Abstract
Purpose
Combined hepatocellular carcinoma and cholangiocarcinoma (cHCC-CC) has wide histologic diversity. This study investigated the effects of cHCC-CC histology, according to the 2010 World Health Organization (WHO) classification, on patient prognosis.
Methods
The medical records of patients who underwent surgical resection for cHCC-CC at our institution between July 2012 and June 2019 were retrospectively evaluated.
Results
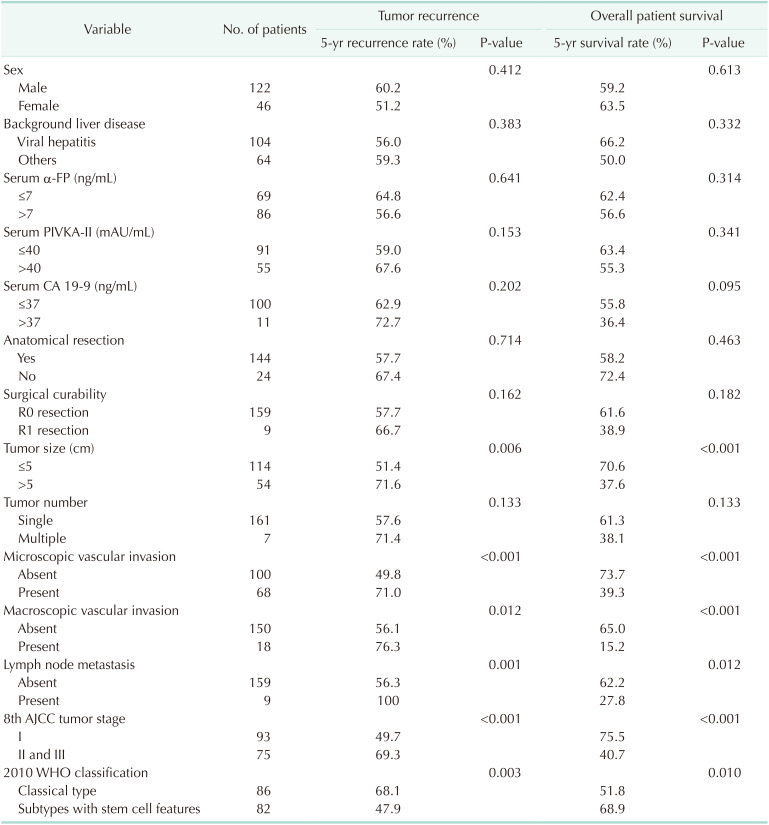
During the study period, 168 patients, 122 males (72.6%) and 46 females (27.4%), underwent surgical resection for cHCC-CC, including 159 patients (94.6%) who underwent R0 resection. Mean tumor diameter was 4.4 ± 2.8 cm, and 161 patients (95.8%) had solitary tumors. Histologically, 86 patients (51.2%) had classical type, and 82 (48.8%) had tumors with stem cell (SC) features, including 33 (19.6%) with intermediate-cell and 23 (13.7%) each with typical SC and cholangiolocellular features; 3 tumors (1.8%) were unclassifiable. At 1, 3, and 5 years, tumor recurrence rates were 31.9%, 49.6%, and 58.1%, respectively, and patient survival rates were 91.0%, 70.2%, and 60.3%, respectively. Univariate analysis showed that tumor size of >5 cm, microscopic and macroscopic vascular invasion, lymph node metastasis, 8th edition of the American Joint Committee on Cancer (AJCC) tumor stage, and 2010 WHO classification were significantly prognostic. Multivariate analysis showed that the 8th AJCC tumor stage and 2010 WHO histologic classification were independently prognostic for tumor recurrence and patient survival. There were no significant prognostic differences among the 3 SC subtypes.
Conclusion
Postresection outcomes are better in patients with SC-type than with classical-type cHCC-CC.
Keywords: Combined tumor, Hepatic, Hepatocellular carcinoma, Progenitor cells, Stem cells
INTRODUCTION
Combined hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (CC) is an uncommon type of primary liver cancer, first described as a distinct disease entity in 1903. This tumor was first classified in 1949 [1] and again in 1985 [2]. The third classification, by the World Health Organization (WHO) in 2010, categorized this tumor according to its origin from hepatic progenitor cells (HPCs) [3]. Thus, combined HCC and CC (cHCC-CC) can be classified into 2 main histological forms, the classical type and subtypes with stem cell (SC) features.
We have previously evaluated the association between pathological characteristics and postresection prognosis of patients with cHCC-CC according to the 2010 WHO classification [4]. Because of the low incidence of cHCC-CC and the relatively recent adoption of the 2010 WHO classification, only a few studies to date have evaluated the pathology-based prognosis of these patients following hepatic resection (HR) [3,4,5,6,7]. The present study investigated the clinical and pathological features, as determined by the 2010 WHO classification, and the postresection outcomes of patients with cHCC-CC who underwent HR.
METHODS
Patients
We searched the liver cancer database of our institution to identify patients with cHCC-CC who were diagnosed according to the 2010 WHO classification from July 2012 to June 2019. Of the 6,504 patients who underwent HR for HCC and intrahepatic CC (ICC) in our institution during the study period [8], 179 (2.8%) underwent HR for cHCC-CC. Patients who had HCC and cHCC-CC concurrently, and those who underwent R2 resection, were excluded. Finally, 168 patients were selected for this study; all were followed up until July 2020 through a review of institutional medical records and with the assistance of the National Health Insurance Service. The study protocol was approved by the Institutional Review Board of Asan Medical Center (No. 2019-1347), which waived the requirement for informed consent due to the retrospective nature of this study. This study was performed in accordance with the ethical guidelines of the World Medical Association Declaration of Helsinki 2013.
Histologic diagnosis according to the 2010 WHO classification
Tumors were categorized as the classical type and as subtypes with SC features based on the 2010 WHO classification [3]. Tumors with SC features were subclassified as typical, intermediate-cell, and cholangiolocellular subtypes. The typical subtype was defined as tumors with a nest of mature hepatocytes surrounded by peripheral clusters of small cells exhibiting morphological and immunohistochemical characteristics of progenitor cells; the intermediate-cell subtype was defined as tumors containing cells with features intermediate between hepatocytes and cholangiocytes, with immunohistochemical markers of both arranged in trabeculae, solid nests, or strands. The cholangiolocellular subtype was defined as tumors composed of cells morphologically mimicking cholangioles arranged in a tubular anastomosing pattern within a dense, sclerotic stroma and expressing progenitor/SC markers.
Tumors were classified into subtypes based on the results of immunohistochemical staining for α-FP, CEA, cytokeratin 7, cytokeratin 19, epithelial cell adhesion molecule (EpCAM), HepPar1, CD10, CD34, KIT (CD117), nuclear cell adhesion molecule (NCAM1/CD56), reticulin, and others [3]. A small number of tumors with SC features could not be immunohistochemically characterized into one of these 3 subtypes and were therefore defined as being of unclassifiable subtype.
Tumor staging according to the 8th edition of the American Joint Committee on Cancer staging system
All cHCC-CCs were staged according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system for ICC because this staging system was found to be equally valid for ICCs, cHCC-CCs, and primary endocrine tumors of the liver [9]. T1 stage is defined as a solitary tumor without vascular invasion (T1a ≤ 5 cm and T1b > 5 cm); T2 stage includes solitary tumors with vascular invasion or multiple tumors; T3 stage is defined as penetration of the visceral peritoneum; and T4 stage is defined as direct invasion of extrahepatic structures.
Statistical analysis
We analyzed the continuous variables by using Student t-tests or analysis of variance depending on their distribution. We compared the categorical variables by using the chi-square test or Fisher exact test. Survival curves were estimated by the Kaplan-Meier method and compared by the log-rank test. Cox proportional hazard regression analysis was used to calculate hazard ratios and 95% confidence intervals. Statistical analyses were performed using IBM SPSS ver. 22 (IBM Corp., Armonk, NY, USA), with P-values of <0.05 regarded as statistically significant.
RESULTS
Clinicopathological features
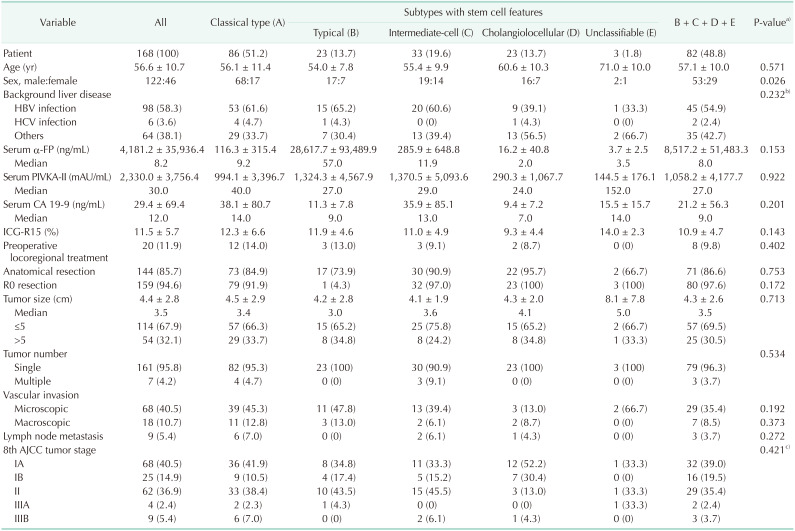
The clinical features of the 168 patients pathologically diagnosed with cHCC-CC are summarized in Table 1. These patients included 122 males (72.6%) and 46 females (27.4%), with mean age of 56.6 ± 10.7 years (range, 30–81 years). Most patients had been preoperatively diagnosed with HCC, with 19 (11.3%) initially undergoing transcatheter arterial chemoembolization (TACE) and 1 (0.6%) undergoing radiofrequency ablation.
Table 1. Clinicopathological features of the 168 patients with combined hepatocellular carcinoma-cholangiocarcinoma according to the 2010 WHO classification.
Values are presented as number (%), mean ± standard deviation, or number only if otherwise specified.
PIVKA-II, proteins induced by vitamin K antagonist or absence-II; ICG-R15, indocyanine green retention test at 15 minutes; MELD, model for end-stage liver disease; AJCC, American Joint Committee on Cancer.
a)A vs. (B + C + D + E). b)Viral hepatitis vs. others. c)Stage I vs. other advanced stages.
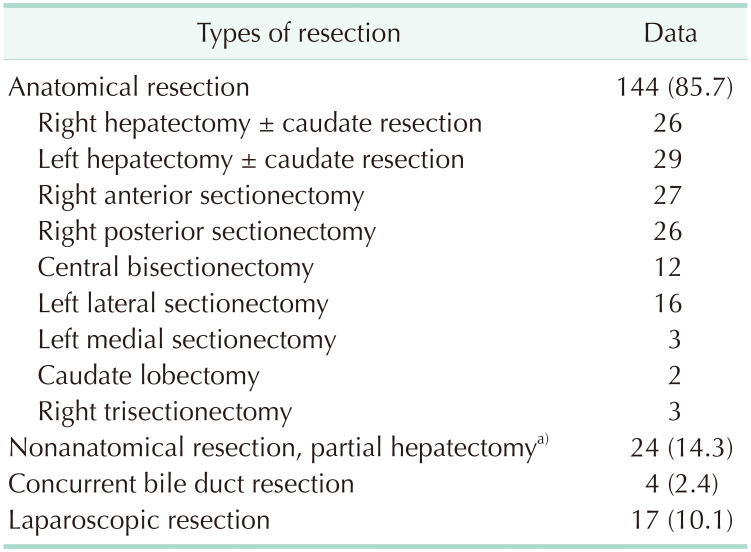
Of these 168 patients, 159 (94.6%) underwent R0 resection and 9 (5.4%) underwent R1 resection. The extents of HR were anatomical resection in 144 (85.7%) and nonanatomical resection including subsegmentectomy and partial hepatectomy in 24 (14.3%). Laparoscopic HR and concurrent bile duct resection were performed in 17 (10.1%) and 4 (2.4%), respectively (Table 2).
Table 2. Extents of hepatic resection.
Values are presented as number (%) or number only.
a)Including subsegmentectomy and nonanatomical partial hepatectomy.
The pathological findings of these patients are summarized in Table 1. Mean tumor diameter was 4.4 ± 2.8 cm, and 161 patients (95.8%) had solitary tumors. Histologically, 86 patients (51.2%) had classical-type cHCC-CCs, whereas 82 (48.8%) had tumors with SC features, including 23 (13.7%) with typical subtype, 33 (19.6%) with intermediate-cell subtype, 23 (13.7%) with cholangiolocellular subtype, and 3 (1.8%) with unclassifiable type. Except for age, there were no statistically significant differences in clinical and pathological features in patients assorted by the 2010 WHO classification (Table 1).
Postresection prognosis
None of these 168 patients died of perioperative complications. During a mean follow-up period of 43.8 ± 24.8 months (range, 3–95 months), 90 patients (53.6%) had recurrent tumors.
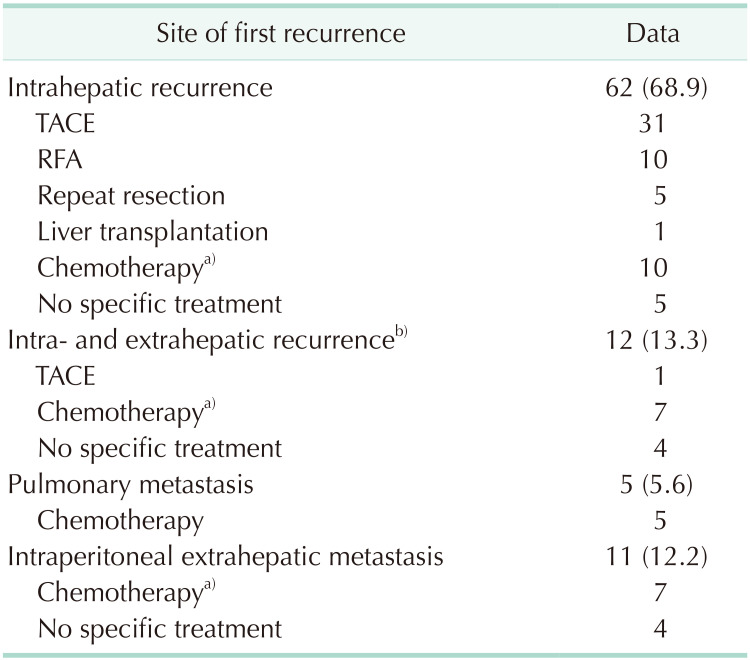
The preferred initial treatments for these recurrent lesions were TACE (n = 31), radiofrequency ablation (n = 10), and systemic chemotherapy (n = 10) for intrahepatic recurrence; and systemic chemotherapy for intra- and extrahepatic recurrence (n = 7), pulmonary metastasis (n = 5), and intraperitoneal extrahepatic metastasis (n = 7). No specific recurrence treatment was provided to 13 patients because of poor general condition and rapid tumor progression (Table 3).
Table 3. Initial treatments for the first recurrence in 90 patients with postresection tumor recurrence.
Values are presented as number (%) or number only.
TACE, transcatheter arterial chemoembolization; RFA, radiofrequency ablation.
a)Chemotherapy regimens included gemcitabine, 5-fluorouracil, sorafenib, and other agents. b)Including lung and intraperitoneal metastases.
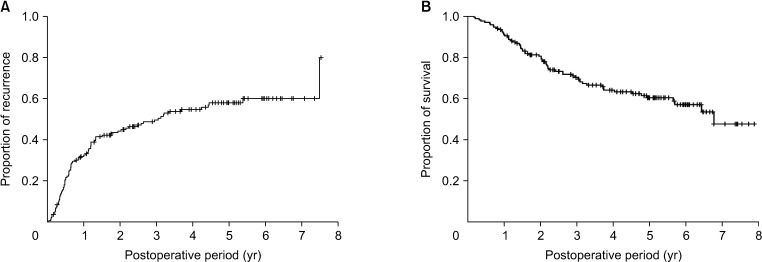
The cumulative 1-, 3-, and 5-year tumor recurrence rates in these patients were 31.9%, 49.6%, and 58.1%, respectively, whereas their 1-, 3-, and 5-year overall patient survival rates were 91.0%, 70.2%, and 60.3%, respectively (Fig. 1).
Fig. 1. Kaplan-Meier analysis of (A) tumor recurrence and (B) overall survival of all 168 patients with combined hepatocellular carcinoma-cholangiocarcinoma.
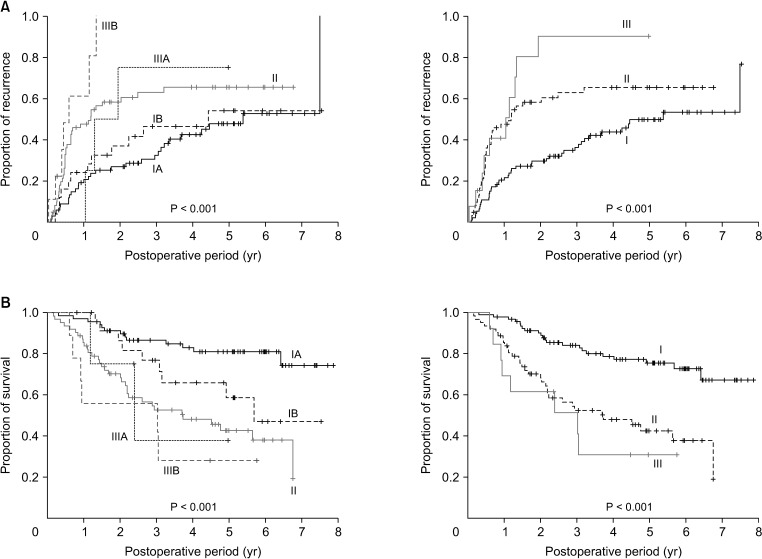
According to the 8th AJCC staging system, 68 patients (40.5%) had stage IA, 25 (14.9%) had stage IB, 62 (36.9%) had stage II, 4 (2.4%) had stage IIIA, and 9 (5.4%) had stage IIIB tumors. Tumor recurrence and patient survival rates showed definite prognostic contrasts according to 8th AJCC tumor stages (all P < 0.001) (Fig. 2).
Fig. 2. Kaplan-Meier analysis of (A) tumor recurrence and (B) overall survival of patients classified according to the 8th edition of American Joint Committee on Cancer tumor staging system.
Risk factor analysis for postresection prognosis
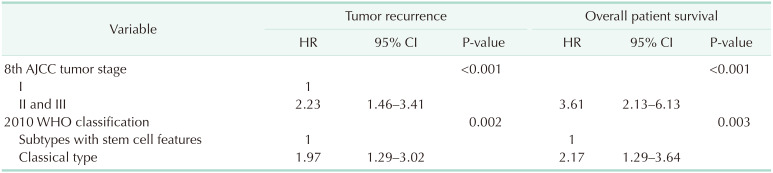
Univariate analyses revealed that significant risk factors for both tumor recurrence and overall patient survival included tumor size of >5 cm, microscopic and macroscopic vascular invasion, lymph node metastasis, 8th AJCC tumor stage, and 2010 WHO histologic classification (Table 4). Because tumor size of >5 cm, microscopic and macroscopic vascular invasion, and lymph node metastasis are essential components of the 8th AJCC tumor staging system, these risk factors can be simplified as the 8th AJCC tumor stage and 2010 WHO histologic classification. Multivariate analysis showed that these 2 factors were also independent prognostic factors for tumor recurrence and overall patient survival (Table 5).
Table 4. Univariate analyses of factors associated with tumor recurrence and patient survival.
PIVKA-II, proteins induced by vitamin K antagonist or absence-II; 8th AJCC, 8th edition of American Joint Committee on Cancer; WHO, World Health Organization.
Table 5. Multivariate analyses of factors associated with tumor recurrence and patient survival.
HR, hazard ratio; CI, confidence interval; 8th AJCC, 8th edition of American Joint Committee on Cancer; WHO, World Health Organization.
Prognostic analysis according to the 2010 World Health Organization classification
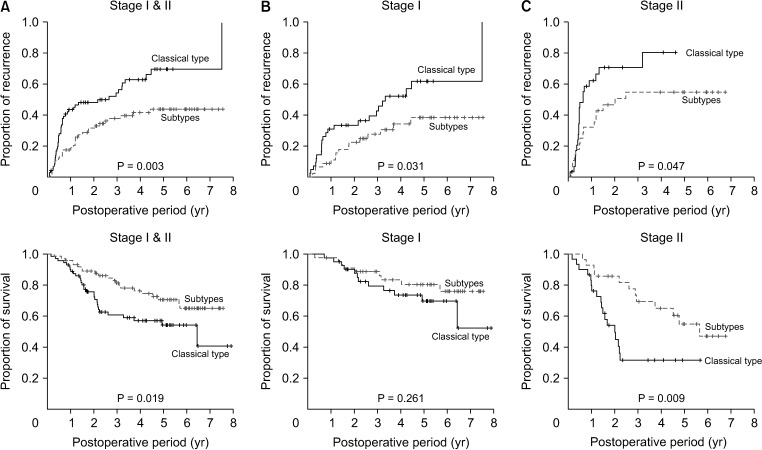
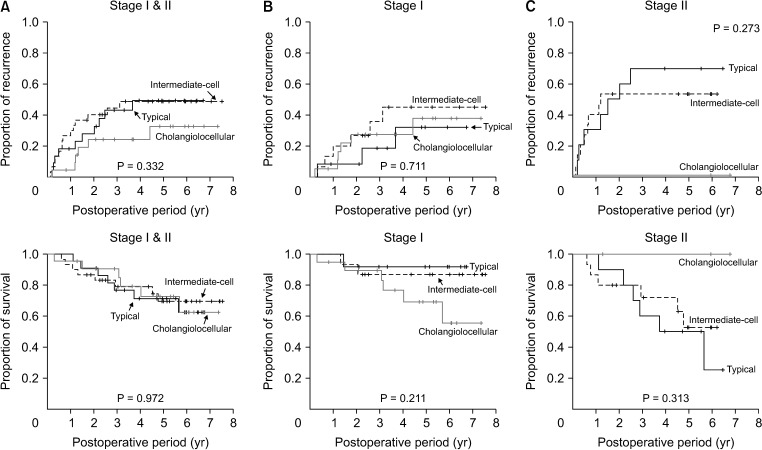
To avoid the confounding effects from less frequent findings, patients with 8th AJCC stage III tumors and unclassifiable subtype, and those who underwent R1 resection, were excluded. Analysis of the 146 patients with 8th AJCC stages I and II cHCC-CCs showed that tumor recurrence rate was significantly higher (P = 0.003) and overall survival significantly lower (P = 0.019) in patients with classical-type cHCC-CCs than in those with tumors with SC feature subtypes (Fig. 3). Furthermore, analysis of the 74 patients with 8th AJCC stages I and II cHCC-CCs with SC features showed no significant differences among these 3 histologic subtypes in tumor recurrence rate (P = 0.331) and patient survival (P = 0.972) (Fig. 4).
Fig. 3. Kaplan-Meier analysis of tumor recurrence and overall survival in patients who underwent R0 resection for classical-type combined hepatocellular carcinoma and cholangiocarcinoma and subtypes with stem cell features according to the 2010 World Health Organization classification. (A) Tumor stages I and II, (B) tumor stage I, and (C) tumor stage II of the 8th edition of American Joint Committee on Cancer.
Fig. 4. Kaplan-Meier analysis tumor recurrence and overall survival in patients who underwent R0 resection for the 3 subtypes of combined hepatocellular carcinoma and cholangiocarcinoma with stem cell features, as determined by the 2010 World Health Organization classification. (A) Tumor stages I and II, (B) tumor stage I, and (C) tumor stage II of the 8th edition of American Joint Committee on Cancer.
DISCUSSION
cHCC-CCs are rare tumors, comprising approximately 2.6% of primary liver malignancies in the present study. This incidence was lower than the 5.8% reported in our previous study [4] but was generally consistent with rates ranging from 0.8% to 14.3% of primary liver malignancies in other patient populations [10,11,12]. A population-level analysis in the United States found that between 1988 and 2009, 52,825 patients had HCC, 7,181 had ICC, and 465 had cHCC-CC, making the proportion of those with cHCC-CC 0.8% [13].
Advances in molecular biology have led to the development of the cancer SC theory of solid neoplasms. Primary liver cancers, including HCC, ICC, and cHCC-CC, are thought to originate from HPCs. HPCs are liver-specific adult SCs that are activated when mature hepatocytes and/or cholangiocytes are damaged. Advances in HPC research have provided insight into the development of cHCC-CCs [14,15,16]. These tumors are to derive from bipotent HPCs, which are intermediate SCs capable of undergoing bidirectional differentiation into hepatocytes or bile duct epithelial cells [17,18,19,20]. Microdissection of cHCC-CCs and DNA extraction showed that both the hepatocellular and cholangiocellular components of these tumors share identical allelic losses, suggesting their monoclonal origin [21]. Gene expression profiling, however, showed that biliary committed cells were precursors of cholangiolocellular type, and biphenotypic progenitor-like cells were precursors of the classical type and other SC subtypes of cHCC-CCs, suggesting that these tumors may derive from more than one cell type [22].
The 2010 WHO classification divides cHCC-CCs into 2 types, the classical type and subtypes with SC features [3]. The classical type, which contains areas typical of both HCC and ICC, was observed in 51.2% of our patients. These tumors are thought to develop from independent and separate HCCs and ICCs. HCCs develop first and transform into ICC or vice versa. Alternatively, malignant changes may occur first in HPCs, followed by their differentiation into HCC and ICC to variable degrees.
Although cHCC-CCs with SC features were initially reported to be rare [6], they are actually relatively common, with 48.8% of patients in the present study having this tumor type, including 13.7% with the typical subtype. The intermediate-cell subtype, observed in 19.6% of patients, corresponds to liver carcinoma of the intermediate (hepatocyte-cholangiocyte) phenotype [23]. Cholangiolocellular carcinoma was classified as a subtype of ICC in previous WHO classifications, but, in 2010, it was classified as a cholangiolocellular subtype of cHCC-CC with SC features [24]. Although considered a rare malignant liver tumor, 13.7% of the patients in this study had cholangiolocellular carcinoma [24]. In addition, tumors in 1.8% of our patients could not be classified. These findings indicate that the classification of various subtypes of cHCC-CC patients with SC features is still challenging and requires further validation [6].
At the time the 2010 WHO classification was introduced, prognosis of patients was thought to be worse in patients with SC features than in patients with HCC. However, the prognosis of patients with cHCC-CC and SC features had not been determined, as findings were based on conflicting evidence from studies that included relatively few patients [3]. Several small-volume studies have assessed the prognosis of patients with cHCC-CC classified according to the 2010 WHO guidelines. Although a Japanese study reported that patients with subtypes with SC features had poorer survival outcomes than patients with classical type [5], another found no significant differences in survival outcomes between these patients with classical type and subtypes with SC features [6]. Moreover, retrospective classification of 63 cHCC-CC specimens, all of which were reported to contain all 3 SC subtypes in various degrees and combinations, according to the 2010 WHO classification, found that 4 (6.3%) could be classified as the classical type, and 3 (4.8%), 28 (44.4%) and 27 (42.9%) as having typical, intermediate-cell and cholangiolocellular subtypes of cHCC-CCs with SC features, respectively [7]. The proportions of these subtypes varied widely in these 3 Japanese studies. Our previous study of postresection prognosis in 100 patients with cHCC-CC found that the presence of SC features was closely associated with favorable tumor biology [4]. However, we found that histologic type according to the 2010 WHO classification was not an independent prognostic factor in that study, primarily because of the relatively small sample number and the short follow-up period.
The results of present study clearly demonstrated that the histological types of cHCC-CC according to the 2010 WHO classification and tumor staging were independently prognostic of tumor recurrence and overall patient survival. Patients having subtypes with SC features showed better prognosis than those with classical-type cHCC-CC, but there were no differences among patients with the 3 subtypes with SC features. To our knowledge, the present study is the largest cohort study of patients who were prospectively diagnosed with cHCC-CC according to the 2010 WHO classification. Our previous comparison of prognosis in patients with cHCC-CC and a propensity score-matched group of patients with ICC showed that postresection tumor recurrence and patient survival were similar in patients with classical-type cHCC-CC and ICC [4], whereas survival outcomes were improved in patients having cHCC-CC subtypes with SC features [5,25]. These results suggested that classical-type cHCC-CC and ICC may share similarly aggressive tumor biology, but that subtypes with SC features may have less aggressive tumor biology.
The recurrence rate of cHCC-CC after HR was high. Methods used to treat recurrent lesions include liver-directed therapy, such as TACE and systemic chemotherapy. TACE is frequently used to treat recurrent cHCC-CC lesions, but its therapeutic effect is unclear because of the histological heterogeneity of cHCC-CCs, with these tumors being more fibrotic and less vascular than HCCs [26]. Studies of the efficacy of TACE in patients with primary unresectable and recurrent cHCC-CCs in our institution found that treatment response and prognosis were highly related to tumor vascularity [27,28]. The role of systemic chemotherapy for unresectable and recurrent cHCC-CCs remains unclear, although it has been associated with unfavorable outcomes. For example, a multicenter study involving 36 patients evaluating several first-line treatments, including gemcitabine/cisplatin, fluorouracil/cisplatin, and sorafenib, showed that overall survival was poorer in patients who received sorafenib monotherapy than in those treated with platinum-containing regimens [29].
This study had several limitations, including its retrospective design, and inclusion of patients at a single center in a HBV-endemic area. Multi-regional, multicenter collective studies are needed to validate the prognostic influence of cHCC-CC subtypes with SC features.
In conclusion, cHCC-CC is a neoplasm with wide histologic diversity, indicating a strong association with HPCs. Patients classified as having cHCC-CC subtypes with SC features have better postresection outcomes than those classified as having classical-type cHCC-CC, suggesting a close association between the postresection outcomes and the histological types according to the 2010 WHO classification.
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: SH.
- Project Administration: SH.
- Formal Analysis: MK, SH, SMH.
- Investigation: CSA, KHK.
- Methodology: DBM, TYH, GWS, DHJ, GCP.
- Writing — Original Draft: SH, MK.
- Writing — Review & Editing: All authors.
References
- 1.Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949;25:647–655. [PMC free article] [PubMed] [Google Scholar]
- 2.Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L. Combined hepatocellular-cholangiocarcinoma: a histologic and immunohistochemical study. Cancer. 1985;55:124–135. doi: 10.1002/1097-0142(19850101)55:1<124::aid-cncr2820550120>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Theise ND, Park YN, Nakanuma Y. Combined hepatocellular-cholangiocarcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. 4th ed. Lyon, France: IARC; 2010. pp. 225–227. [Google Scholar]
- 4.Jung DH, Hwang S, Hong SM, Chung YK, Song GW, Lee YJ, et al. Post-resection prognosis of combined hepatocellular carcinoma-cholangiocarcinoma according to the 2010 WHO classification. World J Surg. 2017;41:1347–1357. doi: 10.1007/s00268-016-3837-y. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda H, Harada K, Sato Y, Sasaki M, Yoneda N, Kitamura S, et al. Clinicopathologic significance of combined hepatocellular-cholangiocarcinoma with stem cell subtype components with reference to the expression of putative stem cell markers. Am J Clin Pathol. 2013;140:329–340. doi: 10.1309/AJCP66AVBANVNTQJ. [DOI] [PubMed] [Google Scholar]
- 6.Akiba J, Nakashima O, Hattori S, Tanikawa K, Takenaka M, Nakayama M, et al. Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am J Surg Pathol. 2013;37:496–505. doi: 10.1097/PAS.0b013e31827332b0. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki M, Sato H, Kakuda Y, Sato Y, Choi JH, Nakanuma Y. Clinicopathological significance of ‘subtypes with stemcell feature’ in combined hepatocellularcholangiocarcinoma. Liver Int. 2015;35:1024–1035. doi: 10.1111/liv.12563. [DOI] [PubMed] [Google Scholar]
- 8.Cho HD, Hwang S, Lee YJ, Park KM, Kim KH, Kim JC, et al. Changes in the types of liver diseases requiring hepatic resection: a single-institution experience of 9,016 cases over a 10-year period. Korean J Hepatobiliary Pancreat Surg. 2016;20:49–52. doi: 10.14701/kjhbps.2016.20.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al., editors. AJCC cancer staging manual. 8th ed. New York: Springer; 2017. [Google Scholar]
- 10.Chantajitr S, Wilasrusmee C, Lertsitichai P, Phromsopha N. Combined hepatocellular and cholangiocarcinoma: clinical features and prognostic study in a Thai population. J Hepatobiliary Pancreat Surg. 2006;13:537–542. doi: 10.1007/s00534-006-1117-1. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Chung GE, Yu SJ, Hwang SY, Kim JS, Kim HY, et al. Long-term prognosis of combined hepatocellular and cholangiocarcinoma after curative resection comparison with hepatocellular carcinoma and cholangiocarcinoma. J Clin Gastroenterol. 2011;45:69–75. doi: 10.1097/MCG.0b013e3181ce5dfa. [DOI] [PubMed] [Google Scholar]
- 12.Tang D, Nagano H, Nakamura M, Wada H, Marubashi S, Miyamoto A, et al. Clinical and pathological features of Allen's type C classification of resected combined hepatocellular and cholangiocarcinoma: a comparative study with hepatocellular carcinoma and cholangiocellular carcinoma. J Gastrointest Surg. 2006;10:987–998. doi: 10.1016/j.gassur.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Garancini M, Goffredo P, Pagni F, Romano F, Roman S, Sosa JA, et al. Combined hepatocellular-cholangiocarcinoma: a population-level analysis of an uncommon primary liver tumor. Liver Transpl. 2014;20:952–959. doi: 10.1002/lt.23897. [DOI] [PubMed] [Google Scholar]
- 14.Kojiro M. Pathomorphology of advanced hepatocellular carcinoma. In: Tobe T, Kameda H, Okudaira M, Ohto M, Endo Y, Mito M, et al., editors. Primary liver cancer in Japan. Tokyo: Springer-Verlag; 1992. pp. 31–37. [Google Scholar]
- 15.Shiraishi M, Takushi Y, Simoji H, Oshiro T, Shinzato S, Tanigawa N, et al. Combined hepatocellular and cholangiocellular carcinoma in a non-cirrhotic liver. J Gastroenterol. 1998;33:593–596. doi: 10.1007/s005350050140. [DOI] [PubMed] [Google Scholar]
- 16.Zhang F, Chen XP, Zhang W, Dong HH, Xiang S, Zhang WG, et al. Combined hepatocellular cholangiocarcinoma originating from hepatic progenitor cells: immunohistochemical and double-fluorescence immunostaining evidence. Histopathology. 2008;52:224–232. doi: 10.1111/j.1365-2559.2007.02929.x. [DOI] [PubMed] [Google Scholar]
- 17.Stavraka C, Rush H, Ross P. Combined hepatocellular cholangiocarcinoma (cHCC-CC): an update of genetics, molecular biology, and therapeutic interventions. J Hepatocell Carcinoma. 2018;6:11–21. doi: 10.2147/JHC.S159805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeh MM. Patholog y of combined hepatocellular-cholangiocarcinoma. J Gastroenterol Hepatol. 2010;25:1485–1492. doi: 10.1111/j.1440-1746.2010.06430.x. [DOI] [PubMed] [Google Scholar]
- 19.Theise ND, Yao JL, Harada K, Hytiroglou P, Portmann B, Thung SN, et al. Hepatic ‘stem cell’ malignancies in adults: four cases. Histopathology. 2003;43:263–271. doi: 10.1046/j.1365-2559.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 20.Wu PC, Fang JW, Lau VK, Lai CL, Lo CK, Lau JY. Classification of hepatocellular carcinoma according to hepatocellular and biliary differentiation markers: clinical and biological implications. Am J Pathol. 1996;149:1167–1175. [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii H, Zhu XG, Matsumoto T, Inagaki M, Tokusashi Y, Miyokawa N, et al. Genetic classification of combined hepatocellularcholangiocarcinoma. Hum Pathol. 2000;31:1011–1017. doi: 10.1053/hupa.2000.9782. [DOI] [PubMed] [Google Scholar]
- 22.Moeini A, Sia D, Zhang Z, Camprecios G, Stueck A, Dong H, et al. Mixed hepatocellular cholangiocarcinoma tumors: cholangiolocellular carcinoma is a distinct molecular entity. J Hepatol. 2017;66:952–961. doi: 10.1016/j.jhep.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Kim H, Park C, Han KH, Choi J, Kim YB, Kim JK, et al. Primary liver carcinoma of intermediate (hepatocyte-cholangiocyte) phenotype. J Hepatol. 2004;40:298–304. doi: 10.1016/j.jhep.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Ariizumi S, Kotera Y, Katagiri S, Nakano M, Nakanuma Y, Saito A, et al. Long-term survival of patients with cholangiolocellular carcinoma after curative hepatectomy. Ann Surg Oncol. 2014;21(Suppl 3):S451–S458. doi: 10.1245/s10434-014-3582-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CH, Hsieh SY, Chang CJ, Lin YJ. Comparison of clinical characteristics of combined hepatocellular-cholangiocarcinoma and other primary liver cancers. J Gastroenterol Hepatol. 2013;28:122–127. doi: 10.1111/j.1440-1746.2012.07289.x. [DOI] [PubMed] [Google Scholar]
- 26.De Vito C, Sarker D, Ross P, Heaton N, Quaglia A. Histological heterogeneity in primary and metastatic classic combined hepatocellular-cholangiocarcinoma: a case series. Virchows Arch. 2017;471:619–629. doi: 10.1007/s00428-017-2196-x. [DOI] [PubMed] [Google Scholar]
- 27.Kim JH, Yoon HK, Ko GY, Gwon DI, Jang CS, Song HY, et al. Nonresectable combined hepatocellular carcinoma and cholangiocarcinoma: analysis of the response and prognostic factors after transcatheter arterial chemoembolization. Radiology. 2010;255:270–277. doi: 10.1148/radiol.09091076. [DOI] [PubMed] [Google Scholar]
- 28.Na SK, Choi GH, Lee HC, Shin YM, An J, Lee D, et al. The effectiveness of transarterial chemoembolization in recurrent hepatocellular-cholangiocarcinoma after resection. PLoS One. 2018;13:e0198138. doi: 10.1371/journal.pone.0198138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi S, Terashima T, Shiba S, Yoshida Y, Yamada I, Iwadou S, et al. Multicenter retrospective analysis of systemic chemotherapy for unresectable combined hepatocellular and cholangiocarcinoma. Cancer Sci. 2018;109:2549–2557. doi: 10.1111/cas.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]