Abstract
Here, we present a case of a 43-year-old patient with a background of active intravenous drug use who was diagnosed with aortic valve endocarditis. This was complicated by extensive acute embolic stroke and acute splenic, renal and liver infarction. This case highlights the difficulties in managing infective endocarditis in intravenous drug users and the importance of a comprehensive approach, addressing both the intracardiac infection and the underlying issue of substance misuse, to ensure best patient outcomes.
Keywords: valvar diseases, cardiovascular medicine, infections
Background
Infective endocarditis (IE) is a multisystem condition resulting from bacterial or fungal infection of the endocardium and usually involves the heart valves. Intravenous drug use (IVDU) is a well-documented risk factor for IE. With an increasing number of individuals injecting drugs worldwide,1 2 the prevalence of associated IE cases are expected to increase. This case has highlighted the difficulties in managing intravenous drug users (IVDUs) with a diagnosis of IE and the challenges faced when complicated by multiple septic emboli. As surgical management in this patient group raises ethical dilemmas, we discuss the decision to operate and the need for further interventions to improve outcomes in the IVDU population.
Case presentation
This 43-year-old female patient presented to the emergency department with pain and swelling affecting her right foot, epigastric pain, vomiting, diarrhoea and a productive cough. Her medical history comprised active IVDU, previous necrotising fasciitis of the right leg, septic arthritis affecting the right ankle, deep vein thrombosis and hepatitis C. She was a known methicillin-resistant Staphylococcus aureus (MRSA) carrier. On initial assessment, the patient had no fever. She was admitted for investigation of potential septic arthritis of the right ankle and an acute abdominal cause of her symptoms. Her blood tests indicated raised inflammatory markers. An ankle X-ray showed no evidence of osteomyelitis. An MRI was unable to be performed due to lack of patient cooperation. Her temperature was later reported to be 38.1°C. Intravenous teicoplanin, ciprofloxacin and metronidazole were initiated as per microbiology guidance. Blood cultures were delayed due to difficulty gaining venous access, and intravenous antibiotics were switched to oral linezolid, ciprofloxacin and metronidazole. On day 3, a CT of the abdomen and pelvis identified wedge-shaped regions of hypoattenuation in the left kidney and spleen representing acute splenic and renal infarction (figure 1). A small pericardial effusion was noted. On day 6, a peripherally inserted central catheter (PICC) line was inserted to aid antibiotic administration. With persisting symptoms of abdominal pain and fever, a repeat CT of the abdomen and pelvis was performed. This demonstrated a new wedge-shaped hypodensity in the left lobe of the liver which likely represented a new embolic infarct (figure 2). There was evidence of pseudo-obstruction of the large bowel, a new right-sided pleural effusion and worsening pericardial effusion. The patient later left the ward unattended and returned with slurred speech and drowsiness. A white substance was aspirated from the PICC line. Blood cultures confirmed MRSA. A transthoracic echocardiogram was performed indicating vegetation on the aortic valve with cusp erosion, resulting in severe aortic regurgitation (figure 3, video 1). The patient deteriorated and subsequently developed a left-sided facial weakness. A CT of the head showed extensive low attenuation change within the right cerebral hemisphere representing acute ischaemic stroke in the distribution of the right middle cerebral artery (MCA) (figure 4). The stroke team advised to give aspirin without any further intervention due to the presence of multiple organ involvement. Prophylactic dose low-molecular-weight heparin was withheld to decrease the risk of haemorrhagic transformation. Cardiology input with cardiothoracic opinion resulted in transfer to the cardiothoracic department for consideration of cardiac surgery. It was decided that she was not fit for surgery due to the risk of haemorrhagic transformation on heparinisation, poor rehabilitation potential and current active IVDU. The patient continued to deteriorate post transfer, developing signs of raised intracranial pressure secondary to her stroke. She was reviewed by neurosurgery but did not fit the criteria for decompressive craniectomy due to late presentation after 72 hours. The patient then developed an aspiration pneumonia and her condition declined despite antibiotic therapy. The decision was made on day 12 to adopt a palliative approach with best supportive care. The patient passed away shortly thereafter.
Figure 1.
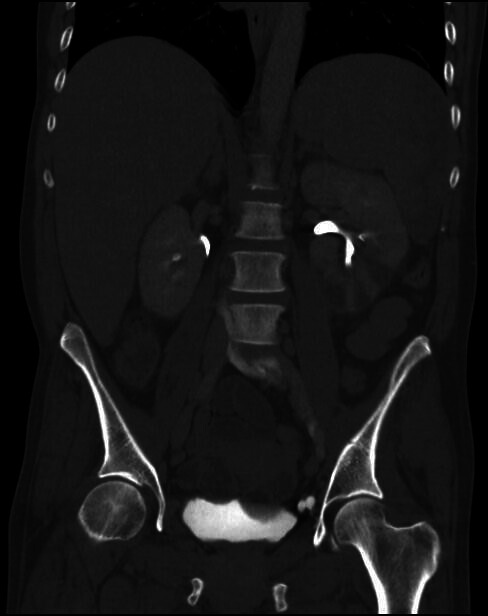
CT abdomen and pelvis image (coronal view) demonstrating a splenic infarct and left kidney infarcts.
Figure 2.
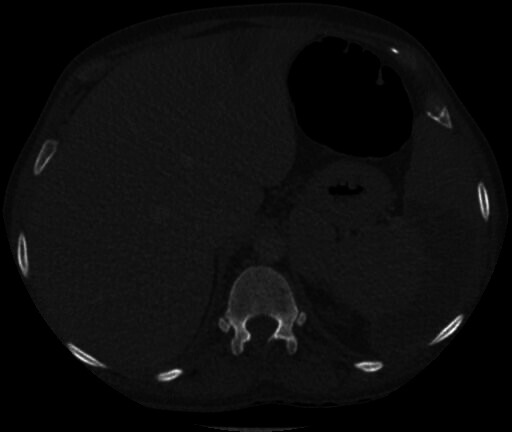
CT abdomen and pelvis image (axial view) demonstrating a splenic infarct and liver infarct.
Figure 3.
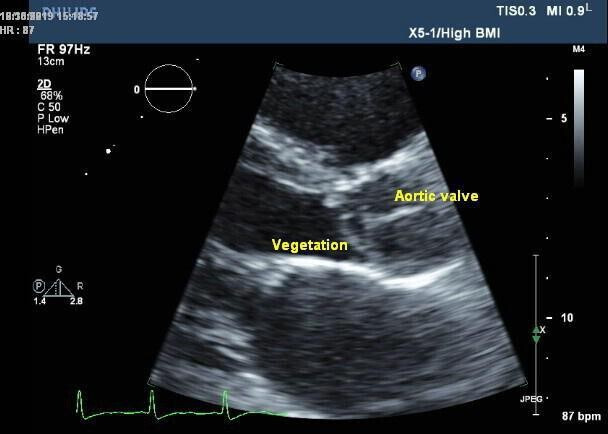
Transthoracic echocardiogram image demonstrating vegetation on the aortic valve with cusp erosion leading to severe aortic regurgitation. There was difficulty performing the scan due to poor patient cooperation.
Video 1.
Figure 4.
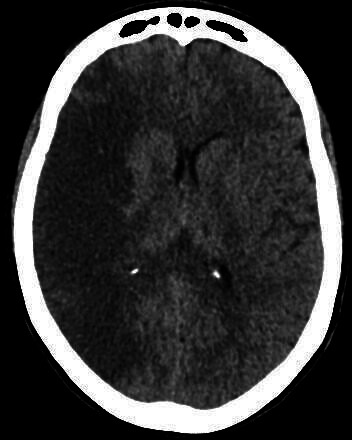
CT head image demonstrating low attenuation in the right middle cerebral artery territory in keeping with acute infarction.
Investigations
CT of the abdomen and pelvis with contrast.
Repeat CT of the abdomen and pelvis with contrast.
Transthoracic echocardiogram.
CT of the head without contrast.
Blood cultures.
Treatment
Intravenous teicoplanin, ciprofloxacin and metronidazole.
Oral option: linezolid, ciprofloxacin and metronidazole.
Intravenous teicoplanin and oral rifampicin.
Supportive care.
The patient was penicillin allergic. An oral antibiotic option was required if intravenous access was lost. Both teicoplanin and linezolid were started to treat a suspected MRSA infection. Due to concerns regarding intra-abdominal infection, ciprofloxacin and metronidazole were also given. Rifampicin was initiated alongside teicoplanin after blood cultures confirmed an MRSA bacteraemia.
Outcome and follow-up
The patient died during the hospital admission.
Discussion
IVDU is a notorious risk factor for IE. The estimated prevalence of individuals who inject drugs worldwide has increased between 2008 and 2017, with evidence of IVDU in 31 more countries than in 2008.1 2 A study conducted in a mid-Western tertiary care centre confirmed an increase in hospital admissions for IE secondary to IVDU by 436% from 2012 to 2017.3 Despite the number of IE cases associated with IVDU becoming an increasing concern, the disease as a complication of injecting drug practice is often overlooked.4
Septic embolisation is a life-threatening complication of IE, seen in 22%–50% of patients5 but falling to 6%–21% after initiation of antibiotic therapy.6–8 The most common microorganism accountable for IE in this patient group is Staphylococcus aureus, responsible for 68% of cases.9 Ruotsalainen et al concluded that extracardiac infection complicated 85% of IVDUs with S. aureus endocarditis.10 Multiple systems may be affected, with up to 65% of cases involving the central nervous system, predominantly in the MCA distribution.11 One study demonstrated that the incidence of stroke in individuals receiving appropriate antibiotic therapy was 4.8/1000 patient days in the first week of treatment, decreasing to 1.7/1000 patient days in the second week, with subsequent further reductions.12 It is evident that early initiation of antimicrobial therapy is key to minimising the risk of systemic emboli.13 At least three sets of blood cultures taken at 30-minute intervals, as proposed by the 2015 European Society of Cardiology (ESC) guidelines, is crucial to target antibiotic treatment and to facilitate a diagnosis of IE using the Modified Duke’s criteria.14 With difficulty obtaining blood cultures, gaining intravenous access and consequently administering antibiotics, the management of this patient was challenging.
The role of cardiac surgery was also considered in this patient’s case. The ESC guidelines advise surgery is indicated in left-sided valve endocarditis with evidence of severe heart failure, uncontrolled infection, including infection secondary to multiresistant organisms, and for the prevention of embolism in patients with prior embolic events or large vegetations.15 Despite this patient meeting these criteria, it has been advised that individuals with neurological complications requiring surgery for IE should delay the procedure for at least 4 weeks.16 With a risk of perioperative hypotension and haemorrhagic conversion following cardiopulmonary bypass, this is necessary to prevent further neurological complications.17 The US IE guidelines additionally recommend to avoid surgery when possible in IVDUs due to the risk of continued intravenous drug misuse and reinfection.18 A systematic review and meta-analysis concluded that IVDUs had a shorter survival and a higher risk for reoperation after cardiac surgery.19 Surgical management in this patient group is controversial and raises ethical considerations. It has been suggested that a holistic approach should be adopted, managing both the substance misuse and the intracardiac infection. Interventions that address substance misuse are in need of further study to improve outcomes in this population.20
Early discussions were carried out with the cardiology team. However, it was at a later stage of the admission when an echocardiogram was performed and the patients care formally taken over by the specialist team. Input was then gained from the Infective Endocarditis Lead at a tertiary centre and the patient was subsequently transferred. The complexity of the case alongside significant multisystem complications highlights the importance of early multidisciplinary team input. Timely transition to an optimal setting, ideally under the care of a specialist team, may have improved this patient’s outcome. It was equally essential to recognise for this patient when supportive care was in her best interests over further interventions.
This case has highlighted the difficulties in managing IVDUs with a diagnosis of IE. Issues with obtaining blood cultures, administration of antibiotics and misuse of long-term intravenous catheters alongside decisions regarding surgical management all occurred throughout admission. With patients more likely to present atypically and often with significant social difficulties, there are both diagnostic and therapeutic challenges in this patient population.21
Learning points.
Intravenous drug use (IVDU) is a significant risk factor for infective endocarditis (IE), with drug-related IE cases increasing in number.
This patient group can be complex to diagnose, investigate and treat, and a high index of clinical suspicion for IE is essential in active intravenous drug users with fever.
Early initiation of antimicrobial therapy is key to minimising the risk of systemic emboli.
Surgical management in this patient group raises ethical dilemmas due to the risk of continued IVDU and reinfection, and the decision to operate should involve careful risk–benefit analysis.
A multidisciplinary team approach is essential to improve outcomes in this patient population, addressing not only the intracardiac infection but also the underlying issue of substance misuse.
Footnotes
Contributors: Both Dr HLH and Professor BKJ have made substantial contributions to the conception and design of the case report alongside the acquisition, analysis and interpretation of data. Both authors have contributed to drafting and revising the case report and have been involved in final approval of the version published. They both agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Mathers BM, Degenhardt L, Phillips B, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet 2008;372:1733–45. 10.1016/S0140-6736(08)61311-2 [DOI] [PubMed] [Google Scholar]
- 2. Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2017;5:e1192–207. 10.1016/S2214-109X(17)30375-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Day S, Patel N, Maffett S. Infective endocarditis trends amongst intravenous drug users: an examination of practice patterns in a MID-WESTERN tertiary care center. J Am Coll Cardiol 2019;73:21. 10.1016/S0735-1097(19)33783-0 [DOI] [Google Scholar]
- 4. Keeshin SW, Feinberg J. Endocarditis as a marker for new epidemics of injection drug use. Am J Med Sci 2016;352:609–14. 10.1016/j.amjms.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanfilippo AJ, Picard MH, Newell JB, et al. Echocardiographic assessment of patients with infectious endocarditis: prediction of risk for complications. J Am Coll Cardiol 1991;18:1191–9. 10.1016/0735-1097(91)90535-H [DOI] [PubMed] [Google Scholar]
- 6. Vilacosta I, Graupner C, San Román JA, et al. Risk of embolization after institution of antibiotic therapy for infective endocarditis. J Am Coll Cardiol 2002;39:1489–95. 10.1016/S0735-1097(02)01790-4 [DOI] [PubMed] [Google Scholar]
- 7. Thuny F, Beurtheret S, Mancini J, et al. The timing of surgery influences mortality and morbidity in adults with severe complicated infective endocarditis: a propensity analysis. Eur Heart J 2011;32:2027–33. 10.1093/eurheartj/ehp089 [DOI] [PubMed] [Google Scholar]
- 8. Steckelberg JM, Murphy JG, Ballard D, et al. Emboli in infective endocarditis: the prognostic value of echocardiography. Ann Intern Med 1991;114:635–40. 10.7326/0003-4819-114-8-635 [DOI] [PubMed] [Google Scholar]
- 9. Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International collaboration on Endocarditis-Prospective cohort study. Arch Intern Med 2009;169:463–73. 10.1001/archinternmed.2008.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruotsalainen E, Sammalkorpi K, Laine J, et al. Clinical manifestations and outcome in Staphylococcus aureus endocarditis among injection drug users and nonaddicts: a prospective study of 74 patients. BMC Infect Dis 2006;6:137. 10.1186/1471-2334-6-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alsip SG, Blackstone EH, Kirklin JW, et al. Indications for cardiac surgery in patients with active infective endocarditis. Am J Med 1985;78:138–48. 10.1016/0002-9343(85)90376-6 [DOI] [PubMed] [Google Scholar]
- 12. Dickerman SA, Abrutyn E, Barsic B, et al. The relationship between the initiation of antimicrobial therapy and the incidence of stroke in infective endocarditis: an analysis from the ice prospective cohort study (ICE-PCS). Am Heart J 2007;154:1086–94. 10.1016/j.ahj.2007.07.023 [DOI] [PubMed] [Google Scholar]
- 13. Chu VH, Sexton DJ, Cabell CH, et al. Repeat infective endocarditis: differentiating relapse from reinfection. Clin Infect Dis 2005;41:406–9. 10.1086/431590 [DOI] [PubMed] [Google Scholar]
- 14. Lee A, Mirrett S, Reller LB, et al. Detection of bloodstream infections in adults: how many blood cultures are needed? J Clin Microbiol 2007;45:3546–8. 10.1128/JCM.01555-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of cardiology (ESC). endorsed by: European association for Cardio-Thoracic surgery (EACTS), the European association of nuclear medicine (EANM). Eur Heart J 2015;36:3075–128. 10.1093/eurheartj/ehv319 [DOI] [PubMed] [Google Scholar]
- 16. Byrne JG, Rezai K, Sanchez JA, et al. Surgical management of endocarditis: the Society of thoracic surgeons clinical practice guideline. Ann Thorac Surg 2011;91:2012–9. 10.1016/j.athoracsur.2011.01.106 [DOI] [PubMed] [Google Scholar]
- 17. Angstwurm K, Borges AC, Halle E, et al. Timing the valve replacement in infective endocarditis involving the brain. J Neurol 2004;251:1220–6. 10.1007/s00415-004-0517-x [DOI] [PubMed] [Google Scholar]
- 18. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American heart association. Circulation 2015;132:1435–86. 10.1161/CIR.0000000000000296 [DOI] [PubMed] [Google Scholar]
- 19. Goodman-Meza D, Weiss RE, Gamboa S, et al. Long term surgical outcomes for infective endocarditis in people who inject drugs: a systematic review and meta-analysis. BMC Infect Dis 2019;19:918. 10.1186/s12879-019-4558-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elbatarny M, Bahji A, Bisleri G, et al. Management of endocarditis among persons who inject drugs: a narrative review of surgical and psychiatric approaches and controversies. Gen Hosp Psychiatry 2019;57:44–9. 10.1016/j.genhosppsych.2019.01.008 [DOI] [PubMed] [Google Scholar]
- 21. Colville T, Sharma V, Albouaini K. Infective endocarditis in intravenous drug users: a review article. Postgrad Med J 2016;92:105–11. 10.1136/postgradmedj-2015-133648 [DOI] [PubMed] [Google Scholar]


