Abstract
Objectives
SLE and/or antiphospholipid syndrome (SLE/APS) are complex and rare systemic autoimmune diseases that predominantly affect women of childbearing age. Women with SLE/APS are at high risk of developing complications during pregnancy. Therefore, clinical practice guidelines recommend that patients with SLE/APS should receive multidisciplinary counselling before getting pregnant. We investigated the clinical effectiveness of implementing a multidisciplinary clinical pathway including prepregnancy counselling of patients with SLE/APS.
Methods
A clinical pathway with specific evaluation and prepregnancy counselling for patients with SLE/APS was developed and implemented in a tertiary, academic hospital setting. Patients were prospectively managed within the clinical pathway from 2014 onwards and compared with a retrospective cohort of patients that was not managed in a clinical pathway. Primary outcome was a combined outcome of disease flares for SLE and thromboembolic events for APS. Secondary outcomes were maternal and fetal pregnancy complications.
Results
Seventy-eight patients with 112 pregnancies were included in this study. The primary combined outcome was significantly lower in the pathway cohort (adjusted OR (aOR) 0.20 (95% CI 0.06 to 0.75)) which was predominantly determined by a fivefold risk reduction of SLE flares (aOR 0.22 (95% CI 0.04 to 1.09)). Maternal and fetal pregnancy complications were not different between the cohorts (respectively, aOR 0.91 (95% CI 0.38 to 2.17) and aOR 1.26 (95% CI 0.55 to 2.88)).
Conclusions
The outcomes of this study suggest that patients with SLE/APS with a pregnancy wish benefit from a multidisciplinary clinical pathway including prepregnancy counselling.
Keywords: systemic lupus erythematosus, lupus nephritis, antiphospholipid syndrome
Key messages.
What is already known about this subject?
Pregnancies in patients with SLE and/or antiphospholipid syndrome (APS) remain high-risk, therefore prepregnancy counselling is considered essential according to current international clinical practice guidelines.
What does this study add?
This study establishes an approach to manage high-risk pregnancies in patients with SLE/APS by implementation of a multidisciplinary clinical pathway including prepregnancy counselling.
Compared with a historical cohort, the implementation of such a clinical pathway significantly reduced a composite end point of disease-related flares (ie, SLE flares or thromboembolic events) during pregnancy.
Pregnancy-related maternal and fetal outcomes were comparable in the historical cohort and clinical pathway cohort.
How might this impact on clinical practice or future developments?
This study provides evidence that the implementation of a multidisciplinary clinical pathway including prepregnancy counselling can positively contribute to optimisation of the care for patients with SLE/APS with a pregnancy wish.
Introduction
SLE is a systemic autoimmune disease with an incidence of 8/100 000 predominantly diagnosed in women of childbearing age.1 2 Similarly, primary antiphospholipid syndrome (APS) is also diagnosed in women of childbearing age and characterised by venous or arterial thrombosis (thrombotic APS) or pregnancy complications (obstetric APS) in combination with the presence of antiphospholipid antibodies (aPL). Co-incidence of APS occurs in 20%–35% of patients with SLE and the overall incidence of APS is estimated to be around 5/100 000.3 4 As such, the desire to have children is a common issue for patients with SLE and APS and therefore a pregnancy wish should be addressed as integral part of the management of patients with SLE and/or APS (SLE/APS).
From a maternal perspective, patients with SLE are at increased risk of flares and patients with APS are at increased risk of thromboembolic events (TEE) during pregnancy.5 6 Additionally, patients with SLE/APS are at higher risk of pregnancy complications such as gestational hypertensive disease (including pre-eclampsia and haemolysis elevated liver enzymes low platelets (HELLP) syndrome) and miscarriage. From a fetal/neonatal perspective, there is an increased risk for prematurity, fetal growth restriction (FGR), stillbirth and neonatal death. Also, infants born to SLE mothers who carry anti-Ro/SSA or anti-La/SSB antibodies have a 1%–2% risk of congenital heart block associated with neonatal lupus erythematosus.6–11
As a consequence, some decades ago pregnancy was actually discouraged in women with SLE because of the potential severe pregnancy complications and disease exacerbations with pregnancy loss up to 43% in 1965.12 Fortunately, increasing insights in the determinants that can negatively impact maternal and fetal outcomes, pregnancy loss in patients with SLE decreased to 17% in 2003.6 11–14 Consequently, pregnancies in patients with SLE are nowadays more and more embraced, rather than discouraged, taking into account the challenges in the management of pregnant patients with SLE/APS.15–17 This view is emphasised within the recently published Joint European League Against Rheumatism, European Renal Association and American College of Rheumatology (EULAR/ERA-EDTA/ACR) recommendations for the management of patients with SLE/APS with a pregnancy wish.18–20 Within these recommendations, preconception counselling is considered essential. It seems self-evident that prepregnancy counselling of patients with SLE/APS requires expertise from different specialisms and could therefore benefit from a multidisciplinary approach by a specialised team.21 22
A strategy for implementation of a multidisciplinary approach for prepregnancy counselling of patients with SLE/APS is the use of a clinical pathway. While clinical practice guidelines have emerged as rigorous means to make clinical studies and research more accessible for practitioners, they are not always sufficient to change practice behaviour, especially in complex diseases such as SLE/APS.23 Therefore, clinical pathways are an important strategy to improve effective knowledge transfer and sharing, promote standardised evidence-based practices and are internationally recognised as a form of quality improvement.23 24 Thus, based on EULAR/ERA-EDTA/ACR recommendations to implement multidisciplinary prepregnancy counselling for patients with SLE/APS, the present study investigated the clinical effectiveness of a clinical pathway with specific evaluation and prepregnancy counselling for patients with SLE/APS.
Materials and methods
Study design and participants
We performed a retrospective analysis of patients with SLE/APS that were prospectively managed in a multidisciplinary clinical pathway focused on prepregnancy counselling at a third-line, academic, referral centre compared with a cohort of patients with SLE/APS that were managed without a clinical pathway. Patients were included in the study over the period of January 2008 to February 2020. In 2014, a multidisciplinary clinical pathway was implemented involving specialists from the Department of Obstetrics, Rheumatology, Nephrology and Thrombosis and Haemostasis at the Leiden University Medical Centre (LUMC). On indication, a pulmonologist, cardiologist, radiologist and social worker were consulted. The study included SLE/APS pregnancies that were managed within the clinical pathway from May 2014 onwards (hereafter referred to as the ‘pathway cohort’) and pregnancies that were managed within our centre before initiation of the clinical pathway (hereafter referred to as ‘historical cohort’). Patients had to meet the following inclusion criteria: any singleton or multiple pregnant woman with a diagnosis of SLE according to the EULAR/ACR revised criteria of 2019 and/or an APS diagnosis according to the Sydney criteria.25 26 There were no exclusion criteria. The clinical pathway is described in more detail in the online supplemental file.
lupus-2020-000472supp001.pdf (146KB, pdf)
Management in the clinical pathway
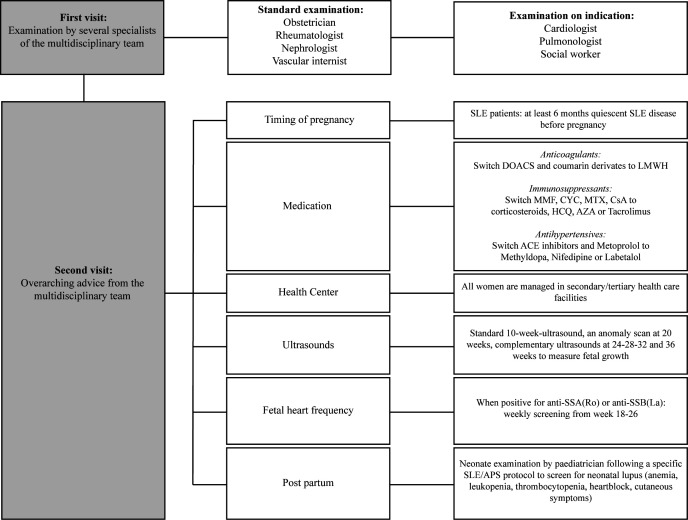
Patients with SLE/APS with a pregnancy wish were enrolled in the clinical pathway from 2014 onwards. During the first visit, patients consulted the individual specialists of the multidisciplinary team in 2 days, with the guidance of a dedicated nurse. The multidisciplinary team included specialists from the Department of Obstetrics, Nephrology, Rheumatology and Thrombosis and Haemostasis at the LUMC. On indication, a pulmonologist, cardiologist, radiologist and social worker were consulted. Every 2 weeks all enrolled patients, before and during pregnancy, were discussed in a multidisciplinary meeting. At this meeting, a personalised, overarching, advice was formed concerning timing of pregnancy, medication policy and complementary medical check-ups and diagnostics for every new patient. Medication policy was established in accordance with guidelines on medication use during pregnancy and lactation.27–29 Figure 1 displays the possible content of the overarching advice in more detail, whereas specific prepregnancy counselling contents at first presentation are shown in online supplemental table S1. One week after the first visit, the women received elaborate counselling and advice. Obstetric routine ultrasound follow-up was advised for all patients: a 10–14 weeks ultrasound, an anomaly scan at 20 weeks of gestation and complementary ultrasounds at 24–28–32–36 weeks of gestation to monitor fetal growth. When patients were positive for anti-RO/SS-A or anti-LA/SS-B, weekly screening for congenital heart block in the framework of neonatal lupus was performed between week 18 and 26 weeks of gestation. For postpartum patients with SLE, paediatricians performed neonate examinations to screen for neonatal lupus. Furthermore, all women in the pathway were advised to use 80 mg acetylsalicylic acid daily, from the moment of a detected heartbeat around 8 weeks until 36 weeks of gestation. Low-molecular-weight heparin (LMWH) was indicated according to risk factors as is explained extensively in online supplemental table S2.
Figure 1.
Management in the clinical pathway. APS, antiphospholipid syndrome; AZA, azathioprine; CsA, ciclosporin A; CYC, cyclophosphamide; DOACS, direct oral anticoagulants; HCQ, hydroxychloroquine; LMWH, low-molecular-weight heparin; MMF, mycophenolate mofetil; MTX, methotrexate.
Data collection and outcomes
Data collection from electronic patient records included: disease-relevant maternal characteristics, obstetric characteristics, disease-relevant SLE/APS history and medication use.
The primary outcome was a combined end point of disease flares for patients with SLE and TEEs for patients with APS. SLE flares were defined as a combination of clinical symptoms, laboratory findings (complement consumption), treating physician’s judgement of a disease flare and the initiation or intensification of immunosuppressive treatment during pregnancy and the postpartum period (≤6 weeks post partum). Major flares were defined as those that involve central nervous system, kidney, lung, vasculitis, myositis, haemolytic anaemia with haemoglobin <8 g/dL, thrombocytopenia <20 000/mm3, addition of prednisone at doses >0.5 mg/kg/day or addition of an immunosuppressive agent. All other flares are considered minor flares. Disease activity was established according to the Systemic Lupus Erythematosus Pregnancy Disease Activity Index (SLEPDAI).30 TEEs were defined as arterial, venous and small vessel thrombosis, other than superficial venous thrombosis, in any tissue or organ.
Secondary outcomes were defined as maternal and fetal outcomes: maternal outcomes included miscarriage (early miscarriage was defined as spontaneous pregnancy loss before 10 weeks and late miscarriage between 10 and 16 weeks of gestation), gestational hypertension and severe hypertensive disease including pre-eclampsia (systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg measured two times and proteinuria ≥300 mg/24 hours), eclampsia and HELLP syndrome. Fetal outcomes included perinatal death (fetal death ≥24 weeks of gestation or neonatal death ≤7 days post partum), FGR (birth weight <10th percentile of the PERINED 2008 dataset), congenital heart block (conduction system disease of the heart which is diagnosed antenatally or within 28 days after birth) and preterm birth (delivery before 37 weeks of gestation).
Statistical methods
The baseline characteristics and outcomes were summarised within the APS and the SLE pregnancies using descriptive statistics and comparisons between the pathway and historical cohort were analysed with Mann-Whitney U test for numerical and χ2 test for categorical variables. Logistic regression with robust SEs to account for clustering of pregnancies within patients (Generalised Estimating Equations) was used to assess the association between the pathway and the primary and secondary outcomes. Disease characteristics at baseline and specific medical history were predefined as possible confounders for the association between attending the clinical pathway and disease/pregnancy outcome. Predefined confounders were EULAR/ACR criteria points and a history of lupus nephritis for patients with SLE; and a history of lupus nephritis, TEE, pre-eclampsia and number of miscarriages for the combined analysis of patients. The small number of events did not allow correction for confounders in the separate analysis of the patients with primary APS. Adjusted ORs are presented from multivariable logistic regression analyses including these possible confounders. Statistical analysis was performed using SPSS V.25.0 software.
Results
Participants
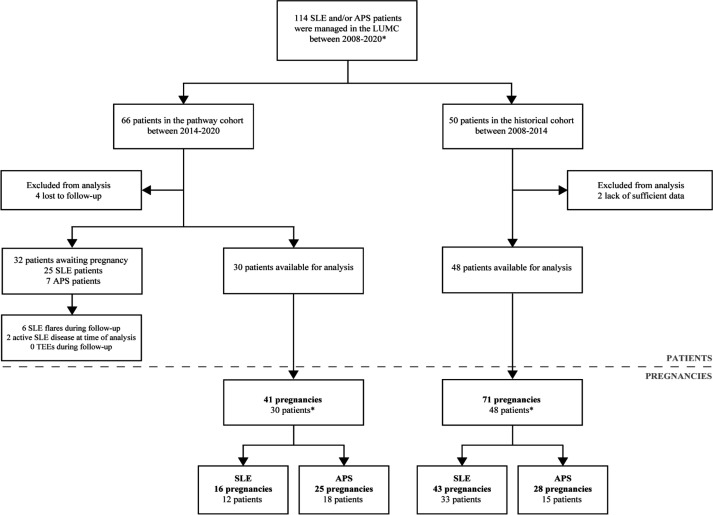
Seventy-eight patients with 112 pregnancies met the inclusion criteria (figure 2). In the pathway cohort, 30 patients with 41 pregnancies were included, 12 patients with SLE (±secondary APS) with 16 pregnancies and 18 patients with primary APS with 25 pregnancies. As illustrated in figure 2, 32 patients in the pathway cohort were awaiting pregnancy at time of analysis, of which 25 patients with SLE and 7 patients with primary APS. Of these 32 patients awaiting pregnancy, 6 patients flared during follow-up (4 major, 2 minor flares), 2 patients had active disease at time of analysis and were advised to postpone conception until stable disease for >6 months was achieved. One patient conceived against medical advice (see online supplemental table S1 and S3 for disease characteristics of all patients and pregnancies in the pathway cohort).
Figure 2.
Flow chart of patient enrolment in the ‘pathway’ and ‘historical’ cohort. APS, antiphospholipid syndrome; LUMC, Leiden University Medical Centre; TEE, thromboembolic event. *Patients could be enrolled with multiple pregnancies.
In the historical cohort, 48 patients with 71 pregnancies were analysed, 33 patients with SLE (±secondary APS) with 43 pregnancies and 15 patients with primary APS with 28 pregnancies. Two patients overlapped as one pregnancy had follow-up in the pathway cohort, while the other received care in the historical cohort. Six patients were excluded from analysis due to lack of sufficient data and follow-up.
Descriptive data
As shown in table 1, overall, for patients with SLE the median disease duration was 9 (5–11) years with a Systemic Lupus International Collaborating Clinics index score of 0 (0–1) that did not differ between the pathway and historical cohort. Patients with SLE in the pathway cohort had a significantly higher score on the item list of EULAR/ACR 2019 criteria, increased frequency of secondary APS and significant higher steroid dosing before pregnancy than in the historical cohort. Furthermore, the pathway cohort included numerically more women with a history of lupus nephritis (63% vs 47%) than the historical cohort, more often displayed complement usage at start of pregnancy and were more often treated with hydroxychloroquine and tacrolimus.
Table 1.
Baseline and disease characteristics in SLE and APS pregnancies
| Pregnancies (n) | SLE | Primary APS | ||
| Pathway N=16 |
Historical N=43 |
Pathway N=25 |
Historical N=28 |
|
| Maternal characteristics | ||||
| Age at conception* | 33 (31–35) | 32 (27–34) | 31 (30–37) | 30 (28–34) |
| Caucasian | 11 (68.8) | 29 (67.4) | 18 (72.0) | 14 (50.0) |
| Smoking during pregnancy | 1 (6.3) | 1 (2.3) | 3 (12.0) | 3 (10.7) |
| BMI (kg/m2)* | 24.9 (23.2–29.2) | 23.5 (21.5–25.8) | 26.2 (21.4–31.6) | 25.9 (19.6–28.3) |
| Chronic hypertension | 4 (25.0) | 6 (14.0) | 2 (8.0) | 0 (0.0) |
| Obstetric characteristics | ||||
| Nulliparous | 12 (75.0) | 21 (48.8) | 17 (68.0) | 12 (42.9) |
| Singleton pregnancy | 16 (100.0) | 42 (97.7) | 24 (96.0) | 27 (96.4) |
| History of miscarriage | 2 (12.5) | 9 (20.9) | 22 (88.0)† | 16 (57.1) |
| History of pre-eclampsia | 2 (12.5) | 7 (16.3) | 4 (16.0) | 6 (21.4) |
| Specific APS history | ||||
| Thrombotic APS | 3 (18.8) | 1 (2.3) | 78 (32.0) | 12 (42.9) |
| Obstetric APS | 1 (6.3) | 1 (2.3) | 14 (56.0)† | 5 (17.9) |
| Thrombotic and obstetric APS | 0 (0.0) | 0 (0.0) | 3 (12.0)† | 11 (39.3) |
| History of thromboembolic events | 4 (25.0) | 6 (14.0) | 10 (40.0)† | 23 (82.1) |
| Lupus anticoagulant | 5 (31.3) | 3 (7.0) | 15 (60.0) | 23 (82.1) |
| Anticardiolipin antibodies | 4 (25.0)† | 0 (0.0) | 10 (40.0)† | 24 (85.7) |
| Anti-β2-glycoprotein-I antibodies | 3 (18.8) | 0 (0.0) | 12 (48.0) | 14 (77.8) |
| Number of positive aPL tests | ||||
| 1 | 6 (37.5)† | 3 (7.0) | 14 (56.0)† | 5 (17.9) |
| 2 | 0 (0.0) | 0 (0.0) | 10 (40.0) | 11 (39.3) |
| 3 | 2 (12.5) | 0 (0.0) | 1 (4.0)† | 12 (42.9) |
| Specific SLE history | ||||
| Duration SLE disease (years)* | 9 (6–11) | 9 (4–12) | – | – |
| SLICC damage index* | 0 (0–1) | 0 (0–1) | – | – |
| EULAR/ACR criteria* | 23 (12–38)† | 17 (10–22) | – | – |
| Secondary APS | 4 (25.0)† | 2 (4.7) | – | – |
| Serological Active Clinically Quiescent | 3 (18.8) | 0 (0.0) | ||
| Clinically active SLE <6 months before | 1 (6.3) | 4 (9.3) | – | – |
| History of LN | 10 (62.5) | 20 (46.5) | – | – |
| I | 0 (0.0) | 2 (4.7) | – | – |
| II | 0 (0.0) | 1 (2.3) | – | – |
| III | 2 (12.5) | 3 (7.0) | – | – |
| IV | 5 (31.3) | 9 (20.9) | – | – |
| V | 2 (12.5) | 5 (11.6) | – | – |
| ANA | 14 (87.5) | 29 (67.4) | – | – |
| Anti-Ro/SS-A | 11 (68.7) | 23 (53.5) | – | – |
| Anti-La/SS-B | 4 (25.0) | 13 (30.2) | – | – |
| Anti-dsDNA | 7 (43.8) | 18 (41.9) | – | – |
| Low C3 before pregnancy‡ | 8 (50.0) | 8 (18.6) | – | – |
| Low C4 before pregnancy‡ | 4 (25.0) | 3 (7.0) | – | – |
| Medication before pregnancy | ||||
| Only HCQ or no immunosuppressants | 3 (18.8) | 21 (48.8) | 25 (100.0) | 28 (100.0) |
| Corticosteroid | 11 (68.8) | 18 (41.9) | – | – |
| Dose in mg* | 10.0 (7.5–10.0)† | 5 (4.4–8.1) | ||
| Hydroxychloroquine | 16 (100.0)† | 25 (58.1) | 2 (8.0) | 1 (3.6) |
| Dose in mg* | 350 (200–400) | 400 (200–400) | 400 (400–400) | 400 (400–400) |
| Tacrolimus | 7 (43.8)† | 1 (2.3) | – | – |
| Dose in mg* | 6 (4–6) | 3 (3) | ||
| Azathioprine | 7 (43.8) | 14 (32.6) | – | – |
| Dose in mg* | 150 (100–150) | 100 (50–125) | ||
| Medication in pregnancy | ||||
| LMWH | 4 (25.0)† | 4 (9.3) | 21 (84.0) | 20 (71.4) |
| Acetylsalicylic acid | 16 (100.0)† | 22 (51.2) | 21 (84.0)† | 16 (57.1) |
Data depicted as numbers (%) unless otherwise specified.
*Median (IQR).
†Shows a significant difference with two-sided α<0.05.
‡Low C3 defined as <0.9 g/L and low C4 defined as <95 mg/L
ACR, American College of Rheumatology; aPL, antiphospholipid antibodies; APS, antiphospholipid syndrome; BMI, body mass index; dsDNA, double stranded DNA; EULAR, European League Against Rheumatism; HCQ, hydroxychloroquine; LMWH, low-molecular-weight heparin; LN, lupus nephritis; SLICC, Systemic Lupus International Collaborating Clinics; triple-positive, positivity for lupus anticoagulant+anticardiolipin+anti-β2-glycoprotein 1.
For patients with APS, the pathway cohort had a significantly higher frequency of women with obstetric APS and a history of miscarriages than the historical cohort. Furthermore, numerically fewer women with thrombotic APS were observed in the pathway cohort (32% vs 43%) with significantly less TEEs in medical history. Moreover, the women in the pathway cohort were less often triple-positive for the aPL than the women in the historical cohort. Interestingly, LMWH use in pregnancy was comparable.
Main results
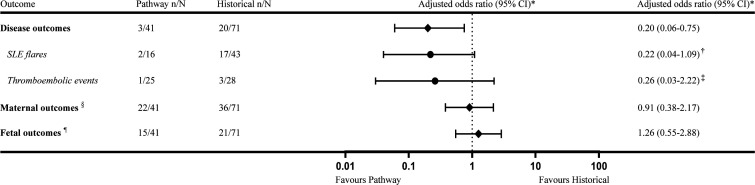
The primary outcome of disease-related events was significantly reduced in the pathway cohort compared with the historical cohort (respectively 7% vs 28%, figure 3). The positive effect in the pathway cohort was mainly determined by a significant reduction in SLE flares (13% vs 40%, table 2). The crude OR for the disease outcomes composite was 0.20 (95% CI 0.06 to 0.73) in favour of the pathway cohort. After correction for predefined confounders, adjusted OR was 0.20 (95% CI 0.06 to 0.75). The crude OR for SLE flares was 0.22 (95% CI 0.05 to 1.05) and 0.26 (95% CI 0.03 to 2.22) for TEEs in primary APS pregnancies, both in favour of the pathway cohort. After adjustment, the OR for SLE flares had a tendency towards a reduced frequency in the pathway cohort, although the result was not statistically significant, 0.22 (95% CI 0.04 to 1.09). The SLEPDAI was 7 (minimum 6 to maximum 8) and 4 (2–16), respectively for SLE flares in the pathway compared with the historical cohort. One patient in the pathway (4%) and three in the historical (11%) cohort suffered from a TEE. Three of these patients did not use LMWH at the time of the TEE. Also, three out of four patients were triple-positive for the aPL.
Figure 3.
Composite outcomes comparing the pathway with the historical cohort. Data depicted as number of pregnancies. *GEE model adjusted for predefined confounders: history of lupus nephritis, thromboembolic events, pre-eclampsia and the number of miscarriages.†GEE model adjusted for predefined confounders: lupus nephritis and EULAR/ACR criteria. ‡Crude OR was presented: the small number of events did not allow adjustment for confounders in the separate analysis of the patients with primary APS. §Composite outcome including miscarriage, gestational hypertension and severe hypertensive disease.¶Composite outcome including perinatal death, fetal growth restriction, congenital heart block, preterm birth <37 weeks, NICU admission. ACR, American College of Rheumatology; EULAR, European League Against Rheumatism; GEE, Generalised Estimating Equations; NICU, neonatal intensive care unit.
Table 2.
Primary and secondary outcomes for patients with SLE/APS in both cohorts
| Pregnancies (n) | SLE (±secondary APS) |
Primary APS (thrombotic+obstetric) |
||
| Pathway N=16 |
Historical N=43 |
Pathway N=25 |
Historical N=28 |
|
| Disease outcomes | ||||
| SLE flare | 2 (12.5) | 17 (39.5) | – | – |
| Major SLE flare | 1 (6.3) | 7 (16.3) | – | – |
| Kidney | 0/1 (0.0) | 6/7 (85.7) | – | – |
| Heart/Lungs | 1/1 (100.0) | 0/7 (0.0) | – | – |
| Nervous system | 0/1 (0.0) | 0/7 (0.0) | – | – |
| Haematological | 0/1 (0.0) | 2/7 (28.6) | – | – |
| Minor SLE flare | 1 (6.3) | 10 (23.3) | – | – |
| Joints | 1/1 (100.0) | 8/10 (80.0) | – | – |
| Skin | 0/1 (0.0) | 4/10 (40.0) | – | – |
| SLEPDAI* | 7 (6–8) | 4 (2–16) | – | – |
| Thromboembolic events | 0 (0.0) | 0 (0.0) | 1 (4.0) | 3 (10.7) |
| Maternal outcomes | ||||
| Miscarriage | 0 (0.0) | 3 (7.0) | 10 (40.0) | 14 (50.0) |
| <10 weeks | 0 (0.0) | 3 (7.0) | 10 (40.0) | 9 (32.1) |
| 10–16 weeks | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (17.9) |
| Gestational hypertension† | 1 (6.3) | 2 (5.0) | 2 (13.3) | 0 (0.0) |
| Severe hypertensive disease† | 5 (31.3) | 13 (32.5) | 4 (26.7) | 4 (28.6) |
| Vaginal delivery† | 11 (68.8) | 25 (62.5) | 7 (46.7) | 9 (64.3) |
| Fetal outcomes | ||||
| Perinatal death† | 1 (6.3) | 1 (2.5) | 0 (0.0) | 0 (0.0) |
| FGR (EFW <p10)† | 3 (18.8) | 8 (20.0) | 1 (6.7) | 1 (7.1) |
| Preterm birth <37 weeks† | 6 (37.5) | 15 (37.5) | 6 (40.0) | 3 (21.4) |
| Preterm birth <32 weeks† | 1 (6.3) | 5 (12.5) | 1 (6.7) | 2 (14.3) |
| Congenital heart block† | 0 (0.0) | 1 (2.5) | 0 (0.0) | 0 (0.0) |
| NICU admission† | 2 (12.5) | 7 (17.5) | 1 (6.7) | 2 (14.3) |
Data depicted as number of pregnancies (% of cohort).
Severe hypertensive disease=pre-eclampsia, eclampsia or HELLP.
*Median (minimum, maximum).
†For calculations miscarriages were excluded.
APS, antiphospholipid syndrome; EFW, estimated fetal weight; FGR, fetal growth restriction; HELLP, haemolysis elevated liver enzymes low platelets; NICU, neonatal intensive care unit; SLEPDAI, Systemic Lupus Erythematosus Pregnancy Disease Activity Index.
With respect to secondary outcomes, both maternal and fetal outcome composites were not significantly different (respectively, adjusted OR 0.91 (95% CI 0.38 to 2.17) and 1.26 (95% CI 0.55 to 2.88)). Incidence of severe hypertensive disease did not differ significantly between the pathway cohort and the historical cohort for both SLE (31% vs 33%) and APS (27% vs 29%). In SLE pregnancies, the number of preterm births was comparable between the cohorts (38%). One case of congenital heart block was observed in the historical cohort needing implantation of a permanent epicardial pacemaker after birth. For patients with primary APS, preterm birth was seen more often in the pathway cohort than the historical cohort (40% vs 21%), although not significant. There were no differences in mode of delivery between the cohorts with a mean caesarean rate of 39%.
Discussion
We are the first to demonstrate beneficial effects of the implementation of a multidisciplinary clinical pathway including prepregnancy counselling of patients with SLE/APS on pregnancy complications. This is to our knowledge the only comparative study that demonstrated a significant reduction in maternal-related complications in patients with SLE/APS that were managed within a clinical pathway. Notably, an almost fivefold reduction in SLE disease flares during pregnancy was achieved. Therefore, in addition to previous studies that extensively demonstrated the benefit of preconception counselling and timing of pregnancy, our study described an added-value managing pregnancies of patients with SLE/APS by a structured, multidisciplinary approach in a clinical pathway.15–22 31 Thus, our study establishes an approach to preconception counselling of patients with SLE/APS that is considered essential according to current international clinical practice guidelines.19
The present study investigated the implementation of a structured, multidisciplinary clinical pathway focused on patients with SLE/APS in the setting of an academic, referral centre. For this patient group with rare disease, evaluating the impact of implementation strategies is often challenging due to many unintended effects that occur by merely changing standard practice. Illustrative are the differences in baseline characteristics in our study where implementation of the clinical pathway may have led to the management of patients with SLE with higher grade of disease, less favourable disease characteristics and a higher number of patients with recurrent miscarriages due to obstetric APS. As such, it is very plausible that the implementation of a clinical pathway influences physicians’ decision to refer patients to that pathway. Nevertheless, even though the patients in the pathway cohort may have been skewed towards more severe SLE disease characteristics, maternal disease outcomes were improved without negative effects on the course of pregnancy. Implementation of the pathway, unfortunately, neither led to decreased pregnancy complications, which is likely associated with the disease characteristics at baseline. Also, for patients with APS, where more patients with thrombotic APS and triple aPL positivity were included in the historical cohort, there was no significant difference observed in TEEs. The incidence of 8% TEE was comparable to the EUROAPS study however.32 Altogether, it remains noteworthy to establish the high incidences of pregnancy and disease complications in patients with SLE/APS, that is, flares, TEEs, severe hypertensive disease, preterm birth and FGR, re-affirming the need of specialised care in a tertiary, academic centre.
Current standard practice on prepregnancy counselling often relies on peer consultation in (pre-)pregnancy, sometimes spread out over different hospitals, similar to the standard practice in the historical cohort of our study. We emphasised a more structured approach for these rare, often complex patients, starting before conception. Therefore, we organised a clinical pathway with a follow-up meeting every 2 weeks that provides an integral, patient-tailored treatment plan for the preconception, pregnancy, delivery and postpartum period based on the expert opinion of a multidisciplinary team. The multidisciplinary setting of the clinical pathway facilitated knowledge transfer, made interdisciplinary dialogue accessible, determined key management issues on a per case basis and created a learning environment on state-of-the-art developments in management of patients with SLE/APS during pregnancy. As such, a clinical pathway was hypothesised to improve pregnancy outcome of patients with SLE/APS.
Importantly, one of the strengths of this study is the effectiveness of the clinical pathway cohort evaluated on clinically relevant outcomes. This is in contrast to the majority of studies evaluating clinical pathways which focused on outcomes as cost issues or reduction in length of hospital stay.33 Also, even though the study’s sample size is small and patients were recruited over a 12-year period, significant improvement in disease outcome could be detected in favour of the clinical pathway. Since no exclusion criteria were used, and because the initiation of a clinical pathway within our hospital organisation provided a unique opportunity to study an ‘experiment of nature’ within our care organisation, we believe the results of this study can be generalised to other hospitals.
An inevitable limitation of this study is its small sample size caused by the rarity of SLE/APS and the negative impact on patients’ pregnancy wish. Although the specific interventions and therapy regimens during pregnancy have not dramatically changed over time, one could argue that some knowledge was increased given temporal trends which could have led to improved management of patients with SLE/APS before and during pregnancy. Therefore, outcomes could have altered over time, besides implementing the clinical pathway. Another limitation of the study was the inability to detect differences on clinically important outcomes for primary APS pregnancies. Furthermore, patients with SLE who were also aPL carriers were not given heparin by protocol in the clinical pathway (online supplemental table S2). Given the recent literature, preconception risk stratification should include aPL profile and heparin may be recommended to those patients with a more severe phenotype.25 34 Also, the recurrence rate of miscarriages was not included in this study that mainly focused on relevant pregnancy outcomes. Lastly, one needs to recognise that this study was conducted in a single centre and therefore careful interpretation of its results is warranted because of confounding factors and single-centre Hawthorne effects.24 33 Indeed, randomised controlled multicentre studies would be ideal for evaluating the effectiveness of the implementation of a clinical pathway, however hardly feasible and unethical in the rare and complex group of patients with SLE/APS with a pregnancy wish.
To conclude, our study demonstrated that patients with SLE/APS could benefit from prepregnancy counselling in a multidisciplinary clinical pathway. The risk of developing a SLE flare was lower even though the pathway cohort skewed towards more severe SLE disease characteristics. One has to recognise that the results of an implementation study that relies on a historical comparator have the inherent limitation that improvements in managing high-risk pregnancies in women with SLE/APS can also reflect temporal trends of improving management. However, because a randomised controlled study setting seems hardly feasible, our study provides evidence that the implementation of a multidisciplinary clinical pathway including prepregnancy counselling could contribute to optimisation of the care for patients with SLE/APS with a pregnancy wish.
Footnotes
Twitter: @LuVaCsL
MW, MH, MS and YKOT contributed equally.
Contributors: Conceptualisation: all authors. Data curation: MW, MH and TJB. Writing of the manuscript: MW, MH, MS and YKOT. Methodology: MW, MH, MS and YKOT. Data analysis: MW, MH and NvG. Critical revision of the manuscript for important intellectual content: JE, CFA, HJL, H-MJS, MKN, NvG, TH, TR and JMMvL. Supervision: MS and YKOT.
Funding: The work of YKOT was supported by the Dutch Kidney Foundation (17OKG04).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The data underlying this article will be shared on reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Approval for the study was obtained by the Medical Ethical Committee of the LUMC.
References
- 1.Rees F, Doherty M, Grainge MJ, et al. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology 2017;56:1945–61. 10.1093/rheumatology/kex260 [DOI] [PubMed] [Google Scholar]
- 2.Pons-Estel GJ, Alarcón GS, Scofield L, et al. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum 2010;39:257–68. 10.1016/j.semarthrit.2008.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Negrini S, Pappalardo F, Murdaca G, et al. The antiphospholipid syndrome: from pathophysiology to treatment. Clin Exp Med 2017;17:257–67. 10.1007/s10238-016-0430-5 [DOI] [PubMed] [Google Scholar]
- 4.Cervera R, Piette J-C, Font J, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum 2002;46:1019–27. 10.1002/art.10187 [DOI] [PubMed] [Google Scholar]
- 5.Erkan D, Yazici Y, Peterson MG, et al. A cross-sectional study of clinical thrombotic risk factors and preventive treatments in antiphospholipid syndrome. Rheumatology 2002;41:924–9. 10.1093/rheumatology/41.8.924 [DOI] [PubMed] [Google Scholar]
- 6.Smyth A, Oliveira GHM, Lahr BD, et al. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol 2010;5:2060–8. 10.2215/CJN.00240110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bundhun PK, Soogund MZS, Huang F. Impact of systemic lupus erythematosus on maternal and fetal outcomes following pregnancy: a meta-analysis of studies published between years 2001-2016. J Autoimmun 2017;79:17–27. 10.1016/j.jaut.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 8.Bouvier S, Cochery-Nouvellon E, Lavigne-Lissalde G, et al. Comparative incidence of pregnancy outcomes in treated obstetric antiphospholipid syndrome: the NOH-APS observational study. Blood 2014;123:404–13. 10.1182/blood-2013-08-522623 [DOI] [PubMed] [Google Scholar]
- 9.Bundhun PK, Soogund MZS, Huang F. Arterial/venous thrombosis, fetal loss and stillbirth in pregnant women with systemic lupus erythematosus versus primary and secondary antiphospholipid syndrome: a systematic review and meta-analysis. BMC Pregnancy Childbirth 2018;18:212. 10.1186/s12884-018-1850-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cimaz R, Spence DL, Hornberger L, et al. Incidence and spectrum of neonatal lupus erythematosus: a prospective study of infants born to mothers with anti-Ro autoantibodies. J Pediatr 2003;142:678–83. 10.1067/mpd.2003.233 [DOI] [PubMed] [Google Scholar]
- 11.Wei S, Lai K, Yang Z, et al. Systemic lupus erythematosus and risk of preterm birth: a systematic review and meta-analysis of observational studies. Lupus 2017;26:563–71. 10.1177/0961203316686704 [DOI] [PubMed] [Google Scholar]
- 12.Clark CA, Spitzer KA, Laskin CA. Decrease in pregnancy loss rates in patients with systemic lupus erythematosus over a 40-year period. J Rheumatol 2005;32:1709–12. [PubMed] [Google Scholar]
- 13.Clowse MEB, Magder LS, Witter F, et al. The impact of increased lupus activity on obstetric outcomes. Arthritis Rheum 2005;52:514–21. 10.1002/art.20864 [DOI] [PubMed] [Google Scholar]
- 14.Buyon JP, Kim MY, Guerra MM, et al. Predictors of pregnancy outcomes in patients with lupus: a cohort study. Ann Intern Med 2015;163:153–63. 10.7326/M14-2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tani C, Zucchi D, Haase I, et al. Are remission and low disease activity state ideal targets for pregnancy planning in systemic lupus erythematosus? A multicentre study. Rheumatology 2021. 10.1093/rheumatology/keab155. [Epub ahead of print: 16 Feb 2021]. [DOI] [PubMed] [Google Scholar]
- 16.Pastore DEA, Costa ML, Surita FG. Systemic lupus erythematosus and pregnancy: the challenge of improving antenatal care and outcomes. Lupus 2019;28:1417–26. 10.1177/0961203319877247 [DOI] [PubMed] [Google Scholar]
- 17.Moroni G, Doria A, Giglio E, et al. Fetal outcome and recommendations of pregnancies in lupus nephritis in the 21st century. A prospective multicenter study. J Autoimmun 2016;74:6–12. 10.1016/j.jaut.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 18.Bertsias G, Ioannidis JPA, Boletis J, et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a task force of the EULAR standing Committee for international clinical studies including therapeutics. Ann Rheum Dis 2008;67:195–205. 10.1136/ard.2007.070367 [DOI] [PubMed] [Google Scholar]
- 19.Andreoli L, Bertsias GK, Agmon-Levin N, et al. EULAR recommendations for women's health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis 2017;76:476–85. 10.1136/annrheumdis-2016-209770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sammaritano LR, Bermas BL, Chakravarty EE, et al. 2020 American College of rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Care Res 2020;72:461–88. 10.1002/acr.24130 [DOI] [PubMed] [Google Scholar]
- 21.Teng YKO, Bredewold EOW, Rabelink TJ. An evidence-based approach to pre-pregnancy counselling for patients with systemic lupus erythematosus. Rheumatology 2017;57. 10.1093/rheumatology/kex374 [DOI] [PubMed] [Google Scholar]
- 22.Knight CL, Nelson-Piercy C. Management of systemic lupus erythematosus during pregnancy: challenges and solutions. Open Access Rheumatol 2017;9:37–53. 10.2147/OARRR.S87828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jabbour M, Newton AS, Johnson D, et al. Defining barriers and enablers for clinical pathway implementation in complex clinical settings. Implement Sci 2018;13:139. 10.1186/s13012-018-0832-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev 2010;3:Cd006632. 10.1002/14651858.CD006632.pub2 [DOI] [PubMed] [Google Scholar]
- 25.Aringer M, Costenbader K, Daikh D, et al. 2019 European League against Rheumatism/American College of rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol 2019;71:1400–12. 10.1002/art.40930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. 10.1111/j.1538-7836.2006.01753.x [DOI] [PubMed] [Google Scholar]
- 27.Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 2016;75:795–810. 10.1136/annrheumdis-2015-208840 [DOI] [PubMed] [Google Scholar]
- 28.Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-Part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology 2016;55:1693–7. 10.1093/rheumatology/kev404 [DOI] [PubMed] [Google Scholar]
- 29.Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-Part II: analgesics and other drugs used in rheumatology practice. Rheumatology 2016;55:1698–702. 10.1093/rheumatology/kev405 [DOI] [PubMed] [Google Scholar]
- 30.Buyon JP, Kalunian KC, Ramsey-Goldman R, et al. Assessing disease activity in SLE patients during pregnancy. Lupus 1999;8:677–84. 10.1191/096120399680411272 [DOI] [PubMed] [Google Scholar]
- 31.Andreoli L, Fredi M, Nalli C, et al. Pregnancy implications for systemic lupus erythematosus and the antiphospholipid syndrome. J Autoimmun 2012;38:J197–208. 10.1016/j.jaut.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 32.Alijotas-Reig J, Ferrer-Oliveras R, Ruffatti A, et al. The European registry on obstetric antiphospholipid syndrome (EUROAPS): a survey of 247 consecutive cases. Autoimmun Rev 2015;14:387–95. 10.1016/j.autrev.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 33.El Baz N, Middel B, van Dijk JP, et al. Are the outcomes of clinical pathways evidence-based? A critical appraisal of clinical pathway evaluation research. J Eval Clin Pract 2007;13:920–9. 10.1111/j.1365-2753.2006.00774.x [DOI] [PubMed] [Google Scholar]
- 34.Lazzaroni M-G, Fredi M, Andreoli L, et al. Triple antiphospholipid (aPL) antibodies positivity is associated with pregnancy complications in aPL carriers: a multicenter study on 62 pregnancies. Front Immunol 2019;10:10. 10.3389/fimmu.2019.01948 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lupus-2020-000472supp001.pdf (146KB, pdf)
Data Availability Statement
Data are available on reasonable request. The data underlying this article will be shared on reasonable request to the corresponding author.