Abstract
This proof-of-principle clinical trial examines use of intraoperative molecular imaging for localizing visually occult parathyroid glands in both anatomic and ectopic locations.
Intraoperative identification of the parathyroid glands is of paramount importance for avoiding injury to normal glands and localizing abnormal or ectopic glands during parathyroidectomy. Intraoperative adjuncts to aid parathyroid identification have long been sought, but none have gained widespread use.1 Here, we harness the emerging field of intraoperative molecular imaging (IMI) and demonstrate that visually occult parathyroid glands can be identified in both anatomic and ectopic locations. Intraoperative molecular imaging is the application of real-time optical imaging during surgery, using targeted fluorescent probes.2 In this pilot study of 5 patients, IMI accurately localized parathyroids in anatomic positions as well as ectopic locations.
Methods
After approval by the University of Pennsylvania institutional review board, 5 patients (3 men, 2 women) undergoing thyroidectomy or parathyroidectomy were enrolled in a prospective, proof-of-principle clinical trial. All patients provided written informed consent prior to enrollment in the study. After exposure of the central neck and identification of parathyroid tissue, gland autofluorescence was assessed with a clinical grade optical imaging device with a λexcitation laser of 785 nm and λemission bandpass filter of 800 to 835 nm (Vision Sense). Patients were then administered 5 mg of indocyanine green (ICG). Fifteen minutes after infusion, IMI was performed to confirm fluorescence of glands in their normal position or to aid the search for ectopic glands. Surgical specimens containing parathyroids were imaged ex vivo for fluorescence. To quantitate fluorescence, ImageJ software (National Institutes of Health) was used to generate signal-to-background ratios (SBRs). Parathyroid fluorescence was based on the entire gland, with background readings taken from surrounding tissue.
Results
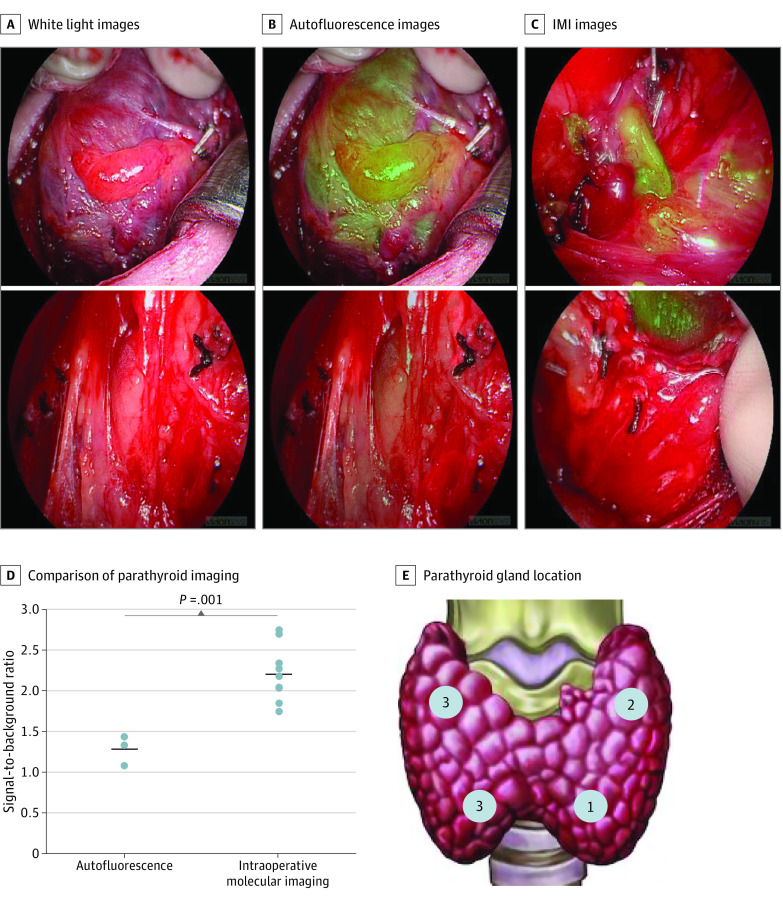
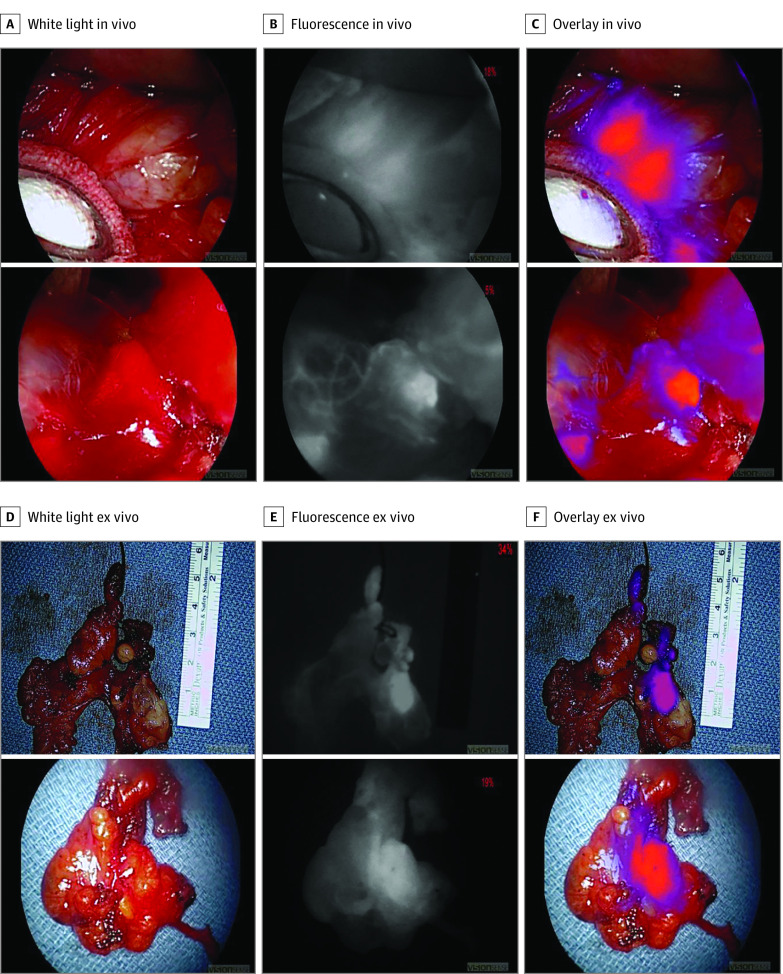
Three male patients and 2 female patients were enrolled, with a mean age of 48.8 years. Three patients underwent thyroidectomy for either Graves disease or thyroid malignant abnormality and 2 patients underwent parathyroidectomy for hyperparathyroidism (HPT). In those undergoing thyroidectomy, IMI confirmed in vivo fluorescence in all (9/9) parathyroid glands located by conventional surgical methods (Figure 1) and demonstrated superior SBR to parathyroid autofluorescence (mean SBR, 2.59 vs 1.50; P = .001). In patients undergoing parathyroidectomy, ectopic parathyroid glands were unable to be located by conventional methods. Intraoperative molecular imaging successfully identified a fluorescent parathyroid gland in the thymus in both cases (Figure 2) (mean in vivo SBR, 2.25; mean ex vivo SBR, 2.10). Parathyroid adenoma was confirmed by pathologic analysis and biochemical cures of HPT were achieved in both cases. One patient had mild asymptomatic hypocalcemia postoperatively but no other complications were observed. There were no adverse effects related to ICG infusion.
Figure 1. Representative Images of Parathyroid Glands Imaged in Situ.
ICG indicates indocyanine green; IMI, intraoperative molecular imaging; NIR, near-infrared. A, The first column shows white light images of parathyroids imaged at the start of each case. B, The middle column shows images of the glands imaged with an NIR imaging device to detect autofluorescence, and (C) the final column shows intraoperative molecular imaging of glands following ICG injection. D, Signal-to-background ratios of parathyroid gland autofluorescence (n = 3) and parathyroid glands imaged with IMI (n = 9). Horizontal lines denote means with standard error bars, and circles indicate individual parathyroid samples. E, Schematic of the location of the parathyroid glands imaged in situ.
Figure 2. Representative Images of Parathyroid Glands Localized in Ectopic Positions.
A, The left column shows in vivo white light images of thymic tissue without clearly identifiable parathyroid glands. B, The middle column shows the same images viewed with a near-infrared imaging device. C, The right column shows the overlay images identifying the ectopic parathyroid glands in the thymus. D, E, and F, Representative ex vivo images of the resected thymuses with intraoperative molecular imaging confirming the presence of intrathymic parathyroid glands in both cases.
Discussion
Results of this proof-of-principle clinical trial suggest that IMI is an accurate and reproducible method of localizing parathyroid glands. Parathyroids demonstrated a high fluorescence signal and IMI identified ectopic glands that were missed by conventional surgical methods. This is the first report, to our knowledge, of the use of IMI to localize ectopic glands that were indistinguishable from surrounding tissue.
Techniques for intraoperative parathyroid identification have been sought for decades. Previous studies using imaging-based technologies have included injection of contrast agents such as methylene blue or intraoperative technetium Tc 99m imaging.1,3 More recent work has focused on using near-infrared imaging devices to detect parathyroid autofluorescence, but the imaging signal from autofluorescence has been limited, with SBRs well below 2.0.4,5,6 This study redemonstrated these limitations, as autofluorescence SBRs were significantly lower than IMI SBRs. Our findings also compare favorably with previous attempts at ICG-based parathyroid localization.1 These improvements may be accounted for by dosing strategy, imaging technique, or advancements in imaging devices.
We acknowledge several limitations in this study. This exploratory study was not randomized and consisted of a small number of patients at a single institution. Nonetheless, this trial demonstrated important potential uses of IMI in parathyroid identification and justifies ongoing studies to confirm its efficacy and move toward clinical implementation.
References
- 1.Wong A, Wong JCY, Pandey PU, Wiseman SM. Novel techniques for intraoperative parathyroid gland identification: a comprehensive review. Expert Rev Endocrinol Metab. 2020;15(6):439-457. doi: 10.1080/17446651.2020.1831913 [DOI] [PubMed] [Google Scholar]
- 2.Tipirneni KE, Warram JM, Moore LS, et al. Oncologic procedures amenable to fluorescence-guided surgery. Ann Surg. 2017;266(1):36-47. doi: 10.1097/SLA.0000000000002127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahramangil B, Berber E. The use of near-infrared fluorescence imaging in endocrine surgical procedures. J Surg Oncol. 2017;115(7):848-855. doi: 10.1002/jso.24583 [DOI] [PubMed] [Google Scholar]
- 4.Squires MH, Jarvis R, Shirley LA, Phay JE. Intraoperative parathyroid autofluorescence detection in patients with primary hyperparathyroidism. Ann Surg Oncol. 2019;26(4):1142-1148. doi: 10.1245/s10434-019-07161-w [DOI] [PubMed] [Google Scholar]
- 5.Squires MH, Shirley LA, Shen C, Jarvis R, Phay JE. Intraoperative autofluorescence parathyroid identification in patients with multiple endocrine neoplasia type 1. JAMA Otolaryngol Head Neck Surg. 2019. doi: 10.1001/jamaoto.2019.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solórzano CC, Thomas G, Baregamian N, Mahadevan-Jansen A. Detecting the near infrared autofluorescence of the human parathyroid: hype or opportunity? Ann Surg. 2020;272(6):973-985. doi: 10.1097/SLA.0000000000003700 [DOI] [PMC free article] [PubMed] [Google Scholar]