Abstract
Background and purpose:
Gauze dressing is a barrier against microbial infection in wounds. The seed gums of Tamarindus indica and Cassia fistula are abundant in tropical countries; we used them as a coating material of cotton gauzes to improve the liquid absorption ability. Moreover, Chromolaena odorata leaf extract was loaded on the gums for antibacterial gauze dressing with hemostatic activity.
Experimental approach:
Crude gums were extracted from T. indica and C. fistula seeds and carboxymethyl gums were then derived and chracterized. C. Odorata ethanolic extract was also prepared by maceration and its antimicrobial and blood clotting activities were determine coated gauze dressing containing different concentrations of carboxymethyl gum was prepared in the presence of various concentrations of C. odorata extract. Then, the physical properties, antibacterial activity, and skin-irritating effects of the coated gauze were analyzed.
Findings/Results:
The results indicated that the amount of carboxymethyl gum affected the physical properties and absorption capacity of the coated gauze. C. odorata extract exhibited better bactericidal activity against Staphylococcus aureus than against Escherichia coli. The blood clotting effects of C. odorata extract indicated that it had dramatic hemostatic efficacy. The coated gauze exhibited bactericidal activity against S. aureus. In the human skin irritation test, the coated gauze caused no adverse effects on human skin.
Conclusion and implication:
Coated gauze has the potential to serve as a prototype for primary hemostasis in first aid for opened wounds such as abrasions and lacerations.
Keywords: Cassia fistula, Chromolaena odorata, Dressing, Hemostasis, Tamarindus indica
INTRODUCTION
Extensive knowledge regarding wound healing has been accumulated (1,2,3,4). Dressing is currently considered to provide the optimal conditions for wound healing. It is designed to be in direct contact with the wound, differing from bandages that hold the dressing in place. The major functions of dressing are facilitating wound healing and preventing further issues such as infection or complications. Dressing is also important for stopping bleeding, promoting clotting, and absorbing any excess blood, plasma, or other fluids to permit wound healing. One of the materials for wound dressing is gauze, which can exhibit antibacterial (5) and hemostatic properties. Hence, the development of antibacterial gauze dressing with hemostatic activity is desired.
Seed gums are natural biopolymers of polysaccharides that have important roles in creating the coating layer of gauze dressing because they are easy to create and abundantly available in nature. Tamarindus indica (tamarind) and Cassia fistula (golden shower) are leguminous plants that contain high amounts of endosperm, a source of water- soluble polysaccharides (6). These polysaccharides are the materials of choice among hydrophilic polymers because they are non-toxic and acceptable for use in pharmaceutical products (7,8). The chemical structure of gum from T. indica is composed of a β-(1-4)-D-glucan backbone substituted with side chains of β(1-4)-D-xylopyranose and (1-6)-linked [β-D-gal actopyranosyl-(1-2)- □ α-D-xylopyranosyl] to glucose residues (9). Conversely, gum from C. fistula seed consists of a straight chain of β-(1-4) linked D- mannopyranose units with a random distribution of a-(1-6)-linked D-galactopyranose units (10). Even if polysaccharide gums have various applications, they have some drawbacks, such as impurity, viscosity, and cold water solubility. To increase the utility and versatility of polysaccharide gums, their chemical structure can be changed via modifications such as carboxymethylation (6,11). Numerous studies have modified polysaccharide gum via carboxymethylation and assessed the influence of the reaction conditions, the alkali concentration, the alkali to sodium monochloroacetate ratio, and the reaction medium, time, and temperature (11,12).
Chromolaena odorata (L.), also known as Siam weed, is an important Thai traditional medicinal herb. It is native to North America and has been introduced to tropical Asia (13,14). The fresh leaves of C. odorata are widely used as a hemostatic agent to stop bleeding and promote wound healing. Substantial research efforts have been directed toward clarifying the pharmacological activities of C. odorata (14,15), especially it’s anti-inflammatory (16), antioxidant (17), and anticancer effects (18) associated with important natural compounds such as essential oils, alkaloids, and flavonoids (19,20,21). In addition, several studies suggested that C. odorata will remain an important agent in wound healing because of its hemostatic and antimicrobial activities (22). The present study examined the development and characterization of cotton gauze coated with biopolymers and herbs. It is expected that the tamarind or golden shower seed gum and C. odorata extract coating on cotton gauze will improve its hemostatic and antibacterial properties. The physical properties, antimicrobial activities, and skin-irritating properties of the coated- cotton gauze were examined.
MATERIALS AND METHODS
Materials
Crude gums were extracted from T. indica and C. fistula seeds collected in Aungtong and Chonburi provinces in Thailand, respectively. Crude gums were ground into a powder form. Carboxymethyl gums were derived from the crude gums of T. indica (CT) and C. fistula (CC) seeds. We chemically modified the crude gum powder in ethanol under reaction conditions of 70 °C for 1 h. Next, sodium hydroxide and monochloroacetic acid in a ratio of 1.78 moles were used to react with 0.56 moles of crude gum. Subsequently, the reaction product was precipitated and washed with ethanol to remove impurities (23). Mature C. odorata leaves were collected from Chonburi province, Thailand. The C. odorata leaves were identified by Dr. Boonyadist Vongsak, Faculty of Pharmaceutical Sciences, Burapha University, Thailand. The voucher specimens (KM No.0415001, KM No.0415002, and KM No.0415003) were deposited at the Faculty of Pharmaceutical Sciences, Burapha University, Thailand. The cotton gauze yarn (24 × 20 mesh) was purchased from a drug store. Four commercial brands of woven cotton fibers were selected for further comparison.
Chemical compositions of seed gums
Moisture and ash contents were determined according to the American Society for Testing and Materials methods (ASTM-D2974-87) (24) and AOAC Official Method 923.03 (32.1.05) (25), respectively. Protein and fat contents were determined according to the AOAC Official Method of Analysis 920.87 (32.1.22) and 922.06 (32.1.14) (25), respectively.
Intrinsic viscosity
The intrinsic or limiting viscosity (η) of dilute solutions of crude and carboxymethyl gums was measured at 25.0 ± 0.1 °C using a Cannon-Fenske Routine viscometer (9721- A53) (ASTM-D2515, ISO 3105, Series 100). Solutions had relative viscosities of approximately 1.2-2.0 to assure good accuracy and linearity of the extrapolation to zero concentration. The intrinsic viscosity was conventionally obtained by double extrapolation to zero concentration using Huggins’ and Kraemer’s equations, respectively, as follows:
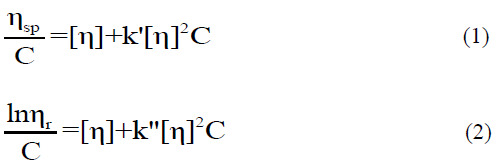
where ηsp and ηrl are the (dimensionless) specific and relative viscosities, respectively, k′ and k″ are the Huggins’ and Kraemer’s coefficients, respectively, and C is the solution concentration.
Water solubility
The water solubility of the crude and carboxymethyl gums was evaluated. Different concentrations of gum sample dispersions were prepared. All aqueous solutions were stirred for 30 min using a magnetic stirrer at ambient temperature. The non-dissolved material was removed via centrifugation at 4000 rpm for 10 min. The supernatant was collected and then dried at 100 °C for 1 h. The solubility (%) was calculated as follows:
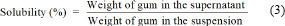
C. odorata extract
The fresh leaves of C. odorata were washed and cut. In total, 100 g of fresh leaves were soaked in 100 mL of 95% (v/v) ethanol at ambient temperature for 18 h. The mixtures were filtered through Whatman filter paper No. 1 and concentrated using a rotary evaporator at 60 °C. The dark brownish-green viscous residues were dried at 60 °C and kept in a tightly closed brown vial at 4 °C until use. Dimethyl sulfoxide (DMSO) was used as a dissolving agent for further testing.
Antimicrobial activity of C. odorata extract
Staphylococcus aureus (ATCC 1466) and Escherichia coli (ATCC 780) were used as representative gram-positive and gramnegative bacteria, respectively, because they are commonly found in the human respiratory system and on the skin. The bacterial stock cultures were incubated at 37 °C for 24 h on nutrient agar. The bacterial suspensions were compared to a 0.5 McFarland turbidity standard to obtain concentrations of 1 × 107 -1 × 108 CFU/mL.
Disk diffusion method
The antimicrobial activity of C. odorata extracts against two pathogenic bacteria was investigated using the agar disk diffusion method (26). The herbal extracts at concentrations of 100, 200, and 300 mg/mL were screened for antibacterial activity against S. aureus and E. coli. The test cultures were spread on nutrient agar plates. Whatman filter paper (No. 1) was cut into disks of 0.6 cm in diameter, soaked in C. odorata extract solutions, and then placed on prepared plates, which were incubated at 37 °C for 18-24 h. DMSO was used as a negative control. The zones of growth inhibition (including the diameter of the disk) were recorded in millimeters. The experiments were conducted in triplicate.
Minimum inhibitory concentration
The minimum inhibitory concentration (MIC) of C. odorata extract was determined using two-fold serial dilutions from 1000 to 1.961 mg/mL. All concentrations of herbal extract were loaded on filter paper on a sterile plate. The plates were prepared in triplicate and then incubated at 37 °C for 18-24 h. The zone of growth inhibition was measured.
Blood clotting test of C. odorata extract
The blood clotting test was a modified version of the protocol used by Dasgupta et al. (27). Cow blood was used for the blood clotting test. Platelets were removed from plasma via centrifugation at 400 rpm for 10 min at room temperature, and the supernatant was removed. The residue was resuspended in normal saline (0.85% NaCl solution) and centrifuged at 4000 rpm for 10 min at room temperature. Then, the supernatant was removed. The processes of dilution with normal saline and centrifugation were repeated until the supernatant was clarified. The platelets were stored at 4 °C for no longer than 5 days until further analysis. For the blood clotting test, the blood sample was diluted in normal saline (1:10 dilution). C. odorata extract at the MIC was mixed at a ratio of 1:1 (v/v). DMSO and normal saline were used as controls. Blood coagulation was observed using a stereomicroscope (Carton CM 400 series).
Preparation of coated-cotton gauze
Different concentrations of carboxymethyl gum aqueous solutions were prepared, namely 1-2.5 wt% for T. indica and 0.5-2 wt% for C. fistula. All aqueous solutions were stirred at ambient temperature for 30 min and centrifuged at 4000 rpm for 10 min at ambient temperature to remove insoluble matter. The coating solution was prepared by adding glycerol (0.5 wt%), and the solution was mixed for 15 min. The herbal extract solution at the MIC was added, and the mixture was stirred for 30 min. The cotton gauze (5 × 5 cm2) was immersed in the coating solution and dried in an air oven for 2 h at 80 °C. The coated-cotton gauze was stored in a desiccator at a constant temperature before testing.
Characterization of the coated-cotton gauze
The appearance of coated-cotton gauze was observed using a ZEISS Stemi 305 stereomicroscope featuring a ZEISS Axiocam 503 color digital camera. The examined physical properties and antimicrobial activities of the coated-cotton gauze were thickness, moisture, water vapor permeability (WVP), adsorption ability, the percent inhibition of bacteria growth, and long-term (6 months) antibacterial activity. The physical properties and antimicrobial activities of the coated- cotton gauze were compared with those of four commercial gauze brands.
Physical properties
The thickness of coated-cotton gauze was measured using a dial indicator (Model 543-257 Mitutoyo Corp., Tokyo, Japan) at five random positions on each specimen to an accuracy of ± 1 μm. Moisture was measured using the American Society for Testing and Materials methods (ASTM-D1439). The WVP of coated- cotton gauze was evaluated using a gravimetric method (ASTM E9-95) with modifications as described by Bonilla et al. (28). The absorption ability of coated-cotton gauze was examined using the modified formula described by Balasubramanian et al. (5). The samples were dried in a conventional oven at 60 °C for 48 h and then stored in the desiccator. Immediately upon cooling, the samples were weighed. The samples were then immersed in pork blood at 37 °C for 24 h. Samples were removed, wiped gently with a lint-free cloth, and weighed. The percentage absorption ability (%) was calculated using the following equation:
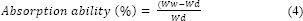
where Ww and Wd are the weights of the sample after and before impregnation, respectively.
Antibacterial testing
The percent inhibition of bacterial growth by C. odorata extract was determined using the colony count method (ISO22196). The microbial inoculum was prepared in 5 mL of nutrient broth at a concentration of 1 × 107 -1 × 108 CFU/mL. Cotton gauze with and without C. odorata extract was cut into 2.5 × 2.5 cm2 squares and sterilized via exposure to ultraviolet radiation for 15 min. Then, the gauze was incubated with the inoculum in nutrient broth at 37 °C for 24 h. Incubated cultures were diluted in 0.85% normal saline. Then, 100 μL of the diluted cultures were poured into nutrient agar and incubated at 37 °C for 48 h. Colonies that grew on a Petri dish were counted. The results of triplicate measurements were averaged. The percent inhibition of bacterial growth was calculated using the following equation:
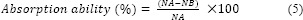
where NA and NB are the amounts of bacteria remaining after exposure to non-coated and C. odorata extract-coated gauze, respectively. The short- and long-term antibacterial activity of coated-cotton gauze was investigated after 2 and 6 months of storage at room temperature, respectively. The antibacterial activity was tested using the agar diffusion test.
Skin irritation assay
The skin irritation test was performed as described by Jirova et al. (29). The selection of volunteers and test methods were conducted in accordance with the ethical principles described in the Declaration of Burapha University (Ethical code: BUU56/2557). In the patch test, coated-cotton gauze was applied to the skin of the upper outer arm of 11 healthy volunteers for up to 4 h. Specifically, the exposure time was progressively increased from 1 to 4 h in 1 h increments with each progressive application at a new skin site, and the irritation reaction was reported. The treatment sites were also assessed at 24, 48, and 72 h after patch test removal. The sites were graded and photographed after patch removal at each time point using a 4-point grading scale (0 = no reaction; +1 = weakly positive reaction, usually characterized by mild erythema and/or dryness across most of the treatment site; +2 = moderately positive reaction, usually distinct erythema and/or dryness, possibly spreading beyond of the treatment site; +3 = strongly positive reaction, strong and often spreading erythema with edema and/or eschar formation) of increasing irritation as described in ISO 10993-10:2002 (E). Sodium lauryl sulfate (SLS, 15% w/v) was used as the positive control, and distilled water was used as the negative control.
Statistical analysis
The data were statistically analyzed using one-way ANOVA followed by Tukey’s multiple comparison test. Significance was defined as P < 0.05.
RESULTS
Chemical compositions
Carboxymethyl or modified gums were developed through a chemical reaction to assess the quality and usability of crude gums from T. indica and C. fistula seeds. The results indicated that compared with the findings for their corresponding crude gums, the carboxymethyl gums had fewer impurities such as protein and fat content and higher polysaccharide content (Table 1). The obtained T. indica and C. fistula gums exhibited degrees of carboxymethyl substitution of 0.1779 and 0.1774, respectively (data not shown).
Table 1.
Chemical compositions of crude and carboxymethyl gums. All values (%) are presented as the mean ± SD, (n = 3).
| Components (%) | Tamarindus indica gum | Cassia fistula gum | ||
|---|---|---|---|---|
| Crude | Modified | Crude | Modified | |
| Moisture | 5.40 ± 0.06 | 3.97 ± 0.16 | 8.17 ± 0.20 | 7.60 ± 0.26 |
| Ash | 0.09 ± 0.00 | 0.00 ± 0.00 | 0.14 ± 0.01 | 0.00 ± 0.00 |
| Protein | 3.58 ± 0.16 | 0.00 ± 0.00 | 1.03 ± 0.14 | 0.00 ± 0.00 |
| Fat | 15.25 ± 0.85 | 2.59 ± 0.42 | 10.04 ± 1.56 | 4.64 ± 0.67 |
| Polysaccharidea | 81.08 ± 3.73 | 97.41 ± 1.43 | 88.79 ± 1.88 | 95.36 ± 2.61 |
aPolysaccharide content (%) = 100 - (protein% + fat%).
Physical properties of seed gums
As previously reported, crude gums from T. indica and C. fistula seeds were carb oxymethylated to improve functional properties such as viscosity, swelling volume, water solubility, and absorption capacity. Hence, the intrinsic viscosity and water solubility of the crude and modified gums were investigated, as presented in Table 2.
Table 2.
Intrinsic viscosity and water solubility of crude and carboxymethyl gums.
| Gum type | [η]H A (dlg−1) | [η]K B (dlg−1) | Water solubility (%) |
|---|---|---|---|
| Tamarindus indica | |||
| Crude gum | 4.45 | 4.44 | 23.7 |
| Carboxymethyl gum | 6.03 | 6.02 | 90.1 |
| Cassia fistula | |||
| Crude gum | 10.72 | 11.08 | 41.9 |
| Carboxymethyl gum | 11.61 | 12.05 | 95.4 |
A[η]H is the intrinsic viscosity from Huggins’ plot (Eq. 1) and B[η]K is the intrinsic viscosity from Kraemer’s plot (Eq. 2).
The carboxymethyl gums had the advantages of greater intrinsic viscosity and water solubility (%) than native gums.
Antibacterial activity of C. odorata extract
Leaves of C. odorata were extracted by maceration using ethanol. The average percentage yield of leaf extracts was 6.9%. The antibacterial activity of the C. odorata extract against Gram-positive (S. aureus ATCC 1466) and Gram-negative bacteria (E. coli ATCC 780) was tested using the disk diffusion method at different concentrations (100, 200, and 300 mg/mL), whereas the MIC was determined using the dilution technique. The results indicated that the C. odorata extract exerted antibacterial activity against S. aureus but not against E. coli. The MIC of C. odorata extract was 31.5 mg/mL with an inhibition zone of 8.0 ±1.0 mm against S. aureus (Table 3).
Table 3.
Zone of inhibition (mm) of Chromolaena odorata extract against Staphylococcus aureus. All values are presented as the mean ± SD, n = 3.
| Concentrations of C. odorata extract (mg/mL) | Zone of inhibition (mm) |
|---|---|
| 1000 | 18.3 ± 2.89 |
| 500 | 15.0 ± 0.00 |
| 250 | 14.0 ± 1.00 |
| 125 | 13.3 ± 2.89 |
| 62.50 | 11.3 ± 2.31 |
| 31.50 | 8.00 ± 1.00 |
| 15.63 | - |
| 7.81 | - |
| 3.91 | - |
| 1.95 | - |
-, No zone of inhibition.
Platelet activation and aggregation
The influence of C. odorata extract on platelet function and coagulation was tested. Because the cow is a mammalian vertebrate similar to humans, cow blood was thus applied to test clot formation following exposure to C. odorata extract at the MIC (approximately 31.5 mg/mL). Figure 1 reveals the appearance of platelets minutes after the addition of the herbal extract. The C. odorata extract enhanced the agglomeration of platelets as illustrated in Fig. 1B. This proves that C. odorata extract is a hemostatic agent that can stop bleeding by promoting coagulation.
Fig. 1.
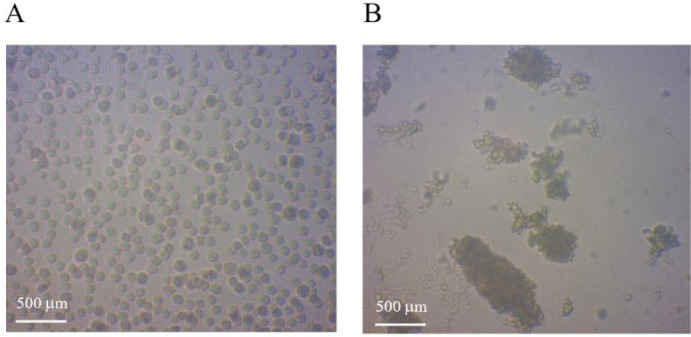
Images of platelets of cow blood after 5 min in the (A) absence and (B) the presence of Chromolaena odorata extract. Images were obtained using a light microscope (×40).
Coated-cotton gauze characteristics
Wound dressing was prepared using different concentrations of C. odorata extract and carboxymethyl gums from T. indica and C. fistula seeds. The appearance of the coated- cotton gauze was investigated using a stereomicroscope as presented in Fig. 2. From the stereomicroscope images, the cotton gauze was covered by the gum loaded C. odorata extract (yellow color). The cotton gauze coated with modified C. fistula seed gum contained smaller pores (Fig. 2B) than that coated with T. indica (Fig. 2A). In addition to evaluating the impact of the gum concentration on the physical properties of the coated-cotton gauze, the physical properties and antibacterial activity of the gauze were studied.
Fig. 2.
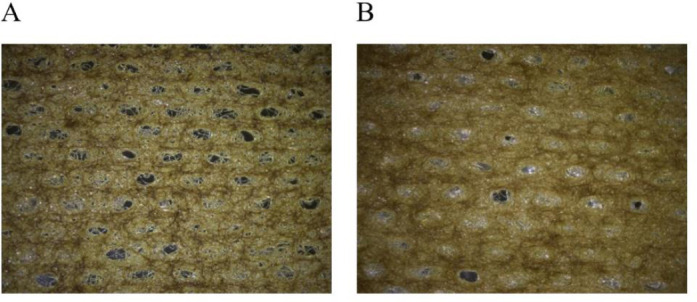
Microscopic observation of cotton gauze coated with 2 wt% carboxymethyl gum of (A) Tamarindus indica and (B) Cassia fistula at a magnification of ×10.
Physical properties
The physical properties of coated-cotton gauze were analyzed by measuring its thickness, moisture content, WVP, and absorption ability. The average thicknesses of coated-cotton gauze at different gum concentrations are presented in Table 4. The data illustrated that the thickness and WVP of the gauze were not significantly changed by increasing the gum concentration. Contrarily, the moisture content and absorption ability of the coated-cotton gauze were significantly increased when the gum content was increased, especially for higher gum concentrations (2.5 and 2.0 %wt for T. indica and C. fistula, respectively).
Table 4.
Thickness, moisture content, and WVP of coated-cotton gauze. Data are presented as the mean ± SD, (n = 3).
| Samples | Concentrations (wt%) | Thickness (mm) | Moisture (%) | WVP × 107 (g·mm·m−2·d−1·kPa−1) | Absorption (%) |
|---|---|---|---|---|---|
| 1.0 | 0.33 ± 0.00a | 2.90 ± 0.72a | 4.77 ± 0.00a | 396.3 ± 21.91a | |
| carboxymethyl gum of T. indica | 1.5 | 0.38 ± 0.01b | 3.19 ± 0.58ab | 5.99 ± 0.00a | 597.0 ± 26.05b |
| 2.0 | 0.38 ± 0.00b | 5.75 ± 0.83c | 6.15 ± 0.00a | 647.2 ± 50.18bc | |
| 2.5 | 0.38 ± 0.01b | 7.32 ± 0.70d | 11.72 ± 0.00a | 785.4 ± 39.31d | |
| 0.5 | 0.32 ± 0.00c | 2.61± 0.34ab | 3.66 ± 0.00a | 457.24 ± 11.43a | |
| Carboxymethyl gum of C. fistula | 1.0 | 0.32 ± 0.00ac | 5.31 ± 0.53c | 6.53 ± 0.00a | 475.32 ± 11.51ae |
| 1.5 | 0.32 ± 0.01ac | 5.97 ± 0.32cd | 8.81 ± 0.00a | 611.28 ± 43.72bc | |
| 2.0 | 0.32 ± 0.01ac | 6.16 ± 0.08cd | 9.81 ± 0.00a | 819.62 ± 80.33d | |
| Commercial product A | - | 0.36 ± 0.00d | 0.02 ± 0.04e | 7.45 ± 0.00a | 87.84 ± 0.20f |
| Commercial product B | - | 0.66 ± 0.00e | 0.03 ± 0.02e | 3.60 ± 0.00a | 84.83 ± 0.33f |
| Commercial product C | - | 0.68 ± 0.00f | 0.04 ± 0.02e | 3.59 ± 0.00a | 82.20 ± 0.52f |
| Commercial product D | - | 0.69 ± 0.00f | 0.03 ± 0.03e | 4.70 ± 0.00a | 87.92 ± 0.21f |
Same superscript letters within a column indicate no significant difference. WVP, water vapor permeability.
Compare to the commercial gauzes (A-D), the thickness of carboxymethylated gum was thinner but contain higher moisture content. The WVP were from 3.59-7.45 which were not different from the coated-cotton gauze. However, the outstanding difference was the blood absorption percent; the modified-gum coated-gauze provided significantly higher than those of the commercial product for 5 to 10 times. This might be because hydrophilic properties of the modified gum that can enhance liquid adsorption.
Antibacterial testing
The coated-cotton gauze containing various concentrations of C. odorata extracts (1.0%-2.5% w/w) was found to possess antibacterial activity against S. aureus in terms of the percent inhibition of bacterial growth, as shown in Table 5.
Table 5.
Percent inhibition of Staphylococcus aureus and the short- and long-term antimicrobial activity of the coatedcotton gauze containing various of Chromolaena odorata extract. All values are presented as the mean ± SD of three parallel measurements.
| Samples | Concentration (wt%) | Inhibition (%) | Diameter of inhibition zone (mm) | ||
|---|---|---|---|---|---|
| 1 Month | 2 Months | 6 Months | |||
| 1.0 | 97.55 ± 3.61 | 14.00 ± 1.15 | 11.67 ± 2.89 | 6.00 ± 0.00 | |
| Coated-cotton gauze with carboxymethyl gums from T. indica | 1.5 | 98.22 ± 2.31 | 15.00 ± 0.00 | 13.00 ± 1.73 | 6.00 ± 0.00 |
| 2.0 | 98.32 ± 6.38 | 13.33 ± 4.04 | 13.00 ± 1.73 | 6.00 ± 0.00 | |
| 2.5 | 99.04 ± 5.51 | 13.33 ± 0.58 | 13.00 ± 1.73 | 6.00 ± 0.00 | |
| 0.5 | 97.84 ± 7.21 | 12.00 ± 0.00 | 12.00 ± 0.00 | 6.00 ± 0.00 | |
| Coated-cotton gauze with carboxymethyl gums from C. fistula | 1.0 | 98.68 ± 7.02 | 12.00 ± 0.00 | 12.00 ± 0.00 | 6.00 ± 0.00 |
| 1.5 | 98.25 ± 4.04 | 12.00 ± 0.00 | 12.00 ± 0.00 | 6.00 ± 0.00 | |
| 2.0 | 98.13 ± 6.24 | 12.00 ± 0.00 | 12.00 ± 0.00 | 6.00 ± 0.00 | |
The percentage inhibition of S. aureus was not significantly different from various concentrations of the C. odorata extract. For the stability of the products, we examined whether their antibacterial activity was affected by the storage time. As shown in Table 5, the zone of inhibition exceeded 12 mm for all freshly prepared samples (cotton gauze coated with seed gums from carboxymethyl gum of T. indica or C. fistula), then the inhibition zone reduced slightly with the cotton gauze coated with modified T. indica gum after 2 months, whereas the cotton gauze coated with modified C. fistula gum provided a similar zone of inhibition. After 6 months, the diameter of inhibition zones of those of both modified gums significantly decreased to 6.00 mm. Last but not least, the four commercial gauzes could not prohibit S. aureus growth (data not shown) at all. This is because commercial gauzes do not contain any antiseptic or antibiotic.
Skin irritation test
Coated-cotton gauze prepared from both types of carboxymethyl gums was applied to human skin for 1, 2, 3, or 4 h. The 4 h human patch test results of 11 volunteers are presented in Table 6. The results revealed that no irritation had occurred by 72 h after removal. Conversely, skin irritation was observed in 1 or 2 volunteers exposed to SLS for 4 h.
Table 6.
Human skin irritation test for 11 volunteers and the grading scale.
| Materials | Time (h) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 24 | 48 | 72 | |
| Number of volunteers (grading scale)* | |||||||
| T. indica coating | 11 (0) | 11 (0) | 11 (0) | 11 (0) | 11 (0) | 11 (0) | 11 (0) |
| C. fistula coating | 11 (0) | 11 (0) | 11 (0) | 11 (0) | 11 (0) | 11 (0) | 11 (0) |
| Positive control (15% (w/v) SLS) | 10 (0) | 9 (0) | 10 (0) | 10 (0) | 11 (0) | 11 (0) | 11 (0) |
| 1 (1+) | 2 (1+) | 1 (1+) | 1 (1+) | ||||
| Negative control (distilled water) | 11 (0) | 11 (0) | 11 (0) | 11 (0) | 11 (0) | 11 (0) | 11 (0) |
*0, No reaction; 1+, weakly positive reaction (usually characterized by mild erythema and/or dryness across most of the treatment site); 2+, moderately positive reaction (usually distinct erythema and/or dryness, possibly spreading beyond of the treatment site); 3+, strongly positive reaction (strong and often spreading erythema with edema and/or eschar formation). SLS, Sodium lauryl sulfate.
DISCUSSION
The carboxy modification of the gums derived from T. indica and C. fistula diminished the amount of protein and fat in the crude gums. This is because protein and fat were dissolved and eluted in ethanol, sodium hydroxide, and monochloroacetic acid, which were solvents used in the reaction (9). Apart from gum purification, the carboxy-modification process also enhanced the water solubility of the native gums. This is because the carboxymethyl gums had higher polysaccharide content (Table 1) and more carboxymethyl groups (6), facilitating hydration in aqueous solutions (9).
The antibacterial activity of C. odorata was evaluated against S. aureus and E. coli. C. odorata could prohibit only Gram-positive bacteria (S. aureus). This result may be because E. coli is Gram-negative; its cell wall contains lipopolysaccharide, preventing the accumulation of antimicrobial agents in the cells. Similar findings were reported by Viral et al. (30), who found that the ethanol extract of C. odorata levels showed inhibitory effects against S. aureus but not against E. coli. However, many researchers reported that the ethanol extract of C. odorata leaves showed inhibitory activity against both S. aureus and E. coli (31,32,33). The C. odorata extract inhibited the growth of S. aureus at a starting concentration of 31.50 mg/mL. This discrepancy may be because the antimicrobial activity of C. odorata extract is related to the amount of natural compounds, such as total phenolic and flavonoid compounds, which are largely dependent on the plant source, part of the plant material extracted, type of solvent used, and extraction procedure (33,34)
The C. odorata extract at MIC concentration could also promote blood clotting as seen in the stereomicroscope image. This hemostasis can be induced through three mechanisms including vascular constriction, the formation of a platelet plug, and blood coagulation. In previous reports, C. odorata extract was found to accelerate hemostasis in Wistar rats (3), promote wound healing (22,33), and reduce the bleeding time in rats (13).
Subsequently, the cotton gauze was coated with C. odorata extract and carboxymethyl gums from T. indica or C. fistula seeds. The modified C. fistula seed gum could cover the cotton gauze better (less porosity, Fig. 2B) than that of T. Indica. This may be due to the high viscosity of carboxymethylated C. fistula seed gum (Table 2) that could coat and cover the cotton fiber well (35). Furthermore, a high concentration of gum increased the moisture content and absorption ability of the coated- cotton gauze, consistent with the findings of Balasubramanian et al. (5) using carboxymethyl-treated cotton fabrics.
The cotton gauze coated with C. odorata extract could efficiently inhibit the growth of S. aureus during the first 2 months. The antibacterial activity declined in the 6th month. This was consistent with a study report by Ngozi et al. (36). This report also recommended the storage of the C. odorata extract in dry conditions to extend its shelf life.
Lastly, the coated-cotton gauze prepared using both gum types did not cause human skin irritation for 72 h, whereas the positive control (15% w/v SLS) caused skin irritation. This is because the gauzes were fabricated from natural raw materials that have been studied before (6,37).
CONCLUSION
This work investigated the preliminary application of a potential medical product. The seed gums of two plants (T. indica and C. fistula) and C. odorata can be used as coating of gauze dressings for first aid purposes based on their antibacterial and hemostatic activities. The adsorption efficiency of carboxymethyl gums from T. indica and C. fistula seeds were prominent in the range of 396.32%-819.62%. C. odorata extracts showed good potential for use as an antibacterial and hemostatic agent that can accelerate the healing of wounds. Moreover, the rate of human skin irritation was very low for the cotton gauze coated with both carboxymethyl gums. In summary, the coated gauze is a potential wound dressing material that is biocompatible and safe for application on human skin.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Authors’ contribution
W. Sittikijyothin and K. Huanbutta conceptualized, planned, conducted, and drafted the manuscript. B. Phonyotin and T. Sangnim critically evaluated the results. All the authors revised the manuscript.
Acknowledgments
B. Phonyotin acknowledges the Shell Centennial Education Fund, Shell Companies in Thailand for the financial support of a research project in 2015. The authors thank Dr. Boonyadist Vongsak for the plant identification. We also thank Enago (enago.com) for the English language review.
REFERENCES
- 1.Shanmugasundaram OL, Gowda RVM. Development and characterization of cotton and organic cotton gauze fabric coated with biopolymers and antibiotic drugs for wound healing. Indian J Fibre Text Res. 2012;37:146–150. [Google Scholar]
- 2.Singh B, Sharma S, Dhiman A. Design of antibiotic containing hydrogel wound dressings: biomedical properties and histological study of wound healing. Int J Pharma. 2013;457(1):82–91. doi: 10.1016/j.ijpharm.2013.09.028. DOI: 10.1016/j.ijpharm.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 3.Pandith H, Zhang X, Liggett J, Min KW, Gritsanapan W, Baek SJ. Hemostatic and wound healing properties of Chromolaena odorata Leaf extract. ISRN Dermatol. 2013;2013:168269,1–8. doi: 10.1155/2013/168269. DOI: 10.1155/2013/168269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai T, Tanaka M, Huang YY, Hamblin MR. Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev Anti Infect Ther. 2011;9(7):857–879. doi: 10.1586/eri.11.59. DOI: 10.1586/eri.11.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balasubramanian E, Balasubramanian V, Babu G, Devika S, Rajendran R. Moist wound dressing fabrications: carboxymethylation of antibacterial cotton gauze. J Eng Fibers Fabr. 2013;8(4):78–87. DOI: 10.1177/155892501300800402. [Google Scholar]
- 6.Huanbutta K, Sittikijyothin W. Use of seed gums from Tamarindus indica and Cassia fistula as controlled-release agents. Asain J Pharma Sci. 2018;13(5):398–408. doi: 10.1016/j.ajps.2018.02.006. DOI: 10.1016/j.ajps.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durai RD, Rajalakshmi G, Joseph J, Kanchalochana S, Hari V. Tamarind seed polysaccharide: a promising natural excipient for pharmaceuticals. Int J Green Pharm. 2012;6(4):270–278. DOI: 10.4103/0973-8258.108205. [Google Scholar]
- 8.Jothy SL, Zakaria Z, Chen Y, Lau YL, Latha LY, Sasidharan S. A cute oral toxicity of methanolic seed extract of Cassia fistula in mice. Molecules. 2011;16(6):5268–5282. doi: 10.3390/molecules16065268. DOI: 10.3390/molecules16065268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huanbutta K, Sangnim T, Sittikijyothin W. Physicochemical characterization of gum from tamarind seed: potential for pharmaceutical application. Asian J Pharm Sci. 2016;11:176–177. DOI: 10.1016/j.ajps.2015.11.051. [Google Scholar]
- 10.Srivastava M, Kapoor VP. Seed galactomannans: an overview. Chem Biodivers. 2005;2(3):295–317. doi: 10.1002/cbdv.200590013. DOI: 10.1002/cbdv.200590013. [DOI] [PubMed] [Google Scholar]
- 11.Dodi G, Hritch D, Popa MI. Carboxymethylation of guar gum: synthesis and characterization. Cellul Chem Technol. 2011;45(3-4):171–176. [Google Scholar]
- 12.Rajput G, Pandey IP, Joshi G. Carboxymethylation of Cassia angustifolia seed gum: synsthesis and rheological study. Carbohydr Polym. 2015;117:494–500. doi: 10.1016/j.carbpol.2014.09.063. DOI: 10.1016/j.carbpol.2014.09.063. [DOI] [PubMed] [Google Scholar]
- 13.Pandith H, Thongpraditchote S, Wongkrajang Y, Gritsanapan W. In vivo and in vitro hemostatic activity of Chromolaena odorata leaf extract. Pharm Biol. 2012;50(9):1073–1077. doi: 10.3109/13880209.2012.656849. DOI: 10.3109/13880209.2012.656849. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty AK, Rambhade S, Patil UK. Chromolaena odorata (L.): an overview. J Pharm Res. 2011;4(3):573–576. [Google Scholar]
- 15.Suksamrarn A, Chotipong A, Suavansri T, Boongird S, Timsuksai P, Vimuttipong S, et al. Antimycobacterial activity and cytotoxicity of flavonoids from the flowers of Chromolaena odorata. Arch Pharm Res. 2004;27(5):507–511. doi: 10.1007/BF02980123. DOI: 10.1007/bf02980123. [DOI] [PubMed] [Google Scholar]
- 16.Hanh TTH, Hang DTT, Van Minh C, Dat NT. Anti- inflammatory effects of fatty acids isolated from Chromolaena odorata. Asian Pac J Trop Med. 2011;4(10):760–763. doi: 10.1016/S1995-7645(11)60189-2. DOI: 10.1016/S1995-7645(11)60189-2. [DOI] [PubMed] [Google Scholar]
- 17.Amatya S, Tuladhar SM. In vitro antioxidant activity of extracts from Eupatorium odoratum L. Res J Med Plant. 2011;5(1):79–84. DOI: 10.3923/rjmp.2011.79.84. [Google Scholar]
- 18.Adedapo AA, Oyagbemi AA, Fagbohun OA, Omobowale TO, Yakubu MA. Evaluation of the anticancer properties of the methanol leaf extract of Chromolaena odorata on HT-29 cell line. FASEB J. 2016;30(S1):1193.6–1193.6. DOI: 10.1096/fasebj.30.1_supplement.6. [Google Scholar]
- 19.Biller A, Boppré M, Witte L, Hartmann T. Pyrrolizidine alkaloids in Chromolaena odorata. Chemical and chemoecological aspects. Phytochemistry. 1994;35(3):615–619. DOI: 10.1016/S0031-9422(00)90573-9. [Google Scholar]
- 20.Pisutthanan N, Liawruangrath B, Liawruangrath S, Baramee A, Apisariyakul A, Korth J, et al. Constituents of the essential oil from aerial parts of Chromolaena odorata from Thailand. Nat Prod Res. 2006;20(6):636–640. doi: 10.1080/14786410500462678. DOI: 10.1080/14786410500462678. [DOI] [PubMed] [Google Scholar]
- 21.Ling SK, Pisar MM, Man S. Platelet-activating factor (PAF) receptor binding antagonist activity of the methanol extracts and isolated flavonoids from Chromolaena odorata (L.) King and Robinson. Biol Pharm Bull. 2007;30(6):1150–1152. doi: 10.1248/bpb.30.1150. DOI: 10.1248/bpb.30.1150. [DOI] [PubMed] [Google Scholar]
- 22.Omotayo MA, Avungbeto O, Sokefun OO, Eleyowo OO. Antibacterial activity of Crassocephalum crepidioides (fireweed) and Chromolaena odorata (Siam weed) hot aqueous leaf extract. Int J Pharm Biol Sci. 2015;5(2):114–122. [Google Scholar]
- 23.Huanbutta K, Sittikijyothin W. Development and characterization of seed gums from Tamarindus indica and Cassia fistula as disintegrating agent for fast disintegrating Thai cordial tablet. Asian J Pharm Sci. 2017;12(4):370–377. doi: 10.1016/j.ajps.2017.02.004. DOI: 10.1016/j.ajps.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preeth K. ATM D 2974-87 standard test methods for mositure, ash, and organic matter of peat and other organic soils. 1993:1–3. [Google Scholar]
- 25.Latimer GW. Official Methods of Analysis of AOAC International. 20th ed. Gaithersburg, Md: AOAC International; 2016. p. 2,5,14. [Google Scholar]
- 26.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6(2):71–79. doi: 10.1016/j.jpha.2015.11.005. DOI: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dasgupta N, Ranjan S, Mohammed SMA, Jadon PS, Melvin SS, Harris AD, et al. Extraction-based blood coagulation activity of marigold leaf: a comparative study. Comp Clin Path. 2014;23(6):1715–1718. DOI: 10.1007/s00580-014-1943-5. [Google Scholar]
- 28.Bonilla J, Atarés L, Vargas M, Chiralt A. Properties of wheat starch film-forming dispersions and films as affected by chitosan addition. J Food Eng. 2013;114(3):303–312. DOI: 10.1016/j.jfoodeng.2012.08.005. [Google Scholar]
- 29.Jirova D, Basketter D, Liebsch M, Bendova H, Kejlova K, et al. Comparison of human skin irritation and photo-irritation patch test data with cellular in vitro assays and animal in vivo data. Contact Dermatitis. 2010;62(2):109–116. doi: 10.1111/j.1600-0536.2009.01640.x. DOI: 10.1111/j.1600-0536.2009.01640.x. [DOI] [PubMed] [Google Scholar]
- 30.Viral PG, Rivera WL. Antimicrobial activity and cytotoxicity of Chromolaena odorata (L.f.) King and Robinson and Uncaria perrottetii (A. Rich) Merr. extracts. J Med Plants Res. 2009;3(7):511–518. [Google Scholar]
- 31.Anyasor GN, Aina DA, Olushola M, Aniyikaye AF. Phytochemical constituent, proximate analysis, antioxidant, antibacterial and wound healing properties of leaf extracts of Chromolaena odorata. Ann Biol Res. 2011;2:441–451. [Google Scholar]
- 32.Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharm Sci. 2011;1(1):98–106. [Google Scholar]
- 33.Hanphakphoom S, Krajangsang S. Antimicrobial activity of Chromolaena odorata extracts against bacterial human skin infections. Mod Appl Sci. 2016;10(2):159–171. DOI: 10.5539/mas.v10n2p159. [Google Scholar]
- 34.Natheer SE, Sekar C, Amutharaj P, Rahman SA, Khan KF. Evaluation of antibacterial activity of Morinda citrifolia, Vitex trifolia and Chromolaena odorata. Afr J Pharm Pharmacol. 2012;6:783–788. DOI: 10.5897/AJPP11.435. [Google Scholar]
- 35.Saha A, Tyagi S, Gupta RK, Tyagi YK. Natural gums of plant origin as edible coatings for food industry applications. Crit Rev Biotechnol. 2017;37(8):959–973. doi: 10.1080/07388551.2017.1286449. DOI: 10.1080/07388551.2017.1286449. [DOI] [PubMed] [Google Scholar]
- 36.Ngozi IM, Jude IC, Catherine C. Chemical profile of Chromolaena odorata L.(King and Robinson) leaves. Pak J Nutr. 2009;8(5):521–524. DOI: 10.3923/pjn.2009.521.524. [Google Scholar]
- 37.Koul O, Isman M. Toxicity of the limonoid allelochemical cedrelone to noctuid larvae. Entomol Exp Appl. 1992;64(3):281–287. DOI: 10.1111/j.1570-7458.1992.tb01618.x. [Google Scholar]


