ABSTRACT
Envenomation and death resulting from snakebites represent a significant public health problem worldwide, particularly in tropical and subtropical regions. The WHO has defined snakebite as a neglected tropical health concern. Bites from Macrovipera lebetina obtusa usually cause life-threatening systemic hemodynamic disturbances, reduced functionality of the kidneys, and other serious symptoms, including hypotension shock, edema, and tissue necrosis, at the bite site. Herein, we highlight five cases of M. l. obtusa envenomation that presented with wide-ranging manifestations. Many recovered cases were left with long-term musculoskeletal disabilities. In a particular case, a 15-year-old male patient was envenomed in his palm by an 80-cm M. l. obtusa. Within 12 hours, swelling extended to near the shoulder. Fasciotomy was performed on the forearm and part of the upper arm of this patient. Symptoms of severe localized pain and swelling, dizziness, weakness, low blood pressure, and itching around the bite area were documented. The patient remained in the hospital for 13 days.
INTRODUCTION
Snakes bite more than five million people annually, resulting in about 400,000 amputations (are left disabled or disfigured by their injuries) and between 20,000 and 100,000 deaths.1 Unfortunately, populations from tropical and underdeveloped countries largely comprise the victims of snakebites, and a significant portion of the victims of snakebite are unable to get life-saving treatment and/or treatment able to prevent permanent damage due to a shortage of antivenoms, poor health services, and difficulties of rapid access to health centers.1,2 Snakebite envenomation was officially added to category A of the neglected tropical diseases by the WHO on June 9, 2017.3 Even with this recent addition, snakebite envenomation remains a hidden health crisis.
Bites of vipers usually result in local extravasation of plasma and blood into the bitten limb, inflammation, and tissue damage. Snakebites cause diverse symptoms such as pain, edema, blistering, bleeding, and necrosis of skin, subcutaneous tissues, and muscle.4 Furthermore, extensive local tissue damage results in permanent tissue damage. Viperidae snake venoms are typically rich in hydrolytic enzymes, contain a high content of zinc-dependent metalloproteinases (SVMPs), phospholipase A2, and serine proteinases, although the relative proportions of these enzymes vary among venoms of different species.5,6 SVMPs playing critical roles in envenomation-related pathogenesis include local and systemic hemorrhage, blistering, dermonecrosis, edema, and coagulopathies, in addition to being allogenic and strongly pro-inflammatory.7,8
Of Middle Eastern countries, Iran has the greatest number of different venomous snakes.9,10 There are around 86 snake species distributed among nine different families in Iran.11,12 Venomous snakes comprise 30% of these and belong to two families: Viperidae, represented by the genera Gloydius, Cerastes, Echis, Eristicophis, Macrovipera, Montivipera, Pseudocerastes, and Vipera; and Elapidae, represented by Bungarus, Naja, Walterinnesia, Hydrophis, and Microcephalophis genera.11,13 Macrovipera lebetina is a highly venomous snake, and bites from this snake result in serious clinical problems in Iran.14 Envenoming by M. lebetina is characterized by prominent local tissue damage and hemorrhage, abnormalities in the blood coagulation system, necrosis, and edema.14 In Iran, M. lebetina has two representatives, Macrovipera lebetina obtusa in western and northwestern parts and M. l. cernovi in northeastern parts.15 Bites from M. l. obtusa have the greatest mortality rate of all Macrovipera bites.16 Between 2002 and 2011, an estimated 53,787 cases of snake bites were reported in Iran17; however, the bulk of these, as represented by 80% in Kashan city (Isfahan Province, Iran), are most likely from nonvenomous snakes.18
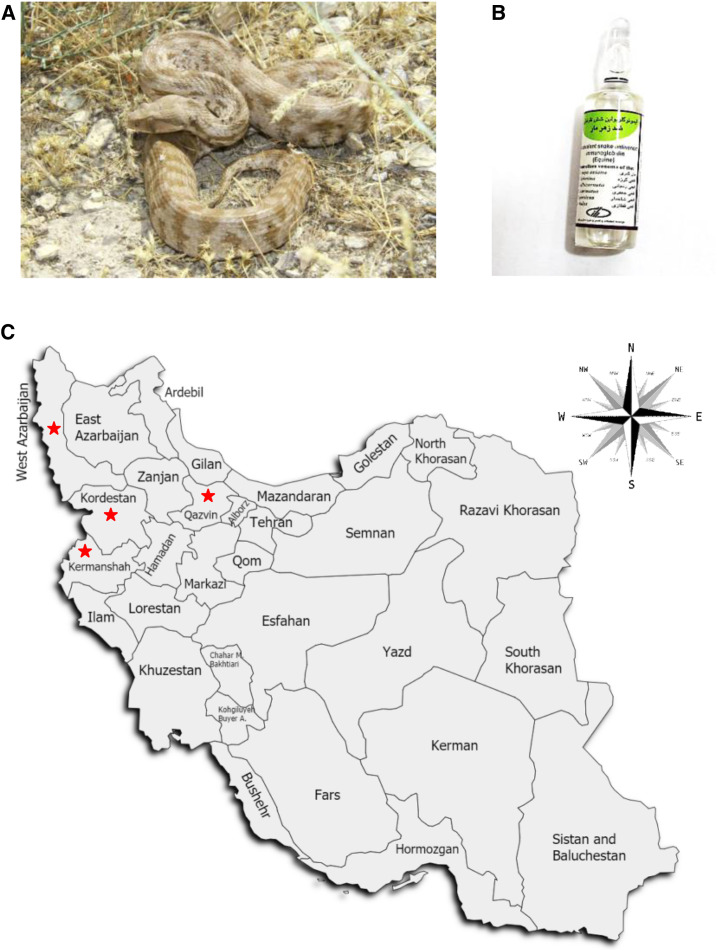
In general, the Levant blunt-nosed viper, M. l. obtusa, is endemic to Asia. Reaching around 1.7 m (5.6 ft) in length, it is found from central Turkey through Syria, Lebanon, Iraq, northern Jordan, the Caucasus region, Azerbaijan, Dagestan, and western and northwestern Iran (Figure 1A).19–21 Human envenomings by these snakes cause life-threatening systemic hemodynamic disturbances, reduced functionality of the kidneys, and ischemia at the bite site.22,23 We are reporting five cases of snakebite by M. l. obtusa. They presented with wide-ranging clinical manifestations. Several victims recovered, but many were left with long-term musculoskeletal disabilities. Razi™ polyvalent snake antivenin (Razi Vaccine and Serum Research Institute, Karaj, Iran) is the only available antivenom for the treatment of snakebite envenomation in Iran (Figure 1B).24–26 It is an equine F(ab’)2 antivenom and neutralizes the venom of Echis carinatus, Naja naja oxiana, Vipera lebetina, Vipera albicornuta, Agkistrodon halys, and Pseudocerastes persicus.26
Figure 1.
Levant blunt-nosed viper Macrovipera lebetina obtusa. (A) Photo of M. l. obtusa captured by Bahman Zangi. (B) Razi™ polyvalent antivenin, the only available antivenom for the treatment of snakebite envenomation in Iran. (C) Map showing the distribution of the five cases of M. l. obtusa envenomation in four provinces of Iran discussed here. Red stars indicate the localities of the examined cases. This figure appears in color at www.ajtmh.org.
CASE PRESENTATIONS
Five cases of snakebite by M. l. obtusa were reported in Kermanshah, Kurdistan, West Azerbaijan, and Qazvin provinces during spring 2019 (Figure 1C).
First case of M. l. obtusa envenomation in Qazvin Province.
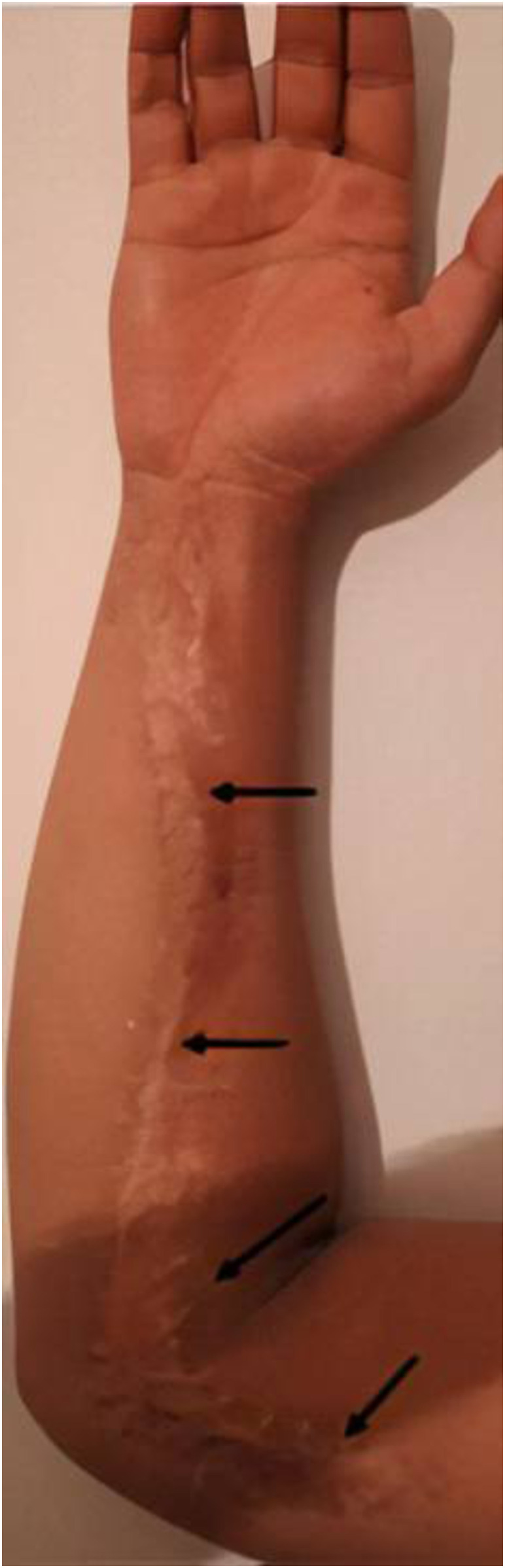
A 15-year-old man envenomed by an 80-cm-long M. l. obtusa presented to the Valie Asr Hospital (Abyek, Qazvin province, Iran) less than 1 hour after the bite. On initial clinical examination, the patient was suffering from severe localized pain and swelling, dizziness, weakness, low blood pressure, and itching around the snakebite areas, which were observed to be in his hand and right forearm. Renal and liver function tests were within normal ranges. The serum electrolytes including sodium, potassium, calcium, and magnesium; and serum and urea creatinine, blood urea nitrogen, urinalysis, acid–base condition, uric acid, creatine phosphokinase, and complete blood count were checked regularly. The whole blood clotting time (20-min WBCT) test was performed every 6 hours. Because of extensive swelling, the patient immediately received an initial dose (given slowly via intravenous) of 1 mL of polyvalent anti-snake venom which was available as lyophilized equine immunoglobulins (Razi Vaccine and Serum Research Institute, Karaj, Iran), in addition to 10-mg dose of loratadine, a tricyclic antihistamine. Twelve hours after the bite, swelling was still progressing and extended the entire length of the arm to near the shoulder. At this time, the patient was given five more doses of the antivenom and moved to the coronary care unit of the hospital for 2 days. Each 1 mL dose of the polyvalent antivenom is diluted in 250-500 mL of normal saline and administered using a peripheral venous catheter in the unaffected extremity. As result of major swelling and high intra-compartmental pressure (more than 45 mmHg) unresponsive to the antivenom, the patient then received urgent fasciotomy on the forearm and part of the upper arm to prevent further complications (Figure 2). Fasciotomy was performed after the coagulopathy is corrected. Two days following this operation, the localized swelling in the remaining part of the arm was reduced. Antibiotics such as cefazolin (Ancef and Kefzol) are given to treat fever, incised wounds, and abscess formation. The patient remained in the hospital for a total of 13 days before discharge and after three normal subsequent WBCT tests. After discharge, two consecutive follow-up visits were recommended every week to check the wound.
Figure 2.
Optimizing wound closure following a fasciotomy. Fasciotomy was performed on the forearm and part of the upper arm to prevent further complications due to Macrovipera lebetina obtusa bite. Black arrows show the site of the medical stitches after fasciotomy along the arm. This figure appears in color at www.ajtmh.org.
Second case of M. l. obtusa envenomation in Qazvin Province.
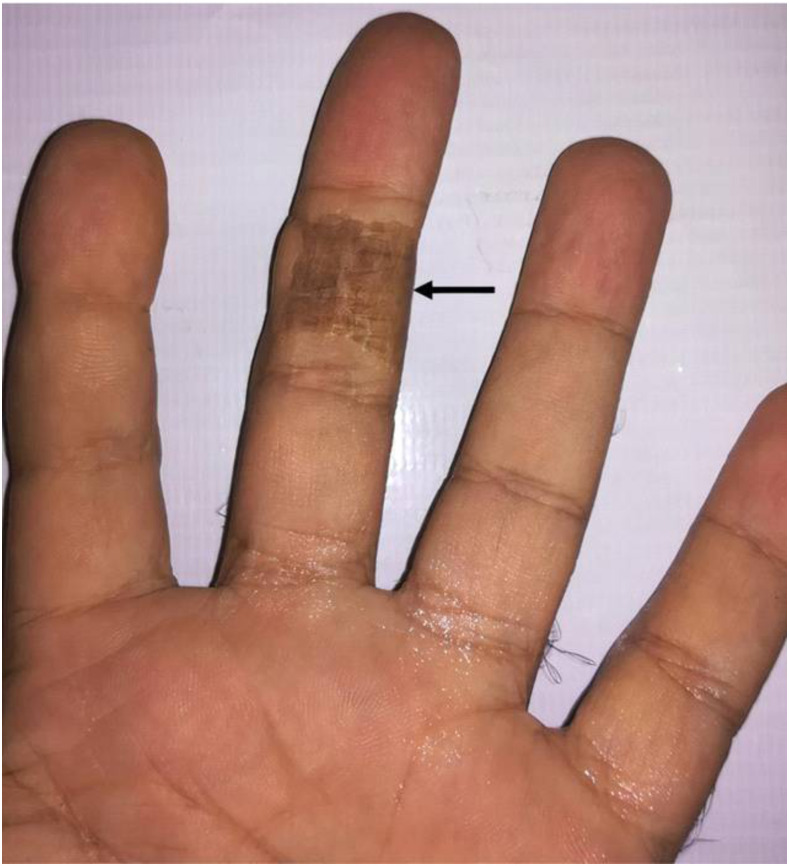
A 29-year-old male was bitten by a 40-cm M. l. obtusa. Eight hours after envenomation, the patient was transferred to Loghman Hakim Hospital, Tehran, Iran. On initial examination, the patient was suffering from severe pain, weakness, blurred vision, sleepiness, weakness, and decreased consciousness. The patient then received five vials (each dose is 1 mL) of Razi polyvalent antivenom: one dose directly injected using a peripheral venous catheter and four doses injected into the serum during blood transfusions. He received blood transfusions twice per day for 3 days. In addition, morphine was provided as a painkiller, and 20-mg of Zaditen (ketotifen), a histamine H1-receptor antagonist, was administrated intravenously to reduce inflammation. The patient responded well to the antivenom, and after 2 days, localized pain and swelling ceased. He was stable with a pulse rate of 100/minute, blood pressure of 120/70 mmHg, and a respiratory rate of 20/minute. The patient remained hospitalized for 5 days and then was released with few signs of the bite: deformities in the middle finger of the left hand (Figure 3).
Figure 3.
Frontal view of local signs of an Macrovipera lebetina obtusa bite after treatment with polyvalent antivenom in the absence of fasciotomy. Black arrow shows the site of necrosis in the middle finger of the patient’s left hand. This figure appears in color at www.ajtmh.org.
Third case of M. l. obtusa envenomation in Kermanshah Province.
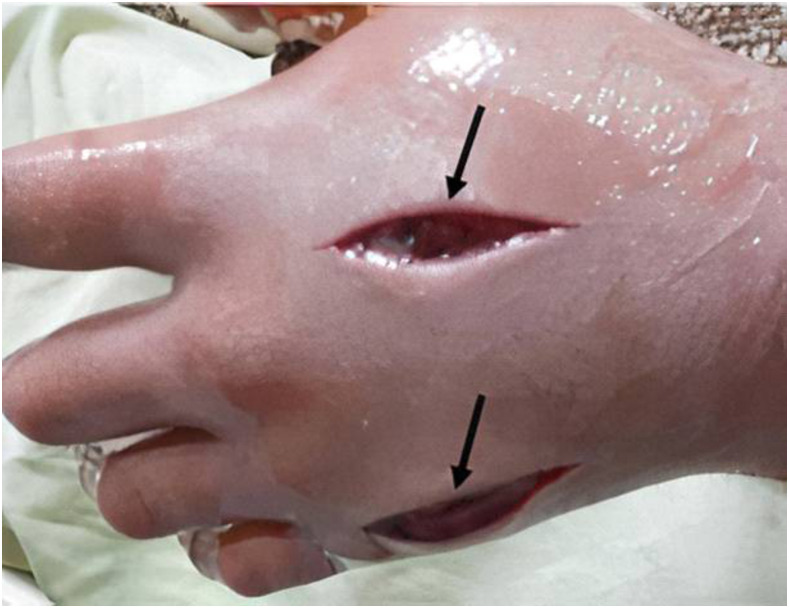
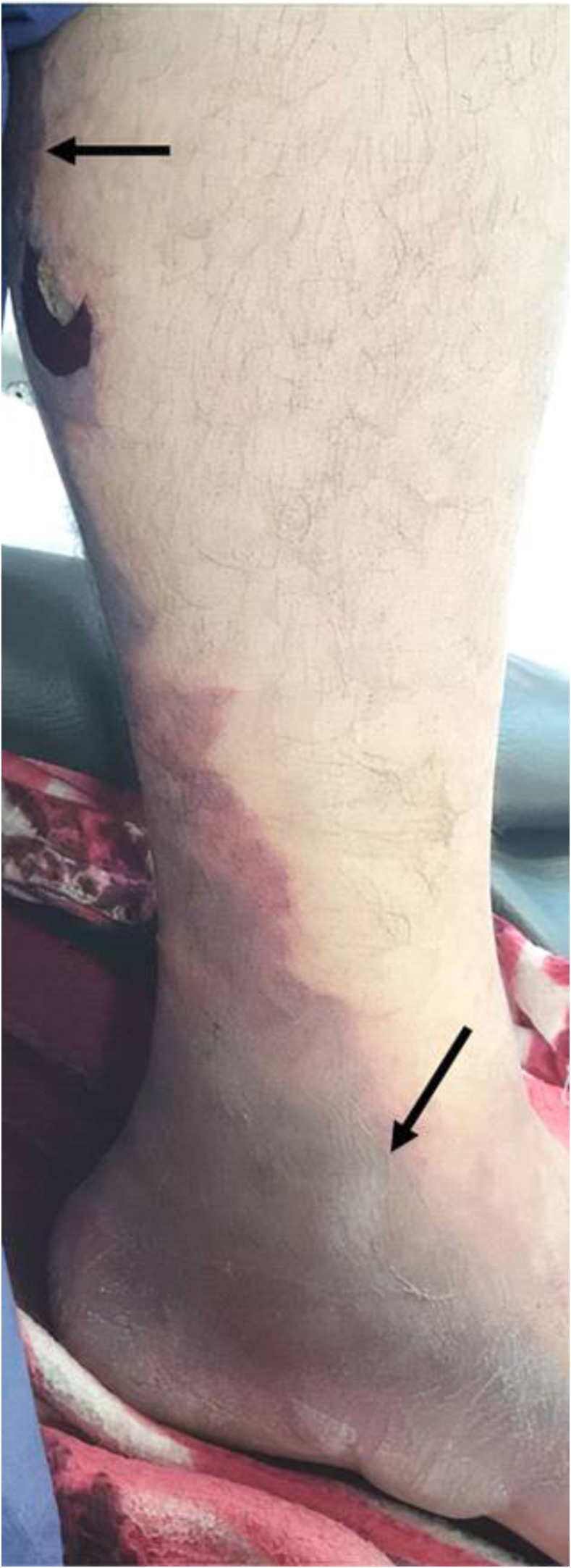
A 36-year-old man was bitten on the back of the hand by a 120-cm M. l. obtusa. Thirty minutes later, he was brought to the emergency department of Imam Khomeini hospital (Kermanshah Province, Iran) complaining of a swelling in his left hand and bruising in the bite area. Initial examination revealed a fully conscious patient with stable vital signs and arterial blood gases. Local examination of the patient’s left hand showed edema, redness, hotness, swelling, and tenderness. The bite wound was cleaned and debrided. The patient was shifted to the intensive care unit (ICU) with fluid therapy, symptomatic support, and continuous monitoring. Snake antivenom was recommended and promptly administered alongside symptomatic care and monitoring. Oral hydrochlorothiazide (Microzide) (12.5-mg) as an anti-edema was given. Five vials (each dose is 1 mL) of Razi polyvalent snake antivenom were given by intravenous infusion with 10-mg of loratadine, an antihistaminic, infusion in another intravenous line. Pain persisted for 2 days after the snakebite, and the patient was feverish (axillary temperature over 38°C) on the first day. The patient received three blood transfusions each day for 2 days. Fasciotomy was performed at Taleqani Hospital (Kermanshah Province, Iran) under general anesthesia with an incision on the dorsum of the left hand (Figure 4). The patient was then moved from the ICU to a general ward after reaching stability and until recovery. The patient was discharged a week after hospitalization. The patient was followed up with the plastic surgery section at Imam Khomeini Hospital for management of the affected hand tissue, and achieved full, functional recovery.
Figure 4.
Fasciotomy after the development of compartment syndrome due to an Macrovipera lebetina obtusa bite. Under general anesthesia, fasciotomy was performed with incision on the dorsum of the left hand. Black arrows show the site of the fasciotomy. This figure appears in color at www.ajtmh.org.
Fourth case of M. l. obtusa envenomation in west Azerbaijan Province.
A 30-year-old man was bitten on the left leg by M. l. obtusa. Ten hours following the snakebite, he was admitted to Shahid Gholi Pur Hospital (Bukan, West Azerbaijan Province, Iran) with acute-onset pain, swelling, severe fever, weakness, delusions, imbalance, and bruising (Figure 5). Immediately, he received two vials (each dose 1 mL) of Razi polyvalent antivenom for initial control of envenomation and oral opioids for pain management before transfer to the ICU. Because of extended edema with blisters and a possible associated compartment syndrome, the patient underwent surgery for fasciotomy on his lower left leg. The patient returned to the operating room every 2–3 days to clean the wound and remove necrotic tissues. Necrosis extended to the lower part of the leg. Seven days later, the leg was amputated at the knee. Afterward, the patient was discharged from the hospital.
Figure 5.
An Macrovipera lebetina obtusa bite caused significant swelling and bruising. Black arrows show significant bruising visible on the patient’s foot, extending to the lower part of the leg to the knee. Subsequent necrosis extending to the entire lower leg leads to amputation at the knee. This figure appears in color at www.ajtmh.org.
Fifth case of M. l. obtusa envenomation in Kurdistan Province.
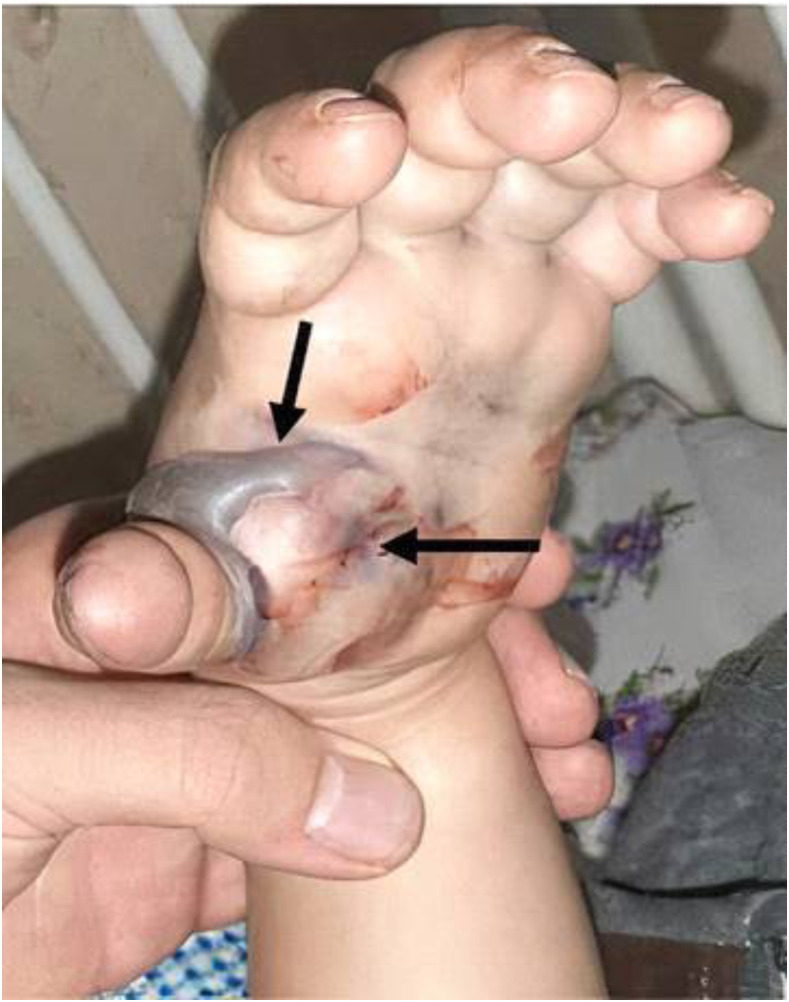
A 7-year-old girl was envenomed by M. l. obtusa. There was intense redness, bleeding, swelling, bruising, and blistering observed at the site of the bite in her left hand (Figure 6). She received two vials (each dose 1 mL) of Razi polyvalent antivenom for initial control of envenomation. The patient remained hospitalized for 6 days and then was released.
Figure 6.
Hemorrhagic blistering and tissue necrosis at the bite site from Macrovipera lebetina. obtusa. Black arrows show the bite area around the thumb finger including swelling and graying. This figure appears in color at www.ajtmh.org.
DISCUSSION
Symptoms of viper bites usually appear within a few minutes to a few hours after a bite and may include swelling and pain spread gradually from the bite site, necrosis, nausea, vomiting, abdominal pain, diarrhea, hypotension, coagulopathy, and bleeding. Renal failure is uncommon, but when it happens, it is due to hypotension, and the deposits of hemoglobin, myoglobin, and fibrin proteins in the renal tubules.26–28 In Iran, Echis and Macrovipera genus comprise the highest number of venomous snakes and cause the most severe cases of envenomations. M. l. obtusa is one of the most common venomous snakes in Iran as well as the neighboring countries of Iran including Iraq, Turkey, Armenia, and Azerbaijan. Serious clinical problems such as edema, hemorrhage, and tissue necrosis are observed in humans following M. l. obtusa envenomation. Based on WHO reports in 2018, M. l. obtusa is of the highest medical importance in Iran, Iraq, Lebanon, Syrian, and Turkey. In Iraq, it was considered the most important cause of snakebite casualties at 50% case fatality.14,29 In addition, fatal cases have been recorded in Cyprus.30
Several proteins and peptides have been isolated and characterized from the venoms of M. lebetina subspecies to include M. l. cernovi, M. l. lebetina, M. l. obtusa, M. l. transmediterranea, and M. l. turanica. These snake species cause most of the human envenomings in Central Asia (Middle East) and North Africa. These active components include Zn2+-metalloproteinases, serine proteinases, L-amino acid oxidase, 5′-nucleotidase, phosphodiesterase, phosphomonoesterase, nucleases, hyaluronidase, phospholipase A2, C-type lectin-like protein, disintegrin, DC-fragment, cysteine-rich secretory protein, proteinase inhibitors, nerve growth factor, vascular endothelial growth factor, and low molecular weight peptides.31 Many of these components target the hemostatic system; inhibit platelet aggregation by different mechanisms; increase the permeability of capillaries, the latter along with natriuretic peptides promoting hypotension; and influences the growth of neurons. The reported cases showed inflammatory and severe tissue-damaging symptoms as a result of the synergistic effects of these active biomolecules.
Clinical symptoms develop soon after envenomation by Macrovipera to include severe pain, swelling, and bruising with lymphangitic lines and painful regional lymphadenopathy spreading rapidly to encompass the envenomed limb and possibly part of the trunk. Serosanguinous blisters may appear preceding local necrosis that may require surgical debridement or amputation. Systemic symptoms include nausea, vomiting, diarrhea, fever, rigors, and trembling. Falling blood pressure and tachycardia can develop into shock. Coagulopathy and thrombocytopenia may lead to extensive ecchymosis, subconjunctival and retinal hemorrhages, hemoptysis, and melena, resulting in severe anemia.22,23,29,32 From the cases reported here, it is clear that several degrees of immune response and tissue necrosis from M. l. obtusa envenomation were noticed. Most of the expected systemic symptoms appear on reported incidents; however, none of these patients suffered major renal failure. The severity of the clinical symptoms and supportive treatments depends on the location of the snakebite and the time duration between the incident and hospitalization. Inside the hospital, several protocols of treatment were given, including blood transfusions, administration of antivenoms, and invasive fasciotomy. Delayed treatment is considered a major factor in the severity of the pathology. Moreover, differences in immune responses may reflect the different ages of these patients. Notably, the availability of antivenoms and blood transfusions were key treatments.
Different biotechnological and medicinal chemistry approaches are merging in the pursuit of developing effective and novel antivenoms.33,34 However, serum-based antivenom derived from immunized animals is currently the only therapeutically effective treatment option for more than 700 types of snake venoms.35,36 Razi polyvalent snake antivenin is the only available antivenom the for treatment of snakebite envenomation in Iran and has been used to treat the five cases of the present study. The total quantity of antivenom required for treatment varies from case to case, the major variables being the first aid methods used, the body weight, and previous health of the victim.
CONCLUSION
In conclusion, five cases were reported of M. l. obtusa envenomation in four different provinces in Iran in the spring of 2019. These case reports signify the importance of education of the people of the pitfalls to avoid after snake bite exposure, and the exigency of fast seeking the medical care. Furthermore, it confirms for clinicians about the importance of timely diagnosis and treatment that is a gold standard in toxicology practice, and it is pivotal in snakebite cases to reach a favorable outcome. Finally, physicians, herpetologists, farmers, children, and snake collectors should be aware of the potential for serious systemic envenomation by the Levant blunt-nosed viper during spring.
ACKNOWLEDGMENTS
We are very grateful to Prof José María Gutiérrez and Prof Jay W. Fox for their helpful comments; Dr. Mahboubeh Sadat Hosseinzadeh, Prof. Zuhair S. Amr, Prof. Jüri Siigur, and Parviz Ghezellou for providing literature; Mohammad Hossein Jahan-Mahin and Shadi Modares for recent information about M. l. obtusa snakebites; and Bahman Zangi, Iranian zoologist, for his image of M. l. obtusa.
REFERENCES
- 1.Williams DJ, et al. 2019. Strategy for a globally coordinated response to a priority neglected tropical disease: snakebite envenoming. PLoS Negl Trop Dis 13: e0007059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutiérrez JM, Williams D, Fan HW, Warrell DA, 2010. Snakebite envenoming from a global perspective: towards an integrated approach. Toxicon 56: 1223–1235. [DOI] [PubMed] [Google Scholar]
- 3.Chippaux JP, 2017. Snakebite envenomation turns again into a neglected tropical disease. J Venom AniToxins Incl Trop Dis 23: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warrell D, Gutiérrez J, 2007. Rabies and Envenomings: A Neglected Public Health Issue: Report of a Consultative Meeting. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 5.Calvete JJ, 2011. Proteomic tools against the neglected pathology of snake bite envenoming. Expert Rev Proteomics 8: 739–758. [DOI] [PubMed] [Google Scholar]
- 6.Lomonte B, Fernández J, Sanz L, Angulo Y, Sasa M, Gutiérrez JM, Calvete JJ, 2014. Venomous snakes of Costa Rica: biological and medical implications of their venom proteomic profiles analyzed through the strategy of snake venomics. J Proteomics 105: 323–339. [DOI] [PubMed] [Google Scholar]
- 7.Gutiérrez JM, Rucavado A, Escalante T, 2010. Snake venom metalloproteinases. Biological roles and participation in the pathophysiology of envenomation. In. Handbook of Venoms and Toxins of Reptiles. Mackessy SP, ed. Boca Raton, FL: CRC Press, 115–138. [Google Scholar]
- 8.Mohamed Abd El-Aziz T, Soares AG, Stockand JD, 2019. Snake venoms in drug discovery: valuable therapeutic tools for life saving. Toxins 11: 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terribile L, de Oliveira G, Albuquerque F, Rodriguez M, Diniz-Filho JA, 2009. Global conservation strategies for two clades of snakes: combining taxon-specific goals with general prioritization schemes. Divers Distrib 15: 841–851. [Google Scholar]
- 10.Terribile LC, Olalla-Tárraga MA, Morales-Castilla I, Rueda M, Vidanes RM, Rodríguez MA, Diniz-Filho JA, 2009. Global richness patterns of venomous snakes reveal contrasting influences of ecology and history in two different clades. Oecologia 159: 617–626. [DOI] [PubMed] [Google Scholar]
- 11.Safaei-Mahroo B, et al. 2015. The herpetofauna of Iran: checklist of taxonomy, distribution and conservation status. Asian Herpetological Res 6: 257–290. [Google Scholar]
- 12.Kazemi SM, Hosseinzadeh M, 2020. High diversity and endemism of herpetofauna in the Zagros Mountains. ECOPERSIA 8: 221–229. [Google Scholar]
- 13.Rezaie-Atagholipour M, Ghezellou P, Hesni MA, Dakhteh SMH, Ahmadian H, Vidal N, 2016. Sea snakes (Elapidae, Hydrophiinae) in their westernmost extent: an updated and illustrated checklist and key to the species in the Persian Gulf and Gulf of Oman. ZooKeys 622: 129–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fatehi-Hassanabad Z, Fatehi M, 2004. Characterisation of some pharmacological effects of the venom from Vipera lebetina. Toxicon 43: 385–391. [DOI] [PubMed] [Google Scholar]
- 15.Afroosheh M, 2011. Macrovipera lebetina Cernovi (Ophidia: Viperidae), a Newcomer to Iran. Abstract published in the 16th European Congress of Herpetology (SEH) and 47 Deutscher Herpetologentag (DGHT) at Luxembourg and Trier, Germany, 25–29 September 2011. [Google Scholar]
- 16.García-Arredondo A, Martínez M, Calderón A, Saldívar A, Soria R, 2019. Preclinical assessment of a new polyvalent antivenom (inoserp Europe) against several species of the subfamily viperinae. Toxins 11: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dehghani R, Fathi B, Shahi MP, Jazayeri M, 2014. Ten years of snakebites in Iran. Toxicon 90: 291–298. [DOI] [PubMed] [Google Scholar]
- 18.Dehghani R, Mehrpour O, Shahi MP, Jazayeri M, Karrari P, Keyler D, Zamani N, 2014. Epidemiology of venomous and semi-venomous snakebites (Ophidia: viperidae, colubridae) in the kashan city of the isfahan province in Central Iran. J Res Med Sci 19: 33–40. [PMC free article] [PubMed] [Google Scholar]
- 19.Mallow D, Ludwig D, Nilson G, 2003. True Vipers: Natural History and Toxinology of Old World Vipers. Malabar, FL: Krieger Publishing Company. [Google Scholar]
- 20.Oraie H, Rastegar Pouyani E, Khosravani A, Moradi N, Akbari A, Sehhatisabet M, Shafiei S, Stümpel N, Joger U, 2018. Molecular and morphological analyses have revealed a new species of blunt-nosed viper of the genus macrovipera in Iran. Salamandra 54: 233–248. [Google Scholar]
- 21.Stümpel N, 2012. Phylogenie und Phylogeographie eurasischer Viperinae unter besonderer Berücksichtigung der orientalischen Vipern der Gattungen Montivipera und Macrovipera. Braunschweig, Germany: Technische Universität Braunschweig. [Google Scholar]
- 22.Göçmen B, Arikan H, Mermer A, Çiçek K, 2006. Clinical, physiological and serological observations of a human following a venomous bite by Macrovipera lebetina lebetina (Reptilia: serpentes). Türkiye Parazitolojii Dergisi/Türkiye Parazitoloji Derneği = Acta Parasitologica Turcica/Turkish Soc Parasitol 30: 158–162. [PubMed] [Google Scholar]
- 23.Sharma LR, Lal V, Simpson ID, 2008. Snakes of medical significance in India: the first reported case of envenoming by the Levantine viper (Macrovipera lebetina). Wilderness Environ Med 19: 195–198. [DOI] [PubMed] [Google Scholar]
- 24.Theakston RD, Warrell DA, 1991. Antivenoms: a list of hyperimmune sera currently available for the treatment of envenoming by bites and stings. Toxicon 29: 1419–1470. [DOI] [PubMed] [Google Scholar]
- 25.Afshari R, Monzavi SM, 2017. AFSHARI’S Clinical Toxicology and Poisoning Emergency Care. Mashhad, Iran: Mashhad University of Medical Sciences Publishing Group. [Google Scholar]
- 26.Monzavi SM, Dadpour B, Afshari R, 2014. Snakebite management in Iran: devising a protocol. J ResMed Sci 19: 153. [PMC free article] [PubMed] [Google Scholar]
- 27.Latifi M, Leviton AE, Zug GR; Amphibians and Reptiles , 1991. The snakes of Iran. Society for the Study of Amphibians and Reptiles. Oxford, OH: Amphibia-Reptilia. [Google Scholar]
- 28.Dehghani R, Rabani D, Panjeh Shahi M, Jazayeri M, Sabahi Bidgoli M, 2012. Incidence of snake bites in Kashan, Iran during an eight year period (2004–2011). Arch Trauma Res 1: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amr ZS, Abu Baker MA, Warrell DA, 2020. Terrestrial venomous snakes and snakebites in the Arab countries of the Middle East. Toxicon 177: 1–15. [DOI] [PubMed] [Google Scholar]
- 30.Warrell D, 2017. Clinical Toxicology of Snakebite in Africa and the Middle East/Arabian Peninsula. [Google Scholar]
- 31.Siigur J, Aaspõllu A, Siigur E, 2019. Biochemistry and pharmacology of proteins and peptides purified from the venoms of the snakes Macrovipera lebetina subspecies. Toxicon 158: 16–32. [DOI] [PubMed] [Google Scholar]
- 32.Esmaeili Jahromi H, Zare A, Kamalzadeh M, 2016. Evaluation of Iranian Snake ‘Macrovipera lebetina’ Venom Cytotoxicity in Kidney Cell Line HEK-293. [Google Scholar]
- 33.El-Aziz TMA, et al. 2017. Efficient functional neutralization of lethal peptide toxins in vivo by oligonucleotides. Sci Rep 7: 7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taiwe GS, et al. 2019. Aptamer Efficacies for in vitro and in vivo modulation of αc-conotoxin PrXA pharmacology. Molecules 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calvete JJ, Sanz L, Angulo Y, Lomonte B, Gutiérrez JM, 2009. Venoms, venomics, antivenomics. FEBS Lett 583: 1736–1743. [DOI] [PubMed] [Google Scholar]
- 36.Gutiérrez JM, León G, Lomonte B, Angulo Y, 2011. Antivenoms for snakebite envenomings. Inflamm Allergy Drug Targets 10: 369–380. [DOI] [PubMed] [Google Scholar]