Abstract
Background
Etiology of and outcomes following idiosyncratic drug-induced liver injury (DILI) vary geographically. We conducted a prospective study of DILI in India, from 2013 to 2018 and summarize the causes, clinical features, outcomes and predictors of mortality.
Methods
We enrolled patients with DILI using international DILI expert working group criteria and Roussel Uclaf causality assessment method. Follow-up was up to 3 months from onset of DILI or until death. Multivariate logistics regression was carried out to determine predictors of non-survival.
Results
Among 1288 patients with idiosyncratic DILI, 51.4% were male, 68% developed jaundice, 68% required hospitalization and 8.2% had co-existing HIV infection. Concomitant features of skin reaction, ascites, and encephalopathy (HE) were seen in 19.5%, 16.4%, and 10% respectively. 32.4% had severe disease. Mean MELD score at presentation was 18.8 ± 8.8. Overall mortality was 12.3%; 65% in those with HE, 17.6% in patients who fulfilled Hy's law, and 16.6% in those that developed jaundice. Combination anti-TB drugs (ATD) 46.4%, complementary and alternative medicines (CAM) 13.9%, anti-epileptic drugs (AED) 8.1%, non-ATD antimicrobials 6.5%, anti-metabolites 3.8%, anti-retroviral drugs (ART)3.5%, NSAID2.6%, hormones 2.5%, and statins 1.4% were the top 9 causes. Univariate analysis identified, ascites, HE, serum albumin, bilirubin, creatinine, INR, MELD score (p < 0.001), transaminases (p < 0.04), and anti-TB drugs (p = 0.02) as predictors of non-survival. Only serum creatinine (p = 0.017), INR (p < 0.001), HE (p < 0.001), and ascites (p = 0.008), were significantly associated with mortality on multivariate analysis. ROC yielded a C-statistic of 0.811 for MELD and 0.892 for combination of serum creatinine, INR, ascites and HE. More than 50 different agents were associated with DILI. Mortality varied by drug class: 15% with ATD, 13.6% with CAM, 15.5% with AED, 5.8% with antibiotics.
Conclusion
In India, ATD, CAM, AED, anti-metabolites and ART account for the majority of cases of DILI. The 3-month mortality was approximately 12%. Hy's law, presence of jaundice or MELD were predictors of mortality.
Keywords: Anti-tuberculosis drugs, Isoniazid, Rifampicin, Pyrazinamide, Traditional medicines, Complimentary medicines, Mortality, Prognosis, Jaundice
Abbreviations: AED, Anti-epileptic drugs; ALF, Acute liver failure; ALT, Alanine aminotransferase; ART, Anti-retroviral drugs; AST, Aspartate aminotransferase; ATD, Anti- tuberculosis drugs; CAM, Complementary and alternative medicine; C.I, Confidence interval; DILI, Drug-induced liver injury; DILIN, Drug induced liver injury network; HE, Hepatic encephalopathy; HIV, Human immunodeficiency virus; INR, International normalised ratio; MELD, Model for end stage liver disease; NSAID, Nonsteroidal anti-inflammatory drugs; OR, Odds ratio; ROC, Receiver operating characteristic; RUCAM, Roussel uclaf causality assessment method; TB, Tuberculosis.; TCM, Traditional chinese medicines.; USA, United states of america; ULN, Upper limit of normal
Introduction
The liver's position at the intersection between the gastrointestinal tract (gut) and systemic circulation exposes it continuously to a myriad of food products, bacterial by-products, drugs and other xenobiotics from birth with minimal or no adverse reaction to the body. However, rarely this default function gets disrupted, either from the direct injurious effect of drugs or toxins or as a result of idiosyncratic reaction to these agents. These reactions can vary from self-limited often asymptomatic liver biochemical test abnormalities to severe liver injury manifesting as jaundice, rarely progressing to acute liver failure.
Drug induced liver injury (DILI) is relatively rare. The reported incidence varies from 14 per 100,000 inhabitants in France1 to 19 per100,000 inhabitants in Iceland.2 In South Korea it was 12 per 100,000 inhabitants,3 while it is higher in China.4 Drug classes causing DILI vary according to geographic regions. In the West, paracetamol (acetaminophen)5 and antimicrobials6 are the leading cause of acute liver failure (ALF) and idiosyncratic DILI respectively. In the East, traditional Chinese medicines (TCM) and anti-tuberculosis DILI are equally prevalent with some geographic variability.4 There is mounting evidence of an increasing burden of DILI related to complementary and alternative medicine (CAM) worldwide, especially in East Asia where TCM is integrated into the health systems.3,4
Information about DILI in India is limited mostly to single centre reports.7,8 Generally, anti-TB drugs are the most common cause of DILI, although there are regional variations with increasing reports of CAM causing DILI.9 Multicenter and nationwide DILI registries such as those in the United States of America (USA) or Spain are lacking.6,10 With a heterogeneous population of 1.3 billion people, India has several unique challenges, from varying disease burden to prescription practices including the widespread use of alternative (Ayurveda/Unani/Siddha/Homeopathic) systems of medicine, the contribution to DILI from which are unclear and under recognized.
Therefore, under the aegis of the Indian National Association for the Study of Liver (INASL), we undertook this nationwide study to evaluate the causes and outcome of DILI and identify predictors of mortality in a large cohort of patients enrolled prospectively from a number of centers across India. We examined and compared the characteristics of common drugs causing DILI, including the subset causing severe DILI resulting in ALF. We also evaluated the utility of established prognostic indices such as model for end stage liver disease (MELD) score11 and identified predictors of outcome.
Methods
The Indian Network for Drug-Induced Liver Injury (INDILI) prospectively collected data pertaining to consecutive cases with DILI, from different centers throughout India over a 5-year period (2013–2018). Patient details were captured on a case record form (Supplement file 1) and sent to a nodal center (St. John's Medical College Hospital, Bangalore). The diagnosis of DILI and its severity were made based on criteria adopted by international DILI Expert Working Group.12 Briefly, patients were considered to have DILI if they met the following criteria: (a) documented drug ingestion resulting in recent onset abnormalities in liver biochemistry tests (rise in bilirubin of at least 2 mg/dl or symptoms of liver injury with aspartate aminotransferase (AST) or alanine aminotransferase (ALT) > 3 times the upper limit of normal or alkaline phosphatase > 2 times the upper limit of normal or (b) AST or ALT >5 times the upper limit of normal without symptoms and exclusion of other competing causes of liver injury, including viral and autoimmune among others, by appropriate serological testing and imaging studies. Severe disease was defined using international DILI expert working group criteria12 i.e. bilirubin >2 gm/dl and INR> 1.5 with ascites or encephalopathy or death. Patterns of liver injury were classified as hepatocellular, cholestatic and mixed based on the R value of ≥5, ≤2, >2 and < 5 respectively, where R= (AST or ALT/ULN)/(ALP/ULN).12 Jaundice was defined as clinically apparent jaundice.
Causality assessment was carried out using the Roussel Uclaf Causality Assessment Method (RUCAM) model.13 Patients with suspected DILI with at least a possible relationship by RUCAM were enrolled. Anti-tuberculosis therapy, including combination regimen with isoniazid, rifampicin with or without pyrazinamide and ethambutol were considered as a single entity.12 The diagnosis of ALF was made when standard criteria were met.14 Patients were followed for 3 months from onset of DILI or until death. We investigated the effect of Hy's law15,16 and clinically apparent jaundice on outcome. We analyzed patients with idiosyncratic DILI, after excluding patients with predictable hepatotoxicity resulting from intentional overdose. Model for end stage liver disease (MELD) score was calculated by standard means.11 The study was approved by institutional review boards of participating centers.
Statistical methods
Descriptive statistics in the form of mean and standard deviations for interval variables and frequency with percentages for categorical variables were calculated. Student t tests (Normal data) or Mann Whitney U tests (non-normal data) were applied to see significant mean or median levels between recovered and non-recovered outcome variables. Chi-square test was used to determine the association between outcomes and demographic/clinical characteristics. Multivariate logistics regression was performed to identify risk factors for non-recovery. ROC curves with C-statistics were calculated to determine predictive accuracy of outcome from MELD score and the predictive probabilities. P value 0.05 (two tailed) was considered for statistically significant levels. SPSS 22.0 statistical package was used for the analysis.
Results
The INDILI enrolled 1373 subjects, of whom 70 subjects were excluded because of incomplete or missing information. We also excluded an additional 15 patients with intrinsic DILI (10 from paracetamol hepatotoxicity and 5 from ferrous sulfate toxicity, both from intentional overdose). Thirty centers and 5 physicians in solo practice participated in the study (Figure 1). We analyzed detailed information on 1288 patients with idiosyncratic DILI. This included 79 children aged <18 years (8%).
Figure 1.
Indian map depicting location of contributing institutions.
The baseline demographic and laboratory characteristics are presented in Table 1. Of the 1288 subjects 51.4% subjects were males, and 8.2% had co-existing HIV infection. Jaundice was noted at presentation in 67.2%, and overall 68.3% were hospitalized. Based on R values calculated using data available on 1217 patients, 362 (29.7%), 521 (42.8%) and 334 (27.4%) were classified as having hepatocellular, cholestatic and mixed hepatitis respectively. Features of hypersensitivity skin reaction were seen in 19.4% and Ascites and encephalopathy on admission or during hospitalization were seen in 16.3% and 10% respectively. A history of regular alcohol consumption was obtained in 10.7% patients and 6.2% had concomitant type 2 diabetes mellitus.
Table 1.
Demographic, and Laboratory Characteristics of 1288 Patients With Idiosyncratic DILI.
| Mean ± Std. Deviation | Range | |
|---|---|---|
| Age (years) | 43 ± 16.5 | 1–86 |
| Sex (males: females) | 661 (51.4%): 627 (48.6%) | – |
| Duration (days) IQR |
27 (11–60) | 1–929 |
| Weight (kg) | 55 ± 14 | 34–106 |
| BMI | 22.0 ± 4.5 | 10.6–43.2 |
| Serum protein (g/dl) | 6.5 ± 2.4 | 2.0–82.0 |
| Serum albumin (g/dl) | 3.1 ± 0.7 | 0.6–5.2 |
| Total bilirubin (mg/dl) | 8.3 ± 10.0 | 0.13–44.0 |
| Direct bilirubin (mg/dl) | 5.6 ± 6.6 | 0.04–32.0 |
| AST IU/L IQR |
220 (119–438) | 28.7–7538 |
| ALT IU/L IQR |
241 (110–519) | 29–9115 |
| ALP IU/L IQR |
180 (123–287) | 25–2986 |
| GGT IU/L IQR |
130 (62.294) | 14–5964 |
| Serum creatinine (mg/dl) | 1.0 ± 0.8 | 0.13–10.2 |
| INR | 1.7 ± 1.5 | 0.50–19.3 |
| Hemoglobin (g/dl) | 11.2 ± 2.1 | 2.7–18.2 |
| WBC (/mm3) IQR |
8600 (6238–11500) | 1100–23020 |
| Platelets 105/dl) | 2.3 ± 1.1 | .15–9.0 |
| MELD | 18.7 ± 8.8 | 6–56 |
Abbreviations: ATD: anti-tuberculosis drugs, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, BMI: Body Mass Index, GGT: gamma glutamyl transferase, INR: International normalized ratio, IQR: inter quartile range, MELD: Model for end stage liver disease, WBC: white blood cells.
Applying RUCAM, 53% were deemed to be definite (highly probable), 31% probable, 16% possible. Almost one third of patients (n = 422; 32.4%) had severe disease (total bilirubin >2 gm/dl and INR> 1.5 or ascites or encephalopathy or death). The mean MELD score was 18.8 ± 8.8.
Drugs associated with DILI
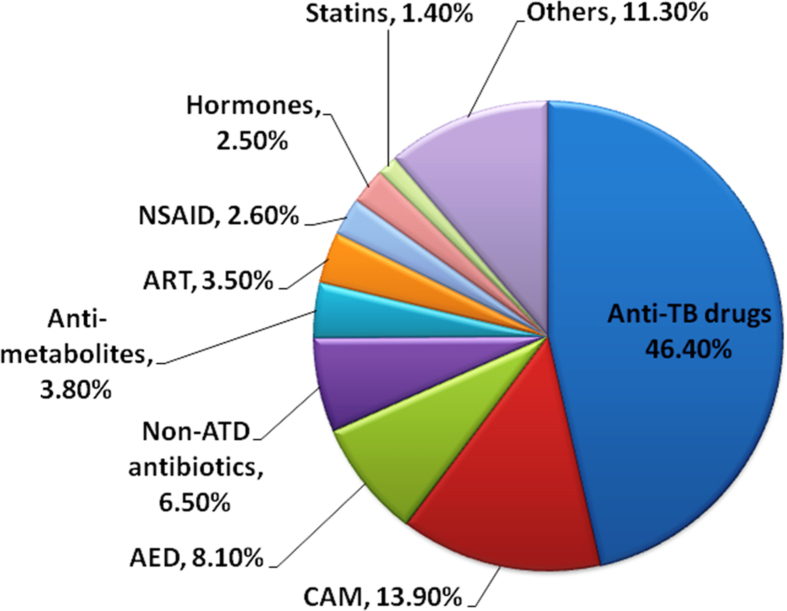
More than 50 different agents/classes were associated with DILI. Combination anti-TB drugs (ATD) was the most common class (46.4%) followed by complementary and alternative medicines (13.9%), anti-epileptic drugs (AED) 8.1%, non-ATD antibiotics 6.5%, anti-metabolites 3.8%, anti-retroviral drugs (ART) 3.5%, NSAID 2.6%, hormones 2.5%, statins 1.4% and others (Figure 3).
Figure 3.
Agents/Classes causing DILI.
Table 3 shows the differences in characteristics between the top 5 drugs. Overall demographic characteristics were similar across all 5 major groups/agents except that indices for severity of liver injury and mortality was low in those patients with DILI caused by antibiotics and NSAIDs. Mortality varied by drug class: 15% with ATD, 13.6% with CAM, 15.5% with AED, 5.8% with antibiotics, and 0% with NSAIDs.
Table 3.
Comparison of Selected Characteristics Among Top 5 Drug Classes Causing DILI.
| ATD | CAM | AED | Antimicrobials | NSAID | |
|---|---|---|---|---|---|
| Female | 49.5% | 38.7% | 47.2% | 49.1% | 47.1% |
| Jaundice | 70.2% | 87.7% | 55.2% | 55.8% | 50% |
| Skin Rash | 9.2% | 16.7% | 61% | 32.7% | 9.1% |
| HE | 13.6% | 8.4% | 9.5% | 0% | 2.9% |
| Ascites | 25.2% | 21.8% | 13.3% | 7.7% | 14.7% |
| Recovery | 85.4% | 86.4% | 84.5% | 94.2% | 100% |
| Severity | 35.8% | 37% | 33% | 18.2% | 17.6% |
| Hy's Law | 59.8% | 58% | 49.1% | 41.8% | 44.1% |
| Age (years) | 43.7 ± 16.6 | 41.7 ± 14.8 | 37.0 ± 16.9 | 44.4 ± 18.6 | 52.3 ± 19.4 |
| Duration of treatment (days) IQR |
25 (12–60) | 21 (6–62) | 31 (15–42) | 7 (5–14) | 15 (6–34) |
| BMI | 21.4 ± 4.4 | 23.4 ± 4.2 | 21.5 ± 4.8 | 22.1 ± 3.8 | 24.3 ± 4.5 |
| Weight (kg) | 53.6 ± 13.1 | 61.7 ± 11.3 | 54.1 ± 14.0 | 50.8 ± 17.6 | 58.6 ± 13.8 |
| Total protein (g/dl) | 6.3 ± 1.0 | 7.3 ± 5.9 | 6.2 ± 0.8 | 6.7 ± 0.9 | 6.8 ± 0.8 |
| Serum albumin (g/dl) | 2.9 ± 0.7 | 3.3 ± 0.7 | 3.1 ± 0.6 | 3.3 ± 0.9 | 3.3 ± 0.6 |
| Total Bilirubin (mg/dl) | 6.8 ± 6.8 | 15.6 ± 12 | 6.8 ± 8.3 | 6.0 ± 8.0 | 5.1 ± 6.1 |
| Direct Bilirubin (mg/dl) | 4.7 ± 5.4 | 10.3 ± 8.8 | 4.7 ± 6.0 | 4.3 ± 6.4 | 3.3 ± 4.1 |
| AST (IU/L) IQR |
236 (131–510) | 235 (133-4450 | 258 (159–761) | 154 (89–322) | 236 (123–396) |
| ALT (IU/L) IQR |
237 (104–525) | 224 (85–561) | 347 (158–761) | 202 (76–419) | 276 (139–660) |
| ALP (IU/L) IQR |
165 (118–250) | 171 (128–255) | 228 (152–346) | 281 (142–423) | 197 (136–313) |
| GGT (IU/L) IQR |
111 (53–213) | 80 (42–162) | 438 (213–1014) | 203 (103–352) | 199 (76–375) |
| Creatinine (mg/dl) | 1.0 ± 0.7 | 1.2 ± 1.3 | 0.9 ± 0.6 | 0.9 ± 0.9 | 1.2 ± 1.1 |
| INR | 1.9 ± 1.7 | 1.6 ± 0.6 | 1.4 ± 0.6 | 1.2 ± 0.4 | 1.2 ± 0.2 |
| Hb (g/dl) | 10.9 ± 2.0 | 11 ± 2.2 | 11.6 ± 2.0 | 11.7 ± 1.6 | 12.1 ± 2.2 |
| WBC (10/dl) IQR |
8270 (6200–11000) | 9200 (7250–11800) | 9450 (6370–13650) | 8950 (6190–12760) | 7800 (7100–14100) |
| Eosinophils (%) | 3.37 ± 4.710 | 3.9 ± 3.8 | 9.57 ± 12.9 | 3.6 ± 3.8 | 3.8 ± 3.6 |
| Platelets (105/dl) | 2.3 ± 1.2 | 2.1 ± 0.9 | 2.4 ± 0.9 | 2.7 ± 1.0 | 2.7 ± 1.7 |
| MELD | 19.1 ± 9.5 | 21.8 ± 7.2 | 16.4 ± 9.6 | 16.2 ± 8.2 | 14.9 ± 9.1 |
Abbreviation.ATD: Anti-TB drugs; CAM: Complimentary alternative medicine; AED: Anti-epileptic drugs; NSAID: Non-steroidal anti inflammatory drug. AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, BMI: Body Mass Index, GGT: gamma glutamyl transferase, INR: International normalized ratio, IQR: inter quartile range, MELD: Model for end stage liver disease, WBC: white blood cells.
Mortality
Information about final outcome was available in 1263 patients, 156 of whom died (12.3%). Mortality was 64.8% (79 of 124) in those with ALF. Clinically apparent jaundice entailed a mortality of 16.6% (135 of 814) compared to 123 (17.6%) of 705 patients who fulfilled Hy's law criteria. Table 2 shows the characteristics between survivors and non-survivors.
Table 2.
Comparison of Characteristics of Survivors and Non-survivors With Idiosyncratic DILI.
| Variable | Survivors (N = 1084) | Non-survivors (N = 153) | P value |
|---|---|---|---|
| Age (Years) | 42 ± 16. | 48 ± 17 | 0.001 |
| Duration of treatment (days) IQR |
26 (10–60) | 24 (12–101) | 0.80 |
| Weight (kg) | 55 ± 14 | 56 ± 14 | 0.50 |
| BMI | 22.0 ± 4 | 22 ± 5 | 0.60 |
| ATD | 85.4% | 14.6% | 0.027 |
| Serum total protein (g/dl) | 6.6 ± 2.5 | 6.0 ± 1.1 | 0.005 |
| Serum albumin (g/dl) | 3.1 ± 0.7 | 2.7 ± 0.8 | 0.001 |
| Total bilirubin (mg/dl) | 7.3 ± 8.6 | 14.2 ± 15.4 | 0.001 |
| Direct bilirubin (mg/dl) | 5.1 ± 6.4 | 8.6 ± 6 | 0.001 |
| AST (IU/L) IQR |
211 (117–700) | 286 (152–758) | 0.004 |
| ALT (IU/L) IQR |
236 (109–480) | 315 (128–731) | 0.04 |
| ALP(IU/L) IQR |
176 (123–276) | 190 (130–315) | 0.40 |
| GGT (IU/L) IQR |
132 (64–296) | 101 (56.207) | 0.40 |
| Serum creatinine (mg/dl) | 0.9 ± 0.8 | 1.4 ± 1 | 0.001 |
| INR | 1.5 ± 1.2 | 2.9 ± 2.5 | 0.001 |
| HB (g/dl) | 11.3 ± 2.1 | 10.8 ± 2 | 0.015 |
| WBC (10/dl) IQR |
8500 (6200–11100) | 9320 (7000–13480) | 0.50 |
| Neutrophils (%) | 68 ± 15 | 73 ± 13 | 0.005 |
| Platelets (105/dl) | 2.4 ± 1.1 | 2.1 ± 1.3 | 0.008 |
| MELD | 16.8 ± 7.5 | 28.3 ± 10.4 | 0.001 |
| Females | 47.6% | 55.6% | 0.064 |
| Admission | 65.7% | 88.3% | 0.001 |
| Jaundice | 63.9% | 88.8% | 0.001 |
| Skin rashes | 20.3% | 15.3% | 0.15 |
| Encephalopathy | 4.1% | 52.7% | 0.001 |
| Ascites | 12.5% | 42.7% | 0.001 |
| Alcohol | 10% | 17% | 0.16 |
| Diabetes | 6% | 8.5% | 0.28 |
| Severe disease | 23% | 93.5% | 0.001 |
Abbreviations: ATD: anti-tuberculosis drugs, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, BMI: Body Mass Index, GGT: gamma glutamyl transferase, INR: International normalized ratio, IQR: inter quartile range, MELD: Model for end stage liver disease, WBC: white blood cells.
Univariate analysis identified the following variables to be independently associated with mortality: age (p < 0.001), ascites (p < 0.001), HE (p < 0.001), serum albumin (p < 0.001), bilirubin (p < 0.001), transaminases (p < 0.04), creatinine (p < 0.001), INR (p < 0.001), MELD score (p < 0.001), and anti-TB drugs (p = 0.02).
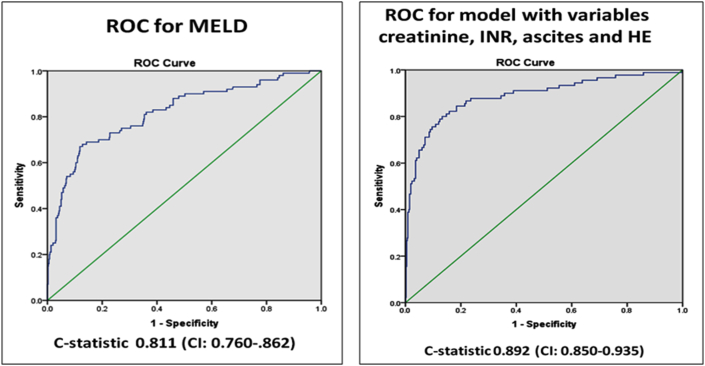
On multivariate analysis only serum creatinine (p = 0.017) (95% CI: 1.06–1.91), INR (p < 0.001) (95% CI: 1.18–1.97), HE (p < 0.001) (95% CI: 5.3–22.8), and ascites (p = 0.002) (95% CI = 1.5–5.9), were significantly associated with mortality. ROC yielded a C-statistic of 0.811 (CI: 0.760-.862) for MELD and 0.892 (CI: 0.850–0.935) for combination of serum creatinine, INR, ascites and HE (Figure 2). Admission MELD score of 19 at presentation was noted to have sensitivity and specificity for mortality of 81% and 65% respectively.
Figure 2.
Receiver operator curve for MELD and combination of factors (creatinine, INR, ascites and hepatic encephalopathy).
Drug-induced acute liver failure
We then analyzed the subset of 124 patients who exhibited features of idiosyncratic drug-induced ALF. Characteristics of patients with and without ALF are illustrated in Table 4. There was a trend towards a greater proportion of women in the ALF group (p = 0.06). Treatment duration was not significant. Of the 124 with idiosyncratic drug-induced ALF, 43 (35.2%) survived and 79 (64.8%) died. MELD score was 28.5 in non-survivors compared to 17 in survivors (? p value). Principal drug classes causing ALF were as follows: anti-TB drugs (N = 78; 63%), CAM (N = 15; 12.1%), AED (N = 10; 8.1%; CNS agents (n = 4; 3.2%), dapsone (n = 3; 2.4%) ADM (N = 1; 0.8%), Antifungal (n = 1; 0.8%), ART (N = 4; 3.2%), chemotherapeutic (N-1; 0.8%), hormone (N = 1; 0.8%) methotrexate (N = 1; 0.8%) NSAID (N = 1; 0.8%), Statin (n = 2; 1.6%) unknown (n = 1; 0.8%).
Table 4.
Clinical and Laboratory Characteristics of Patients With and Without ALF.
| Encephalopathy (ALF) (n = 124; 10%) |
No Encephalopathy (No ALF) (n = 1150; 90%) |
P value | |
|---|---|---|---|
| Females n (%) | 71 (57.3%) | 539 (48.3%) | 0.06 |
| Jaundice n (%) | 116 (93.5%) | 720 (64.7%) | 0.001 |
| Skin rashes n (%) | 20 (16.5%) | 217 (19.9%) | 0.38 |
| Ascites n (%) | 54 (43.5%) | 149 (13.4%) | 0.001 |
| Non-Survivors n (%) | 64.8% | 6.6% | 0.001 |
| Age (years) | 49 ± 16.2 | 42 ± 16.4 | 0.001 |
| Duration of treatment (days) IQR |
32 (12–105) | 27 (11–60) | 0.46 |
| Weight (kg) | 57.5 ± 14.9 | 55.2 ± 13.5 | 0.22 |
| Serum total protein (g/dl) | 6 ± 1 | 6.6 ± 2.6 | 0.010 |
| Serum albumin (g/dl) | 2.6 ± 0.7 | 3.1 ± 0.7 | 0.001 |
| Total bilirubin (mg/dl) | 15.5 ± 16.7 | 7.7 ± 8.8 | 0.001 |
| Direct bilirubin (mg/dl) | 9 ± 5.9 | 5.3 ± 6.6 | 0.001 |
| AST (IU/L) IQR |
342 (170–981) | 210 (104–505) | 0.001 |
| ALT (IU/L) IQR |
312 (162–713) | 235 (104–505) | 0.014 |
| ALP (IU/L) IQR |
187 (132–315) | 176 (122–279) | 0.37 |
| GGT (IU/L) IQR |
101 (53–214) | 132 (64–296) | 0.52 |
| Serum creatinine (mg/dl) | 1.3 ± 1.1 | 0.9 ± 0.8 | 0.003 |
| INR | 3 ± 2.2 | 1.5 ± 1 | 0.001 |
| Hemoglobin (g/dl) | 10.7 ± 2.2 | 11.2 ± 2.1 | 0.027 |
| WBC (10/dl) IQR |
9400 (7000–14850) | 8565 (6200–11200) | 0.051 |
| Neutrophils (%) | 71.3 ± 17 | 68 ± 14 | 0.068 |
| Platelets (105/dl) | 2.1 ± 1.1 | 2.9 ± 1.1 | 0.040 |
| MELD | 28.5 ± 10 | 17 ± 7.5 | 0.001 |
Abbreviations: ATD: anti-tuberculosis drugs, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, BMI: Body Mass Index, GGT: gamma glutamyl transferase, INR: International normalized ratio, IQR: inter quartile range, MELD: Model for end stage liver disease, WBC: white blood cells.
In the drug-induced ALF cohort the mean MELD score in survivors was 20.2 ± 7 9, as compared to 30.7 ± 9.9 in non-survivors (p < 001). Although ascites, bilirubin, INR, creatinine and MELD were significant on univariate analysis, only MELD was noted to be a significant predictor of mortality on multivariate regression (p < 0.001). ROC curve yielded an area under curve of 0.829 (95% CI: 0.743–0.915) (data not shown).
CAM and DILI
Table 5 shows the characteristics of CAM vs prescription drugs. More men than women were linked to CAM associated DILI. DILI secondary to CAM was associated with more severe disease as illustrated by greater frequency of jaundice, ascites and higher MELD scores. CAM, very often consists of undeclared constituents in the form of powders, pastes, leaves, barks and tablets. Very often, a single tablet consists of a number of ingredients and in 1 instance up to 49 ingredient. Furthermore, our case record form does not have provision to capture individual drug or component details. Only in very few instances was the prescription for CAM traced and in one patient with marked jaundice the following combination was identified: kanchhar Guggul, punar nawashtak vati, pratap lankeshwar vati, abhipattikar churna, sup shekhar vati, and triphala churna. However, outcome (mortality) was similar between the CAM (13.6%) and prescription medicine (12%) cohorts.
Table 5.
Characteristics of Patients With Complementary and Alternative Medication (CAM) -Induced DILI vs DILI From Prescription Medications.
| CAM | Prescription drugs | P value | |
|---|---|---|---|
| Gender (Females) (%) | 38.7 | 50.3 | 0.004 |
| Admitted (%) | 69.4 | 67.8 | 0.73 |
| Jaundice (%) | 87.7 | 64.3 | 0.001 |
| Skin rashes (%) | 16.7 | 20 | 0.324 |
| Encephalopathy (%) | 8.4 | 10.3 | 0.433 |
| Ascites (%) | 21.8 | 15.5 | 0.034 |
| Died (%) | 13.6 | 12 | 0.569 |
| Age (years) | 41.7 ± 14.8 | 42.9 ± 16.8 | 0.31 |
| Duration of treatment (days) IQR | 21 (6–62) | 27 (12–60) | 0.95 |
| Weight (kg) | 61.7 ± 11.3 | 54.6 ± 13.7 | 0.001 |
| BMI | 23.4 ± 4.2 | 21.9 ± 4.5 | 0.028 |
| Serum total protein (g/dl) | 7.3 ± 5.9 | 6.4 ± 1.1 | 0.069 |
| Serum albumin (g/dl) | 3.3 ± 0.7 | 3.1 ± 0.7 | 0.004 |
| Total bilirubin mg/dl) | 15.6 ± 12 | 7.1 ± 9.1 | 0.001 |
| Direct bilirubin (mg/dl) | 10.3 ± 8.8 | 4.8 ± 5.7 | 0.001 |
| AST IU/L IQR |
235 (133–445) | 215 (118–435) | 0.347 |
| ALT IU/L QIR |
224 (85–562) | 242 (115–513) | 0.439 |
| ALP IU/L IQR |
171 (128–255) | 181 (122–292) | 0.945 |
| GGT IU/L IQR |
80 (42–162) | 139 (167–320) | 0.001 |
| Serum creatinine (mg/dl) | 1.2 ± 1.3 | 1 ± 0.7 | 0.050 |
| INR | 1.6 ± 0.6 | 1.7 ± 1.6 | 0.032 |
| Hemoglobin (g/dl) | 11 ± 2.2 | 11.2 ± 2.1 | 0.470 |
| WBC (10/L) IQR |
9200 (7250–11800) | 5800 (6190–11400) | 0.018 |
| Platelets (105/L) | 2.1 ± 0.9 | 2.4 ± 1.2 | 0.001 |
| MELD | 21.8 ± 7.2 | 18.2 ± 9 | 0.001 |
Abbreviations: ATD: anti-tuberculosis drugs, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, BMI: Body Mass Index, CAM: Complimentary alternative medicine GGT: gamma glutamyl transferase, INR: International normalized ratio, IQR: inter quartile range, MELD: Model for end stage liver disease, WBC: white blood cells.
Discussion
In this prospective nationwide DILI study from India, anti-TB drugs followed by complementary and alternative medicines and anti-epileptic drugs were the top 3 classes causing DILI. Together with anti-microbials, CAM, AED, anti-metabolites and ART they accounted for 85% of cases. Ten percent of all cases of DILI presented as or progressed to ALF. The overall 3-month mortality was 12% while mortality from NSAID was none and from non-anti-TB anti-microbials was 6%. Presence of clinically apparent jaundice itself was associated with a mortality of 16%%. Hy's law or clinical jaundice and MELD were predictors of mortality. Although more men than women developed DILI, females seemed to have a higher risk of dying from DILI. Worse survival in women than men has been described in paracetamol,17 amoxicillin-clavulanate18 and anti-tuberculosis hepatotoxicity.19 The reasons for increased severity of DILI in women are far from clear, and could relate to greater use of hepatotoxic medications in women,20 and sex based differences in drug metabolism21 and to innate immune response. Furthermore, weight based dosing could result in higher concentration and drug exposure in women. The low mortality of 6% associated with non-anti-TB antimicrobials is similar to the 6% mortality described by Bjornsson and Olsson in a Swedish series.22
Unlike the Western experience, direct (predictable) DILI from paracetamol (acetaminophen) toxicity represented <1% of cases of DILI. Despite its wide and easy availability, paracetamol related DILI is distinctly rare in India. Anti-TB drugs were the major cause of idiosyncratic DILI accounting for 46.4% of cases. This is similar to prior single center series and reflects the disease burden of TB in India. With India home to 22.7% of the world's TB burden23 and with ~5 of all patients with TB developing DILI of any severity,24 it is not surprising that anti-TB DILI is the commonest cause of both DILI and drug-induced ALF in the country. In the reported Spanish and American series, anti-microbials, particularly amoxicillin-clavulanate,6 are the commonest drugs causing idiosyncratic DILI followed by isoniazid in the DILIN series, when used as primary prophylaxis of TB. This is in contrast to India where combination anti-TB drugs for therapy of TB disease are a major cause. Anti-TB DILI is associated with higher than expected mortality due to the greater number of patients presenting with advanced disease such as jaundice, hyperbilirubinemia, ascites, encephalopathy, and MELD score (data not shown).
CAM was the second most common cause of DILI. This picture mirrors the worldwide experience6 and given the ubiquitous use of CAM for all kinds of diseases and promotion of wellness, is not surprising. Unlike the experience in the rest of the world, more men (61%) than women (39%) developed CAM induced DILI (p = 0.004). Although liver injury was more severe than in non-CAM patients (as reflected by bilirubin and MELD score), mortality was similar.
Contrary to reports from the West, our study did not identify age or female gender as risk factors for DILI. Our patients were much younger, with a mean age of 42 years, as compared to 49, 53 and 58 years from USA,6 Spain,10 and Sweden,22 respectively. This is because of the unique demographic characteristics of India with a median age of the population estimated at 28.4 years (statista.com) and also because of the predominance of TB afflicted population in the age group of 35–45 (WHO 2017). Furthermore, the agents responsible for DILI reflect the disease and prescription patterns unique to the Indian population. For example the use of first generation anti-epileptic drugs such as phenytoin, carbamazepine and phenobarbitone because of the low cost and decades of experience lends itself to a higher rate of adverse effects compared to low or negligible risk of hepatotoxicity with newer agents such as levetiracetam and clobazam.25
Details of clinical outcome were available in 1263 patients; of whom 156 (12.4%) died. The presence of clinically apparent jaundice also implied a higher risk of mortality (16.6%), which was similar to the observed mortality in patients fulfilling the Hy's law (17.4%).
The drugs causing ALF mirrored the overall DILI cohort, with anti-TB DILI, CAM and AED being the top 3 drugs. However, anti-TB DILI constituted almost three fourths of cases of DILI-ALF, indicating a propensity to progress to severe disease in anti-TB drug-related liver injury. These results are similar to the experience of previous single center reports.26, 27, 28 It is intuitive to link severity of injury with mortality. Indices of liver function severity such as albumin, bilirubin, INR, and severity index such as MELD score were, not surprisingly, significantly increased in non-survivors. Furthermore, patients that fulfilled severity criteria of international working group12 were also at risk of dying from DILI. These parameters may be used to educate patients and caregivers about DILI and the need for early diagnosis and prompt discontinuation of the offending agent, expedited transfer to centers that perform liver transplantation or potential consideration of treatment options with reported efficacy such as plasmapheresis, in cases of severe DILI.
Admittedly, our study has limitations. Two thirds of our INDILI subjects were enrolled from teaching hospitals and tertiary referral centers. The resulting heterogeneity of patient care across centers needs to be taken into consideration. In addition, details of treatment received were not available in all cases, as this was not captured fully in the case record form. It is likely that patients with severe disease who were extensively worked up were preferentially recruited. Our study is not population based but we believe the causes of DILI are reflective of national trends although there may be regional variations. Since India is home to over a quarter of the world's TB and since the drugs used to treat TB are potentially hepatotoxic, it is not surprising that anti-TB DILI constitutes a major proportion of the patients. Another limitation is the difficulty in determining the type, nature and constituents of CAM, which very often consists of undeclared constituents in the form of powders, pastes, leaves, barks and tablets. Furthermore, our case record form did not have the provision to capture individual drug or component/ingredient in detail. Only in very rare instances was the prescription for CAM traced. The challenges encountered in identifying and analyzing CAM has been highlighted in a recent publication where CAM was the leading cause of drug-induced ACLF in Asia.29 Regardless, the association of liver injury from a common formulation has recently been highlighted from a western series.30 A further limitation is non-feasibility of determining chronic DILI given the lack of long term follow up. Our strengths include the prospective nature of the study with contributions from all regions of the country (see Figure 1).
Authors contribution
HD: Designed, initiated, and supervised the study, enrolled patients, analyzed, updated, and interpreted the data, and drafted, edited, and approved the final draft of the paper. TJ, VVR, MP: supervised the study, enrolled, updated and interpreted the data and approved the final draft of the paper. SPS: Designed, initiated and supervised the study, enrolled patients, revised the manuscript for intellectual content and approved the final draft of the paper. RS: performed statistical analysis, interpreted the data, and revised the manuscript for intellectual content, and approved the final draft of the paper. NSK, JV, VT, BG, GB, PR, RD, AN, S: enrolled patients, revised the manuscript for intellectual content and approved the final draft of the paper. CR, SPS, PS, AG, CEE, AA, VL, GG, RP, ST, AK, PNR, SSK, AKD, JV, AKJ, MW, PR, DJ, PG, SN, GKD, ACK, AJ, PR: enrolled patients, and approved the final draft of the paper.
Conflicts of interest
The authors have none to declare.
Acknowledgements
The authors would like to thank Ms. Edel Quinn for secretarial assistance, Dr. Mohanraj and all fellows in Gastroenterology St. John's Medical College Hospital for data management during the period of the study.
Financial disclosure
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2020.11.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sgro C., Clinard F., Ouazir K. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36:451–455. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- 2.Bjornsson E.S., Bergmann O.M., Bjornsson H.K. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–1425. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Suk K.T., Kim D.J., Kim C.H. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107:1380–1387. doi: 10.1038/ajg.2012.138. [DOI] [PubMed] [Google Scholar]
- 4.Shen T., Liu Y., Shang J. Incidence and Etiology of drug-induced liver injury in mainland China. Gastroenterology. 2019;156:2230–2241. doi: 10.1053/j.gastro.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Ostapowicz G., Fontana R.J., Schiodt F.V. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 6.Chalasani N., Bonkovsky H.L., Fontana R. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148:1340–1352. doi: 10.1053/j.gastro.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devarbhavi H., Dierkhising R., Kremers W.K. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–2404. doi: 10.1038/ajg.2010.287. [DOI] [PubMed] [Google Scholar]
- 8.Rathi C., Pipaliya N., Patel R. Drug induced liver injury at a tertiary hospital in India: Etiology, clinical features and predictors of mortality. Ann Hepatol. 2017;16:442–450. doi: 10.5604/16652681.1235488. [DOI] [PubMed] [Google Scholar]
- 9.Philips C.A., Paramaguru R., Joy A.K. Clinical outcomes, histopathologic patterns and chemical analysis of ayurveda and herbal medicine associated with severe liver injury- A single center experience from South India. Indian J Gastroenterol. 2018;37:9–17. doi: 10.1007/s12664-017-0815-8. [DOI] [PubMed] [Google Scholar]
- 10.Andrade R.J., Lucena M.I., Fernandez M.C. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Kamath P.S., Wiesner R.H., Malinchoc M. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 12.Aithal G.P., Watkins P.B., Andrade R.J. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89:806–815. doi: 10.1038/clpt.2011.58. [DOI] [PubMed] [Google Scholar]
- 13.Danan G., Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 14.O'Grady J.G., Schalm S.W., Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273–275. doi: 10.1016/0140-6736(93)91818-7. [DOI] [PubMed] [Google Scholar]
- 15.Temple R. Hy's law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15:241–243. doi: 10.1002/pds.1211. [DOI] [PubMed] [Google Scholar]
- 16.Senior J.R. How can 'Hy's law' help the clinician? Pharmacoepidemiol Drug Saf. 2006;15:235–239. doi: 10.1002/pds.1210. [DOI] [PubMed] [Google Scholar]
- 17.Rubin J.B., Hameed B., Gottfried M. Acetaminophen-induced acute liver failure is more common and more severe in women. Clin Gastroenterol Hepatol. 2018;16:936–946. doi: 10.1016/j.cgh.2017.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.deLemos A.S., Ghabril M., Rockey D.C. Amoxicillin-clavulanate-induced liver injury. Dig Dis Sci. 2016;61:2406–2416. doi: 10.1007/s10620-016-4121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devarbhavi H. Acute liver failure induced by anti-infectious drugs:causes and management. Current Hepatology Reports. 2017;16:276–285. [Google Scholar]
- 20.Kaufman D.W., Kelly J.P., Rosenberg L. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. Jama. 2002;287:337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 21.Amacher D.E. Female gender as a susceptibility factor for drug-induced liver injury. Hum Exp Toxicol. 2014;33:928–939. doi: 10.1177/0960327113512860. [DOI] [PubMed] [Google Scholar]
- 22.Bjornsson E., Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42:481–489. doi: 10.1002/hep.20800. [DOI] [PubMed] [Google Scholar]
- 23.WHO . vol. 2017. 2017. pp. 1–249. (Global Tuberculosis Report). [Google Scholar]
- 24.Tweed C.D., Wills G.H., Crook A.M. Liver toxicity associated with tuberculosis chemotherapy in the REMoxTB study. BMC Med. 2018;16:46. doi: 10.1186/s12916-018-1033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devarbhavi H., Andrade R.J. Drug-induced liver injury due to antimicrobials, central nervous system agents, and nonsteroidal anti-inflammatory drugs. Semin Liver Dis. 2014;34:145–161. doi: 10.1055/s-0034-1375956. [DOI] [PubMed] [Google Scholar]
- 26.Devarbhavi H., Patil M., Reddy V.V. Drug-induced acute liver failure in children and adults: results of a single-centre study of 128 patients. Liver Int. 2018;38:1322–1329. doi: 10.1111/liv.13662. [DOI] [PubMed] [Google Scholar]
- 27.Kumar R., Bhatia V., Khanal S. Antituberculosis therapy-induced acute liver failure: magnitude, profile, prognosis, and predictors of outcome. Hepatology. 2010;51:1665–1674. doi: 10.1002/hep.23534. [DOI] [PubMed] [Google Scholar]
- 28.Devarbhavi H., Singh R., Patil M. Outcome and determinants of mortality in 269 patients with combination anti-tuberculosis drug-induced liver injury. J Gastroenterol Hepatol. 2013;28:161–167. doi: 10.1111/j.1440-1746.2012.07279.x. [DOI] [PubMed] [Google Scholar]
- 29.Devarbhavi H., Choudhury A.K., Sharma M.K. Drug-induced acute-on-chronic liver failure in asian patients. Am J Gastroenterol. 2019;114:929–937. doi: 10.14309/ajg.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 30.Björnsson H.K., Björnsson E.S., Avula B. Ashwagandha-induced liver injury: a case series from Iceland and the US drug-induced liver injury network. Liver Int. 2020;40:825–829. doi: 10.1111/liv.14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.