Abstract
Background
Percutaneous ablation is an important part of management strategy for liver tumors. While radiofrequency ablation and microwave ablation are the most widely used ablative techniques, cryoablation (CA) has several technical advantages but has been underused till recently. In this study, we report the initial experience with percutaneous CA of liver tumors.
Methods
This was a retrospective evaluation of consecutive patients with liver tumors who underwent percutaneous CA between October 2018 and August 2019. The ablation procedures were performed under combined ultrasound and computed tomography guidance using argon-helium–based CA systems. The baseline tumor characteristics (including size and location), Barcelona Clinic Liver Cancer stage, and Child-Pugh score were recorded. Each patient underwent a follow-up after 1 month and at 3 months subsequently. Technical success, complete response, local tumor progression, and overall survival were evaluated.
Results
Nine patients (mean age, 62.4 years, median age, 66 years, five men and four women) with 10 liver tumors (mean size, 2.22 cm) underwent CA. Seven (77.8%) patients had hepatocellular carcinoma (HCC), and 2 patients had solitary liver metastasis. One patient with HCC had two lesions, while the rest had only one lesion. Of the two metastatic lesions, one was from carcinoma of the cervix and the other was from jejunal neuroendocrine tumor. Five tumors were located adjacent to the gallbladder, two lesions were adjacent to the right portal vein, two lesions were subcapsular, and one lesion was adjacent to the stomach. Technical success was achieved in all the patients. Complete response was achieved in 7 (77.8%) patients. The median follow-up period was 7 months (range, 3–12 months). There was no local tumor progression and no death during the follow-up period. No procedure-related complication was seen.
Conclusion
Percutaneous CA of hepatic tumors is technically feasible and is a safe and effective ablative technique.
Keywords: ablation, cryoablation, hepatocellular carcinoma
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; CA, Cryoablation; CT, Computed Tomography; HCC, Hepatocellular Carcinoma; LTP, Local Tumor Progression; MWA, Microwave Ablation; RFA, Radiofrequency Ablation; US, Ultrasound
The most common primary liver tumor is hepatocellular carcinoma (HCC).1 As most of the HCCs originate in the setting of cirrhosis, resection is possible in only 20–30% of the patients at the time of diagnosis.2 Local ablative therapies are now considered an integral part of management of patients presenting with very early and early-stage HCC, in accordance with the latest Barcelona Clinic Liver Cancer (BCLC) classification.2,3 Because of its rich blood supply, the liver is also the most common site for metastasis.4 Recently, there is a trend for aggressive approach toward solitary or oligometastatic disease in patients with certain malignancies, particularly colorectal metastasis. The preferred method for treating solitary or oligometastatic disease is surgical resection.5 However, when the resection is not possible or deemed risky, ablation may be considered potentially curative.4
There are several local ablative modalities. They are divided into chemical, thermal, and nonthermal methods.2, 6 The most popular among these are the thermal ablation techniques. Thermal techniques are further classified into heat-based (radiofrequency ablation [RFA], microwave ablation [MWA], high intensity focused ultrasound, laser ablation) and cold-based (cryoablation [CA]) techniques. CA has a few advantages as compared with the heat-based modalities.7 These include the ability to see the ice ball formation during the procedure, activation of potent immunological effects, reduced incidence of damage to the blood vessels and other critical structures, and lack of severe pain.7 Despite these advantages, there has been relative underutilization of CA as compared with RFA and MWA. This is due to the concerns regarding serious complications reported in the earlier surgical series.8, 9, 10 However, technical advancements have made CA a safe procedure. CA has now become available in India as well.
We report our initial experience with the CA of liver tumors from a tertiary care interventional radiology centre in North India.
Materials and methods
Patients
We conducted a retrospective evaluation of consecutive patients with liver tumors who underwent percutaneous CA from October 2018 to August 2019. The local ethics committee approved the study, and informed written procedural consent was obtained. Patients having HCC lesions within Milan criteria (i.e. one nodule of size ≤5 cm or up to 3 nodules <3 cm) with no portal vein invasion, extrahepatic metastases, or thrombocytopenia (platelet count <100 × 103/μL) were included. In addition, patients with solitary liver metastasis ≤5 cm were also included. CA was chosen over RFA and MWA due to the subcapsular location of the tumors or due to the vicinity of the lesions to critical structures including the gallbladder or gastrointestinal tract. The tumors that were within 5 mm of the gallbladder, gastrointestinal tract, or a peripheral venous structure ≥3 mm in diameter were included. In addition, tumors located within 3 mm of the liver surface (subcapsular) were included. Lesions located near the liver hilum were excluded. Based on imaging evaluation, the separation of the vital structures from tumor nodules using artificial ascites was not deemed safe or feasible. All these patients could have been treated with irreversible electroporation (IRE) as well but gave consent for CA due to its lower cost and relative ease of the procedure. The diagnosis of HCC was based on multiphasic computed tomography (CT) or magnetic resonance imaging (MRI) in patients with cirrhosis according to the American Association for the Study of Liver Diseases (AASLD) guidelines.11 The diagnosis of metastatic disease was based on ultrasound (US)-guided fine needle aspiration cytology. The presence of multiple metastases was excluded by a 18F-FDG-positron emission tomography scan. The number, size, and location of tumors were recorded. The BCLC stage and the Child-Pugh score were recorded in patients with HCC.
Percutaneous CA procedure
All CA procedures were performed under local anesthesia using combined US and CT guidance. The introduction of cryoprobes was performed under US guidance, while the intraprocedural monitoring was performed using CT fluoroscopy. The commercially available CA system, Endocare Corporation cryosurgery system (Irvine, CA, USA), was used. Based on the tumor size and geometry, single or multiple probes were used. A variable probe (V-probe® cryoprobe, Endocare Corporation) with the shaft diameter of 2.4 mm and length of 15 cm was used in all cases. This probe allows creation of 5 different isotherms ranging in size from 1.5 to 5 cm from the same probe by adjusting the slider. The length of the isotherm in an individual patient was decided based on the size of the lesion. According to the recommended protocol, two freeze-thaw cycles were carried out with an initial freezing for 10 min, followed by passive thawing for 10 min and refreezing for 10 min and then passive thawing. While considering the tumor size, a minimum ablative margin of 0.5 cm beyond the outer most boundary of the tumor was added. Real-time monitoring of the ice ball was performed with US. Intermittently CT was also performed to check the evolving ice ball. The ablation was considered adequate when the ice ball covered the entire tumor along with the margin of the adjacent liver. Immediately after the ablation, contrast-enhanced CT was performed to evaluate vascular and haemorrhagic complications.
Outcome measurement
Technical success was defined as the ability to complete the ablative procedure as planned. Multiphase contrast-enhanced CT was performed one month after the ablation and every 3 months thereafter. The response evaluation was performed using modified response evaluation criteria in solid tumors (mRECIST).12 In patients with residual or recurrent disease, the second line of treatment was planned after discussion with the referring physician.
Results
During the study period, 54 patients underwent percutaneous ablation of liver tumors. Forty-five patients underwent RFA (n = 23), MWA (n = 16), and IRE (n = 6). Nine patients with 10 liver tumors underwent CA (Figure 1, Figure 2, Figure 3). There were 5 men and 4 women. The median age was 66 years (range, 45–76 years; mean, 62.4 years). The demographic characteristics of the subjects are enlisted in Table 1. Seven patients had HCC and two had hepatic metastasis (one from carcinoma of the cervix and one from jejunal neuroendocrine tumor). One patient with HCC had two lesions, while the rest had only one lesion. All patients with HCC had cirrhosis, 4 (57.1%) had Child-Pugh A status, and 3 (42.9%) had Child-Pugh B status. The mean size of tumors was 2.22 cm (range, 1.7–3.2 cm). Based on tumor location, five tumors were located adjacent to the gallbladder, two lesions were located near the right portal vein, two tumors were subcapsular, and one tumor was located adjacent to the stomach.
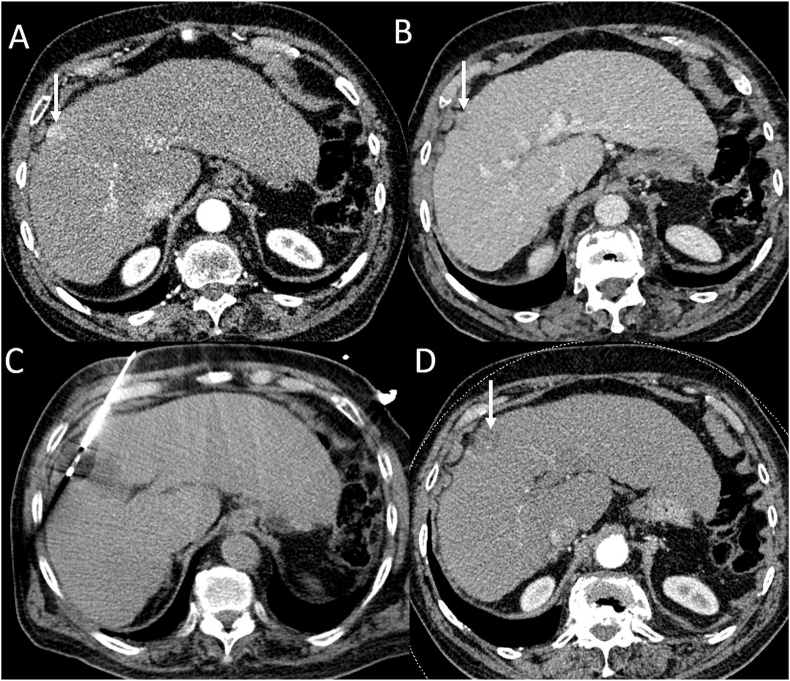
Figure 1.
Cryoablation of subcapsular HCC. (A) Arterial phase CT image shows an arterial enhancing subcapsular nodule in segment 8 (arrow). (B) Portal venous phase CT image shows washout (arrow). (C) CT obtained during the procedure shows a cryoprobe positioned within the lesion. (D) After ablation, CT obtained 1 month after the procedure shows complete ablation. HCC, hepatocellular carcinoma; CT, computed tomography.
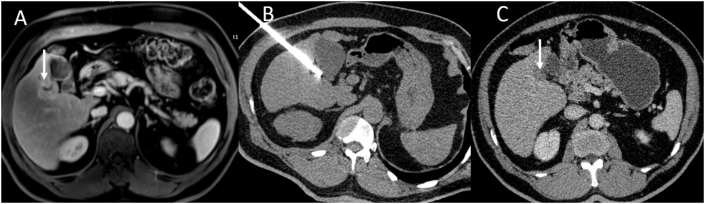
Figure 2.
Cryoablation of HCC near the gallbladder. (A) Arterial phase MRI shows a peripherally enhancing lesion near the gallbladder (arrow). (B) CT obtained during the procedure shows a cryoprobe positioned within the lesion. (C) After ablation, CT obtained 1 month after the procedure shows complete ablation (arrow). HCC, hepatocellular carcinoma; MRI, magnetic resonance imaging; CT, computed tomography.
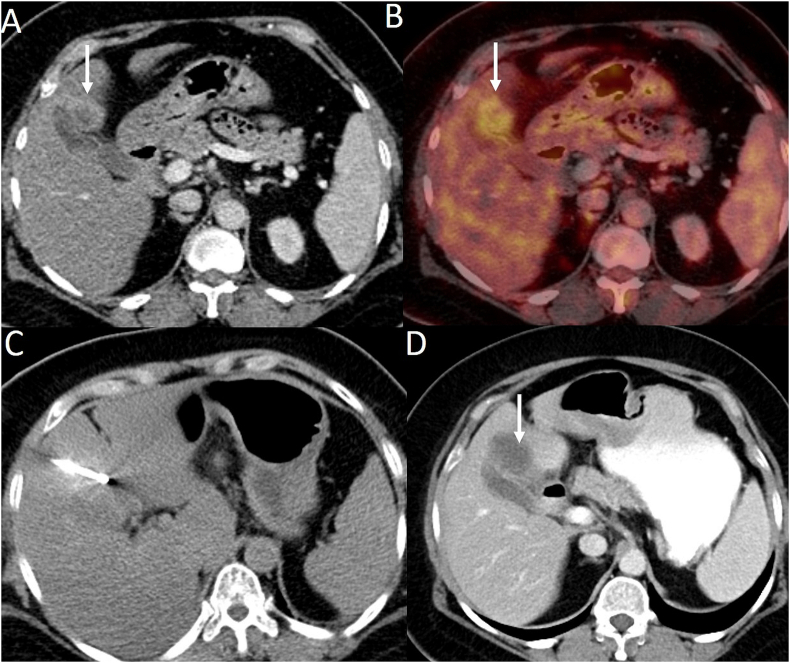
Figure 3.
Cryoablation of metastasis (from carcinoma of the cervix) near the gallbladder. (A) Portal venous phase CT image shows a peripherally enhancing lesion near the gallbladder (arrow). (B) PET-CT image shows avidity in the lesion (arrow). There were no other liver lesions. (C) CT obtained during the procedure shows a cryoprobe positioned within the lesion. (D) After ablation, CT obtained 1 month after the procedure shows complete ablation. PET-CT, positron emission tomography; CT, computed tomography.
Table 1.
Clinical and Demographic Characteristics of the Patients.
| Characteristics | Number |
|---|---|
| Median age in years (range) | 66 (45–76) |
| Male | 5 |
| Female | 4 |
| Type of hepatic lesion (n = 9) | |
| HCC | 7 |
| Metastasis | 2 |
| Primary tumor causing metastasis (n = 2) | |
| Carcinoma cervix | 1 |
| Jejunal neuroendocrine tumor | 1 |
| Chronic liver disease (n = 7) | |
| Hepatitis B virus | 3 |
| Hepatitis C virus | 3 |
| Nonalcoholic steatohepatitis | 1 |
| Child-Pugh class (n = 7) | |
| A | 4 |
| B | 3 |
| α-Fetoprotein (ng/ml) | 393.13 (5.2–852)a |
| Platelet count (lacs/μL) | 1.79 (1.7–2.3)a |
| Total bilirubin (mg/dl) | 1.26 (0.7–2.1)a |
| Albumin (g/dl) | 2.9 (2.4–3.6)a |
| Prothrombin time index (%) | 83.4 (76–94)a |
| Tumor size (cm) | 2.22 (1.7–3.2)a |
| Tumor location (Couinaud segment) (n = 10) | |
| I | 1 |
| III | 1 |
| IVA | 1 |
| IVB/V | 1 |
| V | 5 |
| V/VI | 1 |
HCC, hepatocellular carcinoma.
Values are in mean.
Technical parameters
Technical success was attained in all cases. In 8 patients, a single probe was used for each lesion. Ablation was performed using two cryoprobes for one lesion in one patient. Based on mRECIST, complete response was achieved in 77.8% (7/9) of the patients. Partial response was seen in two patients. The outcomes of tumors are shown in Table 2. There was no difference between the tumor showing partial response versus those showing complete response in terms of size and location. The one with ill-defined areas of residual arterial hyperenhancement underwent transarterial chemoembolization, and the other with a well-defined residual nodule was treated with percutaneous ethanol injection (Figure 4). Complete response was achieved after these adjuvant locoregional therapies. The median follow-up period was 7 months (range, 3–12 months). There was no local tumor progression or death during the follow-up period.
Table 2.
Outcomes in Patients Treated With Cryoablation.
| Characteristic | Number (n = 9) |
|---|---|
| Median follow-up | 7 months (range, 3–12 months). |
| Complete responsea | 7 (77.8%) |
| Partial responsea | 2b (22.2%) |
| Characteristics of tumors with PR | |
| Location | Subcapsular [segment III (n = 1) and V (n = 1)] |
| Size | 2.3 cm and 2.1 cm |
| Adjuvant therapies for residual disease | |
|
1 (11.1%) |
|
1 (11.1%) |
PR, partial response; TACE, transarterial chemoembolization; PEI, percutaneous ethanol injection; modified RECIST, modified response evaluation criteria in solid tumors.
Based on modified RECIST at 1 month.12
Both were hepatocellular carcinoma.
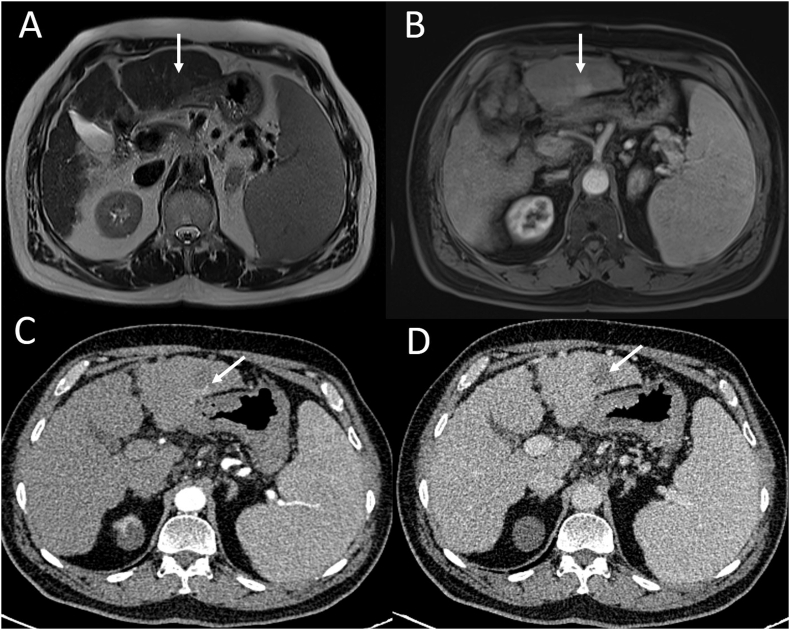
Figure 4.
Imaging of a patient with HCC who had a recurrence after cryoablation. Baseline MRI (A, B). (A) T2-weighted MRI shows a well-defined hyperintense nodule in segment 3 (arrow). (B) Arterial phase MRI shows enhancement within the nodule (arrow). After cryoablation CT (C, D). (C) Arterial phase CT image shows peripheral arterial enhancement (arrow). (D) Venous phase CT image shows washout (arrow). HCC, hepatocellular carcinoma; MRI, magnetic resonance imaging; CT, computed tomography.
Complications
No complications occurred during the CA procedure or during the hospital stay after the procedure.
Discussion
The present study reports the safety and efficacy of CA in a series of nine patients with liver tumors. To the best of our knowledge, this is the first study on CA for hepatic tumors from India. CA was technically successful in all the patients. Complete response was achieved in 7 patients. No major complications occurred in any patient.
The most extensively studied local ablative technique for liver tumors is RFA.4 The major limitations of this technique are heat sink effect and the small size of the ablation zone. Heat sink effect leads to ineffective ablation of perivascular tumors.2,4 In addition, there is a risk of thermal injury to the biliary structures, diaphragm, and bowel.4 While there are methods to address these limitations, these add to the complexity of the RFA. MWA is being increasingly used and has an efficacy similar to that of RFA.12,13 The advantages are a relative lack of heat sink effect and larger ablation zone. Although CA has been existent for a long time, it has been underused as far as liver tumors are concerned.8, 9, 10 This is due to the life-threatening complications of cryoshock and massive hemorrhage resulting from the earlier surgical series.8, 9, 10 However, these studies were performed using the CA systems that used nitrogen as cryogen and probes of larger diameter. In addition, larger tumors were selected for cryosurgery, resulting in a massive cytokine release.
The tissue injury by CA is based on formation of ice ball at the tip of the cryoprobe. When argon gas under high pressure is circulated through the cryoprobe, the temperature drops to −160 °C and results in an ice ball formation at the tip of the cryoprobe.14 Thawing of the ice ball is performed using helium gas. This alternate cycle of freeze and thaw induces direct and indirect cell damage.14 During the freezing part of the cycle, the ice crystals form inside and outside the cell. This causes cell dehydration and damage to the cell membrane. Besides, there is irreversible damage to intracellular organelles such as the mitochondria and endoplasmic reticulum leading to cell death. During thawing, extracellular water enters the cell causing cell swelling and destruction. Indirect tumor cell damage results from ischemia and cell hypoxia. The microvascular thrombosis during CA occurs due to vascular endothelial damage and increased platelet aggregation. CA also induces a potent immunological response due to the release of tumor antigens.15
Recent studies have demonstrated the safety and feasibility of CA with encouraging results in locations that are considered ‘difficult to treat’.16, 17, 18, 19 Kim et al17 reported the results of CA in 45 patients with very early and early HCC. Technical success and complete ablation were achieved in all the patients.17 After a mean follow-up of 28.1 ± 15.6 months, local tumor progression (LTP) was reported in 11.1% of the patients. There were no major complications. Yang et al18 performed CA in 61 patients with 74 HCCs abutting the diaphragm. The mean tumor size was 3.3 ± 1.7 cm.18 After the first session of CA, complete ablation was achieved in 73% of the patients. After a median follow-up of 18.7 months, LTP was reported in 31 tumors. Major complications were reported in 5 patients. However, there was no procedure-related mortality. In a study by Wang et al19, 57 patients with 68 subcapsular HCC nodules were treated with CA. Technical success was achieved in all the patients. Complete ablation was achieved in 97% of the nodules.19 After a median follow-up of 12.8 months, LTP was reported in 16.2% of the lesions. Major complications were reported in 2 patients. In a study comprising 866 patients with 1197 HCC nodules undergoing CA, no procedure-related mortality was reported. Major complications were reported in 2.8% of the patients.20 Our results are comparable with the published literature reporting technical success in most of the patients and complete ablation in 73%–100% of the patients. In our initial experience, technical success was achieved in all cases and complete response was seen in 77.8% of the patients. The residual tumors in two patients may be due to technical factors. The oblong shape of the ice ball may have resulted in an inadequate ablation margin. This underscores the need to sculpt the ablation volume to not only include the tumor but also have an adequate ablation margin.
CA has been shown to have similar efficacy as RFA. The efficacy of CA in early HCC in recent series has been comparable with the data for RFA and MWA.21, 22 In a randomized controlled trial (RCT) by Wang et al21, LTP rates were significantly lower in the CA arm than in the RFA arm. However, the overall survival and disease-free survival rates were similar between the two groups. There was no significant difference in the complication rates between the two groups. However, two meta-analyses report significantly higher complication rates with CA. This is due to the inclusion of the earlier surgical series.23,24 In a study by Ei et al22, CA of HCC was compared with RFA/MWA. Of the 119 patients included in the study, 55 patients underwent CA and 64 patients underwent RFA/MWA. Despite a significantly larger tumor size in patients who underwent CA, there was no significant difference in local recurrence at 2 years. Subgroup analysis including tumors more than 2 cm in diameter showed a higher local recurrence with RFA/MWA.
There were several limitations to our study. It is a single arm, retrospective study. The sample size is small and had a selection bias. There were only two patients with liver metastasis.
In conclusion, CA is a promising relatively new ablative modality for percutaneous treatment of liver tumors. It is technically feasible and safe. Based on our experience, we are not recommending CA as a replacement for RFA/MWA. However, it is a viable ablation technique in ‘difficult-to-treat’ locations. Larger studies and randomized controlled trials including RFA/MWA are required to establish CA as a standard ablative technique for hepatic ablation in our setup.
CRediT authorship contribution statement
Naveen Kalra: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - review & editing. Pankaj Gupta: Writing - Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Tejeshwar Jugpal: Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Shailendra S. Naik: Data curation, Formal analysis, Investigation, Methodology, Writing - review & editing. Ujjwal Gorsi: Data curation, Formal analysis, Investigation, Methodology, Writing - review & editing. Sreedhara B. Chaluvashetty: Data curation, Formal analysis, Investigation, Methodology, Writing - review & editing. Harish Bhujade: Data curation, Formal analysis, Investigation, Methodology, Writing - review & editing. Ajay Duseja: Data curation, Investigation, Methodology, Writing - review & editing. Virendra Singh: Data curation, Investigation, Methodology, Writing - review & editing. Radha K. Dhiman: Data curation, Investigation, Methodology, Writing - review & editing. Manavjit S. Sandhu: Data curation, Writing - review & editing, Supervision.
Conflicts of interest
The authors have none to declare.
References
- 1.Balogh J., Victor D., 3rd, Asham E.H. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41–53. doi: 10.2147/JHC.S61146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalra N., Gupta P., Chawla Y., Khandelwal N. Locoregional treatment for hepatocellular carcinoma: the best is yet to come. World J Radiol. 2015;7:306–318. doi: 10.4329/wjr.v7.i10.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta P., Kalra N., Keshava S.N. Indian society of vascular and interventional radiology expert consensus statements for ablation in hepatocellular carcinoma: Part I. J Clin Interven Radiol ISVIR. 2020;4:98–106. [Google Scholar]
- 4.de Ridder J., de Wilt J.H., Simmer F., Overbeek L., Lemmens V., Nagtegaal I. Incidence and origin of histologically confirmed liver metastases: an explorative case-study of 23,154 patients. Oncotarget. 2016;7:55368–55376. doi: 10.18632/oncotarget.10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim K.H., Yoon Y.S., Yu C.S. Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J Korean Surg Soc. 2011;81:25–34. doi: 10.4174/jkss.2011.81.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalra N., Gupta P., Gorsi U. Irreversible electroporation for unresectable hepatocellular carcinoma: initial experience. Cardiovasc Intervent Radiol. 2019;42:584–590. doi: 10.1007/s00270-019-02164-2. [DOI] [PubMed] [Google Scholar]
- 7.Song K.D. Percutaneous cryoablation for hepatocellular carcinoma. Clin Mol Hepatol. 2016;22:509–515. doi: 10.3350/cmh.2016.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tait I.S., Yong S.M., Cuschieri S.A. Laparoscopic in situ ablation of liver cancer with cryotherapy and radiofrequency ablation. Br J Surg. 2002;89:1613–1619. doi: 10.1046/j.1365-2168.2002.02264.x. [DOI] [PubMed] [Google Scholar]
- 9.Bilchik A.J., Wood T.F., Allegra D. Cryosurgical ablation and radiofrequency ablation for unresectable hepatic malignant neoplasms: a proposed algorithm. Arch Surg. 2000;135:657–662. doi: 10.1001/archsurg.135.6.657. [DOI] [PubMed] [Google Scholar]
- 10.Adam R., Hagopian E.J., Linhares M. A comparison of percutaneous cryosurgery and percutaneous radiofrequency for unresectable hepatic malignancies. Arch Surg. 2002;137:1332–1339. doi: 10.1001/archsurg.137.12.1332. [DOI] [PubMed] [Google Scholar]
- 11.Heimbach J.K., Kulik L.M., Finn R.S. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 12.Lencioni R., Llovet J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Facciorusso A., Di Maso M., Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperther. 2016;32:339–344. doi: 10.3109/02656736.2015.1127434. [DOI] [PubMed] [Google Scholar]
- 14.Tan W., Deng Q., Lin S., Wang Y., Xu G. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperther. 2019;36:264–272. doi: 10.1080/02656736.2018.1562571. [DOI] [PubMed] [Google Scholar]
- 15.Tatli S., Acar M., Tuncali K., Morrison P.R., Silverman S. Percutaneous cryoablation techniques and clinical applications. Diagn Interv Radiol. 2010;16:90–95. doi: 10.4261/1305-3825.DIR.1922-08.0. [DOI] [PubMed] [Google Scholar]
- 16.Aarts B.M., Klompenhouwer E.G., Rice S.L. Cryoablation and immunotherapy: an overview of evidence on its synergy. Insights Imaging. 2019 20;10:53. doi: 10.1186/s13244-019-0727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim D.K., Han K., Won J.Y., Kim G.M., Kwon J.H., Kim M.D. Percutaneous cryoablation in early stage hepatocellular carcinoma: analysis of local tumor progression factors. Diagn Interv Radiol. 2020;26:111–117. doi: 10.5152/dir.2019.19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y., Zhang Y., Wu Y. Efficacy and safety of percutaneous argon-helium cryoablation for hepatocellular carcinoma abutting the diaphragm. J Vasc Interv Radiol. 2020;31:393–400.e1. doi: 10.1016/j.jvir.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Wang F., Ma J., Wu L. Percutaneous cryoablation of subcapsular hepatocellular carcinoma: a retrospective study of 57 cases. Diagn Interv Radiol. 2020;26:34–39. doi: 10.5152/dir.2019.18543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rong G., Bai W., Dong Z. Long-term outcomes of percutaneous cryoablation for patients with hepatocellular carcinoma within Milan criteria. PloS One. 2015;10 doi: 10.1371/journal.pone.0123065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C., Wang H., Yang W. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61:1579–1590. doi: 10.1002/hep.27548. [DOI] [PubMed] [Google Scholar]
- 22.Ei S., Hibi T., Tanabe M. Cryoablation provides superior local control of primary hepatocellular carcinomas of >2 cm compared with radiofrequency ablation and microwave coagulation therapy: an underestimated tool in the toolbox. Ann Surg Oncol. 2015;22:1294–1300. doi: 10.1245/s10434-014-4114-7. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y.Z., Zhou S.C., Zhou H., Tong M. Radiofrequency ablation versus cryosurgery ablation for hepatocellular carcinoma: a meta-analysis. Hepato-Gastroenterology. 2013;60:1131–1135. doi: 10.5754/hge121142. [DOI] [PubMed] [Google Scholar]
- 24.Wu S., Hou J., Ding Y. Cryoablation versus radiofrequency ablation for hepatic malignancies: a systematic review and literature-based analysis. Medicine (Baltim) 2015;94:e2252. doi: 10.1097/MD.0000000000002252. [DOI] [PMC free article] [PubMed] [Google Scholar]