Introduction
Electrocochleography (ECochG) is an electrophysiological technique that records electrical potentials generated by different components of the inner ear and peripheral cochlear nerve in response to acoustic stimulation. Over the past few decades, there have been multiple applications of this tool, with ongoing refinements in technique and updates in the understanding of recorded potentials. Historically, ECochG found its main application in the diagnostic evaluation of Meniere's disease (MD). However, in the last decade, the focus has shifted towards ECochG use during cochlear implantation (CI). The ability to monitor cochlear trauma during CI electrode placement holds promise to improve hearing preservation outcomes and potentially modify surgical techniques and electrode design. The goal of this review is to provide a comprehensive overview of the electrophysiology and history of ECochG, discuss its recent applications in CI, and explore the ongoing research related to this expanding field.
Electrophysiology
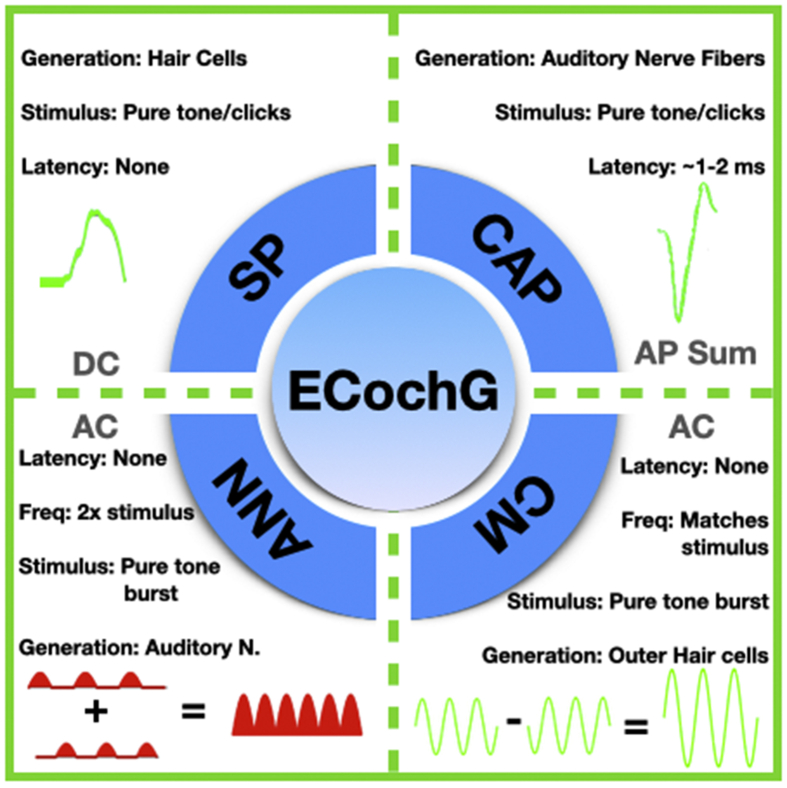
ECochG measures electrophysiological responses from the cochlea and the auditory nerve.1 During ECochG, a brief duration of acoustic stimuli (i.e. clicks or acoustic tone bursts) of alternating polarity (condensation and rarefaction) is used to elicit electrophysiological responses that can be measured using skin electrodes, extra tympanic electrodes, transtympanic electrodes, or intracochlear CI electrodes.2 An acoustic tone burst generates electrophysiological responses from a localized region in the cochlea or the auditory nerve, whereas clicks are known to elicit responses across a broader frequency range.2 ECochG responses can be analyzed into (1) cochlear microphonics (CM), (2) auditory nerve neurophonics (ANN), (3) summating potential (SP), and (4) compound action potential (CAP) (Fig. 1).
Fig. 1.
The four main components of ECochG. Cochlear microphonics (CM) and auditory nerve neurophonics (ANN) are alternating currents elicited from a tone burst stimuli of alternating polarity. CM represents a mechanical signal from the outer hair cells. ANN represents the phase-locked signal from the auditory nerve. The Summating Potential (SP) and the Compound Action Potential (CAP) are direct currents. SP represents responses from all hair cells, while the CAP represents responses from the auditory nerve.
Frequency-specific alternating polarity acoustic tone bursts are used to measure CM and ANN. CM represents the difference (i.e. subtraction) between ECochG responses to the two acoustic stimuli of alternating polarity.3 This potential is believed to primarily represent mechanical movement of the stereocilia on the outer hair cells.4 The CM potential appears as an alternating current (AC) that is phase locked to the stimulus tone.5 Thus, all the CM potentials recorded during ECochG provide real-time feedback. Currently, the CM appears to be the most sensitive detector of trauma during CI insertion.6 The summation (i.e. addition) of the ECochG responses measured from two alternating polarity acoustic stimuli is known as ANN, and is believed to represent the phase locked responses of the auditory nerve. The ANN is an AC potential with a frequency response twice the stimulus frequency.7,8 ANN is not particularly useful for real-time feedback of trauma to the hair cells, as it is not believed to originate from intracochlear structures.
The summating potential (SP) is the response of the inner hair cells at low frequencies, but can be a mixed response from all hair cells at the high frequencies.5,8 The SP is a direct current (DC) signal that arises from an AC stimulus, and represents a sustained depolarization of the hair cells during sound presentation.8 Thus, even in response to tone bursts, the SP appears only as a shift in the baseline, and does not provide immediate feedback.9 As the SP can have multiple sources and be influenced by many factors, it is rarely analyzed in the context of real-time intraoperative recordings during CI. The compound action potential (CAP) refers to the summation of individual action potentials from the auditory nerve fibers.8 The CAP is roughly the same potential as Wave I of an auditory brainstem response (ABR), and has identical latency as the ABR recording.10 In patients with existing hearing loss, the CAP can be absent or highly variable,6 and the CAP's correlation with hearing or speech testing is variable.11
History
The first description of auditory nerve potentials was from Wever and Bray in 1930,12 who studied cats, but laid the foundation for future work in humans.12 In 1935, Fromm et al. described the placement of electrodes through tympanic membrane(TM) perforations to measure electrical potentials, effectively becoming the first researcher to identify human cochlear potentials.13,14 Several other groups in the 1930s/1940s then refined the technique to reliably obtain CM, eventually making adaptations to use a cotton-tipped electrode and recording on the round window (RW) niche through a TM perforation.15,16
The term “cochleogram” was first used in 1947, when Lempert et al measured CM on 11 patients with different ear pathologies and used recordings for diagnostic purposes.13 During the1950s/1960s, Ruben and colleagues refined the clarity of CM waveforms,17,18 recorded the first CAP, and explored the relevance of CAP in MD.19 These advancements made ECochG a more practical and feasible tool for clinical use. Eggermont became the first to suggest the use of transtympanic ECochG as an objective measurement of hearing.20
As ECochG techniques became more refined, investigators set out to understand what the different waveforms in ECochG represented. In 1974, Schmidt et al.22 noted a larger SP in MD.21 SP widening was suggested as an objective tool for the diagnosis of MD, which was challenged by contemporaries.20 In the following decades, MD largely became the focus of debates over the clinical application of ECochG.17 To this day, ECochG remains a controversial tool in the diagnosis of MD,23 with significant skepticism among otolaryngologists.24
Although much of the effort on ECochG research has been given to the diagnosis of MD, there has been some other, less sought after, uses. Historically, ECochG has been used as an objective hearing measurement, but its routine use was largely replaced with the advent of ABR.10 Attempts were also made at using ECochG to diagnose vestibular schwannomas,25 but this proved impractical and unreliable. ECochG has also been proposed as a tool to diagnose auditory neuropathy spectrum disorder.26
ECochG and cochlear implantation
In the modern age of ECochG, there has been a significant shift of focus towards applications to CI. In 1985, the FDA first approved the use of multichannel CIs for adults with post-lingual deafness.27 In 1998, Nucleus introduced the CI24M electrode array, the first capable of performing neural response telemetry (NRT).28 Over the last two decades, the technology surrounding CI has vastly improved and candidacy criteria continues to expand. Refinements in electrode design and surgical technique, including less traumatic electrode insertion, have enabled many implant recipients to maintain significant residual hearing following CI.29
In patients with postoperative residual hearing, combined electric and acoustic stimulation (EAS) has resulted in improved speech perception,30 sound localization,31 and music appreciation.32 Despite efforts to mitigate trauma during electrode insertion, varying degrees of hearing preservation is only achievable in 47%-82% of CI recipients.29,33,34 Cochlear trauma during electrode placement is assumed to be a leading cause for postoperative hearing loss. At present, it is not possible to perform high resolution temporal bone imaging in real-time during electrode placement. ECochG, or more specifically, intraoperative CM measurements, can be used to monitor cochlear trauma during electrode placement.35 Over the past several years, the role and utility of intraoperative ECochG on CI hearing preservation has become a topic of extensive debate and research. ECochG has been used to compare pre-implantation to post-implantation responses, with the most recent advancements being made in real-time feedback during electrode insertion. The details of current knowledge on intraoperative ECochG technique and outcomes are discussed below.
Intraoperative ECochG applications
Extracochlear ECochG in CI
With extracochlear recordings, the recording electrode can be placed on the promontory, the stapes or the tympanic membrane.1,35 In 2010, Campbell et al.36 made the first intraoperative extracochlear ECochG recordings in gerbils. CI electrodes were inserted under direct visualization with an endoscope to identify signs of cochlear trauma.36 Generally, irreversible decreases in the CM correlated visually with direct trauma to cochlear structures, and histologic analysis confirmed these changes.36 This study was one of the first to definitively demonstrate that real-time changes in ECochG potentials directly correlated to trauma, thus laying the foundation for future exploration of real-time ECochG as a feedback tool in human CI.
In humans, the feasibility of extracochlear ECochG in CI was first explored through stapes and RW recordings. In 2011, Harris and colleagues were the first to show that ECochG potentials could be recorded from the stapes during CI.35 In this pilot study, insertion of the CI electrode into the basal turn of the cochlea and suctioning of perilymph was associated with loss of ECochG signal.35 In 2012, Choudhury et al.8 demonstrated that extracochlear ECochG potentials could be measured from the RW even in patients with poor preoperative audiograms and pure tone averages (PTAs) < 100 dB. The success rate of obtaining recordings varied between studies, but overall there was agreement that despite poor preoperative hearing, ECochG waveforms could be reliably obtained in 52%-100% of patients undergoing CI.8,35,37, 38, 39 Though controversial, some have speculated that pre-implantation ECochG might serve as the most reliable predictor of postoperative speech perception compared to other commonly used clinical factors and audiological measures.39,40
The ability for peri-implantation extracochlear ECochG to predict post-operative hearing outcomes varies among studies. Radeloff et al.41 performed RW ECochG in six patients, measuring CM at various time points of electrode insertion. Four patients had postoperative loss of residual hearing, despite preservation of CM thresholds during insertion.41 Dalbert et al.37 made measurements on the promontory pre- and post-implantation. All patients experiencing detectable threshold changes intraoperatively suffered complete loss of residual hearing, though not all with hearing loss experienced threshold changes.37 In another cohort, none of the subjects showed a loss in post-implantation extracochlear ECochG that would suggest insertion trauma, but some still had residual hearing loss after surgery.38 A recent study agreed that those experiencing decreases in ECochG responses intraoperatively showed significantly greater likelihood of hearing loss, but hearing loss was still possible despite maintaining intraoperative extracochlear ECochG thresholds.42 The authors posited that some trauma may be limited to the high-frequency regions of the cochlea, and can be missed on routine low-frequency ECochG recordings.43 This discrepancy between intraoperative preservation of ECochG signal and loss of residual hearing suggests that postoperative inflammatory reactions may also play a role in hearing loss following CI.41 More practically, another study demonstrated that in surgeons who are given real time intraoperative ECochG feedback during electrode insertion, 85% achieved hearing preservation while only 33% of the non-feedback group did.44 Overall, these results suggest that real-time feedback may enable surgeons to preserve hearing in a greater number of cases. Moreover, ECochG likely provides a low sensitivity, but high specificity, when using loss of intraoperative extracochlear ECochG signal as a predictor of postoperative residual hearing loss.
Intracochlear ECochG in CI
The history of intracochlear ECochG is somewhat limited as this technology only came to the forefront approximately 5 years ago. Intracochlear ECochG has been performed by inserting a recording electrode into the cochlea45 or, alternatively, by using one of the CI electrodes as the recording electrode.35 In the most recent implementations, ECochG has been measured using the most apical CI electrode as the recording electrode and an extracochlear case, ring or ball electrode as the return electrode.46
In 2014, Calloway was the first to describe intracochlear ECochG recordings during CI.45 Intracochlear responses were much larger in amplitude than extracochlear responses, as confirmed by other studies.38,47 The authors also observed increasing signal amplitudes with increasing electrode depths in the cochlea, which they attributed to growing proximity to the residual functional neural elements at the low-frequency apex of the cochlea.45 Campbell was the first to prove feasibility of intracochlear recordings using the existing CI electrodes by recording from five subjects with residual hearing after CI.48 Dalbert et al. expanded on this technique further by performing intracochlear recordings using the CI electrode intraoperatively and postoperatively. They showed that intracochlear ECochG signal could continue to decline in the first week after implantation,38 consistent with theories that postoperative inflammation in the cochlea may contribute to the delayed loss of residual hearing. Compared to extracochlear ECochG, intracochlear measurements have been shown to have increased signal to noise ratio.45 However, as the electrode is constantly moving, a stable response cannot necessarily be used to detect trauma.49 Some authors advocate for a mixed approach with dual recording of extra and intracochlear potentials during electrode insertion, and postoperative follow up using continued intracochlear measurements.38
It is only in the last two to three years that real-time intracochlear ECochG during electrode insertion has been explored. Lo et al.50 pioneered continuous intracochlear ECochG in animal studies and showed that ECochG amplitudes tended to increase with insertion depth, with loss of signal correlating with post-implantation hearing loss. Harris and colleagues showed that it was also highly feasible in humans to continuously record CM and ANN during electrode insertion.46 He later characterized the intracochlear ECochG amplitude patterns observed during electrode insertion into 3 types.7 The Harris Type A ECochG pattern is defined as an overall increase in amplitude from the beginning of insertion to completion.7 The Harris Type B pattern has maximum amplitude at the beginning of insertion, with an overall decrease as insertion goes to completion, sometimes to complete signal loss.7 Finally, the Harris type C has similar amplitudes at the beginning and completion of insertion, with a maximum amplitude reached mid-insertion.7 Type A was the most common, followed by Type B, then Type C.7 Ramos-Macias et al.4 examined the correlation between Harris classification and postoperative hearing outcomes. Intraoperatively, the Type B pattern leading to complete loss of signal was associated with complete loss of residual hearing, and may be suggestive of irreversible trauma during insertion.4 In the case of an intracochlear ECochG recording that drops in amplitude but recovers, proposed mechanisms include temporary physical contact between the electrode and cochlear elements, or destructive interference between the hair cell and neural potentials.6
The association between loss of intracochlear ECochG signal and cochlear trauma or electrode positioning remains controversial, and there is active debate regarding what the Harris Type B pattern represents. In Lo et al.‘s animal study, histological studies showed that intraoperative loss of CAP amplitude was associated with higher grades of trauma, but not all cases of post-implantation hearing loss had signs of trauma on pathology.50 In humans, one retrospective case control study used post-operative imaging to identify electrode arrays that translocated into the scala vestibuli as a proxy for trauma to the basilar membrane.51 Similar to the animal studies, the authors found no difference in intraoperative ECochG amplitude between the translocation group and the nontranslocation group, despite drastic differences in hearing preservation between the two groups. In contrast to these studies above, O'Connell et al.52 in 2017 showed that there might be a slight association between electrode translocation and intraoperative ECochG thresholds. In their cohort, there were differences between the intraoperative ECochG thresholds and postoperative PTA thresholds, and these differences were significantly larger for translocated electrodes. However, they were unable to demonstrate a correlation between ECochG and postoperative behavioral thresholds. Another study in support of this association showed that intraoperative ECochG could reliably predict electrode translocation in 82% of patients, with a sensitivity of 100% and specificity of 77%, but only a positive predictive value of 54%.53 As such, the promising data presented by O'Connell et al. regarding the predictive value of intraoperative ECochG needs further investigation, given conflicting evidence correlating ECochG changes to postoperative outcomes.
Postoperative ECochG applications
Postoperatively, intracochlear ECochG has been used to determine the lowest stimulus presentation level that generates CMs or CM thresholds in CI patients with residual hearing. The results show a significant correlation between CM thresholds and behavioral thresholds in CI patients.38,48,52,54, 55, 56, 57 CM thresholds thus offer an objective method to assess hearing sensitivity in CI subjects who cannot participate in behavioral audiometry57 and can even be used to assess air-bone gaps in implanted patients.55 The new AIM system from Advanced Bionics allows providers to perform a quick estimation of the CM thresholds immediately after CI electrode placement, which may be helpful in understanding mechanisms driving the loss of residual hearing immediately after electrode placement.
Combined EAS has been shown to provide the best outcomes in CI, but it is important to appropriately set the frequency boundaries of acoustic and electric stimulation to further refine its efficacy.58 Furthermore, excitotoxicity from high levels of electrical stimulation has been shown to cause delayed postoperative hearing loss in CI patients with initially preserved residual hearing.59 ECochG measurements provide an objective and time efficient method to assess EAS interaction in the cochlea and the auditory nerve.54 CM and ANN responses can be measured in the presence of electrical stimulation to determine the frequency boundaries of acoustic and electrical stimulation and minimize interaction between the two modes of stimulation.
It is also worthwhile to consider the location of the CI electrodes as it determines the place-pitch sensation produced by electrical stimulation of each individual electrode. A mismatch between the frequency information delivered to the CI electrode and the place-pitch sensation produced by electrical stimulation is known to adversely affect CI outcome.60 CM tuning curves can be measured for different acoustic pure tone frequencies by varying the intracochlear recording electrodes and can be used to determine CI electrode location along the basilar membrane.61
Thus, postoperatively, ECochG can be used to measure CM thresholds and predict behavioral auditory thresholds, determine EAS interactions, program the EAS stimulation device, and determine the cochlear location of implant electrodes, all of which may potentially lead to improvements in CI outcomes.
Future application and conclusion
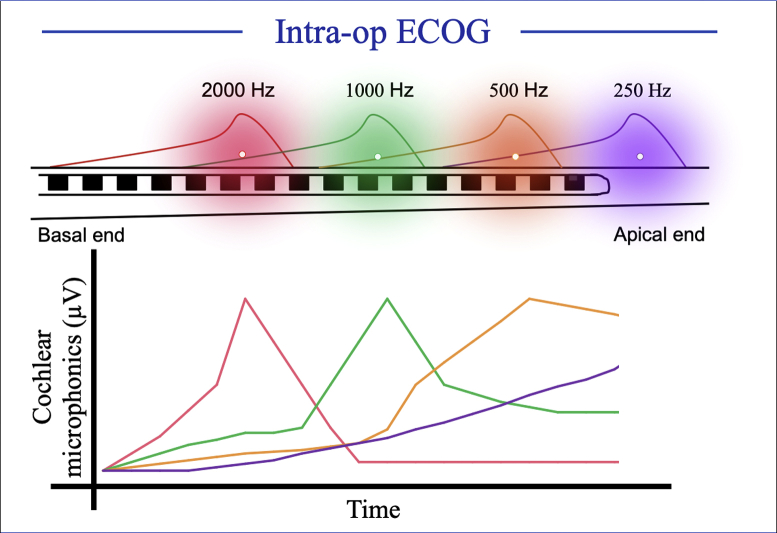
ECochG offers an opportunity to measure frequency specific CMs elicited from a localized region in the cochlea. However, CMs generated for a low frequency tone such as 500 Hz may not be sensitive to cochlear trauma in the basal region during CI electrode placement.43 At our institution, we are using a pure tone complex to elicit CMs such that we can present up to four acoustic pure tone stimuli simultaneously and measure CMs from four different locations along the basilar membrane (Fig. 2). Preliminary results show that these multi-frequency CM measurements can be used to monitor cochlear trauma from different regions in the cochlea.
Fig. 2.
The 4-tone burst technique to measure CM during a CI electrode array insertion.
The discussion of this technology demonstrates that we now have the ability to potentially localize the CI electrode array in real-time -- something that has not been previously possible. At this point, it is unclear if this technology has the capacity to reliably detect cochlear trauma, prevent cochlear trauma in real-time, or predict postoperative hearing loss. The excitement around this renewed use of ECochG should thus be attenuated by the lack of definitive data supporting objective outcomes.
Optimism remains for ECochG's application for hearing preservation, but another underlying benefit is the possibility that this technology can be used to train surgeons in soft insertion techniques. Additionally, we are now given the opportunity to potentially understand when hearing loss may occur, either intraoperatively or in the immediate postoperative period.
Many questions remain in this evolving field. While immediate feedback alerts the surgeon to potential impending trauma, it is unclear exactly how a surgeon should adjust, if adjustments can feasibly be made, and how this would affect hearing outcomes. For example, the tip of an electrode that has been inserted 300° is unlikely to be adjusted in a meaningful manner from outside of the cochlea. Further research is also required to know when and if electrode advancement should be halted in light of ECochG feedback. Moreover, with continued data collection, understanding of exactly where trauma most likely occurs and when hearing loss occurs could lead to the development of new devices such as alternately shaped or drug eluting arrays.
Over the last decade, ECochG has begun to reshape how we think about hearing preservation in CI. With rapid advancements in this technology and continued revisions in CI candidacy, ECochG will likely continue to play a vital role in predicting hearing outcomes, informing surgical technique, understanding postoperative physiology, and providing a deeper understanding of the mechanisms of hearing loss.
Declaration of Competing Interest
Aniket A. Saoji is a consultant for Advanced Bionics and Envoy Medical. He has a research grant from Advanced Bionics and research support from Cochlear Corporation.
Edited by Yu-Xin Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Bakhos D., Marx M., Villeneuve A., Lescanne E., Kim S., Robier A. Electrophysiological exploration of hearing. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134:325–331. doi: 10.1016/j.anorl.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Gibson W.P. The clinical uses of electrocochleography. Front Neurosci. 2017;11:274. doi: 10.3389/fnins.2017.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santarelli R., Scimemi P., Dal Monte E., Arslan E. Cochlear microphonic potential recorded by transtympanic electrocochleography in normally-hearing and hearing-impaired ears. Acta Otorhinolaryngol Ital. 2006;26:78–95. [PMC free article] [PubMed] [Google Scholar]
- 4.Ramos-Macias A., O'Leary S., Ramos-deMiguel A., Bester C., Falcon-Gonzalez J.C. Intraoperative intracochlear electrocochleography and residual hearing preservation outcomes when using two types of slim electrode arrays in cochlear implantation. Otol Neurotol. 2019;40(5S Suppl 1):S29–S37. doi: 10.1097/MAO.0000000000002212. [DOI] [PubMed] [Google Scholar]
- 5.Fontenot T.E., Giardina C.K., Fitzpatrick D.C. A model-based approach for separating the cochlear microphonic from the auditory nerve neurophonic in the ongoing response using electrocochleography. Front Neurosci. 2017;11:592. doi: 10.3389/fnins.2017.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giardina C.K., Brown K.D., Adunka O.F. Intracochlear electrocochleography: response patterns during cochlear implantation and hearing preservation. Ear Hear. 2019;40:833–848. doi: 10.1097/AUD.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris M.S., Riggs W.J., Giardina C.K. Patterns seen during electrode insertion using intracochlear electrocochleography obtained directly through a cochlear implant. Otol Neurotol. 2017;38:1415–1420. doi: 10.1097/MAO.0000000000001559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choudhury B., Fitzpatrick D.C., Buchman C.A. Intraoperative round window recordings to acoustic stimuli from cochlear implant patients. Otol Neurotol. 2012;33:1507–1515. doi: 10.1097/MAO.0b013e31826dbc80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappa A.K., Hutson K.A., Scott W.C. Hair cell and neural contributions to the cochlear summating potential. J Neurophysiol. 2019;121:2163–2180. doi: 10.1152/jn.00006.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minaya C., Atcherson S.R. Simultaneous extratympanic electrocochleography and auditory brainstem responses revisited. Audiol Res. 2015;5:105. doi: 10.4081/audiores.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott W.C., Giardina C.K., Pappa A.K. The compound action potential in subjects receiving a cochlear implant. Otol Neurotol. 2016;37:1654–1661. doi: 10.1097/MAO.0000000000001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wever E.G., Bray C.W. Auditory nerve impulses. Science. 1930;71:215. doi: 10.1126/science.71.1834.215. [DOI] [PubMed] [Google Scholar]
- 13.Lempert J., Wever E.G., Lawrence M. The cochleogram and its clinical application; a preliminary report. Arch Otolaryngol. 1947;45:61–67. doi: 10.1001/archotol.1947.00690010068005. [DOI] [PubMed] [Google Scholar]
- 14.Fromm B., Nylén C.O., Zotterman Y. Studies in the mechanism of the wever and Bray effect. Acta Oto Laryngologica. 1935;22:477–486. [Google Scholar]
- 15.Andreev A.M., Arapova A.A., Gersuni G.V. On the electrical potentials of the human cochlea. J Physiol USSR. 1939;26:205–212. [Google Scholar]
- 16.Perlman H.B., Case T.J. Electrical phenomena of the cochlea in man. JAMA Otolaryngol Head Neck Surg. 1941;34:710–718. [Google Scholar]
- 17.Eggermont J.J. Ups and downs in 75 years of electrocochleography. Front Syst Neurosci. 2017;11:2. doi: 10.3389/fnsys.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruben R.J., Lieberman A.T., Bordley J.E. Some observations on cochlear potentials and nerve action potentials in children. Laryngoscope. 1962;72:545–554. doi: 10.1288/00005537-196205000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Ruben R.J., Walker A.E. The viiith nerve action potential in Meniere's disease. Laryngoscope. 1963;73:1456–1464. doi: 10.1288/00005537-196311000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Eggermont J.J. Summating potentials in Meniere's disease. Arch Otorhinolaryngol. 1979;222:63–75. doi: 10.1007/BF00456340. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt P.H., Eggermont J.J., Odenthal D.W. Study of Meniere's disease by electrocochleography. Acta Otolaryngol. 1974;316:75–84. doi: 10.1080/16512251.1974.11675748. [DOI] [PubMed] [Google Scholar]
- 22.Gibson W.P., Moffat D.A., Ramsden R.T. Clinical electrocochleography in the diagnosis and management of Meneere's disorder. Audiology. 1977;16:389–401. doi: 10.3109/00206097709071852. [DOI] [PubMed] [Google Scholar]
- 23.Lamounier P., Gobbo D.A., Souza T.S., Oliveira C.A., Bahmad F., Jr. Electrocochleography for Meniere's disease: is it reliable? Braz J Otorhinolaryngol. 2014;80:527–532. doi: 10.1016/j.bjorl.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen L.T., Harris J.P., Nguyen Q.T. Clinical utility of electrocochleography in the diagnosis and management of Meniere's disease: AOS and ANS membership survey data. Otol Neurotol. 2010;31:455–459. doi: 10.1097/MAO.0b013e3181d2779c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka F., Tsukasaki N., Nakao Y., Shigeno K., Kobayashi T. Electrocochleographic evaluation of hearing loss in acoustic neuromas. Am J Otol. 1999;20:479–483. [PubMed] [Google Scholar]
- 26.Riggs W.J., Roche J.P., Giardina C.K. Intraoperative electrocochleographic characteristics of auditory neuropathy spectrum disorder in cochlear implant subjects. Front Neurosci. 2017;11:416. doi: 10.3389/fnins.2017.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eshraghi A.A., Nazarian R., Telischi F.F., Rajguru S.M., Truy E., Gupta C. The cochlear implant: historical aspects and future prospects. Anat Rec (Hoboken) 2012;295:1967–1980. doi: 10.1002/ar.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shallop J.K., Facer G.W., Peterson A. Neural response telemetry with the nucleus CI24M cochlear implant. Laryngoscope. 1999;109:1755–1759. doi: 10.1097/00005537-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Carlson M.L., Driscoll C.L.W., Gifford R.H. Implications of minimizing trauma during conventional cochlear implantation. Otol Neurotol. 2011;32:962–968. doi: 10.1097/MAO.0b013e3182204526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfe J., Neumann S., Schafer E., Marsh M., Wood M., Baker R.S. Potential benefits of an integrated electric-acoustic sound processor with children: a preliminary report. J Am Acad Audiol. 2017;28:127–140. doi: 10.3766/jaaa.15133. [DOI] [PubMed] [Google Scholar]
- 31.Irving S., Gillespie L., Richardson R., Rowe D., Fallon J.B., Wise A.K. Electroacoustic stimulation: now and into the future. Biomed Res Int. 2014;2014:350504. doi: 10.1155/2014/350504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brockmeier S.J., Peterreins M., Lorens A. Music perception in electric acoustic stimulation users as assessed by the Mu.S.I.C. test. Adv Otorhinolaryngol. 2010;67:70–80. doi: 10.1159/000262598. [DOI] [PubMed] [Google Scholar]
- 33.Anagiotos A., Hamdan N., Lang-Roth R. Young age is a positive prognostic factor for residual hearing preservation in conventional cochlear implantation. Otol Neurotol. 2015;36:28–33. doi: 10.1097/MAO.0000000000000586. [DOI] [PubMed] [Google Scholar]
- 34.Carlson M.L., Patel N.S., Tombers N.M. Hearing preservation in pediatric cochlear implantation. Otol Neurotol. 2017;38:e128–e133. doi: 10.1097/MAO.0000000000001444. [DOI] [PubMed] [Google Scholar]
- 35.Harris R., Cruise A., Gibson W., Bate K., Sanli H. Preliminary results and technique for electrophysiological intra-operative monitoring of residual hearing during cochlear implantation. Cochlear Implants Int. 2011;12:209–215. doi: 10.1179/146701011X12950038111657. [DOI] [PubMed] [Google Scholar]
- 36.Campbell A.P., Suberman T.A., Buchman C.A., Fitzpatrick D.C., Adunka O.F. Correlation of early auditory potentials and intracochlear electrode insertion properties: an animal model featuring near real-time monitoring. Otol Neurotol. 2010;31:1391–1398. doi: 10.1097/MAO.0b013e3181f6c899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalbert A., Sim J.H., Gerig R., Pfiffner F., Roosli C., Huber A. Correlation of electrophysiological properties and hearing preservation in cochlear implant patients. Otol Neurotol. 2015;36:1172–1180. doi: 10.1097/MAO.0000000000000768. [DOI] [PubMed] [Google Scholar]
- 38.Dalbert A., Pfiffner F., Röösli C. Extra- and intracochlear electrocochleography in cochlear implant recipients. Audiol Neurootol. 2015;20:339–348. doi: 10.1159/000438742. [DOI] [PubMed] [Google Scholar]
- 39.Formeister E.J., McClellan J.H., Merwin W.H. Intraoperative round window electrocochleography and speech perception outcomes in pediatric cochlear implant recipients. Ear Hear. 2015;36:249–260. doi: 10.1097/AUD.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 40.McClellan J.H., Formeister E.J., Merwin W.H. Round window electrocochleography and speech perception outcomes in adult cochlear implant subjects: comparison with audiometric and biographical information. Otol Neurotol. 2014;35:e245–e252. doi: 10.1097/MAO.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 41.Radeloff A., Shehata-Dieler W., Scherzed A. Intraoperative monitoring using cochlear microphonics in cochlear implant patients with residual hearing. Otol Neurotol. 2012;33:348–354. doi: 10.1097/MAO.0b013e318248ea86. [DOI] [PubMed] [Google Scholar]
- 42.Dalbert A., Pfiffner F., Hoesli M. Assessment of cochlear function during cochlear implantation by extra- and intracochlear electrocochleography. Front Neurosci. 2018;12:18. doi: 10.3389/fnins.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalbert A., Huber A., Veraguth D., Roosli C., Pfiffner F. Assessment of cochlear trauma during cochlear implantation using electrocochleography and cone beam computed tomography. Otol Neurotol. 2016;37:446–453. doi: 10.1097/MAO.0000000000000998. [DOI] [PubMed] [Google Scholar]
- 44.Mandalà M., Colletti L., Tonoli G., Colletti V. Electrocochleography during cochlear implantation for hearing preservation. Otolaryngol Head Neck Surg. 2012;146:774–781. doi: 10.1177/0194599811435895. [DOI] [PubMed] [Google Scholar]
- 45.Calloway N.H., Fitzpatrick D.C., Campbell A.P. Intracochlear electrocochleography during cochlear implantation. Otol Neurotol. 2014;35:1451–1457. doi: 10.1097/MAO.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 46.Harris M.S., Riggs W.J., Koka K. Real-time intracochlear electrocochleography obtained directly through a cochlear implant. Otol Neurotol. 2017;38:e107–e113. doi: 10.1097/MAO.0000000000001425. [DOI] [PubMed] [Google Scholar]
- 47.Haumann S., Imsiecke M., Bauernfeind G. Monitoring of the inner ear function during and after cochlear implant insertion using electrocochleography. Trends Hear. 2019;23 doi: 10.1177/2331216519833567. 2331216519833567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campbell L., Kaicer A., Briggs R., O'Leary S. Cochlear response telemetry: intracochlear electrocochleography via cochlear implant neural response telemetry pilot study results. Otol Neurotol. 2015;36:399–405. doi: 10.1097/MAO.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 49.Giardina C.K., Khan T.E., Pulver S.H. Response changes during insertion of a cochlear implant using extracochlear electrocochleography. Ear Hear. 2018;39:1146–1156. doi: 10.1097/AUD.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lo J., Bester C., Collins A. Intraoperative force and electrocochleography measurements in an animal model of cochlear implantation. Hear Res. 2017;358:50–58. doi: 10.1016/j.heares.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Riggs W.J., Dwyer R.T., Holder J.T. Intracochlear electrocochleography: influence of scalar position of the cochlear implant electrode on postinsertion results. Otol Neurotol. 2019;40:e503–e510. doi: 10.1097/MAO.0000000000002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Connell B.P., Holder J.T., Dwyer R.T. Intra- and postoperative electrocochleography may BePredictive of final electrode position and postoperative hearing preservation. Front Neurosci. 2017;11:291. doi: 10.3389/fnins.2017.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koka K., Riggs W.J., Dwyer R. Intra-cochlear electrocochleography during cochear implant electrode insertion is predictive of final scalar location. Otol Neurotol. 2018;39:e654–e659. doi: 10.1097/MAO.0000000000001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koka K., Litvak L.M. Feasibility of using electrocochleography for objective estimation of electro-acoustic interactions in cochlear implant recipients with residual hearing. Front Neurosci. 2017;11:337. doi: 10.3389/fnins.2017.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koka K., Saoji A.A., Attias J., Litvak L.M. An objective estimation of air-bone-gap in cochlear implant recipients with residual hearing using electrocochleography. Front Neurosci. 2017;11:210. doi: 10.3389/fnins.2017.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim J.S., Tejani V.D., Abbas P.J., Brown C.J. Postoperative electrocochleography from hybrid cochlear implant users: an alternative analysis procedure. Hear Res. 2018;370:304–315. doi: 10.1016/j.heares.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koka K., Saoji A.A., Litvak L.M. Electrocochleography in cochlear implant recipients with residual hearing: comparison with audiometric thresholds. Ear Hear. 2017;38:e161–e167. doi: 10.1097/AUD.0000000000000385. [DOI] [PubMed] [Google Scholar]
- 58.Vermeire K., Anderson I., Flynn M., Van de Heyning P. The influence of different speech processor and hearing aid settings on speech perception outcomes in electric acoustic stimulation patients. Ear Hear. 2008;29:76–86. doi: 10.1097/AUD.0b013e31815d6326. [DOI] [PubMed] [Google Scholar]
- 59.Kopelovich J.C., Reiss L.A., Etler C.P. Hearing loss after activation of hearing preservation cochlear implants might Be related to afferent cochlear innervation injury. Otol Neurotol. 2015;36:1035–1044. doi: 10.1097/MAO.0000000000000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rader T., Döge J., Adel Y., Weissgerber T., Baumann U. Place dependent stimulation rates improve pitch perception in cochlear implantees with single-sided deafness. Hear Res. 2016;339:94–103. doi: 10.1016/j.heares.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 61.Campbell L., Bester C., Iseli C. Electrophysiological evidence of the basilar-membrane travelling Wave and frequency place coding of sound in cochlear implant recipients. Audiol Neurootol. 2017;22:180–189. doi: 10.1159/000478692. [DOI] [PubMed] [Google Scholar]