Abstract
Background
Transarterial chemoembolization (TACE) is the most common locoregional therapy for hepatocellular carcinoma (HCC). Postembolization syndrome is not an uncommon complication. At present, there is no specific treatment for management of this complication. We aimed to study the role of N-acetyl cysteine (NAC), an antioxidant, in management of this complication.
Methods
In a prospective observational study, consecutive patients with HCC undergoing TACE from January 2016 to January 2017 were included. Patients with postembolization syndrome, defined as an elevation of transaminase levels more than 3–4 times the upper limit of normal, were administered intravenous NAC for 72 h (150 mg/kg for 1 h, then 12.5 mg/kg/h for 4 h, and continuous infusion 6.25 mg/h for the remaining 67 h). The other group received only supportive standard of care. The primary end point was reduction in post-TACE transaminitis.
Results
Of 112 patients with HCC, 53 (47.3%) received NAC. The majority were cirrhotics in both the groups. Both groups were well matched in demographic, laboratory, and tumor characteristics. In the NAC group, there was significant reduction in Aspartate transaminase (AST) and Alanine transaminase (ALT) levels from day 1 to day 3 (p = 0.000) compared with the non-NAC group, with no significant change in bilirubin or international normalized ratio levels. The duration of hospital stay was similar in both the groups. None had any major adverse events to NAC.
Conclusion
This is a prospective, single-center experience, showing that early initiation of N-acetyl cysteine in those with post-TACE embolization syndrome reduces the transaminase level significantly.
Keywords: chronic liver disease, liver cancer, liver transplant
Abbreviations: AFP, alpha-fetoprotein; ANOVA, analysis of variance; BCLC, Barcelona Clinic Liver Cancer; CT, computed tomography; HCC, hepatocellular carcinoma; IL, interleukin; INR, international normalized ratio; LT, liver transplantation; NAC, N-acetyl cysteine; PES, postembolization syndrome; TACE, Transarterial chemoembolization
Hepatocellular carcinoma (HCC) is the sixth most common neoplasm and the third most frequent cause of cancer death.1 HCC is also the leading cause of death in compensated cirrhosis.2 The age-adjusted incidence rate of HCC in India for men ranges from 0.7 to 7.5 and for women ranges from 0.2 to 2.2 per 100,000 population per year.3
The various therapeutic options available for management of HCC and their outcome depend on tumor characteristics in the form of size, the number of lesions, vascular invasion, host characteristics such as Child's status of liver disease, and the overall performance status of the patient.4 While curative resection and liver transplantation (LT) are the optimal treatment modalities for Barcelona Clinic Liver Cancer (BCLC) stage 0 and A disease, palliation is the only option for BCLC stage B and stage C disease.5 The recommended treatment for the latter two stages is locoregional therapies such as transarterial chemoembolization (TACE) and sorafenib, respectively.6 TACE serves as a bridge to LT to downstage or maintain lesions within the transplant criteria while awaiting a deceased donor liver.7 The procedure also has a potential therapeutic role as an adjuvant procedure for postoperative tumor recurrence.8 As palliative care in unresectable HCC, compared with supportive care alone, TACE has been shown to improve overall survival at one year from 32% to 57%.9 In a recent study from our center, we have shown that TACE in combination with sorafenib in BCLC stage C disease improved the overall survival from 4 months to 9 months.10
Although TACE is relatively safe, postembolization syndrome (PES) is a common adverse reaction,11 manifesting as fever, right upper quadrant discomfort, and mainly significant transaminitis. Less frequent symptoms are nausea and vomiting.12 In an elegant study by Mason et al.,13 PES was reported in 48.6% patients after the first TACE. Furthermore, PES was also associated with reduction in the median overall survival from 25 months to 16 months, with a twofold increase in death rate.13 In the pathogenesis of post-TACE hepatic injury, there is essentially hepatocyte apoptosis or necroptosis. The fading hepatocytes release damage-associated molecular patterns that are recognized by immune cells in the liver as ‘danger’ signals leading to a proinflammatory response.14 There is also release of reactive oxygen species, which further drives the inflammatory cascade and can even precipitate post-TACE liver decompensation.15 To circumvent these inflammatory cascades, today there are some emerging data that antioxidants such as N-acetyl cysteine (NAC) may have a beneficial role.16
With this background, we undertook this study to determine the efficacy of NAC in managing transaminitis after TACE.
Patients and methods
Patient Population
The patient population of this study consisted of subjects with HCC, enrolled prospectively into this open-label study over a one-year period. This single-center study protocol conformed to the Declaration of Helsinki and was approved by the institutional ethics committee. An informed written consent was taken from each patient at the time of enrollment. All authors had access to the study data and have reviewed and approved the final manuscript.
Inclusion Criteria
Patients with HCC, registered in the outpatient department at Gleneagles Global Health City, Chennai, India, from January 2016 to January 2017 were included in this prospective study. Diagnosis of HCC was based on classical findings of arterial enhancement with contrast and an “early washout” during a triple-phase imaging, either by contrast-enhanced computed tomography (CT) or by magnetic resonance imaging.17 Patients with alcohol-related liver disease were advised strict alcohol abstinence, and those with cirrhosis due to viral etiology were continued on antiviral therapy.
Exclusion Criteria
Patients with a Child Turcotte Pugh (CTP) score higher than 8, a total bilirubin level of >3 mg/dL, renal insufficiency with a serum creatinine level of ≥2 mg/dL or creatinine clearance <30 mL/min, significant cardiopulmonary comorbidity, and presence of any other illness that significantly affected survival were excluded.18 Others excluded were patients with advanced tumor characteristics19 such as tumor involving both lobes of the liver, significant arterioportal shunting, large intrahepatic arteriovenous fistula, complete thrombosis of the main portal vein, and evidence of extrahepatic spread and those not willing for TACE or for participation in the study.
Methodology
All patients underwent baseline routine biochemical tests, including complete blood count, liver biochemistry, kidney function tests, international normalized ratio (INR), and alpha-fetoprotein (AFP) estimation. Tumor characterization was performed by contrast-enhanced CT of the abdomen—triple-phase imaging. Enrollment of participants, assessing eligibility, and obtaining consent was carried out by one of the two authors (C.K.K. or S.B.).
After enrollment, patients were assigned to the NAC group if the transaminase levels increased to more than 3 to 4 times the upper limit of normal on day 1 after the procedure. NAC was given intravenously for 72 h at a dose of 150 mg/kg for 1 h followed by 12.5 mg/kg/h for 4 h, then continued at a dose of 6.25 mg/h for the remaining 67 h or until discharge. The remaining age- and gender-matched patients who had TACE during the same period of time were allotted to the non-NAC group. All patients in the study received standard-of-care treatment that included analgesics such as intravenous paracetamol for abdominal pain or fever and intravenous fluids and antiemetics for nausea or vomiting.
Follow-up
Both groups were followed up daily for the next three days or until discharge via liver biochemistry, creatinine, and INR analysis. The patients were asked to maintain a record for any adverse effects, such as pain in the abdomen, vomiting, joint pain, or skin rash.
Primary and Secondary Outcomes
The primary outcome was a noticeable reduction in transaminase levels. The secondary outcomes were as follows: (i) clinical symptomatology, (ii) change in bilirubin and INR levels, (iii) length of hospital stay, and (iv) safety of treatment. Those patients lost to follow-up or who withdrew from the study were censored during the analysis.
Statistical Analyses
The study was time bound. Normally distributed continuous variables were expressed as mean (standard deviation), and the continuous variables with skewed distribution were expressed as median (interquartile range). The chi-square (or Fisher's exact) test compared differences between the groups for categorical variables. Changes in continuous measures between baseline and post-treatment levels were tested by means of the paired t-test, whereas the Wilcoxon signed-rank test was used for nonparametric data. The differences in the changes between the groups were tested using the independent samples t-test, whereas the Mann-Whitney test was used for non-normally distributed data. We compared the change in different liver biochemical parameters between the two treatment groups at different time intervals using the repeated measures analysis of variance (ANOVA) test, followed by post hoc comparison using the Least significant difference (LSD) method. A P-value of <0.05 was considered significant. All statistical tests were performed using SPSS for Windows version 20 (SPSS Inc., Chicago, IL).
Results
A total of 365 patients with HCC were registered in the outpatient clinic during the study period; 112 patients participated in the study (Figure 1). A total of 53 patients received NAC (NAC group), and the remaining 59 patients constituted the non-NAC group. None were lost to follow-up. Table 1 summarizes the baseline characteristics in the two groups. Most patients (NAC group versus non-NAC group) had an underlying cirrhosis (88.7% versus 96.6%). Viral etiology was more common than nonviral etiology. Among those with viral etiology, chronic hepatitis B and C were seen in almost similar proportions. Among those with nonviral etiology, nonalcoholic steatohepatitis/cryptogenic disease was the predominant etiology (NAC group, 22 [41.5%] versus non-NAC group, 23 [39%]). Laboratory data showed well-preserved liver functions in both the groups.
Figure 1.
Flow diagram of the patients in the study. NAC, N-acetyl cysteine; TACE, transarterial chemoembolization; AST, Aspartate transaminase; ALT, Alanine transaminase; CTP, Child Turcotte Pugh.
Table 1.
Baseline Characteristics of the Patients in the Two Groups.
| Parameter | NAC group (N = 53) | Non-NAC group (N = 59) | P value |
|---|---|---|---|
| Age (in years) | 60.6 ± 8.4 | 62 ± 7.9 | 0.4 |
| Male gender | 40 (92.5%) | 50 (84.7%) | 0.2 |
| Cirrhotics | 47 (88.7%) | 57 (96.6%) | 0.1 |
| Etiology of liver disease | |||
|
15 (28.3%) | 14 (23.7%) | 0.2 |
|
16 (30.2%) | 17 (28.8%) | |
|
22 (41.5%) | 28 (47.5%) | |
| Diabetes mellitus | 24 (45.3%) | 29 (49.2%) | 0.7 |
| Tumor characteristics | |||
| |||
| One | 29 (54.7%) | 35 (59.3%) | |
| Two | 10 (18.9%) | 13 (22%) | 0.8 |
| Three | 3 (5.7%) | 1 (1.7%) | |
| More than three | 11 (20.8%) | 10 (16.9%) | |
|
5.7 ± 3.2 | 4.9 ± 3.2 | 0.2 |
|
12 (22.6%) | 15 (25.4%) | 0.7 |
| Platelet count (× 106/mm3) | 1.78 ± 1.3 | 1.26 ± 0.77 | 0.06 |
| Total bilirubin (mg/dl) | 1.2 ± 0.7 | 1.6 ± 0.53 | 0.2 |
| Direct bilirubin (mg/dl) | 0.44 ± 0.45 | 0.54 ± 0.53 | 0.3 |
| AST (IU/L) | 84.5 ± 45.5 | 63.6 ± 24.9 | 0.05 |
| ALT (IU/L) | 59.9 ± 38.5 | 50.6 ± 32.7 | 0.17 |
| ALP (IU/L) | 185.4 ± 148.5 | 155.8 ± 69.9 | 0.2 |
| Albumin (gm/dl) | 3.3 ± 0.65 | 3.2 ± 0.5 | 0.8 |
| INR | 1.2 ± 0.14 | 1.3 ± 0.2 | 0.1 |
| Serum creatinine (mg/dl) | 0.95 ± 0.3 | 0.93 ± 0.3 | 0.8 |
| Alpha-fetoprotein (ng/ml) | 33.5 (IQR = 4.6–823) | 29.1 (IQR = 5.3–951) | 0.14 |
NAC, N-acetyl cysteine; IQR, interquartile range; INR, international normalized ratio; ALP, Alkaline phosphatase; AST, Aspartate transaminase; ALT, Alanine transaminase.
Primary Outcome
After TACE, the AST levels (mentioned as median with interquartile range) in the NAC group reduced significantly from 409 (281.5–901.5) IU/L on day 1 to 196 (111.5–330.5) IU/L on day 3 (p < 0.05). However, in the non-NAC group, the AST levels increased from 116 (82–165) IU/L on day 1 to 165 (115.7–226.5) IU/L on day 3 (p = NS). By using the repeated measures ANOVA test, it was found that the reduction in AST levels from day 1 to day 3 was statistically significant in terms of NAC use (p = 0.000) (Figure 2).
Figure 2.
Change in AST levels at each time points between the two groups. NAC, N-acetyl cysteine; AST, Aspartate transaminase.
The median ALT levels also reduced significantly from 271 (163.5–574.5) IU/L on day 1 to 112 (88–210) IU/L on day 3 (p < 0.05), whereas there was marginal increase in these levels in the non-NAC group (from 99 [50–139] IU/L on day 1 to 134.5 [104.7–220.5] IU/L on day 3 [p = NS]). The reduction in ALT levels from day 1 to day 3 was statistically significant in terms of NAC use (p = 0.000) (Supplementary Figure 1).
Secondary Outcomes
Change in Bilirubin and INR Levels
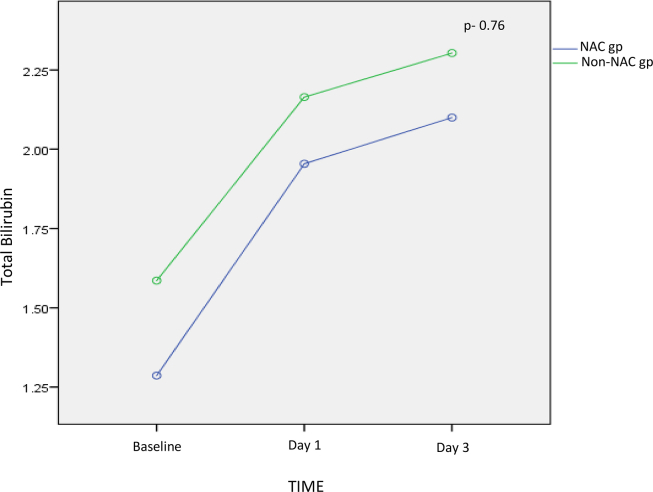
In the NAC group, the mean total bilirubin levels were 1.95 ± 0.94 mg/dl on day 1 and 2.1 ± 0.89 mg/dl on day 3. In the non-NAC group, these were 2 ± 1.03 mg/dl on day 1 and 2.3 ± 1.2 mg/dl on day 3. By using the repeated measures ANOVA test, it was found that there was no statistical change in total bilirubin levels from day 1 to 3 with regard to use of NAC (P = 0.76) (Supplementary Figure 2). The trend remained the same with direct bilirubin levels as well.
In the NAC group, the INR was 1.4 ± 0.13 and 1.3 ± 0.23 on day 1 and day 3, respectively. Similarly, in the non-NAC group, this was 1.4 ± 0.1 and 1.1 ± 0.12 on day 1 and day 3, respectively. After repeated measures ANOVA, use of NAC showed no difference in INR values from day 1 to day 3 (P = 0.4).
Duration of Hospital Stay
The mean duration of in-hospital stay in patients receiving NAC was 2.4 ± 1.1 days, and in the non-NAC group, this was 2.2 ± 2.1 days. The difference was not statistically significant (P = 0.5).
Symptomatology
Between the 2 groups, fever was recorded in 15 (28.3%) and 13 patients (22%). A total of 19 (35.8%) patients in the NAC group had right upper quadrant discomfort, and 5 (9.4%) patients complained of anorexia. In the non-NAC group, abdominal pain needing analgesics was reported in 38 (64.4%), and recurrent vomiting was reported in 3 (5.1%) patients.
Safety of the Therapy
None of the patients had any adverse reaction with NAC, and in addition, there were no untoward complications after TACE procedure.
Discussion
The rationale for TACE is cannulation of the hepatic artery, followed by intra-arterial infusion of a cytotoxic agent such as doxorubicin and then finally embolization of the tumor-feeding blood vessels, with an aim to achieve strong cytotoxic and ischemic effect on the tumor cells.20 However, the procedure is frequently associated with local consequences, mainly in the form of PES. It has been shown that diffuse-type HCC, large tumor size ≥5 cm, lipiodol dose ≥7 mL, and high ALT levels after TACE predicted PES.21 In those with borderline liver functions and large tumor size or multiple tumors, there is also risk of progression of liver disease and post-TACE decompensation. The hepatoma arterial-embolization prognostic (HAP) score based on total bilirubin, albumin, and AFP levels and tumor size has been found to be useful to stratify the risk of post-TACE decompensation.22 In a recent Korean study, the baseline bilirubin level was found to be the single predictor of post-TACE decompensation as per multivariate analysis.23 Hence, conceptually, we can infer that in those with large tumors or multiple tumors or associated cirrhotic background, there will be a poor hepatic reserve, which can get easily overwhelmed if the postembolization sequelae are not treated especially in those with risk factors, leading to progression of liver disease with associated morbidity and mortality.
NAC is the acetylated form of the amino acid l-cysteine. It increases glutathione levels in blood, which acts as an effective antioxidant by attenuating free radical-mediated tissue injury.24 In addition, NAC also has been shown to have anti-inflammatory activity by reducing interleukin (IL) 17 levels.25 It is a recognized antidote for acetaminophen-related toxic hepatitis and liver failure.26 It has also been found to be effective in early non–acetaminophen-related acute liver failure27 and severe alcoholic hepatitis in combination with corticosteroids.28 Recently, intravenous NAC was found to reduce the incidence of PES but not decompensation.16
In our study, we administered NAC in those patients in whom the transaminase levels had increased to more than 3- to 4-fold higher than normal, on day 1, after TACE. In these patients, there was significant reduction of AST and ALT levels. Second, there was no increase in bilirubin or INR levels or were no symptoms of decompensation despite ongoing acute ischemic and cytotoxic hepatitis in these patients, reiterating the beneficial role of early use of NAC. Consequently, the duration of hospital stay was not prolonged in the NAC group. In the non-NAC group, there was no so severe hepatitis after TACE and a similar course of hospital stay. Both groups received supportive treatment for any clinical symptoms after the procedure. None had any obvious adverse events. As seen in patient demographics, most of them were cirrhotics, underscoring the role of NAC in those who had developed severe hepatitis and so possibly preventing decompensation.
The possible limitations of the study are that it was a single-center experience and it had a small patient number. We also had not recorded the dosage of lipiodol administered in the TACE procedure. However, we found that NAC could have a beneficial role in treatment of severe hepatitis after TACE. It would be intriguing to study the levels of inflammatory biomarkers before and after NAC use and whether pre-emptive NAC would have any benefit especially in high-risk groups identified before the procedure. The study was not followed up for further decompensation or mortality.
This was a proof-of-concept study. Further large prospective multicentric randomized placebo controlled studies are needed to validate the use of NAC in post-TACE transaminitis. It will be also be interesting to study the newer molecules such as F-652, a recombinant human IL-22 IgG2 Fc fusion protein,29 or obeticholic acid, FXR agonist,30 in this condition, and whether NAC remains to have an additive role is yet to be seen.
Author Contributions
Chandan K Kedarisetty: Conceptualization, Methodology, original draft preparation. Sipra Bal: Data collection. Subhashree Parida: Data collection. Mayank Jain: Data compilation. Ajeets Bhadoria: Data analysis. Joy Varghese: Supervision. Jayanthi Venkataraman: Reviewing and Editing
Conflicts of interest
The authors have none to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2020.10.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig S1.
Fig S2.
References
- 1.Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: globocan 2008. Int J Canc. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Alazawi W., Cunningham M., Dearden J., Foster G.R. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther. 2010;32:344–355. doi: 10.1111/j.1365-2036.2010.04370.x. [DOI] [PubMed] [Google Scholar]
- 3.Acharya S.K. Epidemiology of hepatocellular carcinoma in India. J Clin Exp Hepatol. 2014;4:S27–S33. doi: 10.1016/j.jceh.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hung H. Treatment modalities for hepatocellular carcinoma. Curr Cancer Drug Targets. 2005;5:131–138. doi: 10.2174/1568009053202063. [DOI] [PubMed] [Google Scholar]
- 5.Llovet J.M., Fuster J., Bruix J of the Barcelona-Clınic Liver Cancer Group The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma liver. Transplantation. 2004;10:S115–S120. doi: 10.1002/lt.20034. [DOI] [PubMed] [Google Scholar]
- 6.Marrero J.A. Multidisciplinary management of hepatocellular carcinoma: where are we today? Semin Liver Dis. 2013;33:S3–S10. doi: 10.1055/s-0033-1333631. [DOI] [PubMed] [Google Scholar]
- 7.Yao F.Y., Kerlan R.K., Hirose R. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819–827. doi: 10.1002/hep.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong Z.R., Zhang P.F., Wang C.H. Postoperative adjuvant transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan criteria: a retrospective analysis. Am J Cancer Res. 2014;5:450–457. [PMC free article] [PubMed] [Google Scholar]
- 9.Lo C.M., Ngan H., Tso W.K. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 10.Varghese J., Kedarisetty C.K., Venkataraman J. Combination of TACE and sorafenib improves survival in BCLC stages B/C of Hepatocellular carcinoma – a single center experience. Ann Hepatol. 2017;16:1–8. doi: 10.5604/16652681.1231583. [DOI] [PubMed] [Google Scholar]
- 11.Pietrosi G., Miraglia R., Luca A. Arterial chemoembolization/embolization and early complications after hepatocellular carcinoma treatment: a safe standardized protocol in selected patients with Child class A and B cirrhosis. J Vasc Intervent Radiol. 2009;20:896–902. doi: 10.1016/j.jvir.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 12.Dhand S., Gupta R. Hepatic transcatheter arterial chemoembolization complicated by postembolization syndrome. Semin Intervent Radiol. 2011;28:207–211. doi: 10.1055/s-0031-1280666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mason M.C., Massarweh N.N., Salami A., Sultenfuss M.A., Anaya D.A. Post embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB. 2015;17:1137–1145. doi: 10.1111/hpb.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malhi H., Gores G.H. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siriwardana R.C., Niriella M.A., Dassanayake A.S. Factors affecting post embolization fever and liver failure after trans-arterial chemoembolization in a cohort without background infective hepatitis – a prospective analysis. BMC Gastroenterol. 2015;15:96. doi: 10.1186/s12876-015-0329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siramolpiwat S., Punjachaipornpon T., Pornthisarn B. N-acetylcysteine prevents post-embolization syndrome in patients with hepatocellular carcinoma following transarterial chemoembolization. Dig Dis Sci. 2019;64:3337–3345. doi: 10.1007/s10620-019-05652-0. [DOI] [PubMed] [Google Scholar]
- 17.Yu N.C., Chaudhari V., Raman S.S. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:161–167. doi: 10.1016/j.cgh.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Omata M., Cheng A.L., Kokudo N. Asia- Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raoul J.L., Sangro B., Forner A. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Canc Treat Rev. 2011;37:212–220. doi: 10.1016/j.ctrv.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Bruix J., Sala M., Llovet J.M. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179–S188. doi: 10.1053/j.gastro.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 21.Jun C.H., Ki H.S., Lee H.K. Clinical significance and risk factors of postembolization fever in patients with hepatocellular carcinoma. World J Gastroenterol. 2013;19:284–289. doi: 10.3748/wjg.v19.i2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadalayil L., Benini R., Pallan L. A simple prognostic scoring system for patients receiving transarterial embolization for hepatocellular cancer. Ann Oncol. 2013;24:2565–2570. doi: 10.1093/annonc/mdt247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park K.H., Kim J.H., Choe W.H. Risk factors for liver function deterioration after transarterial chemoembolization refractoriness in Child Pugh Class A Hepatocellular carcinoma patients. Korean J Gastroenterol. 2020;75:147–156. doi: 10.4166/kjg.2020.75.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bass S., Zook N. Intravenous acetylcysteine for indications other than acetaminophen overdose. Am J Health Syst Pharm. 2013;17:1496–1501. doi: 10.2146/ajhp120645. [DOI] [PubMed] [Google Scholar]
- 25.Stravitz R.T., Sanyal A.J., Reisch J. Effects of N-acetylcysteine on cytokines in non-acetaminophen acute liver failure: potential mechanism of improvement in transplant-free survival. Liver Int. 2013;33:1324–1331. doi: 10.1111/liv.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernal W., Auzinger G., Dhawan A., Wendon J. Acute liver failure. Lancet. 2010;376:190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 27.Lee W.M., Hynan L.S., Rossaro L. Intravenous n-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856–864. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen-Khac E., Thevenot T., Piquet M.A. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365:1781–1789. doi: 10.1056/NEJMoa1101214. [DOI] [PubMed] [Google Scholar]
- 29.Kong X., Feng D., Mathews S., Gao B. Hepatoprotective and anti-fibrotic effects of interleukin – 22: the therapeutic potential for the treatment of alcoholic liver disease. J Gastroenterol Hepatol. 2013;28:56–60. doi: 10.1111/jgh.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas C., Pellicciari R., Pruzanski M., Auwerx J., Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]