Panicker et al. summarize the functions of monogenic Parkinson’s disease genes and the major cell biological pathways that contribute to neurodegeneration.
Abstract
Parkinson’s disease (PD) is a progressive neurodegenerative disorder resulting from the death of dopamine neurons in the substantia nigra pars compacta. Our understanding of PD biology has been enriched by the identification of genes involved in its rare, inheritable forms, termed PARK genes. These genes encode proteins including α-syn, LRRK2, VPS35, parkin, PINK1, and DJ1, which can cause monogenetic PD when mutated. Investigating the cellular functions of these proteins has been instrumental in identifying signaling pathways that mediate pathology in PD and neuroprotective mechanisms active during homeostatic and pathological conditions. It is now evident that many PD-associated proteins perform multiple functions in PD-associated signaling pathways in neurons. Furthermore, several PARK proteins contribute to non–cell-autonomous mechanisms of neuron death, such as neuroinflammation. A comprehensive understanding of cell-autonomous and non–cell-autonomous pathways involved in PD is essential for developing therapeutics that may slow or halt its progression.
Introduction
Parkinson’s disease (PD) is an incurable neurodegenerative disorder characterized by progressive motor deficits including tremor, rigidity, postural instability, and bradykinesia. It is the second most common neurodegenerative disease, and the fastest growing in deaths, disability-adjusted life years, and prevalence among all neurological diseases (Bandres-Ciga et al., 2020). The motor symptoms of PD are caused by death of dopamine (DA)–producing neurons within the substantia nigra pars compacta (SNpc), resulting in DA depletion in the SNpc projection region called the striatum. This depletion of striatal DA is accompanied by the appearance of Lewy bodies (LBs), characteristic intracytoplasmic inclusions within the surviving neurons. Diminished striatal DA results in the dysregulation of basal ganglia circuits, leading to core PD motor deficits (Savitt et al., 2006). While symptomatic therapy through DA replacement has been available since the 1960s (Connolly and Lang, 2014), there are no disease-modifying treatments.
For much of the 20th century, genetics were thought to play a negligible role in PD. However, in the 1990s, observations of Mendelian PD inheritance patterns within certain families led to the identification of SNCA, which codes for the α-synuclein (α-syn) protein, as the causal gene (Polymeropoulos et al., 1996; Polymeropoulos et al., 1997). Subsequently, as many as 90 genes potentially linked to PD have been identified; of these, a small number are able to cause monogenic PD with varying degrees of penetrance (Bandres-Ciga et al., 2020). Even though monogenic PD patients constitute 10–20% of cases, investigating the cell biology of the implicated proteins has led to crucial insights into the mechanisms of both sporadic and familial PD. Furthermore, environmental stressors or other external factors can activate or inhibit proteins implicated in monogenic PD, thereby linking them to sporadic PD. While much previous work has been neuron-centric or cell-type agnostic, recent studies have revealed important roles for these proteins in non–cell-autonomous processes that lead to DA neuron death. Several PD-associated proteins are also expressed by other cell types within the central nervous system (CNS), where they may amplify or dampen inflammatory signaling, thereby modulating PD-associated non–cell-autonomous processes (Hinkle et al., 2019; Kam et al., 2020).
In this review, we summarize the major known functions and pathophysiological mechanisms of monogenic PD genes (Table 1). We further describe the contribution of these genes to glia (dys)function in health and disease, and identify unifying cell biological pathways that contribute to both familial and sporadic PD.
Table 1. Summary of PARK proteins and their roles in PD cell biology.
| Protein (gene) | Native function | Major neurodegenerative pathways | Further reading |
|---|---|---|---|
| α-Syn (SNCA/PARK1) | Regulation of presynaptic function through SNARE complex and synaptic vesicle interactions. | Forms degradation resistant aggregates that disrupt numerous cell biological functions. Posttranslational modifications often promote aggregation. Self-templated spread of α-syn pathology ensues following its cell-to-cell transmission via LAG3-mediated uptake. USP19 mediates LAG3 exocytosis. | Burré et al., 2018; Hijaz and Volpicelli-Daley, 2020; Rocha et al., 2018 |
| β-Glucocerebrosidase (GBA1) | Lysosomal enzyme responsible for glycolipid breakdown. | Loss of function promotes aggregation of α-syn due to impaired endolysosomal function. Furthermore, accumulation of GCase1 substrates is sufficient to induce α-syn fibrillization, though evidence of substrate accumulation in human patients is lacking. | Do et al., 2019; Ryan et al., 2019; Stojkovska et al., 2018 |
| LRRK2 (LRRK2/PARK8) | Multifunctional GTPase, kinase, and signaling scaffold involved in numerous cellular functions. | LRRK2 phosphorylates 4-EBP and the ribosomal subunit protein S15 to increase global protein translation. It associates with β-tubulin to mediate decreased microtubule stability. LRRK2-mediated Rab protein phosphorylation inactivates them, compromising vesicular sorting. | Berwick et al., 2019; Harvey and Outeiro, 2019; Madureira et al., 2020 |
| VPS35 (VPS35) | Component of heterotrimeric retromer complex involved in cargo sorting during vesicular transport. | D620N mutation causes a partial loss of function that disrupts the retromer complex’s sorting function. These defects include impaired endolysosome maturation and autophagy, disrupted recycling of membrane receptors, and impaired formation of mitochondrial-derived vesicles. | Rahman and Morrison, 2019; Sassone et al., 2020; Williams et al., 2017 |
| Parkin (PRKN/PARK2) | E3 ubiquitin ligase that is activated in conjunction with PINK1 in response to mitochondrial stress. Leads to promiscuous ubiquitination of cytosolic and mitochondrial substrates. | PD-associated mutations or c-Abl–mediated Y-phosphorylation abrogates parkin E3 ligase activity, causing an accumulation of its substrates. Accumulation of AIMP2 activates a cell death pathway called parthanatos. Accumulation of PARIS represses mitochondrial biogenesis and function. PINK1 phosphorylates ubiquitin and parkin to mediate parkin activation. Parkin-mediated mitochondrial OMM protein ubiquitination targets mitochondria for clearance via mitophagy. PINK1/parkin signaling maintains a balance between mitochondrial fission and fusion. PINK1/parkin phosphorylate and ubiquitinate (respectively) the protein miro, inhibiting mitochondrial transport. | Bader and Winklhofer, 2020; Ge et al., 2020; Pickrell and Youle, 2015; Quinn et al., 2020; Scarffe et al., 2014 |
| PINK1 (PINK1/PARK6) | Mitochondria-localized protein kinase activated by mitochondrial stress. Co-activates with parkin to mediate mitochondrial quality control. Has parkin-independent role in maintaining ETC. | Major cell biological pathways overlap with Parkin. | Bader and Winklhofer, 2020; Ge et al., 2020; Pickrell and Youle, 2015; Quinn et al., 2020; Scarffe et al., 2014 |
| DJ-1 (PARK7) | Oxidative stress sensor through covalent modification of C106 residue, used for activation of numerous oxidative stress pathways. | Loss of DJ-1 leads to pleiomorphic defects in responses to reactive chemical species such as oxidative and glycative stress. | Biosa et al., 2017; Dolgacheva et al., 2019; van der Vlag et al., 2020 |
α-Syn
Point mutations and gene multiplications of the SNCA gene cause autosomal dominant PD (Kasten and Klein, 2013), while polymorphisms in the SNCA gene locus are implicated in sporadic PD (Nalls et al., 2014). Coupled with evidence that α-syn constitutes a major component of LBs (Spillantini et al., 1998), these findings caused a paradigm shift in our understanding of PD. α-Syn is a 140-aa protein comprising an N terminus that assumes an α-helical secondary structure upon membrane binding, a hydrophobic non-amyloid component domain that can adapt a β-sheet conformation and contributes to aggregation, and a highly negatively charged unstructured C-terminal domain. α-Syn is abundantly expressed in the CNS, and localizes to the vicinity of synaptic vesicles, with further work suggesting it plays a role in synaptic transmission (Fig. 1 A; Burré et al., 2018). Under pathological conditions, α-syn can assume a β-pleated secondary structure that forms the basis for LBs and Lewy neurites (Tuttle et al., 2016). α-Syn monomers coalesce to form string-like protofibrils, which can assemble into larger fibrils. Some studies indicate that the aggregated forms of α-syn are not toxic to neurons and that LBs may, in fact, be a protective mechanism to compartmentalize toxic oligomeric α-syn species (Chartier and Duyckaerts, 2018). These studies raise the issue/relationship between aggregation and toxicity. α-Syn variants that are more prone to form oligomers were shown to exhibit greater neurotoxicity than variants predisposed to forming higher-order fibrils/aggregates (Winner et al., 2011). It is likely that heterogenous populations of α-syn conformations exist within degenerating neurons, which may mediate distinct pathological events such as seeding, aggregation, or neurotoxicity via independent mechanisms (Danzer et al., 2007).
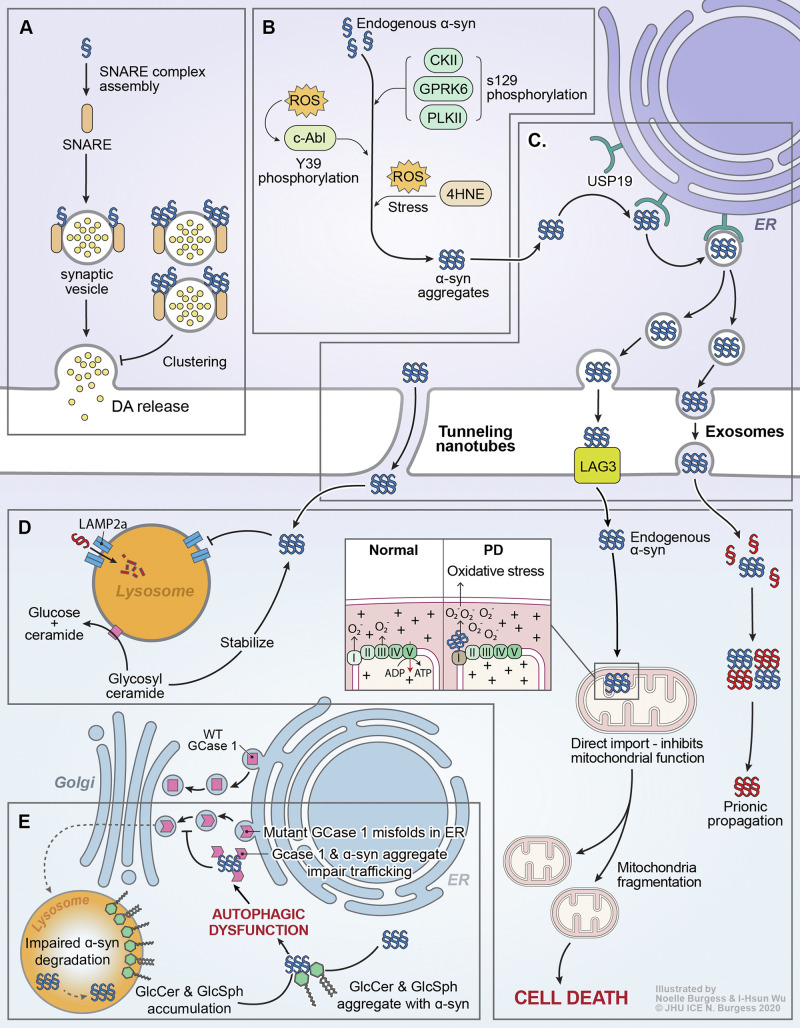
Figure 1.
α-Syn and GBA1 signaling in PD. (A) Monomeric α-syn acts as a chaperone for SNARE proteins, promoting synaptic transmission/DA release. Certain aggregated species of α-syn can reduce DA release by inhibiting synaptic vesicle clustering. (B) Progressive accumulation of α-syn aggregates is a fundamental characteristic of PD progression. Numerous posttranslational modifications have been reported to modulate α-syn aggregation, including pS129 (unclear effect) via multiple kinases, pY39 (pro-aggregation) by c-Abl, and 4-hydroxy-2-nonenal (4HNE) and other modifications mediated by oxidative stress. (C) Aggregated α-syn is incorporated into the ER-transport machinery by USP19 and can propagate from cell to cell via tunneling nanotubes or exosomes, or by direct LAG3-mediated uptake into efferent neurons. (D) Endocytosed α-syn fibrils can seed the templated aggregation of endogenous α-syn and drive prion-like propagation. α-Syn aggregates can also be directly imported into mitochondria, eliciting mitochondrial fragmentation and death. The function of lysosomes, where GCase1 localizes to and metabolizes glycolipids, is also impaired by the presence of α-syn aggregates. (E) GBA1-associated PD is thought to be closely linked to dysregulation of α-syn proteostasis. Likewise, sporadic PD is thought to drive impairments in GCase1 activity. In patients carrying mutant GBA1 alleles, coding mutations in the GCase1 protein may lead to misfolding in the ER, leading to direct coaggregation with α-syn or indirectly causing α-syn aggregation by impairing autophagy. In sporadic PD, loss of GCase1 function may be driven by coaggregation with α-syn, which then serves as a positive feedback loop to accelerate further α-syn aggregation. In addition, accumulation of GCase1 substrates such as GlcCer and glucosylsphingosine (GlcSph) is sufficient to trigger α-syn aggregation.
Posttranslational modifications of α-syn in PD pathology
Posttranslational modifications (PTMs) of proteins are essential for diversifying the proteome and regulating protein function. α-Syn PTMs include enzymatic or spontaneous addition of covalent adducts (e.g., phosphorylation, nitration, ubiquitination, etc.) or direct modification of the peptide sequence itself (truncation; Tenreiro et al., 2014; Zhang et al., 2019). These modifications profoundly affect the development and propagation of pathology by altering α-syn conformation, aggregation kinetics, fibril ultrastructure, subcellular localization, and molecular interactions (Li and Liu, 2021; Tenreiro et al., 2014; Zhang et al., 2019). Of the many α-syn PTMs described in the literature (Tenreiro et al., 2014; Zhang et al., 2019), we highlight a number that have been widely studied (see text box and Fig. 1 B). α-Syn is subject to numerous modifications in both PD and non-PD subjects, and the combinatorial effects of PTMs and other noncovalent cofactors on protein function, fibril structure, and pathological characteristics require further study (Li and Liu, 2021). The PTM landscape of α-syn is further shaped by cell type–specific factors such as presence of different modifying enzymes, chaperones, and reactive chemical species, an area that remains poorly characterized.
Examples of α-syn posttranslational modifications
Phosphorylation of α-syn is one of its most studied covalent, enzyme-mediated modifications and plays a crucial role in its aggregation, propagation, and neurotoxicity. Numerous serine, threonine, and tyrosine residues are phosphorylated, mostly in the C-terminal domain (Zhang et al., 2019). S129 phosphorylation has emerged as a defining hallmark of PD pathology, being almost absent in monomeric α-syn and present in >90% of LBs (Tenreiro et al., 2014). Numerous kinases are reported to phosphorylate α-syn at this site, including CKI/II, GRKs, and PLK2 (Inglis et al., 2009; Okochi et al., 2000; Pronin et al., 2000). However, whether this modification is pathological or protective is unclear; different groups have reported increased while others reported decreased fibril formation or toxicity (Tenreiro et al., 2014; Zhang et al., 2019). While the cause of these discrepancies is largely unknown, some possibilities include species or cell type–dependent expression of other factors influencing α-syn aggregation, the identity of the kinase(s) modifying S129, combinatorial effects from other PTMs, and expression level of α-syn.
α-Syn phosphorylation at Y39 has also attracted substantial attention. Like pS129, pY39 is found to be elevated during aging and accumulates in LBs of sporadic PD patients (Brahmachari et al., 2016). The nonreceptor tyrosine kinase c-Abl phosphorylates α-syn at Y39, leading to accelerated α-syn aggregation and impaired degradation (Brahmachari et al., 2016; Mahul-Mellier et al., 2014). More recent studies have found that Y39-phosphorylation diminishes α-syn’s interaction with chaperone proteins such as HSC70 and HSP90β, resulting in its mitochondrial accumulation and concomitant aggregation (Burmann et al., 2020). Furthermore, cryo-EM studies of synthetic fibrils generated from pY39 α-syn note that they are structurally distinct from WT fibrils and incorporate a greater proportion of the protein including the entire N-terminal region into the fibril core (Li and Liu, 2021; Zhao et al., 2020). These pY39 fibrils seed a greater degree of α-syn pathology and induce greater cytotoxicity than WT fibrils when added to cultured rat neurons (Zhao et al., 2020). As such, phosphorylation at Y39 appears to contribute to α-syn pathology by inducing the formation of more pathogenic fibrillar structures and by disrupting interactions with protein chaperones critical for inhibiting aggregation.
While the two prior examples of PTMs are examples of enzyme-dependent modifications, α-syn can also undergo enzyme-independent modifications by reactive chemical species (Schildknecht et al., 2013). These modifications provide a critical link between α-syn aggregation and the oxidative stress frequently associated with PD (Dias et al., 2013). Oxidative stress can induce oxidation of α-syn at its C terminus (Mirzaei et al., 2006). The byproduct of lipid oxidation, 4-hydroxy-2-nonenal, can associate with and promote β-sheet formation leading to increased soluble oligomeric species generation (Qin et al., 2007). Nitrated α-syn is also known to accumulate in LBs (Giasson et al., 2000) and promotes fibrilization (Hodara et al., 2004). All tyrosine residues in α-syn are vulnerable to nitration, but nitration’s effect on oligomerization depends on residue location (Burai et al., 2015; Sevcsik et al., 2011). Nitration of Y39, for example, accelerates aggregation and leads to robust fibril formation (Danielson et al., 2009). Finally, oxidized DA and DA metabolites can reversibly or irreversibly modify α-syn, leading to altered aggregation characteristics, increased cytotoxicity, and impaired aggregate removal (Post et al., 2018).
Cell biological roles of α-syn at the presynapse, cytoskeleton, and mitochondria
Consistent with its subcellular distribution, several potential pathogenic roles have been ascribed to α-syn pertaining to its function at the synapse (Fig. 1 A). Adeno-associated virus (AAV)–mediated α-syn overexpression leads to a redistribution of the SNARE proteins, which play a role in neurotransmitter release (Chung et al., 2009; Garcia-Reitböck et al., 2010). Membrane-bound multimeric α-syn promotes SNARE complex assembly by acting as an unconventional chaperone for their components (Burré et al., 2014; Burré et al., 2010). Overexpression of α-syn also inhibits neurotransmitter release at the synapse (Nemani et al., 2010). One of the first proteins shown to interact with α-syn was the presynaptic protein synphilin-1 (Engelender et al., 1999), which ameliorates pathology induced by α-syn overexpression through the formation of aggresome-like structures (Smith et al., 2010).
α-Syn also interacts with and perturbs assembly of cytoskeletal proteins (Alim et al., 2002; Lee et al., 2006). Genome-wide association studies (GWAS) show an association of PD with genomic variation at the locus encoding the Alzheimer’s-associated Tau protein, which mediates cytoskeletal stability (Nalls et al., 2011). Tau aggregation is a hallmark of numerous neurodegenerative diseases (Kovacs, 2015). α-Syn and Tau interact and can reciprocally seed each other’s aggregation (Giasson et al., 2003). Hyperphosphorylated Tau has also been observed in a model of α-syn overexpression (Haggerty et al., 2011). Co-expression of α-syn enhanced Tau-induced DA neuron death in drosophila (Roy and Jackson, 2014). α-Syn might also play a role in cytoskeletal assembly; WT and A30P mutant α-syn elicited opposite effects on actin assembly, slowing down and increasing the rate of polymerization, respectively (Sousa et al., 2009).
α-Syn also appears to contribute to mitochondrial dysfunction (Fig. 1 D). Despite lacking a mitochondrial targeting sequence, a pool of endogenous α-syn is present in the mitochondrial membrane of DA neurons (Li et al., 2007). Interaction of oligomeric α-syn with mitochondrial membranes can lead to their fragmentation and Drp1-independent mitochondrial fission (Nakamura et al., 2011). α-Syn also inhibits mitochondrial complex–1 activity (Devi et al., 2008; Liu et al., 2009). Overexpressing the master mitochondrial biogenesis regulator peroxisome proliferator activated receptor γ coactivator 1 α (PGC-1α) prevents A53T α-syn–induced neurotoxicity in rat midbrain primary cultures (Zheng et al., 2010).
Prion-like propagation of α-syn and its potential role in PD pathogenesis
A substantial body of evidence suggests that α-syn aggregates propagate across the CNS in a prion-like manner (Fig. 1 C). PD has been classified into six clinicopathological stages based on the severity and localization of CNS LB pathology. LB pathology seems to initiate in the brainstem and olfactory bulb before appearing in the midbrain and SNpc, and eventually reaching the cortex (Braak et al., 2003). These results suggest the spread of α-syn pathology from one brain region to the other, a notion that received impetus following the finding that fetal midbrain neurons transplanted into PD patients also develop Lewy pathology (Kordower et al., 2008; Li et al., 2008). Moreover, application of exogenous α-syn preformed fibrils (PFFs) to cultured cells and neurons and injection into the brains of WT mice lead to the aggregation of endogenous α-syn and propagation of pathology (Luk et al., 2012; Luk et al., 2009; Volpicelli-Daley et al., 2011). Furthermore, α-syn passive immunotherapy with α-syn monoclonal antibodies reduces PFF-induced pathology and neuron death in vitro and in vivo (Tran et al., 2014), indicating that extracellular transfer is required for PFF-induced disease progression. While there have been concerns about the validity of applying PFFs given their structural differences from in vivo aggregates (Li and Liu, 2021), other studies using aggregates isolated from patient brains also seed pathology in a prion-like manner. α-Syn–containing LB-enriched fractions from PD patient brain tissue seed pathology and initiate progressive neurodegeneration in mice and primates (Recasens et al., 2014), while intracerebral injections of brain homogenates or α-syn isolated from the brains of Dementia with LB or Multisystem Atrophy patients can also initiate endogenous α-syn aggregation and pathology in mice (Masuda-Suzukake et al., 2013; Watts et al., 2013).
The mechanisms by which α-syn aggregates are released or taken up by neurons is an area of active research (Fig. 1 C; Hijaz and Volpicelli-Daley, 2020). Uptake is thought to be mediated through an active, receptor-mediated process, with work suggesting that cell-surface heparan sulfate proteoglycans and neurexin-1β facilitate uptake (Birol et al., 2019; Holmes et al., 2013; Ihse et al., 2017). In addition, lymphocyte-activation gene 3 (LAG3) acts a major receptor that binds to and mediates α-syn PFF internalization into neurons. LAG3-deficient mice are protected from PFF-induced DA neuron loss, striatal DA depletion, and motor deficit onset (Mao et al., 2016). However, there are likely additional receptors or receptor-independent mechanisms through which α-syn is internalized into neurons since LAG3 does not account for all PFF binding. Upon their uptake by neurons, PFFs can activate the cell death pathway parthanatos, resulting in the generation of poly(ADP-ribose) polymers, which cross-seed α-syn aggregation, accelerating α-syn transmission and pathology (Kam et al., 2018). The release of pathological α-syn from neurons is poorly understood, but may involve secretion of externalized vesicles called exosomes in a calcium-dependent manner (Emmanouilidou et al., 2010). The ER-localized deubiquitylase USP19 may mediate export of misfolded cytosolic proteins, including α-syn, providing an alternative mechanism for release (Lee et al., 2016). The transfer of α-syn aggregates may also be accomplished via tunneling nanotubules, slender F-actin channels that connect “donor” and “acceptor” cells and transfer aggregates, which seed fibrilization of endogenous α-syn in the acceptor cell (Abounit et al., 2016).
β-glucocerebrosidase (GCase1)
GBA1 encodes the 497-aa protein β-glucocerebrosidase (GCase1) that localizes to the lysosomal membrane, with the active site in the lysosomal lumen (Do et al., 2019). There, it metabolizes glucosylceramide (GlcCer), regulating ceramide signaling and preventing toxic accumulation of GlcCer (Do et al., 2019). Homozygous or compound heterozygous loss-of-function GBA1 mutations can cause Gaucher disease (GD), a lysosomal storage disorder characterized by lysosomal accumulation of sphingolipids that can be accompanied by CNS pathology (Do et al., 2019). Heterozygous GBA1 mutations are among the most common genetic risk factors for PD, having been identified in 7–12% of patients (Do et al., 2019). GCase1 dysfunction and α-syn pathology appear to be closely linked, as patients with GD or carrying heterozygous GBA1 mutations may exhibit widespread LBs or Lewy neurites (Schneider and Alcalay, 2017). Moreover, GCase1 enzymatic activity is inversely correlated with the severity of α-syn pathology (Murphy et al., 2014), and is reduced in the cerebrospinal fluid and SNpc of GBA1-PD and sporadic PD cases (Balducci et al., 2007; Gegg et al., 2012).
Loss of GCase1 function and α-syn form a pathological positive feedback loop
Loss-of-function GBA1 mutations and loss of GCase1 activity in sporadic PD form a pathological positive feedback loop with α-syn aggregation (Fig. 1 E; Mazzulli et al., 2011). In mice with transgenic or viral-induced α-syn overexpression, loss of GCase1 function through genetic knockout (KO), knock-in of disease mutants, or use of GCase1 inhibitors accelerates disease progression, α-syn pathology, and cell death (Do et al., 2019; Ryan et al., 2019). In cell or neuron culture systems, reduced GCase1 activity is associated with increased α-syn half-life, higher resting levels, and spontaneous formation of pathological oligomers (Du et al., 2015; Kim et al., 2018b; Kim et al., 2018c; Zunke et al., 2018). Moreover, GCase1 inhibition increased vulnerability of cultured neurons to α-syn PFF, and mildly amplifies PFF pathology in vivo, though overall spread of pathology and degree of DA neuron loss seem unaltered (Henderson et al., 2020). Conversely, increasing GCase1 activity through overexpression or treatment with molecular chaperones improves pathology, behavioral abnormalities, and survival in α-syn transgenic mice (Migdalska-Richards et al., 2016; Morabito et al., 2017; Sardi et al., 2011; Sardi et al., 2013).
α-Syn pathology also feeds back to inhibit GCase1 function. In postmortem tissue from PD cases associated with GBA1 mutations, GCase1 is localized to LBs (Goker-Alpan et al., 2008), suggesting that mutant GCase1 may promote LB formation or that LBs may sequester synthesized enzyme. Furthermore, α-syn overexpression or PFF treatment in cell lines, neuron culture, mouse brain, and mouse enteric nervous system reduces GCase1 levels and enzymatic activity (Challis et al., 2020; Henderson et al., 2020; Kim et al., 2018a; Mazzulli et al., 2011). Thus, in both sporadic and GBA1 mutation–associated PD, aggregation of α-syn may compromise GCase1 function, which potentially further drives α-syn aggregation.
Impaired α-syn proteostasis through ER-endolysosomal dysfunction
While loss of GCase1 activity leads to neurodegeneration by aggravating α-syn pathology, the underlying mechanisms remain unclear. One hypothesis is that GBA1 mutations lead to α-syn aggregation by impairing protein processing through the ER-endolysosomal system (Fig. 1 E). Some GBA1 mutations lead to the production of a misfolded protein that then accumulates in the ER and triggers the ER unfolded protein response (Do et al., 2019; Kim et al., 2018a). Furthermore, loss of GCase1 function may impair autophagy through protein phosphatase–2A inactivation, ceramide reduction, Rab8 dysfunction, or failure of autophagic lysosome reformation (Du et al., 2015; Kim et al., 2018b; Magalhaes et al., 2016; Scarlatti et al., 2004). Given the importance of autophagy in mediating the turnover of α-syn, especially that of pathological aggregates resistant to the ubiquitin-proteasome system (Xilouri et al., 2013), these defects may contribute to progressive failure of α-syn proteostasis. GCase1 may also directly play a role in the degradation of α-syn in the lysosome. GCase1 interacts with the C terminus of α-syn at lysosomal pH, and the two proteins colocalize in the lysosome (Yap et al., 2011). Further, α-syn’s nonamyloid component domain may become exposed to the lysosomal lumen upon interacting with GCase1, which may increase its accessibility to lysosomal proteases (Yap et al., 2015). The PD-associated N370S mutant protein shows reduced affinity to α-syn, suggesting that it may directly impair lysosomal degradation (Yap et al., 2011). By disrupting ER-endolysomal function at multiple levels, mutations in GBA1 may compromise α-syn turnover and lead to the accumulation of pathological aggregates.
It is unclear whether GCase1 regulates monomer levels or mediates removal of pathogenic aggregates. While some studies find that monomer levels are elevated when GCase1 activity is lost in transgenic cells, Drosophila melanogaster, and mouse models (Du et al., 2015; Magalhaes et al., 2016; Do et al., 2019), other studies fail to report changes in monomer levels in mice lacking α-syn overexpression (Henderson et al., 2020; Ikuno et al., 2019; Kim et al., 2018a; Tayebi et al., 2017). This suggests that elevated monomer levels associated with GCase1 loss may in part be an overexpression artifact, while endogenous levels of α-syn may not require GCase1 for turnover. A recent study reports that loss of GCase1 activity amplifies PFF-induced pathology more at low doses in culture and in brain regions normally associated with low α-syn pathology, suggesting that loss of GCase1 activity may increase the likelihood of seeding pathology at the earliest disease stages (Henderson et al., 2020). Such a model posits that GCase1 may modulate risk of disease initiation or amplify an ongoing disease process rather than rather than triggering initiation directly, which is consistent with findings that GBA1 mutations increase the risk of developing PD rather than causing highly penetrant monogenic disease. Moreover, GCase1’s role in lysosome function suggests it may be more critical for removal of aggregates than for regulating monomer levels (Xilouri et al., 2013). If this model proves to be consistent with further investigations, future studies that identify which oligomer species are the major target of GCase1-dependent clearance mechanisms may yield insight into the α-syn strains that drive disease initiation and propagation.
GCase1 substrate accumulation and α-syn aggregation
Another emerging hypothesis is that accumulation of glycolipids resulting from loss of GCase1 activity may drive lipid-induced misfolding and aggregation of α-syn (Fig. 1 E). Incubating GlcCer and glucosylsphingosine with purified α-syn is sufficient to convert α-syn into degradation-resistant, neurotoxic aggregates that can seed further monomer aggregation (Mazzulli et al., 2011; Suzuki et al., 2015; Taguchi et al., 2017; Zunke et al., 2018). Furthermore, inhibiting GlcCer synthase can rescue α-syn pathology either associated with or independent of GBA1 mutations (Kim et al., 2018c; Sardi et al., 2017; Zunke et al., 2018). However, while immortalized human cell lines, induced human neurons, and the brains of transgenic mice carrying PD-associated GBA1 mutations show substantial GlcCer and glucosylsphingosine accumulation (Kim et al., 2018c; Mazzulli et al., 2011; Suzuki et al., 2015), evidence that such substrate accumulation occurs in human PD patients is lacking (Boutin et al., 2016; Gegg et al., 2015). While it is clear that substrate accumulation alone may be sufficient to induce α-syn pathology, it is still uncertain whether it plays a critical role in the human disease.
Leucine-rich repeat kinase 2 (LRRK2)
LRRK2 encodes a 2,527-aa multidomain protein containing N-terminal armadillo and Akyrin repeats, leucine-rich repeats, a central catalytic tridomain (composed of GTPase [Ras of complex proteins; ROC], a C-terminal of ROC linker, and kinase domains), and a C-terminal WD-40 repeat region (Berwick et al., 2019; Madureira et al., 2020). LRRK2 mutations account for roughly 1% of sporadic and 4% of familial PD cases, showing incomplete, age-dependent penetrance (Berwick et al., 2019; Madureira et al., 2020). Furthermore, the LRRK2 gene locus was identified in GWAS as a genetic risk for sporadic PD (Nalls et al., 2011).
LRRK2 activity is governed by a complex intramolecular interplay between the GTPase and kinase domains (Berwick et al., 2019). P-loop mutations in the GTPase domain abrogate kinase activity (Ito et al., 2007; Smith et al., 2006). Since LRRK2 autophosphorylates the ROC domain, it is likely that its kinase activity regulates GTPase activity. Consistent with this model, mutating these autophosphorylation sites reduced LRRK2 GTPase activity (Webber et al., 2011), and LRRK2 kinase–enhanced mutants exhibited reduced GTPase hydrolysis (Xiong et al., 2010; Xiong et al., 2012). LRRK2 activity may alternately be regulated by GTPase-activating proteins (GAPs) or guanine nucleotide exchange factors. Notably, ADP ribosylation factor (Arf)GAP binds LRRK2, and its inhibition or KO ameliorates LRRK2 induced neuron death (Xiong et al., 2012).
LRRK2 pathogenic mutations lead to kinase overactivity
LRRK2-induced neuronal toxicity is dependent on kinase activity (Smith et al., 2006; West et al., 2007; Xiong et al., 2018). Most pathogenic LRRK2 mutations appear to increase its kinase activity through domain-specific mechanisms (Berwick et al., 2019; Madureira et al., 2020). Kinase domain G2019S and I2020T LRRK2 mutations lead to an increase in kinase activity (Rudenko and Cookson, 2014). Effects of the other PD-associated LRRK2 mutations on its kinase activity are more contentious, with most but not all groups suggesting that they have increased kinase activities (Martin et al., 2014a). In contrast, a risk variant (G2385R) mutation results in reduced kinase activity, rendering it unclear how this mutation increases disease risk (Rudenko et al., 2012). The G2385R substitution may cause LRRK2 misfolding, facilitating its ubiquitination and proteasomal degradation, suggesting that loss of LRRK2 also can cause PD pathogenesis (Rudenko et al., 2017).
LRRK2 mutations lead to prominent deficits in protein translation, cytoskeletal dynamics, and vesicular trafficking
Studies using LRRK2 transgenic mice to probe mechanisms of SNpc DA neuron death have found inconsistent disease phenotypes. In mice engineered to express human WT, G2019S, or R1441C LRRK2, no DA neuron death occurred in the SNpc, though LRRK2-mediated deficits in neuronal architecture and DA transmission were observed (Li et al., 2009; Melrose et al., 2010; Tong et al., 2009). However, G2019S LRRK2 transgenic mice do show DA neuron loss (Chen et al., 2012; Ramonet et al., 2011). Conditional overexpression of G2019S LRRK2 in tyrosine hydroxylase (TH) positive dopamine neurons leads to robust kinase-dependent degeneration of DA neurons (Xiong et al., 2018). Several factors may contribute to these discrepancies, including transgene expression levels and neuroinflammation, given that a significant fraction of LRRK2-mediated neurotoxicity may be exerted through non–cell-autonomous avenue (discussed in subsequent sections). Viral overexpression of G2019S LRRK2 also elicits kinase-dependent loss of SNpc DA neurons (Lee et al., 2010a).
Within cells, LRRK2 localizes with vesicular components including ER-Golgi, endolysosomes, and multivesicular bodies (Roosen and Cookson, 2016). LRRK2 is a regulatory node for intracellular signaling networks. It serves as a scaffold for protein–protein interactions through its repeat regions, and as a direct regulator through its kinase activity for diverse signaling pathways including WNT, MAPKKKs, PKA, MAPK, β-catenin, disheveled, and LRP6 (Berwick et al., 2019; Harvey and Outeiro, 2019; Madureira et al., 2020). Through these pathways, LRRK2 regulates a wide number of cellular processes including protein translation (Gehrke et al., 2015; Imai et al., 2008; Kim et al., 2020; Martin et al., 2014b), cytoskeletal organization (Kett et al., 2012; Law et al., 2014), and endolysosomal vesicular sorting and trafficking (Berwick et al., 2019; Madureira et al., 2020). In the subsequent sections, we discuss the actions of LRRK2 on these key cell biological functions (Fig. 2, A–C).
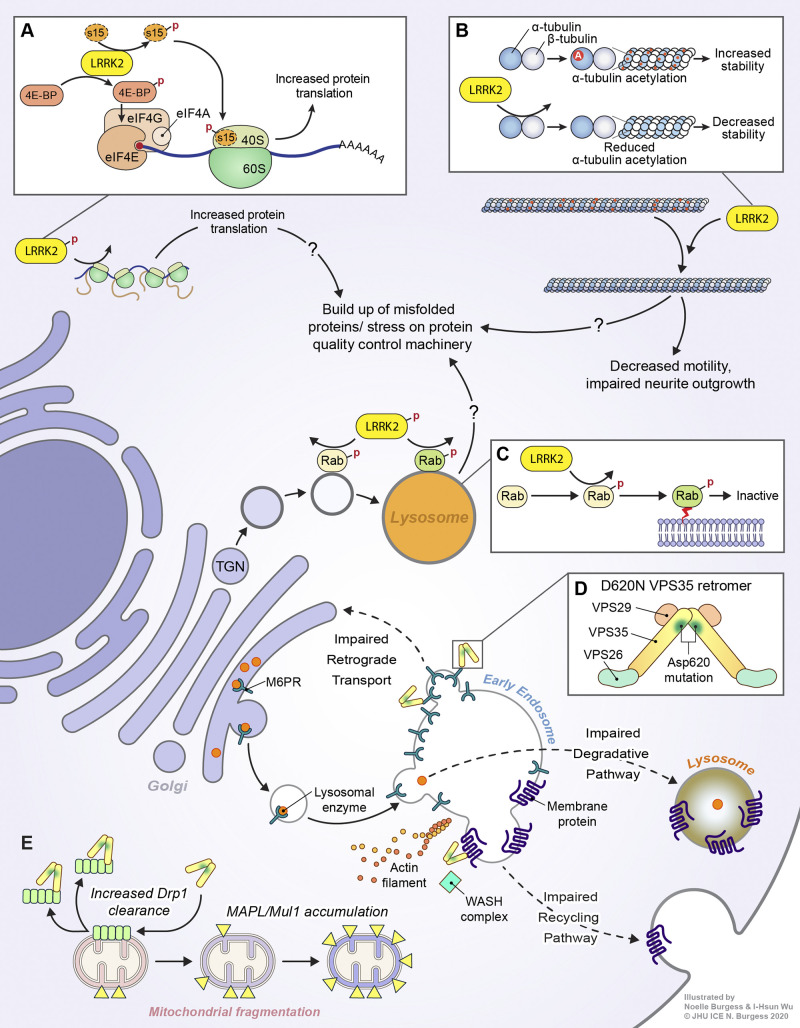
Figure 2.
LRRK2 and VPS35 signaling in PD. (A) LRRK2 may facilitate misfolded protein buildup via increasing global protein translation. It does so by phosphorylating (depicted by a red P) 4E-BP and the 40S ribosomal subunit S15. (B) LRRK2 can associate with β-tubulin via its Roc domain, physically impeding α-tubulin acetylation (depicted by a red A), which may lead to decreased microtubule stability and impaired neurite outgrowth. (C) LRRK2 can phosphorylate multiple RAB proteins within their switch II domains, inactivating them and promoting their membrane targeting. Increased LRRK2 activity may compromise vesicular sorting machinery in neurons, leading to the accumulation of misfolded proteins. (D) The VPS 35 retromer forms a dimer of trimers comprising VPS26/29/35, with the D620N mutation in VPS35 disrupting an acidic residue present at the region critical for retromer complex dimerization, potentially impairing proper assembly of the retromer dimer of trimers. The D620N mutation has been associated with impaired retrograde transport of Golgi-endosome cargo receptors, leading to a deficiency of receptors at the Golgi and impairing forward transport of lysosomal enzymes. D620N VPS35 also exhibits deficiencies in endosome-plasma membrane recycling of surface membrane proteins due to impaired association with the WASH complex. (E) VPS35 is also involved in regulation of mitochondrial dynamics. The D620N mutation can lead to mitochondrial fragmentation through MAPL/Mul1 accumulation or increased Drp1 clearance.
Protein synthesis (Fig. 2 A)
Aberrations in protein translation have been linked to neurodegenerative disorders including PD (Martin et al., 2014b; Polymenidou et al., 2011; Tollervey et al., 2011). An early study suggested that LRRK2 de-represses translation by phosphorylating eukaryotic initiation factor 4 binding protein (Imai et al., 2008), though the validity of eukaryotic initiation factor 4 binding protein as an LRRK2 target in vivo has been questioned (Trancikova et al., 2012). LRRK2 can antagonize the effects of translational repressor microRNAs and increase protein translation (Gehrke et al., 2010), and may also interact with translation elongation factor eEF1A (Gillardon, 2009). Using unbiased tandem affinity purification and mass spectrometry, a large number of LRRK2-binding proteins and phosphosubstrates including the 40S ribosomal subunits s11, s15, and s27 were identified as LRRK2 phosphorylation substrates (Martin et al., 2014b). Eukaryotic mRNAs contain a modified guanine residue on the 5' end called a cap. LRRK2 phosphorylation of s15 enhances cap-dependent as well as cap-independent bulk protein translation, which leads to DA neuron toxicity in Drosophila as well as human DA neurons. Phosphodeficient T136A s15 shows substantial neuroprotection in LRRK2 transgenic flies and human DA neurons, linking LRRK2 phosphorylation of RPS15 to DA neuron death (Martin et al., 2014b). Ribosome profiling of human DA neurons derived from G2019S LRRK2 induced pluripotent stem cells (IPSCs) reveals alterations in the translation of genes regulating calcium (Kim et al., 2020), consistent with previous literature implicating mutant LRRK2 signaling in altered neuronal calcium handling (Cherra et al., 2013; Verma et al., 2017). These alterations are triggered by LRRK2 phosphorylation of RPS15, which enhances the translation of mRNAs with complex 5′ UTR secondary structure (Kim et al., 2020).
Cytoskeletal assembly (Fig. 2 B)
LRRK2 also interacts with cytoskeletal components. LRRK2 associates with microtubules, which requires its kinase activity and WD-40 domain, and PD-associated LRRK2 mutants have greater microtubule association (Kett et al., 2012). LRRK2 also interacts with β-tubulin via its ROC domain, an interaction that is inhibited by mutant R1441G LRRK2. Overexpressing LRRK2 alters growth cone dynamics in neuronal cells via interaction with β-tubulin, preventing microtubule acetylation (Law et al., 2014). Fibroblasts cultured from human G2019S LRRK2 patients show altered cell migration kinetics (Caesar et al., 2013). LRRK2 inhibits neurite outgrowth by phosphorylating the ezrin, radixin, and moesin family proteins, which regulate cytoskeletal dynamics by tethering actin to the cell membrane (Jaleel et al., 2007; Parisiadou et al., 2009). The microtubule-associated protein Tau has also been found to be a LRRK2 substrate (Bailey et al., 2013; Kawakami et al., 2012). Taken together, these studies suggest that mutant LRRK2 may contribute to neuron dysfunction in PD by disrupting regulation of synaptic vesicle endocytosis and protein trafficking between intracellular compartments. Further, LRRK2 dysfunction may engender defective motility and neuronal process outgrowth via cytoskeletal phosphorylation. How these signaling events actually lead to DA neuron death in PD is unclear.
Vesicular trafficking (Fig. 2 C)
LRRK2 is implicated in various facets of vesicular trafficking. Early studies mapped the localization of LRRK2 to membrane-bound organelles such as multivesicular bodies and lysosomes (Alegre-Abarrategui et al., 2009). LRRK2 phosphorylates endophilin A, a protein with important roles in synaptic vesicle endocytosis, which reduces its membrane association. Increasing or decreasing LRRK2-mediated endophilin A phosphorylation reduced synaptic vesicle recycling (Matta et al., 2012). LRRK2-deficient neurons exhibit synaptic vesicle redistribution and increased postsynaptic currents (Piccoli et al., 2011). LRRK2 may also regulate autophagy, the process of cargo delivery to lysosomes for degradation, as primary fibroblasts from LRRK2 mutation carriers show deficits in autophagy induction following starvation (Manzoni et al., 2013). LRRK2 regulation of autophagy is preceded by its dimerization and membrane translocation (Schapansky et al., 2014). In Caenorhabditis elegans, expression of pathogenic LRRK2 variants hastened the age-dependent decline of autophagy (Saha et al., 2015), while kidneys of LRRK2-deficient mice have altered autophagy (Tong et al., 2010). Membrane-associated LRRK2 was shown to reduce autophagy by binding to the autophagy regulatory protein beclin-1 (Takagawa et al., 2018).
The Rab family of GTPases regulates multiple aspects of vesicle trafficking (Kiral et al., 2018). Rabs switch between active GTP-bound and inactive GDP-bound states, which are mediated by their respective guanine nucleotide exchange factors and GAPs. WT LRRK2 associates with Rab7 and negatively regulates Rab7-dependent lysosomal positioning, whereas G2019S LRRK2 has the opposite effect (Dodson et al., 2012), suggesting that LRRK2 mutations may engender vesicular sorting defects in neurons. GWAS implicate the PARK16 locus as a risk factor for PD development, and among the genes at this locus is the GTPase Rab7L1 (Nalls et al., 2014; Satake et al., 2009; Simón-Sánchez et al., 2009). Rab7L1 has also been identified as a LRRK2-interacting protein using an unbiased screen (Beilina et al., 2014). Rab7L1 antagonizes mutant LRRK2-mediated neurite shortening. Overexpression of Rab7L1 compensates for pathogenic LRRK2-mediated lethality and DA neuron loss via restoration of LRRK2-induced sorting defects (MacLeod et al., 2013). A phosphoproteomic screen of HEK-293 cells overexpressing LRRK2 revealed that LRRK2 can phosphorylate multiple Rab GTPases, thus maintaining them in an inactive state. Therefore, LRRK2 hyperactivation may bring about vesicular sorting defects via Rab GTPase inactivation (Steger et al., 2016). Recently, it was shown that a specific subset of LRRK2 Rab GTPase phosphosubstrates (Rab1a, 3c, and 35) may be linked to LRRK2 toxicity (Jeong et al., 2018).
VPS35
The endolysosomal system comprises diverse tubulovesicular organelles critical for nutrient uptake, intracellular trafficking, protein processing, and autophagy (Vidyadhara et al., 2019). Within the endolysosomal system, the early endosome serves as a critical hub for cargo sorting of internalized plasma membrane proteins and newly synthesized proteins received from the TGN. Contents of the early endosome ultimately undergo two distinct fates: the degradative path in which the early endosome matures into the late endosome and fuses with the lysosome for cargo destruction, or a recycling path in which select targets are trafficked to various end-organelle/membrane targets (Burd and Cullen, 2014; Vidyadhara et al., 2019).
A key player in the sorting function of the endolysosomal system is the heterotrimeric retromer complex, comprising Vps26, Vps29, and Vps35, which interacts with sorting nexin proteins to facilitate binding to membrane-localized cargo (Chen et al., 2019a; Kovtun et al., 2018; Lucas et al., 2016). Cargo-bound retromer forms a dimer (or multimer) of trimers via a VPS35 homodimerization domain located at the C terminus, forming a structural scaffold around endosomal cargo that facilitates their selective trafficking (Fig. 2 D; Kendall et al., 2020; Kovtun et al., 2018; Lucas et al., 2016). Among the retromer’s most well-characterized roles is retrograde recycling of transport receptors, such as the cation-independent mannose-6-phosphate receptor (CI-M6PR) required for the delivery of lysosomal hydrolases (Williams et al., 2017).
PD-associated D620N mutation leads to partial loss of function
Exome sequencing of familial PD cohorts identified the D620N mutation in VPS35 as a cause of autosomal dominant PD (Vilariño-Güell et al., 2011; Zimprich et al., 2011). The precise consequence of this mutation on protein function is unclear given inconsistent findings in different model systems and the relatively mild phenotype of D620N mutant animals and cells; D620N knock-in mice show no gross defects or evidence of Golgi, mitochondrial, or endolysosomal dysfunction (Ishizu et al., 2016), and the D620N mutation has no effect in VPS35 localization or interactions with VPS26/29 (Ishizu et al., 2016; McGough et al., 2014; Tsika et al., 2014; Zavodszky et al., 2014). The inheritance pattern of VPS35 PD suggests that the D620N mutation may lead to three effects on protein function: gain of function, dominant negative, or hypomorphic function. In support of a gain-of-function or dominant negative effect, overexpressing the D620N mutant leads to disease phenotypes (McGough et al., 2014; Tang et al., 2015a; Tsika et al., 2014; Wang et al., 2016b). However, toxicity may be an overexpression artifact to some extent, as similar pathologies are observed by overexpressing WT VPS35 (Miura et al., 2014; Tsika et al., 2014; Wang et al., 2016b).
Several lines of evidence suggest D620N is a partial loss-of-function mutation. Structural data indicate that the D620N mutation is located near a highly acidic region of VPS35 critical for retromer dimerization and therefore cargo trafficking (Kendall et al., 2020; Kovtun et al., 2018). A mutation from an acidic aspartate to a neutral asparagine could disrupt this critical interaction, though conclusive evidence is still lacking. Functionally, the D620N mutation phenocopies many defects observed with VPS35 loss (Miura et al., 2014; Tang et al., 2015a; Tang et al., 2015b; Wang et al., 2016a; Zavodszky et al., 2014). Furthermore, D620N VPS35 expression fails to fully rescue VPS35-KO or knockdown phenotypes (Dhungel et al., 2015; Inoshita et al., 2017; Malik et al., 2015; Tang et al., 2015b; Wang et al., 2016a), while D620N overexpression fails to confer gain of functions associated with WT VPS35 overexpression (MacLeod et al., 2013; Munsie et al., 2015; Wang et al., 2016a). The dominant negative phenotype observed by some groups could be an overexpression artifact, because the mutant protein disrupts the strict 1:1:1 stoichiometry of VPS26/29/35 (Hierro et al., 2007; Seaman et al., 2009) and may compete with endogenous WT VPS35 to form hypofunctional retromer complexes. This effect is unlikely to occur in humans, as no evidence would suggest that the mutant allele is more highly expressed than the WT one.
Loss of VPS35 disrupts proteostasis through endosomal sorting defects
Though numerous D620N-associated defects have been identified, it is unclear which contribute to PD pathology. One line of work suggests that mutant VPS35 compromises lysosomal function and may lead to α-syn aggregation (Fig. 2 D). D620N overexpression or knock-in cell models indicate that the D620N mutant may impair CI-M6PR recycling, disrupt lysosomal morphology, impair cathepsin D processing, and reduce Lamp2a levels (Follett et al., 2014; MacLeod et al., 2013; McGough et al., 2014; Yun et al., 2017). An elegant genetic screening study in yeast, coupled with investigations in C. elegans, and mice has shown that knockdown of VPS35 increases vulnerability to increased protein translation caused by overexpression of eIF4G1 (Dhungel et al., 2015), consistent with the lysosome’s known role in maintaining proteostasis. D620N-induced lysosomal dysfunction worsens accumulation of α-syn aggregates in cell lines, yeast, C. elegans, and mice overexpressing α-syn (Dhungel et al., 2015; Miura et al., 2014; Tang et al., 2015a; Tang et al., 2015b).
Though these findings provide a compelling link between pathogenic VPS35 mutations and known PD pathways, they have not been consistently detected across all model systems. Some studies expressing D620N VPS35 in cell lines or in mice fail to find CI-M6PR and LAMP1 mislocalization, changes in cathepsin D expression, or increased susceptibility to autophagy inhibitor bafilomycin A1 (Chen et al., 2019b; Ishizu et al., 2016; Tsika et al., 2014; Zavodszky et al., 2014). Moreover, D620N knock-in mice do not exhibit α-syn accumulation without the presence of an α-syn transgene (Chen et al., 2019b; Ishizu et al., 2016), complicating the question of whether VPS35 plays a role in α-syn proteostasis at endogenous expression levels. To the best of our knowledge, none have reported on the postmortem pathology of PD patients with the D620N mutation, rendering it unclear whether α-syn aggregation is a pathological feature of VPS35 PD.
Disruption of WASH complex–dependent endosomal protein sorting
The D620N mutation also impairs the interaction between VPS35 and FAM21, a component of the WASH complex (Fig. 2 D; McGough et al., 2014; Zavodszky et al., 2014). The WASH complex stimulates Arp2/3 recruitment of filamentous actin to endosomal microdomains in order to drive cargo sorting before transport, contributing to protein trafficking to the plasma membrane and other cellular compartments (Seaman et al., 2013). Because retromer is important for recycling internalized neuronal cell surface proteins (Choy et al., 2014; Hussain et al., 2014; Munsie et al., 2015; Wang et al., 2016a), impaired interaction with the WASH complex could significantly alter synaptic function. However, whether the D620N mutation affects membrane receptor recycling has only been confirmed for AMPAR subunits and DRD1 (Munsie et al., 2015; Wang et al., 2016a; Zavodszky et al., 2014). Other findings suggest that WASH defects contribute to other phenotypes such as impaired endosomal maturation, defective autophagosome formation, and ATG9 mislocalization (Follett et al., 2014; Zavodszky et al., 2014). However, cell lines expressing D620N VPS35 do not always exhibit Fam21 mislocalization (Follett et al., 2014; McGough et al., 2014), suggesting that trafficking defects may depend on context or cell type. How these defects ultimately lead to neurodegeneration is an open area of investigation.
Novel role of VPS35 in mitochondrial quality control
Finally, retromer may also regulate mitochondrial dynamics (Fig. 2 E). VPS35 mediates the formation of mitochondrial-derived vesicles (MDVs) to remove MAPL (MUL1) from the mitochondria (Braschi et al., 2010). Because MAPL promotes fission through Drp1 (DLP1) SUMOylation, a post-translational modification involving the addition of SUMOs (small ubiquitin-like modifiers), and suppresses fusion through Mfn ubiquitination (Braschi et al., 2009; Peng et al., 2016; Yun et al., 2014), these findings implicate VPS35 in regulation of mitochondrial fission/fusion dynamics. Loss of VPS35 or expression of D620N VPS35 leads to the accumulation of MAPL on mitochondria and subsequent reduction of Mfn2, mitochondrial fragmentation, and degeneration of SNpc DA neurons in mice (Tang et al., 2015b). These results suggest that VPS35 promotes mitochondrial fusion, with loss-of-function D620N triggering excessive fission. A separate study likewise found evidence that the D620N mutation increases mitochondrial fission (Wang et al., 2016b). However, this study reports that WT VPS35 promotes mitochondrial fission by removing inactive Drp1 complexes from mitochondria, and that the D620N mutation may lead to gain of function via increased interaction with Drp1 (Wang et al., 2017; Wang et al., 2016b). Thus, two lines of investigation indicate that D620N leads to mitochondrial dysfunction through increased fission, consistent with findings that D620N VPS35 leads to complex I and II defects that can be rescued by inhibiting mitochondrial fission (Zhou et al., 2017).
Given the integral role of VPS35 in regulating vesicle formation and trafficking and given the newly appreciated role of MDVs in removing damaged mitochondrial proteins (Ge et al., 2020; Sugiura et al., 2014), a provocative possibility is that VPS35 contributes to mitochondrial quality control through MDV formation, with the D620N mutation compromising this function. Whether this impairment stems from a gain or loss of VPS35 function and whether previously observed defects in WASH complex binding or endolysosomal function may contribute to these mitochondrial pathologies remain areas of future investigation.
Parkin
Ubiquitination is an essential PTM that controls the function and fates of proteins, relying on the successive actions of ubiquitin-activating, conjugating (E2), and ligase (E3) enzymes (Zheng and Shabek, 2017). Parkin is a 465-aa E3 ubiquitin ligase that can conjugate mono- or polyubiquitin chains onto substrate proteins via K48, K63, and other linkages. Parkin contains a C-terminal really interesting new gene (RING) box domain consisting of two RING finger motifs separated by an in-between-RING finger (IBR) motif and an N-terminal ubiquitin-like (Ubl) domain. The repressor element of parkin region separates the IBR and RING2 domains (Panicker et al., 2017).
Loss-of-function PRKN mutations are the most common known cause of autosomal recessive (AR) PD (Kasten et al., 2018). Structural and biochemical studies have shown that parkin is an autoinhibited protein. Its Ubl domain as well as REP region bind the RING1 domain and prevent access to the parkin E2 enzyme-binding site (Kumar et al., 2015; Sauvé et al., 2015), while the RING0 domain directly occludes access to the catalytic cysteine (Wauer and Komander, 2013). These negatively regulating interdomain interactions need to be overcome during parkin activation. Mitochondrial stressors—membrane depolarization, complex I deficiency, mTOR inhibition, mitochondrial DNA mutations, and misfolded proteins—cause parkin to localize to the mitochondria in a phosphatase and tensin homologue (PTEN)-induced putative kinase 1 (PINK1)–dependent manner (Pickrell and Youle, 2015). Once recruited to the mitochondria, parkin ubiquitinates many targets on the outer mitochondrial membrane (OMM). This is followed by ubiquitination of inner mitochondrial membrane (IMM) proteins (Rose et al., 2016; Rüb et al., 2017; Sarraf et al., 2013). Parkin also ubiquitinates many cytosolic targets, a topic that will be discussed in the subsequent section.
PINK1
PINK1 encodes a 581-aa serine/threonine kinase with a N-terminal IMM localization signal, IMM stop-transfer sequence, Ser/Thr kinase domain, and C-terminal OMM retention signal (Pickrell and Youle, 2015; Rüb et al., 2017). In the absence of damage, PINK1 is transferred across the OMM until the stop-transfer sequence. At this point, the kinase domain and C terminus protrude out into the cytosol. PINK1 is then cleaved at A103 and F104 by IMM-bound proteases to a 52-kD fragment, which is released into the cytosol and degraded via ubiquitination (Liu et al., 2017; Pickrell et al., 2015; Rüb et al., 2017). As such, basal levels of PINK1 are undetectable. Mitochondrial stresses such as membrane depolarization, electron transport chain (ETC) complex dysfunction, mutagenic stress, and proteotoxic stress impair transport through the OMM, thereby preventing proteolysis. This results in the accumulation of PINK1 on the OMM, leading to dimerization and activation of the kinase domain (Pickrell and Youle, 2015; Rüb et al., 2017). Thus, PINK1 acts as a mitochondrial damage sensor that activates mitochondrial quality control pathways. Because these aforementioned studies on PINK1 cleavage and stability were performed in mammalian systems, it is less clear whether PINK1 cleavage or signaling works in a similar manner in other model systems such as Drosophila.
Mutations in PINK1 are the second most common cause of AR PD (Kasten et al., 2018). Most of the 60 pathogenic loss-of-function PINK1 mutations abolish kinase activity or disrupt the PINK1–ubiquitin binding interface (Rüb et al., 2017). While PINK1 is best known for cooperating with parkin to regulate mitochondrial quality control, it also promotes mitochondrial function by maintaining complex I activity in a parkin-independent fashion (Vilain et al., 2012). Inactivating PINK1 leads to decreased mitochondrial membrane potential, ETC defects, increased reactive oxygen species (ROS) production, and reduced ATP levels (Amo et al., 2014; Devireddy et al., 2015; Morais et al., 2014; Morais et al., 2009). Circumventing complex I by overexpressing a yeast electron carrier or treating with electron carrier vitamin K2 rescues PINK1-KO defects (Vilain et al., 2012; Vos et al., 2012). These data indicate that PINK1 plays a key role in maintaining complex I activity and protecting ETC integrity in a parkin-independent manner.
The PINK1-parkin pathway in mitochondrial quality control
Mitochondria are the primary site of oxidative energy generation, serve as key hubs of cellular signaling networks, metabolize and construct biological macromolecules, and buffer cytoplasmic Ca2+ (Spinelli and Haigis, 2018). Reactive intermediates generated by these processes can damage nucleic acids, proteins, and lipids. These agents are especially dangerous to neurons, which have high energetic demands and rely extensively on calcium signaling (Misgeld and Schwarz, 2017). Mitochondrial dysfunction has long been recognized as a key mechanism in familial and sporadic PD (Dawson and Dawson, 2003; Pickrell and Youle, 2015). Mitochondrial quality control is an encompassing term that includes maintenance of mitochondrial function, mitochondrial turnover via mitophagy, and the generation of new mitochondria. Mechanisms of mitochondrial quality control can be broadly categorized into mechanisms that degrade damaged mitochondria (e.g., mitophagy), fission and fusion, and mechanisms that mediate the production of new mitochondria (e.g., biogenesis). Here, we describe the role of parkin and PINK1 as central coordinators that regulate multiple facets of mitochondrial quality control (Fig. 3; further reviewed in Ge et al., 2020; Pickrell and Youle, 2015).
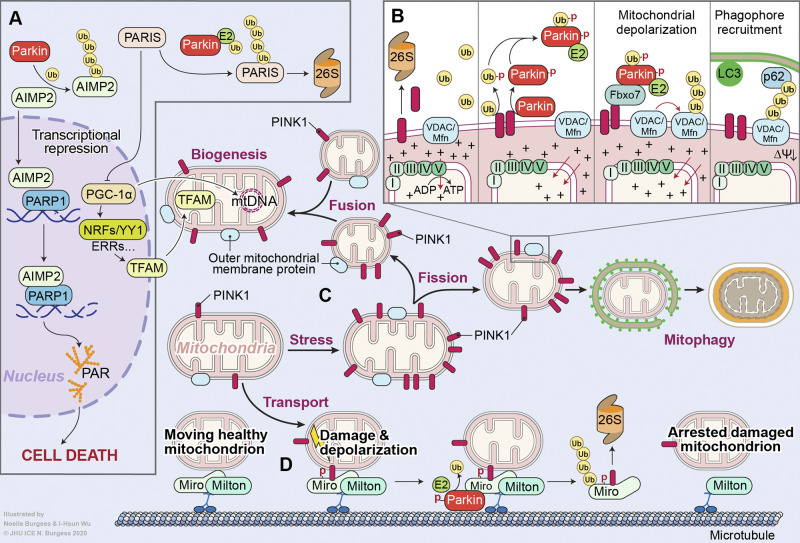
Figure 3.
Parkin and PINK1 signaling in PD. (A) Loss of parkin E3 ligase activity via PD-associated mutations or PTM results in the accumulation of its substrates. Accrual of the amino-acyl tRNA cofactor AIMP2 results in poly(ADP-ribose) polymerase-1 (PARP1)–dependent neuron death. Buildup of PARIS/ZNF746, a transcription inhibitor, prevents mitochondrial biogenesis by repressing the expression of PGC-1α, the master mitochondrial biogenesis regulator. (B) PINK1 is a serine/threonine kinase that phosphorylates (depicted by a red PD) both ubiquitin and parkin at S65, allowing parkin to be recruited to damaged mitochondria. This is followed by the parkin-mediated polyubiquitination of OMM proteins, culminating in the clearance of damaged mitochondria via mitophagy. (C) PINK1/parkin signaling maintains a balance between mitochondrial fission and fusion. (D) Finally, PINK1/parkin inhibit mitochondrial transport by phosphorylating and ubiquitinating the protein Miro, causing mitochondrial detachment from the microtubule. Ub, ubiquitin.
PINK1/parkin inhibit mitochondrial transport and promote recycling of mitochondrial components
PINK1/parkin mediate the segregation of damaged mitochondria by regulating fission/fusion dynamics and generating MDVs (Fig. 3 C). In Drosophila, PINK1 and parkin were shown to play a role in the same genetic pathway that contributes to mitochondrial health in dopaminergic neurons, with PINK1 activation upstream of parkin activation (Clark et al., 2006; Park et al., 2006). These studies also indicated that the defective mitochondrial phenotypes were caused by reduced fission. While some subsequent studies support these findings (Yang et al., 2008), others find contrary results in mammalian cells, showing that PINK1 signaling promotes fusion in some systems (Dagda et al., 2009; Rojas-Charry et al., 2014). This could occur due to the different model systems used, indicating that PINK1 and parkin could have antipodal roles in inducing mitochondrial fusion and fission in different cell types. Alternatively, they may play a role in maintaining the balance between these two processes, and their loss or inactivation could promote either process depending on the local environment or cell type. PINK1 and parkin promote degradation of mitofusins 1/2, which mediate mitochondrial fusion (Gegg et al., 2010; Glauser et al., 2011; Poole et al., 2010; Rakovic et al., 2011; Sarraf et al., 2013; Yu et al., 2011; Ziviani et al., 2010). In addition, PINK1 promotes mitochondrial fission by recruiting Drp1 to mitochondria (Buhlman et al., 2014; Pryde et al., 2016). PINK1 phosphorylation of miro (which links kinesin to mitochondria) facilitates its ubiquitination by parkin, thereby preventing mitochondrial transport and segregating damaged mitochondria before their clearance (Fig. 3 D; Wang et al., 2011).
Parkin/PINK1 also mediate the generation of MDVs, which incorporate oxidized OMM, IMM, and matrix proteins. These MDVs are targeted to and subsequently fuse with endosomes via a syntaxin-17/SNAP29/VAMP6 SNARE complex (McLelland et al., 2016; Sugiura et al., 2014). In contrast to mitochondrial fission, this pathway is Drp1-independent (Sugiura et al., 2014). There also exist other subtypes of MDVs targeted to a variety of other subcellular compartments such as peroxisomes (Sugiura et al., 2014), but it is unclear which subtypes parkin/PINK1 regulate. Overall, MDV-mediated removal of oxidized mitochondrial proteins may represent a selective mechanism by which parkin/PINK1 can remove damaged mitochondrial components without compromising overall mitochondrial function.
PINK1/parkin promote mitophagy
Parkin translocates to mitochondria in a PINK1-dependent manner following treatment of cells with carbonyl cyanide m-chlorophenylhydrazone (CCCP; Fig. 3 B), which inhibits mitochondrial function by uncoupling the proton gradient established across the mitochondrial membrane used to synthesize ATP (Narendra et al., 2008; Narendra et al., 2010; Vives-Bauza et al., 2010). OMM proteins are polyubiquitinated by parkin, resulting in the clearance of damaged mitochondria via mitophagy (Chan et al., 2011; Ordureau et al., 2014; Sarraf et al., 2013). Cleaved PINK1 localizes to the cytosol, where it interacts with parkin to repress its mitochondrial localization (Fedorowicz et al., 2014), suggesting that PINK1 cleavage serves as an inhibitory mechanism preventing parkin mitochondrial localization. While mitochondrial PINK1 is constitutively degraded in cells with healthy mitochondria, it accumulates on the OMM upon depolarization (Matsuda et al., 2010; Narendra et al., 2010). PINK1 phosphorylates the OMM GTPase mitofusin2 (Mfn2), which then serves as a docking site for parkin selectively on dysfunctional mitochondria (Chen and Dorn, 2013). In addition, phosphorylated ubiquitin chains mediate parkin recruitment to damaged mitochondria (Okatsu et al., 2015; Ordureau et al., 2014; Shiba-Fukushima et al., 2012). PINK1 also phosphorylates parkin at the S65 residue of its Ubl domain (Woodroof et al., 2011). As discussed, this domain possesses auto-inhibitory properties, by binding to a region between the IBR and RING1 domains, preventing the association of E2s with parkin (Chaugule et al., 2011). Furthermore, PINK1 is an ubiquitin kinase, phosphorylating ubiquitin at S65, which can then mediate the mitochondrial recruitment of parkin via an interaction with its RING1 and IBR domains (Kane et al., 2014; Kazlauskaite et al., 2014; Koyano et al., 2014; Wauer et al., 2015).
Another PARK protein, F-box only protein-7 (FBXO7), can participate in recruiting mitochondrial substrates to parkin. F-box domain–containing proteins have traditionally been shown to recruit substrates to Skp1 Cul1 FBOX 3 ligase complexes (Skowyra et al., 1997). FBXO7 knockdown prevented the mitochondrial recruitment of parkin and its ectopic expression rescued TH neuron loss and climbing deficits in parkin mutant drosophila. FBXO7 also promotes the parkin-mediated ubiquitination of the parkin substrate, Mfn2 (Burchell et al., 2013).
Recently, the in vivo significance of mitophagy induction studies has been called into question, since they have relied on cell culture overexpression systems, non-mammalian models, and chemical agents to induce profound levels of mitochondrial dysfunction that probably do not occur in PD (Grenier et al., 2013). Furthermore, endogenous neuronal parkin cannot bring about increased mitophagy, even upon CCCP treatment (Van Laar et al., 2011). Studies using patient-derived IPSCs are also contentious; while some investigators were not able to detect parkin mitochondrial translocation in IPSC-derived neurons (Rakovic et al., 2013), others demonstrated valinomycin induced PINK1-dependent parkin mitochondrial translocation (Seibler et al., 2011). Neurodegeneration in the mitoPARK (Dat-Cre +/− TfamloxP/loxP) mice model, in which the mitochondrial transcription factor TFAM is selectively knocked out in dopaminergic neurons, was found to be parkin aindependent (Sterky et al., 2011). However, TH neuron loss in mitochondrial Mutator mice, which harbor a mutation in the DNA polymerase γ gene (a mutation that causes progressive mitochondrial dysfunction), was exacerbated upon parkin deletion (Pickrell et al., 2015). An important caveat, however, is that it is unclear whether human patients experience the same severity of mutagenic stress present in these mutant mice.
A number of in vivo studies of mitophagy using pH-sensitive fluorescent indicators found that basal mitophagy does occur in the mature CNS in mouse and Drosophila (Cornelissen et al., 2018; Kim et al., 2019; Lee et al., 2018; McWilliams et al., 2016; McWilliams et al., 2018; Sun et al., 2015). However, the role of PINK1 and parkin in basal mitophagy in vivo is unclear, as three of these studies found that PINK1/parkin-KO does not influence rates of in vivo mitophagy (Kim et al., 2019; Lee et al., 2018; McWilliams et al., 2018). While there are alternative parkin-independent mechanisms of mitophagy (reviewed in Villa et al., 2018), further investigation is needed to identify the ones that contribute to mitophagy in the mammalian CNS. Studies in human DA neurons derived from human IPSCs lacking parkin indicate that the mitochondrial deficits are due to defects in mitochondrial biogenesis and not mitophagy (Kumar et al., 2020).
The mitochondrial PINK1 and phospho-ubiquitin–mediated parkin activation model suggests that parkin is maintained in a basally auto-inhibited state via multiple inter-domain interactions (Tang et al., 2017). Following mitochondrial damage, PINK1 is stabilized at the OMM, and phosphorylates ubiquitin. Phospho-ubiquitin binding to the RING1 domain and PINK1-mediated S65 phosphorylation within its Ubl domain allow it to attain maximum ubiquitin-ligase activity. However, there is a distinct lack of studies in vertebrate model systems that links mitophagy defects in contributing to PD neuron death. PINK1 was also demonstrated to initiate parkin-independent mitophagy by recruiting multiple autophagy receptors to damaged mitochondria via ubiquitin phosphorylation, but this study was performed in HeLa cells using chemical agents that generate massive mitochondrial damage (Lazarou et al., 2015). Moving forward, it is important to recognize the limitations of using nonneuronal cell lines to propose neuronal functions of PINK1/parkin, given their substantial metabolic differences and the absence of neuron-specific cell biological features (Grenier et al., 2013). For example, recent studies have demonstrated that PINK1 may play an important role in promoting the neuron-specific function of dendrite arborization and survival, in part through regulation of mitochondrial trafficking (Dagda et al., 2014; Liu et al., 2020; Matenia et al., 2012; Wang et al., 2018). The molecular players identified in CCCP using assays will have to be demonstrated to contribute to DA neuron death in mouse models of parkin/PINK1 inactivation for their roles in disease progression to be solidified.
Parkin/PINK1 drive mitochondrial biogenesis
The removal of damaged mitochondria must be followed by the synthesis of new mitochondria. Parkin/PINK1 directly promote mitochondrial biogenesis through degradation of PARIS (ZNF746), a KRAB and zinc finger protein that represses the transcription of PGC-1α, a master regulator of mitochondrial biogenesis (Fig. 3 A; Shin et al., 2011). PINK1 phosphorylates PARIS, priming it for ubiquitination by parkin and proteasomal degradation (Lee et al., 2017). In models of PD, PARIS accumulates due to the loss or inactivation of parkin, leading to down-regulation of PGC-1α, impairment of mitochondrial biogenesis, and degeneration of SNpc DA neurons. Overexpressing PGC-1α restores mitochondrial biogenesis and prevents the loss of DA neurons (Shin et al., 2011; Siddiqui et al., 2016; Stevens et al., 2015). Loss of PINK1 also leads to accumulation of PARIS, down-regulation of PGC-1α, and the selective degeneration of SNpc DA neurons, which can be rescued by reducing PARIS levels (Lee et al., 2017). In Drosophila lacking parkin or PINK1, defects in mitochondrial biogenesis due to the up-regulation of PARIS and repression of PGC-1α drive the loss of DA neurons (Pirooznia et al., 2020). In the context of increased mitochondrial turnover, the regulation of PARIS and PGC-1α by PINK1/parkin is likely essential for maintaining sufficient mitochondrial content to meet the metabolic demands.
In addition to increasing PGC-1α levels, parkin/PINK1 can promote localized translation of critical nuclear-encoded electron chain components in Drosophila. PINK1/parkin mediate the localization of nuclear-encoded electron chain component mRNAs to the OMM and promote their translation by recruiting translation initiation proteins and ubiquitinate the translational repressor Glo (hnRNP-F; Gehrke et al., 2015). In addition, parkin has been suggested to directly ubiquitinate translocase of outer mitochondrial proteins Tom70 and Tom20 (Jacoupy et al., 2019; Martinez et al., 2017; Rose et al., 2016; Sarraf et al., 2013), increasing import of newly synthesized mitochondrial proteins (Jacoupy et al., 2019; Martinez et al., 2017). Therefore, parkin/PINK1 may complement their role in PGC-1α–dependent mitochondrial biogenesis by inducing the local synthesis and import of mitochondrial proteins.
Cytosolic substrates of parkin and PINK1
The dominant PINK1/parkin activation model postulates that cytosolic parkin (the bulk of parkin in the cell) is functionally inactive and can only be activated upon mitochondrial localization following PINK1-mediated ubiquitin phosphorylation. However, several studies have documented cytosolic parkin substrates, which may indicate alternate parkin activation mechanisms or suggest that phospho-ubiquitin may exist in cytosolic niches (Fig. 3 A; Panicker et al., 2017). In addition to PARIS, another cytosolic parkin substrate that may play a role in the pathogenesis of PD is the amino-acyl tRNA cofactor AIMP2 (Corti et al., 2003). AIMP2 accumulated in the brains of PD brains, as well as in parkin KO mouse brains (Ko et al., 2010). Overexpression or accumulation of AIMP2 elicits neuron death via poly(ADP-ribose) polymerase-1–dependent parthanatos activation (Lee et al., 2013). Cytosolic parkin can be activated through several potential mechanisms. First, posttranslational modifications such as S-nitrosylation or sulfhydration have been shown to activate parkin (Ozawa et al., 2013; Vandiver et al., 2013). Furthermore, phospho-ubiquitin is present in cytosolic niches (Lee et al., 2017) and thus likely participates in the activation of cytosolic parkin.
Cytosolic parkin substrates accumulate following inactivation of parkin through several mechanisms. In addition to activating parkin, S-nitrosylation can inhibit or prevent parkin activation (Chung et al., 2004). Oxidation as well as covalent modification by DA reduce its E3 ligase activity (LaVoie et al., 2005; Meng et al., 2011). c-Abl–mediated tyrosine phosphorylation of parkin can also inhibit its activity (Ko et al., 2010). Reducing c-Abl phosphorylation of parkin maintains it in a catalytically active state in pathological α-syn models of neurodegeneration preventing the neurodegeneration by reducing the levels of PARIS and AIMP2 (Brahmachari et al., 2019). The propensity of parkin activity to be inhibited by ROS and c-Abl activation suggests that parkin dysfunction may also contribute to sporadic PD-associated neuronal deficits (Dawson and Dawson, 2014).
A number of cytosolic PINK1 substrates have also been identified. PARIS has been shown to be a PINK1 kinase substrate (Lee et al., 2017). Cytosolic PINK1 has also been reported to phosphorylate the catalytic subunit of protein kinase A via a signaling complex involving valosin-containing protein (VCP) and p47, thereby promoting dendritic arborization through enhanced mitochondrial transport (Dagda et al., 2014; Wang et al., 2018). Cytosolically localized PINK1 likewise has been shown to protect against a variety of cell stressors including proteosome inhibitor–induced protein aggregation, rotenone, and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; Gao et al., 2016; Haque et al., 2008; Murata et al., 2011). Some of these studies identify the putative cytosolic PINK1 substrate involved, such as the ubiquitin receptor sequestosome 1 (Gao et al., 2016). Others identify downstream consequences, such as activation of Akt via mTORC2, without confirming PINK1’s direct target (Murata et al., 2011). Future studies into the disease-relevant conditions that trigger PINK1 accumulation in the cytosol and its subsequent cytosolic substrates may yield further insights into stress responses regulated by PINK1.
DJ-1
DJ-1 (PARK7) is an evolutionarily ancient gene and is unique among PD-linked genes in that it is conserved in prokaryotes and eukaryotes (Cookson, 2012). It encodes a small, 189-aa protein with three oxidation-sensitive cysteines (C46, 53, and 106), which exists as a homodimer localized to the cytosol, mitochondria, and nucleus (Biosa et al., 2017; Dolgacheva et al., 2019). PARK7 PD is inherited in an AR fashion, with loss of function mutations disrupting DJ-1 protein stability and homodimerization (Malgieri and Eliezer, 2008).
DJ-1 as a dose-dependent detector of oxidative stress
Though DJ-1 is involved in many cellular functions (further reviewed in Biosa et al., 2017; Dolgacheva et al., 2019; van der Vlag et al., 2020), it is generally accepted that its primary function is to protect against oxidative damage. Its loss in cells and animals causes progressive, age-dependent oxidative stress, antioxidant response deficiencies, and ROS elevation (Andres-Mateos et al., 2007; Canet-Avilés et al., 2004; Taira et al., 2004). The C106 residue is critical for this function, and mutation of this residue eliminates DJ-1’s ability to protect against toxic insults. Upon exposure to mild ROS levels, C106 (-SH) is oxidized to sulfinic acid (-SOOH), a modification essential for DJ-1 function (Andres-Mateos et al., 2007; Canet-Avilés et al., 2004; Meulener et al., 2006). At higher ROS levels, DJ-1 is oxidized to sulfonic acid (SO3H), which inactivates its protective activity (Gautier et al., 2012; Meulener et al., 2006; Wang et al., 2014). As such, the stepwise modification of C106 under progressively higher levels of ROS allows DJ-1 to function as a dosage-sensitive oxidative stress sensor (Cookson, 2012; Wang et al., 2014).
DJ-1 protection against protein glycation: An unresolved debate
DJ-1 may also protect against glycation, the spontaneous and unregulated formation of covalent modifications on proteins, nucleic acids, and lipids by reactive aldehydes and reducing sugars (Ott et al., 2014). Extensive protein glycation leads to loss of protein function and aggregation in neurodegenerative diseases (Ott et al., 2014). Two primary defenses against glycation include glyoxalases that convert reactive aldehydes into less reactive products, and deglycases that remove glycation adducts from proteins (Pfaff et al., 2017a; Richarme et al., 2017).
While DJ-1 has been consistently found to protect against glycation, whether it acts as a glyoxylase or deglycase is a matter of vigorous debate (reviewed by Jun and Kool, 2020). Initial evidence suggested that DJ-1 may function as a glyoxalase for methylglyoxal (Hasim et al., 2014; Lee et al., 2012; Matsuda et al., 2017). However, other groups found that DJ-1’s activity appears more consistent with that of a protein deglycase (Richarme and Dairou, 2017; Richarme et al., 2015; Zheng et al., 2019). Further work by one of these groups suggested that DJ-1 could also deglycate guanine nucleotides in DNA and RNA (Richarme et al., 2017). These findings have been contradicted by follow-up studies suggesting that the reported deglycase activity of DJ-1 may result from a buffer artifact or spontaneous breakdown of glycated molecules (Andreeva et al., 2019; Pfaff et al., 2017a; Pfaff et al., 2017b). While it is generally agreed that DJ-1 plays a role in protecting cells from glycation damage, further work is required to identify whether its primary role is to detoxify the unconjugated reactive molecules as a glyoxylase or to undo their damage to endogenous biomolecules as a deglycase (Jun and Kool, 2020).
DJ-1 signaling activates diverse protective responses against oxidative stress
DJ-1 is thought to possess a degree of intrinsic antioxidant function and activate other protective pathways in response to oxidative stress. These diverse pathways converge on defense against damage by reactive chemical species such as ROS, reactive aldehyde species, and reducing sugar molecules. DJ-1 has been reported to serve as an atypical peroxiredoxin-like peroxidase (Andres-Mateos et al., 2007), increase mitochondrial uncoupling protein expression (Guzman et al., 2010), and modulate the function of antioxidant proteins peroxiredoxin 2 and paraoxonase-2 through protein–protein interactions (Fernandez-Caggiano et al., 2016; Parsanejad et al., 2014). Furthermore, DJ-1 may promote the transcription of Nrf2-controlled antioxidant stress genes by causing Nrf2 to dissociate from its inhibitor, KEAP (Clements et al., 2006; Im et al., 2012). Treatment with a short DJ-1–derived peptide has been shown to activate the Nrf2 pathway (Lev et al., 2015). Ablation of PTEN, a negative regulator of Akt activation, is protective in various PD mouse models (Domanskyi et al., 2011), and DJ-1 can inhibit PTEN activity by transferring a nitric oxide group from C106 to PTEN (Choi et al., 2014; Kim et al., 2005). Finally, DJ-1 has also been shown to protect against protein aggregation, a major consequence of oxidative stress (Tyedmers et al., 2010), through protein chaperone activity (Kthiri et al., 2010; Le et al., 2012; Zhou et al., 2006; Zondler et al., 2014).
At a high level, the role of DJ-1 seems to converge on protecting cellular macromolecules from damage by reactive chemical species. However, it is unclear how a small protein of 189 amino acids can have such a staggeringly diverse range of enzymatic, transcriptional, and chaperone activities (see text box). One possible explanation for these findings is that DJ-1 may behave differently between species or cell types. For example, DJ-1’s role in Nrf2 pathway regulation may be cell type–dependent; it appears to be dispensable in astrocytes (Gan et al., 2010), but not in other cell lines (Clements et al., 2006). Further characterization is required to understand how DJ-1 function depends on cell type differences in gene expression or stress exposure. Another possibility is that DJ-1 functions primarily as an oxidative stress sensor that works in concert with numerous binding partners or signaling pathways to activate diverse anti-stress pathways. While individual binding partners have been identified for DJ-1, systematic interrogation of DJ-1’s interactome may yield further insights.
Is DJ-1 a moonlighting protein?
Given DJ-1’s striking evolutionary conservation and staggering array of biochemical and cellular functions, we and others (Biosa et al., 2017) speculate that it should be classified as a moonlighting protein. Moonlighting proteins are a growing group of proteins that are highly evolutionarily conserved and have multiple distinct biochemical activities and functions (Jeffery, 2003; Singh and Bhalla, 2020). These proteins include cytochrome C, a core component of the ETC that also induces apoptosis; and α crystallins, the transparent proteins of the cornea and lens that also exhibit chaperone activity (Singh and Bhalla, 2020). One common hypothesis for the emergence of such multifunctional proteins is the chance interaction hypothesis, whereby novel functions are formed and reinforced by serendipitous protein–protein interactions that are evolutionarily advantageous (Copley, 2014; Singh and Bhalla, 2020). Consistent with this hypothesis, many moonlighting proteins have features that increase their probability of random interactions: ubiquitous expression, localization to multiple subcellular compartments, ability to undergo PTMs, and interactions with diverse binding partners (Copley, 2014; Singh and Bhalla, 2020). DJ-1 shares all of these features. DJ-1 is expressed across nearly all major CNS cell types, brain regions, and organs of the adult mouse (Bader et al., 2005; Han et al., 2018; Lein et al., 2007; Saunders et al., 2018), localizes to multiple subcellular compartments (Canet-Avilés et al., 2004; Taira et al., 2004; Zhang et al., 2005a), undergoes oxidative modifications essential for its function (Andres-Mateos et al., 2007; Canet-Avilés et al., 2004; Meulener et al., 2006), and carries many of its functions through binding partners such as PTEN, peroxiredoxin 2, and paraoxonase-2 (Choi et al., 2014; Dolgacheva et al., 2019; Fernandez-Caggiano et al., 2016; Parsanejad et al., 2014). Thus, we speculate that DJ-1’s dose-dependent modification under oxidative stress was co-opted by different stress-response systems as an activation signal.
What does this mean for future research into DJ-1’s role in PD? First, if DJ-1 has evolved as an oxidative stress sensor present in diverse eukaryotic and prokaryotic species, we reason that it elicits vastly different adaptive responses between organisms due to the different stressors they experience. This becomes an important consideration given that some proposed DJ-1 functions were initially uncovered by studies using YajL—an Escherichia coli orthologue of DJ-1 sharing a mere 40% peptide sequence identity with human DJ-1—or by expressing human DJ-1 in nonmammalian cell lines (Biosa et al., 2017; Gautier et al., 2012; Le et al., 2012; Pfaff et al., 2017a; Richarme et al., 2017). Furthermore, gene regulation may also greatly differ between species; for example, Drosophila carries two separate DJ-1 orthologues (Biosa et al., 2017). As such, the disease relevance of reported DJ-1 functions must be validated through expression of human/mammalian DJ-1 in appropriate mammalian cellular contexts (preferably in vivo). Furthermore, identifying downstream signaling factors is essential in understanding DJ-1’s function. Future studies are needed to identify the full diversity of DJ-1's interactome, and how it varies between organisms and cell types, in order to identify disease-relevant pathways downstream of DJ-1.
Non–cell-autonomous pathways contributing to neuronal death in PD
PD research has largely focused on mechanisms that cause neuron dysfunction and death, but it has now become apparent that neurodegeneration results from both cell-autonomous mechanisms occurring within neurons and non–cell-autonomous processes driven by other cell types. Neuroinflammation mediated by microglia and astrocytes is a prominent feature of PD, with evidence of increased number and activity of microglia accompanying the loss of DA neurons in the substantia nigra (Bartels and Leenders, 2007; Mosley et al., 2006; Whitton, 2007). We now review evidence from the human disease and animal models of nonneuronal contributions to disease, mechanisms by which α-syn triggers neuroimmune activation, and insights into glial cell biology through monogenic PD-linked genes (Fig. 4).
Figure 4.
Non-autonomous signaling pathways in PD. (A) Hyperactivated microglia, reactive A1 astrocytes, and cytotoxic T cells can independently or cooperatively act to mediate DA neuron death in PD. (B) α-Syn oligomers or aggregates released from dysfunctional DA neurons can activate the NF-κB pathway in microglia. LRRK2 may play a role in this process. This results in a pro-inflammatory cytokine response. Endocytosed α-syn oligomers or PFFs can also mediate mitochondrial dysfunction, resulting in the production of mitochondria-derived reactive oxygen species (mitoROS), which activates the NLRP3 inflammasome, resulting in IL-1β processing and secretion. Microglia-released pro-inflammatory cytokines and ROSs can directly elicit DA neuron death. Parkin/PINK1 signaling dampens inflammasome activation by preventing mitoROS release. (C) Activated microglia can produce IL-1α, TNF-α, and C1Q to elicit the formation of reactive A1 astrocytes, which directly kill neurons via an unknown mechanism. (D) Aberrant auto-processing of α-syn in DA neurons can result in the generation of α-syn antigenic epitopes, which, when presented by MHC class I molecules, can drive autoimmune cytotoxic T cell responses that result in DA neuron death.
Evidence of neuroinflammation from the human disease and PD models
DA neuron death occurs in part through microglia activation (Fig. 4 A). Microglia produce potentially neurotoxic factors, including pro-inflammatory cytokines such as TNF-α and IL-1β, ROS, and nitric oxide (Glass et al., 2010). The risk of PD is increased in individuals with polymorphisms in the TNF-α and IL-1β genes (Håkansson et al., 2005; Krüger et al., 2000; McGeer et al., 2002). The involvement of these molecules strongly implicates microglia in PD pathogenesis, as their release of IL-1α, TNF-α, and complement protein C1Q can induce astrocytes to adopt a pro-inflammatory “A1” fate, which means they do not partake in synapse formation, lose their phagocytic activity, and are directly neurotoxic (Fig. 4 C; Liddelow et al., 2017).
Given that activated microglia are antigen-presenting cells that can activate T cells (Aloisi, 2001; Gehrmann et al., 1995), a subsequent question is whether the adaptive immune system may also contribute to PD. GWAS have found that polymorphisms in the human leukocyte antigen (HLA) genes involved in antigen presentation are associated with a greater risk of PD development (Hamza et al., 2010; Nalls et al., 2011). Furthermore, postmortem pathology studies show CD8+ and CD4+ T cell infiltration in PD brains (Brochard et al., 2009). The offending antigen in this case is hypothesized to be misfolded/nitrated α-syn. Administration of MPTP results in α-syn drainage into lymph nodes, where it elicits a T cell response (Reynolds et al., 2009). Furthermore, α-syn–derived peptides act as antigenic epitopes and mediate helper and cytotoxic T cell responses in PD patients (Sulzer et al., 2017). This finding, coupled with the discovery that human DA neurons express MHC-I and can attract a cytotoxic T cell attack (Cebrián et al., 2014), could account for T cell–mediated DA neuron death (Fig. 4 D).
Further evidence supporting the role of the adaptive immune system is the finding that preventing adaptive immune activation is neuroprotective in PD models. Regulatory T cells are a subset of CD4+ T cells that can actively dampen immune cell hyperactivation via immunosuppressive cytokine secretion (Josefowicz et al., 2012). Adoptive transfer of regulatory T cells was able to strongly ameliorate microglial activation and prevent DA neuron death in MPTP-injected mice (Reynolds et al., 2007). Recently, an intriguing study in macrophages showed that parkin and PINK1 may repress mitochondrial antigen presentation, thereby preventing T cell activation (Matheoud et al., 2016). Loss of PINK1 leads to mitochondrial antigen presentation, triggering cytotoxic T cells to infiltrate the brain and directly attack DA neurons (Matheoud et al., 2019). As such, parkin/PINK1 may play a critical role in limiting adaptive immune attacks on SNpc DA neurons (further reviewed below and in Ge et al., 2020), a function that may be compromised in disease.
It is notable that many PD transgenic knock-in/KO mouse models do not exhibit DA neuron death in the SNpc. DA neuron-specific overexpression of A53T or C-terminally truncated, 115-aa α-syn also fails to elicit SNpc neuronal death (Daher et al., 2009). While expressing longer 130-aa α-syn does induce DA neuron loss, this occurs during embryogenesis and is not progressive (Wakamatsu et al., 2008). On the other hand, PD model systems that entail non–cell-autonomous pathologies, such as the PFF model, and AAV-mediated α-syn overexpression do elicit DA neuron death (Luk et al., 2012; Oliveras-Salvá et al., 2013). We speculate that the latter set of model systems may be more effective at mediating neuron loss since they entail a glial response component, whereas the pathology of the former set of models is largely restricted to neurons through cell type–specific expression. Microgliosis has been documented in virtually every mouse model of PD that exhibits TH neuron loss in the SNpc, including the MPTP, 6-hydroxydopamine, α-syn AAV, and A53T α-syn overexpression models (Fourgeaud et al., 2016; Panicker et al., 2015; Theodore et al., 2008). The signaling mechanisms that link chronic microglial activation to neurodegeneration in PD remain an active area of investigation.
Glial activation by aggregated/misfolded α-syn
As discussed, the prion-like spread of α-syn is a non–cell-autonomous process (Fig. 4 B). α-Syn aggregates can be taken up not only by neurons but also by many other major CNS cell types. Neuron-released α-syn can be endocytosed by astrocytes, which then assume a pro-inflammatory phenotype (Lee et al., 2010c). Oligodendrocytes can also endocytose both monomeric and, to a lesser extent, fibrillar α-syn (Reyes et al., 2014). Microglial uptake of α-syn is well-documented. Aggregated forms of α-syn are able to potently activate microglial cells, leading to the robust production of pro-inflammatory cytokines and the activation of the NLRP3 inflammasome (Gordon et al., 2018; Lee et al., 2010b; Zhang et al., 2005b). The mechanisms through which this mediates pro-inflammatory signaling are still being elucidated; Toll-like receptor 2 and CD36 were shown to play a crucial role in the microglial uptake of α-syn, as well as in initiating pro-inflammatory signaling and the production of various inflammatory mediators (Kim et al., 2013a; Panicker et al., 2019).
The role of monogenic PD genes in glial biology
Several other PARK proteins have well-documented roles in glial cell biology that may contribute to PD progression. Apart from neurons, LRRK2 is expressed by microglia, astrocytes, and oligodendrocytes. Its microglial expression is lipopolysaccharide (LPS)–inducible, and inhibiting it pharmacologically or through gene silencing attenuated neuroinflammatory responses in microglia (Moehle et al., 2012). LRRK2-deficient microglia have reduced pro-inflammatory responses compared with WT microglia (Russo et al., 2015). Microglia isolated from mice harboring the LRRK2 R1441G mutation exhibited increased pro-inflammatory cytokine production (Gillardon et al., 2012). LRRK2-deficient rats were found to be resistant to intracranial LPS and α-syn–mediated DA neurodegeneration. Within the midbrain, LRRK2 expression is inducible in myeloid cells, and the neuroprotection in LRRK2-deficient rats can be attributed to attenuated myeloid cell recruitment (Daher et al., 2014). Compared with age-matched controls, LRRK2 expression is increased in B cells, T cells, and monocytes from PD patients (Cook et al., 2017). Consistent with its pro-inflammatory role, polymorphisms around LRRK2 are associated with an increased risk of Crohn’s disease and leprosy, two inflammatory diseases (Barrett et al., 2008; Zhang et al., 2009). We further speculate that the central role of LRRK2 in vesicle trafficking may also contribute to mediating pro-inflammatory responses, given the essential role of the secretory system in releasing pro-inflammatory cytokines (Stow et al., 2009). However, to the best of our knowledge, this hypothesis has not been directly tested.
Loss of parkin function in non-dopaminergic cells may also contribute to PD pathology. It is expressed in microglia and down-regulated following pro-inflammatory NF-κB activation upon LPS or TNF-α stimulation. Parkin-deficient macrophages show hyperactivated pro-inflammatory responses (Tran et al., 2011). As mentioned previously, parkin−/− mice do exhibit spontaneous DA neuron loss in the SNpc. However, repeated, low-dose systemic treatment with the inflammation inducer LPS caused SNpc DA neuron death in parkin KO, but not WT mice, suggesting that parkin deficiency might sensitize mice to neuroinflammatory insults and that parkin might contribute to non–cell-autonomous processes linked to PD (Frank-Cannon et al., 2008). Activation of the NLRP3 inflammasome leads to parkin inactivation via proteolytic cleavage, which inhibits mitophagy. This leads to an increased mitochondrial ROS generation and subsequent activation of the NLRP3 inflammasome in macrophages (Yu et al., 2014). There are further data suggesting that parkin regulates inflammatory signaling in vivo; parkin-KO microglia and astrocytes display more severe mitochondrial deficits compared with their WT counterparts. In fact, the degree of mitochondrial defects seen in the glia was more severe than that observed in parkin-KO neurons (Schmidt et al., 2011). Cortical brain slices from PINK1-deficient mice showed increased basal cytokine production when compared with brain slices from WT mice (Kim et al., 2013b). The accumulation of mitochondrial DNA was found to contribute to significant neuroinflammation and cytokine production, resulting in DA neuron death in mice lacking parkin and PINK1. Deletion of the STING, which is required for the type 1 interferon response, abrogated neuroinflammation and neuron loss in these mice (Sliter et al., 2018). Finally, intestinal infection of PINK1-deficient mice with Gram-negative bacteria was sufficient to reduce striatal DA innervation, suggesting that PINK1-mediated repression of immune function may contribute to its role in preventing PD onset (Matheoud et al., 2019).
As described above, DJ-1 is expressed nearly ubiquitously across cell types in the CNS. Its expression can be increased by oxidative stress in astrocytes (Yanagida et al., 2009). Furthermore, DJ-1 can regulate interferon-γ–mediated pro-inflammatory signaling in both microglia and astrocytes by mediating the interaction between STAT1 and its phosphatase SHP1, leading to reduced production of microglial nitrite and astrocytic induction of interferon regulatory factor-1 (Kim et al., 2013c).
Hence, PD-associated proteins play fundamental roles in glial cell biology, and the degree to which their glial roles contribute to PD disease phenotype is an area of active investigation. With the recent development of numerous transgenic mice permitting selective gene KO in microglial lineages (Goldmann et al., 2013; Kaiser and Feng, 2019; McKinsey et al., 2020), the glia-specific roles of PD-associated proteins can be investigated in PD mouse models with greater acuity. It is possible that glial-specific parkin or PINK1 KO mice may exhibit spontaneous inflammation or exacerbated inflammation when subjected to inflammogen treatment. The inverse results would be expected when using microglia-specific LRRK2 KO mice.
Concluding remarks
Prior to the discovery of the first PD-associated gene, toxin-induced murine models of PD, such as MPTP and 6-hydroxydopamine, dominated the field and remain popular today. These models have been invaluable in identifying stages of the disease, translate to primate model systems (Eslamboli et al., 2005; Porras et al., 2012), and are amenable to preclinical testing of therapeutic agents. However, the acute DA neuron death induced by these toxins does not resemble the slow, progressive neurodegeneration in PD and fails to recapitulate other neuropathological hallmarks of PD, such as formation of LBs (Tieu, 2011). Perhaps as a result of these limitations, these model systems have not yielded a disease-modifying treatment for PD.
The discovery of monogenic PD genes and characterization of their protein products have provided important insights into the pathogenesis of familial and sporadic PD. Among these classic PD genes are α-syn, a presynaptic protein that disrupts cellular functions by forming aggregates, which spread in a prion-like manner. It can also elicit deficits in synaptic transmission and autophagy. Pathogenic mutations of LRRK2, a kinase and GTPase, cause DA neuron death through cytoskeletal rearrangements, vesicular sorting defects, and shifts in global protein translation. VPS35 is part of the retromer complex, and is critical for maintaining lysosomal function and mitochondrial dynamics through retromer’s role in vesicular transport. Parkin is an E3 ubiquitin ligase, and its substrates accumulate following its deletion or mutation. Accumulation of these substrates drives neuron death through parthanatos and defects in mitochondrial biogenesis. PINK1 is a mitochondrial protein kinase that cooperates with parkin to regulate mitochondrial quality control, and maintains ETC function in a parkin-independent fashion. DJ-1 acts a multi-function protein, detecting ROSs and activating neuroprotective signaling pathways.
Collective evidence implicates a diverse array of cell biological processes that go awry in PD. Many lines of evidence converge upon failure of proteostasis, defects in vesicular transport and endolysosomal function, and failure of mitochondrial quality control/oxidative stress responses as core, interconnected mechanisms of neurodegeneration (see text box). While we summarize these pathways in separate sections, it is important to recognize that they are highly interconnected. For example, failures in endolysosomal function can inhibit proper protein turnover (e.g., VPS35 and GCase1 mutations causing α-syn aggregation), while proteostatic failure and vesicular trafficking defects will disrupt mitochondrial quality control (e.g., α-syn, LRRK2, and VPS35 causing mitochondrial defects). Furthermore, it is becoming increasingly evident that many of the pathways discovered in genetic models play a critical role in both familial and sporadic forms of PD. Further studies into the functions of monogenic PD genes may thus lead to the identification of promising therapeutic targets that can benefit most if not all PD patients. While much work has gone into understanding the neuronal function of these genes, it is also becoming clear that many PD-associated proteins have non–cell-autonomous functions in nonneuronal cells such as microglia, which could contribute to the progression of PD. Further work may shed important insights into mechanisms of non–cell-autonomous neurodegeneration in PD.
Unifying cell biological pathways of PD genes
Defects in protein degradation and loss of proteostasis
The clearest example of this is the aggregation of α-syn triggered by gene mutation, increased gene copy number, posttranslational modifications, or seeded by extrinsic aggregates. Numerous PARK genes likely also contribute to impaired α-syn proteostasis. Loss of GCase1 leads to lysosomal and autophagy defects that impair defense against α-syn aggregates, while accumulation of its lipid substrates may also promote aggregation. LRRK2 kinase also indirectly aggravates α-syn pathology in a kinase-dependent manner (reviewed in O’Hara et al., 2020) through mechanisms such as including accelerated aggregation of α-syn into inclusions following α-syn fibril treatment and increased transcellular propagation (Bae et al., 2018; Volpicelli-Daley et al., 2016). Furthermore, VPS35’s role in supporting endolysosomal function by regulating vesicular trafficking has also been reported to regulate α-syn levels, while DJ-1 has been reported to function as a protein chaperone to ameliorate the aggregation of proteins such as α-syn. Beyond α-syn alone, loss of VPS35 greatly aggravates proteotoxic stress induced by eIF4G1 overexpression, while LRRK2 promotes protein translation through multiple mechanisms, suggesting that PD genes that modulate α-syn pathology may have more general roles in maintaining proteostasis.
Disruption of vesicular trafficking and endolysosomal function
LRRK2 and VPS35 play an essential role in regulating vesicular trafficking. While LRRK2 and VPS35 both have independent functions at different levels of the endolysosomal system, numerous studies have proposed a strong genetic interaction between both proteins (Inoshita et al., 2017; Linhart et al., 2014; MacLeod et al., 2013; Mir et al., 2018). Further studies propose either a direct interaction between the two proteins or regulation of a common molecular target (MacLeod et al., 2013; Mir et al., 2018), indicating some degree of convergence upon shared cellular pathways. Furthermore, GCase1 also may impair these pathways through aggregation of mutant GCase1 in the ER or through lysosomal dysfunction (see main text). GCase1 function and localization require intact vesicular trafficking, a process that can be disrupted by expression of mutant LRRK2 and rescued by LRRK2 inhibition (Sanyal et al., 2020; Ysselstein et al., 2019). These collective defects lead to a cascade of cellular failures such as impaired clearance of damaged proteins and organelles, inability to meet metabolic demands, and failure of synaptic function (Hunn et al., 2015). Critically, just as these defects can disrupt proteostasis and contribute to α-syn pathology, α-syn aggregates themselves can also feedback to disrupt vesicular trafficking and endolysosomal function (Cooper et al., 2006; Hunn et al., 2015; Mazzulli et al., 2016).
Mitochondrial dysfunction and oxidative stress
Parkin and PINK1 are two classic examples of closely interacting proteins contributing to PD pathogenesis, functioning together as a detector of mitochondrial damage and trigger of mitochondrial quality control pathways. Given the role of DJ-1 in oxidative stress responses, it is unsurprising that loss of DJ-1 causes numerous mitochondrial defects (Biosa et al., 2017; Cookson, 2012). Furthermore, numerous studies have found evidence of a bidirectional genetic interaction between parkin/PINK1 and DJ-1 (Hao et al., 2010; Haque et al., 2012; Irrcher et al., 2010; Thomas et al., 2011), suggesting that they may act together or in parallel to preserve mitochondrial function. α-Syn aggregation can also trigger mitochondrial defects in part by inducing c-Abl activation, which phosphorylates and inactivates parkin and leads to the accumulation of parkin substrates (Brahmachari et al., 2016; Brahmachari et al., 2019; Ko et al., 2010). Disease-associated LRRK2 mutations further lead to mitochondrial defects through numerous mechanisms such as kinase-dependent inactivation of PINK1/parkin (Bonello et al., 2019; Singh et al., 2019). Finally, given the known roles of VPS35 and PINK1/parkin in driving MDV formation and regulating fission/fusion dynamics, an intriguing possibility is that both pathways may functionally or moleculary converge on the same molecular targets. Given the unique energetic challenges imposed by the structural polarization, energy demand, and lifespan of CNS cells (Misgeld and Schwarz, 2017), they are uniquely vulnerable to mitochondrial damage and thus rely on these protective mechanisms for their survival.
Acknowledgments
We thank Deborah Plana at Harvard Medical School and Audrey H. Effenberger at Massachusetts Institute of Technology for discussions and editorial comments during the preparation of the paper. The figures for this article were illustrated by Noelle Burgess and I-Hsun Wu.
This work was supported by grants from the National Institutes of Health/National Institute of Neurological Disorders and Stroke (NS38377, NS097049, and NS102035), the National Institutes of Health/National Institute on Aging (AG059686), the JPB Foundation, the Michael J. Fox Foundation , and the RMS Family Foundation. The authors acknowledge the joint participation by the Adrienne Helis Malvin Medical Research Foundation and the Diana Helis Henry Medical Research Foundation through their direct engagement in the continuous active conduct of medical research in conjunction with the Johns Hopkins Hospital and the Johns Hopkins University School of Medicine and the Foundation’s Parkinson’s Disease Programs (M-1, M-2, M-2013, M-2014, M-2016, M-2017, M-2018, M-2019, and H-2014). N. Panicker is supported by a Pathway to Independence grant from the National Institute on Aging (K99AG066862). P. Ge is supported by a National Institutes of Health Medical Scientist Training Program grant (T32GM007753) and by the Fred (1960) and Sandra Holubow Fellowship at Massachusetts Institute of Technology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper.
T.M. Dawson owns stock in American Gene Technologies International Inc.; T.M. Dawson owns stock options in Mitokinin; T.M. Dawson and V.L. Dawson own stock options Inhibikase Therapeutics Inc.; T.M. Dawson and V.L. Dawson are founders of Valted, LLC, and hold an ownership equity interest in the company. T.M. Dawson and V.L. Dawson are inventors of technology of Neuraly, Inc., that has optioned from Johns Hopkins University. T.M. Dawson and V.L. Dawson are founders of, and hold shares of stock options as well as equity in, Neuraly, Inc., which is now a subsidiary of D&D Pharmatech; T.M. Dawson and V.L. Dawson hold shares of stock options as well as equity in D&D Pharmatech; T.M. Dawson and V.L. Dawson are founders of and hold equity in Valted Seq Inc. These arrangements have been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies. The remaining authors declare no competing financial interests.
Author contributions: N. Panicker, P. Ge, V.L. Dawson, and T.M. Dawson conceived and designed the manuscript. N. Panicker, P. Ge, V.L. Dawson, and T.M. Dawson wrote the manuscript. N. Panicker, V.L. Dawson, and T.M. Dawson secured funding.
References
- Abounit, S., Bousset L., Loria F., Zhu S., de Chaumont F., Pieri L., Olivo-Marin J.C., Melki R., and Zurzolo C.. 2016. Tunneling nanotubes spread fibrillar α-synuclein by intercellular trafficking of lysosomes. EMBO J. 35:2120–2138. 10.15252/embj.201593411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegre-Abarrategui, J., Christian H., Lufino M.M., Mutihac R., Venda L.L., Ansorge O., and Wade-Martins R.. 2009. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum. Mol. Genet. 18:4022–4034. 10.1093/hmg/ddp346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alim, M.A., Hossain M.S., Arima K., Takeda K., Izumiyama Y., Nakamura M., Kaji H., Shinoda T., Hisanaga S., and Ueda K.. 2002. Tubulin seeds alpha-synuclein fibril formation. J. Biol. Chem. 277:2112–2117. 10.1074/jbc.M102981200 [DOI] [PubMed] [Google Scholar]
- Aloisi, F. 2001. Immune function of microglia. Glia. 36:165–179. 10.1002/glia.1106 [DOI] [PubMed] [Google Scholar]
- Amo, T., Saiki S., Sawayama T., Sato S., and Hattori N.. 2014. Detailed analysis of mitochondrial respiratory chain defects caused by loss of PINK1. Neurosci. Lett. 580:37–40. 10.1016/j.neulet.2014.07.045 [DOI] [PubMed] [Google Scholar]
- Andreeva, A., Bekkhozhin Z., Omertassova N., Baizhumanov T., Yeltay G., Akhmetali M., Toibazar D., and Utepbergenov D.. 2019. The apparent deglycase activity of DJ-1 results from the conversion of free methylglyoxal present in fast equilibrium with hemithioacetals and hemiaminals. J. Biol. Chem. 294:18863–18872. 10.1074/jbc.RA119.011237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres-Mateos, E., Perier C., Zhang L., Blanchard-Fillion B., Greco T.M., Thomas B., Ko H.S., Sasaki M., Ischiropoulos H., Przedborski S., et al. 2007. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc. Natl. Acad. Sci. USA. 104:14807–14812. 10.1073/pnas.0703219104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader, V., and Winklhofer K.F.. 2020. PINK1 and Parkin: team players in stress-induced mitophagy. Biol. Chem. 401:891–899. 10.1515/hsz-2020-0135 [DOI] [PubMed] [Google Scholar]
- Bader, V., Ran Zhu X., Lübbert H., and Stichel C.C.. 2005. Expression of DJ-1 in the adult mouse CNS. Brain Res. 1041:102–111. 10.1016/j.brainres.2005.02.006 [DOI] [PubMed] [Google Scholar]
- Bae, E.J., Kim D.K., Kim C., Mante M., Adame A., Rockenstein E., Ulusoy A., Klinkenberg M., Jeong G.R., Bae J.R., et al. 2018. LRRK2 kinase regulates α-synuclein propagation via RAB35 phosphorylation. Nat. Commun. 9:3465. 10.1038/s41467-018-05958-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, R.M., Covy J.P., Melrose H.L., Rousseau L., Watkinson R., Knight J., Miles S., Farrer M.J., Dickson D.W., Giasson B.I., and Lewis J.. 2013. LRRK2 phosphorylates novel tau epitopes and promotes tauopathy. Acta Neuropathol. 126:809–827. 10.1007/s00401-013-1188-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balducci, C., Pierguidi L., Persichetti E., Parnetti L., Sbaragli M., Tassi C., Orlacchio A., Calabresi P., Beccari T., and Rossi A.. 2007. Lysosomal hydrolases in cerebrospinal fluid from subjects with Parkinson’s disease. Mov. Disord. 22:1481–1484. 10.1002/mds.21399 [DOI] [PubMed] [Google Scholar]
- Bandres-Ciga, S., Diez-Fairen M., Kim J.J., and Singleton A.B.. 2020. Genetics of Parkinson’s disease: An introspection of its journey towards precision medicine. Neurobiol. Dis. 137:104782. 10.1016/j.nbd.2020.104782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, J.C., Hansoul S., Nicolae D.L., Cho J.H., Duerr R.H., Rioux J.D., Brant S.R., Silverberg M.S., Taylor K.D., Barmada M.M., et al. Wellcome Trust Case Control Consortium . 2008. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat. Genet. 40:955–962. 10.1038/ng.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels, A.L., and Leenders K.L.. 2007. Neuroinflammation in the pathophysiology of Parkinson’s disease: evidence from animal models to human in vivo studies with [11C]-PK11195 PET. Mov. Disord. 22:1852–1856. 10.1002/mds.21552 [DOI] [PubMed] [Google Scholar]
- Beilina, A., Rudenko I.N., Kaganovich A., Civiero L., Chau H., Kalia S.K., Kalia L.V., Lobbestael E., Chia R., Ndukwe K., et al. North American Brain Expression Consortium . 2014. Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proc. Natl. Acad. Sci. USA. 111:2626–2631. 10.1073/pnas.1318306111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick, D.C., Heaton G.R., Azeggagh S., and Harvey K.. 2019. LRRK2 Biology from structure to dysfunction: research progresses, but the themes remain the same. Mol. Neurodegener. 14:49. 10.1186/s13024-019-0344-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biosa, A., Sandrelli F., Beltramini M., Greggio E., Bubacco L., and Bisaglia M.. 2017. Recent findings on the physiological function of DJ-1: Beyond Parkinson’s disease. Neurobiol. Dis. 108:65–72. 10.1016/j.nbd.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Birol, M., Wojcik S.P., Miranker A.D., and Rhoades E.. 2019. Identification of N-linked glycans as specific mediators of neuronal uptake of acetylated α-Synuclein. PLoS Biol. 17:e3000318. 10.1371/journal.pbio.3000318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonello, F., Hassoun S.M., Mouton-Liger F., Shin Y.S., Muscat A., Tesson C., Lesage S., Beart P.M., Brice A., Krupp J., et al. 2019. LRRK2 impairs PINK1/Parkin-dependent mitophagy via its kinase activity: pathologic insights into Parkinson’s disease. Hum. Mol. Genet. 28:1645–1660. 10.1093/hmg/ddz004 [DOI] [PubMed] [Google Scholar]
- Boutin, M., Sun Y., Shacka J.J., and Auray-Blais C.. 2016. Tandem Mass Spectrometry Multiplex Analysis of Glucosylceramide and Galactosylceramide Isoforms in Brain Tissues at Different Stages of Parkinson Disease. Anal. Chem. 88:1856–1863. 10.1021/acs.analchem.5b04227 [DOI] [PubMed] [Google Scholar]
- Braak, H., Del Tredici K., Rüb U., de Vos R.A., Jansen Steur E.N., and Braak E.. 2003. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 24:197–211. 10.1016/S0197-4580(02)00065-9 [DOI] [PubMed] [Google Scholar]
- Brahmachari, S., Ge P., Lee S.H., Kim D., Karuppagounder S.S., Kumar M., Mao X., Shin J.H., Lee Y., Pletnikova O., et al. 2016. Activation of tyrosine kinase c-Abl contributes to α-synuclein-induced neurodegeneration. J. Clin. Invest. 126:2970–2988. 10.1172/JCI85456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmachari, S., Lee S., Kim S., Yuan C., Karuppagounder S.S., Ge P., Shi R., Kim E.J., Liu A., Kim D., et al. 2019. Parkin interacting substrate zinc finger protein 746 is a pathological mediator in Parkinson’s disease. Brain. 142:2380–2401. 10.1093/brain/awz172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braschi, E., Zunino R., and McBride H.M.. 2009. MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep. 10:748–754. 10.1038/embor.2009.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braschi, E., Goyon V., Zunino R., Mohanty A., Xu L., and McBride H.M.. 2010. Vps35 mediates vesicle transport between the mitochondria and peroxisomes. Curr. Biol. 20:1310–1315. 10.1016/j.cub.2010.05.066 [DOI] [PubMed] [Google Scholar]
- Brochard, V., Combadière B., Prigent A., Laouar Y., Perrin A., Beray-Berthat V., Bonduelle O., Alvarez-Fischer D., Callebert J., Launay J.M., et al. 2009. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Invest. 119:182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhlman, L., Damiano M., Bertolin G., Ferrando-Miguel R., Lombès A., Brice A., and Corti O.. 2014. Functional interplay between Parkin and Drp1 in mitochondrial fission and clearance. Biochim. Biophys. Acta. 1843:2012–2026. 10.1016/j.bbamcr.2014.05.012 [DOI] [PubMed] [Google Scholar]
- Burai, R., Ait-Bouziad N., Chiki A., and Lashuel H.A.. 2015. Elucidating the Role of Site-Specific Nitration of α-Synuclein in the Pathogenesis of Parkinson’s Disease via Protein Semisynthesis and Mutagenesis. J. Am. Chem. Soc. 137:5041–5052. 10.1021/ja5131726 [DOI] [PubMed] [Google Scholar]
- Burchell, V.S., Nelson D.E., Sanchez-Martinez A., Delgado-Camprubi M., Ivatt R.M., Pogson J.H., Randle S.J., Wray S., Lewis P.A., Houlden H., et al. 2013. The Parkinson’s disease-linked proteins Fbxo7 and Parkin interact to mediate mitophagy. Nat. Neurosci. 16:1257–1265. 10.1038/nn.3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd, C., and Cullen P.J.. 2014. Retromer: a master conductor of endosome sorting. Cold Spring Harb. Perspect. Biol. 6:a016774. 10.1101/cshperspect.a016774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmann, B.M., Gerez J.A., Matečko-Burmann I., Campioni S., Kumari P., Ghosh D., Mazur A., Aspholm E.E., Šulskis D., Wawrzyniuk M., et al. 2020. Regulation of α-synuclein by chaperones in mammalian cells. Nature. 577:127–132. 10.1038/s41586-019-1808-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burré, J., Sharma M., Tsetsenis T., Buchman V., Etherton M.R., and Südhof T.C.. 2010. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 329:1663–1667. 10.1126/science.1195227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burré, J., Sharma M., and Südhof T.C.. 2014. α-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc. Natl. Acad. Sci. USA. 111:E4274–E4283. 10.1073/pnas.1416598111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burré, J., Sharma M., and Südhof T.C.. 2018. Cell Biology and Pathophysiology of α-Synuclein. Cold Spring Harb. Perspect. Med. 8:a024091. 10.1101/cshperspect.a024091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar, M., Zach S., Carlson C.B., Brockmann K., Gasser T., and Gillardon F.. 2013. Leucine-rich repeat kinase 2 functionally interacts with microtubules and kinase-dependently modulates cell migration. Neurobiol. Dis. 54:280–288. 10.1016/j.nbd.2012.12.019 [DOI] [PubMed] [Google Scholar]
- Canet-Avilés, R.M., Wilson M.A., Miller D.W., Ahmad R., McLendon C., Bandyopadhyay S., Baptista M.J., Ringe D., Petsko G.A., and Cookson M.R.. 2004. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. USA. 101:9103–9108. 10.1073/pnas.0402959101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebrián, C., Zucca F.A., Mauri P., Steinbeck J.A., Studer L., Scherzer C.R., Kanter E., Budhu S., Mandelbaum J., Vonsattel J.P., et al. 2014. MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nat. Commun. 5:3633. 10.1038/ncomms4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis, C., Hori A., Sampson T.R., Yoo B.B., Challis R.C., Hamilton A.M., Mazmanian S.K., Volpicelli-Daley L.A., and Gradinaru V.. 2020. Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat. Neurosci. 23:327–336. 10.1038/s41593-020-0589-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, N.C., Salazar A.M., Pham A.H., Sweredoski M.J., Kolawa N.J., Graham R.L., Hess S., and Chan D.C.. 2011. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum. Mol. Genet. 20:1726–1737. 10.1093/hmg/ddr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier, S., and Duyckaerts C.. 2018. Is Lewy pathology in the human nervous system chiefly an indicator of neuronal protection or of toxicity? Cell Tissue Res. 373:149–160. 10.1007/s00441-018-2854-6 [DOI] [PubMed] [Google Scholar]
- Chaugule, V.K., Burchell L., Barber K.R., Sidhu A., Leslie S.J., Shaw G.S., and Walden H.. 2011. Autoregulation of Parkin activity through its ubiquitin-like domain. EMBO J. 30:2853–2867. 10.1038/emboj.2011.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., and Dorn G.W. II. 2013. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 340:471–475. 10.1126/science.1231031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.Y., Weng Y.H., Chien K.Y., Lin K.J., Yeh T.H., Cheng Y.P., Lu C.S., and Wang H.L.. 2012. (G2019S) LRRK2 activates MKK4-JNK pathway and causes degeneration of SN dopaminergic neurons in a transgenic mouse model of PD. Cell Death Differ. 19:1623–1633. 10.1038/cdd.2012.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, K.E., Healy M.D., and Collins B.M.. 2019a. Towards a molecular understanding of endosomal trafficking by Retromer and Retriever. Traffic. 20:465–478. 10.1111/tra.12649 [DOI] [PubMed] [Google Scholar]
- Chen, X., Kordich J.K., Williams E.T., Levine N., Cole-Strauss A., Marshall L., Labrie V., Ma J., Lipton J.W., and Moore D.J.. 2019b. Parkinson’s disease-linked D620N VPS35 knockin mice manifest tau neuropathology and dopaminergic neurodegeneration. Proc. Natl. Acad. Sci. USA. 116:5765–5774. 10.1073/pnas.1814909116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherra, S.J. III, Steer E., Gusdon A.M., Kiselyov K., and Chu C.T.. 2013. Mutant LRRK2 elicits calcium imbalance and depletion of dendritic mitochondria in neurons. Am. J. Pathol. 182:474–484. 10.1016/j.ajpath.2012.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, M.S., Nakamura T., Cho S.J., Han X., Holland E.A., Qu J., Petsko G.A., Yates J.R. III, Liddington R.C., and Lipton S.A.. 2014. Transnitrosylation from DJ-1 to PTEN attenuates neuronal cell death in parkinson’s disease models. J. Neurosci. 34:15123–15131. 10.1523/JNEUROSCI.4751-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy, R.W., Park M., Temkin P., Herring B.E., Marley A., Nicoll R.A., and von Zastrow M.. 2014. Retromer mediates a discrete route of local membrane delivery to dendrites. Neuron. 82:55–62. 10.1016/j.neuron.2014.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, K.K., Thomas B., Li X., Pletnikova O., Troncoso J.C., Marsh L., Dawson V.L., and Dawson T.M.. 2004. S-nitrosylation of parkin regulates ubiquitination and compromises parkin’s protective function. Science. 304:1328–1331. 10.1126/science.1093891 [DOI] [PubMed] [Google Scholar]
- Chung, C.Y., Koprich J.B., Siddiqi H., and Isacson O.. 2009. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J. Neurosci. 29:3365–3373. 10.1523/JNEUROSCI.5427-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, I.E., Dodson M.W., Jiang C., Cao J.H., Huh J.R., Seol J.H., Yoo S.J., Hay B.A., and Guo M.. 2006. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 441:1162–1166. 10.1038/nature04779 [DOI] [PubMed] [Google Scholar]
- Clements, C.M., McNally R.S., Conti B.J., Mak T.W., and Ting J.P.. 2006. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl. Acad. Sci. USA. 103:15091–15096. 10.1073/pnas.0607260103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly, B.S., and Lang A.E.. 2014. Pharmacological treatment of Parkinson disease: a review. JAMA. 311:1670–1683. 10.1001/jama.2014.3654 [DOI] [PubMed] [Google Scholar]
- Cook, D.A., Kannarkat G.T., Cintron A.F., Butkovich L.M., Fraser K.B., Chang J., Grigoryan N., Factor S.A., West A.B., Boss J.M., and Tansey M.G.. 2017. LRRK2 levels in immune cells are increased in Parkinson’s disease. NPJ Parkinsons Dis. 3:11. 10.1038/s41531-017-0010-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson, M.R. 2012. Parkinsonism due to mutations in PINK1, parkin, and DJ-1 and oxidative stress and mitochondrial pathways. Cold Spring Harb. Perspect. Med. 2:a009415. 10.1101/cshperspect.a009415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, A.A., Gitler A.D., Cashikar A., Haynes C.M., Hill K.J., Bhullar B., Liu K., Xu K., Strathearn K.E., Liu F., et al. 2006. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 313:324–328. 10.1126/science.1129462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copley, S.D. 2014. An evolutionary perspective on protein moonlighting. Biochem. Soc. Trans. 42:1684–1691. 10.1042/BST20140245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen, T., Vilain S., Vints K., Gounko N., Verstreken P., and Vandenberghe W.. 2018. Deficiency of parkin and PINK1 impairs age-dependent mitophagy in Drosophila. eLife. 7:e35878. 10.7554/eLife.35878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti, O., Hampe C., Koutnikova H., Darios F., Jacquier S., Prigent A., Robinson J.C., Pradier L., Ruberg M., Mirande M., et al. 2003. The p38 subunit of the aminoacyl-tRNA synthetase complex is a Parkin substrate: linking protein biosynthesis and neurodegeneration. Hum. Mol. Genet. 12:1427–1437. 10.1093/hmg/ddg159 [DOI] [PubMed] [Google Scholar]
- Dagda, R.K., Cherra S.J. III, Kulich S.M., Tandon A., Park D., and Chu C.T.. 2009. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J. Biol. Chem. 284:13843–13855. 10.1074/jbc.M808515200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda, R.K., Pien I., Wang R., Zhu J., Wang K.Z., Callio J., Banerjee T.D., Dagda R.Y., and Chu C.T.. 2014. Beyond the mitochondrion: cytosolic PINK1 remodels dendrites through protein kinase A. J. Neurochem. 128:864–877. 10.1111/jnc.12494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher, J.P., Ying M., Banerjee R., McDonald R.S., Hahn M.D., Yang L., Flint Beal M., Thomas B., Dawson V.L., Dawson T.M., and Moore D.J.. 2009. Conditional transgenic mice expressing C-terminally truncated human alpha-synuclein (alphaSyn119) exhibit reduced striatal dopamine without loss of nigrostriatal pathway dopaminergic neurons. Mol. Neurodegener. 4:34. 10.1186/1750-1326-4-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher, J.P., Volpicelli-Daley L.A., Blackburn J.P., Moehle M.S., and West A.B.. 2014. Abrogation of α-synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proc. Natl. Acad. Sci. USA. 111:9289–9294. 10.1073/pnas.1403215111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson, S.R., Held J.M., Schilling B., Oo M., Gibson B.W., and Andersen J.K.. 2009. Preferentially increased nitration of alpha-synuclein at tyrosine-39 in a cellular oxidative model of Parkinson’s disease. Anal. Chem. 81:7823–7828. 10.1021/ac901176t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer, K.M., Haasen D., Karow A.R., Moussaud S., Habeck M., Giese A., Kretzschmar H., Hengerer B., and Kostka M.. 2007. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J. Neurosci. 27:9220–9232. 10.1523/JNEUROSCI.2617-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson, T.M., and Dawson V.L.. 2003. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 302:819–822. 10.1126/science.1087753 [DOI] [PubMed] [Google Scholar]
- Dawson, T.M., and Dawson V.L.. 2014. Parkin plays a role in sporadic Parkinson’s disease. Neurodegener. Dis. 13:69–71. 10.1159/000354307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi, L., Raghavendran V., Prabhu B.M., Avadhani N.G., and Anandatheerthavarada H.K.. 2008. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J. Biol. Chem. 283:9089–9100. 10.1074/jbc.M710012200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devireddy, S., Liu A., Lampe T., and Hollenbeck P.J.. 2015. The Organization of Mitochondrial Quality Control and Life Cycle in the Nervous System In Vivo in the Absence of PINK1. J. Neurosci. 35:9391–9401. 10.1523/JNEUROSCI.1198-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhungel, N., Eleuteri S., Li L.B., Kramer N.J., Chartron J.W., Spencer B., Kosberg K., Fields J.A., Stafa K., Adame A., et al. 2015. Parkinson’s disease genes VPS35 and EIF4G1 interact genetically and converge on α-synuclein. Neuron. 85:76–87. 10.1016/j.neuron.2014.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias, V., Junn E., and Mouradian M.M.. 2013. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 3:461–491. 10.3233/JPD-130230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do, J., McKinney C., Sharma P., and Sidransky E.. 2019. Glucocerebrosidase and its relevance to Parkinson disease. Mol. Neurodegener. 14:36. 10.1186/s13024-019-0336-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson, M.W., Zhang T., Jiang C., Chen S., and Guo M.. 2012. Roles of the Drosophila LRRK2 homolog in Rab7-dependent lysosomal positioning. Hum. Mol. Genet. 21:1350–1363. 10.1093/hmg/ddr573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgacheva, L.P., Berezhnov A.V., Fedotova E.I., Zinchenko V.P., and Abramov A.Y.. 2019. Role of DJ-1 in the mechanism of pathogenesis of Parkinson’s disease. J. Bioenerg. Biomembr. 51:175–188. 10.1007/s10863-019-09798-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanskyi, A., Geissler C., Vinnikov I.A., Alter H., Schober A., Vogt M.A., Gass P., Parlato R., and Schütz G.. 2011. Pten ablation in adult dopaminergic neurons is neuroprotective in Parkinson’s disease models. FASEB J. 25:2898–2910. 10.1096/fj.11-181958 [DOI] [PubMed] [Google Scholar]
- Du, T.T., Wang L., Duan C.L., Lu L.L., Zhang J.L., Gao G., Qiu X.B., Wang X.M., and Yang H.. 2015. GBA deficiency promotes SNCA/α-synuclein accumulation through autophagic inhibition by inactivated PPP2A. Autophagy. 11:1803–1820. 10.1080/15548627.2015.1086055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidou, E., Melachroinou K., Roumeliotis T., Garbis S.D., Ntzouni M., Margaritis L.H., Stefanis L., and Vekrellis K.. 2010. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 30:6838–6851. 10.1523/JNEUROSCI.5699-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelender, S., Kaminsky Z., Guo X., Sharp A.H., Amaravi R.K., Kleiderlein J.J., Margolis R.L., Troncoso J.C., Lanahan A.A., Worley P.F., et al. 1999. Synphilin-1 associates with alpha-synuclein and promotes the formation of cytosolic inclusions. Nat. Genet. 22:110–114. 10.1038/8820 [DOI] [PubMed] [Google Scholar]
- Eslamboli, A., Georgievska B., Ridley R.M., Baker H.F., Muzyczka N., Burger C., Mandel R.J., Annett L., and Kirik D.. 2005. Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson’s disease. J. Neurosci. 25:769–777. 10.1523/JNEUROSCI.4421-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorowicz, M.A., de Vries-Schneider R.L., Rüb C., Becker D., Huang Y., Zhou C., Alessi Wolken D.M., Voos W., Liu Y., and Przedborski S.. 2014. Cytosolic cleaved PINK1 represses Parkin translocation to mitochondria and mitophagy. EMBO Rep. 15:86–93. 10.1002/embr.201337294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Caggiano, M., Schröder E., Cho H.J., Burgoyne J., Barallobre-Barreiro J., Mayr M., and Eaton P.. 2016. Oxidant-induced Interprotein Disulfide Formation in Cardiac Protein DJ-1 Occurs via an Interaction with Peroxiredoxin 2. J. Biol. Chem. 291:10399–10410. 10.1074/jbc.M115.699850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett, J., Norwood S.J., Hamilton N.A., Mohan M., Kovtun O., Tay S., Zhe Y., Wood S.A., Mellick G.D., Silburn P.A., et al. 2014. The Vps35 D620N mutation linked to Parkinson’s disease disrupts the cargo sorting function of retromer. Traffic. 15:230–244. 10.1111/tra.12136 [DOI] [PubMed] [Google Scholar]
- Fourgeaud, L., Través P.G., Tufail Y., Leal-Bailey H., Lew E.D., Burrola P.G., Callaway P., Zagórska A., Rothlin C.V., Nimmerjahn A., and Lemke G.. 2016. TAM receptors regulate multiple features of microglial physiology. Nature. 532:240–244. 10.1038/nature17630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Cannon, T.C., Tran T., Ruhn K.A., Martinez T.N., Hong J., Marvin M., Hartley M., Treviño I., O’Brien D.E., Casey B., et al. 2008. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J. Neurosci. 28:10825–10834. 10.1523/JNEUROSCI.3001-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, L., Johnson D.A., and Johnson J.A.. 2010. Keap1-Nrf2 activation in the presence and absence of DJ-1. Eur. J. Neurosci. 31:967–977. 10.1111/j.1460-9568.2010.07138.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, J., Li M., Qin S., Zhang T., Jiang S., Hu Y., Deng Y., Zhang C., You D., Li H., et al. 2016. Cytosolic PINK1 promotes the targeting of ubiquitinated proteins to the aggresome-autophagy pathway during proteasomal stress. Autophagy. 12:632–647. 10.1080/15548627.2016.1147667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Reitböck, P., Anichtchik O., Bellucci A., Iovino M., Ballini C., Fineberg E., Ghetti B., Della Corte L., Spano P., Tofaris G.K., et al. 2010. SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain. 133:2032–2044. 10.1093/brain/awq132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier, V., Le H.T., Malki A., Messaoudi N., Caldas T., Kthiri F., Landoulsi A., and Richarme G.. 2012. YajL, the prokaryotic homolog of the Parkinsonism-associated protein DJ-1, protects cells against protein sulfenylation. J. Mol. Biol. 421:662–670. 10.1016/j.jmb.2012.01.047 [DOI] [PubMed] [Google Scholar]
- Ge, P., Dawson V.L., and Dawson T.M.. 2020. PINK1 and Parkin mitochondrial quality control: a source of regional vulnerability in Parkinson’s disease. Mol. Neurodegener. 15:20. 10.1186/s13024-020-00367-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg, M.E., Cooper J.M., Chau K.Y., Rojo M., Schapira A.H., and Taanman J.W.. 2010. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum. Mol. Genet. 19:4861–4870. 10.1093/hmg/ddq419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg, M.E., Burke D., Heales S.J., Cooper J.M., Hardy J., Wood N.W., and Schapira A.H.. 2012. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann. Neurol. 72:455–463. 10.1002/ana.23614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg, M.E., Sweet L., Wang B.H., Shihabuddin L.S., Sardi S.P., and Schapira A.H.. 2015. No evidence for substrate accumulation in Parkinson brains with GBA mutations. Mov. Disord. 30:1085–1089. 10.1002/mds.26278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke, S., Imai Y., Sokol N., and Lu B.. 2010. Pathogenic LRRK2 negatively regulates microRNA-mediated translational repression. Nature. 466:637–641. 10.1038/nature09191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke, S., Wu Z., Klinkenberg M., Sun Y., Auburger G., Guo S., and Lu B.. 2015. PINK1 and Parkin control localized translation of respiratory chain component mRNAs on mitochondria outer membrane. Cell Metab. 21:95–108. 10.1016/j.cmet.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann, J., Matsumoto Y., and Kreutzberg G.W.. 1995. Microglia: intrinsic immuneffector cell of the brain. Brain Res. Brain Res. Rev. 20:269–287. 10.1016/0165-0173(94)00015-H [DOI] [PubMed] [Google Scholar]
- Giasson, B.I., Duda J.E., Murray I.V., Chen Q., Souza J.M., Hurtig H.I., Ischiropoulos H., Trojanowski J.Q., and Lee V.M.. 2000. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 290:985–989. 10.1126/science.290.5493.985 [DOI] [PubMed] [Google Scholar]
- Giasson, B.I., Forman M.S., Higuchi M., Golbe L.I., Graves C.L., Kotzbauer P.T., Trojanowski J.Q., and Lee V.M.. 2003. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 300:636–640. 10.1126/science.1082324 [DOI] [PubMed] [Google Scholar]
- Gillardon, F. 2009. Interaction of elongation factor 1-alpha with leucine-rich repeat kinase 2 impairs kinase activity and microtubule bundling in vitro. Neuroscience. 163:533–539. 10.1016/j.neuroscience.2009.06.051 [DOI] [PubMed] [Google Scholar]
- Gillardon, F., Schmid R., and Draheim H.. 2012. Parkinson’s disease-linked leucine-rich repeat kinase 2(R1441G) mutation increases proinflammatory cytokine release from activated primary microglial cells and resultant neurotoxicity. Neuroscience. 208:41–48. 10.1016/j.neuroscience.2012.02.001 [DOI] [PubMed] [Google Scholar]
- Glass, C.K., Saijo K., Winner B., Marchetto M.C., and Gage F.H.. 2010. Mechanisms underlying inflammation in neurodegeneration. Cell. 140:918–934. 10.1016/j.cell.2010.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser, L., Sonnay S., Stafa K., and Moore D.J.. 2011. Parkin promotes the ubiquitination and degradation of the mitochondrial fusion factor mitofusin 1. J. Neurochem. 118:636–645. 10.1111/j.1471-4159.2011.07318.x [DOI] [PubMed] [Google Scholar]
- Goker-Alpan, O., Lopez G., Vithayathil J., Davis J., Hallett M., and Sidransky E.. 2008. The spectrum of parkinsonian manifestations associated with glucocerebrosidase mutations. Arch. Neurol. 65:1353–1357. 10.1001/archneur.65.10.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann, T., Wieghofer P., Müller P.F., Wolf Y., Varol D., Yona S., Brendecke S.M., Kierdorf K., Staszewski O., Datta M., et al. 2013. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 16:1618–1626. 10.1038/nn.3531 [DOI] [PubMed] [Google Scholar]
- Gordon, R., Albornoz E.A., Christie D.C., Langley M.R., Kumar V., Mantovani S., Robertson A.A.B., Butler M.S., Rowe D.B., O’Neill L.A., et al. 2018. Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 10:eaah4066. 10.1126/scitranslmed.aah4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier, K., McLelland G.L., and Fon E.A.. 2013. Parkin- and PINK1-Dependent Mitophagy in Neurons: Will the Real Pathway Please Stand Up? Front. Neurol. 4:100. 10.3389/fneur.2013.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman, J.N., Sanchez-Padilla J., Wokosin D., Kondapalli J., Ilijic E., Schumacker P.T., and Surmeier D.J.. 2010. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 468:696–700. 10.1038/nature09536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggerty, T., Credle J., Rodriguez O., Wills J., Oaks A.W., Masliah E., and Sidhu A.. 2011. Hyperphosphorylated Tau in an α-synuclein-overexpressing transgenic model of Parkinson’s disease. Eur. J. Neurosci. 33:1598–1610. 10.1111/j.1460-9568.2011.07660.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson, A., Westberg L., Nilsson S., Buervenich S., Carmine A., Holmberg B., Sydow O., Olson L., Johnels B., Eriksson E., and Nissbrandt H.. 2005. Interaction of polymorphisms in the genes encoding interleukin-6 and estrogen receptor beta on the susceptibility to Parkinson’s disease. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 133B:88–92. 10.1002/ajmg.b.30136 [DOI] [PubMed] [Google Scholar]
- Hamza, T.H., Zabetian C.P., Tenesa A., Laederach A., Montimurro J., Yearout D., Kay D.M., Doheny K.F., Paschall J., Pugh E., et al. 2010. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat. Genet. 42:781–785. 10.1038/ng.642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, X., Wang R., Zhou Y., Fei L., Sun H., Lai S., Saadatpour A., Zhou Z., Chen H., Ye F., et al. 2018. Mapping the Mouse Cell Atlas by Microwell-Seq. Cell. 172:1091–1107.e17. 10.1016/j.cell.2018.02.001 [DOI] [PubMed] [Google Scholar]
- Hao, L.Y., Giasson B.I., and Bonini N.M.. 2010. DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function. Proc. Natl. Acad. Sci. USA. 107:9747–9752. 10.1073/pnas.0911175107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque, M.E., Thomas K.J., D’Souza C., Callaghan S., Kitada T., Slack R.S., Fraser P., Cookson M.R., Tandon A., and Park D.S.. 2008. Cytoplasmic Pink1 activity protects neurons from dopaminergic neurotoxin MPTP. Proc. Natl. Acad. Sci. USA. 105:1716–1721. 10.1073/pnas.0705363105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque, M.E., Mount M.P., Safarpour F., Abdel-Messih E., Callaghan S., Mazerolle C., Kitada T., Slack R.S., Wallace V., Shen J., et al. 2012. Inactivation of Pink1 gene in vivo sensitizes dopamine-producing neurons to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and can be rescued by autosomal recessive Parkinson disease genes, Parkin or DJ-1. J. Biol. Chem. 287:23162–23170. 10.1074/jbc.M112.346437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey, K., and Outeiro T.F.. 2019. The role of LRRK2 in cell signalling. Biochem. Soc. Trans. 47:197–207. 10.1042/BST20180464 [DOI] [PubMed] [Google Scholar]
- Hasim, S., Hussin N.A., Alomar F., Bidasee K.R., Nickerson K.W., and Wilson M.A.. 2014. A glutathione-independent glyoxalase of the DJ-1 superfamily plays an important role in managing metabolically generated methylglyoxal in Candida albicans. J. Biol. Chem. 289:1662–1674. 10.1074/jbc.M113.505784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, M.X., Sedor S., McGeary I., Cornblath E.J., Peng C., Riddle D.M., Li H.L., Zhang B., Brown H.J., Olufemi M.F., et al. 2020. Glucocerebrosidase Activity Modulates Neuronal Susceptibility to Pathological α-Synuclein Insult. Neuron. 105:822–836.e7. 10.1016/j.neuron.2019.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierro, A., Rojas A.L., Rojas R., Murthy N., Effantin G., Kajava A.V., Steven A.C., Bonifacino J.S., and Hurley J.H.. 2007. Functional architecture of the retromer cargo-recognition complex. Nature. 449:1063–1067. 10.1038/nature06216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijaz, B.A., and Volpicelli-Daley L.A.. 2020. Initiation and propagation of α-synuclein aggregation in the nervous system. Mol. Neurodegener. 15:19. 10.1186/s13024-020-00368-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle, J.T., Dawson V.L., and Dawson T.M.. 2019. The A1 astrocyte paradigm: New avenues for pharmacological intervention in neurodegeneration. Mov. Disord. 34:959–969. 10.1002/mds.27718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodara, R., Norris E.H., Giasson B.I., Mishizen-Eberz A.J., Lynch D.R., Lee V.M., and Ischiropoulos H.. 2004. Functional consequences of alpha-synuclein tyrosine nitration: diminished binding to lipid vesicles and increased fibril formation. J. Biol. Chem. 279:47746–47753. 10.1074/jbc.M408906200 [DOI] [PubMed] [Google Scholar]
- Holmes, B.B., DeVos S.L., Kfoury N., Li M., Jacks R., Yanamandra K., Ouidja M.O., Brodsky F.M., Marasa J., Bagchi D.P., et al. 2013. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl. Acad. Sci. USA. 110:E3138–E3147. 10.1073/pnas.1301440110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunn, B.H., Cragg S.J., Bolam J.P., Spillantini M.G., and Wade-Martins R.. 2015. Impaired intracellular trafficking defines early Parkinson’s disease. Trends Neurosci. 38:178–188. 10.1016/j.tins.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain, N.K., Diering G.H., Sole J., Anggono V., and Huganir R.L.. 2014. Sorting Nexin 27 regulates basal and activity-dependent trafficking of AMPARs. Proc. Natl. Acad. Sci. USA. 111:11840–11845. 10.1073/pnas.1412415111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihse, E., Yamakado H., van Wijk X.M., Lawrence R., Esko J.D., and Masliah E.. 2017. Cellular internalization of alpha-synuclein aggregates by cell surface heparan sulfate depends on aggregate conformation and cell type. Sci. Rep. 7:9008. 10.1038/s41598-017-08720-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuno, M., Yamakado H., Akiyama H., Parajuli L.K., Taguchi K., Hara J., Uemura N., Hatanaka Y., Higaki K., Ohno K., et al. 2019. GBA haploinsufficiency accelerates alpha-synuclein pathology with altered lipid metabolism in a prodromal model of Parkinson’s disease. Hum. Mol. Genet. 28:1894–1904. 10.1093/hmg/ddz030 [DOI] [PubMed] [Google Scholar]
- Im, J.Y., Lee K.W., Woo J.M., Junn E., and Mouradian M.M.. 2012. DJ-1 induces thioredoxin 1 expression through the Nrf2 pathway. Hum. Mol. Genet. 21:3013–3024. 10.1093/hmg/dds131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, Y., Gehrke S., Wang H.Q., Takahashi R., Hasegawa K., Oota E., and Lu B.. 2008. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J. 27:2432–2443. 10.1038/emboj.2008.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis, K.J., Chereau D., Brigham E.F., Chiou S.S., Schöbel S., Frigon N.L., Yu M., Caccavello R.J., Nelson S., Motter R., et al. 2009. Polo-like kinase 2 (PLK2) phosphorylates alpha-synuclein at serine 129 in central nervous system. J. Biol. Chem. 284:2598–2602. 10.1074/jbc.C800206200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoshita, T., Arano T., Hosaka Y., Meng H., Umezaki Y., Kosugi S., Morimoto T., Koike M., Chang H.Y., Imai Y., and Hattori N.. 2017. Vps35 in cooperation with LRRK2 regulates synaptic vesicle endocytosis through the endosomal pathway in Drosophila. Hum. Mol. Genet. 26:2933–2948. 10.1093/hmg/ddx179 [DOI] [PubMed] [Google Scholar]
- Irrcher, I., Aleyasin H., Seifert E.L., Hewitt S.J., Chhabra S., Phillips M., Lutz A.K., Rousseaux M.W., Bevilacqua L., Jahani-Asl A., et al. 2010. Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum. Mol. Genet. 19:3734–3746. 10.1093/hmg/ddq288 [DOI] [PubMed] [Google Scholar]
- Ishizu, N., Yui D., Hebisawa A., Aizawa H., Cui W., Fujita Y., Hashimoto K., Ajioka I., Mizusawa H., Yokota T., and Watase K.. 2016. Impaired striatal dopamine release in homozygous Vps35 D620N knock-in mice. Hum. Mol. Genet. 25:4507–4517. 10.1093/hmg/ddw279 [DOI] [PubMed] [Google Scholar]
- Ito, G., Okai T., Fujino G., Takeda K., Ichijo H., Katada T., and Iwatsubo T.. 2007. GTP binding is essential to the protein kinase activity of LRRK2, a causative gene product for familial Parkinson’s disease. Biochemistry. 46:1380–1388. 10.1021/bi061960m [DOI] [PubMed] [Google Scholar]
- Jacoupy, M., Hamon-Keromen E., Ordureau A., Erpapazoglou Z., Coge F., Corvol J.C., Nosjean O., Mannoury la Cour C., Millan M.J., Boutin J.A., et al. 2019. The PINK1 kinase-driven ubiquitin ligase Parkin promotes mitochondrial protein import through the presequence pathway in living cells. Sci. Rep. 9:11829. 10.1038/s41598-019-47352-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaleel, M., Nichols R.J., Deak M., Campbell D.G., Gillardon F., Knebel A., and Alessi D.R.. 2007. LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson’s disease mutants affect kinase activity. Biochem. J. 405:307–317. 10.1042/BJ20070209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery, C.J. 2003. Moonlighting proteins: old proteins learning new tricks. Trends Genet. 19:415–417. 10.1016/S0168-9525(03)00167-7 [DOI] [PubMed] [Google Scholar]
- Jeong, G.R., Jang E.H., Bae J.R., Jun S., Kang H.C., Park C.H., Shin J.H., Yamamoto Y., Tanaka-Yamamoto K., Dawson V.L., et al. 2018. Dysregulated phosphorylation of Rab GTPases by LRRK2 induces neurodegeneration. Mol. Neurodegener. 13:8. 10.1186/s13024-018-0240-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz, S.Z., Lu L.F., and Rudensky A.Y.. 2012. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30:531–564. 10.1146/annurev.immunol.25.022106.141623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun, Y.W., and Kool E.T.. 2020. Small Substrate or Large? Debate Over the Mechanism of Glycation Adduct Repair by DJ-1. Cell Chem. Biol. 27:1117–1123. 10.1016/j.chembiol.2020.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser, T., and Feng G.. 2019. Tmem119-EGFP and Tmem119-CreERT2 Transgenic Mice for Labeling and Manipulating Microglia. eNeuro. 6:ENEURO.0448-18.2019. 10.1523/ENEURO.0448-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam, T.I., Mao X., Park H., Chou S.C., Karuppagounder S.S., Umanah G.E., Yun S.P., Brahmachari S., Panicker N., Chen R., et al. 2018. Poly(ADP-ribose) drives pathologic α-synuclein neurodegeneration in Parkinson’s disease. Science. 362:eaat8407. 10.1126/science.aat8407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam, T.I., Hinkle J.T., Dawson T.M., and Dawson V.L.. 2020. Microglia and astrocyte dysfunction in parkinson’s disease. Neurobiol. Dis. 144:105028. 10.1016/j.nbd.2020.105028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane, L.A., Lazarou M., Fogel A.I., Li Y., Yamano K., Sarraf S.A., Banerjee S., and Youle R.J.. 2014. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J. Cell Biol. 205:143–153. 10.1083/jcb.201402104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten, M., and Klein C.. 2013. The many faces of alpha-synuclein mutations. Mov. Disord. 28:697–701. 10.1002/mds.25499 [DOI] [PubMed] [Google Scholar]
- Kasten, M., Hartmann C., Hampf J., Schaake S., Westenberger A., Vollstedt E.J., Balck A., Domingo A., Vulinovic F., Dulovic M., et al. 2018. Genotype-Phenotype Relations for the Parkinson’s Disease Genes Parkin, PINK1, DJ1: MDSGene Systematic Review. Mov. Disord. 33:730–741. 10.1002/mds.27352 [DOI] [PubMed] [Google Scholar]
- Kawakami, F., Yabata T., Ohta E., Maekawa T., Shimada N., Suzuki M., Maruyama H., Ichikawa T., and Obata F.. 2012. LRRK2 phosphorylates tubulin-associated tau but not the free molecule: LRRK2-mediated regulation of the tau-tubulin association and neurite outgrowth. PLoS One. 7:e30834. 10.1371/journal.pone.0030834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskaite, A., Kondapalli C., Gourlay R., Campbell D.G., Ritorto M.S., Hofmann K., Alessi D.R., Knebel A., Trost M., and Muqit M.M.. 2014. Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem. J. 460:127–139. 10.1042/BJ20140334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall, A.K., Xie B., Xu P., Wang J., Burcham R., Frazier M.N., Binshtein E., Wei H., Graham T.R., Nakagawa T., and Jackson L.P.. 2020. Mammalian Retromer Is an Adaptable Scaffold for Cargo Sorting from Endosomes. Structure. 28:393–405.e4. 10.1016/j.str.2020.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kett, L.R., Boassa D., Ho C.C., Rideout H.J., Hu J., Terada M., Ellisman M., and Dauer W.T.. 2012. LRRK2 Parkinson disease mutations enhance its microtubule association. Hum. Mol. Genet. 21:890–899. 10.1093/hmg/ddr526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, R.H., Peters M., Jang Y., Shi W., Pintilie M., Fletcher G.C., DeLuca C., Liepa J., Zhou L., Snow B., et al. 2005. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 7:263–273. 10.1016/j.ccr.2005.02.010 [DOI] [PubMed] [Google Scholar]
- Kim, C., Ho D.H., Suk J.E., You S., Michael S., Kang J., Joong Lee S., Masliah E., Hwang D., Lee H.J., and Lee S.J.. 2013a. Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 4:1562. 10.1038/ncomms2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J., Byun J.W., Choi I., Kim B., Jeong H.K., Jou I., and Joe E.. 2013b. PINK1 Deficiency Enhances Inflammatory Cytokine Release from Acutely Prepared Brain Slices. Exp. Neurobiol. 22:38–44. 10.5607/en.2013.22.1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.H., Choi D.J., Jeong H.K., Kim J., Kim D.W., Choi S.Y., Park S.M., Suh Y.H., Jou I., and Joe E.H.. 2013c. DJ-1 facilitates the interaction between STAT1 and its phosphatase, SHP-1, in brain microglia and astrocytes: A novel anti-inflammatory function of DJ-1. Neurobiol. Dis. 60:1–10. 10.1016/j.nbd.2013.08.007 [DOI] [PubMed] [Google Scholar]
- Kim, D., Hwang H., Choi S., Kwon S.H., Lee S., Park J.H., Kim S., and Ko H.S.. 2018a. D409H GBA1 mutation accelerates the progression of pathology in A53T α-synuclein transgenic mouse model. Acta Neuropathol. Commun. 6:32. 10.1186/s40478-018-0538-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M.J., Jeon S., Burbulla L.F., and Krainc D.. 2018b. Acid ceramidase inhibition ameliorates α-synuclein accumulation upon loss of GBA1 function. Hum. Mol. Genet. 27:1972–1988. 10.1093/hmg/ddy105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S., Yun S.P., Lee S., Umanah G.E., Bandaru V.V.R., Yin X., Rhee P., Karuppagounder S.S., Kwon S.H., Lee H., et al. 2018c. GBA1 deficiency negatively affects physiological α-synuclein tetramers and related multimers. Proc. Natl. Acad. Sci. USA. 115:798–803. 10.1073/pnas.1700465115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.Y., Um J.H., Yoon J.H., Kim H., Lee D.Y., Lee Y.J., Jee H.J., Kim Y.M., Jang J.S., Jang Y.G., et al. 2019. Assessment of mitophagy in mt-Keima Drosophila revealed an essential role of the PINK1-Parkin pathway in mitophagy induction in vivo. FASEB J. 33:9742–9751. 10.1096/fj.201900073R [DOI] [PubMed] [Google Scholar]
- Kim, J.W., Yin X., Jhaldiyal A., Khan M.R., Martin I., Xie Z., Perez-Rosello T., Kumar M., Abalde-Atristain L., Xu J., et al. 2020. Defects in mRNA Translation in LRRK2-Mutant hiPSC-Derived Dopaminergic Neurons Lead to Dysregulated Calcium Homeostasis. Cell Stem Cell. 27:633–645.e7. 10.1016/j.stem.2020.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiral, F.R., Kohrs F.E., Jin E.J., and Hiesinger P.R.. 2018. Rab GTPases and Membrane Trafficking in Neurodegeneration. Curr. Biol. 28:R471–R486. 10.1016/j.cub.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, H.S., Lee Y., Shin J.H., Karuppagounder S.S., Gadad B.S., Koleske A.J., Pletnikova O., Troncoso J.C., Dawson V.L., and Dawson T.M.. 2010. Phosphorylation by the c-Abl protein tyrosine kinase inhibits parkin’s ubiquitination and protective function. Proc. Natl. Acad. Sci. USA. 107:16691–16696. 10.1073/pnas.1006083107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower, J.H., Chu Y., Hauser R.A., Freeman T.B., and Olanow C.W.. 2008. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 14:504–506. 10.1038/nm1747 [DOI] [PubMed] [Google Scholar]
- Kovacs, G.G. 2015. Invited review: Neuropathology of tauopathies: principles and practice. Neuropathol. Appl. Neurobiol. 41:3–23. 10.1111/nan.12208 [DOI] [PubMed] [Google Scholar]
- Kovtun, O., Leneva N., Bykov Y.S., Ariotti N., Teasdale R.D., Schaffer M., Engel B.D., Owen D.J., Briggs J.A.G., and Collins B.M.. 2018. Structure of the membrane-assembled retromer coat determined by cryo-electron tomography. Nature. 561:561–564. 10.1038/s41586-018-0526-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano, F., Okatsu K., Kosako H., Tamura Y., Go E., Kimura M., Kimura Y., Tsuchiya H., Yoshihara H., Hirokawa T., et al. 2014. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 510:162–166. 10.1038/nature13392 [DOI] [PubMed] [Google Scholar]
- Krüger, R., Hardt C., Tschentscher F., Jäckel S., Kuhn W., Müller T., Werner J., Woitalla D., Berg D., Kühnl N., et al. 2000. Genetic analysis of immunomodulating factors in sporadic Parkinson’s disease. J. Neural Transm. (Vienna). 107:553–562. 10.1007/s007020070078 [DOI] [PubMed] [Google Scholar]
- Kthiri, F., Le H.T., Gautier V., Caldas T., Malki A., Landoulsi A., Bohn C., Bouloc P., and Richarme G.. 2010. Protein aggregation in a mutant deficient in yajL, the bacterial homolog of the Parkinsonism-associated protein DJ-1. J. Biol. Chem. 285:10328–10336. 10.1074/jbc.M109.077529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A., Aguirre J.D., Condos T.E., Martinez-Torres R.J., Chaugule V.K., Toth R., Sundaramoorthy R., Mercier P., Knebel A., Spratt D.E., et al. 2015. Disruption of the autoinhibited state primes the E3 ligase parkin for activation and catalysis. EMBO J. 34:2506–2521. 10.15252/embj.201592337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, M., Acevedo-Cintrón J., Jhaldiyal A., Wang H., Andrabi S.A., Eacker S., Karuppagounder S.S., Brahmachari S., Chen R., Kim H., et al. 2020. Defects in Mitochondrial Biogenesis Drive Mitochondrial Alterations in PARKIN-Deficient Human Dopamine Neurons. Stem Cell Reports. 15:629–645. 10.1016/j.stemcr.2020.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie, M.J., Ostaszewski B.L., Weihofen A., Schlossmacher M.G., and Selkoe D.J.. 2005. Dopamine covalently modifies and functionally inactivates parkin. Nat. Med. 11:1214–1221. 10.1038/nm1314 [DOI] [PubMed] [Google Scholar]
- Law, B.M., Spain V.A., Leinster V.H., Chia R., Beilina A., Cho H.J., Taymans J.M., Urban M.K., Sancho R.M., Blanca Ramírez M., et al. 2014. A direct interaction between leucine-rich repeat kinase 2 and specific β-tubulin isoforms regulates tubulin acetylation. J. Biol. Chem. 289:895–908. 10.1074/jbc.M113.507913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou, M., Sliter D.A., Kane L.A., Sarraf S.A., Wang C., Burman J.L., Sideris D.P., Fogel A.I., and Youle R.J.. 2015. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 524:309–314. 10.1038/nature14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, H.T., Gautier V., Kthiri F., Malki A., Messaoudi N., Mihoub M., Landoulsi A., An Y.J., Cha S.S., and Richarme G.. 2012. YajL, prokaryotic homolog of parkinsonism-associated protein DJ-1, functions as a covalent chaperone for thiol proteome. J. Biol. Chem. 287:5861–5870. 10.1074/jbc.M111.299198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H.J., Khoshaghideh F., Lee S., and Lee S.J.. 2006. Impairment of microtubule-dependent trafficking by overexpression of alpha-synuclein. Eur. J. Neurosci. 24:3153–3162. 10.1111/j.1460-9568.2006.05210.x [DOI] [PubMed] [Google Scholar]
- Lee, B.D., Shin J.H., VanKampen J., Petrucelli L., West A.B., Ko H.S., Lee Y.I., Maguire-Zeiss K.A., Bowers W.J., Federoff H.J., et al. 2010a. Inhibitors of leucine-rich repeat kinase-2 protect against models of Parkinson’s disease. Nat. Med. 16:998–1000. 10.1038/nm.2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, E.J., Woo M.S., Moon P.G., Baek M.C., Choi I.Y., Kim W.K., Junn E., and Kim H.S.. 2010b. Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J. Immunol. 185:615–623. 10.4049/jimmunol.0903480 [DOI] [PubMed] [Google Scholar]
- Lee, H.J., Kim C., and Lee S.J.. 2010c. Alpha-synuclein stimulation of astrocytes: Potential role for neuroinflammation and neuroprotection. Oxid. Med. Cell. Longev. 3:283–287. 10.4161/oxim.3.4.12809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.Y., Song J., Kwon K., Jang S., Kim C., Baek K., Kim J., and Park C.. 2012. Human DJ-1 and its homologs are novel glyoxalases. Hum. Mol. Genet. 21:3215–3225. 10.1093/hmg/dds155 [DOI] [PubMed] [Google Scholar]
- Lee, Y., Karuppagounder S.S., Shin J.H., Lee Y.I., Ko H.S., Swing D., Jiang H., Kang S.U., Lee B.D., Kang H.C., et al. 2013. Parthanatos mediates AIMP2-activated age-dependent dopaminergic neuronal loss. Nat. Neurosci. 16:1392–1400. 10.1038/nn.3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.G., Takahama S., Zhang G., Tomarev S.I., and Ye Y.. 2016. Unconventional secretion of misfolded proteins promotes adaptation to proteasome dysfunction in mammalian cells. Nat. Cell Biol. 18:765–776. 10.1038/ncb3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y., Stevens D.A., Kang S.U., Jiang H., Lee Y.I., Ko H.S., Scarffe L.A., Umanah G.E., Kang H., Ham S., et al. 2017. PINK1 Primes Parkin-Mediated Ubiquitination of PARIS in Dopaminergic Neuronal Survival. Cell Rep. 18:918–932. 10.1016/j.celrep.2016.12.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.J., Sanchez-Martinez A., Martinez Zarate A., Benincá C., Mayor U., Clague M.J., and Whitworth A.J.. 2018. Basal mitophagy is widespread in Drosophila but minimally affected by loss of Pink1 or parkin. J. Cell Biol. 217:1613–1622. 10.1083/jcb.201801044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein, E.S., Hawrylycz M.J., Ao N., Ayres M., Bensinger A., Bernard A., Boe A.F., Boguski M.S., Brockway K.S., Byrnes E.J., et al. 2007. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 445:168–176. 10.1038/nature05453 [DOI] [PubMed] [Google Scholar]
- Lev, N., Barhum Y., Ben-Zur T., Aharony I., Trifonov L., Regev N., Melamed E., Gruzman A., and Offen D.. 2015. A DJ-1 Based Peptide Attenuates Dopaminergic Degeneration in Mice Models of Parkinson’s Disease via Enhancing Nrf2. PLoS One. 10:e0127549. 10.1371/journal.pone.0127549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, D., and Liu C.. 2021. Hierarchical chemical determination of amyloid polymorphs in neurodegenerative disease. Nat. Chem. Biol. 17:237–245. 10.1038/s41589-020-00708-z [DOI] [PubMed] [Google Scholar]
- Li, W.W., Yang R., Guo J.C., Ren H.M., Zha X.L., Cheng J.S., and Cai D.F.. 2007. Localization of alpha-synuclein to mitochondria within midbrain of mice. Neuroreport. 18:1543–1546. 10.1097/WNR.0b013e3282f03db4 [DOI] [PubMed] [Google Scholar]
- Li, J.Y., Englund E., Holton J.L., Soulet D., Hagell P., Lees A.J., Lashley T., Quinn N.P., Rehncrona S., Björklund A., et al. 2008. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 14:501–503. 10.1038/nm1746 [DOI] [PubMed] [Google Scholar]
- Li, Y., Liu W., Oo T.F., Wang L., Tang Y., Jackson-Lewis V., Zhou C., Geghman K., Bogdanov M., Przedborski S., et al. 2009. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nat. Neurosci. 12:826–828. 10.1038/nn.2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow, S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Münch A.E., Chung W.S., Peterson T.C., et al. 2017. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 541:481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhart, R., Wong S.A., Cao J., Tran M., Huynh A., Ardrey C., Park J.M., Hsu C., Taha S., Peterson R., et al. 2014. Vacuolar protein sorting 35 (Vps35) rescues locomotor deficits and shortened lifespan in Drosophila expressing a Parkinson’s disease mutant of Leucine-Rich Repeat Kinase 2 (LRRK2). Mol. Neurodegener. 9:23. 10.1186/1750-1326-9-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, G., Zhang C., Yin J., Li X., Cheng F., Li Y., Yang H., Uéda K., Chan P., and Yu S.. 2009. alpha-Synuclein is differentially expressed in mitochondria from different rat brain regions and dose-dependently down-regulates complex I activity. Neurosci. Lett. 454:187–192. 10.1016/j.neulet.2009.02.056 [DOI] [PubMed] [Google Scholar]
- Liu, Y., Guardia-Laguarta C., Yin J., Erdjument-Bromage H., Martin B., James M., Jiang X., and Przedborski S.. 2017. The Ubiquitination of PINK1 Is Restricted to Its Mature 52-kDa Form. Cell Rep. 20:30–39. 10.1016/j.celrep.2017.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., Lear T.B., Verma M., Wang K.Z., Otero P.A., McKelvey A.C., Dunn S.R., Steer E., Bateman N.W., Wu C., et al. 2020. Chemical inhibition of FBXO7 reduces inflammation and confers neuroprotection by stabilizing the mitochondrial kinase PINK1. JCI Insight. 5:e131834. 10.1172/jci.insight.131834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas, M., Gershlick D.C., Vidaurrazaga A., Rojas A.L., Bonifacino J.S., and Hierro A.. 2016. Structural Mechanism for Cargo Recognition by the Retromer Complex. Cell. 167:1623–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk, K.C., Song C., O’Brien P., Stieber A., Branch J.R., Brunden K.R., Trojanowski J.Q., and Lee V.M.. 2009. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. USA. 106:20051–20056. 10.1073/pnas.0908005106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk, K.C., Kehm V., Carroll J., Zhang B., O’Brien P., Trojanowski J.Q., and Lee V.M.. 2012. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 338:949–953. 10.1126/science.1227157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod, D.A., Rhinn H., Kuwahara T., Zolin A., Di Paolo G., McCabe B.D., Marder K.S., Honig L.S., Clark L.N., Small S.A., and Abeliovich A.. 2013. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson’s disease risk. Neuron. 77:425–439. 10.1016/j.neuron.2012.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madureira, M., Connor-Robson N., and Wade-Martins R.. 2020. “LRRK2: Autophagy and Lysosomal Activity”. Front. Neurosci. 14:498. 10.3389/fnins.2020.00498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes, J., Gegg M.E., Migdalska-Richards A., Doherty M.K., Whitfield P.D., and Schapira A.H.. 2016. Autophagic lysosome reformation dysfunction in glucocerebrosidase deficient cells: relevance to Parkinson disease. Hum. Mol. Genet. 25:3432–3445. 10.1093/hmg/ddw185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahul-Mellier, A.L., Fauvet B., Gysbers A., Dikiy I., Oueslati A., Georgeon S., Lamontanara A.J., Bisquertt A., Eliezer D., Masliah E., et al. 2014. c-Abl phosphorylates α-synuclein and regulates its degradation: implication for α-synuclein clearance and contribution to the pathogenesis of Parkinson’s disease. Hum. Mol. Genet. 23:2858–2879. 10.1093/hmg/ddt674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgieri, G., and Eliezer D.. 2008. Structural effects of Parkinson’s disease linked DJ-1 mutations. Protein Sci. 17:855–868. 10.1110/ps.073411608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik, B.R., Godena V.K., and Whitworth A.J.. 2015. VPS35 pathogenic mutations confer no dominant toxicity but partial loss of function in Drosophila and genetically interact with parkin. Hum. Mol. Genet. 24:6106–6117. 10.1093/hmg/ddv322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni, C., Mamais A., Dihanich S., McGoldrick P., Devine M.J., Zerle J., Kara E., Taanman J.W., Healy D.G., Marti-Masso J.F., et al. 2013. Pathogenic Parkinson’s disease mutations across the functional domains of LRRK2 alter the autophagic/lysosomal response to starvation. Biochem. Biophys. Res. Commun. 441:862–866. 10.1016/j.bbrc.2013.10.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, X., Ou M.T., Karuppagounder S.S., Kam T.I., Yin X., Xiong Y., Ge P., Umanah G.E., Brahmachari S., Shin J.H., et al. 2016. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science. 353:aah3374. 10.1126/science.aah3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, I., Kim J.W., Dawson V.L., and Dawson T.M.. 2014a. LRRK2 pathobiology in Parkinson’s disease. J. Neurochem. 131:554–565. 10.1111/jnc.12949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, I., Kim J.W., Lee B.D., Kang H.C., Xu J.C., Jia H., Stankowski J., Kim M.S., Zhong J., Kumar M., et al. 2014b. Ribosomal protein s15 phosphorylation mediates LRRK2 neurodegeneration in Parkinson’s disease. Cell. 157:472–485. 10.1016/j.cell.2014.01.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez, A., Lectez B., Ramirez J., Popp O., Sutherland J.D., Urbé S., Dittmar G., Clague M.J., and Mayor U.. 2017. Quantitative proteomic analysis of Parkin substrates in Drosophila neurons. Mol. Neurodegener. 12:29. 10.1186/s13024-017-0170-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Suzukake, M., Nonaka T., Hosokawa M., Oikawa T., Arai T., Akiyama H., Mann D.M., and Hasegawa M.. 2013. Prion-like spreading of pathological α-synuclein in brain. Brain. 136:1128–1138. 10.1093/brain/awt037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matenia, D., Hempp C., Timm T., Eikhof A., and Mandelkow E.M.. 2012. Microtubule affinity-regulating kinase 2 (MARK2) turns on phosphatase and tensin homolog (PTEN)-induced kinase 1 (PINK1) at Thr-313, a mutation site in Parkinson disease: effects on mitochondrial transport. J. Biol. Chem. 287:8174–8186. 10.1074/jbc.M111.262287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheoud, D., Sugiura A., Bellemare-Pelletier A., Laplante A., Rondeau C., Chemali M., Fazel A., Bergeron J.J., Trudeau L.E., Burelle Y., et al. 2016. Parkinson’s Disease-Related Proteins PINK1 and Parkin Repress Mitochondrial Antigen Presentation. Cell. 166:314–327. 10.1016/j.cell.2016.05.039 [DOI] [PubMed] [Google Scholar]
- Matheoud, D., Cannon T., Voisin A., Penttinen A.M., Ramet L., Fahmy A.M., Ducrot C., Laplante A., Bourque M.J., Zhu L., et al. 2019. Intestinal infection triggers Parkinson’s disease-like symptoms in Pink1-/- mice. Nature. 571:565–569. 10.1038/s41586-019-1405-y [DOI] [PubMed] [Google Scholar]
- Matsuda, N., Sato S., Shiba K., Okatsu K., Saisho K., Gautier C.A., Sou Y.S., Saiki S., Kawajiri S., Sato F., et al. 2010. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 189:211–221. 10.1083/jcb.200910140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda, N., Kimura M., Queliconi B.B., Kojima W., Mishima M., Takagi K., Koyano F., Yamano K., Mizushima T., Ito Y., and Tanaka K.. 2017. Parkinson’s disease-related DJ-1 functions in thiol quality control against aldehyde attack in vitro. Sci. Rep. 7:12816. 10.1038/s41598-017-13146-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta, S., Van Kolen K., da Cunha R., van den Bogaart G., Mandemakers W., Miskiewicz K., De Bock P.J., Morais V.A., Vilain S., Haddad D., et al. 2012. LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron. 75:1008–1021. 10.1016/j.neuron.2012.08.022 [DOI] [PubMed] [Google Scholar]
- Mazzulli, J.R., Xu Y.H., Sun Y., Knight A.L., McLean P.J., Caldwell G.A., Sidransky E., Grabowski G.A., and Krainc D.. 2011. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 146:37–52. 10.1016/j.cell.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli, J.R., Zunke F., Isacson O., Studer L., and Krainc D.. 2016. α-Synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc. Natl. Acad. Sci. USA. 113:1931–1936. 10.1073/pnas.1520335113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer, P.L., Yasojima K., and McGeer E.G.. 2002. Association of interleukin-1 beta polymorphisms with idiopathic Parkinson’s disease. Neurosci. Lett. 326:67–69. 10.1016/S0304-3940(02)00300-2 [DOI] [PubMed] [Google Scholar]
- McGough, I.J., Steinberg F., Jia D., Barbuti P.A., McMillan K.J., Heesom K.J., Whone A.L., Caldwell M.A., Billadeau D.D., Rosen M.K., and Cullen P.J.. 2014. Retromer binding to FAM21 and the WASH complex is perturbed by the Parkinson disease-linked VPS35(D620N) mutation. Curr. Biol. 24:1670–1676. 10.1016/j.cub.2014.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey, G.L., Lizama C.O., Keown-Lang A.E., Niu A., Santander N., Larpthaveesarp A., Chee E., Gonzalez F.F., and Arnold T.D.. 2020. A new genetic strategy for targeting microglia in development and disease. eLife. 9:e54590. 10.7554/eLife.54590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLelland, G.L., Lee S.A., McBride H.M., and Fon E.A.. 2016. Syntaxin-17 delivers PINK1/parkin-dependent mitochondrial vesicles to the endolysosomal system. J. Cell Biol. 214:275–291. 10.1083/jcb.201603105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams, T.G., Prescott A.R., Allen G.F., Tamjar J., Munson M.J., Thomson C., Muqit M.M., and Ganley I.G.. 2016. mito-QC illuminates mitophagy and mitochondrial architecture in vivo. J. Cell Biol. 214:333–345. 10.1083/jcb.201603039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams, T.G., Prescott A.R., Montava-Garriga L., Ball G., Singh F., Barini E., Muqit M.M.K., Brooks S.P., and Ganley I.G.. 2018. Basal Mitophagy Occurs Independently of PINK1 in Mouse Tissues of High Metabolic Demand. Cell Metab. 27:439–449.e5. 10.1016/j.cmet.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose, H.L., Dächsel J.C., Behrouz B., Lincoln S.J., Yue M., Hinkle K.M., Kent C.B., Korvatska E., Taylor J.P., Witten L., et al. 2010. Impaired dopaminergic neurotransmission and microtubule-associated protein tau alterations in human LRRK2 transgenic mice. Neurobiol. Dis. 40:503–517. 10.1016/j.nbd.2010.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, F., Yao D., Shi Y., Kabakoff J., Wu W., Reicher J., Ma Y., Moosmann B., Masliah E., Lipton S.A., and Gu Z.. 2011. Oxidation of the cysteine-rich regions of parkin perturbs its E3 ligase activity and contributes to protein aggregation. Mol. Neurodegener. 6:34. 10.1186/1750-1326-6-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulener, M.C., Xu K., Thomson L., Ischiropoulos H., and Bonini N.M.. 2006. Mutational analysis of DJ-1 in Drosophila implicates functional inactivation by oxidative damage and aging. Proc. Natl. Acad. Sci. USA. 103:12517–12522. 10.1073/pnas.0601891103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migdalska-Richards, A., Daly L., Bezard E., and Schapira A.H.. 2016. Ambroxol effects in glucocerebrosidase and α-synuclein transgenic mice. Ann. Neurol. 80:766–775. 10.1002/ana.24790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir, R., Tonelli F., Lis P., Macartney T., Polinski N.K., Martinez T.N., Chou M.Y., Howden A.J.M., König T., Hotzy C., et al. 2018. The Parkinson’s disease VPS35[D620N] mutation enhances LRRK2-mediated Rab protein phosphorylation in mouse and human. Biochem. J. 475:1861–1883. 10.1042/BCJ20180248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei, H., Schieler J.L., Rochet J.C., and Regnier F.. 2006. Identification of rotenone-induced modifications in alpha-synuclein using affinity pull-down and tandem mass spectrometry. Anal. Chem. 78:2422–2431. 10.1021/ac051978n [DOI] [PubMed] [Google Scholar]
- Misgeld, T., and Schwarz T.L.. 2017. Mitostasis in Neurons: Maintaining Mitochondria in an Extended Cellular Architecture. Neuron. 96:651–666. 10.1016/j.neuron.2017.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura, E., Hasegawa T., Konno M., Suzuki M., Sugeno N., Fujikake N., Geisler S., Tabuchi M., Oshima R., Kikuchi A., et al. 2014. VPS35 dysfunction impairs lysosomal degradation of α-synuclein and exacerbates neurotoxicity in a Drosophila model of Parkinson’s disease. Neurobiol. Dis. 71:1–13. 10.1016/j.nbd.2014.07.014 [DOI] [PubMed] [Google Scholar]
- Moehle, M.S., Webber P.J., Tse T., Sukar N., Standaert D.G., DeSilva T.M., Cowell R.M., and West A.B.. 2012. LRRK2 inhibition attenuates microglial inflammatory responses. J. Neurosci. 32:1602–1611. 10.1523/JNEUROSCI.5601-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morabito, G., Giannelli S.G., Ordazzo G., Bido S., Castoldi V., Indrigo M., Cabassi T., Cattaneo S., Luoni M., Cancellieri C., et al. 2017. AAV-PHP.B-Mediated Global-Scale Expression in the Mouse Nervous System Enables GBA1 Gene Therapy for Wide Protection from Synucleinopathy. Mol. Ther. 25:2727–2742. 10.1016/j.ymthe.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais, V.A., Verstreken P., Roethig A., Smet J., Snellinx A., Vanbrabant M., Haddad D., Frezza C., Mandemakers W., Vogt-Weisenhorn D., et al. 2009. Parkinson’s disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol. Med. 1:99–111. 10.1002/emmm.200900006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais, V.A., Haddad D., Craessaerts K., De Bock P.J., Swerts J., Vilain S., Aerts L., Overbergh L., Grünewald A., Seibler P., et al. 2014. PINK1 loss-of-function mutations affect mitochondrial complex I activity via NdufA10 ubiquinone uncoupling. Science. 344:203–207. 10.1126/science.1249161 [DOI] [PubMed] [Google Scholar]
- Mosley, R.L., Benner E.J., Kadiu I., Thomas M., Boska M.D., Hasan K., Laurie C., and Gendelman H.E.. 2006. Neuroinflammation, Oxidative Stress and the Pathogenesis of Parkinson’s Disease. Clin. Neurosci. Res. 6:261–281. 10.1016/j.cnr.2006.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsie, L.N., Milnerwood A.J., Seibler P., Beccano-Kelly D.A., Tatarnikov I., Khinda J., Volta M., Kadgien C., Cao L.P., Tapia L., et al. 2015. Retromer-dependent neurotransmitter receptor trafficking to synapses is altered by the Parkinson’s disease VPS35 mutation p.D620N. Hum. Mol. Genet. 24:1691–1703. 10.1093/hmg/ddu582 [DOI] [PubMed] [Google Scholar]
- Murata, H., Sakaguchi M., Jin Y., Sakaguchi Y., Futami J., Yamada H., Kataoka K., and Huh N.H.. 2011. A new cytosolic pathway from a Parkinson disease-associated kinase, BRPK/PINK1: activation of AKT via mTORC2. J. Biol. Chem. 286:7182–7189. 10.1074/jbc.M110.179390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, K.E., Gysbers A.M., Abbott S.K., Tayebi N., Kim W.S., Sidransky E., Cooper A., Garner B., and Halliday G.M.. 2014. Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson’s disease. Brain. 137:834–848. 10.1093/brain/awt367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, K., Nemani V.M., Azarbal F., Skibinski G., Levy J.M., Egami K., Munishkina L., Zhang J., Gardner B., Wakabayashi J., et al. 2011. Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J. Biol. Chem. 286:20710–20726. 10.1074/jbc.M110.213538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls, M.A., Plagnol V., Hernandez D.G., Sharma M., Sheerin U.M., Saad M., Simón-Sánchez J., Schulte C., Lesage S., Sveinbjörnsdóttir S., et al. International Parkinson Disease Genomics Consortium . 2011. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet. 377:641–649. 10.1016/S0140-6736(10)62345-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls, M.A., Pankratz N., Lill C.M., Do C.B., Hernandez D.G., Saad M., DeStefano A.L., Kara E., Bras J., Sharma M., et al. Alzheimer Genetic Analysis Group . 2014. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 46:989–993. 10.1038/ng.3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra, D., Tanaka A., Suen D.F., and Youle R.J.. 2008. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183:795–803. 10.1083/jcb.200809125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra, D.P., Jin S.M., Tanaka A., Suen D.F., Gautier C.A., Shen J., Cookson M.R., and Youle R.J.. 2010. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 8:e1000298. 10.1371/journal.pbio.1000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani, V.M., Lu W., Berge V., Nakamura K., Onoa B., Lee M.K., Chaudhry F.A., Nicoll R.A., and Edwards R.H.. 2010. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 65:66–79. 10.1016/j.neuron.2009.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara, D.M., Pawar G., Kalia S.K., and Kalia L.V.. 2020. LRRK2 and α-Synuclein: Distinct or Synergistic Players in Parkinson’s Disease? Front. Neurosci. 14:577. 10.3389/fnins.2020.00577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okatsu, K., Koyano F., Kimura M., Kosako H., Saeki Y., Tanaka K., and Matsuda N.. 2015. Phosphorylated ubiquitin chain is the genuine Parkin receptor. J. Cell Biol. 209:111–128. 10.1083/jcb.201410050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okochi, M., Walter J., Koyama A., Nakajo S., Baba M., Iwatsubo T., Meijer L., Kahle P.J., and Haass C.. 2000. Constitutive phosphorylation of the Parkinson’s disease associated alpha-synuclein. J. Biol. Chem. 275:390–397. 10.1074/jbc.275.1.390 [DOI] [PubMed] [Google Scholar]
- Oliveras-Salvá, M., Van der Perren A., Casadei N., Stroobants S., Nuber S., D’Hooge R., Van den Haute C., and Baekelandt V.. 2013. rAAV2/7 vector-mediated overexpression of alpha-synuclein in mouse substantia nigra induces protein aggregation and progressive dose-dependent neurodegeneration. Mol. Neurodegener. 8:44. 10.1186/1750-1326-8-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordureau, A., Sarraf S.A., Duda D.M., Heo J.M., Jedrychowski M.P., Sviderskiy V.O., Olszewski J.L., Koerber J.T., Xie T., Beausoleil S.A., et al. 2014. Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis. Mol. Cell. 56:360–375. 10.1016/j.molcel.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott, C., Jacobs K., Haucke E., Navarrete Santos A., Grune T., and Simm A.. 2014. Role of advanced glycation end products in cellular signaling. Redox Biol. 2:411–429. 10.1016/j.redox.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa, K., Komatsubara A.T., Nishimura Y., Sawada T., Kawafune H., Tsumoto H., Tsuji Y., Zhao J., Kyotani Y., Tanaka T., et al. 2013. S-nitrosylation regulates mitochondrial quality control via activation of parkin. Sci. Rep. 3:2202. 10.1038/srep02202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker, N., Saminathan H., Jin H., Neal M., Harischandra D.S., Gordon R., Kanthasamy K., Lawana V., Sarkar S., Luo J., et al. 2015. Fyn Kinase Regulates Microglial Neuroinflammatory Responses in Cell Culture and Animal Models of Parkinson’s Disease. J. Neurosci. 35:10058–10077. 10.1523/JNEUROSCI.0302-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker, N., Dawson V.L., and Dawson T.M.. 2017. Activation mechanisms of the E3 ubiquitin ligase parkin. Biochem. J. 474:3075–3086. 10.1042/BCJ20170476 [DOI] [PubMed] [Google Scholar]
- Panicker, N., Sarkar S., Harischandra D.S., Neal M., Kam T.I., Jin H., Saminathan H., Langley M., Charli A., Samidurai M., et al. 2019. Fyn kinase regulates misfolded α-synuclein uptake and NLRP3 inflammasome activation in microglia. J. Exp. Med. 216:1411–1430. 10.1084/jem.20182191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisiadou, L., Xie C., Cho H.J., Lin X., Gu X.L., Long C.X., Lobbestael E., Baekelandt V., Taymans J.M., Sun L., and Cai H.. 2009. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J. Neurosci. 29:13971–13980. 10.1523/JNEUROSCI.3799-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J., Lee S.B., Lee S., Kim Y., Song S., Kim S., Bae E., Kim J., Shong M., Kim J.M., and Chung J.. 2006. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 441:1157–1161. 10.1038/nature04788 [DOI] [PubMed] [Google Scholar]
- Parsanejad, M., Bourquard N., Qu D., Zhang Y., Huang E., Rousseaux M.W., Aleyasin H., Irrcher I., Callaghan S., Vaillant D.C., et al. 2014. DJ-1 interacts with and regulates paraoxonase-2, an enzyme critical for neuronal survival in response to oxidative stress. PLoS One. 9:e106601. 10.1371/journal.pone.0106601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, J., Ren K.D., Yang J., and Luo X.J.. 2016. Mitochondrial E3 ubiquitin ligase 1: A key enzyme in regulation of mitochondrial dynamics and functions. Mitochondrion. 28:49–53. 10.1016/j.mito.2016.03.007 [DOI] [PubMed] [Google Scholar]
- Pfaff, D.H., Fleming T., Nawroth P., and Teleman A.A.. 2017a. Evidence Against a Role for the Parkinsonism-associated Protein DJ-1 in Methylglyoxal Detoxification. J. Biol. Chem. 292:685–690. 10.1074/jbc.M116.743823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff, D.H., Fleming T., Nawroth P., and Teleman A.A.. 2017b. Reply to Richarme: Evidence against a role of DJ-1 in methylglyoxal detoxification. J. Biol. Chem. 292:12784–12785. 10.1074/jbc.L117.798579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli, G., Condliffe S.B., Bauer M., Giesert F., Boldt K., De Astis S., Meixner A., Sarioglu H., Vogt-Weisenhorn D.M., Wurst W., et al. 2011. LRRK2 controls synaptic vesicle storage and mobilization within the recycling pool. J. Neurosci. 31:2225–2237. 10.1523/JNEUROSCI.3730-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell, A.M., and Youle R.J.. 2015. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 85:257–273. 10.1016/j.neuron.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell, A.M., Huang C.H., Kennedy S.R., Ordureau A., Sideris D.P., Hoekstra J.G., Harper J.W., and Youle R.J.. 2015. Endogenous Parkin Preserves Dopaminergic Substantia Nigral Neurons following Mitochondrial DNA Mutagenic Stress. Neuron. 87:371–381. 10.1016/j.neuron.2015.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirooznia, S.K., Yuan C., Khan M.R., Karuppagounder S.S., Wang L., Xiong Y., Kang S.U., Lee Y., Dawson V.L., and Dawson T.M.. 2020. PARIS induced defects in mitochondrial biogenesis drive dopamine neuron loss under conditions of parkin or PINK1 deficiency. Mol. Neurodegener. 15:17. 10.1186/s13024-020-00363-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymenidou, M., Lagier-Tourenne C., Hutt K.R., Huelga S.C., Moran J., Liang T.Y., Ling S.C., Sun E., Wancewicz E., Mazur C., et al. 2011. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat. Neurosci. 14:459–468. 10.1038/nn.2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos, M.H., Higgins J.J., Golbe L.I., Johnson W.G., Ide S.E., Di Iorio G., Sanges G., Stenroos E.S., Pho L.T., Schaffer A.A., et al. 1996. Mapping of a gene for Parkinson’s disease to chromosome 4q21-q23. Science. 274:1197–1199. 10.1126/science.274.5290.1197 [DOI] [PubMed] [Google Scholar]
- Polymeropoulos, M.H., Lavedan C., Leroy E., Ide S.E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., et al. 1997. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 276:2045–2047. 10.1126/science.276.5321.2045 [DOI] [PubMed] [Google Scholar]
- Poole, A.C., Thomas R.E., Yu S., Vincow E.S., and Pallanck L.. 2010. The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PLoS One. 5:e10054. 10.1371/journal.pone.0010054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras, G., Li Q., and Bezard E.. 2012. Modeling Parkinson’s disease in primates: The MPTP model. Cold Spring Harb. Perspect. Med. 2:a009308. 10.1101/cshperspect.a009308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post, M.R., Lieberman O.J., and Mosharov E.V.. 2018. Can Interactions Between α-Synuclein, Dopamine and Calcium Explain Selective Neurodegeneration in Parkinson’s Disease? Front. Neurosci. 12:161. 10.3389/fnins.2018.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronin, A.N., Morris A.J., Surguchov A., and Benovic J.L.. 2000. Synucleins are a novel class of substrates for G protein-coupled receptor kinases. J. Biol. Chem. 275:26515–26522. 10.1074/jbc.M003542200 [DOI] [PubMed] [Google Scholar]
- Pryde, K.R., Smith H.L., Chau K.Y., and Schapira A.H.. 2016. PINK1 disables the anti-fission machinery to segregate damaged mitochondria for mitophagy. J. Cell Biol. 213:163–171. 10.1083/jcb.201509003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, Z., Hu D., Han S., Reaney S.H., Di Monte D.A., and Fink A.L.. 2007. Effect of 4-hydroxy-2-nonenal modification on alpha-synuclein aggregation. J. Biol. Chem. 282:5862–5870. 10.1074/jbc.M608126200 [DOI] [PubMed] [Google Scholar]
- Quinn, P.M.J., Moreira P.I., Ambrósio A.F., and Alves C.H.. 2020. PINK1/PARKIN signalling in neurodegeneration and neuroinflammation. Acta Neuropathol. Commun. 8:189. 10.1186/s40478-020-01062-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, A.A., and Morrison B.E.. 2019. Contributions of VPS35 Mutations to Parkinson’s Disease. Neuroscience. 401:1–10. 10.1016/j.neuroscience.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakovic, A., Grünewald A., Kottwitz J., Brüggemann N., Pramstaller P.P., Lohmann K., and Klein C.. 2011. Mutations in PINK1 and Parkin impair ubiquitination of Mitofusins in human fibroblasts. PLoS One. 6:e16746. 10.1371/journal.pone.0016746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakovic, A., Shurkewitsch K., Seibler P., Grünewald A., Zanon A., Hagenah J., Krainc D., and Klein C.. 2013. Phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1)-dependent ubiquitination of endogenous Parkin attenuates mitophagy: study in human primary fibroblasts and induced pluripotent stem cell-derived neurons. J. Biol. Chem. 288:2223–2237. 10.1074/jbc.M112.391680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramonet, D., Daher J.P., Lin B.M., Stafa K., Kim J., Banerjee R., Westerlund M., Pletnikova O., Glauser L., Yang L., et al. 2011. Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS One. 6:e18568. 10.1371/journal.pone.0018568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recasens, A., Dehay B., Bové J., Carballo-Carbajal I., Dovero S., Pérez-Villalba A., Fernagut P.O., Blesa J., Parent A., Perier C., et al. 2014. Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann. Neurol. 75:351–362. 10.1002/ana.24066 [DOI] [PubMed] [Google Scholar]
- Reyes, J.F., Rey N.L., Bousset L., Melki R., Brundin P., and Angot E.. 2014. Alpha-synuclein transfers from neurons to oligodendrocytes. Glia. 62:387–398. 10.1002/glia.22611 [DOI] [PubMed] [Google Scholar]
- Reynolds, A.D., Banerjee R., Liu J., Gendelman H.E., and Mosley R.L.. 2007. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson’s disease. J. Leukoc. Biol. 82:1083–1094. 10.1189/jlb.0507296 [DOI] [PubMed] [Google Scholar]
- Reynolds, A.D., Stone D.K., Mosley R.L., and Gendelman H.E.. 2009. Nitrated alpha-synuclein-induced alterations in microglial immunity are regulated by CD4+ T cell subsets. J. Immunol. 182:4137–4149. 10.4049/jimmunol.0803982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richarme, G., and Dairou J.. 2017. Parkinsonism-associated protein DJ-1 is a bona fide deglycase. Biochem. Biophys. Res. Commun. 483:387–391. 10.1016/j.bbrc.2016.12.134 [DOI] [PubMed] [Google Scholar]
- Richarme, G., Mihoub M., Dairou J., Bui L.C., Leger T., and Lamouri A.. 2015. Parkinsonism-associated protein DJ-1/Park7 is a major protein deglycase that repairs methylglyoxal- and glyoxal-glycated cysteine, arginine, and lysine residues. J. Biol. Chem. 290:1885–1897. 10.1074/jbc.M114.597815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richarme, G., Liu C., Mihoub M., Abdallah J., Leger T., Joly N., Liebart J.C., Jurkunas U.V., Nadal M., Bouloc P., et al. 2017. Guanine glycation repair by DJ-1/Park7 and its bacterial homologs. Science. 357:208–211. 10.1126/science.aag1095 [DOI] [PubMed] [Google Scholar]
- Rocha, E.M., De Miranda B., and Sanders L.H.. 2018. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 109(pt B):249–257. 10.1016/j.nbd.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Rojas-Charry, L., Cookson M.R., Niño A., Arboleda H., and Arboleda G.. 2014. Downregulation of Pink1 influences mitochondrial fusion-fission machinery and sensitizes to neurotoxins in dopaminergic cells. Neurotoxicology. 44:140–148. 10.1016/j.neuro.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosen, D.A., and Cookson M.R.. 2016. LRRK2 at the interface of autophagosomes, endosomes and lysosomes. Mol. Neurodegener. 11:73. 10.1186/s13024-016-0140-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, C.M., Isasa M., Ordureau A., Prado M.A., Beausoleil S.A., Jedrychowski M.P., Finley D.J., Harper J.W., and Gygi S.P.. 2016. Highly Multiplexed Quantitative Mass Spectrometry Analysis of Ubiquitylomes. Cell Syst. 3:395–403.e4. 10.1016/j.cels.2016.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, B., and Jackson G.R.. 2014. Interactions between Tau and α-synuclein augment neurotoxicity in a Drosophila model of Parkinson’s disease. Hum. Mol. Genet. 23:3008–3023. 10.1093/hmg/ddu011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüb, C., Wilkening A., and Voos W.. 2017. Mitochondrial quality control by the Pink1/Parkin system. Cell Tissue Res. 367:111–123. 10.1007/s00441-016-2485-8 [DOI] [PubMed] [Google Scholar]
- Rudenko, I.N., and Cookson M.R.. 2014. Heterogeneity of leucine-rich repeat kinase 2 mutations: genetics, mechanisms and therapeutic implications. Neurotherapeutics. 11:738–750. 10.1007/s13311-014-0284-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko, I.N., Kaganovich A., Hauser D.N., Beylina A., Chia R., Ding J., Maric D., Jaffe H., and Cookson M.R.. 2012. The G2385R variant of leucine-rich repeat kinase 2 associated with Parkinson’s disease is a partial loss-of-function mutation. Biochem. J. 446:99–111. 10.1042/BJ20120637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko, I.N., Kaganovich A., Langston R.G., Beilina A., Ndukwe K., Kumaran R., Dillman A.A., Chia R., and Cookson M.R.. 2017. The G2385R risk factor for Parkinson’s disease enhances CHIP-dependent intracellular degradation of LRRK2. Biochem. J. 474:1547–1558. 10.1042/BCJ20160909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo, I., Berti G., Plotegher N., Bernardo G., Filograna R., Bubacco L., and Greggio E.. 2015. Leucine-rich repeat kinase 2 positively regulates inflammation and down-regulates NF-κB p50 signaling in cultured microglia cells. J. Neuroinflammation. 12:230. 10.1186/s12974-015-0449-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, E., Seehra G., Sharma P., and Sidransky E.. 2019. GBA1-associated parkinsonism: new insights and therapeutic opportunities. Curr. Opin. Neurol. 32:589–596. 10.1097/WCO.0000000000000715 [DOI] [PubMed] [Google Scholar]
- Saha, S., Ash P.E., Gowda V., Liu L., Shirihai O., and Wolozin B.. 2015. Mutations in LRRK2 potentiate age-related impairment of autophagic flux. Mol. Neurodegener. 10:26. 10.1186/s13024-015-0022-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal, A., DeAndrade M.P., Novis H.S., Lin S., Chang J., Lengacher N., Tomlinson J.J., Tansey M.G., and LaVoie M.J.. 2020. Lysosome and Inflammatory Defects in GBA1-Mutant Astrocytes Are Normalized by LRRK2 Inhibition. Mov. Disord. 35:760–773. 10.1002/mds.27994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi, S.P., Clarke J., Kinnecom C., Tamsett T.J., Li L., Stanek L.M., Passini M.A., Grabowski G.A., Schlossmacher M.G., Sidman R.L., et al. 2011. CNS expression of glucocerebrosidase corrects alpha-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc. Natl. Acad. Sci. USA. 108:12101–12106. 10.1073/pnas.1108197108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi, S.P., Clarke J., Viel C., Chan M., Tamsett T.J., Treleaven C.M., Bu J., Sweet L., Passini M.A., Dodge J.C., et al. 2013. Augmenting CNS glucocerebrosidase activity as a therapeutic strategy for parkinsonism and other Gaucher-related synucleinopathies. Proc. Natl. Acad. Sci. USA. 110:3537–3542. 10.1073/pnas.1220464110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi, S.P., Viel C., Clarke J., Treleaven C.M., Richards A.M., Park H., Olszewski M.A., Dodge J.C., Marshall J., Makino E., et al. 2017. Glucosylceramide synthase inhibition alleviates aberrations in synucleinopathy models. Proc. Natl. Acad. Sci. USA. 114:2699–2704. 10.1073/pnas.1616152114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarraf, S.A., Raman M., Guarani-Pereira V., Sowa M.E., Huttlin E.L., Gygi S.P., and Harper J.W.. 2013. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature. 496:372–376. 10.1038/nature12043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone, J., Reale C., Dati G., Regoni M., Pellecchia M.T., and Garavaglia B.. 2020. The Role of VPS35 in the Pathobiology of Parkinson’s Disease. Cell. Mol. Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake, W., Nakabayashi Y., Mizuta I., Hirota Y., Ito C., Kubo M., Kawaguchi T., Tsunoda T., Watanabe M., Takeda A., et al. 2009. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat. Genet. 41:1303–1307. 10.1038/ng.485 [DOI] [PubMed] [Google Scholar]
- Saunders, A., Macosko E.Z., Wysoker A., Goldman M., Krienen F.M., de Rivera H., Bien E., Baum M., Bortolin L., Wang S., et al. 2018. Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell. 174:1015–1030.e16. 10.1016/j.cell.2018.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvé, V., Lilov A., Seirafi M., Vranas M., Rasool S., Kozlov G., Sprules T., Wang J., Trempe J.F., and Gehring K.. 2015. A Ubl/ubiquitin switch in the activation of Parkin. EMBO J. 34:2492–2505. 10.15252/embj.201592237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitt, J.M., Dawson V.L., and Dawson T.M.. 2006. Diagnosis and treatment of Parkinson disease: molecules to medicine. J. Clin. Invest. 116:1744–1754. 10.1172/JCI29178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarffe, L.A., Stevens D.A., Dawson V.L., and Dawson T.M.. 2014. Parkin and PINK1: much more than mitophagy. Trends Neurosci. 37:315–324. 10.1016/j.tins.2014.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlatti, F., Bauvy C., Ventruti A., Sala G., Cluzeaud F., Vandewalle A., Ghidoni R., and Codogno P.. 2004. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J. Biol. Chem. 279:18384–18391. 10.1074/jbc.M313561200 [DOI] [PubMed] [Google Scholar]
- Schapansky, J., Nardozzi J.D., Felizia F., and LaVoie M.J.. 2014. Membrane recruitment of endogenous LRRK2 precedes its potent regulation of autophagy. Hum. Mol. Genet. 23:4201–4214. 10.1093/hmg/ddu138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildknecht, S., Gerding H.R., Karreman C., Drescher M., Lashuel H.A., Outeiro T.F., Di Monte D.A., and Leist M.. 2013. Oxidative and nitrative alpha-synuclein modifications and proteostatic stress: implications for disease mechanisms and interventions in synucleinopathies. J. Neurochem. 125:491–511. 10.1111/jnc.12226 [DOI] [PubMed] [Google Scholar]
- Schmidt, S., Linnartz B., Mendritzki S., Sczepan T., Lübbert M., Stichel C.C., and Lübbert H.. 2011. Genetic mouse models for Parkinson’s disease display severe pathology in glial cell mitochondria. Hum. Mol. Genet. 20:1197–1211. 10.1093/hmg/ddq564 [DOI] [PubMed] [Google Scholar]
- Schneider, S.A., and Alcalay R.N.. 2017. Neuropathology of genetic synucleinopathies with parkinsonism: Review of the literature. Mov. Disord. 32:1504–1523. 10.1002/mds.27193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman, M.N., Harbour M.E., Tattersall D., Read E., and Bright N.. 2009. Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J. Cell Sci. 122:2371–2382. 10.1242/jcs.048686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman, M.N., Gautreau A., and Billadeau D.D.. 2013. Retromer-mediated endosomal protein sorting: all WASHed up! Trends Cell Biol. 23:522–528. 10.1016/j.tcb.2013.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibler, P., Graziotto J., Jeong H., Simunovic F., Klein C., and Krainc D.. 2011. Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. J. Neurosci. 31:5970–5976. 10.1523/JNEUROSCI.4441-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevcsik, E., Trexler A.J., Dunn J.M., and Rhoades E.. 2011. Allostery in a disordered protein: oxidative modifications to α-synuclein act distally to regulate membrane binding. J. Am. Chem. Soc. 133:7152–7158. 10.1021/ja2009554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba-Fukushima, K., Imai Y., Yoshida S., Ishihama Y., Kanao T., Sato S., and Hattori N.. 2012. PINK1-mediated phosphorylation of the Parkin ubiquitin-like domain primes mitochondrial translocation of Parkin and regulates mitophagy. Sci. Rep. 2:1002. 10.1038/srep01002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, J.H., Ko H.S., Kang H., Lee Y., Lee Y.I., Pletinkova O., Troconso J.C., Dawson V.L., and Dawson T.M.. 2011. PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson’s disease. Cell. 144:689–702. 10.1016/j.cell.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui, A., Rane A., Rajagopalan S., Chinta S.J., and Andersen J.K.. 2016. Detrimental effects of oxidative losses in parkin activity in a model of sporadic Parkinson’s disease are attenuated by restoration of PGC1alpha. Neurobiol. Dis. 93:115–120. 10.1016/j.nbd.2016.05.009 [DOI] [PubMed] [Google Scholar]
- Simón-Sánchez, J., Schulte C., Bras J.M., Sharma M., Gibbs J.R., Berg D., Paisan-Ruiz C., Lichtner P., Scholz S.W., Hernandez D.G., et al. 2009. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat. Genet. 41:1308–1312. 10.1038/ng.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, N., and Bhalla N.. 2020. Moonlighting Proteins. Annu. Rev. Genet. 54:265–285. 10.1146/annurev-genet-030620-102906 [DOI] [PubMed] [Google Scholar]
- Singh, A., Zhi L., and Zhang H.. 2019. LRRK2 and mitochondria: Recent advances and current views. Brain Res. 1702:96–104. 10.1016/j.brainres.2018.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowyra, D., Craig K.L., Tyers M., Elledge S.J., and Harper J.W.. 1997. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 91:209–219. 10.1016/S0092-8674(00)80403-1 [DOI] [PubMed] [Google Scholar]
- Sliter, D.A., Martinez J., Hao L., Chen X., Sun N., Fischer T.D., Burman J.L., Li Y., Zhang Z., Narendra D.P., et al. 2018. Parkin and PINK1 mitigate STING-induced inflammation. Nature. 561:258–262. 10.1038/s41586-018-0448-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, W.W., Pei Z., Jiang H., Dawson V.L., Dawson T.M., and Ross C.A.. 2006. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat. Neurosci. 9:1231–1233. 10.1038/nn1776 [DOI] [PubMed] [Google Scholar]
- Smith, W.W., Liu Z., Liang Y., Masuda N., Swing D.A., Jenkins N.A., Copeland N.G., Troncoso J.C., Pletnikov M., Dawson T.M., et al. 2010. Synphilin-1 attenuates neuronal degeneration in the A53T alpha-synuclein transgenic mouse model. Hum. Mol. Genet. 19:2087–2098. 10.1093/hmg/ddq086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa, V.L., Bellani S., Giannandrea M., Yousuf M., Valtorta F., Meldolesi J., and Chieregatti E.. 2009. alpha-synuclein and its A30P mutant affect actin cytoskeletal structure and dynamics. Mol. Biol. Cell. 20:3725–3739. 10.1091/mbc.e08-03-0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini, M.G., Crowther R.A., Jakes R., Hasegawa M., and Goedert M.. 1998. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. USA. 95:6469–6473. 10.1073/pnas.95.11.6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli, J.B., and Haigis M.C.. 2018. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 20:745–754. 10.1038/s41556-018-0124-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger, M., Tonelli F., Ito G., Davies P., Trost M., Vetter M., Wachter S., Lorentzen E., Duddy G., Wilson S., et al. 2016. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. eLife. 5:e12813. 10.7554/eLife.12813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterky, F.H., Lee S., Wibom R., Olson L., and Larsson N.G.. 2011. Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo. Proc. Natl. Acad. Sci. USA. 108:12937–12942. 10.1073/pnas.1103295108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, D.A., Lee Y., Kang H.C., Lee B.D., Lee Y.I., Bower A., Jiang H., Kang S.U., Andrabi S.A., Dawson V.L., et al. 2015. Parkin loss leads to PARIS-dependent declines in mitochondrial mass and respiration. Proc. Natl. Acad. Sci. USA. 112:11696–11701. 10.1073/pnas.1500624112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojkovska, I., Krainc D., and Mazzulli J.R.. 2018. Molecular mechanisms of α-synuclein and GBA1 in Parkinson’s disease. Cell Tissue Res. 373:51–60. 10.1007/s00441-017-2704-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow, J.L., Low P.C., Offenhäuser C., and Sangermani D.. 2009. Cytokine secretion in macrophages and other cells: pathways and mediators. Immunobiology. 214:601–612. 10.1016/j.imbio.2008.11.005 [DOI] [PubMed] [Google Scholar]
- Sugiura, A., McLelland G.L., Fon E.A., and McBride H.M.. 2014. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J. 33:2142–2156. 10.15252/embj.201488104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer, D., Alcalay R.N., Garretti F., Cote L., Kanter E., Agin-Liebes J., Liong C., McMurtrey C., Hildebrand W.H., Mao X., et al. 2017. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature. 546:656–661. 10.1038/nature22815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, N., Yun J., Liu J., Malide D., Liu C., Rovira I.I., Holmström K.M., Fergusson M.M., Yoo Y.H., Combs C.A., and Finkel T.. 2015. Measuring In Vivo Mitophagy. Mol. Cell. 60:685–696. 10.1016/j.molcel.2015.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, M., Fujikake N., Takeuchi T., Kohyama-Koganeya A., Nakajima K., Hirabayashi Y., Wada K., and Nagai Y.. 2015. Glucocerebrosidase deficiency accelerates the accumulation of proteinase K-resistant α-synuclein and aggravates neurodegeneration in a Drosophila model of Parkinson’s disease. Hum. Mol. Genet. 24:6675–6686. 10.1093/hmg/ddv372 [DOI] [PubMed] [Google Scholar]
- Taguchi, Y.V., Liu J., Ruan J., Pacheco J., Zhang X., Abbasi J., Keutzer J., Mistry P.K., and Chandra S.S.. 2017. Glucosylsphingosine Promotes α-Synuclein Pathology in Mutant GBA-Associated Parkinson’s Disease. J. Neurosci. 37:9617–9631. 10.1523/JNEUROSCI.1525-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira, T., Saito Y., Niki T., Iguchi-Ariga S.M., Takahashi K., and Ariga H.. 2004. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 5:213–218. 10.1038/sj.embor.7400074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagawa, T., Kitani A., Fuss I., Levine B., Brant S.R., Peter I., Tajima M., Nakamura S., and Strober W.. 2018. An increase in LRRK2 suppresses autophagy and enhances Dectin-1-induced immunity in a mouse model of colitis. Sci. Transl. Med. 10:eaan8162. 10.1126/scitranslmed.aan8162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, F.L., Erion J.R., Tian Y., Liu W., Yin D.M., Ye J., Tang B., Mei L., and Xiong W.C.. 2015a. VPS35 in Dopamine Neurons Is Required for Endosome-to-Golgi Retrieval of Lamp2a, a Receptor of Chaperone-Mediated Autophagy That Is Critical for α-Synuclein Degradation and Prevention of Pathogenesis of Parkinson’s Disease. J. Neurosci. 35:10613–10628. 10.1523/JNEUROSCI.0042-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, F.L., Liu W., Hu J.X., Erion J.R., Ye J., Mei L., and Xiong W.C.. 2015b. VPS35 Deficiency or Mutation Causes Dopaminergic Neuronal Loss by Impairing Mitochondrial Fusion and Function. Cell Rep. 12:1631–1643. 10.1016/j.celrep.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, M.Y., Vranas M., Krahn A.I., Pundlik S., Trempe J.F., and Fon E.A.. 2017. Structure-guided mutagenesis reveals a hierarchical mechanism of Parkin activation. Nat. Commun. 8:14697. 10.1038/ncomms14697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayebi, N., Parisiadou L., Berhe B., Gonzalez A.N., Serra-Vinardell J., Tamargo R.J., Maniwang E., Sorrentino Z., Fujiwara H., Grey R.J., et al. 2017. Glucocerebrosidase haploinsufficiency in A53T α-synuclein mice impacts disease onset and course. Mol. Genet. Metab. 122:198–208. 10.1016/j.ymgme.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenreiro, S., Eckermann K., and Outeiro T.F.. 2014. Protein phosphorylation in neurodegeneration: friend or foe? Front. Mol. Neurosci. 7:42. 10.3389/fnmol.2014.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore, S., Cao S., McLean P.J., and Standaert D.G.. 2008. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J. Neuropathol. Exp. Neurol. 67:1149–1158. 10.1097/NEN.0b013e31818e5e99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, K.J., McCoy M.K., Blackinton J., Beilina A., van der Brug M., Sandebring A., Miller D., Maric D., Cedazo-Minguez A., and Cookson M.R.. 2011. DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum. Mol. Genet. 20:40–50. 10.1093/hmg/ddq430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieu, K. 2011. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 1:a009316. 10.1101/cshperspect.a009316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollervey, J.R., Curk T., Rogelj B., Briese M., Cereda M., Kayikci M., König J., Hortobágyi T., Nishimura A.L., Zupunski V., et al. 2011. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat. Neurosci. 14:452–458. 10.1038/nn.2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, Y., Yamaguchi H., Giaime E., Boyle S., Kopan R., R.J. Kelleher, 3rd, and Shen J.. 2010. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci U S A. 107:9879–9884. 10.1073/pnas.1004676107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, Y., Pisani A., Martella G., Karouani M., Yamaguchi H., Pothos E.N., and Shen J.. 2009. R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc. Natl. Acad. Sci. USA. 106:14622–14627. 10.1073/pnas.0906334106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, T.A., Nguyen A.D., Chang J., Goldberg M.S., Lee J.K., and Tansey M.G.. 2011. Lipopolysaccharide and tumor necrosis factor regulate Parkin expression via nuclear factor-kappa B. PLoS One. 6:e23660. 10.1371/journal.pone.0023660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, H.T., Chung C.H., Iba M., Zhang B., Trojanowski J.Q., Luk K.C., and Lee V.M.. 2014. Α-synuclein immunotherapy blocks uptake and templated propagation of misfolded α-synuclein and neurodegeneration. Cell Rep. 7:2054–2065. 10.1016/j.celrep.2014.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trancikova, A., Mamais A., Webber P.J., Stafa K., Tsika E., Glauser L., West A.B., Bandopadhyay R., and Moore D.J.. 2012. Phosphorylation of 4E-BP1 in the mammalian brain is not altered by LRRK2 expression or pathogenic mutations. PLoS One. 7:e47784. 10.1371/journal.pone.0047784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsika, E., Glauser L., Moser R., Fiser A., Daniel G., Sheerin U.M., Lees A., Troncoso J.C., Lewis P.A., Bandopadhyay R., et al. 2014. Parkinson’s disease-linked mutations in VPS35 induce dopaminergic neurodegeneration. Hum. Mol. Genet. 23:4621–4638. 10.1093/hmg/ddu178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle, M.D., Comellas G., Nieuwkoop A.J., Covell D.J., Berthold D.A., Kloepper K.D., Courtney J.M., Kim J.K., Barclay A.M., Kendall A., et al. 2016. Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nat. Struct. Mol. Biol. 23:409–415. 10.1038/nsmb.3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyedmers, J., Mogk A., and Bukau B.. 2010. Cellular strategies for controlling protein aggregation. Nat. Rev. Mol. Cell Biol. 11:777–788. 10.1038/nrm2993 [DOI] [PubMed] [Google Scholar]
- van der Vlag, M., Havekes R., and Heckman P.R.A.. 2020. The contribution of Parkin, PINK1 and DJ-1 genes to selective neuronal degeneration in Parkinson’s disease. Eur. J. Neurosci. 52:3256–3268. 10.1111/ejn.14689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laar, V.S., Arnold B., Cassady S.J., Chu C.T., Burton E.A., and Berman S.B.. 2011. Bioenergetics of neurons inhibit the translocation response of Parkin following rapid mitochondrial depolarization. Hum. Mol. Genet. 20:927–940. 10.1093/hmg/ddq531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandiver, M.S., Paul B.D., Xu R., Karuppagounder S., Rao F., Snowman A.M., Ko H.S., Lee Y.I., Dawson V.L., Dawson T.M., et al. 2013. Sulfhydration mediates neuroprotective actions of parkin. Nat. Commun. 4:1626. 10.1038/ncomms2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma, M., Callio J., Otero P.A., Sekler I., Wills Z.P., and Chu C.T.. 2017. Mitochondrial Calcium Dysregulation Contributes to Dendrite Degeneration Mediated by PD/LBD-Associated LRRK2 Mutants. J. Neurosci. 37:11151–11165. 10.1523/JNEUROSCI.3791-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidyadhara, D.J., Lee J.E., and Chandra S.S.. 2019. Role of the endolysosomal system in Parkinson’s disease. J. Neurochem. 150:487–506. 10.1111/jnc.14820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilain, S., Esposito G., Haddad D., Schaap O., Dobreva M.P., Vos M., Van Meensel S., Morais V.A., De Strooper B., and Verstreken P.. 2012. The yeast complex I equivalent NADH dehydrogenase rescues pink1 mutants. PLoS Genet. 8:e1002456. 10.1371/journal.pgen.1002456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilariño-Güell, C., Wider C., Ross O.A., Dachsel J.C., Kachergus J.M., Lincoln S.J., Soto-Ortolaza A.I., Cobb S.A., Wilhoite G.J., Bacon J.A., et al. 2011. VPS35 mutations in Parkinson disease. Am. J. Hum. Genet. 89:162–167. 10.1016/j.ajhg.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa, E., Marchetti S., and Ricci J.E.. 2018. No Parkin Zone: Mitophagy without Parkin. Trends Cell Biol. 28:882–895. 10.1016/j.tcb.2018.07.004 [DOI] [PubMed] [Google Scholar]
- Vives-Bauza, C., Zhou C., Huang Y., Cui M., de Vries R.L., Kim J., May J., Tocilescu M.A., Liu W., Ko H.S., et al. 2010. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl. Acad. Sci. USA. 107:378–383. 10.1073/pnas.0911187107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley, L.A., Luk K.C., Patel T.P., Tanik S.A., Riddle D.M., Stieber A., Meaney D.F., Trojanowski J.Q., and Lee V.M.. 2011. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 72:57–71. 10.1016/j.neuron.2011.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley, L.A., Abdelmotilib H., Liu Z., Stoyka L., Daher J.P., Milnerwood A.J., Unni V.K., Hirst W.D., Yue Z., Zhao H.T., et al. 2016. G2019S-LRRK2 Expression Augments α-Synuclein Sequestration into Inclusions in Neurons. J. Neurosci. 36:7415–7427. 10.1523/JNEUROSCI.3642-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos, M., Esposito G., Edirisinghe J.N., Vilain S., Haddad D.M., Slabbaert J.R., Van Meensel S., Schaap O., De Strooper B., Meganathan R., et al. 2012. Vitamin K2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science. 336:1306–1310. 10.1126/science.1218632 [DOI] [PubMed] [Google Scholar]
- Wakamatsu, M., Ishii A., Iwata S., Sakagami J., Ukai Y., Ono M., Kanbe D., Muramatsu S., Kobayashi K., Iwatsubo T., and Yoshimoto M.. 2008. Selective loss of nigral dopamine neurons induced by overexpression of truncated human alpha-synuclein in mice. Neurobiol. Aging. 29:574–585. 10.1016/j.neurobiolaging.2006.11.017 [DOI] [PubMed] [Google Scholar]
- Wang, X., Winter D., Ashrafi G., Schlehe J., Wong Y.L., Selkoe D., Rice S., Steen J., LaVoie M.J., and Schwarz T.L.. 2011. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 147:893–906. 10.1016/j.cell.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y.T., Piyankarage S.C., Williams D.L., and Thatcher G.R.. 2014. Proteomic profiling of nitrosative stress: protein S-oxidation accompanies S-nitrosylation. ACS Chem. Biol. 9:821–830. 10.1021/cb400547u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C., Niu M., Zhou Z., Zheng X., Zhang L., Tian Y., Yu X., Bu G., Xu H., Ma Q., and Zhang Y.W.. 2016a. VPS35 regulates cell surface recycling and signaling of dopamine receptor D1. Neurobiol. Aging. 46:22–31. 10.1016/j.neurobiolaging.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W., Wang X., Fujioka H., Hoppel C., Whone A.L., Caldwell M.A., Cullen P.J., Liu J., and Zhu X.. 2016b. Parkinson’s disease-associated mutant VPS35 causes mitochondrial dysfunction by recycling DLP1 complexes. Nat. Med. 22:54–63. 10.1038/nm.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W., Ma X., Zhou L., Liu J., and Zhu X.. 2017. A conserved retromer sorting motif is essential for mitochondrial DLP1 recycling by VPS35 in Parkinson’s disease model. Hum. Mol. Genet. 26:781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K.Z.Q., Steer E., Otero P.A., Bateman N.W., Cheng M.H., Scott A.L., Wu C., Bahar I., Shih Y.T., Hsueh Y.P., and Chu C.T.. 2018. PINK1 Interacts with VCP/p97 and Activates PKA to Promote NSFL1C/p47 Phosphorylation and Dendritic Arborization in Neurons. eNeuro. 5:ENEURO.0466-18.2018. 10.1523/ENEURO.0466-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts, J.C., Giles K., Oehler A., Middleton L., Dexter D.T., Gentleman S.M., DeArmond S.J., and Prusiner S.B.. 2013. Transmission of multiple system atrophy prions to transgenic mice. Proc. Natl. Acad. Sci. USA. 110:19555–19560. 10.1073/pnas.1318268110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauer, T., and Komander D.. 2013. Structure of the human Parkin ligase domain in an autoinhibited state. EMBO J. 32:2099–2112. 10.1038/emboj.2013.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauer, T., Simicek M., Schubert A., and Komander D.. 2015. Mechanism of phospho-ubiquitin-induced PARKIN activation. Nature. 524:370–374. 10.1038/nature14879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber, P.J., Smith A.D., Sen S., Renfrow M.B., Mobley J.A., and West A.B.. 2011. Autophosphorylation in the leucine-rich repeat kinase 2 (LRRK2) GTPase domain modifies kinase and GTP-binding activities. J. Mol. Biol. 412:94–110. 10.1016/j.jmb.2011.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, A.B., Moore D.J., Choi C., Andrabi S.A., Li X., Dikeman D., Biskup S., Zhang Z., Lim K.L., Dawson V.L., and Dawson T.M.. 2007. Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum. Mol. Genet. 16:223–232. 10.1093/hmg/ddl471 [DOI] [PubMed] [Google Scholar]
- Whitton, P.S. 2007. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br. J. Pharmacol. 150:963–976. 10.1038/sj.bjp.0707167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, E.T., Chen X., and Moore D.J.. 2017. VPS35, the Retromer Complex and Parkinson’s Disease. J. Parkinsons Dis. 7:219–233. 10.3233/JPD-161020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner, B., Jappelli R., Maji S.K., Desplats P.A., Boyer L., Aigner S., Hetzer C., Loher T., Vilar M., Campioni S., et al. 2011. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc. Natl. Acad. Sci. USA. 108:4194–4199. 10.1073/pnas.1100976108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodroof, H.I., Pogson J.H., Begley M., Cantley L.C., Deak M., Campbell D.G., van Aalten D.M., Whitworth A.J., Alessi D.R., and Muqit M.M.. 2011. Discovery of catalytically active orthologues of the Parkinson’s disease kinase PINK1: analysis of substrate specificity and impact of mutations. Open Biol. 1:110012. 10.1098/rsob.110012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xilouri, M., Brekk O.R., and Stefanis L.. 2013. α-Synuclein and protein degradation systems: a reciprocal relationship. Mol. Neurobiol. 47:537–551. 10.1007/s12035-012-8341-2 [DOI] [PubMed] [Google Scholar]
- Xiong, Y., Coombes C.E., Kilaru A., Li X., Gitler A.D., Bowers W.J., Dawson V.L., Dawson T.M., and Moore D.J.. 2010. GTPase activity plays a key role in the pathobiology of LRRK2. PLoS Genet. 6:e1000902. 10.1371/journal.pgen.1000902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, Y., Yuan C., Chen R., Dawson T.M., and Dawson V.L.. 2012. ArfGAP1 is a GTPase activating protein for LRRK2: reciprocal regulation of ArfGAP1 by LRRK2. J. Neurosci. 32:3877–3886. 10.1523/JNEUROSCI.4566-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, Y., Neifert S., Karuppagounder S.S., Liu Q., Stankowski J.N., Lee B.D., Ko H.S., Lee Y., Grima J.C., Mao X., et al. 2018. Robust kinase- and age-dependent dopaminergic and norepinephrine neurodegeneration in LRRK2 G2019S transgenic mice. Proc. Natl. Acad. Sci. USA. 115:1635–1640. 10.1073/pnas.1712648115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida, T., Tsushima J., Kitamura Y., Yanagisawa D., Takata K., Shibaike T., Yamamoto A., Taniguchi T., Yasui H., Taira T., et al. 2009. Oxidative stress induction of DJ-1 protein in reactive astrocytes scavenges free radicals and reduces cell injury. Oxid. Med. Cell. Longev. 2:36–42. 10.4161/oxim.2.1.7985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Ouyang Y., Yang L., Beal M.F., McQuibban A., Vogel H., and Lu B.. 2008. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc. Natl. Acad. Sci. USA. 105:7070–7075. 10.1073/pnas.0711845105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap, T.L., Gruschus J.M., Velayati A., Westbroek W., Goldin E., Moaven N., Sidransky E., and Lee J.C.. 2011. Alpha-synuclein interacts with Glucocerebrosidase providing a molecular link between Parkinson and Gaucher diseases. J. Biol. Chem. 286:28080–28088. 10.1074/jbc.M111.237859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap, T.L., Jiang Z., Heinrich F., Gruschus J.M., Pfefferkorn C.M., Barros M., Curtis J.E., Sidransky E., and Lee J.C.. 2015. Structural features of membrane-bound glucocerebrosidase and α-synuclein probed by neutron reflectometry and fluorescence spectroscopy. J. Biol. Chem. 290:744–754. 10.1074/jbc.M114.610584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ysselstein, D., Nguyen M., Young T.J., Severino A., Schwake M., Merchant K., and Krainc D.. 2019. LRRK2 kinase activity regulates lysosomal glucocerebrosidase in neurons derived from Parkinson’s disease patients. Nat. Commun. 10:5570. 10.1038/s41467-019-13413-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, W., Sun Y., Guo S., and Lu B.. 2011. The PINK1/Parkin pathway regulates mitochondrial dynamics and function in mammalian hippocampal and dopaminergic neurons. Hum. Mol. Genet. 20:3227–3240. 10.1093/hmg/ddr235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J., Nagasu H., Murakami T., Hoang H., Broderick L., Hoffman H.M., and Horng T.. 2014. Inflammasome activation leads to Caspase-1-dependent mitochondrial damage and block of mitophagy. Proc. Natl. Acad. Sci. USA. 111:15514–15519. 10.1073/pnas.1414859111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, J., Puri R., Yang H., Lizzio M.A., Wu C., Sheng Z.H., and Guo M.. 2014. MUL1 acts in parallel to the PINK1/parkin pathway in regulating mitofusin and compensates for loss of PINK1/parkin. eLife. 3:e01958. 10.7554/eLife.01958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, S.P., Kim H., Ham S., Kwon S.H., Lee G.H., Shin J.H., Lee S.H., Ko H.S., and Lee Y.. 2017. VPS35 regulates parkin substrate AIMP2 toxicity by facilitating lysosomal clearance of AIMP2. Cell Death Dis. 8:e2741. 10.1038/cddis.2017.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavodszky, E., Seaman M.N., Moreau K., Jimenez-Sanchez M., Breusegem S.Y., Harbour M.E., and Rubinsztein D.C.. 2014. Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. Nat. Commun. 5:3828. 10.1038/ncomms4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L., Shimoji M., Thomas B., Moore D.J., Yu S.W., Marupudi N.I., Torp R., Torgner I.A., Ottersen O.P., Dawson T.M., and Dawson V.L.. 2005a. Mitochondrial localization of the Parkinson’s disease related protein DJ-1: implications for pathogenesis. Hum. Mol. Genet. 14:2063–2073. 10.1093/hmg/ddi211 [DOI] [PubMed] [Google Scholar]
- Zhang, W., Wang T., Pei Z., Miller D.S., Wu X., Block M.L., Wilson B., Zhang W., Zhou Y., Hong J.S., and Zhang J.. 2005b. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 19:533–542. 10.1096/fj.04-2751com [DOI] [PubMed] [Google Scholar]
- Zhang, F.R., Huang W., Chen S.M., Sun L.D., Liu H., Li Y., Cui Y., Yan X.X., Yang H.T., Yang R.D., et al. 2009. Genomewide association study of leprosy. N. Engl. J. Med. 361:2609–2618. 10.1056/NEJMoa0903753 [DOI] [PubMed] [Google Scholar]
- Zhang, J., Li X., and Li J.D.. 2019. The Roles of Post-translational Modifications on α-Synuclein in the Pathogenesis of Parkinson’s Diseases. Front. Neurosci. 13:381. 10.3389/fnins.2019.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, K., Lim Y.J., Liu Z., Long H., Sun Y., Hu J.J., Zhao C., Tao Y., Zhang X., Li D., et al. 2020. Parkinson’s disease-related phosphorylation at Tyr39 rearranges α-synuclein amyloid fibril structure revealed by cryo-EM. Proc. Natl. Acad. Sci. USA. 117:20305–20315. 10.1073/pnas.1922741117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, N., and Shabek N.. 2017. Ubiquitin Ligases: Structure, Function, and Regulation. Annu. Rev. Biochem. 86:129–157. 10.1146/annurev-biochem-060815-014922 [DOI] [PubMed] [Google Scholar]
- Zheng, B., Liao Z., Locascio J.J., Lesniak K.A., Roderick S.S., Watt M.L., Eklund A.C., Zhang-James Y., Kim P.D., Hauser M.A., et al. Global PD Gene Expression (GPEX) Consortium . 2010. PGC-1α, a potential therapeutic target for early intervention in Parkinson’s disease. Sci. Transl. Med. 2:52ra73. 10.1126/scitranslmed.3001059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Q., Omans N.D., Leicher R., Osunsade A., Agustinus A.S., Finkin-Groner E., D’Ambrosio H., Liu B., Chandarlapaty S., Liu S., and David Y.. 2019. Reversible histone glycation is associated with disease-related changes in chromatin architecture. Nat. Commun. 10:1289. 10.1038/s41467-019-09192-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, W., Zhu M., Wilson M.A., Petsko G.A., and Fink A.L.. 2006. The oxidation state of DJ-1 regulates its chaperone activity toward alpha-synuclein. J. Mol. Biol. 356:1036–1048. 10.1016/j.jmb.2005.12.030 [DOI] [PubMed] [Google Scholar]
- Zhou, L., Wang W., Hoppel C., Liu J., and Zhu X.. 2017. Parkinson’s disease-associated pathogenic VPS35 mutation causes complex I deficits. Biochim. Biophys. Acta Mol. Basis Dis. 1863:2791–2795. 10.1016/j.bbadis.2017.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich, A., Benet-Pagès A., Struhal W., Graf E., Eck S.H., Offman M.N., Haubenberger D., Spielberger S., Schulte E.C., Lichtner P., et al. 2011. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am. J. Hum. Genet. 89:168–175. 10.1016/j.ajhg.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziviani, E., Tao R.N., and Whitworth A.J.. 2010. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc. Natl. Acad. Sci. USA. 107:5018–5023. 10.1073/pnas.0913485107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondler, L., Miller-Fleming L., Repici M., Gonçalves S., Tenreiro S., Rosado-Ramos R., Betzer C., Straatman K.R., Jensen P.H., Giorgini F., and Outeiro T.F.. 2014. DJ-1 interactions with α-synuclein attenuate aggregation and cellular toxicity in models of Parkinson’s disease. Cell Death Dis. 5:e1350. 10.1038/cddis.2014.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunke, F., Moise A.C., Belur N.R., Gelyana E., Stojkovska I., Dzaferbegovic H., Toker N.J., Jeon S., Fredriksen K., and Mazzulli J.R.. 2018. Reversible Conformational Conversion of α-Synuclein into Toxic Assemblies by Glucosylceramide. Neuron. 97:92–107.e10. 10.1016/j.neuron.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]