Abstract
Acute genital ulcers (AGU), known as Lipschütz ulcers, are painful vulvar ulcers typically affecting non-sexually active girls and women. AGU have been associated with viral infections, namely, Epstein-Barr virus (EBV). Here, we describe a case of AGU in the setting of SARS-CoV-2 in a non-sexually active adolescent girl hospitalised for pain control and urinary retention, who failed a course of oral corticosteroids and then improved with colchicine. Testing for herpes simplex virus, EBV and Behcet’s syndrome were all negative. Testing for SARS-CoV-2 was positive. COVID-19 increases cytokines such as tumour necrosis factor alpha, which has been shown to affect endothelial cell adhesion and neutrophil chemotaxis, leading to aphthosis.
Keywords: genital ulcers, vulvovaginal disorders, infectious diseases, dermatology
Background
Primary acute aphthous genital ulcers, also known as Lipschütz ulcers, ulcus vulvae acutum or acute genital ulcers (AGU), are painful vulvar ulcers without an identifiable aetiology. This rare ulcerative condition occurs most frequently in virginal women and girls and is not associated with sexually transmitted infection. Lipschütz ulcer is a diagnosis of exclusion, after ruling out sexually transmitted infection, Behçet’s syndrome, anogenital Crohn’s disease and other specific ulcerative diagnoses.
AGU have been linked to infection, mainly Epstein-Barr virus (EBV) in the paediatric population,1 2 but also mycoplasma pneumoniae,3 cytomegalovirus (CMV),4 parvovirus B19,5 influenza A and B6 7 and adenovirus.6 8 It is not well understood how these infections may lead to the formation of genital ulceration. Here, we describe a case of primary acute aphthous genital ulcers in the setting of SARS-CoV-2 in a non-sexually active adolescent girl.
Case presentation
A 13-year-old Caucasian (Northern European/Italian American) girl presented with severe ulceration and oedema of the vulva. Her vulvar symptoms began with stinging and burning of the external genitalia, with vulvar oedema starting 1 day later, followed by increased swelling, ulceration and severe vulvar pain. She also noted pain with urination. Her symptom began 3 days following systemic symptoms of fevers, chills, sore throat and decreased taste due to COVID-19, for which she and her sister both tested positive. She is not sexually active. Menarche was age 13 and she was not menstruating at that time of symptoms.
Her medical history is significant for a minor deletion of the SHOX gene and she takes human growth hormone. Otherwise, her medical and surgical history is negative. She has no allergies to medications. She has a history of recurrent oral aphthae. Her sister has a history of Lipschütz ulcers from EBV.
Investigations
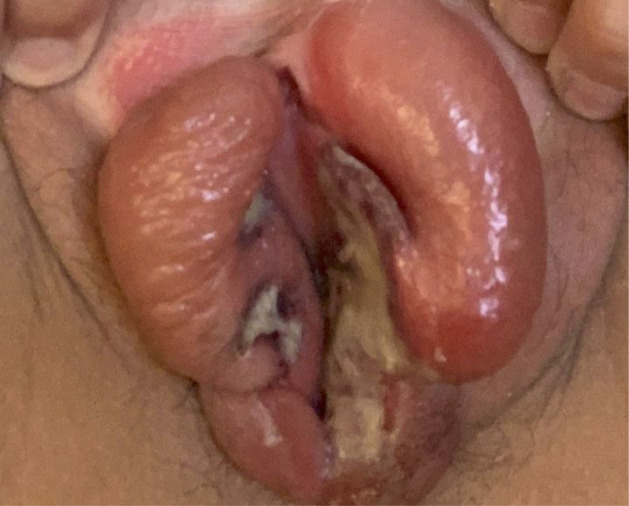
Physical examination revealed multiple, shallow ulcers with raised and sharply demarcated borders located on the medial aspects of the labia minora. Grey exudate and eschar are noted. The surrounding vulvar tissue was erythematous and markedly oedematous (figure 1).
Figure 1.
Acute aphthous ulcers with raised, sharply demarcated borders and grey exudate located classically on the medial aspect of the labia minora, with surrounding oedema.
Differential diagnosis
Herpes simplex virus (HSV) type 1 and 2 were negative, as well as rapid plasma reagin screening for syphilis and HIV testing. Evaluation for Behcet’s syndrome was also performed, revealing negative C reactive protein (CRP), erythrocyte sedimentation rate (ESR) and human leucocyte antigen-B51 (HLA-B51). Testing for EBV was negative. A nasopharyngeal swab for SARS-CoV-2 using reverse transcription PCR (RT-PCR) was positive.
Treatment
She presented to the hospital emergency department and was initially prescribed a steroid cream and topical lidocaine; however, she was unable to use the steroid cream due to extreme pain and was started on an oral corticosteroid regimen of prednisone 45 mg daily for 4 days, 40 mg daily for 4 days, 20 mg daily for 4 days and then 10 mg daily for 2 days. Six days after initial vulvar symptoms, she began to have severe dysuria for which she started Pyridium. She returned to the hospital on day 7 for pain management and oral steroids. HSV, EBV, HIV and syphilis testing at that time were all negative. She was hospitalised for pain control for 5 days, completing a 14-day course of oral steroids. She applied topical lidocaine 5% ointment and benzocaine 20% spray for pain control. The genital oedema had decreased, but ulcers and vulvar pain were still present. She was started on colchicine 1.2 mg daily and was noted to have further improvement of oedema and pain within 1 week. Subsequent evaluation by Rheumatology was not consistent with Behcet’s syndrome, revealing negative CRP, ESR and HLA-B51.
Outcome and follow-up
Ulceration and oedema resolved within 6 weeks; however, she was noted to have agglutination of the labia minora. Adhesions were refractory to a trial of topical clobetasol propionate 0.05% ointment and she underwent lysis of labial adhesions under sedation. Postoperative evaluation revealed normal anatomy, no evidence of scarring and resolution of pain.
Discussion
Lipschütz first described AGU in 1913 in a series of virginal adolescent young women.9 AGU in non-sexually active girls have been associated with infection, autoimmune disorders and idiopathic aphthosis. In a review of five case series describing a total of 62 non-sexually active girls, 71% presented with mild prodromal systemic symptoms and 47% had a history of oral aphthous ulcers, although most oral ulcers were not present at the time of genital ulceration.1 4 10–13 Twenty-two percent of patients were hospitalised due to AGU. Hospitalisation is often necessary in cases of acute urinary retention and severe pain not controlled by oral pain medications.13
EBV is noted to be the most cited infectious aetiology for AGU, followed by CMV infection.1 2 5 14 Over 40 cases of AGU have been linked to EBV in the literature and it is estimated that EBV is implicated in about 30% of AGU cases.1 2 15 There have been two hypotheses regarding the mechanism of genital ulceration in the setting of viral infection. The first is formation of immune complex deposition causing a type III hypersensitivity reaction that leads to microthrombosis and necrosis in the genital area. The second is virus-provoked cytolysis resulting after haematological spread of virus-infected lymphocytes, autoinoculation through self-contact or, in the case of sexually active patients, through genital–genital or oral–genital contact.1 3 16 Cases of AGU have also been described in influenza A and B virus and adenovirus,6–8 but at the time of publication, there have been no described cases of AGU associated with SAR-CoV-2.
Oral aphthous ulcers have been reported in four patients related to COVID-19 in Spain.17 All four patients presented with typical COVID-19 symptoms, including anosmia, fever, headache, malaise and dyspnoea. COVID-19 was confirmed with a positive nasopharyngeal swab for SARS-CoV-2 using RT-PCR. Testing for secondary causes of aphthosis, including HSV, syphilis, hepatitis, HIV, EBV and CMV, were all negative in these cases. Dominguez-Santas et al 17 postulated that COVID-19 increases cytokines, inciting tumour necrosis factor alpha (TNF-alpha), leading neutrophils to disrupt the oral mucosa. Elevated TNF-alpha has been found in patients with recurrent aphthae and affects endothelial cell adhesion and neutrophil chemotaxis.17 18
In addition, one report describes COVID-19 identified in a vulvar lesion by RT-PCR in a pregnant patient.19 The lesion was described as a 3 mm ulceration on the right posterior fourchette of the vulva; however, the report did not describe pain associated with the lesion, only vaginitis symptoms consistent with vulvovaginal candidiasis. In this case, a viral swab was collected from a vulvar lesion and inadvertently tested positive for SARS-CoV-2 RNA and Pan-SARS RNA. The vulvar lesion tested negative for HSV-1 and HSV-2. The patient had upper respiratory symptoms, including cough, fatigue, myalgias and anosmia, consistent with COVID-19 infection at the time of diagnosis. This case demonstrates SARS-CoV-2 viral shedding detected in a vulvar lesion.19 However, it is unclear whether the lesion was associated with concurrent COVID-19 infection or whether viral shedding may occur in any disruption of the mucous membrane of any origin, and it happened to be detected with testing.
AGU are a self-limiting condition. Treatment generally involves supportive care and symptomatic pain relief in the form of oral and topical medications.20 21 In general, non-steroidal anti-inflammatory drugs are avoided, as they have been associated with recurrent oral and genital aphthous ulcers in one case.22 A course of systemic corticosteroids is often used in treatment. In the case series of non-sexually active girls with AGU described by Rosman et al, there were insufficient data to determine whether oral corticosteroids shortened the course of AGU; however, ultrapotent topical steroids, such as clobetasol propionate 0.05% ointment, have shown benefit in the treatment of oral aphthous ulcers and may also be beneficial for AGU.13 20 21 Colchicine has been reported as a treatment for AGU associated with EBV infection in the setting of previously failed treatment, including topical and systemic corticosteroids and topical imiquimod.15 Colchicine has been used in other ulcerative conditions, such as recurrent aphthous stomatitis and Behçet’s disease.23 24 The proposed mechanisms of action are through inhibition of chemotaxis and phagocytosis of neutrophils.25
Patient’s perspective.
This was an extremely traumatic and painful ordeal for our daughter. The swelling/ulcers came on very suddenly after a fairly mild case of COVID-19. Her COVID-19 symptoms lasted approximately 1 week, but the ulcers lasted for about 6–8 weeks with a minor surgery at the end to help repair her vagina. It was the length of the healing process that was the most frustrating, along with the continued pain with urination. She also had a lot of problems walking or moving around in general due to the pain.
Once she was diagnosed with the ulcers, she started on a course of oral steroids. However, after this initial course of steroids was done, the healing process seemed stalled for a long period of time. Another frustrating aspect of this was that at this point, several doctors were not sure what the next steps would be to continue the healing process (and she was still in severe pain). Also, given her COVID-19 diagnosis, doctors did not necessarily want to see her in person. Getting her seen in person and with specialists was key to her recovery. The entire ordeal was very tough on a patient of this age, especially the constant viewing of the affected area.
Learning points.
Less common than other forms of genital ulcers, acute genital ulcers (AGU) are essential to recognise and consider in the differential diagnosis of painful vulvar ulceration, especially in the paediatric and non-sexually active adolescent populations.
Although this condition is self-limited, acute urinary retention and pain control are important considerations in regard to management and need for hospitalisation. Cases of AGU not responsive to systemic corticosteroids may be candidates for treatment with colchicine. Labial agglutination is a potential complication of AGU and can be resolved by topical corticosteroids or surgically.
The relationship between SARS-CoV-2 and genital aphthae is unknown. AGU have been associated with other viral infections, such as Epstein-Barr virus and influenza, and the mechanism of ulceration may be similar. Reports such as these alert clinicians to possible conditions and presentations associated with COVID-19 as we continue to learn about this novel coronavirus.
Footnotes
Twitter: @jillkrapfmd
Contributors: JMK was involved in conception and design, analysis and interpretation of data, and drafting the article. RKC was involved in acquisition of data, analysis and interpretation of data, and revising the manuscript critically for important intellectual content. ATG was involved in conception and design, acquisition of data, analysis and interpretation of data, and revising the manuscript critically for important intellectual content. All authors approved the final version published. All authors agree to be accountable for the article and to ensure that all questions regarding the accuracy or integrity of the article are investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Farhi D, Wendling J, Molinari E, et al. Non–Sexually related acute genital ulcers in 13 pubertal girls. Arch Dermatol 2009;145:38–45. 10.1001/archdermatol.2008.519 [DOI] [PubMed] [Google Scholar]
- 2. Sárdy M, Wollenberg A, Niedermeier A, et al. Genital ulcers associated with Epstein-Barr virus infection (Ulcus vulvae acutum). Acta Derm Venereol 2011;91:55–9. 10.2340/00015555-0979 [DOI] [PubMed] [Google Scholar]
- 3. Vieira-Baptista P, Machado L, Costa AR. Mycoplasma pneumoniae: a rare cause of vulvar ulcers or an undiagnosed one? J Low Genit Tract Dis 2013;17:00Y00. [DOI] [PubMed] [Google Scholar]
- 4. Huppert JS, Gerber MA, Deitch HR, et al. Vulvar ulcers in young females: a manifestation of aphthosis. J Pediatr Adolesc Gynecol 2006;19:195–204. 10.1016/j.jpag.2006.02.006 [DOI] [PubMed] [Google Scholar]
- 5. Vieira-Baptista P, Lima-Silva J, Beires J, et al. Lipschütz ulcers: should we rethink this? an analysis of 33 cases. Eur J Obstet Gynecol Reprod Biol 2016;198:149–52. 10.1016/j.ejogrb.2015.07.016 [DOI] [PubMed] [Google Scholar]
- 6. Haidari G, MacMahon E, Tong CYW, et al. Genital ulcers: it is not always simplex …. Int J STD AIDS 2015;26:72–3. 10.1177/0956462414541241 [DOI] [PubMed] [Google Scholar]
- 7. Wetter DA, Bruce AJ, MacLaughlin KL, et al. Ulcus vulvae acutum in a 13-year-old girl after influenza A infection. Skinmed 2008;7:95–8. 10.1111/j.1751-7125.2008.07273.x [DOI] [PubMed] [Google Scholar]
- 8. Swenson PD, Lowens MS, Celum CL, et al. Adenovirus types 2, 8, and 37 associated with genital infections in patients attending a sexually transmitted disease clinic. J Clin Microbiol 1995;33:2728–31. 10.1128/JCM.33.10.2728-2731.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lipschütz B. Über eine eigenartige Geschwürsform des weiblichen Genitales (Ulcus vulvae acutum). Arch. f. Dermat. 1912;114:363–96. 10.1007/BF01973166 [DOI] [Google Scholar]
- 10. Lehman JS, Bruce AJ, Wetter DA, et al. Reactive nonsexually related acute genital ulcers: review of cases evaluated at Mayo clinic. J Am Acad Dermatol 2010;63:44–51. 10.1016/j.jaad.2009.08.038 [DOI] [PubMed] [Google Scholar]
- 11. Cebesoy FB, Balat O, Inaloz S. Premenarchal vulvar ulceration: is chronic irritation a causative factor? Pediatr Dermatol 2009;26:514–8. 10.1111/j.1525-1470.2008.00805.x [DOI] [PubMed] [Google Scholar]
- 12. Deitch HR, Huppert J, Adams Hillard PJ. Unusual vulvar ulcerations in young adolescent females. J Pediatr Adolesc Gynecol 2004;17:13–16. 10.1016/j.jpag.2003.11.015 [DOI] [PubMed] [Google Scholar]
- 13. Rosman IS, Berk DR, Bayliss SJ, et al. Acute genital ulcers in nonsexually active young girls: case series, review of the literature, and evaluation and management recommendations. Pediatr Dermatol 2012;29:147–53. 10.1111/j.1525-1470.2011.01589.x [DOI] [PubMed] [Google Scholar]
- 14. Halvorsen JA, Brevig T, Aas T, et al. Genital ulcers as initial manifestation of Epstein-Barr virus infection: two new cases and a review of the literature. Acta Derm Venereol 2006;86:439–42. 10.2340/00015555-0140 [DOI] [PubMed] [Google Scholar]
- 15. Nouchi A, Monsel G, Lafon-Desmurs B, et al. Epstein-Barr virus-related acute genital ulcer successfully treated with colchicine. Acta Derm Venereol 2018;98:134–5. 10.2340/00015555-2761 [DOI] [PubMed] [Google Scholar]
- 16. Leigh R, Nyirjesy P. Genitourinary manifestations of Epstein-Barr virus infections. Curr Infect Dis Rep 2009;11:449–56. 10.1007/s11908-009-0065-8 [DOI] [PubMed] [Google Scholar]
- 17. Dominguez-Santas M, Diaz-Guimaraens B, Fernandez-Nieto D, et al. Minor aphthae associated with SARS-CoV-2 infection. Int J Dermatol 2020;59:1022–3. 10.1111/ijd.15004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–4. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rubin ES, Sansone SA, Hirshberg A. Detection of COVID-19 in a vulvar lesion. Amer J Perinatol 2020;37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huppert JS. Lipschutz ulcers: evaluation and management of acute genital ulcers in women. Dermatol Ther 2010;23:533–40. 10.1111/j.1529-8019.2010.01356.x [DOI] [PubMed] [Google Scholar]
- 21. Bandow GD. Diagnosis and management of vulvar ulcers. Dermatol Clin 2010;28:753–63. 10.1016/j.det.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 22. Healy CM, Thornhill MH. An association between recurrent oro-genital ulceration and non-steroidal anti-inflammatory drugs. J Oral Pathol Med 1995;24:46–8. 10.1111/j.1600-0714.1995.tb01129.x [DOI] [PubMed] [Google Scholar]
- 23. Yurdakul S, Mat C, Tüzün Y, et al. A double-blind trial of colchicine in Behçet's syndrome. Arthritis Rheum 2001;44:2686–92. [DOI] [PubMed] [Google Scholar]
- 24. Pakfetrat A, Mansourian A, Momen-Heravi F, et al. Comparison of colchicine versus prednisolone in recurrent aphthous stomatitis: a double-blind randomized clinical trial. Clin Invest Med 2010;33:189–95. 10.25011/cim.v33i3.13725 [DOI] [PubMed] [Google Scholar]
- 25. Altenburg A, Abdel-Naser MB, Seeber H, et al. Practical aspects of management of recurrent aphthous stomatitis. J Eur Acad Dermatol Venereol 2007;21:1019–26. 10.1111/j.1468-3083.2007.02393.x [DOI] [PubMed] [Google Scholar]