ABSTRACT
In Pakistan, the treatment of multidrug-resistant tuberculosis (MDR-TB) with a shorter treatment regimen (STR), that is, 4–6 months of amikacin, moxifloxacin (Mfx), ethionamide, clofazimine (Cfz), pyrazinamide (Z), ethambutol (E), and high-dose isoniazid, followed by 5 months of Mfx, Cfz, Z, and E, was initiated in 2018. However, there is a lack of information about its effectiveness in Pakistani healthcare settings. Therefore, this retrospective record review of MDR-TB patients treated with STR at eight treatment sites in Pakistan aimed to fill this gap. Data were analyzed using SPSS 23. Multivariate binary logistic regression (MVBLR) analysis was conducted to find factors associated with death and treatment failure, and lost to follow-up (LTFU). A P-value < 0.05 was considered statistically significant. Of 912 MDR-TB patients enrolled at the study sites, only 313 (34.3%) eligible patients were treated with STR and included in the current study. Of them, a total of 250 (79.9%) were cured, 12 (3.8%) completed treated, 31 (9.9%) died, 16 (5.1%) were LTFU, and four (1.3%) were declared as treatment failures. The overall treatment success rate was 83.7%. In MVBLR analysis, patients’ age of 41–60 (odds ratio [OR] = 4.9, P-value = 0.020) and > 60 years (OR = 3.6, P-value = 0.035), being underweight (OR = 2.7, P-value = 0.042), and previous TB treatment (OR = 0.4, P-value = 0.042) had statistically significant association with death and treatment failure, whereas patients’ age of > 60 years (OR = 5.4, P-value = 0.040) and previous TB treatment (OR = 0.2, P-value = 0.008) had statistically significant association with LTFU. The treatment success rate of STR was encouraging. However, to further improve the treatment outcomes, special attention should be paid to the patients with identified risk factors.
INTRODUCTION
The emergence and spread of multidrug-resistant tuberculosis (MDR-TB) caused by a strain of Mycobacterium tuberculosis (MTB) concurrently resistant to both rifampicin (R) and isoniazid (H) pose a significant threat to the successful control of TB.1,2 In 2018, an approximately 0.5 million people (range, 417,000–556,000) developed R-resistant TB worldwide, of which 78% had MDR-TB.2 As MDR-TB patients are concurrently resistant to the two most effective, well-tolerated, and cheap first-line anti-TB drugs (FLDs), the WHO initially recommended to treat all MDR-TB patients for a minimum of 20 months with a longer treatment regimen (LTR) that mainly comprised comparatively less effective, more toxic, and expensive second-line anti-TB drugs (SLDs).3–5 However, the treatment of MDR-TB patients with LTR had the major problems of comparatively lower treatment success (56%), higher incidence of adverse events, high treatment cost, and the acquisition of additional drug resistance during treatment.1,2,6,7 To overcome the aforementioned problems associated with LTR, in 2016, the WHO recommended a new, shorter, and cheaper regimen known as shorter treatment regimen (STR) for treating MDR-TB under specific conditions. Shorter treatment regimen comprised 4–6 months of amikacin (Am), moxifloxacin (Mfx), ethionamide (Eto), clofazimine (Cfz), pyrazinamide (Z), ethambutol (E), and high-dose H followed by 5 months of Mfx, Cfz, Z, and E.3 The treatment of eligible MDR-TB patients with STR under operational conditions has produced comparatively better treatment outcomes under elsewhere. The reported treatment success rate of MDR-TB patients treated with STR ranged from 68.7% to 89.2%.8–14
Unfortunately, Pakistan is a DR-TB high burden country. With an estimated incidence of 28,000 MDR-TB patients with 4.2% (95% CI = 3.2–5.3) in new and 16% (95% CI = 15–17) in previously treated TB patients, Pakistan currently ranks fourth globally with and first in the east Mediterranean region of the WHO.2 Pakistan adopted the programmatic management of drug-resistant TB (PMDT) in the year 2010,15 and currently, there are 32 functional PMDT units across the country. Since the inception of PMDT in the country, MDR-TB patients were treated with the WHO-recommended and National TB Control Program (NTP) adopted LTR. In Pakistan, the reported treatment success rate among MDR-TB patients treated with LTR ranged from 40.5% to 76.9%.7,15–18 However, after the proven benefits of STR in terms of treatment success rate, shorter treatment duration, and low cost, NTP published a protocol and started the treatment of eligible MDR-TB patients with STR at selective PMDT sites.19 Evaluating the treatment outcomes of a cohort of patients is an established, widely used, and effective method for analyzing the effectiveness of a treatment regimen under operational conditions.15,16 However, to the best of our knowledge, information about effectiveness of STR among MDR-TB patients treated is lacking from Pakistan. Therefore, the current study is conducted to evaluate the treatment outcomes and factors associated with unsuccessful outcomes among MDR-TB patients treated with STR. This study will assess the effectiveness of STR in Pakistani MDR-TB patients and help the clinicians to recognize those patients who are at high risk of unsuccessful treatment outcomes before or early in the course of MDR-TB treatment.
MATERIALS AND METHODS
Study population, settings, and design.
This is a retrospective record review of eligible MDR-TB patients treated with STR at the following eight PMDT units: 1) Lady Reading Hospital, Peshawar; 2) Saidu Teaching Hospital, Swat; 3) Medical Teaching Institute, Mardan; 4) Rawalpindi Leprosy Hospital, Rawalpindi; 5) District Head Quarter Hospital, Faisalabad; 6) Nishter Hospital, Multan; 7) Sheikh Zaid Hospital, Rahimyar Khan; and 8) Jinnah Hospital, Lahore. All newly diagnosed, culture-confirmed, pulmonary MDR-TB patients who received STR at the aforementioned sites between January 1, 2018 and July 31, 2019 were included in the study.
Diagnosis and treatment of MDR-TB patients.
At all the study sites, in line with the guideline recommendations,19 presumed pulmonary MDR-TB patients who had no documented history of using SLDs for ≥ 1 month were initially screened for MTB, and R and H resistance with two sputum samples by direct sputum smear microscopy using Ziehl–Neelsen stain, Xpert MTB/R (Cepheid, Sunnyvale, CA), and line probe assay (LPA), respectively. After positive results of sputum smear microscopy, Xpert MTB/R, and LPA, patients were assessed for treatment eligibility with STR, and their sputum samples were sent to reference laboratories for drug susceptibility testing (DST) against anti-TB drugs through conventional culture and DST. The protocols of conducting culture and DST at these reference laboratories have been reported elsewhere.15,20,21 In summary, DST at the reference laboratories was carried out by using the Agar proportion method on enriched Middlebrook 7H10 medium (BBL; Beckton Dickinson, Sparks, MD) at the following concentrations: H (0.2 μg/mL), R (1 μg/mL), E (5 μg/mL), streptomycin (2 μg/mL), kanamycin (5 μg/mL), Am (4 μg/mL), capreomycin (4 μg/mL), ofloxacin (2 μg/mL), levofloxacin (1 μg/mL), and Eto (5 μg/mL). In compliance with the manufacturer’s instructions, DST for Z was conducted at 100 μg/mL by using BACTEC Mycobacterial Growth Indicator Tube (BBL; Beckton Dickinson, Sparks, MD). Meanwhile, MDR-TB patients fulfilling the eligibility criteria of no documented history of treatment with any SLD for ≥ 1 month, no confirmed resistance or suspected ineffectiveness to any SLD in STR, and no intolerance or risk of toxicity to any medicine included in STR were enrolled on STR. In addition to patients who were not fulfilling the aforementioned criteria, those patients were also not enrolled on STR who belonged to anyone of the following categories: clinically severe patients like those suffering from disseminated and extrapulmonary TB such as tubercular meningitis, clinically diagnosed MDR-TB patients, patients with advanced pulmonary diseases (bilateral lung cavitation or lung lesions in more than three zones), coinfected with HIV, had alanine transaminase/aspartate transaminase > 5 times upper normal limit (UNL), creatinine > 2 times of UNL or creatinine clearance < 50 mL/minute, the corrected QT interval > 500 ms, or who were pregnant. The STR regimen comprised 4–6 months with Am-Mfx-Eto-Cfz-Z high-dose H and E, followed by 5 months treatment with Mfx-Cfz-Z-E. The intensive phase of treatment in which the regimen contained an injectable SLD lasted for 4 months. However, if there was no sputum culture conversion (SCC) defined as “two consecutive negative sputum cultures taken at least 30 days apart following a positive culture”18 by month 4 of treatment, the intensive phase was extended to up to 6 months. The continuation phase lasted for fixed 5 months post-injectable SLD stoppage. However, on positive phenotypic DST finding of resistance to fluoroquinolones (FQs) and/or injectable SLDs, patients were switched from STR to LTR. From the baseline visit, all patients were treated as outpatients. Patients’ adherence with treatment regimen was monitored by trained treatment supporters, assessed by the doctors at monthly visits and ensured by a home DOTS linkage facilitator by paying home visits, linking the patients, PMDT units, the district TB officers, and the nearest healthcare facilities. In addition to free of cost treatment, monthly food rations and treatment allowances were given to all patients and their treatment supporters.
Data collection.
All PMDT units in the country share the MDR-TB patients’ data through electronic nominal recording and reporting system (ENRS) with NTP on monthly basis. A purpose-designed data collection form based on the information present in NTP guidelines and previous published articles was used to extract the patients’ sociodemographic, microbiological, and clinical data from the ENRS shared with NTP. Treatment outcomes of patients were categorized in line with WHO guidelines3 (Supplementary File 1). The outcomes of death, treatment failure, and lost to follow-up (LTFU) were grouped together as unsuccessful treatment outcomes, whereas cured and treatment completed were grouped together as successful treatment outcomes.
Statistical analysis.
Data were analyzed using SPSS version 23 (IBM Corp., Armonk, NY). Univariate analysis was conducted to find associations between independent variables and the outcomes of death and treatment failure, and LTFU. All independent variables tested in the univariate analysis were based on previously published articles and their biologically plausible association with death and treatment failure, and LTFU in MDR-TB patients. To obtain the final model of factors associated with death and treatment failure, and LTFU, all independent variables with a P-value < 0.2 were assessed for collinearity and entered in multivariate binary logistic regression (MVBLR) analysis. Independent variables with high correlation (variance inflation factor 10 and/or tolerance value < 0.1) were not entered in multivariate analysis. Discrimination power of final models for evaluating death and treatment failure, and LTFU was examined by using receiver operating characteristic curve (ROC) analysis. Statistical significance was taken at a P-value of < 0.05.
Ethical approval.
The study was approved by the Research and Ethics Committee of the Faculty of Pharmacy and Health Sciences, the University of Balochistan Quetta, and permission to conduct the study was granted by NTP via letter Ref No: DSA 2901/2020. Being a retrospective record review, it was not possible to trace all the study participants for taking written or oral consent, so the aforementioned institutions approved consent waiver. All the study participants’ information was anonymized before conducting analysis.
RESULTS
Patients’ characteristics.
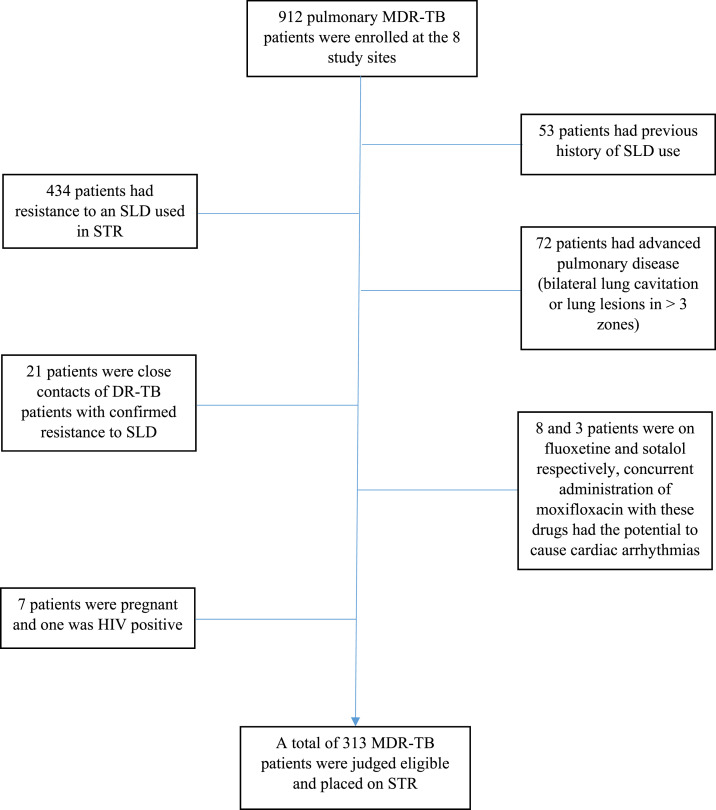
During the study period (January 1, 2018 to July 31, 2019), a total of 912 pulmonary MDR-TB patients were enrolled for treatment at the eight PMDT sites. Among them, 313 (34.3%) eligible MDR-TB patients were treated with STR and included in the current study. Of the remaining patients who were not treated with STR, 434 (47.6%) had resistance to an SLD (mostly a FQ) included in STR, 72 had advanced pulmonary disease (7.9%), 53 (5.8%) had a documented history of previous use of SLDs, 21 (2.3) were close contacts of DR-TB patients with confirmed resistance to SLDs, 11 (1.2%) were receiving drugs with potential to cause toxicity on coadministration with Mfx, seven patients (0.7%) were pregnant, and one was HIV positive (Figure 1). The baseline sociodemographic, microbiological, and clinical characteristics of the study participants are given in Table 1. The study participants were 33.7 ± 16.5 years old. The majority of them were females (50.2%), had a history of TB treatment (70%), were nonsmokers (94.2%), and did not suffer from any comorbidity (81.8%). The study participants were resistant to a median of 2 (range 2–5) drugs. A total of 69.1% patients were resistant to two, 20.1% to three, 8.6% to four, and 2.2% to all five FLDs, respectively. No amplification of drug resistance was observed during the course of treatment.
Figure 1.
Consort diagram of patients’ inclusion and exclusion in the study. This figure appears in color at www.ajtmh.org.
Table 1.
Patients’ baseline sociodemographic and clinical characteristics
| Variable | No. (%) |
|---|---|
| PMDT unit | |
| Lady Reading Hospital, Peshawar | 60 (19.2) |
| Saidu Teaching Hospital, Swat | 12 (3.8) |
| Medical Teaching Institute, Mardan | 17 (5.4) |
| Rawalpindi Leprosy Hospital, Rawalpindi | 32 (10.2) |
| District Head Quarter Hospital, Faisalabad | 77 (24.6) |
| Nishter Hospital, Multan | 51 (16.3) |
| Sheikh Zaid Hospital, Rahimyar Khan | 39 (12.5) |
| Jinnah Hospital, Lahore | 25 (8.0) |
| Gender | |
| Female | 157 (50.2) |
| Male | 156 (49.8) |
| Age-group (years) | |
| ≤ 20 | 77 (24.6) |
| 21–40 | 142 (45.4) |
| 41–60 | 67 (21.4) |
| > 60 | 27 (8.6) |
| Body mass index (kg/m2) | |
| Normal (18.5–22.9) | 90 (28.8) |
| Underweight (< 18.5) | 142 (45.4) |
| Overweight (23–27.5) | 43 (13.7) |
| Obese (> 27.5) | 38 (12.1) |
| Smoking | |
| No | 295 (94.2) |
| Yes | 18 (5.8) |
| Comorbidity | |
| No | 256 (81.8) |
| Yes | 57 (18.2) |
| Type of comorbidity | |
| Chronic liver diseases | 4 (1.3) |
| Chronic obstructive pulmonary disease | 2 (0.6) |
| Depression | 5 (1.6) |
| Diabetes mellitus | 37 (11.8) |
| Hepatitis C | 5 (1.6) |
| Hypertension | 2 (0.6) |
| Others | 6 (1.9) |
| Previous TB treatment | |
| No | 94 (30.0) |
| Yes | 219 (70.0) |
| Previous TB treatment regimen | |
| New | 94 (30.0) |
| Cat-I | 178 (56.9) |
| Cat-II | 19 (6.1) |
| Others | 22 (7.0) |
| Previous TB treatment centre | |
| No history of TB treatment | 94 (30.0) |
| Public | 86 (27.5) |
| Private | 109 (34.8) |
| Public–private mix | 12 (3.8) |
| Unknown | 12 (3.8) |
| Baseline sputum smear grading | |
| Negative | 31 (9.9) |
| Scanty (1–9 AFB/100 HPF) | 42 (13.4) |
| +1 (10–99 AFB/100 HPF) | 134 (42.8) |
| +2 (1–9 AFB/HPF) | 77 (24.6) |
| +3 (> 9 AFB/HPF) | 29 (9.3) |
| Lung lesions at baseline chest X-ray | |
| No lesions | 15 (4.8) |
| One zone | 113 (36.1) |
| 2–3 zones | 175 (55.9) |
| Not available | 10 (3.2) |
| Resistance pattern | |
| Resistance to ethambutol | 43 (13.7) |
| Resistance to pyrazinamide | 70 (22.4) |
| Resistance to streptomycin | 27 (8.6) |
| Resistance to two drugs | 216 (69.1) |
| Resistance to three drugs | 63 (20.1) |
| Resistance to four drugs | 27 (8.6) |
| Resistance to all five first line drugs | 7 (2.2) |
| Number of resistant drugs | 2 (2–5)* |
AFB = acid-fast bacilli; HPF = high power field; PMDT = programmatic management of drug-resistant tuberculosis; TB = tuberculosis.
Median (range).
Sputum culture conversion.
A total of 272/313 (86.9%) patients achieved SCC. Of the 41 patients who did not achieve SCC, 22 died and 16 were LTFU before SCC, whereas the remaining three patients had no information about sputum culture results. The median time to SCC was 1 month (interquartile range 1–2 months), and 221 (70.6%) patients were culture negative by the end of second month of treatment. In the current study, a total of 270 patients received STR for ≥ 4 months. Among these 270 patients, 133 (49.2%) received injectable SLDs for > 4 months (extended intensive phase). In the univariate analysis, no independent factor had statistically significant association with extended intensive phase.
Treatment outcomes.
Of 313 patients, 250 (79.9%) were declared cured, 12 (3.8%) treatment completed, 31 (9.9%) died, 16 (5.1%) LTFU, and four (1.3%) patients as treatment failures. The overall treatment success rate was 83.7%. Of the 31 patients who died, 16 (51.6%) died in the first 3 months of treatment, and the remaining survived for a median of 6 months (range 4–11 months). Of 31 patients who died, completed mortality review form was available for 19 patients. Among these 19 deceased patients, respiratory failure and cardiopulmonary arrest were, respectively, documented as the immediate cause of death in 10 and nine patients.
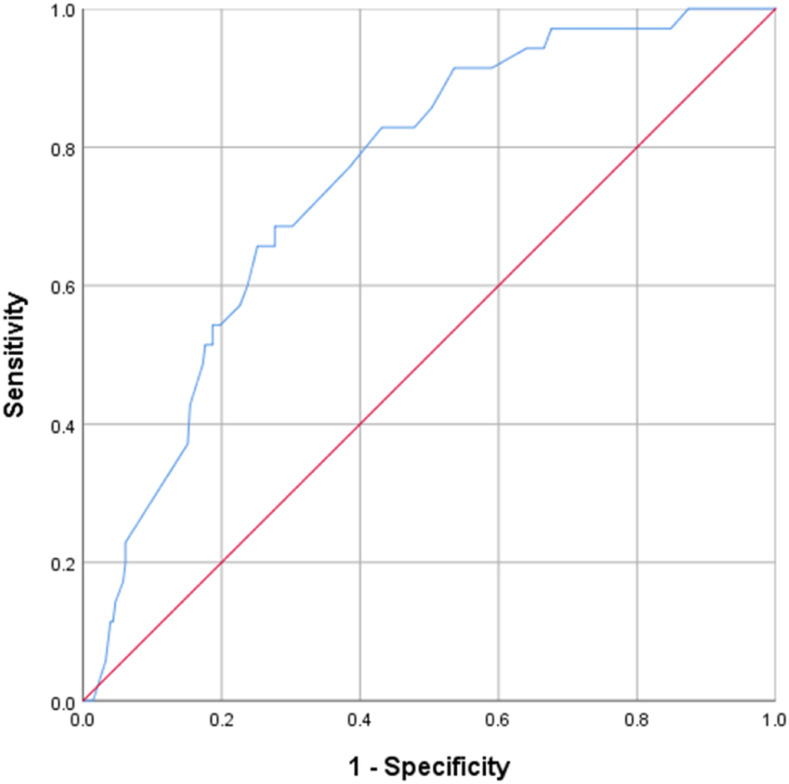
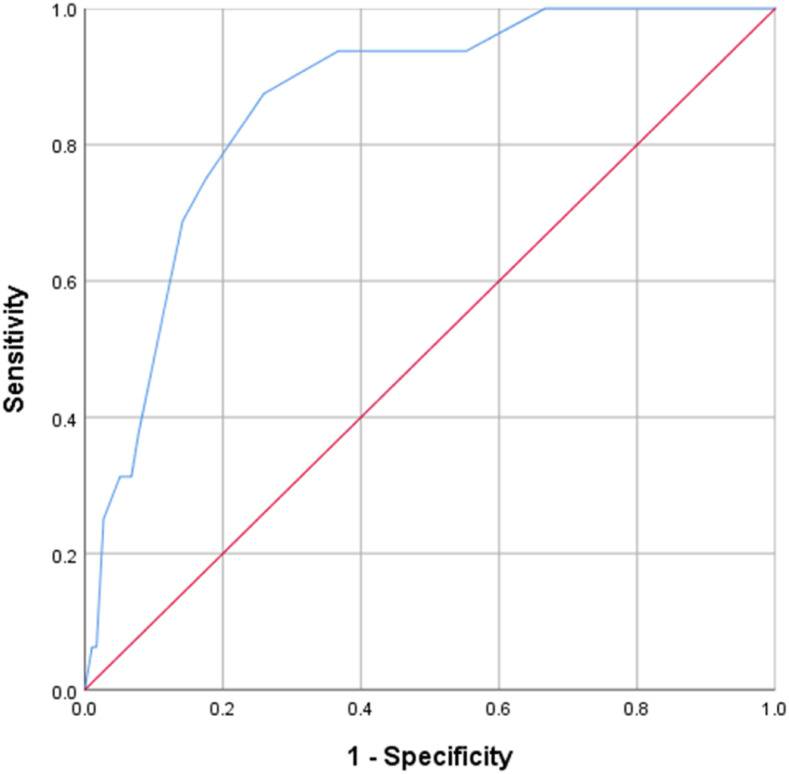
The results of MVBLR analysis revealed that patients’ age of 41–60 (odds ratio [OR] = 4.9, P-value = 0.020) and > 60 years (OR = 3.6, P-value = 0.035), being underweight (OR = 2.7, P-value = 0.042), and the history of previous TB treatment (OR = 0.4, P-value = 0.042) had statistically significant association with death and treatment failure (Table 2), whereas patients’ age of > 60 years (OR = 5.4, P-value = 0.040) and the history of previous TB treatment (OR = 0.2, P-value = 0.008) had statistically significant association with LTFU (Table 3). Receiver operating characteristic curve analysis by the nonparametric method revealed a fair discrimination power of the final model predicting factors associated with death and treatment failure (area under curve [AUC] = 0.754, 95% CI: 0.679–0.830, P-value < 0.001) (Figure 2) and good discrimination power of the final model predicting factors associated LTFU (AUC = 0.860, 95% CI: 0.783–0.937, P-value < 0.001) (Figure 3).
Table 2.
Factors associated with death and treatment failure
| Variable | Death and treatment failure, no. (%) | Univariate analysis OR (95% CI) | P-value | Multivariate analysis OR (95% CI) | P-value* | |
|---|---|---|---|---|---|---|
| No | Yes | |||||
| Gender | 0.605 | – | – | |||
| Female | 138 (87.9) | 19 (12.1) | Referent | |||
| Male | 140 (89.7) | 16 (10.3) | 0.830 (0.410–1.681) | |||
| Age-group (years) | ||||||
| ≤ 20 | 73 (93.6) | 5 (6.4) | Referent | Referent | ||
| 21–40 | 125 (88.0) | 17 (12.0) | 1.986 (0.703–5.607) | 0.195 | 2.688 (0.904–7.872) | 0.075 |
| 41–60 | 52 (86.6) | 9 (13.4) | 2.266 (0.720–7.128) | 0.162 | 4.923 (1.279–18.943) | 0.020 |
| > 60 | 28 (84.6) | 4 (15.4) | 2.655 (0.656–10.749) | 0.171 | 3.614 (1.183–17.020) | 0.035 |
| Body mass index | ||||||
| Normal (18.5–22.9) | 71 (91.0) | 7 (9.0) | Referent | Referent | – | |
| Underweight (< 18.5) | 118 (84.3) | 22 (15.7) | 1.891 (0.769–4.651) | 0.165 | 2.741 (1.038–7.239) | 0.042 |
| Overweight (23–27.5) | 38 (92.7) | 3 (7.3) | 0.801 (0.196–3.276) | 0.757 | 0.980 (0.227–4.230) | 0.979 |
| Obese (> 27.5) | 34 (91.9) | 3 (8.1) | 0.895 (0.218–3.676) | 0.878 | 1.242 (0.285–5.407) | 0.772 |
| Not available | 17 (100) | – | Non-computable | – | Non-computable | – |
| Smoking | 0.029 | 0.180 | ||||
| No | 265 (89.8) | 30 (10.2) | Referent | Referent | ||
| Yes | 13 (72.2) | 5 (27.8) | 3.397 (1.133–10.189) | 2.330 (0.676–8.025) | ||
| Comorbidity | 0.771 | – | – | |||
| No | 228 (89.1) | 28 (10.9) | Referent | |||
| Yes | 50 (87.7) | 7 (12.3) | 1.140 (0.471–2.757) | |||
| Previous TB treatment | 0.083 | 0.028 | ||||
| No | 79 (84.0) | 15 (16.0) | Referent | Referent | ||
| Yes | 199 (90.9) | 20 (9.1) | 0.529 (0.258–1.086) | 0.417 (0.191–0.911) | ||
| Baseline sputum smear grading | – | – | ||||
| Negative | 29 (93.5) | 2 (6.5) | Referent | |||
| Scanty (1–9 AFB/100 HPF) | 35 (83.3) | 7 (16.7) | 2.900 (0.559–15.051) | 0.205 | ||
| +1 (10–99 AFB/100 HPF) | 124 (92.5) | 10 (7.5) | 1.169 (0.243–5.627) | 0.845 | ||
| +2 (1–9 AFB/HPF) | 65 (84.4) | 12 (15.6) | 2.677 (0.563–12.734) | 0.216 | ||
| +3 (> 9 AFB/HPF) | 25 (86.2) | 4 (13.8) | 2.320 (0.391–13.753) | 0.354 | ||
| Lung lesions at baseline chest X-ray | ||||||
| No lesions | 13 (86.7) | 2 (13.3) | Referent | |||
| One zone | 103 (91.2) | 10 (8.8) | 0.631 (0.124–3.202) | 0.579 | ||
| 2–3 Zones | 153 (87.4) | 22 (12.6) | 0.935 (0.198–4.423) | 0.932 | ||
| Information not available | 9 (90.9) | 1 (10.0) | 0.722 (0.057–9.217) | 0.802 | ||
| Number of resistant drugs | 0.111 | 0.024 | ||||
| 2 | 196 (90.7) | 20 (9.3) | Referent | Referent | ||
| > 2 | 82 (84.5) | 15 (15.5) | 1.793 (0.875–3.673) | 2.518 (1.130–5.611) | ||
| Resistance to ethambutol | 0.536 | – | – | |||
| No | 241 (89.3) | 29 (10.7) | Referent | |||
| Yes | 37 (86.0) | 6 (14.0) | 1.348 (0.524–3.466) | |||
| Resistance to Z | 0.352 | – | – | |||
| No | 218 (89.7) | 25 (10.3) | Referent | |||
| Yes | 60 (85.7) | 10 (14.3) | 1.453 (0.662–3.193) | |||
| Resistance to streptomycin | 0.533 | – | – | |||
| No | 255 (89.2) | 31 (10.8) | Referent | |||
| Yes | 23 (85.2) | 4 (14.8) | 1.431 (0.464–4.408) | |||
AFB = acid-fast bacilli; HPF = high power field; OR = odds ratio; TB = tuberculosis. Model fit was based on the nonsignificant Hosmer–Lemeshow test (P-value = 0.917) and overall percentage = 88.2% from the classification table.
Bold P-values indicate statistically significant.
Table 3.
Factors associated with lost to follow-up
| Variable | Lost to follow-up, no. (%) | Univariate analysis OR (95% CI) | P-value | Multivariate analysis OR (95% CI) | P-value* | |
|---|---|---|---|---|---|---|
| No | Yes | |||||
| Gender | 0.600 | – | – | |||
| Female | 150 (95.5) | 7 (4.5) | Referent | |||
| Male | 47 (94.2) | 9 (5.8) | 1.312 (0.476–3.615) | |||
| Age-group (years) | ||||||
| ≤ 20 | 75 (96.2) | 3 (3.8) | Referent | – | Referent | – |
| 21–40 | 141 (99.3) | 1 (0.7) | 0.177 (0.018–1.734) | 0.137 | 0.193 (0.019–1.920) | 0.160 |
| 41–60 | 60 (89.6) | 7 (10.4) | 2.917 (0.723–11.762) | 0.132 | 3.969 (0.851–18.520) | 0.079 |
| > 60 | 21 (80.8) | 5 (19.2) | 5.952 (1.314–26.970) | 0.021 | 5.373 (1.082–26.677) | 0.040 |
| Body mass index | – | – | ||||
| Normal (18.5–22.9) | 73 (93.6) | 5 (6.4) | Referent | – | ||
| Underweight (< 18.5) | 135 (96.4) | 5 (3.6) | 0.541 (0.152–1.929) | – | ||
| Overweight (23–27.5) | 39 (95.1) | 2 (4.9) | 0.749 (0.139–4.039) | 0.343 | ||
| Obese (> 27.5) | 33 (89.2) | 4 (10.8) | 1.770 (0.446–7.017) | 0.736 | ||
| Not available | 17 (100) | – | Non-computable | 0.417 | ||
| Smoking | 0.930 | – | – | |||
| No | 280 (94.9) | 15 (5.1) | Referent | |||
| Yes | 17 (94.4) | 1 (5.6) | 1.098 (0.137–8.812) | |||
| Comorbidity | 0.011 | 0.155 | ||||
| No | 247 (96.5) | 9 (3.5) | Referent | Referent | ||
| Yes | 50 (87.7) | 7 (12.3) | 3.842 (1.367–10.799) | 2.289 (0.732–7.160) | ||
| Previous TB treatment | 0.025 | 0.008 | ||||
| No | 85 (90.4) | 9 (9.6) | Referent | Referent | ||
| Yes | 212 (96.8) | 7 (3.2) | 0.312 (0.113–0.864) | 0.203 (0.063–0.657) | ||
| Baseline sputum smear grading | – | – | ||||
| Negative | 28 (90.3) | 3 (9.7) | Referent | – | ||
| Scanty (1–9 AFB/100 HPF) | 39 (92.9) | 3 (7.1) | 0.718 (0.135–3.823) | 0.698 | ||
| +1 (10–99 AFB/100 HPF) | 128 (95.5) | 6 (4.5) | 0.438 (0.103–1.856) | 0.262 | ||
| +2 (1–9 AFB/HPF) | 74 (96.1) | 3 (3.9) | 0.378 (0.072–1.897) | 0.251 | ||
| +3 (> 9 AFB/HPF) | 28 (96.6) | 1 (3.4) | 0.333 (0.033–3.402) | 0.354 | ||
| Number of resistant drugs | 0.286 | – | – | |||
| 2 | 203 (94.0) | 13 (6.0) | Referent | |||
| > 2 | 94 (96.9) | 3 (3.1) | 0.498 (0.139–1.791) | |||
| Resistance to Z | 0.722 | – | – | |||
| No | 230 (94.7) | 13 (5.3) | Referent | |||
| Yes | 67 (95.7) | 3 (7.3) | 0.792 (0.219–2.862) | |||
AFB = acid-fast bacilli; HPF = high power field; OR = odds ratio; TB = tuberculosis; Z = pyrazinamide. Model fit was based on the nonsignificant Hosmer–Lemeshow test (P-value = 0.519) and overall percentage = 94.9% from the classification table.
Bold P-values indicate statistically significant.
Figure 2.
Receiver operating characteristic curve of discriminatory power of final model predicting death and treatment failure. This figure appears in color at www.ajtmh.org.
Figure 3.
Receiver operating characteristic curve of discriminatory power of final model predicting loss to follow-up. This figure appears in color at www.ajtmh.org.
DISCUSSION
To the best of our knowledge, this is the first study to evaluate the treatment outcomes and factors associated with unsuccessful outcomes among MDR-TB patients treated with STR in Pakistani healthcare settings. We found that an overwhelming majority of MDR-TB patients (65.7%) enrolled for treatment at the study sites were not eligible for treatment with STR. This finding is in line with the previous reports from Pakistan,21 Brazil,22 Southeast Asia,23 Europe,24 and Mexico.25 In compliance with the findings of the aforementioned studies,21–25 the major reason for patients’ ineligibility to be treated with STR at the current study sites was resistance to an SLD (47.6%). In the current study, a significant proportion of patients (30%) had no documented history of previous TB treatment. This was well above the previously reported estimated incidence of new MDR-TB patients (4.2%, 95% CI = 3.2–5.3%) in the country.2 However, it was in compliance with the recent reports which state that new patients make up more than half of DR-TB incidence26–28 and advocate for better infection control measures to interrupt the transmission. As a minimum, soon after the diagnosis of an index case, all household contacts should be identified and screened for active TB disease, and rapid DST should be performed for all coincident cases.29
The treatment success rate of STR observed in the current study (83.7%) complemented those reported by an individual patient data meta-analysis (83%),11 and studies conducted in nine African countries (82%),13 Bangladesh (85%),8 and Cameroon (89%).12 But it was superior to the global treatment success rate (56%) of MDR-TB patients treated with LTR.2 Similarly, it was also better than the range of treatment success rate (40.5–76.9%) reported among MDR-TB patients treated with LTR in Pakistani settings.7,15–18 The proportion deaths (9.9%) in the current study was in compliance with the mortality range (5.6–7.8%) reported among MDR-TB patients treated with STR elsewhere,10–13 but was well below the mortality range (18.7–25.1%) observed among MDR-TB patients treated with LTR in Pakistani settings.7,15–18 Furthermore, the LTFU rate (5.1%) in the current cohort was in the range reported among MDR-TB patients treated with STR (4.8–7.7%) elsewhere10–13 and LTR (1.1–18.3%) in Pakistani settings.7,15–17 However, while comparing the current treatment success rate with various cohorts of MDR-TB patients treated with LTR in Pakistani settings,7,15–18 we observed a stark difference in the pattern of drug resistance between the two sets of patients. The current study participants were resistant to a median of two drugs, and no single patient had resistance to any SLD. On the contrary, the previously reported MDR-TB patients treated with LTR in Pakistani settings were resistant to a median of 4–5 drugs, with SLD resistance ranging from 28.5% to 55.7%.7,15–18 Moreover, among SLDs, resistance was highest for FQs (29.1–55.7%),7,15–18 which was reported as a risk factor for unsuccessful treatment outcomes in these studies.7,15,17,18 Furthermore, as mentioned earlier, only those MDR-TB patients were treated with STR who had no documented history of treatment with any SLD for ≥ 1 month, no confirmed resistance or suspected ineffectiveness to any SLD and no intolerance or risk of toxicity to any medicine in the STR, had no advanced pulmonary disease and clinically severe TB, and who were not coinfected with HIV. This made the current cohort a comparatively healthier group of patients than those who are treated with LTR, and hence attained the expected better outcomes. A comparative analysis of the effectiveness of STR and LTR between MDR-TB patients matched for the aforementioned factors is suggested to know about the relatively better treatment success rate of STR in Pakistani settings.
In the current study, the multivariate analysis revealed that patients who were > 40 years old, underweight, had no history of previous TB treatment, and resistant to more than two drugs were at significantly greater risk of death and treatment failure. Furthermore, patients who were > 60 years old and had no history of previous TB treatment were at significantly greater risk of LTFU. In the studies conducted elsewhere, the univariate analysis showed a statistically significant positive association between older age and unsuccessful treatment outcomes in MDR-TB patients treated with STR, but this significance was not retained in multivariate analysis.10,12 However, in compliance with our finding, older age has been reported a risk factor for unsuccessful outcomes among MDR-TB patients treated with LTR in studies conducted in Pakistan15–18 and elsewhere.30,31 The significantly higher rates of death and treatment failure, and LTFU in elderly MDR-TB patients could be due to the combination of multiple risk factors such as physical deterioration, concurrent comorbidities and complex medication schedule, compromised immunity, and difficulty in monthly visits to the PMDT units.17,32 The current finding of lower body mass index (< 18.5 kg/m2) as a risk factor for death and treatment failure in MDR-TB patients is in line with the previous studies.7,15,16,31 The poor bioavailability of oral anti-TB drugs due to poor gastrointestinal absorption and possible insufficient dosing in malnourished underweight patients could have led to subtherapeutic serum concentration of anti-TB drugs and consequently a high rate of death and treatment failure in these patients.7,15,16,31 Our finding of additional drug resistance as a risk factor for death and treatment failure is in compliance with previous reports where additional resistance to FLDs and SLDs was associated with unsuccessful outcomes in MDR-TB patients.7,15,33 In the current study, patients with a previous history of TB treatment were significantly less likely to develop the outcomes of death and treatment failure, and LTFU. The studies conducted elsewhere have found no significant association between the previous TB treatment and treatment outcomes among MDR-TB patients treated with STR.10–13 However, opposite to our finding, the history of TB treatment had a statistically significant positive association with unsuccessful treatment outcomes among MDR-TB patients treated with LTR.34,35 The high rates of drug resistance in the previously treated TB patients in these studies34,35 could be a possible reason for unsuccessful treatment outcomes. Furthermore, for the presence of a significantly negative association between the history of previous TB treatment and LTFU, we speculate that the lack of awareness about the total treatment duration and the serious nature of MDR-TB, relief of symptoms after initiating STR, first time experience, and fragility toward the adverse effects of the drugs included in STR could be some of the possible reasons for the significantly high rate of LTFU among the new MDR-TB patients in the current cohort. On the other hand, experiencing the beneficial effects of an effective regimen after their past experiences of long and complicated pathways of care before being diagnosed with MDR-TB and awareness that this is their last treatment resort might have made the patients with the history of TB treatment more adherent and less prone to LTFU.
CONCLUSION
The high treatment success rate of standardized STR among eligible MDR-TB patients at multiple Pakistani PMDT units is encouraging. However, the ineligibility of overwhelming majority of MDR-TB patients (65.7%) to be treated with STR limits its applicability in Pakistani healthcare settings. Providing special attention and enhanced clinical management to MDR-TB patients who are > 40 years old, underweight, had additional resistance to anti-TB drugs, and no history of previous TB treatment may further improve the treatment outcomes. Therapeutic drug monitoring in underweight MDR-TB patients may help in the dose adjustment and manipulation anti-TB therapy in these patients. Although the current study is the first one which reported the effectiveness of STR among a large number of MDR-TB patients treated at eight different PMDT units in Pakistan, its observational design and retrospective data collection are its major limitations. Whereas no modification in the treatment regimen of a single study participant can be taken as a proxy marker for the nonoccurrence of clinically significant adverse events and tolerability of STR in the current cohort, the lack of information about the nature, frequency, and management of adverse events and relapse is another major limitation of the current study.
Supplemental table
ACKNOWLEDGMENT
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Note: Supplemental table appears at www.ajtmh.org.
REFERENCES
- 1.The Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment–2017 Ahmad N, et al. 2018. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet 392: 821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization , 2019. Global Tuberculosis Report 2019. Geneva, Switzerland: WHO. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 3.World Health Organization , 2016. WHO Treatment Guidelines for Drug-Resistant Tuberculosis: 2016 Update. Geneva, Switzerland: WHO. WHO/HTM/TB/2016.04. [PubMed] [Google Scholar]
- 4.World Health Organization , 2011. Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis: 2011 Update. Geneva, Switzerland: WHO. WHO/HTM/TB/2011.6. [PubMed] [Google Scholar]
- 5.World Health Organization , 2014. Companion Handbook to the WHO Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. Geneva, Switzerland: WHO. WHO/HTM/TB/2014.11. [PubMed] [Google Scholar]
- 6.Lan Z, et al. 2020. Drug-associated adverse events in the treatment of multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir Med 8: 383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Javaid A, Shaheen Z, Shafqat M, Khan AH, Ahmad N, 2017. Risk factors for high death and loss-to-follow-up rates among patients with multidrug-resistant tuberculosis at a programmatic management unit. Am J Infect Control 45: 190–193. [DOI] [PubMed] [Google Scholar]
- 8.Deun AV, Aung KJM, Salim MAH, Das PK, Sarker MR, Daru P, Rieder HL, 2010. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med 182: 684–692. [DOI] [PubMed] [Google Scholar]
- 9.Piubello A, Harouna SH, Souleymane M, Boukary I, Morou S, Daouda M, Hanki Y, Deun AV, 2014. High cure rate with standardised short-course multidrug-resistant tuberculosis treatment in Niger: no relapses. Int J Tuberc Lung Dis 18: 1188–1194. [DOI] [PubMed] [Google Scholar]
- 10.Aung KJM, Deun AV, Declercq E, Sarker MR, Das PK, Hossain MA, Rieder HL, 2014. Successful ‘9-month Bangladesh regimen’ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis 18: 1180–1187. [DOI] [PubMed] [Google Scholar]
- 11.Khan FA, et al. 2017. Effectiveness and safety of standardised shorter regimens for multidrug-resistant tuberculosis: individual patient data and aggregate data meta-analyses. Eur Respir J 50: 1700061. [DOI] [PubMed] [Google Scholar]
- 12.Kuaban C, Noeske J, Rieder HL, Aït-Khaled N, Abena Foe JL, Trébucq A, 2015. High effectiveness of a 12-month regimen for MDR-TB patients in Cameroon. Int J Tuberc Lung Dis 19: 517–524. [DOI] [PubMed] [Google Scholar]
- 13.Trébucq A, et al. 2018. Treatment outcome with a short multidrug-resistant tuberculosis regimen in nine African countries. Int J Tuberc Lung Dis 22: 17–25. [DOI] [PubMed] [Google Scholar]
- 14.Du Y, et al. 2020. Treatment outcome of a shorter regimen containing clofazimine for multidrug-resistant tuberculosis: a randomized control trial in China. Clin Infect Dis 71: 1047–1054. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad N, Javaid A, Basit A, Afridi AK, Khan MA, Ahmad I, Sulaiman SAS, Khan AH, 2015.Management and treatment outcomes of MDR-TB: results from a setting with high rates of drug resistance. Int J Tuberc Lung Dis 19: 1109–1114. [DOI] [PubMed] [Google Scholar]
- 16.Khan I, Ahmad N, Khan S, Muhammad S, Khan SA, Ahmad I, Khan A, Gulalai, Atif M, 2019. Evaluation of treatment outcomes and factors associated with unsuccessful outcomes in multidrug resistant tuberculosis patients in Baluchistan province of Pakistan. J Infect Public Heal 12: 809–815. [DOI] [PubMed] [Google Scholar]
- 17.Atif M, Ahmad W, Ahmad N, Malik I, Sarwar S, 2020. Treatment outcomes among multidrug-resistant TB patients in Bahawal Victoria hospital, Bahawalpur, Pakistan: a retrospective record review. Trans R Soc Trop Med Hyg 114: 733–741. [DOI] [PubMed] [Google Scholar]
- 18.Javaid A, Ahmad N, Afridi AK, Basit A, Khan AH, Ahmad I, Atif M, 2018. Validity of time to sputum culture conversion to predict cure in patients with multidrug-resistant tuberculosis: a retrospective single-center study. Am J Trop Med Hyg 98: 1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Tuberculosis Control Program . Protocol for Treating MDR-TB/RR-TB with Shorter Treatment Regimen (STR). NTP 217. Available at: http://ntp.gov.pk/ntp-old/uploads/NTP_Protocol_Shorter_Treatment_Regimen_STR.pdf. Accessed September 8, 2020. [Google Scholar]
- 20.Ahmad N, Javaid A, Sulaiman SAS, Meng LC, Ahmad I, Khan AH, 2016. Resistance patterns, prevalence, and predictors of fluoroquinolones resistance in multidrug resistant tuberculosis patients. Braz J Infect Dis 20: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Javaid A, Ahmad N, Khan AH, Shaheen Z, 2017. Applicability of the World Health Organization recommended new shorter regimen in a multidrug-resistant tuberculosis high burden country. Eur Respir J 49: 1601967. [DOI] [PubMed] [Google Scholar]
- 22.Dalcolmo M, et al. 2017. Resistance profile of drugs composing the “shorter” regimen for multidrug-resistant tuberculosis in Brazil, 2000–2015. Eur Respir J 49: 1602309. [DOI] [PubMed] [Google Scholar]
- 23.Chee CB, KhinMar KW, Sng LH, Jureen R, Cutter J, Lee VJ, Wang YT, 2017. The shorter multidrug-resistant tuberculosis treatment regimen in Singapore: are patients from south-east Asia eligible? Eur Respir J 50: 1700753. [DOI] [PubMed] [Google Scholar]
- 24.van der Werf MJ, Ködmön C, Catchpole M, 2017. Shorter regimens for multidrug-resistant tuberculosis should also be applicable in Europe. Eur Respir J 49: 1700463. [DOI] [PubMed] [Google Scholar]
- 25.Munoz-Torrico M, Salazar MA, Millán MdJM, Orozco JAM, Diaz LAN, Del Pilar MS, Visca D, D'Ambrosio L, Centis R, Migliori GB, 2018. Eligibility for the shorter regimen for multidrug-resistant tuberculosis in Mexico. Eur Respir J 51: 1702267. [DOI] [PubMed] [Google Scholar]
- 26.Claiborne A, English R, Olson S, 2011. The New Profile of Drug-Resistant Tuberculosis in Russia: A Global and Local Perspective: Summary of a Joint Workshop by the Institute of Medicine and the Russian Academy of Medical Science. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 27.Leung ECC, Leung CC, Kam KM, Yew WW, Chang KC, Leung WM, Tam CM, 2013. Transmission of multidrug-resistant and extensively drug-resistant tuberculosis in a metropolitan city. Eur Respir J 41: 901–908. [DOI] [PubMed] [Google Scholar]
- 28.Shah NS, et al. 2017. Transmission of extensively drug-resistant tuberculosis in South Africa. N Eng J Med 376: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore DA, 2016. What can we offer to 3 million MDRTB household contacts in 2016? BMC Med 14: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khaliaukin A, Kumar A, Skrahina A, Hurevich H, Rosuvich V, Gadoev J, Falzon D, Khogali M, de Colombani P, 2014. Poor treatment outcomes among multidrug-resistant tuberculosis patients in Gomel region, Republic of Belarus. Public Health Action 4: S24–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurbatova EV, et al. 2012. Predictors of poor outcomes among patients treated for multidrug-resistant tuberculosis at DOTS-plus projects. Tuberculosis 92: 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ananthakrishnan R, Kumar K, Ganesh M, Kumar AMV, Krishnan N, Swaminathan S, Edginton M, Arunagiri K, Gupta D, 2013. The profile and treatment outcomes of the older (aged 60 years and above) tuberculosis patients in Tamilnadu, south India. PLoS One 8: e67288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leveri TH, Lekule I, Mollel E, Lyamuya F, Kilonzo K, 2019. Predictors of treatment outcomes among multidrug resistant tuberculosis patients in Tanzania. Tuberc Res Treat 2019: 3569018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kliiman K, Altraja A, 2009. Predictors of poor treatment outcome in multi-and extensively drug-resistant pulmonary TB. Eur Respir J 33: 1085–1094. [DOI] [PubMed] [Google Scholar]
- 35.Milanov V, Falzon D, Zamfirova M, Varleva T, Bachiyska E, Koleva A, Dara M, . 2015. Factors associated with treatment success and death in cases with multidrug-resistant tuberculosis in Bulgaria, 2009–2010. Int J Mycobacteriol 4: 131–137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.