Abstract
Background and purpose:
Erynginum billardieri has been used to control diabetes in traditional medicine. This research was performed to study the antidiabetic, hepatoprotective, and hypolipidemic effects of E. billardieri root extract (EBRE) on streptozotocin/nicotinamide-induced type 2 diabetic male rats.
Experimental approach:
Type two diabetic animals were treated by three different doses of EBRE (100, 200, and 400 mg/kg), orally administered for 4 weeks. Ultimately, after anesthesia, the glucose, insulin, lipid profiles, hepatic enzyme levels in the blood and liver, and pancreas tissues of the animals were analyzed.
Findings/Results:
Induction of diabetes caused a diminution in insulin level, high-density lipoprotein (HDL), and significantly enhanced the level of other lipid profiles, glucose, and liver enzymes (P < 0.05). Administration of the EBRE to diabetic-male rats significantly reduced glucose level, lipid profiles, and liver enzymes, and increased the level of HDL to near normal.
Conclusion and implications:
The results of the present study showed that E. billardieri had a positive effect on diminishing the lipid profiles, liver enzymes, and controlling diabetes. The most effective dose was found to be 100 mg/kg.
Keywords: Antidiabetic, Eryngium billardieri, Nicotinamide, Streptozotocin
INTRODUCTION
Diabetes syndrome is a widespread metabolic disturbance in the world, which is marked by an abnormal lipid profile, inadequate secretion, or insulin dysfunction (1,2). This disease is the most common chronic disorder in the whole world. It is estimated that by 2030, the number of people suffering from the disease will reach more than 370 million (3). Increased generation of reactive oxygen species and the occurrence of oxidative stress led to the destruction of insulin-secreting beta cells of the islets of Langerhans. All of these play key roles in the induction and progression of diabetes syndrome (4). Free radicals are produced uncontrollably in diabetic patients by glucose oxidation, non-enzymatic glycation of proteins, followed by oxidative damage of glycosylated proteins (5).
Increased levels of free radicals and the simultaneous reduction of defense mechanisms can lead to tissue and enzyme damage, followed by increased lipid peroxidation and insulin resistance in diabetic patients (6). Although anti-diabetic drugs such as biguanides and sulphonylureas are available today, they lead to many complications such as lactic acidosis, gastrointestinal disturbance, blood dyscrasia, and hypersensitivity reactions (7). Hypoglycemic traditional herbs have been of great interest in recent years given their low cost, several varieties of effective combinations, lower side effects in comparison to synthetic drugs, and the recommendations of the World Health Organization (8). E. billardieri is among the many herbs, which have been recommended for the cure of diabetes. Also known as Shkay and Shocked Al Bayda, E. billardieri has been reported for its antidiabetic effects (9). E. billardieri is a clear herbaceous plant with a stem height of 1 to 2 m covered with white cotton cords. Some of its properties include low branch stem, wing-like side, lobe and thorn in the length of the main stem, and its branched and long oval leaves with edges of the lobe and serrated end to the strong horn and sharp-pointed. Its fruits are hazelnut painted gray to black scales and end to a bunch of dark brown color. This plant grows in the north of Iran in the provinces of Gorgan, Mazandaran, Azerbayejan, and Hamedan (10). The plant contains compounds such as alkaloids, saponins, flavonoids, terpenoids, coumarone, chlorogenic acid, caffeic acid, and beta carotene. Results of previous studies show that this plant has biological effects including anti-inflammatory, antioxidant, cytotoxic, anti- apoptotic, antibacterial and fungal, and antimalaria. The antiradical and antioxidant compounds found in the plant can improve the function of the liver in hypercholesterolemia mice without adverse side effects on renal function. In addition, the alkaloid compounds in the plant help to reduce fat absorption from the intestine (9,11). In spite of the active ingredients in this herb, which have probably important effects on the control of blood glucose and fat, few works have been carried out in this field. Because of Eryngium species roots effects against various inflammatory conditions and a wide range of ailments are well-known in the traditional medicines worldwide (12), the aim of this study was to evaluate the antidiabetic and hypolipidemic effects of the E. billardieri root extract (EBRE) on streptozotocin/ nicotinamide-induced diabetic rats. In this study, we applied the streptozotocin/ nicotinamide in order to mild impairment in the secretion of insulin hormone that is more closely resembled in the induction of type 2 diabetes (13).
MATERIALS AND METHODS
Plant preparation and extract
Fresh roots of E. billardieri were bought from medicinal plants bazaar in Qom province in Iran and authenticated scientifically by the Department of Medicinal Plant of Qom University of Medical Sciences. The roots were then dried and turned into a powder by an electric mill. To prepare the extract, 50 g of the powdered plants were soaked in 200 mL of hydroalcoholic solvent (distilled water:ethanol, 96% mixture; 60:40) and kept for 72 h in a closed dish at room temperature. The roots were then filtered (No. 1 Whatman filter paper) at the end. After removing the solvent and drying at room temperature, about 7 g (with a purity of 15%) of a semisolid mass were obtained, which were kept at 4 °C prior to use (14). A voucher specimen of (No. TMRC. 2291) E. billardieri is deposited in the herbarium of Traditional Medicine Research Center (TMRC) of Shahid Beheshti University of Medical Sciences, Tehran, I.R. Iran.
Animals
Thirty-six Wistar male rats weighting 200-240 g were used. During the research, all animals in groups had free availability for feeding and were kept in a house with controlled conditions (temperature: 20-24 °C, humidity: 40-45%, and luminosity: 12/12-h light/dark). Ethical principles (Ethic No. IR.MUQ.REC.1395.10) of work on animals were taken in accordance with the agenda of the Ethics Committee of the Medical Sciences University, Qom, I.R. Iran.
Study groups and interventions
Male rats were randomly distributed into six groups (n = 6). The groups included the control (group 1), streptozotocin/nicotinamide-induced diabetic with no treatment (group 2) who received normal saline orally, diabetic rats with oral administration of glibenclamide (GLI; 0.25 mg/kg BW, Sigma Aldrich Co., USA) (group 3), and diabetic rats orally fed with EBRE at 100, 200, and 400 mg/kg daily (9) for 28 days (15)(groups 4-6). The experiments were carried out over a period of four weeks.
Induction of type two model of diabetes
For inducing diabetes, the male rats fasted overnight and received intraperitoneally an injection of nicotinamide (Merck Co., Germany) dissolved in normal saline at a dose of 110 mg/kg. After 15 min, streptozotocin (Sigma Co, USA) dissolved in citrate buffer, pH 4.5 (Sigma Aldrich Co., USA) was injected intraperitoneally at 60 mg/kg. The fasting blood glucose levels of the rats were measured after 72 h of streptozotocin administration and the rats with blood glucose levels of above 200 mg/dL were regarded as diabetic (15).
Biochemical assessment
The blood glucose levels were determined using an Elegance glucometer (CT-X10, Convergent Technologies, Germany). In order to evaluate biochemical factors, serum was separated by blood centrifugation for 10 min at 2000 g, 4 °C and stored at -20 °C until analysis (16). To evaluate the serum insulin levels, a commercial ELISA kit (Monobind, California, USA) was used according to the manufacturer’s instructions. The homeostatic model assessment function of pancreatic β-cell (HOMA-β) and insulin resistance (HOMA-IR) were then obtained through the following equations (17):
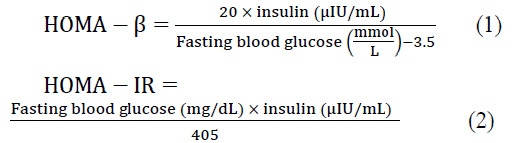
The low-density lipoprotein cholesterol (LDL-c), serum level of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-c), the level of alkaline phosphatase (ALP), serum glutamic pyruvic transaminase (SGPT), and glutamic oxaloacetic transaminase (SGOT) were assessed using commercial kits (Pars Azmoon, Iran).
In addition, the levels of very low-density lipoprotein cholesterol (VLDL-c), which equals one-fifth of TG content, were determined using the Norbert formula (18). As the zone of atherogenic risk, the atherogenic index of plasma (AIP = Log (TG/HDL-C)) was assessed (19).
Histopathological evaluation
For the histological examination, the samples (liver and endocrine pancreas) were fixed in 10% formalin (20) for at least 24 h, then tissues were dehydrated with a sequence of ethanol solutions, embedded in paraffin, cut into 5 μm sections and stained with hematoxylin and eosin dye (H&E stain). Slides were observed under a light microscope (Olympus, Tokyo, Japan) connected to a camera (Digital Microscope BMZ-04-DZ) in a blind manner (21). Six microscope slides per animal were examined for assessment of histological changes. The fat deposit and nuclear pyknosis indices were assessed for the liver. For semi-quantitative evaluation of these indices following scoring system was used: 0 = normal, 1 = mild, 2 = moderate, and 3 = severe, and the total score was calculated by averaging all scores of each group (22). Morphological changes related to degenerated cells were counted in five fields of each tissue section of the endocrine pancreas. The degree of injuries was expressed as the percentage mean of lesions in five different fields (zigzag manner) in each section (23).
Statistical analysis
Acquired data were demonstrated as mean ± SEM. Statistical assessment by analysis of variance (one-way ANOVA) followed by Tukey’s test was performed by SPSS Statistics, V. 17.01 (SPSS Inc, Chicago, USA). P < 0.05 was considered statistically significant.
RESULTS
Effect of EBRE on blood glucose
As indicated in Table 1, the induction of diabetes resulted in elevated blood glucose and the insulin concentration decreased, remarkably compared with the control group (P < 0.001).
Table 1.
Effect of EBRE on insulin, fasting plasma glucose levels, HOMA-IR, and HOMA-β in streptozotocin-induced diabetic rat. Results are expressed as mean ± SEM, n = 6.
| Groups | Glucose (mg/dL) | Insulin (μIU/mL) | HOMA-IR | HOMA-β |
|---|---|---|---|---|
| Control | 99.00 ± 6.30 | 2.70 ± 0.25 | 0.69 ± 0.09 | 156.06 ± 21.1 2 |
| Diabetic | 211.57 ± 10.7### | 1.63 ± 0.13### | 0.85 ± 0.08 | 0.42 ± 2.16### |
| Diabetic+ glibenclamide | 132.16 ±9.38* | 2.53 ± 0.14*** | 0.82 ± 0.06 | 72.26 ± 10.3###,* |
| Diabetic + EBRE (100 mg/kg) | 118.80 ± 9.48*** | 2.84 ± 0.14*** | 0.84 ± 0.08 | 98.96 ± 11.2#, *** |
| Diabetic + EBRE (200 mg/kg) | 197.40 ± 16.5### | 2.40 ± 0.17 | 1.19 ± 0.15## | 34.66 ± 4.84### |
| Diabetic + EBRE (400 mg/kg) | 178.28 ± 12.1### | 1.91 ± 0.17# | 0.83 ± 0.07 | 34.31 ± 8.42### |
EBRE, Eryngium billardieri root extract; HOMA-β, homeostatic model assessments of pancreatic β-cell function; HOMA-IR, homeostatic model assessment of insulin resistance. *P < 0.05 and ***P < 0.001 indicate significant differences compared to the diabetic group; #P < 0.05, ##P < 0.01, and ###P < 0.001 versus control group.
Daily administration of EBRE for 4 weeks to diabetic rats at the dose of 100 mg/kg significantly reversed these changes (P < 0.001). In group 3, taking GLI, the blood glucose level reduced and the level of insulin increased, remarkably (P < 0.05 and P < 0.001, respectively) in comparison to the diabetic group. Moreover, according to the HOMA-IR evaluation criteria, the insulin resistance in the diabetic group was enhanced in comparison to the control group, but it was not significant. Based on HOMA-β criteria, the beta cells function diminished significantly in group 2 compared with the control intact group (P < 0.001). In addition, 28-day treatment of diabetic animals with GLI and EBRE at 100 mg/kg, significantly increased HOMA-β compared to the diabetic group (P < 0.05 and P < 0.001, respectively).
Effect of EBRE on body weight
As indicated in Fig. 1, after induction of diabetes, the rats’ body weight was significantly decreased compared with the control group (P < 0.05). Although the body weight increased in other groups than the diabetic group, the changes were not significant.
Fig. 1.
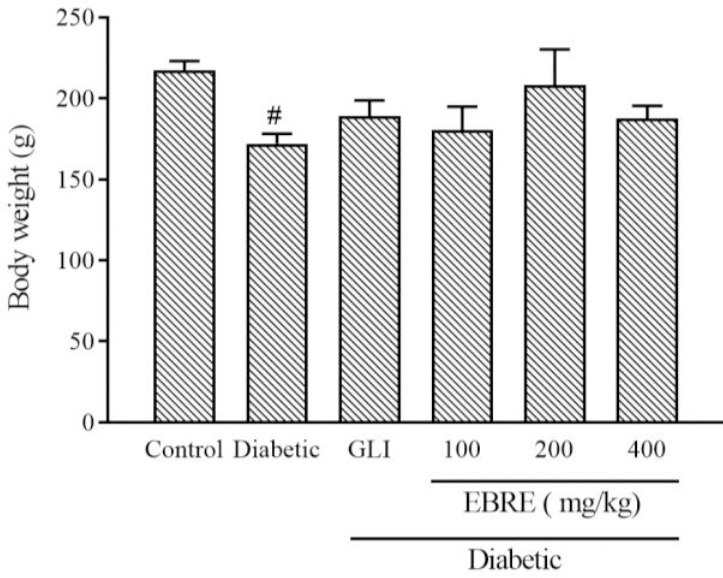
Effect of EBRE on the body weight after 4 weeks in streptozotocin-induced diabetic rat. Results are expressed as mean ± SEM, n = 6. #P < 0.05 Indicates significant differences versus the control group. GLI, Glibenclamide; EBRE, Eryngium billardieri root extract.
Effect of EBRE on lipid profile level
As shown in Table 2, after induction of diabetes in rats, the HDL level diminished (P < 0.01) and the levels of VLDL, TG, LDL and cholesterol increased (P < 0.01 and P < 0.05, respectively) significantly compared with the control group. According to the experimental results listed in Table 2, 2 8 -day treatment of diabetic rats with EBRE at 100 and 400 mg/kg, significantly decreased the levels of LDL (P < 0.01) and chol esterol (P < 0.05) relative to the diabetic group. The concentration of HDL increased in the diabetic groups, which received 200 and 400 mg/kg of EBRE (P < 0.01). Treatment of diabetic rats with EBRE, remarkably reduced the VLDL and TG levels in doses of 100 mg/kg (P < 0.01), 200 and 400 mg/kg (P < 0.05) compared with the diabetic group.
Table 2.
Effect of EBRE on lipid profile (mg/dL) and atherogenic index in streptozotocin-induced diabetic rat. Results are expressed as mean ± SEM, n = 6.
| Groups | Cholesterol (mg/dL) | TG (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | VLDL (mg/dL) | Atherogenic index |
|---|---|---|---|---|---|---|
| Control | 46.71 ± 5.59 | 40.57 ± 4.5 | 35.42 ± 3.50 | 2.81 ± 0.32 | 8.11 ± 0.90 | 0.34 ± 0.04 |
| Diabetic | 68.57 ± 5.8# | 68.14 ± 4.91## | 16.78 ± 1.61## | 4.26 ± 0.30# | 13.62 ± 0.98## | 0.74 ± 0.07### |
| Diabetic + GLI | 55.33 ± 3.92 | 58.16 ± 7.92 | 23.33 ± 1.42 | 3.64 ± 0.31 | 11.63 ± 1.58 | 0.55 ± 0.04* |
| Diabetic + EBRE (100 mg/kg) | 43.8 ± 3.35* | 38.4 ± 4.92** | 20.4 ± 3.15 | 2.33 ± 0.36** | 7.68 ± 0.98** | 0.49 ± 0.09** |
| Diabetic + EBRE (200 mg/kg) | 48.0 ± 3.19 | 42,4 ± 4.53* | 35.6 ± 4.74** | 3.66 ± 0.21 | 8.48 ± 0.90* | 0.37 ± 0.05*** |
| Diabetic + EBRE (400 mg/kg) | 47.42 ± 5.16* | 46.14 ± 3.88* | 36.42 ± 4.69** | 2.69 ± 0.25** | 9.22 ± 0.77* | 0.31 ± 0.06*** |
EBRE, Eryngium billardieri root extract; GLI, glibenclamide; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglycerides; VLDL, very low-density lipoprotein. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate significant differences in comparison with the diabetic group; #P < 0.05, ##P < 0.01, and ###P < 0.001 versus control group.
Effect of EBRE on level of liver enzymes
Induction of diabetes in rats significantly increased their serum level of liver enzymes compared to the intact rats (P < 0.001). According to the acquired data shown in Table 3, the treatment of diabetic rats with EBRE, significantly reduced the liver enzymes at 100 and 200 mg/kg (P < 0.01 and P < 0.05, respectively). Furthermore, the serum level of SGPT diminished in diabetic rats after four- week administration of GLI and a dose of 400 mg/kg EBRE (P < 0.05).
Table 3.
Effect of EBRE on SGOT and SGPT (U/L) levels in streptozotocin-induced diabetic rat. Results are expressed as mean ± SEM; n = 3.
| Groups | SGPT (U/L) | SGOT (U/L) |
|---|---|---|
| Control | 167.7 ± 12.6 | 289.71 ± 20.9 |
| Diabetic | 251.3 ± 6.62### | 494.71 ± 28.3### |
| Diabetic + glibenclamide | 193.3 ± 5.56* | 386.50 ± 32.8 |
| Diabetic + EBRE (100 mg/kg) | 170.8 ± 8.97** | 331.60 ± 21.6** |
| Diabetic + EBRE (200 mg/kg) | 187.6 ± 11.9* | 354.00 ± 32.5* |
| Diabetic + EBRE (400 mg/kg) | 193.1 ± 9.90* | 399.42 ± 26.8 |
EBRE, Eryngium billardieri root extract; SGOT, serum glutamic-oxaloacetic transaminase; SGPT, serum glutamicpyruvic transaminase. *P < 0.05 and **P < 0.01 indicate significant differences compared to the diabetic group; ###P < 0.001 versus control group.
Effect of EBRE on histopathological changes of liver tissue
In order to evaluate the effect of EBRE on histopathology of the liver, H&E staining was performed. The intact control group displayed a normal liver cytoarchitecture (Fig. 2A and Table 4). Microscopic examination of liver sections of the untreated diabetic group revealed significant injuries represented by fat deposit and nuclear pyknosis (P < 0.001; Fig. 2B and Table 4). Liver sections from animals treated with GLI (0.25 mg/kg) showed remarkable improvement in liver fat deposit and nuclear pyknosis (P < 0.001 and P < 0.05; Fig. 2C and Table 4). Treatment of animals with EBRE extract, reduced nuclear pyknosis at dose 400 mg/kg (P < 0.01) and fat deposit at doses 200 and 400 mg/kg (P < 0.01 and P < 0.05; Fig. 2D-F, and Table 4).
Fig. 2.
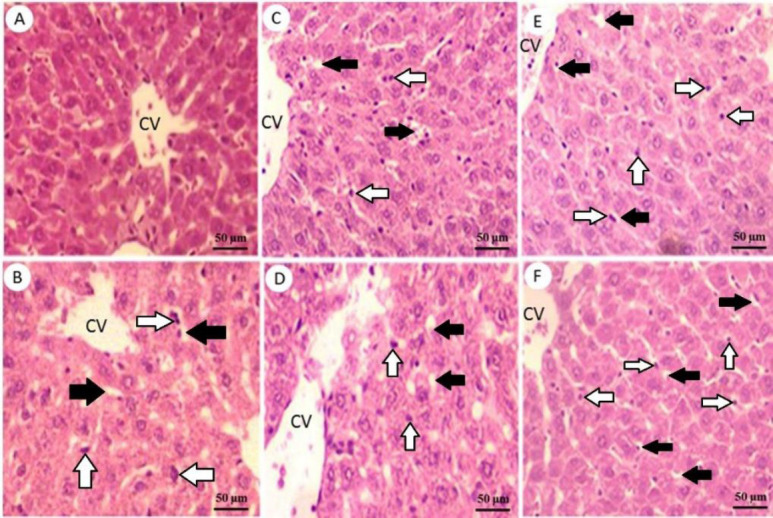
Liver sections stained with hematoxylin and eosin. (A) Normal histological view of the liver in control group rats; (B) microscopic examination of liver tissue of untreated diabetic group showing some pathological changes and injury including fat deposit and nuclear pyknosis, which displaying degenerative changes compared to the control normal rats; (C) liver section from an animal treated with glibenclamide (0.25 mg/kg) showing a significant reduction in nuclear pyknosis and fat deposit; (D-F) liver of animals treated with 100, 200, and 400 mg/kg doses of Eryngium billardieri root extract, respectively which indicating the improvement of pathological injury. Magnification: 200⨯. Black arrows, fat deposit; white arrows, nuclear pyknosis.
Table 4.
The quantified histopathological changes of rat livers in hematoxylin and eosin stained sections. Results are expressed as mean ± SEM; n = 3.
| Groups | Nuclear pyknosis | Fat deposit |
|---|---|---|
| Control | 0.08 ± 0.02 | 0.12 ± 0.01 |
| Diabetic | 1.64 ± 0.21### | 2.06 ± 0.15### |
| Diabetic + glibenclamide | 1.10 ± 0.07###,* | 1.40 ± 0.09###,*** |
| Diabetic + EBRE (100 mg/kg) | 1.20 ± 0.14### | 1.82 ± 0.11### |
| Diabetic + EBRE (200 mg/kg) | 1.14 ± 0.10### | 1.64 ± 0.08###,* |
| Diabetic + EBRE (400 mg/kg) | 1.02 ± 0.07###,** | 1.50 ± 0.06###,** |
EBRE, Eryngium billardieri root extract. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate significant differences compared to the diabetic group; ###P < 0.001 versus control group.
Effect of EBRE on histopathological changes of pancreas tissue
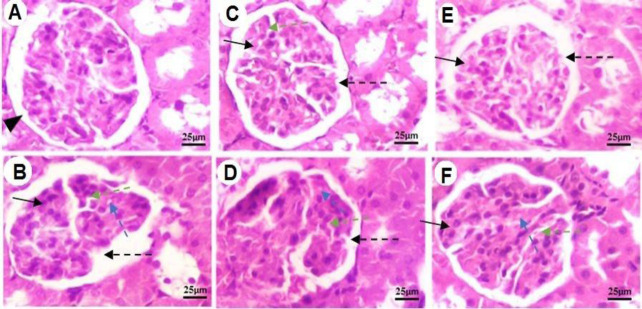
The histological appearance of the pancreatic islets of the control group showed normal endocrine and acinar architecture (Fig. 3A and Fig. 4). Microscopic examination of pancreatic sections of the untreated-diabetic group revealed significant injuries and pathological changes, represented by vacuolar swelling, necrotic cells, disruption of normal endocrine architecture, shrinkage and degeneration of cells (P < 0.001; Fig. 3B; Fig. 4). Pancreas sections from animals treated with GLI (0.25 mg/kg) showed remarkable improvement in pancreatic islets endocrine structure, cellular degeneration, and necrosis reduction (P < 0.05; (Fig. 3C; Fig. 4). Treatment of animals with 200 and 400 mg/kg doses of EBRE, significantly decreased the percentage of degenerative and necrotic cells and vacuolar changes and restored endocrine structure (P < 0.05; Fig. 2D, E, F; Fig. 4).
Fig. 3.
Pancreas sections stained with hematoxylin and eosin. (A) The histological study of pancreas sections shows the normal appearance of pancreatic islets (black triangle) in the control group; (B) microscopic examination of pancreatic tissue of untreated diabetic group shows some pathological changes and injury including degeneration and shrinkage (irregular space in the islets of Langerhans, dashed-green arrows), disruption of normal endocrine architecture (dashed- black arrows), and necrotic cells with pyknotic nuclei (black arrows), swelling (spaces in the islets of Langerhans, dashed- blue arrows) which all of them displaying degenerative changes compared to the control normal rats; (C) pancreas section from an animal treated with glibenclamide (0.25 mg/kg) showing necrosis reduction (black arrows) and cellular degeneration (dashed-green arrows), significant endocrine structure restoration (dashed-black arrows); (D-F) pancreas of animals treated with EBRE at 100, 200, and 400 mg/kg, respectively. Treatment of animals whit EBRE at 100 mg/kg shows a slight decrease in endocrine structure disruption (dashed-black arrows), cellular degeneration (dashed-green arrows), and swelling (dashed-blue arrows). Treatment of rats whit EBRE at 200 and 400 mg/kg indicates a remarkable reduction in degeneration and shrinkage (dashed-green arrows), disruption of normal endocrine structure (dashed-black arrows), minimum vacuolar swelling (dashed-blue arrows), and necrosis (black arrows). Magnification: 200⨯. EBRE, Eryngium billardieri root extract
Fig. 4.
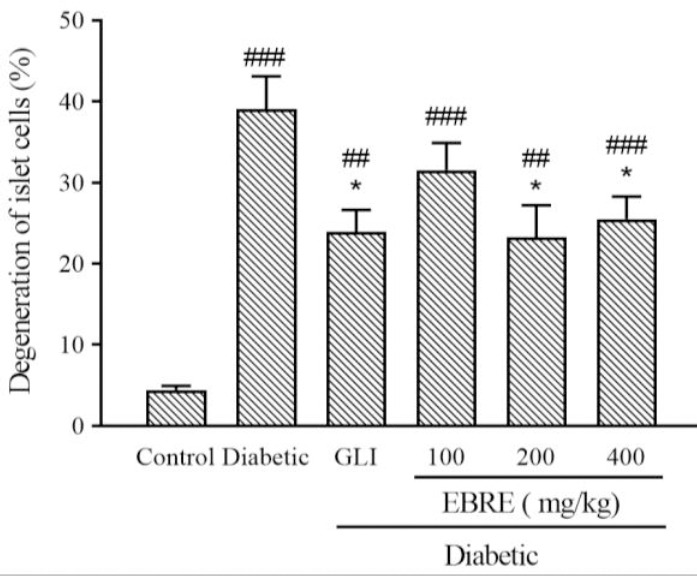
Effect of EBRE on percentage of total damaged cells (degeneration of islet cells) in streptozotocin- induced diabetic rat. Results are expressed as mean ± SEM, n = 6. *P < 0.05 indicates significant differences in comparison with the diabetic group; ##P < 0.01, and ###P < 0.001 versus control group. GLI, Glibenclamide; EBRE, Eryngium billardieri root extract.
DISCUSSION
In our research remarkable diminish in insulin level and increase in blood glucose level was observed in streptozotocin/nicotinamide- induced diabetic animals in comparison to the control group rats. The level of insulin in type II diabetes mellitus rats in this research implied that some insulin secretary β cells are healthy and can still produce and secrete insulin. One of the anti-hyperglycemic mechanisms is elevating insulin production and decreasing glucose absorption from the intestine surface (24). The result of our study showed that treatment with EBRE can significantly reduce blood glucose levels and elevate serum levels of insulin in diabetic rats. Some antioxidant ingredients of the herb such as flavonoids and carotene can probably reduce the level of oxidative stress in beta cells and improve their function to produce insulin and subsequently decrease blood glucose level (9).
Some insulin-related biomarkers such as HOMA-IR and HOMA-β assay are used to reveal the health of insulin generating cells and in sulin function in T2DM (25). In this study, the induction of diabetes elevated the level of HOMA-IR and HOMA-β in diabetic rats. However, only HOMA-β, significantly increased. After treatment with EBRE, the level of HOMA-B reduced remarkably compared with the diabetic group. This can be due to the effect of the plant extract on B-cell and improved insulin production. The results of the hormonal analysis were also supported by histopathological findings of the pancreas as histopathological changes reached up in the diabetic group relative to the control group.
Metabolic disorders related to insulin insensitivity, insulin secretion, and hyperglycemia is reflected by changes in the islet structure, size, or function. This subject in our research demonstrated severe injuries represented by the islets shrinkage (atrophy), irregular islets cellular swelling and degeneration, and the presence of necrotic cells in the diabetic animals (23). The severity of these pathological changes in diabetic rats was remarkably decreased after treatment of extracts and GLI (Fig. 4).
Our findings indicated that the average body weight was significantly diminished in the rats after induction of diabetes by streptozotocin. Our results match those of other researches, demonstrating that streptozotocin-induced diabetes leads to severe loss in body weight (26). Diabetes is concomitant with the decrease of body weight due to excessive lysis of tissue proteins and wasting muscle mass because of insulin shortage (27). Administration of EBRE for diabetic rats led to increased body weight in comparison to diabetic control rats which the change was not significant. In our study, the diabetic control group showed an elevation in the levels of LDL, VLDL, TG, and a decrease in the levels of HDL, which are in agreement with the findings of Farsani et al. (28). After treatment with EBRE, remarkable decreases were observed in the levels of LDL, VLDL, and TG and an elevation was observed in the level of HDL. The mechanisms involved in this process are probably the inhibition of cholesterol synthesis, increased consumption of it, decreased fat absorption in the intestines, increase bowel movements and reduce fat absorption. The hypoglycemic and hypolipidemic properties of some compounds present in E. billardieri extract such as flavonoids and tannins have also been reported (29). In addition, saponin as one of the active components of E. billardieri can modulate lipid profiles by suppressing cholesterol luminal absorption and increasing cholesterol secretion through biliary excretion (30). Alkaloids as another component of extract that also inhibit cholesterol synthesis (31). In addition to the mechanisms mentioned above, a significant reduction in serum lipid levels in the diabetic rats treated with the extract was probably caused as a consequence of increased insulin levels. It is well recognized that insulin shortage reduces lipoprotein lipase activity and leads to hypertriglyceridemia and an increase in plasma free fatty acid concentration (32).
One of the important insulin-dependent tissues is the liver, which is damaged during type II diabetes mellitus (33).
In our study in streptozotocin/nicotinamide- induced diabetic rats, serum levels of SGOT and SGPT elevated indicating liver dysfunction. EBRE led to a remarkable reduction in SGPT and SGOT in the treated groups, which indicated this herb could have a preservative effect on the liver. In addition to serum assessment, also in this research evaluation of the histological appearance of the liver indicated a striking increase in fat deposit and nuclear pyknosis in liver tissue of the diabetic group compared to the intact group which this subject represents the liver injury (22). Treatment of diabetic rats with EBRE, by lessening the severity of injuries, improved liver structure.
Previous studies have proven that flavonoids, one of the components of E. billardieri, are able to quench nitric oxide synthase and those ingredients, which are able to diminish the production of nitric oxide in the liver have hepatoprotective effects (34). On the other hand, the hepatoprotective effect of EBRE on the liver may be related to the antidiabetic properties of the plant. Investigations demonstrated that insulin acts as a hormone-sensitive lipase inhibitor (35). Thus, the activation of hormone-sensitive lipase during insulin shortage is accompanied by the increase of the lipolysis cycle, the liberation of free fatty acids, and stimulation of the conversion of fatty acids into phospholipids and cholesterol in the plasma and liver (36). Furthermore, flavonoids, tannins, and terpenoids in E. billardieri have anti-inflammatory and antioxidant properties and help to protect the liver against oxidative stress- induced factors, which cause hepatotoxicity (9).
CONCLUSION
The results of this study indicated that E. billardieri root extract has hypoglycemic and hepatoprotective effects and can modulate lipid profiles. Therefore, this plant can be useful in type II diabetes mellitus treatment. However, more research is needed to clarify the exact mechanisms of the antidiabetic effects of E. billardieri.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Authors’ contribution
H. Heidari supervised and conducted the study and reviewed the manuscript. S. Khani and M. Abdollahi analyzed the hormonal and tissue data. Z. Asadi and M. Nazeri helped with the literature search prepared the proposal and carried out the laboratory studies. M.A. Nasiri drafted the manuscript and helped in laboratory studies. H. Yusefi prepared the extract and blood sample collecting and data acquisition. A. Moghadam also has interpreted the tissue data. All authors have read and approved the final manuscript.
Acknowledgements
This work has been financially supported by the Vice-Chancellor of Research Affairs of Qom University of Medical Sciences under Grant No. IR.MUQ.REC.1395.10. The authors are also deeply thankful for the technical collaboration of the Qom Danesh Laboratory.
REFERENCES
- 1.Ahangarpour A, Shabani R, Farbood Y. The effect of betulinic acid on leptin, adiponectin, hepatic enzyme levels and lipid profiles in streptozotocin- nicotinamide-induced diabetic mice. Res Pharm Sci. 2018;13(2):142–148. doi: 10.4103/1735-5362.223796. DOI: 10.4103/1735-5362.223796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahangarpour A, Zamaneh HT, Jabari A, Nia HM, Heidari H. Antidiabetic and hypolipidemic effects of Dorema aucheri hydroalcoholic leave extract in streptozotocin-nicotinamide induced type 2 diabetes in male rats. Iran J Basic Med Sci. 2014;17(10):808–814. [PMC free article] [PubMed] [Google Scholar]
- 3.Khazaei M, Pazhouhi M. Protective effect of hydroalcoholic extracts of Trifolium pratense L. on pancreatic ß cell line (RIN-5F) against cytotoxicty of streptozotocin. Res Pharm Sci. 2018;13(4):324–331. doi: 10.4103/1735-5362.235159. DOI: 10.4103/1735-5362.235159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moezi L, Arshadi SS, Motazedian T, Seradj SH, Dehghani F. Anti-diabetic effects of Amygdalus Lycioides spach in streptozocin-induced diabetic rats. Iran J Pharm Res. 2018;17(1):353–364. [PMC free article] [PubMed] [Google Scholar]
- 5.Doustar Y, Mohajeri D. Antioxidant effect of extract of the grape seed in streptozotocin induced diabetic rats. Zahedan J Res Med Sci. 2010;12(1):e94345,1–14. [Google Scholar]
- 6.Alipour M, Salehi I, Soufi FG. Effect of exercise on diabetes-induced oxidative stress in the rat hippocampus. Iran Red Crescent Med J. 2012;14(4):222–228. [PMC free article] [PubMed] [Google Scholar]
- 7.Shewasinad A, Bhoumik D, Zero Hishe H, Masresha B. Antidiabetic activity of methanol extract and fractions of Thymus schimperi ronniger leaves in normal and streptozotocin induce diabetic mice. Iranian J Pharmacol Ther. 2018;16(1):1–8. [Google Scholar]
- 8.Jalili-Nik M, Soukhtanloo M, Javanshir S, Yazdi AJ, Esmaeilizadeh M, Jafarian AH, et al. Effects of ethanolic extract of Ferula gummosa oleo-resin in a rat model of streptozotocin-induced diabetes. Res Pharm Sci. 2019;14(2):138–145. doi: 10.4103/1735-5362.253361. DOI: 10.4103/1735-5362.253361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarei A, Ashtiyani SC, Hamidizadeh S, Rezaei A. The study of the effects hydro-alcoholic extract of Eryngium billardieri on lipid profiles levels and liver and renal functions tests in hypercholesterolemic rats. J Chem Pharm Res. 2015;7(2):200–206. [Google Scholar]
- 10.Zargari A. Pharmaceutical plants (persian) 8th ed. Tehran: Tehran University Press; 2015. pp. 212–214. [Google Scholar]
- 11.Esmaeili S, Irani M, Moazzeni Zehan H, Keramatian B, Tavakoli Harandi Z, Hamzeloo-Moghadam M. Cytotoxic activity of some ethnic medicinal plants from southwest of Iran. Res J Pharmacogn. 2016;3(1):43–47. [Google Scholar]
- 12.Küpeli E, Kartal M, Aslan S, Yesilada E. Comparative evaluation of the anti-inflammatory and antinociceptive activity of Turkish Eryngium species. J Ethnopharmacol. 2006;107(1):32–37. doi: 10.1016/j.jep.2006.02.005. DOI: 10.1016/j.jep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Han X, Shaligram S, Zhang R, Anderson L, Rahimian R. Sex-specific vascular responses of the rat aorta: effects of moderate term (intermediate stage) streptozotocin-induced diabetes. Can J Physiol Pharmacol. 2016;94(4):408–415. doi: 10.1139/cjpp-2015-0272. DOI: 10.1139/cjpp-2015-0272. [DOI] [PubMed] [Google Scholar]
- 14.Ahangarpour A, Heidari H, Oroojan AA, Mirzavandi F, Esfehani KN, Mohammadi ZD. Antidiabetic, hypolipidemic and hepatoprotective effects of Arctium lappa root’s hydro-alcoholic extract on nicotinamide-streptozotocin induced type 2 model of diabetes in male mice. Avicenna J Phytomed. 2017;7(2):169–179. [PMC free article] [PubMed] [Google Scholar]
- 15.Ahangarpour A, Heidari H, Junghani MS, Absari R, Khoogar M, Ghaedi E. Effects of hydroalcoholic extract of Rhus coriaria seed on glucose and insulin related biomarkers, lipid profile, and hepatic enzymes in nicotinamide-streptozotocin-induced type II diabetic male mice. Res Pharm Sci. 2017;12(5):416–424. doi: 10.4103/1735-5362.213987. DOI: 10.4103/1735-5362.213987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehrzadi S, Bahrami N, Mehrabani M, Motevalian M, Mansouri E, Goudarzi M. Ellagic acid: a promising protective remedy against testicular toxicity induced by arsenic. Biomed Pharmacother. 2018;103:1464–1472. doi: 10.1016/j.biopha.2018.04.194. DOI: 10.1016/j.biopha.2018.04.194. [DOI] [PubMed] [Google Scholar]
- 17.Ahangarpour A, Alboghobeish S, Rezaei M, Khodayar MJ, Oroojan AA, Zainvand M. Evaluation of diabetogenic mechanism of high fat diet in combination with Arsenic exposure in male mice. Iran J Pharm Res. 2018;17(1):164–183. [PMC free article] [PubMed] [Google Scholar]
- 18.Mousavi SE, Shahriari A, Ahangarpour A, Vatanpour H, Jolodar A. Effects of Teucrium polium ethyl acetate extract on serum, liver and muscle triglyceride content of sucrose-induced insulin resistance in rat. Iran J Pharm Res. 2012;11(1):347–355. [PMC free article] [PubMed] [Google Scholar]
- 19.Rafieian-Kopaei M, Shahinfard N, Rouhi-Boroujeni H, Gharipour M, Darvishzadeh-Boroujeni P. Effects of Ferulago angulata extract on serum lipids and lipid peroxidation. Evid Based Complement Alternat Med. 2014;2014:680856,1–5. doi: 10.1155/2014/680856. DOI: 10.1155/2014/680856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Javadi I, Rashidi Nooshabadi M, Goudarzi M, Roudbari R. Protective effects of celery (Apium graveloens) seed extract on bleomycin-induced pulmonary fibrosis in rats. J Babol Univ Med Sci. 2015;17(1):70–76. [Google Scholar]
- 21.Safaei F, Mehrzadi S, Khadem Haghighian H, Hosseinzadeh A, Nesari A, Dolatshahi M, et al. Protective effects of gallic acid against methotrexate- induced toxicity in rats. Acta Chir Belg. 2018;118(3):152–160. doi: 10.1080/00015458.2017.1394672. DOI: 10.1080/00015458.2017.1394672. [DOI] [PubMed] [Google Scholar]
- 22.Mehrzadi S, Fatemi I, Esmaeilizadeh M, Ghaznavi H, Kalantar H, Goudarzi M. Hepatoprotective effect of berberine against methotrexate induced liver toxicity in rats. Biomed Pharmacother. 2018;97:233–239. doi: 10.1016/j.biopha.2017.10.113. DOI: 10.1016/j.biopha.2017.10.113. [DOI] [PubMed] [Google Scholar]
- 23.Motshakeri M, Ebrahimi M, Goh YM, Othman HH, Hair-Bejo M, Mohamed S. Effects of brown seaweed (Sargassum polycystum) extracts on kidney, liver, and pancreas of type 2 diabetic rat model. Evid Based Complement Alternat Med. 2014;2014:379407,1–11. doi: 10.1155/2014/379407. DOI: 10.1155/2014/379407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ovalle-Magallanes B, Medina-Campos ON, Pedraza- Chaverri J, Mata R. Hypoglycemic and antihyperglycemic effects of phytopreparations and limonoids from Swietenia humilis. Phytochemistry. 2015;110:111–119. doi: 10.1016/j.phytochem.2014.11.004. DOI: 10.1016/j.phytochem.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Briet C, Ilie MD, Kuhn E, Maione L, Brailly-Tabard S, Salenave S, et al. Changes in metabolic parameters and cardiovascular risk factors after therapeutic control of acromegaly vary with the treatment modality. Endocrine. 2019;63(2):348–360. doi: 10.1007/s12020-018-1797-8. DOI: 10.1007/s12020-018-1797-8. [DOI] [PubMed] [Google Scholar]
- 26.Ghamarian A, Abdollahi M, Su X, Amiri A, Ahadi A, Nowrouzi A. Effect of chicory seed extract on glucose tolerance test (GTT) and metabolic profile in early and late stage diabetic rats. DARU. 2012;20(1):56–54. doi: 10.1186/2008-2231-20-56. DOI: 10.1186/2008-2231-20-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balamurugan R, Ignacimuthu S. Antidiabetic and hypolipidemic effect of methanol extract of Lippia nodiflora L. in streptozotocin induced diabetic rats. Asian Pac J Trop Biomed. 2011;1(1):S30–S36. DOI: 10.1016/S2221-1691(11)60117-2. [Google Scholar]
- 28.Farsani MK, Amraie E, Kavian P, Keshvari M. Effects of aqueous extract of alfalfa on hyperglycemia and dyslipidemia in alloxan-induced diabetic Wistar rats. Interv Med Appl Sci. 2016;8(3):103–108. doi: 10.1556/1646.8.2016.3.5. DOI: 10.1556/1646.8.2016.3.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oyedemi S, Bradley G, Afolayan A. Antidiabetic activities of aqueous stem bark extract of Strychnoshenningsii Gilg in streptozotocin- nicotinamide type 2 diabetic rats. Iran J Pharm Res. 2012;11(1):221–228. [PMC free article] [PubMed] [Google Scholar]
- 30.Banda M, Nyirenda J, Muzandu K, Sijumbila G, Mudenda S. Antihyperglycemic and antihyperlipidemic effects of aqueous extracts of Lannea edulis in alloxan-induced diabetic rats. Front Pharmacol. 2018;9:1099–1106. doi: 10.3389/fphar.2018.01099. DOI: 10.3389/fphar.2018.01099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eguchi K, Fujiwara Y, Hayashida A, Horlad H, Kato H, Rotinsulu H, et al. Manzamine A, a marine- derived alkaloid, inhibits accumulation of cholesterol ester in macrophages and suppresses hyperlipidemia and atherosclerosis in vivo. Bioorg Med Chem. 2013;21(13):3831–3838. doi: 10.1016/j.bmc.2013.04.025. DOI: 10.1016/j.bmc.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 32.Kumar R, Patel DK, Prasad SK, Laloo D, Krishnamurthy S, Hemalatha S. Type 2 antidiabetic activity of bergenin from the roots of Caesalpinia digyna Rottler. Fitoterapia. 2012;83(2):395–401. doi: 10.1016/j.fitote.2011.12.008. DOI: 10.1016/j.fitote.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Ogar I, Egbung GE, Nna VU, Atangwho IJ, Itam EH. Hyptis verticillata attenuates dyslipidaemia, oxidative stress and hepato-renal damage in streptozotocin-induced diabetic rats. Life Sci. 2019;219:283–293. doi: 10.1016/j.lfs.2019.01.027. DOI: 10.1016/j.lfs.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Gentile D, Fornai M, Colucci R, Pellegrini C, Tirotta E, Benvenuti L, et al. The flavonoid compound apigenin prevents colonic inflammation and motor dysfunctions associated with high fat diet-induced obesity. PLoS One. 2018;13(4):e0195502,1–19. doi: 10.1371/journal.pone.0195502. DOI: 10.1371/journal.pone.0195502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Movahedian A, Zolfaghari B, Sajjadi SE, Moknatjou R. Antihyperlipidemic effect of Peucedanum pastinacifolium extract in streptozotocin-induced diabetic rats. Clinics (Sao Paulo) 2010;65(6):629–633. doi: 10.1590/S1807-59322010000600011. DOI: 10.1590/S1807-59322010000600011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie J, Wang Y, Jiang WW, Luo XF, Dai TY, Peng L, et al. Moringa oleifera leaf petroleum ether extract inhibits lipogenesis by activating the AMPK signaling pathway. Front Pharmacol. 2018;9:1447–1458. doi: 10.3389/fphar.2018.01447. DOI: 10.3389/fphar.2018.01447. [DOI] [PMC free article] [PubMed] [Google Scholar]