Abstract
Haemothorax is an accumulation of blood in the pleural space. Retained haemothorax refers to blood that cannot be drained from the pleural cavity and is associated with an increased risk of empyema and fibrothorax often necessitating surgical evacuation. We describe our experience of using intrapleural fibrinolytic therapy in three patients with different bleeding risk and acute non‐traumatic retained haemothorax. The first was a 41‐year‐old female with disseminated Candida guilliermondii sepsis and an iatrogenic haemothorax, second was a 48‐year‐old female with transfusion‐dependent acute myeloid leukaemia and spontaneous haemothorax, and the third was a 72‐year‐old female with spontaneous haemothorax from newly diagnosed lung cancer. All patients received one to two doses of intrapleural alteplase without any bleeding complications and resolution of retained haemothorax. This case series demonstrates the successful application and safety of this approach as an alternative to surgery in a well‐resourced environment with close monitoring and ready access to blood transfusion.
Keywords: Acute haemothorax, intrapleural fibrinolysis, retained haemothorax
We describe our experience of using intrapleural fibrinolytic therapy in three patients with different bleeding risk and acute non‐traumatic retained haemothorax.

Introduction
Haemothorax is defined as a collection of blood within the pleural space, and is often the result of sharp or blunt trauma to the chest. The exact incidence of haemothorax is unclear and has been estimated to occur in approximately 60% of all polytrauma cases. In the United States alone, this approaches 300,000 cases annually [1].
Compared to traumatic haemothorax, non‐traumatic haemothorax occurs much less frequently and are either spontaneous (e.g. bleeding from pleural malignancy) or iatrogenic in nature (e.g. complication of pleural procedure).
First‐line treatment of haemothorax is drainage through the placement of a large‐calibre (>28 Fr) intercostal drain (ICD) [2]. Clotted or retained haemothorax is often not amenable to conventional drainage and is associated with an increased risk of empyema and fibrothorax. Consequently, surgical evacuation of the pleural space is often required.
Intrapleural fibrinolytic therapy (IPFT) has been increasingly used in the management of complex parapneumonic effusions and empyema [3]. Whether there is a role for this approach in the management of non‐traumatic haemothorax is yet to be established.
We report three consecutive patients with acute non‐traumatic retained haemothorax and different bleeding risk successfully treated with IPFT. In this case series, we demonstrate the successful application and safety of this approach.
Case Series
Case 1
A 41‐year‐old female with intestine–liver–pancreas transplant on maintenance tacrolimus and prednisolone was admitted to intensive care with septic shock secondary to disseminated Candida guilliermondii a week after her nephrostomy tube was changed. Nephrectomy undertaken for source control was complicated by post‐operative haemorrhage from the wound bed. This was managed conservatively with blood product support. Two weeks later, therapeutic aspiration of a left‐sided pleural effusion was complicated by a self‐limited haemothorax associated with a haemoglobin drop from 77 to 54 g/L. This was managed with blood product support and an ICD. Despite this, subsequent chest X‐ray (CXR) showed minimal improvement in the effusion (Fig. 1A). Chest computed tomography (CT) was performed. This showed an incorrectly positioned ICD in the left upper lobe and haemorrhage within the left lung and pleural space (Fig. 1B). Bedside thoracic sonography (TUS) showed a complex and highly septated collection (Fig. 1C). The misplaced ICD was removed and replaced with a 12‐Fr ICD inserted into the complex collection under ultrasound guidance. Pleural fluid/serum haematocrit ratio was 0.7 suggestive of a haemothorax. To promote clot breakdown and evacuation of the pleural space, two doses of alteplase 10 mg was instilled intrapleurally 24 h apart with the first dose administered 6 h after ICD insertion. This resulted in drainage of 2000 mL of dark sanguineous fluid. Follow‐up imaging showed no residual effusion (Fig. 1D–F) and haemoglobin remained stable (Fig. 4A).
Figure 1.

Chest X‐ray, chest computed tomography, and thoracic ultrasound images for case 1 before (A–C) and after (D–F) intrapleural fibrinolysis. Red, yellow, and green asterisks indicate septated effusion, normal lung, and spleen, respectively.
Figure 4.
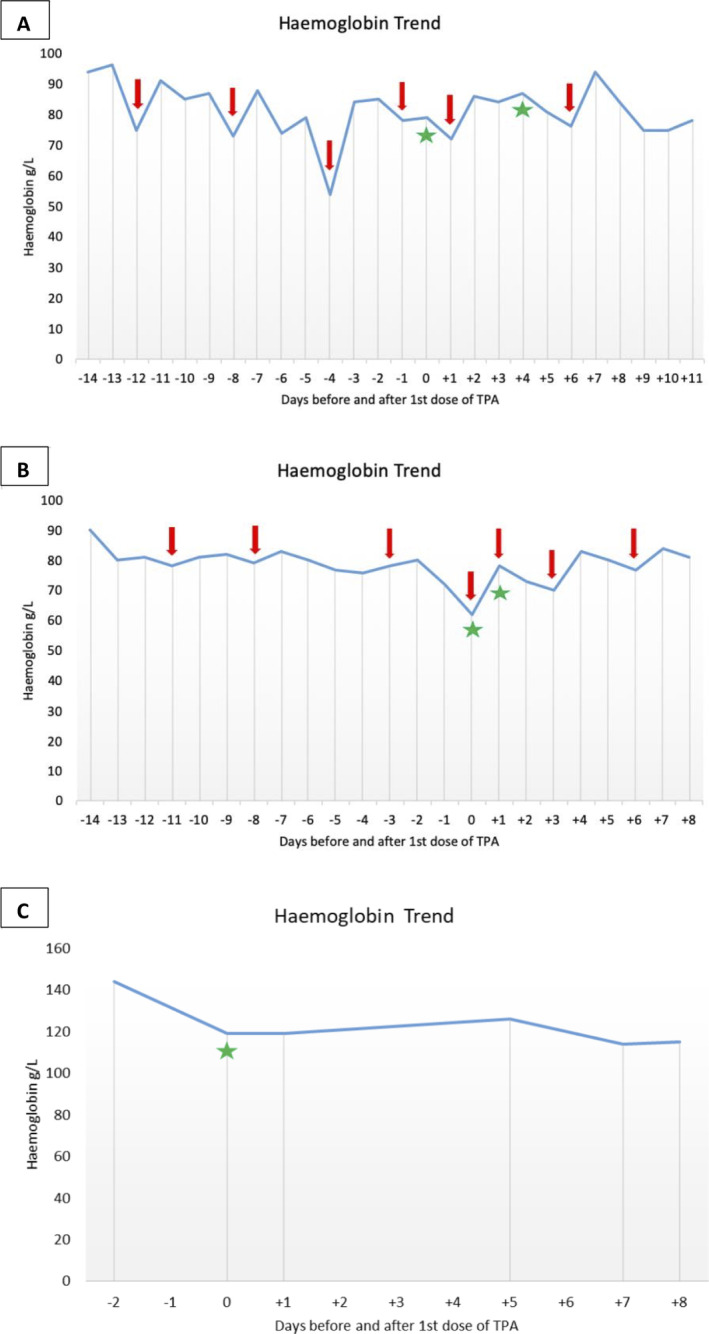
Haemoglobin trend before and after intrapleural alteplase in case 1 (A), case 2 (B), and case 3 (C). Red arrows indicate blood product transfusion. Green asterisks indicate alteplase administration.
Case 2
A 48‐year‐old female was admitted to hospital for management of acute myeloid leukaemia. Chemotherapy was commenced resulting in severe pancytopenia requiring regular red blood cell and platelet transfusions and transfer to intensive care. CXR performed to investigate new pyrexia showed a left‐sided pleural effusion that was simple in appearance on TUS. Sanguineous fluid (1200 mL) was aspirated uneventfully under ultrasound guidance. Despite this, CXR performed the next day showed minimal reduction in the size of the pleural effusion and suggestions of loculations that were confirmed on chest CT (Fig. 2A, B). This was found to be a highly complex and septated collection with features of adjacent haematoma on TUS (Fig. 2C). Platelet count was 20 × 109/L and 1 U of pooled platelets was transfused prior to insertion of an ultrasound‐guided 18‐Fr ICD into the complex collection. This drained 600 mL of sanguineous fluid before tapering off. Pleural fluid/serum haematocrit ratio was 0.67 suggestive of recent pleural haemorrhage. TUS showed large residual collection. To promote clot breakdown and evacuation of the pleural space, two doses of intrapleural alteplase 10 mg were instilled 24 h apart with the first dose administered 4 h after ICD insertion resulting in further drainage of 4500 mL of similar coloured fluid. Platelet count at the time of alteplase administration was 45 × 109/L. Follow‐up imaging showed no residual effusion (Fig. 2D–F) and haemoglobin remained stable (Fig. 4B).
Figure 2.
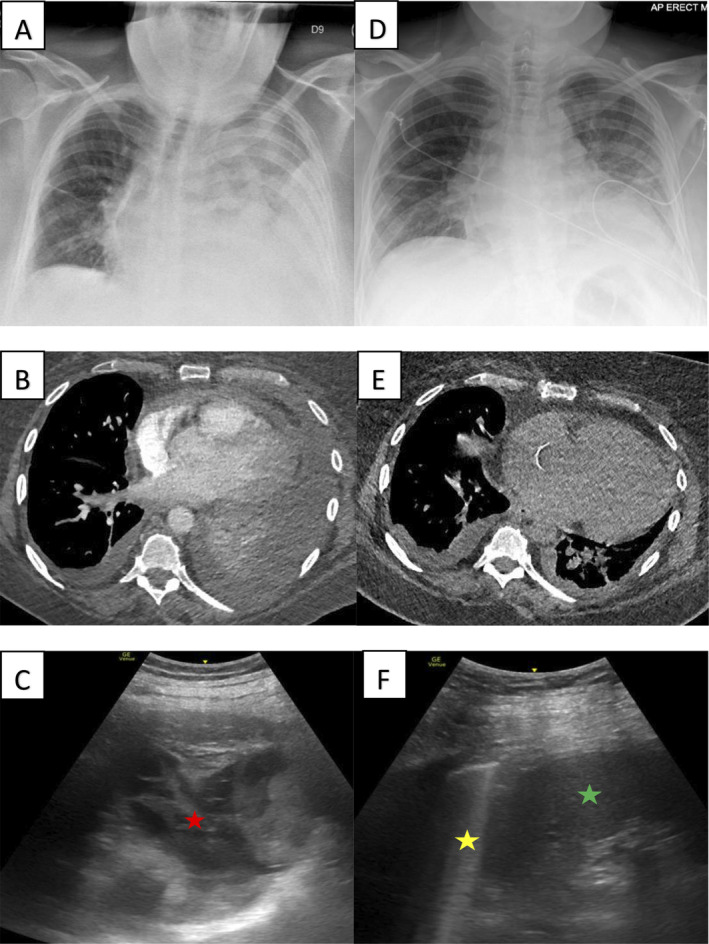
Chest X‐ray, chest computed tomography, and thoracic ultrasound images for case 2 before (A–C) and after (D–F) intrapleural fibrinolysis. Red, yellow, and green asterisks indicate septated effusion, normal lung, and spleen, respectively.
Case 3
A 72‐year‐old female with no significant medical history was referred to the ambulatory pleural service for evaluation of a suspected malignant right‐sided pleural effusion. Chest CT showed circumferential nodular right‐sided pleural thickening with associated pleural effusion and a 2‐cm nodule in the right lower lobe. TUS showed a simple effusion and 500 mL of dark brown fluid (degraded haemoglobin) was aspirated. Three days later, she re‐presented with chest pain. CXR showed reaccumulation of the pleural effusion confirmed on CT (Fig. 3A, B). This was heavily septated on TUS (Fig. 3C). Under ultrasound guidance, a 12‐Fr ICD was inserted into the septated collection. This drained minimal dark sanguineous fluid with a pleural fluid/serum haematocrit ratio of 0.58 suggestive of interval pleural haemorrhage. Given minimal drain output, a single dose of intrapleural alteplase 10 mg was given 30 min after ICD insertion resulting in a further 1400 mL of drain output. Repeat CXR showed a significant reduction in the volume of effusion (Fig. 3D) and revealed underlying trapped lung. Fluid cytology was positive for lung adenocarcinoma and haemoglobin remained stable (Fig. 4C).
Figure 3.

Chest X‐ray, chest computed tomography, and thoracic ultrasound images for case 3 before (A–C) and after (D) intrapleural fibrinolysis. Red and yellow asterisks indicate septated effusion and trapped lung, respectively.
Discussion
The initial management of haemothorax includes resuscitation and prompt drainage. Drainage is often achieved by a basally inserted large‐calibre (>28 Fr) ICD, as this allows fluid and some clots to be evacuated. Intercostal drainage facilitates rapid emptying of the pleural space allowing expansion of the underlying lung and permits accurate assessment of the rate of blood loss, a critical factor in the decision regarding early surgical or radiological intervention [4].
Clotted or retained haemothorax is a known sequela of haemothorax, and is estimated to occur in 4–20% of traumatic haemothorax and an unknown proportion of non‐traumatic cases [5]. Retained haemothorax is often defined as >500 mL residual blood in the pleural space, blood occupying more than one‐third of the thoracic cavity, or residual blood that cannot be drained after 72 h of ICD placement [5]. TUS may have an emerging role in the diagnosis of retained haemothorax, although chest CT remains the current gold standard [6].
If left untreated, retained haemothorax may reabsorb over time, become infected, or progress to fibrothorax. Approximately 25% of retained haemothorax develop empyema, especially if left for more than seven days. Similarly, angiofibroblastic proliferation in response to retained clot has been shown to occur by the seventh day [7]. Hence, it is imperative that retained haemothorax be evacuated promptly.
Current guidelines recommend the use of video‐assisted thoracoscopy surgery (VATS) for the treatment of traumatic retained haemothorax. However, studies have shown that about 25% required a second VATS procedure, and 5% a third [8]. Furthermore, close to 20% of VATS procedures required conversion to thoracotomy [9], increasing post‐operative morbidity and the risk of chronic intercostal neuralgia [10]. Elderly patients and those with multiple comorbidities are also likely to be excluded from surgery due to their higher peri‐operative risk.
IPFT has been shown to improve pleural fluid drainage and reduce the need for surgical intervention in patients with parapneumonic effusions and empyema, although its role in the management of retained haemothorax remains controversial [11]. Fibrinolytics such as alteplase not only facilitates breakdown of fibrinous septations through its activation of plasmin, but have also been shown to stimulate significant pleural fluid formation contributing to a lavage effect of the pleural cavity [11, 12]. Theoretically, this should improve evacuation of retained collections but studies have demonstrated mixed results [13]. Similarly, IPFT when compared to VATS has been shown to be inferior in the management of traumatic retained haemothorax in one study [14], and of similar efficacy in another [13, 15]. While a recent meta‐analysis concluded that the use of IPFT in traumatic retained haemothorax resulted in 81–92% of patients being discharged without further surgical intervention, the authors acknowledged the limited number and poor quality of studies included [16]. Due to limited data on non‐traumatic haemothorax, management approaches have been derived from traumatic haemothorax.
All three patients in our clinical series developed retained haemothorax from non‐traumatic aetiologies. In the first case, this was due to an iatrogenic injury to the intercostal vessels and lung parenchyma. In the second and third cases, spontaneous bleeding resulted from thrombocytopenia secondary to haematological malignancy and pleural malignancy, respectively. Given their high peri‐operative risk, all three patients were treated using IPFT with successful outcomes.
Bleeding risk following IPFT is a concern for many clinicians despite reassuring data from studies examining patients with parapneumonic effusions or empyema [11]. For instance, of 344 patients who received intrapleural alteplase and dornase alfa for the management of pleural infection across five major published studies, only 11 (3.2%) cases of pleural bleed were reported, all of whom were managed with transfusion support [17]. Furthermore, no cases of pleural haemorrhage were reported in 165 patients with pleural effusions of various aetiologies who received single‐agent intrapleural alteplase [11].
The safety of IPFT in individuals with high bleeding risk (e.g. acute bleed, dysregulated clotting, and recent major surgery) remains uncertain, and is often quoted as a contraindication to IPFT. In all our cases, IPFT was administered within three days of bleeding onset with no immediate bleeding complications or significant drop in haemoglobin over the subsequent days, demonstrating that IPFT can be administered, even in patients at high bleeding risk.
Little is known about the dose and dosing interval of IPFT in haemothorax. The empirical use of a 10‐mg dose at 24 h intervals is based on our experience in using IPFT in pleural infections. Emerging data suggest that an individual's response to IPFT may be dependent on factors such as pleural fluid plasminogen activator inhibitor‐1 activity levels and that in future a more personalized approach may be possible by evaluating the individual's “fibrinolytic potential” [18].
In summary, we describe a small case series demonstrating the safe and effective use of IPFT in three cases of non‐traumatic retained haemothorax in patients with high bleeding risk. Whilst in no way definitive, the lack of anticipated complications would support a randomized trial evaluating the efficacy, safety, and optimal dosing of IPFT. It is our view that in a well‐resourced environment, IPFT should be considered alongside surgical interventions for non‐traumatic haemothorax with the decision made in a multidisciplinary team setting involving at least a respiratory physician, thoracic surgeon, and a chest radiologist.
Disclosure Statement
Appropriate written informed consent was obtained for publication of this manuscript and accompanying images.
Author Contribution Statement
Chuan T. Foo and Jurgen Herre were involved in the conceptualization, data curation, and writing, reviewing and editing of the manuscript.
Foo, CT , Herre, J . (2021) Intrapleural fibrinolysis in acute non‐traumatic retained haemothorax. Respirology Case Reports, 9(6), e00760. 10.1002/rcr2.760
Associate Editor: John Wrightson
References
- 1. Richardson JD, Miller FB, Carrillo EH, et al. 1996. Complex thoracic injuries. Surg. Clin. North Am. 76(4):725–748. [DOI] [PubMed] [Google Scholar]
- 2. Meyer DM. 2007. Hemothorax related to trauma. Thorac. Surg. Clin. 17(1):47–55. [DOI] [PubMed] [Google Scholar]
- 3. Rahman NM, Maskell NA, West A, et al. 2011. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N. Engl. J. Med. 365(6):518–526. [DOI] [PubMed] [Google Scholar]
- 4. Boersma WG, Stigt JA, and Smit HJ. 2010. Treatment of haemothorax. Respir. Med. 104(11):1583–1587. [DOI] [PubMed] [Google Scholar]
- 5. Smith JW, Franklin GA, Harbrecht BG, et al. 2011. Early VATS for blunt chest trauma: a management technique underutilized by acute care surgeons. J. Trauma 71(1):102–105; discussion 105–7. [DOI] [PubMed] [Google Scholar]
- 6. Broderick SR. 2013. Hemothorax: etiology, diagnosis, and management. Thorac. Surg. Clin. 23(1):89–96 vi‐vii. [DOI] [PubMed] [Google Scholar]
- 7. Azfar Ali H, Lippmann M, Mundathaje U, et al. 2008. Spontaneous hemothorax: a comprehensive review. Chest 134(5):1056–1065. [DOI] [PubMed] [Google Scholar]
- 8. DuBose J, Inaba K, Demetriades D, et al. 2012. Management of post‐traumatic retained hemothorax: a prospective, observational, multicenter AAST study. J. Trauma Acute Care Surg. 72(1):11–22; discussion 22−4; quiz 316. [DOI] [PubMed] [Google Scholar]
- 9. Landreneau RJ, Keenan RJ, Hazelrigg SR, et al. 1996. Thoracoscopy for empyema and hemothorax. Chest 109(1):18–24. [DOI] [PubMed] [Google Scholar]
- 10. Furrer M, Rechsteiner R, Eigenmann V, et al. 1997. Thoracotomy and thoracoscopy: postoperative pulmonary function, pain and chest wall complaints. Eur. J. Cardiothorac. Surg. 12(1):82–87. [DOI] [PubMed] [Google Scholar]
- 11. Piccolo F, Popowicz N, Wong D, et al. 2015. Intrapleural tissue plasminogen activator and deoxyribonuclease therapy for pleural infection. J. Thorac. Dis. 7(6):999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lansley SM, Cheah HM, Varano Della Vergiliana JF, et al. 2015. Tissue plasminogen activator potently stimulates pleural effusion via a monocyte chemotactic protein‐1‐dependent mechanism. Am. J. Respir. Cell Mol. Biol. 53(1):105–112. [DOI] [PubMed] [Google Scholar]
- 13. Bozzay JD, and Bradley MJ. 2019. Management of post‐traumatic retained hemothorax. Dent. Traumatol. 21(1):14–20. [Google Scholar]
- 14. Oguzkaya F, Akcali Y, and Bilgin M. 2005. Videothoracoscopy versus intrapleural streptokinase for management of post traumatic retained haemothorax: a retrospective study of 65 cases. Injury 36(4):526–529. [DOI] [PubMed] [Google Scholar]
- 15. Kumar S, Rathi V, Rattan A, et al. 2015. VATS versus intrapleural streptokinase: a prospective, randomized, controlled clinical trial for optimum treatment of post‐traumatic residual hemothorax. Injury 46(9):1749–1752. [DOI] [PubMed] [Google Scholar]
- 16. Hendriksen BS, Kuroki MT, Armen SB, et al. 2019. Lytic therapy for retained traumatic hemothorax: a systematic review and meta‐analysis. Chest 155(4):805–815. [DOI] [PubMed] [Google Scholar]
- 17. Popowicz N, Bintcliffe O, De Fonseka D, et al. 2017. Dose de‐escalation of intrapleural tissue plasminogen activator therapy for pleural infection. The Alteplase Dose Assessment for Pleural Infection Therapy project. Ann. Am. Thorac. Soc. 14(6):929–936. [DOI] [PubMed] [Google Scholar]
- 18. Idell S, Florova G, Shetty S, et al. 2017. Precision‐guided, personalized intrapleural fibrinolytic therapy for empyema and complicated parapneumonic pleural effusions: the case for the fibrinolytic potential. Clin. Pulm. Med. 24(4):163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]


