Abstract
Fasting induces lipid accumulation in the liver, while the mechanisms by which fasting dysregulates liver fatty acid oxidation are not clear. Fatty acid ω-oxidation is induced in the fasting state, and administration of dicarboxylic acids to fasting animals decreases plasma ketone bodies. We hypothesized that endogenous dicarboxylic acids might play a role in controlling mitochondrial β-oxidation in fasting animals. A peroxisome proliferator-activated receptor-alpha agonist and an inhibitor for peroxisomal β-oxidation were administered to the fasting rats to investigate the role of dicarboxylic acids in liver fatty acid oxidation and lipid homeostasis. We observed that excessive β-oxidation of endogenous dicarboxylic acids by peroxisomes generated considerable levels of succinate in the liver. Excessive succinate oxidation subsequently increased the mitochondrial NADH/NAD+ ratio and led to an accumulation of 3-OH-CoA and 2-enoyl-CoA intermediates in the liver. This further induced feedback suppression of mitochondrial β-oxidation and promoted hepatic lipid deposition and steatosis. Specific inhibition of peroxisomal β-oxidation attenuated fasting-induced lipid deposition in the liver by reducing succinate production and enhancing mitochondrial fatty acid oxidation. We conclude that suppression of mitochondrial β-oxidation by oxidation of dicarboxylic acids serves as a mechanism for fasting-induced hepatic lipid accumulation and identifies cross talk between peroxisomal and mitochondrial fatty acid oxidation.
Keywords: fatty acid oxidation, ketogenesis, peroxisome, dicarboxylic acid, peroxisome proliferator activator receptor α
Abbreviations: 3-OH-CoA, 3-hydroxyacyl-CoA; ABCD1, peroxisomal ATP-binding cassette transporter D; AcAc, acetoacetate; ACS, acyl-CoA synthetase; ACOX1, acyl-CoA oxidase-1; βOHB, β-hydroxybutyrate; C12, laurate; C12-CoA, CoA thioester of laurate; CFB, clofibrate; CPT1, carnitine palmitoyltransferase-1; DCA, dicarboxylic acid; DCA12, decanedicarboxylic acid; DC12-CoA, mono-CoA thioester of decanedicarboxylic acid; FFA, free fatty acid; KB, ketone body; L-BP, L-bifunctional protein; LC-acyl-CoA, long-chain acyl-CoA; LCAD, long-chain acyl-CoA dehydrogenase; LCADH, long-chain alcohol dehydrogenase; MCAD, medium-chain acyl-CoA dehydrogenase; NAFLD, nonalcoholic fatty liver disease; PPARα, peroxisome proliferator activator receptor α isoform; TAG, triacylglyceride; TBARS, thiobarbituric acid reactive substances; TDYA, 10,12-tricosadiynoic acid; thiolase, peroxisomal 3-oxoacyl-CoA thiolase
The regulation of ketogenesis by carbohydrate has been established, and malonyl-CoA was identified to be the molecule responsible for the inhibition of carnitine palmitoyltransferase-1a (CPT1a), the critical enzyme in the transfer of long-chain fatty acids into mitochondria, thereby inhibiting ketogenesis and stimulating fatty acid synthesis (1, 2). However, it is striking to note that under the condition of fasting when liver malonyl-CoA is depleted and fatty acid synthesis is suppressed (3), excessive uptake of free fatty acids (FFAs) induced lipid accumulation in the liver (4, 5, 6); therefore, a mechanism might work for the suppression of mitochondrial β-oxidation that is independent of malonyl-CoA when the supply of carbohydrate is deficient.
To investigate the potential mechanism, we focused on dicarboxylic acids (DCAs), the product of mono fatty acid that subjected to ω-oxidation as discovered in animals in the 1930s (7). In mammalians, DCAs are generated from mono fatty acids that are catalyzed firstly by CYP4A1, followed by alcohol dehydrogenase and aldehyde dehydrogenase (8). It was reported that exogenous administration of long-chain DCAs to fasting rats rapidly and robustly decreased plasma ketone body, although the mechanism of action is unclear (9, 10, 11), indicating that endogenous DCAs might play a role in regulating mitochondrial β-oxidation.
It is generally accepted that DCAs are almost exclusively metabolized by peroxisomal β-oxidation system (12, 13, 14). Both fatty acid ω-oxidation and peroxisomal fatty acid oxidation are extensively induced in fasting animals (15, 16, 17), indicating accelerated oxidation of endogenous DCAs under ketogenic condition. Previous report indicated that peroxisomal β-oxidation and mitochondrial fatty acid metabolism system are mutually competitive, inhibition of peroxisomal β-oxidation stimulated mitochondrial β-oxidation (18). We hypothesized that peroxisomal oxidation of DCAs might suppress mitochondrial β-oxidation and lead to lipid accumulation in the liver of the fasting animals.
This study investigated the effect of peroxisomal DCA oxidation on mitochondrial β-oxidation as well as the potential mechanism by which prolonged fasting induced hepatic lipid accumulation.
Results
Generation of DCAs and peroxisomal β-oxidation were induced in the liver of the fasting rats
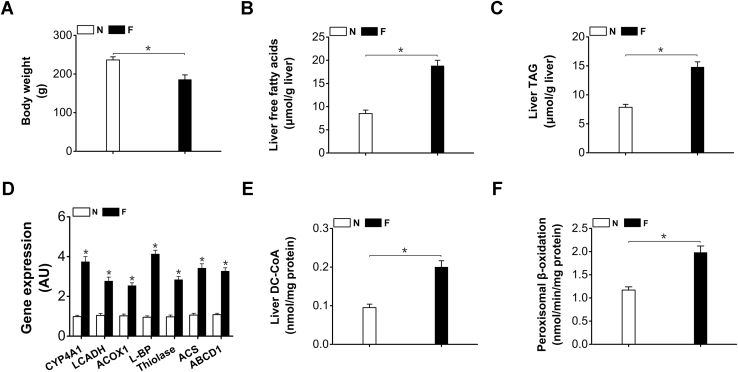
Body weight of the rats decreased after 48-h fasting (Fig. 1A). Liver FFAs and triacylglyceride (TAG) increased significantly in the fasting rats (Fig. 1, B and C). The gene expressions of the enzymes involved in fatty acids ω-oxidation and peroxisomal β-oxidation were upregulated in the livers of the fasting rats (Fig. 1D). Liver dicarboxylyl-CoA (DC-CoA) increased considerably in the fasting rats (by 111% versus normal control) (Fig. 1E). Peroxisomal β-oxidation was enhanced in fasting rats (by 69% versus normal control) (Fig. 1F). The results indicated that in fasting state the generation of DCAs and peroxisomal β-oxidation were induced simultaneously, as DCAs showed antiketogenic activity and were proposed to be metabolized in peroxisomes, induction of peroxisomal β-oxidation of DCAs in the fasting animals might play a role in controlling mitochondrial fatty acid oxidation.
Figure 1.
A, body weight loss in the fasting rats.B, liver free fatty acids were remarkably increased in the fasting rats. C, liver TAG increased significantly in the fasting rats. D, gene expression of fatty acids ω-oxidation and peroxisomal β-oxidation in liver were upregulated by fasting. E, liver DC-CoA was significantly higher in fasting rats. F, liver peroxisomal β-oxidation was robustly induced in fasting rats. ABCD1, peroxisomal ATP-binding cassette transporter D; ACOX1, acyl-CoA oxidase-1; ACS, acyl-CoA synthetase; L-BP, L-bifunctional protein; LCADH, long-chain alcohol dehydrogenase; Thiolase, peroxisomal 3-oxoacyl-CoA thiolase. Mean ± SEM, n = 8, ∗p < 0.05 by t-test between paired conditions.
DCAs are exclusively oxidized by peroxisomal β-oxidation system
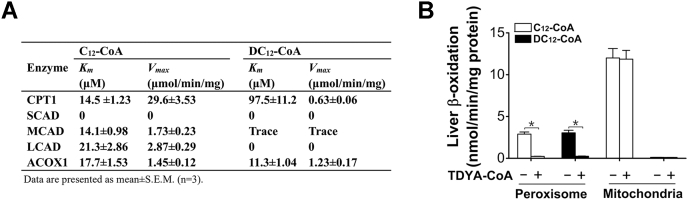
To get a conclusive proof on the cellular compartmentation of DCA oxidation, the kinetic constants of enzymes involved in mitochondrial or peroxisomal β-oxidation for DC12-CoA (mono-CoA thioester of dodecanedioic acid) and C12-CoA (CoA thioester of dodecanoic acid) were summarized in Figure 2A. For CPT1, the enzyme responsible for the transportation of long-chain fatty acids into mitochondria, its Vmax value for DC12-CoA was obviously lower than that of C12-CoA. Moreover, its Km value for DC12-CoA was much higher (97.5 μM versus 14.5 μM of C12-CoA). For short-chain (SCAD), medium-chain (MCAD), and long-chain acyl-CoA dehydrogenases (LCAD), which catalyze the first step of mitochondrial β-oxidation, no significant activities for DC12-CoA were observed. ACOX1, the rate-limiting enzyme of peroxisomal β-oxidation, had comparable Km and Vmax values for DC12-CoA and C12-CoA. Using isolated liver peroxisomes and mitochondria, the capacity of mitochondrial or peroxisomal β-oxidation for DC12- and C12-CoA was studied (Fig. 2B). Peroxisomal β-oxidation capacity for DC12-CoA was equivalent to that for C12-CoA, whereas mitochondrial β-oxidation system showed no significant activity toward DC12-CoA. The activities of peroxisomal β-oxidation for both DC12- and C12-CoA were completely abolished by pretreatment with TDYA-CoA, a specific inhibitor for ACOX1 (18). These results confirmed that DCAs are exclusively oxidized by peroxisomal β-oxidation system.
Figure 2.
A, kinetic parameters of key enzymes involved in peroxisomal and mitochondrial β-oxidation with C12-CoA and DC12-CoA as substrates. Mean ± SEM, n = 3. B, liver peroxisomal and mitochondrial β-oxidation activities with C12-CoA and DC12-CoA as a substrate respectively. ACOX1, acyl-CoA oxidase-1; CPT1, carnitine palmitoyltransferase-1; LCAD, long-chain acyl-CoA dehydrogenase; MCAD, medium-chain acyl-CoA dehydrogenase; SCAD, short-chain acyl-CoA dehydrogenase. Mean ± SEM, n = 6, ∗p < 0.05 by t-test between paired conditions.
Peroxisomal oxidation of DCAs suppressed mitochondrial β-oxidation in liver homogenate
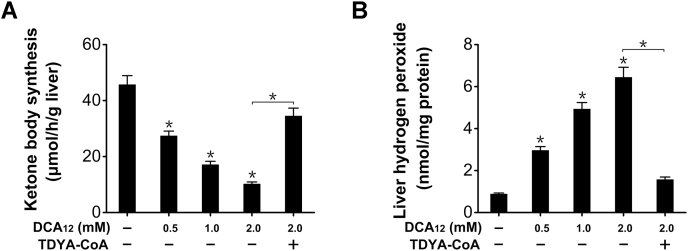
Since DCAs are endogenous substrates for peroxisomal fatty acid β-oxidation (FAO), increased DCAs will be rapidly metabolized by peroxisomes. Addition of dodecanedioic acid (DCA12) into liver homogenates was used to study the effect of DCA metabolism on mitochondrial β-oxidation and ketogenesis in vitro. Ketone body synthesis from hexanoate (C6) by liver homogenate was suppressed by DCA12 in a dose-dependent manner (Fig. 3A). Addition of DCA12 into liver homogenates increased generation of hydrogen peroxide, a by-product of peroxisomal β-oxidation, as reduced by TDYA-CoA (Fig. 3B). The results supported the hypothesis that peroxisomal β-oxidation of DCAs suppressed mitochondrial β-oxidation and ketogenesis.
Figure 3.
A, ketone body synthesis by liver homogenate was suppressed in the presence of DCA12.B, DCA12 addition elevated hydrogen peroxide content in liver homogenate, as lowered by pretreatment with TDYA-CoA. Mean ± SEM, n = 6, ∗p < 0.05 by one-way ANOVA with Dunnett’s T3 test compared with the control or t-test between paired conditions.
Peroxisomal oxidation of DCAs generated succinate and led to accumulation of 3-OH-CoA/2-enoyl-CoA intermediates by elevating mitochondrial NADH/NAD+ ratio
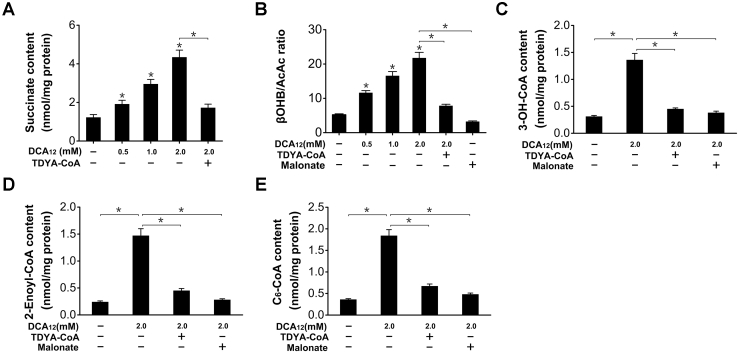
To address the mechanism by which DCAs caused diminished mitochondrial β-oxidation, we noted that mitochondrial β-oxidation flux is controlled by mitochondrial NADH/NAD+ ratio, and high NADH/NAD+ ratio suppresses mitochondrial β-oxidation (19, 20). It was striking to note that succinate is the ultimate product of DCAs subjected to peroxisomal β-oxidation (21, 22), and excessive succinate oxidation caused robust elevation in mitochondrial NADH/NAD+ ratio and suppression of mitochondrial fatty acid oxidation (19, 23, 24, 25, 26, 27, 28). Therefore, we proposed that the diminished mitochondrial β-oxidation as caused by DCAs was attributed to generation of excessive succinate and increased NADH/NAD+ ratio within mitochondria. As expected, addition of DCA12 to liver homogenates generated succinate dose-dependently, as completely abolished by the treatment with TDYA-CoA, a specific inhibitor for peroxisomal β-oxidation (Fig. 4A). With hexanoate as a substrate, addition of DCA12 strongly elevated β-hydroxybutyrate (βOHB)/acetoacetate (AcAc) ratio (by 316% versus control), an indicator for mitochondrial NADH/NAD+ ratio, as completely abolished by the pretreatment with TDYA-CoA and malonate, a specific inhibitor for succinate dehydrogenase (29) (Fig. 4B). The activity of mitochondrial 3-hydroxyacyl-CoA dehydrogenase is strongly dependent of NADH/NAD+ ratio (30), which might cause accumulation of fatty acid oxidation intermediates. Addition of DCA12 into liver homogenates resulted in accumulation of 3-hydroxyacyl-CoA (3-OH-CoA) and 2-enoyl-CoA intermediates in the presence of hexanoate (Fig. 4, C and D), which were reduced by pretreatment with TDYA-CoA or malonate. As 3-OH-CoA and 2-enoyl-CoA are feedback inhibitors for mitochondrial β-oxidation (31, 32, 33), DCA12 treatment also caused accumulation of hexanoyl-CoA (C6-CoA) in liver homogenates with hexanoate as a substrate, as abolished by TDYA-CoA or malonate (Fig. 4E). The results suggested that the diminished mitochondrial β-oxidation and ketogenesis caused by DCAs were attributed to succinate generation, excessive succinate oxidation robustly elevated mitochondrial NADH/NAD+ ratio and led to accumulation of fatty acid oxidation intermediates.
Figure 4.
A, addition of DC12-CoA into liver homogenate generated succinate dose-dependently, as completely abolished by pretreatment with TDYA-CoA.B, DCA12 addition elevated βOHB/AcAc ratio dose-dependently in liver homogenate. C and D, addition of DCA12 into liver homogenate led to accumulation of 3-OH-CoA (C) and 2-enoyl-CoA (D) intermediates with hexanoate as a substrate, as reduced by pretreatment with TDYA-CoA or malonate. E, addition of DCA12 into liver homogenates led to accumulation of hexanoyl-CoA (C6-CoA) with hexanoate as a substrate, as lowered by pretreatment with TDYA-CoA or malonate. Mean ± SEM, n = 6, ∗p < 0.05 by one-way ANOVA with Dunnett’s T3 test compared with the control or t-test between paired conditions.
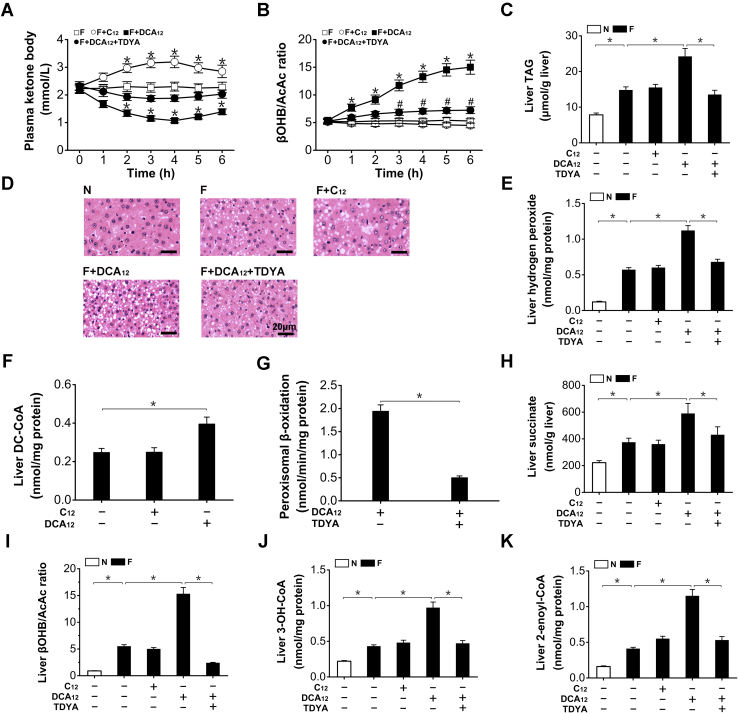
Administration of DCAs suppressed ketogenesis and exacerbated lipid deposition in the fasting rats
To increase hepatic peroxisomal dicarboxylic acid oxidation, DCA12 was administered to fasting rats. Administration of DCA12 to fasting rats caused a rapid and sharp decrease in plasma ketone body (KB) (by 51% after treatment for 4-h), as abolished by pretreatment with TDYA, while dodecanoic acid (C12) treatment significantly elevated plasma KB in the fasting rats (Fig. 5A). DCA12 significantly elevated plasma βOHB/AcAc ratio (by 183% versus fasting control after treatment for 6-h), as decreased by TDYA, and no significant effect was observed in C12-treated rats (Fig. 5B). Administration of DCA12 exacerbated hepatic TAG accumulation in the fasting rats (increased by 64% versus fasting control), as reduced by pretreatment with TDYA, while no significant alteration in C12-treated rats (Fig. 5C). The diameters of fat droplets in the liver sections of fasting rats (1.34 ± 0.19 μm) were increased by DCA12 treatment (3.87 ± 0.68 μm), as reduced by TDYA (1.32 ± 0.17 μm) (Fig. 5D). Hepatic hydrogen peroxide content in the livers of the fasting rats was significantly increased, further elevated by DCA12 treatment (by 96% versus fasting control), as reduced by TDYA (Fig. 5E). The administration of exogenous DCA12 significantly elevated hepatic content of DC-CoA (Fig. 5F). Peroxisomal β-oxidation was effectively inhibited by TDYA, a specific inhibitor for the rate-limiting enzyme ACOX1 (Fig. 5G). Liver succinate level was significantly higher in the fasting rats compared with the fed rats, DCA12 treatment greatly stimulated succinate formation in the fasting rats while TDYA reduced liver succinate generation, C12 treatment did not affect liver succinate level (Fig. 5H). Liver βOHB/AcAc ratio was elevated significantly by fasting, as further enhanced by DCA12 and decreased by TDYA (Fig. 5I). Liver 3-OH-CoA and 2-enoyl-CoA contents were significantly higher in the fasting rats than that of the fed rats, administration of DCA12 caused accumulation of 3-OH-CoA and 2-enoyl-CoA intermediates, which were reduced by TDYA, while no significant alteration in C12-treated rats (Fig. 5, J and K). The results suggested that excessive oxidation of DCAs suppressed mitochondrial β-oxidation and led to lipid deposition in the fasting rats through generation of succinate and elevation in βOHB/AcAc ratio, which caused accumulation of fatty acid oxidation intermediates.
Figure 5.
A, changes in plasma ketone body after treatment with DCA12, C12, and TDYA in fasting rats.B, DCA12 robustly elevated plasma βOHB/AcAc ratio in the fasting rats, as reduced by TDYA. C, DCA12 treatment exacerbated hepatic TAG accumulation in the fasting rats, as reduced by TDYA. D, effects of DCA12 or TDYA upon liver histological changes induced by fasting. Magnification: 200×. Scale bar = 20 μm. E, DCA12 treatment increased hydrogen peroxide generation in the livers of the fasting rats, as decreased by TDYA. F, DCA12 treatment increased liver DC-CoA content in the fasting rats. G, liver peroxisomal β-oxidation was strongly suppressed by TDYA in the fasting rats. H, liver succinate increased in the fasting rats, as further elevated by treatment with DCA12 and reduced by TDYA. I, fasting significantly elevated liver βOHB/AcAc ratio compared with the fed rats, DCA12 treatment further enhanced βOHB/AcAc ratio in the liver of the fasting rats, as lowered by TDYA. J and K, DCA12 treatment led to hepatic accumulation of fatty acid oxidation intermediates 3-OH-CoA (J) and 2-enoyl-CoA (K), as reduced by pretreatment with TDYA. Mean ± SEM, n = 8, ∗p < 0.05 by t-test between paired conditions.
Peroxisomal oxidation of endogenous DCAs led to hepatic lipid deposition in the fasting rats
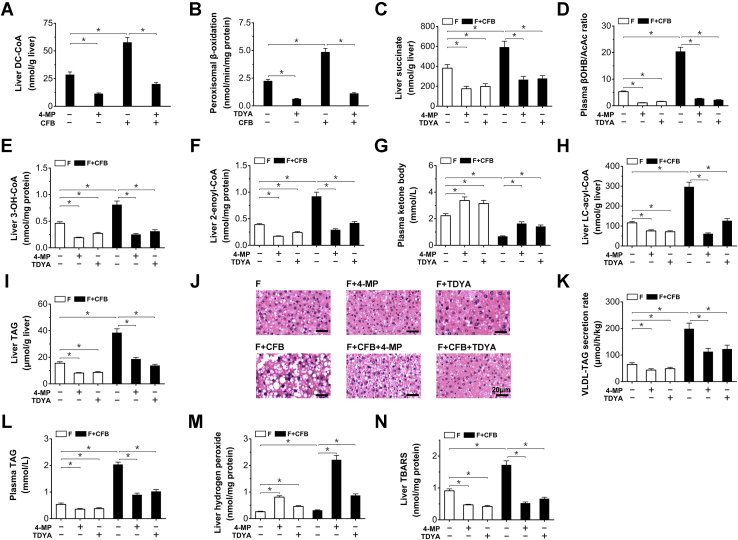
Under ketogenic conditions such as fasting, the hepatic uptake of FFA was remarkably enhanced. To enhance the endogenous generation and oxidation of DCA, Clofibrate (CFB) as a classic peroxisome proliferator-activated receptor α (PPARα) agonist was administered to the fasting rats to induce fatty acid ω-oxidation and peroxisomal β-oxidation (34, 35, 36, 37). 4-Methypyrazole (4-MP), an inhibitor for alcohol dehydrogenase (38), was administered to inhibit endogenous DCA generation.
Liver DC-CoA content in the fasting rats was increased by CFB treatment and decreased by the treatment with 4-MP (Fig. 6A). Peroxisomal β-oxidation was robustly elevated in fasting rats receiving CFB (by 116% versus fasting control) and lowered by TDYA (Fig. 6B). Liver succinate increased significantly in the fasting rats treated with CFB, as reduced by the 4-MP or TDYA (Fig. 4C). CFB treatment robustly increased plasma βOHB/AcAc ratio in the fasting rats (by 285% versus fasting control), as reduced by 4-MP or TDYA (Fig. 6D). Liver 3-OH-CoA and 2-enoyl-CoA contents were significantly higher after CFB treatment, as reduced by TDYA or 4-MP (Fig. 6, E and F). Administration of TDYA or 4-MP to the fasting rats led to increased KB formation, CFB treatment strongly decreased plasma KB in the fasting rats (by 70% versus control rats), as recovered by TDYA or 4-MP (Fig. 6G). Liver long-chain acyl-CoAs were further elevated in CFB treated rats and reduced by the treatment with 4-MP or TDYA (Fig. 6H). Liver TAG seriously accumulated after treatment with CFB (increased by 148% versus fasting control), as reduced by pretreatment with TDYA or 4-MP (Fig. 6I). The densities of fat droplets in the liver sections of fasting rats (5.2 ± 0.9/1000 μm2) were increased by CFB treatment (22.4 ± 4.1/1000 μm2), as reduced by TDYA or 4-MP (13.5 ± 1.3/1000 μm2 and 14.7 ± 1.5/1000 μm2 for F+CFB + TDYA and F+CFB+4-MP group, respectively). Liver lipid droplets in CFB-treated group were mainly macrovascular (with average diameters of 4.74 ± 1.32 μm) while the lipid droplets of 4-MP or TDYA-treated rats were microvascular (with average diameters of 1.79 ± 0.32 and 1.64 ± 0.21 μm for F+CFB+4-MP and F+CFB + TDYA group, respectively) (Fig. 6J). VLDL-TAG secretion rate increased significantly in the fasting rats treated with CFB (Fig. 6K), which led to elevated plasma TAG (Fig. 6L), 4-MP or TDYA treatment decreased VLDL-TAG secretion rate and plasma TAG level in the fasting rats. Administration of CFB stimulated hydrogen peroxide generation in the livers of fasting rats, as abolished by pretreatment with 4-MP or TDYA (Fig. 6M). Liver lipid peroxidation estimated by thiobarbituric acid reactive substances (TBARS) was increased by CFB and reduced by the treatment with 4-MP or TDYA (Fig. 6N). The results indicated that peroxisomal oxidation of endogenous DCAs suppressed mitochondrial β-oxidation and led to hepatic lipid deposition in the fasting rats.
Figure 6.
A, liver DC-CoA content was increased by CFB treatment in the fasting rats, as reduced by 4-MP.B, liver peroxisomal β-oxidation activity was enhanced by the treatment with CFB and inhibited by TDYA. C, CFB treatment increased succinate generation in the liver of the fasting rats, as reduced by 4-MP or TDYA. D, CFB robustly elevated plasma βOHB/AcAc ratio in the fasting rats, as reduced by 4-MP or TDYA. E and F, CFB treatment resulted in hepatic accumulation of 3-OH-CoA (E) and 2-enoyl-CoA (F) intermediates in the fasting rats, as reduced by treatment with 4-MP or TDYA. G, TDYA or 4-MP treatment stimulated ketone body formation in the fasting rats, while CFB decreased plasma ketone body. H, liver LC-acyl-CoA was significantly higher in CFB treated rats and reduced by the treatment with 4-MP or TDYA. I, CFB treatment led to hepatic TAG accumulation in the fasting rats, as reduced by 4-MP or TDYA. J, Effects of CFB, 4-MP or TDYA upon hepatic steatosis in the fasting rats. Magnification: 200×. Scale bar = 20 μm. K, CFB treatment increased VLDL-TAG secretion rate in the fasting rats, as reduced by the treatment with 4-MP or TDYA. L, CFB treatment increased plasma TAG level in the fasting rats, as reduced by 4-MP or TDYA. M, CFB treatment increased hydrogen peroxide generation in the liver of the fasting rat, as reduced by 4-MP or TDYA. N, liver TBARS content increased significantly by CFB and reduced by the treatment with 4-MP or TDYA. Mean ± SEM, n = 8, ∗p < 0.05 by t-test between paired conditions.
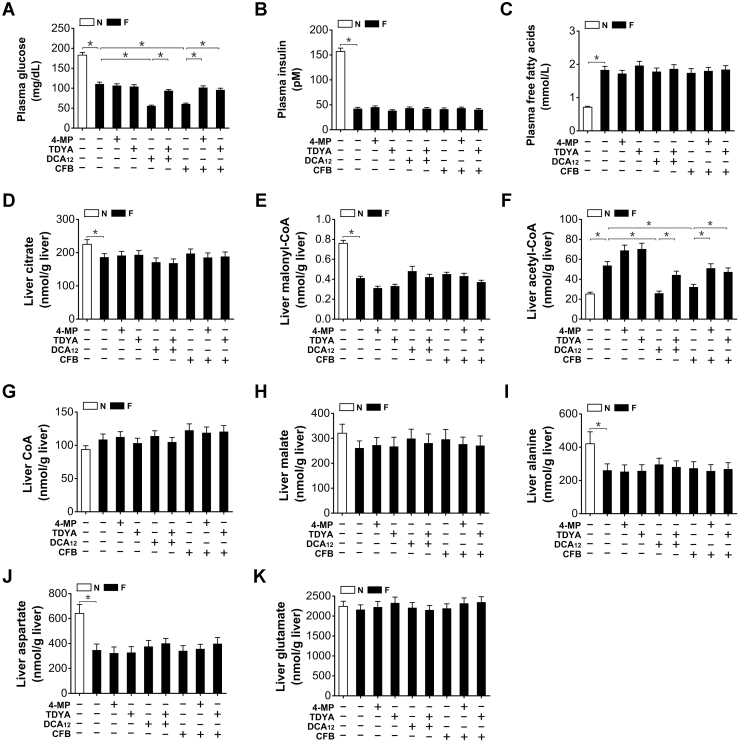
CFB treatment further reduced the plasma glucose level of fasting rats (48-h) while no significant alteration for TDYA (Fig. 7A). Plasma insulin and FFAs were not significantly altered in fasting rats after treatment with CFB, DCA12, or TDYA (Fig. 7, B and C). In the meantime, hepatic citrate or malonyl-CoA content was not significantly altered by DCA12 or CFB treatment (Fig. 7, D and E), which suggested that malonyl-CoA was not involved in DCA-induced suppression of mitochondrial β-oxidation and lipid accumulation. Liver acetyl-CoA increased significantly in the fasting rats, administration of CFB or DCA12 remarkably reduced acetyl-CoA generation in the liver of the fasting rats, as increased by pretreatment with 4-MP or TDYA (Fig. 7F). Liver free CoA, malate, alanine, aspartate, and glutamate levels in the fasting rats were not significantly altered after treatment with CFB, DCA12, 4-MP, or TDYA (Fig. 7, G–K).
Figure 7.
(A), Plasma glucose, (B) insulin, and (C) free fatty acids levels in the fasting rats after treatment with CFB, DCA12, 4-MP, and TDYA. D, liver citrate and (E) malonyl-CoA content were not significantly altered in the fasting rats after treatment with CFB, DCA12, 4-MP, and TDYA. F, liver acetyl-CoA content was reduced in the fasting rats after treatment with CFB or DCA12. (G) Liver free CoA, (H) malate, (I) alanine, (J) aspartate, and (K) glutamate levels in the fasting rats were not significantly altered after treatment with CFB, DCA12, 4-MP, or TDYA. Mean ± SEM, n = 8, ∗p < 0.05 by t-test between paired conditions.
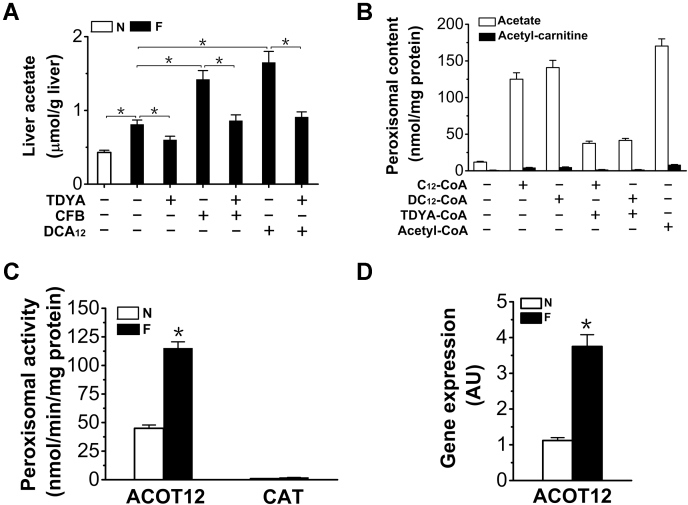
Peroxisomal β-oxidation of DCA generated free acetate as the major final product
Liver acetate was significantly higher in the fasting rats (increased by 88% versus normal rats), as further elevated in DCA12 and CFB-treated rats and reduced by the treatment with TDYA (Fig. 8A), which suggested that peroxisomal metabolism of DCAs stimulated acetate formation. To address whether peroxisomal β-oxidation generated free acetate, liver peroxisomes were incubated with DC12-CoA, C12-CoA, or acetyl-CoA, the results indicated that acetate rather than acetyl-carnitine was the major final product after peroxisomal β-oxidation of DC12-CoA and C12-CoA, nearly 85% of the added acetyl-CoA was converted to acetate, while acetyl-carnitine level was much lower (Fig. 8B). Peroxisomal carnitine acetyltransferase (CAT) and acetyl-CoA hydrolase (ACOT12) activities were assayed (Fig. 8C). ACOT12 activity was markedly induced by fasting and the activity of ACOT12 was much higher than that of CAT (by 58-fold). Gene expression of ACOT12 was upregulated in the livers of the fasting rats (235% versus normal rats) (Fig. 8D). Therefore, due to the high acetyl-CoA hydrolase activity in peroxisomes, most of the acetyl-CoA generate from peroxisomal β-oxidation of DCAs was hydrolyzed to free acetate and released into the cytosol.
Figure 8.
A, liver acetate generation was stimulated by CFB or DCA12treatment and reduced by TDYA in the fasting rats. Mean ± SEM, n = 8, ∗p < 0.05 by t-test between paired conditions. B, generation of acetate and acetyl-carnitine with C12-CoA, DC12-CoA or acetyl-CoA as a substrate by isolated peroxisomes. Mean ± SEM, n = 6, ∗p < 0.05 by t-test between paired conditions. C, peroxisomal activity of ACOT12 was increased in the livers of the fasting rats. D, gene expression of liver ACOT12 was upregulated by fasting. Mean ± SEM, n = 8, ∗p < 0.05 by t-test between paired conditions.
Discussion
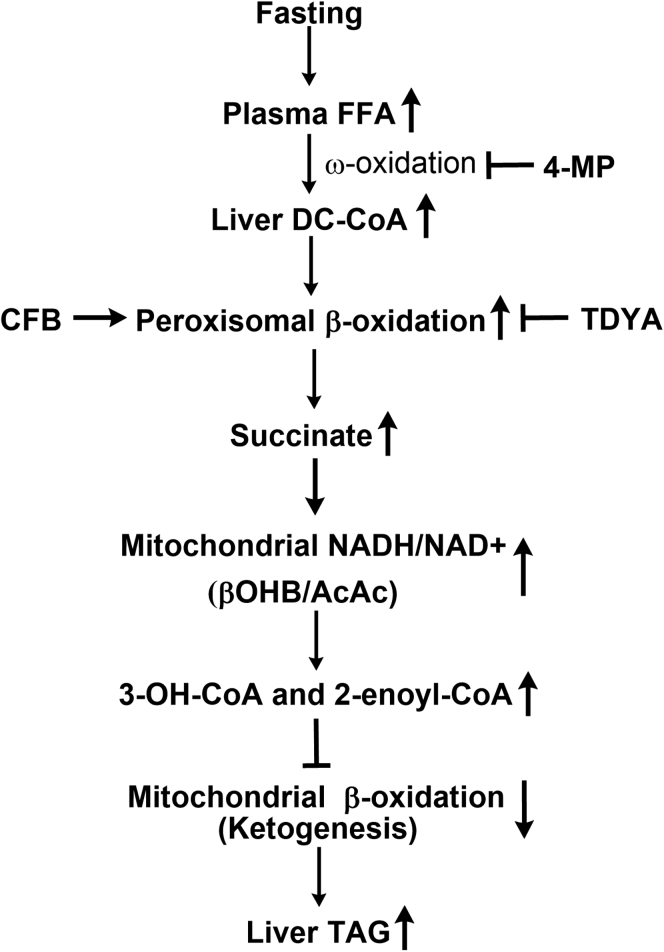
This research revealed a novel mechanism by which liver mitochondrial β-oxidation was regulated when the supply of carbohydrate was deficient and established a cross talk between peroxisomal and mitochondrial fatty acid oxidation. The proposed mechanism was shown in Figure 9. In fasting rats, plasma FFAs elevated significantly due to the accelerated lipolysis, increased hepatic fatty acids uptake resulted in activation of PPARα, and the gene expressions involved in fatty acid ω-oxidation and peroxisomal β-oxidation were extensively induced. Excessive β-oxidation of DCAs by peroxisomes generated considerable succinate in the liver, which remarkably increased mitochondrial NADH/NAD+ ratio and led to accumulation of 3-OH-CoA and 2-enoyl-CoA intermediates in the liver, thereby suppressing mitochondrial β-oxidation. Specific inhibition of peroxisomal β-oxidation reduced liver succinate formation and enhanced mitochondrial fatty acid oxidation and attenuated fasting-induced liver lipid deposition.
Figure 9.
Proposed mechanism by which peroxisomal oxidation of dicarboxylic acids suppresses mitochondrial β-oxidation and induces hepatic lipid accumulation in fasting animals.
The results also demonstrated the potential physiological function of fatty acid ω-oxidation. It was reported that 10% fatty acids were subjected to ω-oxidation, which further proposed that the generation of DCAs may facilitate the oxidation (39). DCAs were also considered to be gluconeogenic precursors through generation of succinate in rats (10, 11). Our results suggested that the physiological role of fatty acid ω-oxidation was to provide substrates for oxidation in peroxisomes for the purpose of generating succinate and regulating mitochondrial fatty acid oxidation under starvation condition.
As mitochondrial β-oxidation is under the control of the redox state of NAD+ (30), elevation in mitochondrial NADH/NAD+ ratio leads to suppression of mitochondrial fatty acid oxidation (19, 25, 26, 27, 28). Our results indicated that metabolism of endogenously generated DCAs negatively regulated mitochondrial β-oxidation by elevating mitochondrial NADH/NAD+ ratio and inducing accumulation of 3-OH-CoA/2-enoyl-CoA intermediates. The elevated mitochondrial NADH/NAD+ ratio as caused by DCAs was attributed to generation of succinate from oxidation of DCAs (19, 25, 26, 27, 28). Previous reports indicated that peroxisomal oxidation of long-chain DCAs generated succinate as the final product (21, 22), our results confirmed this both in vitro and in vivo. Excessive succinate oxidation causes robust reduction of mitochondrial NAD+ by blocking the electron flow from the NADH to the cytochromes as extensively studied by Chance and Krebs (23, 24), which caused accumulation of 3-OH-CoA/2-enoyl-CoA intermediates and suppression of mitochondrial fatty acid oxidation (31, 32, 33). It was reported that CFB treatment mildly but significantly increased NADH/NAD+ ratio in fed rats (40), the results in this research indicated that CFB robustly enhanced mitochondrial redox state of NAD+ in the fasting rats, indicating that the supply of DC-CoA for peroxisomal β-oxidation and subsequent succinate generation was critical for the control of mitochondrial NADH/NAD+ ratio and β-oxidation.
Both clinical and preclinical data indicate that intermittent fasting improves lipid metabolism and ameliorates hepatic steatosis in human subjects as well as animal models (41, 42). However, our results suggested that acute fasting induced hepatic lipid accumulation by stimulating DCA oxidation in rodents. There are two possible causes that lead to the discrepancy between a single acute fast in this study and intermittent fasting. First, the duration of fasting is different. The duration of normal intermittent fasting in human subjects is less than 16 h, plasma FFA and ketone body elevated mildly (43). However, plasma FFA and liver dicarboxylic acid oxidation elevated significantly in human subjects after prolonged fasting (>24-h). For example, it was reported that in human subjects, fasting for 12 h did not lead to significant elevation in plasma FFA and lipid deposition in the liver, while plasma FFA elevated remarkably and significantly hepatic steatosis was observed after 36-h fasting (4). Our results also showed that liver TAG content did not increase significantly in rats after 12-h fasting, while elevated significantly after 24-h fasting (unpublished observation). Therefore, the duration in intermittent fasting is an important factor for consideration in practice, and longer-time fasting (>16 h) might cause hepatic steatosis and liver oxidative injury especially in severe obesity and diabetes when plasma FFA is already very high. Second, the physiological response and metabolic alterations in intermittent fasting (with repeated fasting–refeeding cycles) and a single acute fast are different. It was reported that acute fasting imposed systemic metabolic stress in animals (44), our results suggested that acute fasting exerted redox stress on mitochondria and led to diminished mitochondrial fatty acid oxidation in liver by stimulating metabolism of DCAs. However, fasting followed by refeeding led to metabolic reprogramming and restored metabolic homeostasis in liver, which was accompanied by metabolic alterations that protect against subsequent periods of food deprivation in humans and animals (44).
PPARα agonists such as fibrate have been developed and used as clinical drugs in treating hyperlipidemia and nonalcoholic fatty liver disease (NAFLD) in humans (45). Under ketogenic conditions, excessive hepatic uptake of fatty acids results in activation of PPARα because long-chain fatty acids are identified to be endogenous ligands for PPARα and triggers downstream transcription of the target genes, including the genes involved in mitochondrial fatty acid oxidation, fatty acid ω-oxidation, and peroxisomal β-oxidation in rodents (17). Our results suggested that a potential regulatory mechanism existed for the control of mitochondrial β-oxidation and ketogenesis by metabolism of DCAs in fasting animals. Activation of ω-oxidation and peroxisomal β-oxidation under ketogenic conditions greatly stimulated DCAs turnover in peroxisomes. CFB as a classical activator for PPARα extensively induced fatty acids ω-oxidation and peroxisomal β-oxidation. However, we noted that CFB treatment did not suppress mitochondrial fatty acid oxidation and cause significant hepatic steatosis in the fed state, while mitochondrial β-oxidation was seriously inhibited in fasting rats, indicating that excessive fatty acids supply was another critical factor. When plasma FFAs are elevated under ketogenic conditions such as fasting and diabetes, administration of hypolipidemic drugs will greatly stimulate DCA oxidation and lead to excessive succinate formation, cause strong suppression of mitochondrial β-oxidation, suggesting a risk of fatty liver in rodents and possibly in humans.
It is interesting to note that CFB as well as DCAs treatments resulted in hypoglycemia in fasting rats, which might be caused by the lack of acetyl-CoA for the activation of pyruvate carboxylase, the key enzyme in gluconeogenesis in fasting rats due to the diminished mitochondrial β-oxidation. On the other hand, the importance of fatty acids and ketone bodies as sources of energy was well established. When fatty acids oxidation, as well as ketogenesis, is inhibited, compensatory glucose uptake and utilization are often observed to fix the energy shortage of extrahepatic tissues under the condition of fasting, led to the so-called hypoketotic hypoglycemia in animals as well as humans (46, 47, 48, 49). However, it was reported that there were great differences in PPARα activation on transcription of its target genes between rodents and humans (50). In rodents, PPARα is highly expressed in the liver and activation of PPARα induces large number of genes involved in various metabolic pathways. Compared with rodents, a much lower expression of PPARα in the human liver is observed, and PPARα agonists such as fibrates have only a minor effect on peroxisome proliferation in humans (50, 51). Therefore, it should be noted that PPARα-induced metabolic disorder in this study may not work in the same manner in humans. Clinical investigations are required to make sure whether administration of PPARα agonists to humans under ketogenic conditions contributes to fatty liver and hypoglycemia.
Peroxisomal oxidation of DCAs considerably suppressed ketone body generation, we therefore assumed that the acetyl-CoA generated from peroxisomal oxidation of DCAs was nonketogenic. Because peroxisomes are not permeable to acetyl-CoA (52), it is well accepted that there are two pathways for acetyl-group transfer from peroxisome to the cytosol, one way is to hydrolyze acetyl-CoA to acetate via peroxisomal acetyl-CoA hydrolase, the other way is to transform the acetyl-CoA to acetyl-carnitine via CAT (53). We analyzed the final product of DCAs that subjected to peroxisomal β-oxidation, the results clearly indicated that peroxisomal oxidation of DCAs as well as mono-carboxylic acid generated free acetate as the predominant product, which were in well agreement with previous report (9). The formation of acetate from acetyl-CoA was attributed to high level of expression and activity of ACOT12 in rat liver. On the other hand, the activity of CAT was very low in rat liver, while its expression and activity were high in the heart and muscle (54). Cytosolic free acetate could not be effectively metabolized in the liver due to lack of a specific acetyl-CoA synthetase in liver mitochondria (55). The acetate that released from peroxisomal β-oxidation of DCAs may be used for biosynthesis such as de novo fatty acid synthesis or cholesterol biosynthesis (56). It was reported that administration of PPARα agonist stimulated hepatic fatty acid synthesis (57). In this circumstance, we noted the fact that acetate generated from ethanol oxidation stimulated liver fatty acid synthesis and led to significant hepatic steatosis (58).
It was reported that liver generation of DCAs and peroxisomal β-oxidation were upregulated under the condition of high fat diet feeding or obesity in animals (59, 60, 61), indicating accelerated liver DCA turnover and succinate formation in obesity. Therefore, the DCA–succinate–fatty liver axis might be considered as a mechanism for obesity-induced fatty liver. Insufficient ketogenesis has been well considered to be an important cause that leads to NAFLD and related metabolic disorder (62). Evidences show that liver βOHB/AcAc ratio and 3-OH-CoA/2-enoyl-CoA increase significantly in NAFLD patients (63), and specific inhibition of peroxisomal β-oxidation significantly enhances mitochondrial fatty acid oxidation and improves hepatic steatosis in high fat diet–fed rats (18), which well supports the proposed mechanism. It is well accepted that hepatic lipid plays a critical role in the development of insulin resistance and related metabolic diseases (64). Small molecules specifically targeting peroxisomal β-oxidation such as TDYA or fatty acid ω-oxidation might be promising agents in treating obesity-induced NAFLD and related metabolic diseases by reducing succinate generation and enhancing mitochondrial β-oxidation.
Experimental procedures
Materials
Palmitoyl-L-carnitine, malonate, L-carnitine, acyl-CoAs (C12:0), coenzyme A sodium salt, Percoll, cytochrome c, 5,5-dithio-bis(2-nitrobenzoic acid) (DTNB), and defatted bovine serum albumin (BSA) were purchased from Sigma (St Louis, MO., USA). Clofibrate (CFB), 10,12-tricosadiynoic acid (TDYA), 4-methylpyrazole (4-MP), dodecanedioic acid (DCA12), and dodecanoic acid (C12) were from Tokyo Chemical Industry (Tokyo, Japan). The mono-CoA thioesters of dodecanedioic acid (DC12-CoA) and dodecanoic acid (C12-CoA) were enzymatically prepared by a microsomal acyl-CoA synthetase and purified by high-performance liquid chromatography as previously described (65, 66). TDYA-CoA as an irreversible inhibitor for peroxisomal acyl-CoA oxidase-1 (ACOX) was prepared according to the method of Li et al. (67, 68). All other chemical reagents used were of analytical grade or better.
Animal studies
Male Sprague-Dawley (SD) rats were purchased from Slac Laboratory Animal Co Ltd (Changsha, China). Standard rodent diet (12% fat by calories) was supplied by Slac Laboratory Animal Co Ltd (Changsha, China). All animals were housed in single cage with free access to food and water under controlled temperature (22 °C) and light (12 h of light and 12 h of dark).
For fasting experiments, male SD rats at the age of 8 weeks (200–220g) were deprived of food for 48-h with free access to water. For the study of acute effect of DCA12 on liver fatty acid oxidation, the rats after a 48-h fast were treated with DCA12 at a dose of 2 g/kg by gavage, blood samples were collected at indicated time. For inhibitor intervention, TDYA (200 mg/kg) was administered to rats 120 min prior to DCA12 dosing. For the study of endogenous DCAs on liver fatty acid metabolism, fasting animals were treated with CFB (200 mg/kg) or TDYA (200 mg/kg) by gavage twice per day and 4-MP (100 mg/kg) by intraperitoneal injection once per day. Normal group (N) was fed standard rodent diet throughout the study. After the experiments, all the rats were bled from the eyes and then sacrificed at indicated time as shown in the figures. Livers from 48-h fasted rats and normal rats were removed quickly and stored in liquid nitrogen immediately.
All the animal studies were approved by the Animal Care Committee of Hunan University of Science and Technology.
Histological analysis
Definite parts of right lobe were cut quickly from of the livers of the killed rats and immediately fixed with 4% paraformaldehyde. Paraffin sections were prepared and cut into 5–7 μm-thick sections and stained by hematoxylin-eosin (HE). Hepatic steatosis was observed by optical microscope, four samples were used for each group to observe the lipid droplets in liver tissues.
Isolation of liver subcellular fractions
Mitochondria from rat liver were isolated by differential centrifugation in 0.25 M sucrose as described previously (69), the mitochondrial pellet was washed three times in 0.25 M sucrose at 4 °C and finally suspended in the same medium at a concentration of ∼40 mg/ml for subsequent analysis. The integrity of isolated mitochondria was examined by a commercial mitochondrial staining kit from Sigma. For the isolation of peroxisomes, light mitochondrial fraction (L) after differential centrifugation was further isolated by a Percoll gradient according to the method described before (61). In total, 15 mg of L fraction sample was layered on 5 ml of a 50% (v/v) solution of Percoll containing 250 mM sucrose, 2 mM Mops, 1 mM EGTA, and 0.1% (v/v) ethanol at pH 7.2. After centrifugation at 85,000g for 30 min on a Beckman Optima MAX-XP ultracentrifuge with a MLN80 rotor, the fractions were collected for catalase activity assay. The pooled peak fractions were diluted with 250 mM sucrose and centrifuged at 35,000 g for 15 min to recover sediment containing purified peroxisomes.
Characteristics of the enzymes involved in DCA β-oxidation
Mitochondrial medium-chain acyl-CoA dehydrogenase (MCAD), LCAD, and peroxisomal acyl-CoA oxidase-1 (ACOX) were expressed in Escherichia coli and purified by nickel metal affinity chromatography according to conventional methods. Mitochondrial carnitine palmitoyltransferase-1 (CPT1) was expressed in Saccharomyces cerevisiae and purified as described before (70). Acyl-CoA dehydrogenases were measured as described previously using 2,6-dichloroindophenol (DCIP) as the final electron receptor (71). ACOX1 activity was assayed spectrophotometrically by directly measuring the formation of H2O2 product (18). CPT1 activity was assayed by measuring the formation of free CoA by a DTNB method (70).
Measurement of peroxisomal and mitochondrial β-oxidation
Mitochondrial β-oxidation was determined spectrophotometrically by the method of Osmundsen and Bremer (72), with 100 μM palmitoyl-L-carnitine as a substrate. When using 100 μM DC12-CoA or C12-CoA as substrate, 2.5 mM L-carnitine was added to the reaction solution along with acyl-CoAs. Peroxisomal β-oxidation was assayed by acyl-CoA-dependent NAD+ reduction in the presence of KCN as developed by Lazarow PB (73), with 100 μM DC12-CoA or C12-CoA as a substrate.
Measurement of hepatic DC-CoAs
Extraction of DC-CoAs from liver homogenate was performed as described before (74). The extracts were combined and dried under N2 for assay. DC-CoAs were transformed to succinyl-CoA by a multienzyme reaction system containing 10 U/ml recombinant rat liver ACOX1, 5 U/ml recombinant rat L-bifunctional protein (L-BP), 5 U/ml recombinant rat 3-oxoacyl-CoA thiolase, 5 U/ml catalase (Sigma), 10 U/ml acetyl-CoA hydrolase, and 18 U/ml lactate dehydrogenase (Sigma). The reaction medium contained 50 mM Hepes, pH 7.4, 1 mM NAD+, 5 mM pyruvate, 2 mM CoA, 0.5 mg/ml BSA, 10 mM MgCl2, and 50 μM FAD in a total volume of 1 ml. After incubation at 37 °C for 30 min, the mixture was deproteinized by adding 0.1 m ice-cold perchloric acid (70%, w/v). After neutralization, succinyl-CoA was assayed based on the reaction of DTNB with CoA liberated from succinyl-CoA in the presence of succinyl-CoA synthetase and monitored the absorbance at 412 nm (75). The amount of succinyl-CoA represented the sum of DC-CoAs in the sample.
Ketone body synthesis by liver homogenate in the presence of DCA12
Preparation of rat liver homogenate and ketone body synthesis by liver homogenate with hexanoate as a substrate were performed as described by McGarry (76). The reaction mixture contained 0.1 M Tris-HCl (pH 8.5), 5 mM MgCl2, 5 mM ATP, 0.3 mM CoA, 0.66 mM NAD+, 0.5 mg/ml defatted BSA, 2 mM hexanoate, 0.5–2 mM DCA12, and 0.5 ml liver homogenate in a total volume of 2 ml. In some cases, just before DCA12 treatment, 100 μM of TDYA-CoA was added to inhibit peroxisomal oxidation of DCA12. After reaction for 10 min at 37 °C, the mixture was deproteinized by adding 0.1 ml ice-cold perchloric acid (70%, w/v), the neutralized supernatant-contained 3-hydroxybutyric acid (βOHB) and acetoacetate (AcAc) were analyzed enzymatically.
Studies of DCA12 on mitochondrial β-oxidation in liver homogenate
The reaction mixture contained 50 mM Hepes, pH 7.4, 1 mM NAD+, 5 mM pyruvate, 2 mM CoA, 5 mM ATP, 0.5 mg/ml defatted BSA, 10 mM MgCl2, 50 μM FAD, 20 mg liver homogenate, and DCA12 at a concentration of 0.5 mM, 1.0 mM, and 2.0 mM, respectively. In some cases, just 5 min before DCA12 treatment, 100 μM of TDYA-CoA was added to inhibit peroxisomal oxidation of DCA12. After reaction at 37 °C for 30 min, mitochondria were isolated from the homogenate and β-oxidation was then analyzed.
Measurement of 3-OH-CoA and 2-enoyl-CoA intermediates of β-oxidation
Mitochondria were isolated from the tissue homogenate and reaction mixture by differential centrifugation as described above. The isolated mitochondria were resuspended in 50 mM potassium phosphate, pH 7.2 contained 50 % (v/v) isopropanol. After acidification with acetate (to a final concentration of 3 %, v/v), the solution was extracted with petroleum ether saturated with 50 % aqueous isopropanol. After phase separation, saturated (NH4)2SO4 was added and acyl-CoAs were extracted by chloroform/methanol (1:2; v/v). After centrifugation, the pellet was washed with chloroform/methanol and the supernatants were combined and evaporated. The residue was redissolved in 50 mM potassium phosphate, pH 7.2, and 0.05 % Triton X-100 and added to the reaction mixture contained 80 mM Tris-HCl, pH 9.0, 1 mM NAD+, 10 mU recombinant rat 3-hydroxyacyl-CoA dehydrogenase in a total volume of 0.2 ml (77). For the assay of 2-enoyl-CoA, extra 1.2 mU recombinant rat 2-enoyl-CoA hydratase was needed (78). After incubation at 25 °C for 60 min, 20 μl of 0.6 M NaOH was added and another incubation at 80 °C for 60 min was performed. After partially neutralization by 20 μl of 0.5 M HCl, 20 μl reaction solution was added to cycling solution contained 197 mM Tris-HCl, pH 8.0, 1.75 mM oxaloacetate, 0.29 M ethanol, 2.8 μM bovine serum albumin, 17.5 U/ml alcohol dehydrogenase, and 8.5 U/ml malic dehydrogenase (Sigma) in a total volume of 0.2 ml. 3-OH-CoA and 2-enoyl-CoA in samples are in linear correlation with the amount of malate formed, which could be assayed by the coupled reaction of malic dehydrogenase and glutamic-oxaloacetic transaminase (79).
Quantitative real-time PCR
Total RNA was extracted from liver tissues with TRIzol reagent (Life Technologies Corporation, Carlsbad, CA). RNA was reverse-transcribed with standard reagents (High Capacity Reverse Transcription Kits, Applied Biosystems) using random primers. Complementary DNA was amplified in a 7500 Fast Real-time PCR System using 2×SYBR Green Supermix (Applied Biosystems). The following primers were used: CYP4A1, 5'-CCCGACACAGCCACTCATTC-3'(F) and 5'-CCTTCAGCTCTTCATGGCAAAC T-3' (R); ACOX1, 5'-TGGAGAGCCCTCAGC TATGG-3' (F) and 5'-CGTTTCACCGCCTC GTAAG-3' (R); LCADH, 5'-GGCTGGTT AAGTGATCTCGTGAT-3' (F) and 5'-TCTCC ACCAAAAAGAGGCTAATG -3' (R); ACS, 5'-GGCTCTAGGAGTAAAGGCTGACGT-3' (F) and 5'-TCCTTTCGTTCTAGCTAGCTCCGT-3'(R); L-BP, 5'-AAATACAGAGATACCAGA AGCCG-3' (F) and 5'-AAGAAT CCCCAGTGTGACTTC-3'(R); Thiolase, 5'- CCTGACATCATGGGCATCG-3' (F) and 5'-A GTCAGCCCTGCTTTCTGCA-3'(R); ABCD1, 5'-GGGCCTAAAGCAAC AGTCTCA-3' (F) and 5'-GGGCAACATACACAGACAGGAA-3'(R); ACOT12, 5'-GGAGATTACCACCACC TTGG-3' (F) and 5'-TTCAACCTTAACAG ATATGGCATC3'(R); 18S rRNA, 5'-GTTATGGTCTTTGGTCGC-3' (F) and 5'-CGTCTGCCCTATCAACTTTC-3' (R). mRNA expression levels normalized to 18S rRNA were expressed using the comparative delta CT method.
Biochemical analysis
Plasma FFA concentration was determined using a colorimetric kit based on ACS-ACOX method (Wako Pure Chemical Corporation, Osaka, Japan). Plasma TAG was determined by a commercial kit (Wako, Osaka, Japan). Liver long-chain acyl-CoAs were extracted and determined by the method of Tubbs and Garland (80). Liver TAG was extracted by the method of Bligh and Dyer (81) and determined using a commercial kit (Wako, Osaka, Japan). Liver VLDL-TAG secretion rate was determined by the method as described previously (82). Briefly, rats were injected intravenously with Triton WR1339 at 500 mg/kg, then blood samples were taken at 0, 1, 2, 3, and 4 h after injection and plasma TAG was measured, liver VLDL-TAG secretion rate was calculated from the slope of the curve and expressed as μmol/h/kg. Plasma and liver βOHB and AcAc were determined enzymatically (83), total ketone body was expressed as the sum of βOHB and AcAc. Liver acetate, alanine, malate, aspartate, glutamate, succinate, and citrate were determined using commercial kits according to the manufacturer’s instructions (Sigma, St Louis, MO). Liver malonyl-CoA was analyzed by HPLC as described previously (84). Liver hydrogen peroxide and TBARS were measured by assay kits from Sigma. Plasma glucose was measured using a glucometer (Lifescan, Johnson and Johnson). Liver acetyl-CoA was determined enzymatically using commercial assay kit (Solarbio, Beijing). Liver acetyl-carnitine was assayed by a combined reaction catalyzed by carnitine acetyl transferase, L-malate dehydrogenase, and citrate synthase (85). Liver free CoA content was assayed by a 2-oxoglutarate dehydrogenase method according to Tubbs and Garland (86). Plasma insulin was measured by a rat/mouse insulin ELISA kits from Merck Millipore (Billerica, MA., USA). Protein concentration was measured by Bio-Rad DC protein assay kit (Hercules, CA., USA). C6-CoA was measured by UPLC-MS/MS as described before (87).
Peroxisomal acetyl-CoA thioesterase (ACOT12) was measured spectrophotometrically by a DTNB assay using isolated peroxisomes (88), with acetyl-CoA as a substrate at a concentration of 100 μM. Peroxisomal CAT activity was assayed as described before using acetyl-carnitine as substrate at a concentration of 100 μM (87).
Statistical analysis
Data are presented as mean±SEM. n=6 to 8 for all the groups. The significance of differences was evaluated using one way ANOVA with Dunnett’s T3 test or Student's t-test by SPSS 18.0. p < 0.05 was considered statistically significant.
Data availability
All data are contained within the article.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
This work was supported by the National Natural Science Fund of P. R. China (30900024), Natural Science Fund of Hunan Province (2020JJ5157), Distinguished Professor Funds from Hunan University of Science and Technology, and Project of graduate innovation in Hunan province (CX20190814).
Author contributions
J. Z. conceived the idea for the project and wrote the paper. T. G., S. W. D., and X. Z. conducted most of the animal experiments and analyzed the results. K. C. and L. S. conducted assay of enzymes activity. X. J. C. conducted real-time PCR. Z. L. C. conducted hydrogen peroxide assay. X. C. C. and P. L. conducted β-oxidation assay.
Edited by Dennis Voelker
References
- 1.McGarry J.D., Mannaerts G.P., Foster D.W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J. Clin. Invest. 1977;60:265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGarry J.D., Foster D.W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu. Rev. Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- 3.Hillgartner F.B., Salati L.M., Goodridge A.G. Physiological and molecular mechanisms involved in nutritional regulation of fatty acid synthesis. Physiol. Rev. 1995;75:47–76. doi: 10.1152/physrev.1995.75.1.47. [DOI] [PubMed] [Google Scholar]
- 4.Moller L., Stodkilde-Jorgensen H., Jensen F.T., Jorgensen J.O. Fasting in healthy subjects is associated with intrahepatic accumulation of lipids as assessed by 1H-magnetic resonance spectroscopy. Clin. Sci. 2008;114:547–552. doi: 10.1042/CS20070217. [DOI] [PubMed] [Google Scholar]
- 5.Yokota S.I., Nakamura K., Ando M., Kamei H., Hakuno F., Takahashi S.I., Shibata S. Acetylcholinesterase (AChE) inhibition aggravates fasting-induced triglyceride accumulation in the mouse liver. FEBS Open Bio. 2014;4:905–914. doi: 10.1016/j.fob.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khare P., Jagtap S., Jain Y., Baboota R.K., Mangal P., Boparai R.K., Bhutani K.K., Sharma S.S., Premkumar L.S., Kondepudi K.K., Chopra K., Bishnoi M. Cinnamaldehyde supplementation prevents fasting-induced hyperphagia, lipid accumulation, and inflammation in high-fat diet-fed mice. Biofactors. 2016;42:201–211. doi: 10.1002/biof.1265. [DOI] [PubMed] [Google Scholar]
- 7.Verkade P.E., Lee V.D.J. Researches on fat metabolism. II. Biochem. J. 1934;28:31–40. doi: 10.1042/bj0280031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjorkhem J. On the mechanism of regulation of ω-oxidation of fatty acids. J. Biol. Chem. 1976;251:5259–5266. [PubMed] [Google Scholar]
- 9.Leighton F., Bergseth S., Rørtveit T., Christiansen E.N., Bremer J. Free acetate production by rat hepatocytes during peroxisomal fatty acid and dicarboxylic acid oxidation. J. Biol. Chem. 1989;264:10347–10350. [PubMed] [Google Scholar]
- 10.Wada F., Usami M. Studies on fatty acid omega-oxidation. Antiketogenic effect and gluconeogenicity of dicarboxylic acids. Biochem. Biophys. Acta. 1977;487:261–268. [PubMed] [Google Scholar]
- 11.Mortensen P.B. The possible antiketogenic and gluconeogenic effect of the ω-oxidation of fatty acids in rats. Biochem. Biophys. Acta. 1980;620:177–185. doi: 10.1016/0005-2760(80)90199-x. [DOI] [PubMed] [Google Scholar]
- 12.Vamecq J., Draye J.P. Peroxisomal and mitochondrial β-oxidation of monocarboxylyl-CoA, ω-hydroxymonocarboxylyl-CoA and dicarboxylyl-CoA esters in tissues from untreated and clofibrate-treated rats. J. Biochem. 1989;106:216–222. doi: 10.1093/oxfordjournals.jbchem.a122835. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki H., Yamada J., Watanabe T., Suga T. Compartmentation of dicarboxylic acid β-oxidation in rat liver: Importance of peroxisomes in the metabolism of dicarboxylic acids. Biochem. Biophys. Acta. 1989;990:25–30. doi: 10.1016/s0304-4165(89)80007-8. [DOI] [PubMed] [Google Scholar]
- 14.Bergseth S., Poisson J.P., Bremer J. Metabolism of dicarboxylic acids in rat hepatocytes. Biochem. Biophys. Acta. 1990;1042:182–187. doi: 10.1016/0005-2760(90)90005-i. [DOI] [PubMed] [Google Scholar]
- 15.Leone T.C., Weinheimer C.J., Kelly D.P. A critical role for the peroxisome proliferator-activated receptor α (PPARα) in the cellular fasting response: The PPARα-null mouse as a model of fatty acid oxidation disorders. P. Natl. Acad. Sci. U. S. A. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kersten S., Seydoux J., Peters J.M., Gonzalez F.J., Desvergne B., Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forman B.M., Chen J., Evans R.M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. P. Natl. Acad. Sci.U. S. A. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng J., Deng S., Wang Y., Li P., Tang L., Pang Y. Specific inhibition of Acyl-CoA oxidase-1 by an acetylenic acid improves hepatic lipid and reactive oxygen species (ROS) metabolism in rats fed a high fat diet. J. Biol. Chem. 2017;292:3800–3809. doi: 10.1074/jbc.M116.763532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lumeng L., Bremer J., Davis E.J. Suppression of the mitochondrial oxidation of (-)-palmitylcarnitine by the malate-aspartate and alpha-glycerophosphate shuttles. J. Biol. Chem. 1976;251:277–284. [PubMed] [Google Scholar]
- 20.Eaton S. Control of mitochondrial β-oxidation flux. Prog. Lipid. Res. 2002;41:197–239. doi: 10.1016/s0163-7827(01)00024-8. [DOI] [PubMed] [Google Scholar]
- 21.Tserng K.Y., Jin S.J. Metabolic conversion of dicarboxylic acids to succinate in rat liver homogenates. A stable isotope tracer study. J. Biol. Chem. 1991;266:2924–2929. [PubMed] [Google Scholar]
- 22.Osmundsen H., Bremer J., Pedersen J.I. Metabolic aspects of peroxisomal β-oxidation. Biochem. Biophys. Acta. 1991;1085:141–158. doi: 10.1016/0005-2760(91)90089-z. [DOI] [PubMed] [Google Scholar]
- 23.Chance B., Hollunger G. Energy-linked reduction of mitochondrial pyridine nucleotide. Nature. 1960;185:666–672. doi: 10.1038/185666a0. [DOI] [PubMed] [Google Scholar]
- 24.Krebs H.A. The physiological role of the ketone bodies. Biochem. J. 1961;80:225–233. doi: 10.1042/bj0800225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eaton S., Turnbull D.M., Bartlett K. Redox control of β-oxidation in rat liver mitochondria. Eur. J. Biochem. 1994;220:671–681. doi: 10.1111/j.1432-1033.1994.tb18668.x. [DOI] [PubMed] [Google Scholar]
- 26.Ciman M., Rossi C.R., Siliprandi N. On the mechanism of the antiketogenic action of propionate and succinate in isolated rat liver mitochondria. FEBS Lett. 1972;22:8–10. doi: 10.1016/0014-5793(72)80205-9. [DOI] [PubMed] [Google Scholar]
- 27.Bremer J., Wojtczak A.B. Factors controlling the rate of fatty acid β-oxidation in rat liver mitochondria. Biochem. Biophys. Acta. 1972;280:515–530. doi: 10.1016/0005-2760(72)90131-2. [DOI] [PubMed] [Google Scholar]
- 28.Bremer J. Comparison of acylcarnitines and pyruvate as substrates for rat-liver mitochondria. Biochem. Biophys. Acta. 1966;116:1–11. doi: 10.1016/0005-2760(66)90087-7. [DOI] [PubMed] [Google Scholar]
- 29.Zeevalk G.D., Derr-Yellin E., Nicklas W.J. Relative vulnerability of dopamine and GABA neurons in mesencephalic culture to inhibition of succinate dehydrogenase by malonate and 3-nitropropionic acid and protection by NMDA receptor blockade. J. Pharmacol. Exp. Ther. 1995;275:1124–1130. [PubMed] [Google Scholar]
- 30.Wang H.Y., Baxter C.F., Jr., Schulz H. Regulation of fatty acid β-oxidation in rat heart mitochondria. Arch. Biochem. Biophys. 1991;289:274–280. doi: 10.1016/0003-9861(91)90472-u. [DOI] [PubMed] [Google Scholar]
- 31.He X.Y., Yang S.Y., Schulz H. Inhibition of enoyl-CoA hydratase by long-chain L-3-hydroxyacyl-CoA and its possible effect on fatty acid oxidation. Arch. Biochem. Biophys. 1992;298:527–531. doi: 10.1016/0003-9861(92)90445-3. [DOI] [PubMed] [Google Scholar]
- 32.Powell P.J., Lau S.M., Killian D., Thorpe C. Interaction of acyl coenzyme A substrates and analogues with pig kidney medium-chain acyl-coA dehydrogenase. Biochemistry. 1987;26:3704–3710. doi: 10.1021/bi00386a066. [DOI] [PubMed] [Google Scholar]
- 33.Eaton S., Bartlett K., Pourfarzam M. Mammalian mitochondrial β-oxidation. Biochem. J. 1996;320:345–357. doi: 10.1042/bj3200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddy J.K., Hashimoto T. Peroxisomal β-oxidation and peroxisome proliferator-activated receptor α: An adaptive metabolic system. Annu. Rev. Nutr. 2001;21:193–230. doi: 10.1146/annurev.nutr.21.1.193. [DOI] [PubMed] [Google Scholar]
- 35.Lazarow P.B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. P. Natl. Acad. Sci. U. S. A. 1976;73:2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reich N.O., De Montellano P.R.O. Dissociation of increased lauric acid ω-hydroxylase activity from the antilipidemic action of clofibrate. Biochem. Pharmacol. 1986;35:1227–1233. doi: 10.1016/0006-2952(86)90264-9. [DOI] [PubMed] [Google Scholar]
- 37.Kaikaus R.M., Chan W.K., Lysenko N., Ray R., De Montellano P.R.O., Bass N.M. Induction of peroxisomal fatty acid beta-oxidation and liver fatty acid-binding protein by peroxisome proliferators. Mediation via the cytochrome P-450IVA1 omega-hydroxylase pathway. J. Biol. Chem. 1993;268:9593–9603. [PubMed] [Google Scholar]
- 38.Grunnet N., Kondrup J. The effect of ethanol on the β-oxidation of fatty acids. Alcohol. Clin. Exp. Res. 1986;10:64S–68S. doi: 10.1111/j.1530-0277.1986.tb05182.x. [DOI] [PubMed] [Google Scholar]
- 39.Preiss B., Bloch K. ω-Oxidation of long chain fatty acids in rat liver. J. Biol. Chem. 1964;239:85–88. [PubMed] [Google Scholar]
- 40.Cederbaum A.I., Rubin E. Effects of clofibrate on mitochondrial function. Biochem. Pharmacol. 1974;23:1985–1996. doi: 10.1016/0006-2952(74)90257-3. [DOI] [PubMed] [Google Scholar]
- 41.Antoni R., Johnston K.L., Collins A.L., Robertson M.D. Effects of intermittent fasting on glucose and lipid metabolism. Proc. Nutr. Soc. 2017;76:361–368. doi: 10.1017/S0029665116002986. [DOI] [PubMed] [Google Scholar]
- 42.Lamos E.M., Malek R., Munir K.M. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 2019;381:2541–2551. doi: 10.1056/NEJMra1905136. [DOI] [PubMed] [Google Scholar]
- 43.Cahill G.F. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 44.Secor S.M., Carey H.V. Integrative physiology of fasting. Compr. Physiol. 2016;6:773–825. doi: 10.1002/cphy.c150013. [DOI] [PubMed] [Google Scholar]
- 45.Pawlak M., Lefebvre P., Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015;62:720–733. doi: 10.1016/j.jhep.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 46.Erguven M., Yılmaz O., Koc S., Cakı S., Ayhan Y., Donmez M., Dolunay G. A case of early diagnosed carnitine deficiency presenting with respiratory symptoms. Ann. Nutr. Metab. 2007;51:331–334. doi: 10.1159/000107675. [DOI] [PubMed] [Google Scholar]
- 47.Teijema H.L., Giesberts G.M.A.H. Hypoketosis as a cause of symptoms in childhood hypoglycemia. Eur. J. Pediatr. 1980;134:51–55. doi: 10.1007/BF00442403. [DOI] [PubMed] [Google Scholar]
- 48.Derks T.G., van Dijk T.H., Grefhorst A., Rake J.P., Smit G.P.A., Kuipers F., Reijngoud D.J. Inhibition of mitochondrial fatty acid oxidation in vivo only slightly suppresses gluconeogenesis but enhances clearance of glucose in mice. Hepatology. 2008;47:1032–1042. doi: 10.1002/hep.22101. [DOI] [PubMed] [Google Scholar]
- 49.Houten S.M., Herrema H., Te Brinke H., Denis S., Ruiter J.P., van Dijk T.H., Argmann C.A., Ottenhoff R., Müller M., Groen A.K., Kuipers F., Dirk-Jan R., Wanders R.J.A. Impaired amino acid metabolism contributes to fasting-induced hypoglycemia in fatty acid oxidation defects. Hum. Mol. Genet. 2013;22:5249–5261. doi: 10.1093/hmg/ddt382. [DOI] [PubMed] [Google Scholar]
- 50.Holden P.R., Tugwood J.D. Peroxisome proliferator-activated receptor α: Role in rodent liver cancer and species differences. J. Mol. Endocrinol. 1999;22:1–8. doi: 10.1677/jme.0.0220001. [DOI] [PubMed] [Google Scholar]
- 51.Maryam R., Guido H., Müller M., Sander K., Vincent L. Comparative analysis of gene regulation by the transcription factor PPARα between mouse and human. Plos ONE. 2009;4:e6796. doi: 10.1371/journal.pone.0006796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antonenkov V.D., Hiltunen J.K. Peroxisomal membrane permeability and solute transfer. Biochem. Biophys. Acta.-Mol. Cell. Res. 2006;1763:1697–1706. doi: 10.1016/j.bbamcr.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 53.Westin M.A.K., Hunt M.C., Alexson S.E.H. Short-and medium-chain carnitine acyltransferases and acyl-CoA thioesterases in mouse provide complementary systems for transport of β-oxidation products out of peroxisomes. Cell. Mol. Life Sci. 2008;65:982–990. doi: 10.1007/s00018-008-7576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi Y.R., Fogle P.J., Clarke P.R., Bieber L.L. Quantitation of water-soluble acylcarnitines and carnitine acyltransferases in rat tissues. J. Biol. Chem. 1977;252:7930–7931. [PubMed] [Google Scholar]
- 55.Fujino T., Kondo J., Ishikawa M., Morikawa K., Yamamoto T.T. Acetyl-CoA synthetase 2, a mitochondrial matrix enzyme involved in the oxidation of acetate. J. Biol. Chem. 2001;276:11420–11426. doi: 10.1074/jbc.M008782200. [DOI] [PubMed] [Google Scholar]
- 56.Faust P.L., Kovacs W.J. Cholesterol biosynthesis and ER stress in peroxisome deficiency. Biochimie. 2014;98:75–85. doi: 10.1016/j.biochi.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 57.Capuzzi D.M., Lackman R.D., Uberti M.O., Reed M.A. Stimulation of hepatic triglyceride synthesis and secretion by clofibrate. Biochem. Bioph. Res. Co. 1974;60:1499–1508. doi: 10.1016/0006-291x(74)90367-2. [DOI] [PubMed] [Google Scholar]
- 58.Lieber C.S., Schmid R. The effect of ethanol on fatty acid metabolism; stimulation of hepatic fatty acid synthesis in vitro. J. Clin. Invest. 1961;40:394–399. doi: 10.1172/JCI104266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nilsson A., Arey H., Pedersen J.I., Christiansen E.N. The effect of high-fat diets on microsomal lauric acid hydroxylation in rat liver. Biochem. Biophys. Acta. 1986;879:209–214. doi: 10.1016/0005-2760(86)90104-9. [DOI] [PubMed] [Google Scholar]
- 60.Fruchart J.C. Peroxisome proliferator-activated receptor-alpha (PPARα): At the crossroads of obesity, diabetes and cardiovascular disease. Atherosclerosis. 2009;205:1–8. doi: 10.1016/j.atherosclerosis.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 61.Neat C.E., Thomassen M.S., Osmundsen H. Induction of peroxisomal beta-oxidation in rat liver by high-fat diets. Biochem. J. 1980;186:369–371. doi: 10.1042/bj1860369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cotter D.G., Ercal B., Huang X., Leid J.M., d’Avignon D.A., Graham M.J., Crawford P.A. Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia. J. Clin. Invest. 2014;124:5175–5190. doi: 10.1172/JCI76388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eaton S., Zaitoun A.M., Record C.O., Bartlett K. β-Oxidation in human alcoholic and non-alcoholic hepatic steatosis. Clin. Sci. 1996;90:307–313. doi: 10.1042/cs0900307. [DOI] [PubMed] [Google Scholar]
- 64.Perry R.J., Samuel V.T., Petersen K.F., Shulman G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vamecq J., De Hoffmann E., Van Hoof F. The microsomal dicarboxylyl-CoA synthetase. Biochem. J. 1985;230:683–693. doi: 10.1042/bj2300683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vamecq J., Draye J.P. Interactions between the ω-and β-oxidations of fatty acids. J. Biochem. 1987;102:225–234. doi: 10.1093/oxfordjournals.jbchem.a122035. [DOI] [PubMed] [Google Scholar]
- 67.Li D., Agnihotri G., Dakoji S., Oh E., Lantz M., Liu H.W. The toxicity of methylenecyclopropylglycine: Studies of the inhibitory effects of (methylenecyclopropyl) formyl-CoA on enzymes involved in fatty acid metabolism and the molecular basis of its inactivation of enoyl-CoA hydratases. J. Am. Chem. Soc. 1999;121:9034–9042. [Google Scholar]
- 68.Zeng J., Wu L., Zhang X., Liu Y., Deng G., Li D. Oct-2-en-4-ynoyl-CoA as a specific inhibitor of acyl-CoA oxidase. Org. Lett. 2008;10:3877–3880. doi: 10.1021/ol801571n. [DOI] [PubMed] [Google Scholar]
- 69.De Duve C., Pressman B., Gianetto R., Wattiaux R., Appelmans F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem. J. 1955;60:604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prip-Buus C., Cohen I., Kohl C., Esser V., McGarry J.D., Girard J. Topological and functional analysis of the rat liver carnitine palmitoyltransferase 1 expressed in Saccharomyces cerevisiae. FEBS Lett. 1998;429:173–178. doi: 10.1016/s0014-5793(98)00584-5. [DOI] [PubMed] [Google Scholar]
- 71.Thorpe C., Matthews R.G., Williams C.H., Jr. Acyl-coenzyme A dehydrogenase from pig kidney. Purification and properties. Biochem. 1979;18:331–337. doi: 10.1021/bi00569a016. [DOI] [PubMed] [Google Scholar]
- 72.Osmundsen H., Bremer J. A spectrophotometric procedure for rapid and sensitive measurements of β-oxidation. Demonstration of factors that can be rate-limiting for β-oxidation. Biochem. J. 1977;164:621–633. doi: 10.1042/bj1640621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lazarow P.B. Assay of peroxisomal β-oxidation of fatty acids. Method Enzymol. 1981;72:315–319. doi: 10.1016/s0076-6879(81)72021-4. [DOI] [PubMed] [Google Scholar]
- 74.Pourfarzam M., Bartlett K. [26] synthesis, purification, and characterization of dicarboxylylmono-coenzyme a esters. Method. Enzymol. 1997;279:240–254. doi: 10.1016/s0076-6879(97)79028-1. [DOI] [PubMed] [Google Scholar]
- 75.Quant P.A., Tubbs P.K., Brand M.D. Treatment with rats with glucagon or mannoheptulose increases mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase activity and decreases succinyl-CoA content in liver. Biochem. J. 1989;262:159–164. doi: 10.1042/bj2620159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGarry J.D., Foster D.W. Ketogenesis in vitro. Biochem. Biophys. Acta.-Gen. Subjects. 1969;177:35–41. doi: 10.1016/0304-4165(69)90061-0. [DOI] [PubMed] [Google Scholar]
- 77.Liu X., Chu X., Yu W., Li P., Li D. Expression and purification of his-tagged rat mitochondrial short-chain 3-hydroxyacyl-coa dehydrogenase wild-type and ser137 mutant proteins. Protein Expres. Purif. 2004;37:344–351. doi: 10.1016/j.pep.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 78.Wu L., Lin S., Li D. Comparative inhibition studies of enoyl-coa hydratase 1 and enoyl-coa hydratase 2 in long-chain fatty acid oxidation. Org. Lett. 2008;10:3355–3358. doi: 10.1021/ol801267e. [DOI] [PubMed] [Google Scholar]
- 79.Latipää P.M., Hassinen I.E., Hiltunen J.K. Enzymatic assay for 3-hydroxyacyl-CoA and 2-trans-enoyl-CoA intermediates of β-oxidation. Anal. Biochem. 1988;171:67–72. doi: 10.1016/0003-2697(88)90125-x. [DOI] [PubMed] [Google Scholar]
- 80.Tubbs P.K., Garland P.B. Variations in tissue contents of coenzyme A thioesters and possible metabolic implications. Biochem. J. 1964;93:550. doi: 10.1042/bj0930550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Physiol. Pharm. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 82.Kuipers F., Jong M.C., Lin Y., Eck M.V., Havinga R., Bloks V., Verkade H.J., Hofker M.H., Moshage H., van Berkel T.J.C., Vonk R.J., Havekes L.M. Impaired secretion of very low density lipoprotein-triglycerides by apolipoprotein E-deficient mouse hepatocytes. J. Clin. Invest. 1997;100:2915–2922. doi: 10.1172/JCI119841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Williamson D., Mellanby J., Krebs H. Enzymic determination of D (-)-β-hydroxybutyric acid and acetoacetic acid in blood. Biochem. J. 1962;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.King M.T., Reiss P.D., Cornell N.W. Determination of short-chain coenzyme A compounds by reversed-phase high-performance liquid chromatography. Methods Enzymol. 1988;166:70–79. doi: 10.1016/s0076-6879(88)66012-5. [DOI] [PubMed] [Google Scholar]
- 85.Pearson D.J., Tubbs P.K. Acetyl-carnitine in heart and liver. Nature. 1964;202:91. doi: 10.1038/202091a0. [DOI] [PubMed] [Google Scholar]
- 86.Tubbs P.K., Garland P.B. Assay of coenzyme a and some acyl derivatives. Method. Enzymol. 1969;13:535–551. [Google Scholar]
- 87.Bloom K., Mohsen A.W., Karunanidhi A., El Demellawy D., Reyes-Múgica M., Wang Y., Ghaloul-Gonzalez L., Otsubo C., Tobita K., Muzumdar R., Gong Z., Tas E., Basu S., Chen J., Bennett M. Investigating the link of ACAD10 deficiency to type 2 diabetes mellitus. J. Inherit. Metab. Dis. 2018;41:49–57. doi: 10.1007/s10545-017-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hunt M.C., Solaas K., Kase B.F., Alexson S.E. Characterization of an acyl-coA thioesterase that functions as a major regulator of peroxisomal lipid metabolism. J. Biol. Chem. 2002;277:1128–1138. doi: 10.1074/jbc.M106458200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained within the article.