Abstract
Limited evidence is available regarding the long-term effects of fine particulate (PM2.5) air pollution on hypertension in developing countries. This study aimed to explore the associations of long-term exposure to PM2.5 with hypertension prevalence and blood pressure (BP) in China. We conducted a cross-sectional study based on a nationally representative survey (13,975 participants). We estimated the long-term average exposure to PM2.5 for all subjects during the study period (June 2011 to March 2012) by a satellite-based model with a spatial resolution of 10 × 10 km. We applied multivariable logistic regression models to evaluate the associations between PM2.5 and hypertension prevalence and linear regression models for the associations between PM2.5 and systolic BP and diastolic BP. We also explored potential effect modification by stratification analyses. There were 5715 cases of hypertension, accounting for 40.9% of the study population in this analysis. The annual mean exposure to PM2.5 for all participants was 72.8 μg/m3 on average. An interquartile range increase (IQR, 41.7 μg/m3) in PM2.5 was associated with higher prevalence of hypertension with an odds ratio of 1.11 [95% confidence interval (CI): 1.05, 1.17]. Systolic BP increased by 0.60 mmHg (95% CI: 0.05, 1.15) per an IQR increase in PM2.5. The effects of PM2.5 on hypertension prevalence were stronger among middle-aged, obese and urban participants. This national study indicated that long-term exposure to PM2.5 was associated with increased prevalence of hypertension and slightly higher systolic BP in China.
Keywords: Hypertension, Blood pressure, Fine particulate matter, Ambient air pollution, Cross-sectional study
Graphical Abstract
1. Introduction
High blood pressure (BP) is a well-established risk factor for cardiovascular morbidity and mortality (Brook et al., 2010; Lawes et al., 2008; Pope and Dockery, 2006). It was evidenced that high BP was a leading single risk factor for the global burden of diseases, and there were two-thirds of adults with hypertension living in developing countries (Lim et al., 2012). Recent studies revealed that the average level of systolic blood pressure (SBP) was declining in developed countries but increasing in low-income and middle-income countries (Bromfield and Muntner, 2013). Therefore, identifying the potential risk factors for hypertension is of great significance to global public health.
Epidemiological studies have reported that short-term exposure to fine particulate matter (PM2.5) was significantly associated with hypertension and BP variations (Auchincloss et al., 2008; Dai et al., 2016; Dvonch et al., 2009). However, the long-term effects of PM2.5 on hypertension were less reported and the results were inconsistent. For example, two cross-sectional studies in Asia found significant associations of long-term PM10 exposure with hypertension prevalence and increased BP (Chuang et al., 2011; Dong et al., 2013). However, another cross-sectional study in Germany found insignificant associations between PM2.5 exposure and hypertension prevalence (Fuks et al., 2011).
China is a developing country with severe air pollution problems. Several national surveys have revealed that hypertension prevalence was rapidly increasing and the number of hypertension patients was estimated to exceed 300 million by 2025 (He, 2016; Kearney et al., 2005; Li et al., 2016; Li et al., 2012). Thus, it is of great public health importance to explore the long-term health effects of PM2.5 on BP in China. Therefore, we aimed to explore the associations between long-term exposure to ambient PM2.5 and hypertension prevalence and BP. This is a cross-sectional study based on the China Health and Retirement Longitudinal Study (CHARLS) project.
2. Material and methods
2.1. Study population
We obtained data from the baseline survey of the CHARLS project, which started in June 2011 and finished in March 2012. Details of this project have been documented in previous publications (Zhao et al., 2014). In brief, to ensure the national representativeness of the project, the study populations were selected by a four-stage, stratified and cluster sampling method from 28 provinces (150 counties or districts) of China. A total of 17,708 middle-aged and elderly (age range: 35–100 years) residents from urban and rural areas were enrolled. Locations of the study sites were shown in Fig. 1. Face-to-face interviews were performed using a standard questionnaire to collect basic information on socio-demography (home address, age, sex, educational level), housing conditions (types of energy used for cooking and heating) and health status. Non-response rate of the survey was 19.5%, which was due to refusal (8.8%), inaccessibility of contact (8.2%) and other reasons (2.5%).
Fig. 1.
Location of the study sites in the CHARLS project.
2.2. Health data
The health survey consisted of a self-reported questionnaire and a physical examination. Overall, 13,975 participants completed the whole survey. Information were collected on smoking status, alcohol consumption and whether they had physician-diagnosed hypertension. Standardized resting BP measurements were performed by trained nurses using electronic sphygmomanometers (OMRON Corporation, HEM-7200). All electronic sphygmomanometers were factory calibrated before physical examinations. Participants were told not to smoke, eat or drink alcohol within 30 min before the test. Each participant was instructed to rest for 10 min after arrival. Left upper arm BP at sitting position was measured 3 times under the guidance of our staff. We considered the measurements unstable if the differences of the last two readings were over 5 mmHg, and another one to three measurements were taken until the differences were within 5 mmHg. The second and third measurements were averaged to calculate the SBP and DBP. In this analysis, hypertension was defined as: (1) individuals who reported having diagnosed hypertension or (2) had an average measured SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, or both.
2.3. Exposure assessment
The average PM2.5 concentrations at participants’ addresses over the study period were generated by a satellite-based exposure assessment model. Detailed information on the model specifications has been documented elsewhere (Ma et al., 2016). Monthly PM2.5 concentrations in China were estimated at a resolution of 10 km × 10 km, and were then averaged over the study period. Results of cross-validation showed that this model could well capture the monthly and seasonal variation of historical PM2.5 (R2 = 0.79 and relative prediction error = 35.6%).
To allow for the adjustment of ozone when assessing the effects of PM2.5 on BP, we obtained data on annual-average ozone concentration at a 10 × 10 km resolution estimated by the 2013 Global Burden of Disease project (Brauer et al., 2016). We also derived daily mean temperature of each study site from China’s National Meteorological Information Center, and then averaged the data over the study period.
At last, we geocoded the participants’ home addresses and assigned exposure measurements in ArcGIS software (ESRI Corporation). To be specific, the averaged concentration in each grid cell (10 km × 10 km) was merged with the geographic shape files with information on the official region boundaries of China. The estimates of exposure were then equally assigned to the participants that resided in the same grid.
2.4. Statistical analysis
We applied multivariable logistic regression models to explore the health effects of PM2.5 on the prevalence of hypertension. In the basic model, we controlled for the following variables as potential confounders based on previous studies in this field (Chan et al., 2015; Chen et al., 2015; Dong et al., 2013): sex, age, educational level (low: illiterate; medium: ≤ 6 years; high: > 6 years), BMI, smoking history (current smokers; ex-smokers, quitted smoking ≥3 years; non-smokers), pack-years (years of smoking multiplied by packs per day) for current smokers, frequency of alcohol consumption (none, less than or once a month, more than once a month), types of heating resources (clean: solar power, electricity, natural gas, central heating; unclean: coal and biomass; others) and types of energy for cooking (clean and unclean). In the fully-adjusted model (the main model), we further controlled for annual mean temperature because a number of studies had reported an inverse association between temperature and BP (Su et al., 2014; Wang et al., 2017). We also added a factor variable of season (i.e., summer, winter and spring) into the main model to account for potential seasonal variations in BP (Lewington et al., 2012; Woodhouse et al., 1993). At last, we introduced ozone into the main model because it was reported to affect BP (Chuang et al., 2011; Hoffmann et al., 2012).
We used multivariate linear regression models to examine the associations between PM2.5 and BP. The covariates in this model were exactly the same as those in above-mentioned logistic regression models.
We conducted a stratification analysis to test whether the above associations could be modified by age (less than or equals 60 years and over 60 years), sex (males and females), educational level (low, ≤ 6 years; high >6 years), BMI (< 24 kg/m2 and ≥24 kg/m2, in accordance with the Chinese criteria on defining normal-weight and over-weight) (Zhou, 2002), smoking status (yes; no: never and former), drinking (yes and no), heating energy (clean and unclean) and cooking energy (clean and unclean). The statistical significance of effect modification was tested by including an interaction term between PM2.5 and a potential modifier.
We examined the sensitivity of our results by using the alternative definitions of hypertension. In addition to the main definition, we used self-reported hypertension and diagnostic hypertension with SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg that was measured in this survey.
All the statistical analyses were conducted in SPSS Statistics 22.0 (IBM Corporation). We used two-sided statistical tests, and P values smaller than 0.05 were considered to be statistically significant. The effect estimates for hypertension prevalence were presented as ORs and their 95% confidence interval (CIs) per an interquartile range (IQR) increase in PM2.5 concentrations. The effect estimates for SBP and DBP were presented as the absolute mean changes and their 95% CIs associated with an IQR increase in PM2.5 concentrations.
3. Results
The basic characteristics of study participants are summarized in Table 1. Our study population consisted of 13,975 middle-aged or older residents with a mean age of 59.3 years and an approximately equal sex distribution (47.2% males and 52.8% females). Nearly 65% participants had educational attainment <6 years. The averaged BMI was 23.9 kg/m2. A total of 29.2% participants were current smokers and 24.2% drunk more than once a month. Generally, there were weak or moderate correlations among the covariates included in the main model (data not shown). There were 5715 hypertension patients, accounting for 40.9% of the study population. The averaged SBP and DBP were 130.6 mmHg and 75.9 mmHg, respectively.
Table 1.
Descriptive statistics of the study participants (n = 13.974).
| Variables | Value |
|---|---|
| Sociodemographic characteristics | |
| Age (years, mean ± SD) | 59.3 ± 9.9 |
| Sex (%) | |
| Male | 46.7 |
| Female | 53.3 |
| Educational level (%) | |
| Low | 28.6 |
| Medium | 40.7 |
| High | 30.7 |
| BMI (kg/m2, mean ± SD) | 23.9 ± 3.6 |
| Smoking status (%) | |
| Current | 30.6 |
| Former | 8.9 |
| Never | 60.4 |
| Pack-years of cigarette for current smokers | 23.4 ± 21.0 |
| Alcohol-consumption frequency (%) | |
| ≥1/month | 24.4 |
| <1/month | 7.8 |
| Never | 67.8 |
| Residence (%) | |
| Urban | 37.1 |
| Rural | 62.9 |
| Type of heating energy (%) | |
| Clean (solar energy, electricity, central heating) | 26.1 |
| Unclean (coal or biomass) | 56.4 |
| Others | 17.5 |
| Type of cooking energy (%) | |
| Clean (solar energy, electricity, natural gas) | 43.6 |
| Unclean (coal or biomass) | 56.4 |
| Prevalence of hypertension (%) | 40.9 |
| Blood pressure | |
| Systolic (mm Hg, mean ± SD) | 129.6 ± 21.5 |
| Diastolic (mm Hg, mean ± SD) | 75.5 ± 12.2 |
Abbreviations: SD, standard deviation; BMI, body mass index.
Table 2 shows the summary statistics on environmental variables. The annual average exposure to PM2.5 varied greatly among study participants. The mean of residential PM2.5 exposure was 72.8 μg/m3, which is much higher than the interim target-3 (35 μg/m3) of the Air Quality Guidelines issued by the World Health Organization (WHO, 2006). The annual mean O3 concentration (average: 62.6 μg/m3) and temperature (average: 14.1 °C) also varied appreciably in this analysis.
Table 2.
Summary statistics on PM2.5, O3 and temperature during the study period.
| Variables | Mean | SD | Min | Median | Max | IQR |
|---|---|---|---|---|---|---|
| PM2.5a (μg/m3) | 72.8 | 27.4 | 25.5 | 69.6 | 127.9 | 41.7 |
| O3b (μg/m3) | 62.6 | 5.9 | 43.2 | 63.6 | 79.1 | 4.7 |
| Temperaturec (°C) | 14.1 | 4.9 | 0.7 | 14.7 | 23.2 | 4.8 |
Abbreviation: IQR, interquartile range; SD, standard deviation; PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 μm; O3, ozone.
Data derived from a study by Ma et al. and used in the main analyses.
Data derived from the database of the 2013 Global Burden of Disease project.
Data derived from China’s National Meteorological Information Center.
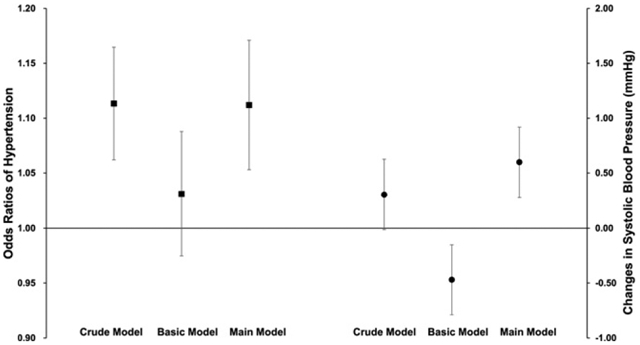
As shown in Table 3, the OR of hypertension per an IQR increment in PM2.5 (41.7 μg/m3) was 1.11 (95%CI: 1.06, 1.16) in the crude (unadjusted) model. The effect estimate was positive but statistically insignificant (OR = 1.03, 95%CI: 0.97,1.09) in the basic models and turned to be statistically significant in the fully-adjusted (main) model (OR = 1.11, 95%CI: 1.05, 1.17).
Table 3.
Odds ratio (and its 95% confidence interval) of hypertension, increments (mmHg, mean and 95% CI) in systolic BP and diastolic BP associated with an interquartile range increase in PM2.5.
| Model | Covariates | OR | Systolic BP | Diastolic BP |
|---|---|---|---|---|
| Crude | Unadjusted | 1.11 (1.06,1.16) | 0.31 (−0.24,0.85) | 0.18 (−0.13,0.49) |
| Basic | Area, age, sex, education, BMI, smoking status, pack-years, alcohol consumption, heating energy and cooking energy | 1.03 (0.97,1.09) | −0.47 (−1.00,0.06) | −0.43 (−0.75,−0.12) |
| Main | Basic model + temperature + O3 + season | 1.11 (1.05,1.17) | 0.60 (0.05,1.15) | 0.02 (−0.30,0.34) |
Abbreviations: OR, odds ratio; PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 μm; CI: confidence interval; IQR, interquartile range; BP, blood pressure.
The specifications for the crude, basic and main model were the same for OR, systolic BP and diastolic BP.
Table 3 also summarizes the associations between PM2.5 and BP measurements. The effects of PM2.5 on SBP was statistically significant only in the main model, in which an IQR increase in PM2.5 was associated with an increment of 0.60 (95%CI: 0.05, 1.15) mmHg. However, we did not observe a significant association between PM2.5 and DBP in all models.
Table 4 presents the results of stratification analyses. For hypertension prevalence, the ORs were significantly larger among those who were ≤60 years old, had a BMI ≥ 24 kg/m2 or resided in urban areas.
Table 4.
Odds ratios of hypertension and estimated absolute increase in systolic BP and diastolic BP (mmHg) (with 95% confidence intervals) associated with an interquartile range (41.7 μg/m3) increase in PM2.5 stratified by potential modifiers.
| Variables | Categories | N (%) | ORs | P for interaction | Systolic BP (mmHg) | Diastolic BP (mmHg) |
|---|---|---|---|---|---|---|
| Age | ≤60 | 6898 (49.4) | 1.15 (1.07,1.22) | 0.008 | 1.31 (0.66,1.96) | 0.41 (0.01,0.82) |
| >60 | 7074 (50.6) | 1.07 (0.97,1.16) | −0.33 (−1.33,0.66) | −0.58 (−1.08,0.08) | ||
| Sex | Males | 6528 (46.7) | 1.17 (1.08,1.26) | 0.164 | 0.96 (0.17,1.75) | 0.35 (−0.13,0.82) |
| Females | 7436 (53.3) | 1.06 (0.98,1.14) | 0.47 (−0.30,1.25) | −0.26 (−0.69,0.16) | ||
| Educational level | Low | 9672 (69.2) | 1.10 (1.03,1.17) | 0.160 | 0.77 (0.07,1.47) | 0.05 (−0.34,0.44) |
| High | 4295 (30.8) | 1.11 (0.99,1.21) | 0.40 (−0.51,1.30) | −0.09 (−0.66,0.49) | ||
| BMI | <24 kg/m2 | 7282 (53.4) | 1.08 (0.99,1.16) | 0.011 | 0.33 (−0.42,1.07) | −0.33 (−0.75,0.09) |
| ≥24 kg/m2 | 6347 (46.6) | 1.16 (1.07,1.24) | 1.17 (0.33,2.01) | 0.48 (0.02,0.97) | ||
| Smoking status | Yes | 4272 (30.6) | 1.16 (1.05,1.27) | 0.423 | 0.48 (−0.19,1.14) | 0.07 (−0.53,0.67) |
| No | 9686 (69.4) | 1.09 (1.02,1.16) | 1.19 (0.19,2.19) | −0.01 (−0.38,0.37) | ||
| Drinking status | Yes | 4493 (32.2) | 1.21 (1.10,1.31) | 0.482 | 0.80 (−0.15,1.75) | 0.27 (−0.31,0.85) |
| No | 9472 (67.8) | 1.07 (0.99,1.14) | 0.65 (−0.03,1.33) | −0.10 (−0.48,0.28) | ||
| Heating energy | Clean | 6065 (43.6) | 1.04(0.94,1.13) | 0.474 | −1.06 (−2.09,0.04) | −0.59 (−1.11,−0.08) |
| Unclean | 7835 (56.4) | 1.17 (1.09,1.25) | 2.01 (1.26,2.76) | 0.58 (0.15,1.01) | ||
| Cooking energy | Clean | 6072 (46.6) | 1.12 (1.03,1.21) | 0.797 | 0.06 (−0.5,0.63) | 0.07 (−0.37,0.51) |
| Unclean | 7857 (56.4) | 1.10 (1.02,1.18) | 1.11 (0.34,1.89) | −0.14 (−0.62,0.34) | ||
| Location | Rural | 8794 (62.9) | 1.05 (0.98,1.13) | 0.001 | 0.76 (0.06,1.46) | 0.01 (−0.39,0.42) |
| Urban | 5180 (37.1) | 1.18 (1.08,1.28) | 0.44 (−0.5,1.39) | 0.03 (−0.51,0.56) |
Abbreviations: BP, blood pressure; OR, odds ratio; CI, confidence interval; BMI: body mass index; PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 μm; IQR, interquartile range.
Categories for variables were dichotomized: educational level (low ≤6 years; high >6 years); heating and cooking energy (clean: electricity, solar power or natural gas; unclean: coal or firewood).
As shown in Table S1, the use of alternative definitions resulted in an appreciably lower prevalence of hypertension (24.3% or 30.8%) than the main definition (40.9%). Accordingly, the estimated OR per an IQR increase of PM2.5 was also appreciably changed, but was still statistically significant.
4. Discussion
This cross-sectional study demonstrated that long-term exposure to ambient PM2.5 was significantly associated with increased prevalence of hypertension and slightly elevated levels of SBP in China. Our estimate was relatively robust to different definitions of hypertension. In addition, the effects of PM2.5 on hypertension were particularly stronger among middle-aged, obese and urban residents. Up to our knowledge, this is the first nationwide study in China to explore the long-term effects of air pollution on hypertension and BP.
Although there are abundant evidence in short-term studies to assess the effects of air pollution on BP, limited knowledge is available on the long-term associations. In our study, we estimated an OR of 1.11 (95%CI: 1.05, 1.17) in hypertension prevalence associated with an IQR (41.7 μg/m3) increase in long-term average PM2.5 concentrations. Our finding was comparable to another cross-sectional study in three northern cities of China, which reported an OR of 1.12 (95%CI: 1.08, 1.16) per an IQR (19 μg/m3) increase in PM10 (Dong et al., 2013). However, a similar study in Germany failed to find an association of one-year PM2.5 exposure with hypertension prevalence (Fuks et al., 2011). The inconsistency may be explained by the differences in PM levels and composition as well as the susceptibility of populations.
We found a weak but statistically significant association between PM2.5 and SBP in the fully-adjusted model. The previous findings on the association between PM2.5 and BP were mixed. For example, Chan et al. reported in the Sister study that a 10 μg/m3 increase in long-term PM2.5 was significantly associated with 1.4 mmHg (95% CI: 0.6, 2.3) higher SBP, but not with DBP (Chan et al., 2015). A cross-sectional study involving 27,752 elderly residents in Taiwan reported an increment of 0.73 mmHg (95%CI: 0.44, 1.03) in DBP per 10 μg/m3 increase in PM10, but null association with SBP (Chen et al., 2015). However, another similar study in Taiwan found significant effects of PM2.5 on both SBP and DBP (Chuang et al., 2011). These inconsistent findings might be due to the heterogeneity in PM mixture, population characteristics (age structure, ethnicity, lifestyle, etc.), exposure assessment methodology, as well as the adjustment for confounders and the in-between collinearity. For example, the use of fixed-site monitoring data as in the aforementioned studies may cause exposure misclassification and eventually bias the results (Auchincloss et al., 2008; Dong et al., 2013).
Identification of potentially susceptible subgroups was crucial to reduce the adverse effects of air pollution. In stratification analyses, PM2.5 had stronger effect on urban residents, probably because they were more likely to be exposed to higher levels of PM2.5. Also, consistent with a previous study (Zhang et al., 2016), we found larger effects of PM2.5 in middle-aged participants (45–60 years). The sympathetic and autonomic nervous system might be less responsive to external stimuli in the elderly (Cohen et al., 2012). Old residents may also spend more time indoors, reducing their exposure to ambient PM2.5. In addition, our inability to adjust for antihypertensive medication led to an imprecise estimate among the elderly who had a large proportion of hypertension patients (40% in the present study). Besides, we observed stronger associations between PM2.5 and hypertension and BP in obese participants, which were consistent with two other studies (Dong et al., 2015; Zhao et al., 2013). This susceptibility might be caused by the inherent inflammation state and higher inhalation rate in obese people (Brochu et al., 2014; Dubowsky et al., 2006).
Although the exact mechanisms behind the association between PM2.5 and elevated BP were unclear, the proposed biological pathways were plausible. The activation of pulmonary reflexes induced by inhalation of PM2.5 may lead to autonomic nervous system imbalance (Brook et al., 2009). Hypertrophic remodeling of resistance vessels may cause medial thickness, which will also result in BP elevations (Valavanidis et al., 2008). Besides, PM2.5 could induce systematic inflammation, oxidative stress, endothelial dysfunction and DNA methylation (Brook et al., 2010; Pope and Dockery, 2006; Wang et al., 2016), resulting in elevated BP.
Our study had two strengths. First, this is the first nationwide study in China to explore the association between long-term exposure to PM2.5 and hypertension prevalence and blood pressure. Second, we utilized a satellite-based spatial statistical model to predict the exposure of PM2.5, which was especially valuable in areas without regular air quality monitors.
Some limitations should also be noted. First, this was a cross-sectional study design, and thus a causal relationship between PM2.5 and BP could not be obtained. Second, exposure misclassification was inevitable because individual-level monitoring was impractical and the spatial resolution of our exposure model was still not high enough which would probably lead to an underestimate on the associations (Sheppard et al., 2012). Third, the data on the use of antihypertensive agents was not available in this study, which might have confounded our estimations on PM2.5 and BP values. Fourth, potential individual-level confounders such as physical activity, dietary structure, occupational history and time-location activity pattern were not evaluated in this study due to the lack of data. Fifth, traffic noise was another confounder that we failed to control. This might not be a big problem because this is a nationwide study covering urban and rural areas, and only a very small fraction of participants was supposed to reside near main roads.
5. Conclusions
Our study demonstrated an association of long-term exposure to PM2.5 with higher prevalence of hypertension and slightly increased SBP in China. The effects of PM2.5 on hypertension were particularly stronger among middle-aged, obese and urban participants. Our findings added to the existing evidence with regard to the long-term effects of PM2.5 on hypertension from a large developing country with severe air pollution problems.
Supplementary Material
Acknowledgements
The study was supported by the Public Welfare Research Program of National Health and Family Planning Commission of China (201502003), the BSR division of the National Institute on Aging (1R01AG037031-03S and R03AG049144), National Natural Science Foundation of China (91643205, 71130002 and 71450001), China Medical Board Collaborating Program (13-152), and Cyrus Tang Foundation (CTF-FD2014001).
The authors thank all the staff of the CHARLS project for collecting the health data. We thank Dr. Michael Brauer at the University of British Columbia for providing the data of O3 from the 2013 Global Burden of Disease project.
The following is the supplementary data related to this article.Table S1. Odds ratios (point estimates and 95% CIs) of hypertension using different definitions associated with an interquartile range increase (41.7 μg/m3) in PM2.5 in the fully-adjusted model. Table S1 provided the results of sensitivity analysis to test the robustness of different definitions of hypertension. Supplementary data associated with this article can be found in the online version, at http://dx.doi.org/10.1016/j.scitotenv.2017.01.133.
Footnotes
Competing financial interests
The authors declare they have no actual or potential competing financial interests.
References
- Auchincloss AH, Diez Roux AV, Dvonch JT, Brown PL, Barr RG, Daviglus ML, et al. , 2008. Associations between recent exposure to ambient fine particulate matter and blood pressure in the multi-ethnic study of atherosclerosis (MESA). Environ. Health Perspect 116, 486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer M, Freedman G, Frostad J, van Donkelaar A, Martin RV, Dentener F, et al. , 2016. Ambient air pollution exposure estimation for the global burden of disease 2013. Environ. Sci. Technol 50, 79–88. [DOI] [PubMed] [Google Scholar]
- Brochu P, Bouchard M, Haddad S, 2014. Physiological daily inhalation rates for health risk assessment in overweight/obese children, adults, and elderly. Risk Anal. 34, 567–582. [DOI] [PubMed] [Google Scholar]
- Bromfield S, Muntner P, 2013. High blood pressure: the leading global burden of disease risk factor and the need for worldwide prevention programs. Curr. Hypertens. Rep 15, 134–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, et al. , 2009. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension 54, 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. , 2010. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121, 2331–2378. [DOI] [PubMed] [Google Scholar]
- Chan SH, Van Hee VC, Bergen S, Szpiro AA, DeRoo LA, London SJ, et al. , 2015. Long-term air pollution exposure and blood pressure in the sister study. Environ. Health Perspect 123, 951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Wu CF, Lee JH, Hoffmann B, Peters A, Brunekreef B, et al. , 2015. Associations between long-term air pollutant exposures and blood pressure in elderly residents of Taipei City: a cross-sectional study. Environ. Health Perspect 123, 779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang KJ, Yan YH, Chiu SY, Cheng TJ, 2011. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in Taiwan. Occup. Environ. Med 68, 64–68. [DOI] [PubMed] [Google Scholar]
- Cohen L, Curhan GC, Forman JP, 2012. Influence of age on the association between lifestyle factors and risk of hypertension. J. Am. Soc. Hypertens 6, 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Koutrakis P, Coull BA, Sparrow D, Vokonas PS, Schwartz JD, 2016. Use of the adaptive LASSO method to identify PM2.5 components associated with blood pressure in elderly men: the veterans affairs normative aging study. Environ. Health Perspect 124, 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong GH, Qian ZM, Xaverius PK, Trevathan E, Maalouf S, Parker J, et al. , 2013. Association between long-term air pollution and increased blood pressure and hypertension in China. Hypertension 61, 578–584. [DOI] [PubMed] [Google Scholar]
- Dong GH, Wang J, Zeng XW, Chen L, Qin XD, Zhou Y, et al. , 2015. Interactions between air pollution and obesity on blood pressure and hypertension in Chinese children. Epidemiology 26, 740–747. [DOI] [PubMed] [Google Scholar]
- Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR, 2006. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ. Health Perspect 114, 992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvonch JT, Kannan S, Schulz AJ, Keeler GJ, Mentz G, House J, et al. , 2009. Acute effects of ambient particulate matter on blood pressure: differential effects across urban communities. Hypertension 53, 853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuks K, Moebus S, Hertel S, Viehmann A, Nonnemacher M, Dragano N, et al. , 2011. Long-term urban particulate air pollution, traffic noise, and arterial blood pressure. Environ. Health Perspect 119, 1706–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, 2016. Hypertension in China: a large and increasing public health challenge. J. Hypertens 34, 29–31. [DOI] [PubMed] [Google Scholar]
- Hoffmann B, Luttmann-Gibson H, Cohen A, Zanobetti A, de Souza C, Foley C, et al. , 2012. Opposing effects of particle pollution, ozone, and ambient temperature on arterial blood pressure. Environ. Health Perspect 120, 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J, 2005. Global burden of hypertension: analysis of worldwide data. Lancet 365, 217–223. [DOI] [PubMed] [Google Scholar]
- Lawes CM, Vander Hoorn S, Rodgers A, 2008. Global burden of blood-pressure-related disease, 2001. Lancet 371, 1513–1518. [DOI] [PubMed] [Google Scholar]
- Lewington S, Li L, Sherliker P, Guo Y, Millwood I, Bian Z, et al. , 2012. Seasonal variation in blood pressure and its relationship with outdoor temperature in 10 diverse regions of China: the China Kadoorie Biobank. J. Hypertens 30, 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Wang LM, Jiang Y, Li XY, Zhang M, Hu N, 2012. Prevalence of hypertension among Chinese adults in 2010. Zhonghua Yu Fang Yi Xue Za Zhi 46, 409–413. [PubMed] [Google Scholar]
- Li W, Gu H, Teo KK, Bo J, Wang Y, Yang J, et al. , 2016. Hypertension prevalence, awareness, treatment, and control in 115 rural and urban communities involving 47,000 people from China. J. Hypertens 34, 39–46. [DOI] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, et al. , 2012. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 380, 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Hu X, Sayer AM, Levy R, Zhang Q, Xue Y, et al. , 2016. Satellite-based spatio-temporal trends in PM2.5 concentrations: China, 2004–2013. Environ. Health Perspect 124, 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA 3rd, Dockery DW, 2006. Health effects of fine particulate air pollution: lines that connect. J. Air Waste Manage. Assoc 56, 709–742. [DOI] [PubMed] [Google Scholar]
- Sheppard L, Burnett RT, Szpiro AA, Kim SY, Jerrett M, Pope CA 3rd, et al. , 2012. Confounding and exposure measurement error in air pollution epidemiology. Air Qual. Atmos. Health 5, 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su D, Du H, Zhang X, Qian Y, Chen L, Chen Y, et al. , 2014. Season and outdoor temperature in relation to detection and control of hypertension in a large rural Chinese population. Int. J. Epidemiol 43, 1835–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valavanidis A, Fiotakis K, Vlachogianni T, 2008. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health. C Environ. Carcinog. Ecotoxicol. Rev 26, 339–362. [DOI] [PubMed] [Google Scholar]
- Wang C, Chen R, Cai J, Shi J, Yang C, Tse LA, et al. , 2016. Personal exposure to fine particulate matter and blood pressure: a role of angiotensin converting enzyme and its DNA methylation. Environ. Int 94, 661–666. [DOI] [PubMed] [Google Scholar]
- Wang Q, Li C, Guo Y, Barnett AG, Tong S, Phung D, et al. , 2017. Environmental ambient temperature and blood pressure in adults: a systematic review and meta-analysis. Sci. Total Environ 575, 276–286. [DOI] [PubMed] [Google Scholar]
- WHO, 2006. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005: Summary of Risk Assessment.
- Woodhouse PR, Khaw KT, Plummer M, 1993. Seasonal variation of blood pressure and its relationship to ambient temperature in an elderly population. J. Hypertens 11, 1267–1274. [PubMed] [Google Scholar]
- Zhang Z, Laden F, Forman JP, Hart JE, 2016. Long-term exposure to particulate matter and self-reported hypertension: a prospective analysis in the Nurses’ health study. Environ. Health Perspect [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Qian ZM, Wang J, Vaughn MG, Liu YQ, Ren WH, et al. , 2013. Does obesity amplify the association between ambient air pollution and increased blood pressure and hypertension in adults? Findings from the 33 communities Chinese health study. Int. J. Cardiol 168, e148–e150. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hu Y, Smith JP, Strauss J, Yang G, 2014. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int. J. Epidemiol 43, 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BF, 2002. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults - study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed. Environ. Sci 15, 83–96. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


