Abstract
Objective
To describe the procedure and results of an adapted closure and reconstruction technique for translabyrinthine surgery that focuses on identifying and managing potential pathways for CSF egress to the middle ear and Eustachian tube.
Methods
Retrospective review of a cohort of translabyrinthine acoustic neuroma cases that were reconstructed using this technique.
Results
In addition to meticulous packing of potential conduits using soft tissue, hydroxyapatite cement is used to seal opened air cell tracts prior to obliteration of the mastoid defect using adipose tissue. Early results of a small patient cohort using this technique are encouraging and there were no wound infections. There was a single case of CSF rhinorrhea associated with incomplete sealing of opened petrous apex cells, with no recurrence after appropriate implementation of the described protocol during revision surgery.
Conclusion
Proactive management of potential conduits of CSF egress including opened air cell tracts has a high likelihood of reducing rates of rhinorrhea and need for revision surgery after the translabyrinthine approach to the posterior fossa.
Keywords: Translabyrinthine approach, Cerebrospinal fluid leak, Rhinorrhea, Air cell tract, Petrous apex, Eustachian tube, Hydroxyapatite cement
Introduction
Developed and popularized by House1 in the 1960s, the translabyrinthine approach for vestibular schwannoma resection is a direct route through the temporal bone with access to the entire internal auditory canal (IAC) and adjacent cerebellopontine angle without need for brain retraction. One of the most common complications of this surgery has been postoperative leakage of cerebrospinal fluid (CSF) with consequences that include prolonged hospital stay, need for re-operation, and meningitis. Manifesting primarily as rhinorrhea or rarely otorrhea, incomplete re-establishment of a watertight seal at the skull base can also lead to CSF collections in the temporal wound that can either remain self-contained and sterile or leak through the incision line. Key elements of the reconstruction technique that have incrementally lowered the incidence of this complication over many years have included the use of abdominal fat instead of temporalis muscle to close the skull base defect,2 and layered wound closure and pressure dressing.3 Whereas post-auricular collections and leaks can be prevented or addressed with appropriate mastoid packing and wound closure, these methods are not sufficient to prevent rhinorrhea, which results from the flow of CSF through mastoid and skull base defects to the middle ear and Eustachian tube.
Tamura et al4 have described air cell tracts that directly connect the petrous apex to the antrum, middle ear, and Eustachian tube as likely pathways of CSF egress following petrosectomy. The failure to identify and/or seal these potential CSF conduits is likely responsible for the 6.5% CSF rhinorrhea rate reported in a meta-analysis on over 3000 patients with vestibular schwannoma who under went translabyrinthine surgery.5 A number of techniques have been proposed to reduce the likelihood of CSF leak, yet, to quote Merkus et al6 in their report, “The ultimate method to avoid a CSF leak has not been published yet”. A major point of distinction between the various approaches is the detailed attention to obstructing potential pathways of CSF transmission at their origin within the skull base defect, versus obliteration and closure of distal sites including the middle ear, external meatus, and Eustachian tube.
Merkus et al6 have described a meticulous approach to obstructing these pathways where they are opened within the surgical defect, thereby achieving proximal control of CSF flow into the middle ear space or Eustachian tube lumen from petrous apex, perilabyrinthine, and other cell tracts or openings. They have reported excellent results after careful obliteration of air cells with bone wax, and packing of the middle ear space with fascia. Hydroxyapatite has been used since the early 1990s for cranioplasty and skull base reconstruction including following translabyrinthine surgery.7 Its superiority over bone wax in sealing bony defects and cell tracts in the petrous apex has resulted in a marked reduction in CSF leaks associated with the retrosigmoid approach.8
The aim of this paper is to describe an adaptation of the method described by Merkus et al6 using tissue grafts and hydroxyapatite to carefully obliterate all air cell tracts of the petrous apex, mastoid, attic, and root of zygoma prior to dural closure and mastoid obliteration with abdominal fat. Though the efficacy of this approach has been previously demonstrated,6,7 the primary goal of this paper is to provide greater and more specific technical details based on the authors’ modification of published methods, as well as early results.
Reconstruction procedure
Harvest tissue that will be used to seal bony openings that may serve as potential pathways for CSF flow to the middle ear or Eustachian tube. Areolar tissue overlying the temporalis fascia is first harvested followed by the true fascia. It is important to leave a centimeter of intact fascia inferiorly over the muscle to allow for a watertight closure of the deep layer of the wound. These two grafts are pressed for 3–5 min and allowed to dry. Periosteum and sternocleidomastoid muscle and tendon are harvested from the mastoid tip and kept moist until needed. Abdominal fat is harvested in preparation for mastoid obliteration.
The reconstruction phase of the translabyrinthine approach starts with a systematic and thorough survey of all bony areas from the root of zygoma to petrous apex, for any “at-risk” cell tracts or opening that may convey CSF to the middle ear or Eustachian tube (Fig. 1). This process consists of the following steps: 1. Exenteration of at-risk air cells; 2. Soft tissue packing of bony openings and remaining air cell tracts; 3. Application of fascial grafts over the aditus ad antrum and IAC defect; 4. Careful application of small aliquots of freshly made hydroxyapatite cement and resurfacing over open air cells; 5. Packing of mastoid cavity with strips of fat; 6. Layered incision closure with or without Titanium mesh. These steps are facilitated by first achieving and maintaining good hemostasis. Closure or narrowing of the dural defect ahead of time also limits the volume of CSF flow into the mastoid field while shielding the intracranial compartment. Additional details follow:
-
1.
Remove all air cell tracts along the tegmen leaving only cortical bone or exposed dura from the superior petrosal sinus to the root of zygoma. Remaining air cells in the retrofacial region and inferior to the external auditory canal may also serve as conduits to the middle ear and should be exenterated or as described in the following steps, sealed off with soft tissue packing and hydroxyapatite cement.
-
2.
At the root of zygoma larger air cells are packed with soft tissue (Fig. 1A and B). Attention is then turned to the attic, which is tightly packed with soft tissue around the ossicles. Removal of the incus may be considered with care not to disarticulate the stapes thereby creating an additional pathway for CSF communication through the oval window. As pieces of periosteum, fascia, and tendinous tissue are packed into the attic and mesotympanum, note is taken of any egress of fluid from adjacent cell tracts such as the facial recess, as these may serve as conduits for CSF flow to the middle ear if not addressed. If large enough, these cells should be packed with small pieces of soft tissue using a blunt instrument such as the annulus elevator. They will then be sealed with limited application of hydroxyapatite cement in the next step. The vestibule is lightly packed with soft tissue to prevent leakage through the oval window (Fig. 1C). Any air cells in the petrous apex are likewise packed with soft tissue. Pressed absorbable gelatin sponge (Gelfoam) is placed over the facial nerve within the internal auditory canal and porusacusticus for additional protection against contact with hydroxyapatite in the next step. Pressed fascial grafts are draped over the IAC (Figs. 1D and 2) and the aditus ad antrum.
-
3.
Now that cell tracts have been identified and packed with soft tissue, they must also be capped with bone cement, which has a better chance of infiltrating and sealing remaining small openings than does bone wax. The precise and controlled application of small amounts of hydroxyapatite cement is best accomplished using a syringe with catheter of sufficient length. Authors have primarily used Hydroset by Stryker Corporation, with which these implements are provided. The administration of small amounts of material at appropriate locations followed by local resurfacing using a Freer elevator should be accomplished while still soft and within 2–3 min of mixing the ingredients. We start medially with administration at the openings of the petrous apex cells and/or at the superior and inferior edges of the fascia graft overlying the IAC defect to help keep it in place (Fig. 3A). Moving laterally cement is applied to any remaining inferior and retrofacial air cell tracts (Fig. 3B), and to the root of zygoma (Fig. 3C). A Freer elevator or similar instrument works well to distribute the cement over the openings of all cell tracts at each at-risk site (Fig. 3D). The cement is then given the appropriate time to cure according to the manufacturer's instructions before filling the cavity with abdominal fat.
-
4.
When possible, the dural defect is narrowed by primary closure of the dural flaps. The remaining opening is either covered by a layer of fascia, or is plugged with the shallow insertion of a strip of fat in a dumbbell fashion.9 The abdominal fat is then placed in strips within the mastoid cavity, completely filling the defect to the rim of the mastoid cavity.
-
5.
Attention is then turned to wound closure with or without Titanium cranioplasty. If a cranioplasty is desired, thin Titanium mesh is fashioned to cover the defect. It is important that the mesh does not protrude anteriorly through the ear canal skin. It is also helpful to preserve a rim of cortical bone at the mastoid tip to secure the mesh inferiorly (Fig. 4). The wound is then closed using a watertight closure of the periosteal/fascia layer followed by tight closure of the subcutaneous layer and a third layer of closure for the skin. A firm mastoid dressing is applied.
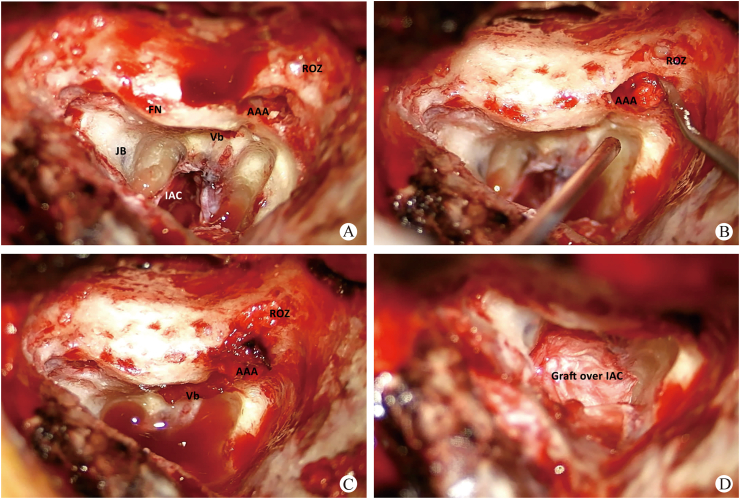
Fig. 1.
Soft tissue is used to pack potential conduits of CSF drainage. A: The surgical defect following translabyrinthine approach to the IAC. Cortical bone and exposed dura remain along the tegmen; open air cells and aditus ad antrum (AAA) remain as potential paths for CSF to the middle ear. B,C: Soft tissue is used to pack the attic (medial and lateral to the ossicles), open air cells at the root of the zygoma (ROZ) and the vestibule (Vb). D: After petrous cells are packed, pressed Gelfoam is placed over remaining contents of the IAC and fascial graft is draped over the bony defect (FN: facial nerve; JB: jugular bulb).
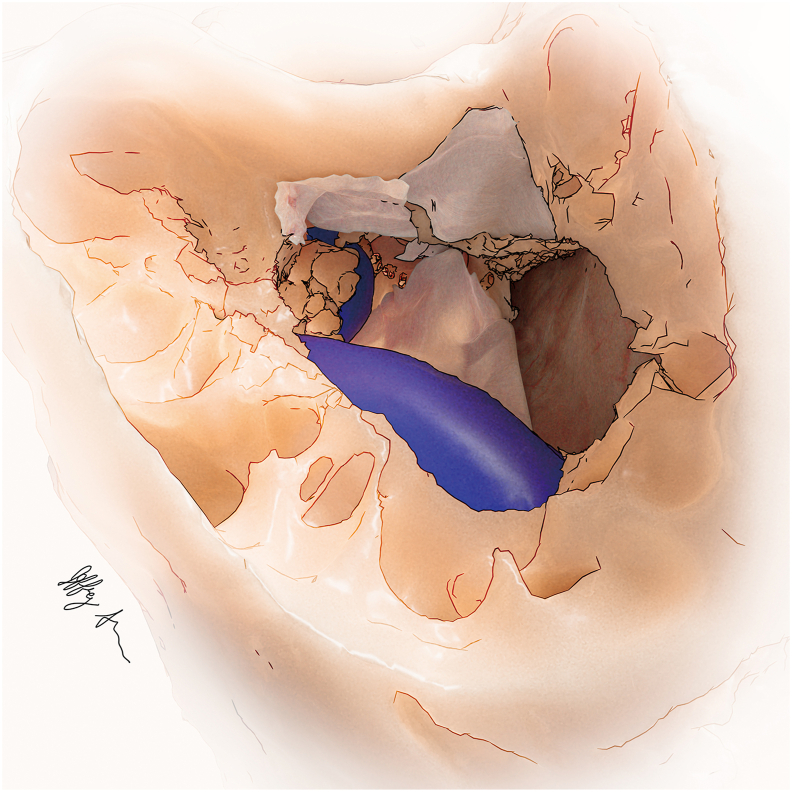
Fig. 2.
Illustration of fascial grafts overlying the packed aditus ad antrum and Gelfoam-lined IAC. Soft tissue has been used to pack the vestibule and open air cells in the retrofacial area.
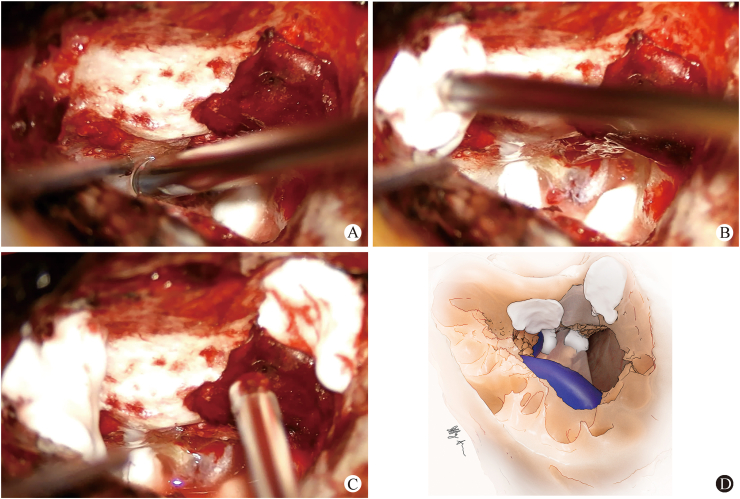
Fig. 3.
Small applications of hydroxyapatite cement to A: The petrous apex and/or at the superior and inferior edges of the fascial graft overlying the IAC; B: Inferior and retrofacial cell tracts; C: The root of zygoma; D: Illustration of typical hydroxyapatite placement after resurfacing with Freer elevator.
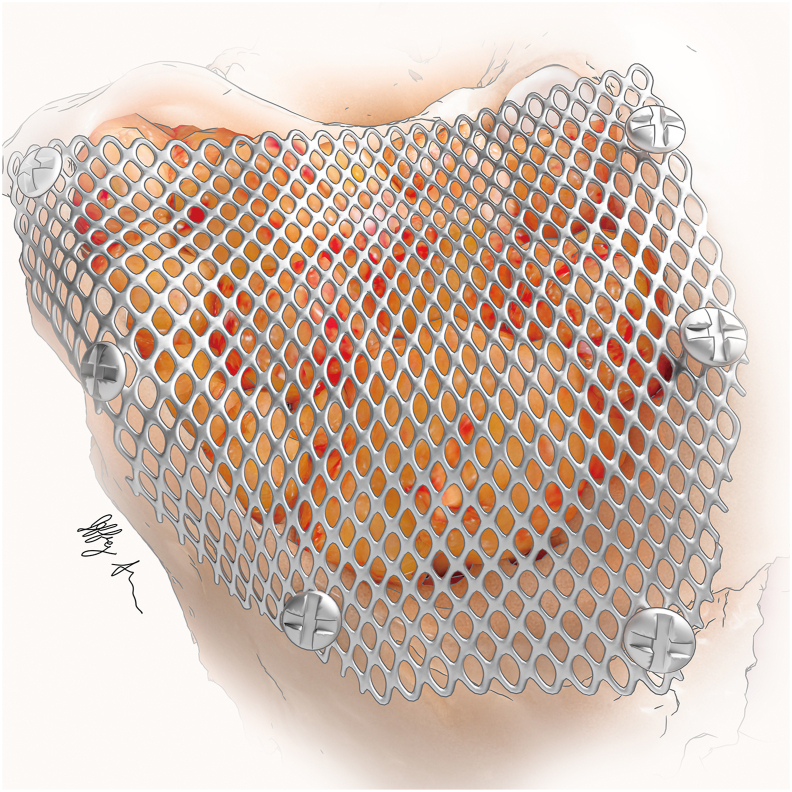
Fig. 4.
The mastoid cavity has been obliterated with adipose tissue and a cranioplasty performed with titanium mesh and screws prior to multi-layered wound closure.
Methods
Arestrospective review was conducted of cases performed using the described technique at Duke University, approved for exemption by the institutional review board (Pro00105223). This approval included a Data Transfer Agreement with Johns Hopkins University where a retrospective review was also approved by the institutional review board (IRB00244722). Data were collected on thirteen patients from Johns Hopkins and ten patients at Duke who all underwent surgery with the senior author using the presented technique between the years of 2014 and 2020.
Results
A total of 23 patients were identified who underwent translabyrinthine approach for removal of vestibular schwannoma with subsequent closure using the presented technique. Fourteen patients were female and 9 were male. The mean age at surgery was 57 years with a range from 39 to 74, and the mean follow up was 21 months. The tumor was located on the left side for 12 patients and the right side for 11 patients. There were no occurrences of wound infection. One of the 23 patients had a postoperative course complicated by CSF rhinorrhea (4.3% rate). At revision surgery, patent air cells were noted in the petrous apex that had not been adequately packed with soft tissue or resurfaced with hydroxyapatite cement possibly providing a route for CSF drainage to the middle ear. The incus was also removed, and the attic and mesotympanum were carefully repacked with fascia strips. All exposed air cells were resurfaced with hydroxyapatite cement and the mastoid cavity was re-packed with adipose tissue. There was a full resolution of the CSF rhinorrhea.
Discussion
The past 60 years has seen a progression ofthe translabyrinthine approach, particularly the technique for reconstruction and closure. As new ideas and techniques have been described in the literature, CSF leak rates have continued to decline, but there continue to be patients who have this costly complication. The successful use of hydroxyapatite cement inretrosigmoid cases to seal open cell tracts in the petrous apex has influenced the evolution of our approach to skull base reconstruction in translabyrinthine cases.8 The 5-fold reduction of CSF rhinorrhea in retrosigmoid cases using this reconstruction technique also suggests the likely role of the petrous apex as a source of CSF rhinorrhea in translabyrinthine procedures, which is further supported by the work of Tamura et al.4 The efficacy of hydroxyapatite cement as a sealant of these surgically opened cell tracts has also influenced our approach to the management of the petrous apex and other potential conduits originating in the petrosectomy defect. Though we do not assess this here, it is our general experience that Titanium cranioplastyas proposed by Fayad et al,10 appears to reduce CSF collections and incisional leaksbut has minimal impact on the rate of rhinorrhea.
The systematic approach to packing and sealing all potential pathways as described by Merkus et al6 provides a logical and reproducible technique associated with a less than 1% leak rate in their hands, even with the use of bone wax. This low rate has not, however, been easily reproduced by others. Hydroxyapatite cement has demonstrated superior performance over bone wax as a sealant of petrous apex cells opened during the retrosigmoid approach.8 Its use may therefore produce more reliable CSF leak control across multiple groups, and we therefore advocate its use as a modification of the technique described by Merkus et al.6
Hydroxyapatite cement has been shown to maintain stability over a full two years,12 and over time it can undergo osseointegration and remodeling that results in its complete replacement by new bone.13 These properties make it an excellent material for reconstruction producing physical integrity similar to that of bone within hours after surgery, without weakening over time. The wound must be monitored for signs of infection however, and if suspected, the cement should be removed. In their large series of translabyrinthine patients that underwent cranioplasty using hydroxyapatite cement, Volsky et al7 report a 1.9% CSF leak rate and identical wound infection rate at a median of 3.6 years. In cases of infection, the hydroxyapatite was drilled away without the de-novo occurrence of CSF leaks. Kveton and Coelho11 reported successful repair of skull base defects in 97% of 109 cases and a 2.8% rate of infection.
A single case of rhinorrhea in our small series of patients undergoing this approach likely resulted from inadequate attention to sealing off open petrous apex cells, and may also point to the potential benefit of removing the incus to accomplish a more thorough packing of the attic and middle ear. Due to the small cohort of only twenty three patients in this study, one patient with postoperative rhinorrhea resulted in a 4.3% rate of CSF rhinorrhea. We acknowledge that the efficacy over other series is not shown by this report, but we emphasize the technical steps and principles needed to approach the favorable outcomes previously reported by Merkus et al6 and Volsky et al.7 This paper does not address CSF collections or leakage associated with the temporal wound and incision. Factors leading to this distinct category of post-operative CSF fistula include firmness and stability of adipose packing aided by placement of Titanium mesh to apply constant pressure against the dural defect; water-tight, multilayered closure of temporal incision; and management of intracranial pressure.6,10
Conclusion
A systematic approach to sealing bony conduits for CSF egress leading to post-operative rhinorrhea is advocated during the reconstructive phase of the translabyrinthine operation. With meticulous attention to identifying and eliminating or obstructing these potential pathways with the appropriate materials, CSF rhinorrhea can become a rare complication of this surgical workhorse.
Author's contribution
Matthew W Cooper: Writing – Data Curation; Original draft,
Bryan K Ward: Data Curation; Writing – Review & Editing,
Jeffery Sharon: Visualization,
Howard W Francis: Conceptualization, Writing – Review & Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have None.
Edited by Yi Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.House W.F. Translabyrinthine approach. In: House W.F., Leutje C.M., editors. vol. II. University Park Press; Management. Baltimore, MD: 1979. pp. 43–87. (Acoustic Tumors). [Google Scholar]
- 2.Montgomery W.W. Translabyrinthine resection of the small acoustic neuroma. Arch Otolaryngol. 1969;89:319–325. doi: 10.1001/archotol.1969.00770020321017. [DOI] [PubMed] [Google Scholar]
- 3.Tos M., Thomsen J. Cerebrospinal fluid leak after translabyrinthine surgery for acoustic neuroma. Laryngoscope. 1985;95:351–354. doi: 10.1288/00005537-198503000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Tamura R., Tomio R., Mohammad F., Toda M., Yoshida K. Analysis of various tracts of mastoid air cells related to CSF leak after the anterior transpetrosal approach. J Neurosurg. 2018;130:360–367. doi: 10.3171/2017.9.JNS171622. [DOI] [PubMed] [Google Scholar]
- 5.Selesnick S.H., Liu J.C., Jen A., Newman J. The incidence of cerebrospinal fluid leak after vestibular schwannoma surgery. Otol Neurotol. 2004;25:387–393. doi: 10.1097/00129492-200405000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Merkus P., Taibah A., Sequino G., Sanna M. Less than 1% cerebrospinal fluid leakage in 1,803 translabyrinthine vestibular schwannoma surgery cases. Otol Neurotol. 2010;31:276–283. doi: 10.1097/MAO.0b013e3181cc06ad. [DOI] [PubMed] [Google Scholar]
- 7.Volsky P.G., Hillman T.A., Stromberg K.J. Hydroxyapatite cement cranioplasty following translabyrinthine approach: long-term study of 369 cases. Laryngoscope. 2017;127:2120–2125. doi: 10.1002/lary.26403. [DOI] [PubMed] [Google Scholar]
- 8.Baird C.J., Hdeib A., Suk I. Reduction of cerebrospinal fluid rhinorrhea after vestibular schwannoma surgery by reconstruction of the drilled porus acusticus with hydroxyapatite bone cement. J Neurosurg. 2007;107:347–351. doi: 10.3171/JNS-07/08/0347. [DOI] [PubMed] [Google Scholar]
- 9.Goddard J.C., Oliver E.R., Lamber P.R. Prevention of cerebrospinal fluid leak after translabyrinthine approach for vestibular schwannoma. OtolNeurotol. 2010;31:473–477. doi: 10.1097/MAO.0b013e3181cdd8fc. [DOI] [PubMed] [Google Scholar]
- 10.Fayad J.N., Schwartz M.S., Slattery W.H., Brackmann D.E. Prevention and treatment of cerebrospinal fluid leak after translabyrinthine acoustic tumor removal. Otol Neurotol. 2007;28:387–390. doi: 10.1097/01.mao.0000265188.22345.d4. [DOI] [PubMed] [Google Scholar]
- 11.Kveton J.F., Coelho D.H. Hydroxyapatite cement in temporal bone surgery: a 10 year experience. Laryngoscope. 2004;114:33–37. doi: 10.1097/00005537-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Kveton J.F., Friedman C.D., Costantino P.D. Indications for hydroxyapatite cement reconstruction in lateral skull base surgery. Am J Otol. 1995;16:465–469. [PubMed] [Google Scholar]
- 13.Costantino P.D., Friedman C.D., Jones K., Chow L.C., Pelzer H.J., Sr S.G.A. Hydroxyapatite cement. I. Basic chemistry and histologic properties. Arch Otolaryngol Head Neck Surg. 1991;117:379–384. doi: 10.1001/archotol.1991.01870160033004. [DOI] [PubMed] [Google Scholar]