Abstract
Background Vaccination is the most important way out of the novel coronavirus disease 2019 (COVID-19) pandemic. Vaccination practices have started in different countries for community immunity. In this process, health authorities in different countries have preferred different type of COVID-19 vaccines. Inactivated COVID-19 vaccine is one of these options and has been administered to more than 7 million people in Turkey. Inactivated vaccines are generally considered safe. Kounis syndrome (KS) is a rare clinical condition defined as the co-existence of acute coronary syndromes and allergic reactions.
Case Report We present the case of a 41-year-old woman with no cardiovascular risk factors who was admitted at our emergency department with flushing, palpitation, dyspnea, and chest pain 15 min after the first dose of inactivated CoronaVac (Sinovac Life Sciences, Beijing, China). Electrocardiogram (ECG) showed V4-6 T wave inversion, and echocardiography revealed left ventricular wall motion abnormalities. Troponin-I level on arrival was elevated. Coronary angiography showed no sign of coronary atherosclerosis. She was diagnosed with type 1 KS. The patient's symptoms resolved and she was discharged from hospital in a good condition.
Why Should an Emergency Physician Be Aware of This? To the best of our knowledge, this is the first case of allergic myocardial infarction secondary to inactivated coronavirus vaccine. This case demonstrates that KS can occur after inactivated virus vaccine against COVID-19. Although the risk of severe allergic reaction after administration of CoronaVac seems to be very low, people who developed chest pain after vaccine administration should be followed by ECG and troponin measurements.
Keywords: allergic reaction, COVID-19, Kounis syndrome, inactivated vaccine
Introduction
Vaccines seem to be the greatest hope to eradicate severe acute respiratory syndrome coronavirus‐2 (SARS-CoV-2) infection, which causes novel coronavirus disease 2019 (COVID-19) pandemic. Different COVID-19 vaccines are being used for immunity around the world. These vaccines include inactive-attenuated virus vaccines, protein subunit-based vaccines, nonreplicating viral vector vaccines, and DNA-based or RNA-based vaccines (1). RNA-based COVID-19 vaccines (such as Pfizer-BioNTech, Moderna, and Oxford-AstraZeneca) are administered in European Union countries and the United States. So far, approximately 7.7 million people have been vaccinated with inactivated coronavirus vaccine in Turkey (2). Based on the available data, although there are important differences in effectiveness rates, no major safety concern was reported for these vaccines (3, 4, 5, 6).
Kounis syndrome (KS) is defined as the coincidental occurrence of acute coronary events and hypersensitivity reactions caused by vasospastic mediators after an allergic reaction (7). This syndrome has been associated with several diseases, a variety of drugs, or environmental exposures (8). Although vaccine ingredients can be considered potential inducers of allergic events, KS induced by vaccines is a very rare clinical condition (9). Only a few cases of allergic myocardial infarction due to tetanus and influenza vaccines have been reported previously (10,11). However, KS precipitated by inactivated coronavirus vaccine has not been reported before. In this report, we describe a 41-year-old woman who developed type 1 KS induced by the first dose of inactivated coronavirus vaccine. With this case presentation, we aimed to keep in mind that KS may occur after inactivated virus vaccine against COVID-19.
Case Report
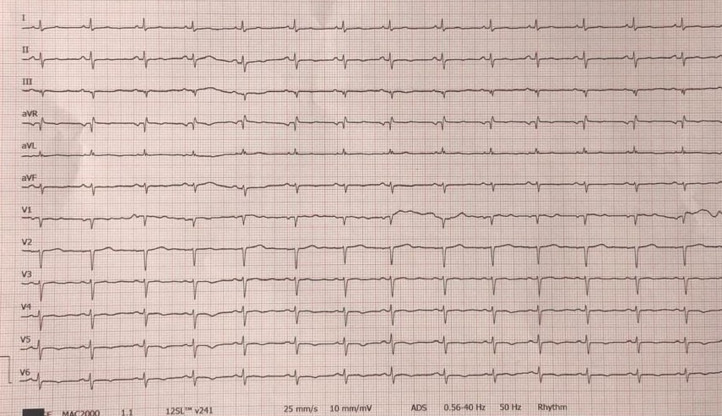
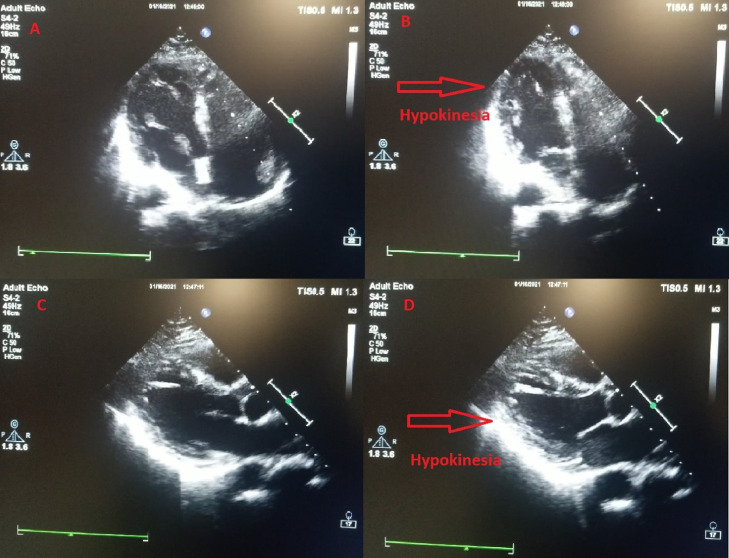
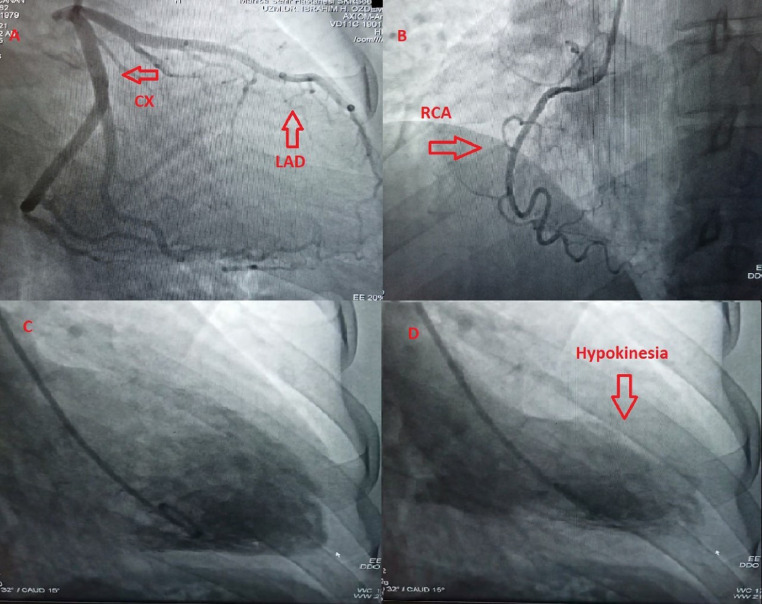
A 41-year-old woman with no cardiovascular risk factors or any chronic disease was admitted at Manisa City Hospital emergency department (ED) with flushing, palpitation, lip and tongue swelling, shortness of breath, and chest pain 15 min after the first dose of inactivated coronavirus vaccine (CoronaVac, Sinovac Life Sciences, Beijing, China). The patient denied any history of asthma or allergic reaction. The patient also stated that she did not have any cardiac or noncardiac symptoms or history before vaccination. On ED arrival, physical examination demonstrated the following vital signs: respiratory rate 22 breaths/min, heart rate 108 beats/min, temperature 36.1°C, pulse oximetry 96% on room air, and blood pressure 110/75 mm Hg. Cardiac examination showed regular rate with normal S1 and S2 without murmurs, rubs, or gallops. She had bronchospasm findings in lung auscultation. She had erythematous appearance in face and there was edema in her lips and uvula. She reported typical chest pain. On admission, her electrocardiogram (ECG) showed poor R wave progression in precordial leads, V4-6 T wave inversion, and fragmented QRS in aVL (Figure 1 ). Transthoracic echocardiography (TTE) performed in the ED showed posterior and apicolateral wall hypokinesia with a left ventricular ejection fraction (LVEF) of 55% (Figure 2 ). Troponin-I estimated on arrival was 0.068 ng/mL (reference: < 0.023 ng/mL), creatine kinase-MB fraction was 5.01 ng/mL (reference: < 4.88 ng/mL). Complete blood count was consistent with eosinophilia (620 μL, reference: < 400 μL). Blood glucose, urea, creatinine, alanine aminotransferase, aspartate aminotransferase, electrolyte parameters, C-reactive protein, procalcitonin, and hemoglobin levels were within normal ranges. N-terminal pro-brain natriuretic peptide (NT-proBNP) was 323 pg/mL (reference: < 125 pg/mL). Intravenous pheniramine maleate 4.5 mg, dexamethasone 8 mg, oxygen treatment, continuous salbutamol by nebulizer, and epinephrine 0.5 mg intramuscular were administered in the ED. Coronary angiography (CAG) was performed to exclude coronary artery disease, and showed no sign of coronary atherosclerosis (Figures 3 A and 3B). However, ventriculography revealed apical and apicolateral wall hypokinesia (Figures 3C and 3D). Troponin-I estimated 6 h after admission was 0.034 ng/mL and decreased to 0.025 ng/mL the next day. The patient was diagnosed to have KS type I variant, secondary to the first dose of inactivated coronavirus vaccine. She was treated with aspirin, oral antihistamines, diltiazem, and corticosteroid for 4 days. Two days later, the repeated cardiac markers were within normal limits, with resolution of electrocardiographic abnormalities and echocardiographic changes with a LVEF of 65%. The patient was discharged from hospital in a good condition, and after 7 days, at a follow-up visit, she was doing well.
Figure 1.
Electrocardiogram demonstrating poor R wave progression in precordial leads, V4-6 T wave inversion, and fragmented QRS in aVL.
Figure 2.
(A) Apical four-chamber echocardiography imaging in diastole. (B) Apical four-chamber echocardiography imaging in systole. (C) Parasternal long axis echocardiography imaging in diastole. (D) Parasternal long axis echocardiography imaging in systole.
Figure 3.
Coronary angiography shows normal coronary arteries (A) left anterior descending (LAD) and circumflex (Cx); (B) right coronary artery (RCA); ventriculography demonstrating apical and apicolateral wall hypokinesia (C) diastole; (D) systole.
Discussion
To the best of our knowledge, this is the first case of allergic myocardial infarction secondary to inactivated coronavirus vaccine. KS was defined by Kounis and Zafras in 1991 (7). KS is defined as the co-existence of acute coronary syndromes, including coronary spasm, and allergic reactions associated with mast cell and platelet activation. This syndrome is caused by inflammatory mediators, such as histamine, platelet-activating factor, arachidonic acid products, and various cytokines and chemokines released during the allergic activation process (12). It is also defined as allergic angina due to development after an allergic reaction. Various allergens, such as foods, environmental exposures, and drugs, have been described in the literature that may cause this syndrome (13, 14, 15). Three variants of the syndrome have been identified (Table 1 ) (16, 17, 18). The type 1 variant includes patients with normal coronary arteries without predisposing factors for coronary artery disease. The release of inflammatory mediators may cause an increase in cardiac enzymes due to coronary artery spasm progressing to acute myocardial infarction. Type 2 is defined as the presence of coronary spasm due to inflammatory mediators together with the erosion or rupture of the pre-existing atherosclerotic plaque. Normal cardiac enzymes and troponin are observed in type 2. Type 3 includes patients with coronary stent thrombosis as a result of an allergic reaction (17,18). In patients with allergic myocardial infarction, ECG may be normal, or some nonspecific ST-T wave changes, ST segment elevation, and ST segment depression may be seen. Cardiac biomarkers, complete blood count, d-dimer, NT-proBNP, serum tryptase, and eosinophil levels could be helpful to identify this syndrome. TTE may also show regional wall motion abnormalities in the distribution of the affected artery, which usually resolve in a few days or weeks without any complication after the acute phase of the disease. CAG is usually needed for patients with suspected KS to assess the coronary anatomy and to make a differential diagnosis (18). The type I variant has a better prognosis (19). The therapeutic management of KS is a procedure that requires treating both cardiac and allergic symptoms at the same time. In patients with type I variant, treatment of the allergic event can relieve symptoms. Corticosteroids and antihistamines are recommended to be used for the treatment of allergic reactions. Vasodilators, such as calcium channel blockers, may treat hypersensitivity induced vasospasm (19). In type II and III variants, the acute coronary syndrome protocol should be applied (18).
Table 1.
Summary of Allergic Acute Coronary Syndromes
| Allergic Acute Coronary Syndromes |
|||
|---|---|---|---|
| Variable | Type I | Type II | Type III |
| Definition | Patients with no underlying IHD | Patients with underlying asymptomatic IHD | Mast cells/eosinophils in coronary or stent thrombus |
| Trigger factors | Drugs, conditions, food consumption, environmental exposures, vaccines | ||
| Clinical features | Acute chest pain, dyspnea, vomiting, nausea, palpitations, tachycardia, pruritus, urticaria, diaphoresis | ||
| Diagnostic tools | Physical examination, ECG, echocardiography, cardiac enzymes (troponin), serum IgE, tryptase, eosinophils, coronary angiography | ||
| Treatment | Oxygen, fluid resuscitation, antihistamines, epinephrine, steroids, mast cell membrane stabilizers | Antiaggregants, anticoagulants, statins, β-blockers, renin–angiotensin system blockers, and revascularization | |
ECG = electrocardiogram; IHD = ischemic heart disease.
Vaccines represent the most powerful weapons against viral epidemic diseases. However, as with any medication, allergic events may occur during vaccination. Fortunately, allergic events triggered by the vaccine are neither serious nor frequent. Nevertheless, it is important to report major adverse events, such as allergic myocardial infarction, even though it is very rare. All COVID-19 vaccines currently in use are generally considered safe (3, 4, 5, 6). Because these vaccines have just started to be administered, they are closely monitored for possible serious adverse events. In a recently published article, it was reported that the risk of serious allergic reaction or anaphylaxis of the RNA-based COVID-19 vaccine is very low (20). According to this report, 1,893,360 first doses of Pfizer-BioNTech COVID-19 vaccine had been administered in the United States. Among these, anaphylaxis occurred in 21 cases, including 17 in patients with a documented history of allergic events, 7 of whom had a history of anaphylaxis. However, no allergic myocardial infarction was reported in this analysis (20). CoronaVac is an inactivated SARS-CoV-2 vaccine. The phase 1 and 2 results of the CoronaVac have been published, but the phase 3 results have not yet been presented (21). Pain at the injection site has been reported as the most common adverse reaction in phase 1 and 2 studies. Only one case of acute hypersensitivity with manifestation of urticaria 48 h after the first dose was reported in the phase 1 study. No serious adverse reaction or allergic myocardial infarction was reported in the phase 2 study (21). With the publication of phase 3 results, we will understand more clearly the safety profile of this vaccine in a real-life practice.
Why Should an Emergency Physicisian Be Aware of This?
Because inactivated vaccines have been used to prevent different infectious diseases for many years, their safety profile is generally known to be good. According to the data in current literature, the risk of serious allergic reaction after administration of CoronaVac seems to be very low. However, this case shows that type 1 KS is among these rare serious adverse events. Physicians should be aware that KS induced by inactivated coronavirus vaccine is a rare but important reaction. Persons who developed post-vaccination chest pain or serious allergic reaction should be followed by ECG, echocardiography, and troponin measurements, and should be observed for a sufficient period of time or hospitalized if necessary.
Footnotes
The patient has given informed consent to the publication of this case report.
REFERENCES
- 1.Yadav T, Srivastava N, Mishra G, et al. Recombinant vaccines for COVID-19. Hum Vaccin Immunother. 2020;16:2905–2912. doi: 10.1080/21645515.2020.1820808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.TR Ministry of Health. COVID-19 vaccine information platform. Available at: https://covid19asi.saglik.gov.tr. Accessed March 8, 2021.
- 3.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Report on Evaluation of Safety, Efficacy and quality of CoronaVac COVID-19 vaccine (Vero Cell) inactivated. Available at: https://www.fhb.gov.hk/download/our_work/health/201200/e_evaluation_report_CoronaVac.pdf. Accessed February 22, 2021.
- 7.Kounis NG, Zavras GM. Histamine-induced coronary artery spasm: the concept of allergic angina. Br J Clin Pract. 1991;45:121–128. [PubMed] [Google Scholar]
- 8.Özlek B, Özlek E, Çelik O, Çil C, Doğan V, Biteker M. Allergic myocardial infarction following recombinant human insulin. Heart Lung. 2018;47:360–362. doi: 10.1016/j.hrtlng.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Kounis NG, Koniari I, de Gregorio C, et al. Allergic reactions to current available COVID-19 vaccinations: pathophysiology, causality, and therapeutic considerations. Vaccines. 2021;9:221. doi: 10.3390/vaccines9030221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia AG, Romero EC, Martinez S., et al. Kounis syndrome in a patient after administration of influenza vaccine. Allergy. 2013;68:514–515. abstract. 1429. [Google Scholar]
- 11.Kundi H, Gök M, Kızıltunç E, Çetin M. A rarely seen type-I Kounis syndrome caused by tetanus vaccine. Koşuyolu Heart J. 2018;21:82–84. [Google Scholar]
- 12.Kounis NG. Coronary hypersensitivity disorder: the Kounis syndrome. Clin Ther. 2013;35:563–571. doi: 10.1016/j.clinthera.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Kounis NG, Kouni SN, Koutsojannis CM. Myocardial infarction after aspirin treatment, and Kounis syndrome. J R Soc Med. 2005;98:296. doi: 10.1258/jrsm.98.6.296-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cevik C, Nugent K, Shome GP, Kounis NG. Treatment of Kounis syndrome. Int J Cardiol. 2010;143:223–226. doi: 10.1016/j.ijcard.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 15.Memon S, Chhabra L, Masrur S, Parker MW. Allergic acute coronary syndrome (Kounis syndrome) Proc (Bayl Univ Med Cent) 2015;28:358–362. doi: 10.1080/08998280.2015.11929274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsigkas G, Chouchoulis K, Theodoropoulos K, Kounis NG, Alexopoulos D. Allergic reaction reveals a non-lethal late stent thrombosis. A new subtype of Kounis syndrome? Int J Cardiol. 2011;149:281–282. doi: 10.1016/j.ijcard.2011.02.060. [DOI] [PubMed] [Google Scholar]
- 17.Kounis NG. Kounis syndrome: an update on epidemiology, pathogenesis, diagnosis and therapeutic management. Clin Chem Lab Med. 2016;54:1545–1559. doi: 10.1515/cclm-2016-0010. [DOI] [PubMed] [Google Scholar]
- 18.Biteker M. Current understanding of Kounis syndrome. Expert Rev Clin Immunol. 2010;6:777–788. doi: 10.1586/eci.10.47. [DOI] [PubMed] [Google Scholar]
- 19.Mori E, Ikeda H, Ueno T, et al. Vasospastic angina induced by nonsteroidal anti-inflammatory drugs. Clin Cardiol. 1997;20:656–658. doi: 10.1002/clc.4960200713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC COVID-19 Response Team. Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine–United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]