoxia/Reoxygenation Injury. Mol Med Rep (2017) 15:1682–92. 10.3892/mmr.2017.6195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Börgeson E, Docherty NG, Murphy M, Rodgers K, Ryan A, O’Sullivan TP, et al. Lipoxin A 4 and Benzo-Lipoxin A 4 Attenuate Experimental Renal Fibrosis. FASEB J (2011) 25:2967–79. 10.1096/fj.11-185017 [DOI] [PubMed] [Google Scholar]
- 139. Brennan EP, Nolan KA, Börgeson E, Gough OS, McEvoy CM, Docherty NG, et al. Lipoxins Attenuate Renal Fibrosis by Inducing let-7c and Suppressing TGF β R1. J Am Soc Nephrol (2013) 24:627–37. 10.1681/ASN.2012060550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Goicoechea M, Sanchez-Niño MD, Ortiz A, García de Vinuesa S, Quiroga B, Bernis C, et al. Low Dose Aspirin Increases 15-Epi-Lipoxin A4 Levels in Diabetic Chronic Kidney Disease Patients. Prostaglandins Leukot Essent Fatty Acids (2017) 125:8–13. 10.1016/j.plefa.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 141. Brennan EP, Mohan M, McClelland A, Tikellis C, Ziemann M, Kaspi A, et al. Lipoxins Regulate the Early Growth Response–1 Network and Reverse Diabetic Kidney Disease. J Am Soc Nephrol (2018) 29:1437–48. 10.1681/ASN.2017101112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal Diseases. Nat Rev Dis Primers (2017) 3:17038. 10.1038/nrdp.2017.38 [DOI] [PubMed] [Google Scholar]
- 143. Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, et al. Reduced Inflammation and Tissue Damage in Transgenic Rabbits Overexpressing 15-Lipoxygenase and Endogenous Anti-Inflammatory Lipid Mediators. J Immunol (2003) 171:6856–65. 10.4049/jimmunol.171.12.6856 [DOI] [PubMed] [Google Scholar]
- 144. Van Dyke TE. Pro-Resolving Mediators in the Regulation of Periodontal Disease. Mol Aspects Med (2017) 58:21–36. 10.1016/j.mam.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Pouliot M, Clish CB, Petasis NA, Van Dyke TE, Serhan CN. Lipoxin A4 Analogues Inhibit Leukocyte Recruitment to Porphyromonas Gingivalis: A Role for Cyclooxygenase-2 and Lipoxins in Periodontal Disease. Biochemistry (2000) 39:4761–8. 10.1021/bi992551b [DOI] [PubMed] [Google Scholar]
- 146. Doğan B, Fentoğlu Ö, Kırzıoğlu FY, Kemer ES, Köroğlu BK, Aksu O, et al. Lipoxin A4 and Neutrophil/Lymphocyte Ratio: A Possible Indicator in Achieved Systemic Risk Factors for Periodontitis. Med Sci Monit (2015) 21:2485–93. 10.12659/MSM.895115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Doğan ESK, Doğan B, Fentoğlu Ö, Kirzioğlu FY. The Role of Serum Lipoxin A4 Levels in the Association Between Periodontal Disease and Metabolic Syndrome. J Periodontal Implant Sci (2019) 49:105–13. 10.5051/jpis.2019.49.2.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Tobón-Arroyave SI, Isaza-Guzmán DM, Gómez-Ortega J, Flórez-Alzate AA. Salivary Levels of Specialized Pro-Resolving Lipid Mediators as Indicators of Periodontal Health/Disease Status. J Clin Periodontol (2019) 46:978–90. 10.1111/jcpe.13173 [DOI] [PubMed] [Google Scholar]
- 149. Pouliot M, Serhan CN. Lipoxin A4 and Aspirin-Triggered 15-Epi-LXA4 Inhibit Tumor Necrosis Factor-α-Initiated Neutrophil Responses and Trafficking: Novel Regulators of a Cytokine-Chemokine Axis Relevant to Periodontal Diseases. J Periodontal Res (1999) 34:370–3. 10.1111/j.1600-0765.1999.tb02268.x [DOI] [PubMed] [Google Scholar]
- 150. Ali M, Yang F, Jansen JA, Walboomers XF. Lipoxin Suppresses Inflammation Via the TLR4/Myd88/NF-κb Pathway in Periodontal Ligament Cells. Oral Dis (2019) 26(2):429–38. 10.1111/odi.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Börgeson E, Lönn J, Bergström I, Brodin VP, Ramström S, Nayeri F, et al. Lipoxin A4 Inhibits Porphyromonas Gingivalis-Induced Aggregation and Reactive Oxygen Species Production by Modulating Neutrophil-Platelet Interaction and CD11b Expression. Infect Immun (2011) 79:1489–97. 10.1128/IAI.00777-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Gaudin A, Tolar M, Peters OA. Lipoxin A4 Attenuates the Inflammatory Response in Stem Cells of the Apical Papilla Via ALX/FPR2. Sci Rep (2018) 8:8921. 10.1038/s41598-018-27194-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Yang YH, Morand E, Leech M. Annexin A1: Potential for Glucocorticoid Sparing in RA. Nat Rev Rheumatol (2013) 9:595–603. 10.1038/nrrheum.2013.126 [DOI] [PubMed] [Google Scholar]
- 154. Baschant U, Lane NE, Tuckermann J. The Multiple Facets of Glucocorticoid Action in Rheumatoid Arthritis. Nat Rev Rheumatol (2012) 8:645–55. 10.1038/nrrheum.2012.166 [DOI] [PubMed] [Google Scholar]
- 155. Zhang L, Zhang X, Wu P, Li H, Jin S, Zhou X, et al. BML-111, a Lipoxin Receptor Agonist, Modulates the Immune Response and Reduces the Severity of Collagen-Induced Arthritis. Inflamm Res (2008) 57:157–62. 10.1007/s00011-007-7141-z [DOI] [PubMed] [Google Scholar]
- 156. Conte FP, Menezes-De-Lima OJ, Verri WA, Cunha FQ, Penido C, Henriques MG. Lipoxin A 4 Attenuates Zymosan-Induced Arthritis by Modulating Endothelin-1 and its Effects. Br J Pharmacol (2010) 161:911–24. 10.1111/j.1476-5381.2010.00950.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kronke G, Katzenbeisser J, Uderhardt S, Zaiss MM, Scholtysek C, Schabbauer G, et al. 12/15-Lipoxygenase Counteracts Inflammation and Tissue Damage in Arthritis. J Immunol (2009) 183:3383–9. 10.4049/jimmunol.0900327 [DOI] [PubMed] [Google Scholar]
- 158. Sodin-Semrl S, Spagnolo A, Barbaro B, Varga J, Fiore S. Lipoxin A4 Counteracts Synergistic Activation of Human Fibroblast-Like Synoviocytes. Int J Immunopathol Pharmacol (2004) 17:15–25. 10.1177/039463200401700103 [DOI] [PubMed] [Google Scholar]
- 159. Fiore S, Antico G, Aloman M, Sodin-Semrl S. Lipoxin A4 Biology in the Human Synovium. Role of the ALX Signaling Pathways in Modulation of Inflammatory Arthritis. Prostaglandins Leukot Essent Fatty Acids (2005) 73:189–96. 10.1016/j.plefa.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 160. Habouri L, El Mansouri FE, Ouhaddi Y, Lussier B, Pelletier JP, Martel-Pelletier J, et al. Deletion of 12/15-Lipoxygenase Accelerates the Development of Aging-Associated and Instability-Induced Osteoarthritis. Osteoarthr Cartil (2017) 25:1719–28. 10.1016/j.joca.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 161. Yang Y, Wang Y, Kong Y, Zhang X, Zhang H, Gang Y, et al. The Therapeutic Effects of Lipoxin A 4 During Treadmill Exercise on Monosodium Iodoacetate-Induced Osteoarthritis in Rats. Mol Immunol (2018) 103:35–45. 10.1016/j.molimm.2018.08.027 [DOI] [PubMed] [Google Scholar]
- 162. Das UN. Lipoxins as Biomarkers of Lupus and Other Inflammatory Conditions. Lipids Health Dis (2011) 10:76. 10.1186/1476-511X-10-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Das UN. Current and Emerging Strategies for the Treatment and Management of Systemic Lupus Erythematosus Based on Molecular Signatures of Acute and Chronic Inflammation. J Inflamm Res (2010) 3:143–70. 10.2147/JIR.S9425 [DOI] [PMC free article] [PubMed] [Google Scholar]