Abstract
Cerebrospinal fluid (CSF) fistulae originating from the fallopian canal of the facial nerve is hypothesized to arise due to atypical patterns of subarachnoid space extension into the geniculate ganglion or more distal regions along the intratemporal course of the facial nerve, but its pathogenesis remains poorly understood. Although a rare etiology of CSF fistulae of the temporal bone, there are significant clinical ramifications due to the risk of recurrent meningitis, difficulty in identifying the anatomic location of the CSF leak, and technical challenges associated with surgical repair. We present three clinical cases of arachnoid cysts within the geniculate fossa with or without CSF fistulization and provide histopathologic correlates of this rare clinical phenomenon from a human temporal bone collection. The pediatric and adult patients presented suggest differential pathophysiologic mechanisms associated with CSF fistulae. Temporal bone histology reveals atypical patterns of subarachnoid space extension in the fallopian canal that may underlie arachnoid cyst formation and overt CSF leak from the geniculate region.
Keywords: Geniculate ganglion, Arachnoid cyst, Fallopian canal, Facial nerve, Cerebrospinal fluid otorrhea, Cerebrospinal fluid leak, Subarachnoid space
Introduction
Due to its location in the skull base and porous architecture, the temporal bone has a predilection for fistulae with the subarachnoid space leading to cerebrospinal fluid (CSF) leakage. Anatomic, physiologic, and congenital processes such as incomplete ossification and elevated intracranial pressure most commonly underlie the development of CSF fistulae. Anomalous placement of arachnoid mater and associated granulations in the lateral skull base can also lead to cerebrospinal fistulae and meningoencephaloceles of the temporal bone.1 Dilation of ectopic arachnoid granulations, possibly facilitated by the cumulative effects of sustained or intermittently elevated intracranial pressures, lead to osseous erosion of the temporal bone, which has been previously studied in histologic specimens.2,3 Intracranial borders of the temporal bone with pre-existing thin bone, such as the tegmen tympani, are the most common sites of occurrence.2,4, 5, 6
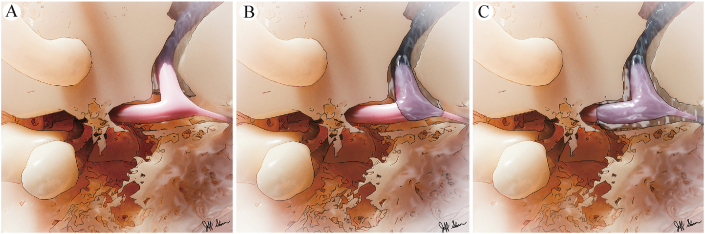
A much rarer phenomenon is the CSF leak that originates from the fallopian canal within the temporal bone, which has only occasionally been described in case reports and small case series.2,7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 Arachnoid cysts of the geniculate fossa have also been rarely reported, both with and without CSF leaks.19 As the facial nerve pierces the meninges at the meatal foramen of the labyrinthine segment, a fibrous ring tightly seals around the nerve to prevent CSF egress into the peripheral nerve sheath.1 The work by Gacek7 describing variations of arachnoid extension distal to the meatal foramen, provides a basis for understanding how arachnoid cysts and CSF leaks in the fallopian canal, and particularly the perigeniculate region, may arise. Type I represents the most common finding where the subarachnoid space terminates proximal to the geniculate fossa within the labyrinthine segment of the fallopian canal (Fig. 1). Distal extension of the subarachnoid space into the substance of the geniculate ganglion characterizes type II, whereas extension beyond the lateral surface of the geniculate ganglion into the tympanic segment defines type III. Therefore, variations in arachnoid placement may serve as a risk factor for the development of arachnoid cysts within the geniculate fossa, with potential consequences that include CSF fistulization into adjacent cell tracts or the middle ear space, and disruption of adjacent labyrinthine structures with expansion.
Fig. 1.
Illustration of three patterns of subarachnoid space extension. A: Type I, subarachnoid space limited to the termination of the petrosal fallopian canal. B: Type II, SAS extension into the substance of the geniculate ganglion and surrounding nerve fascicles. C: Type III, extension into the tympanic segment of the fallopian canal.
Limited by both the rarity of this clinical phenomenon and availability of post-mortem temporal bone histopathology, the relationship between arachnoid cysts and CSF fistulae of the perigeniculate region and histologic patterns of arachnoid mater extension have not been studied since Gacek’s initial description. In this report, we present three clinical cases of arachnoid cysts associated with the geniculate ganglion, and conduct a histologic survey of the Johns Hopkins Temporal Bone collection for potential histopathologic correlates. By adding to a limited repertoire of published cases, we aim to enhance our cumulative understanding of how these rare cases may present clinically, and to further examine the spectrum of histopathologic features in the peri-genicular region that may predispose to them.
Methods
We present three case reports that detail the clinical findings in patients with lesions of the geniculate ganglion that are consistent with arachnoid cysts. We then present observations made in a temporal bone survey focused on studying the patterns of arachnoid mater and extension of the subarachnoid space within the fallopian canal. Temporal bones used in this study are part of the Johns Hopkins Temporal Bone Collection, which was established by Dr. Samuel Crowe and Dr. Stacy Guild in 1924 and expanded by Dr. George Nager.21,22 Details about the harvesting and processing of temporal bones in our collection have been reported previously.2,23 In brief, temporal bones were vertically sectioned at a slice thickness of 24 μm; every tenth section was mounted and stained with hematoxylin and eosin. Autopsy records and demographic data were available for some but not all specimens in these collections. The specimens selected for this study were part of a previous histologic survey for anomalies of the middle fossa skull base in over 300 temporal bones.2
Selected specimens were examined using light microscopy at 2.5× magnification for patterns of extension of arachnoid mater and the subarachnoid space into the labyrinthine, geniculate, and tympanic segments of fallopian canal. Gacek7 defined the subarachnoid space as one that is lined by nucleated cells forming a trabecular network sometimes containing characteristic psammoma bodies and nucleated cells, and in continuity with the subarachnoid space of the IAC. In the present study however, limitations in temporal bone processing rendered this impractical. For instance, cell nuclei could not be consistently visualized due to limits of H&E staining, and contiguity of the subarachnoid space was interrupted by staining of every 10th section. Therefore, for the purposes of this study, we defined subarachnoid space extension to the geniculate fossa by either a demonstrable contiguity with the subarachnoid space of the IAC, or the splitting of geniculate fascicles by a space often containing a trabecular pattern. Trabecular spaces that surround the geniculate ganglion without penetrating its substance and without confirmed continuity with the IAC subarachnoid space were not categorized as an arachnoid extension as they could also arise as the result of tissue shrinkage during processing.
Sections demonstrating atypical arachnoid patterns at the geniculate ganglion were categorized as type I (subarachnoid space limited to the termination of the labyrinthine fallopian canal), type II (extension into the substance of the geniculate ganglion and surrounding nerve fascicles), or type III (extension lateral to the geniculate into tympanic facial nerve segment) patterns in accordance with Gacek’s classification scheme7 (Fig. 1). Serial sections were captured with an Optronics digital camera (Optronics Corp., Goleta, CA) affixed to the microscope (Eclipse E600, Nikon Corp., Tokyo, Japan). Photomicrographs of the contralateral ear of each specimen were taken and served as negative controls. Where available, demographic and clinical data were extracted from records.
Results
Clinical capsules
Case 1
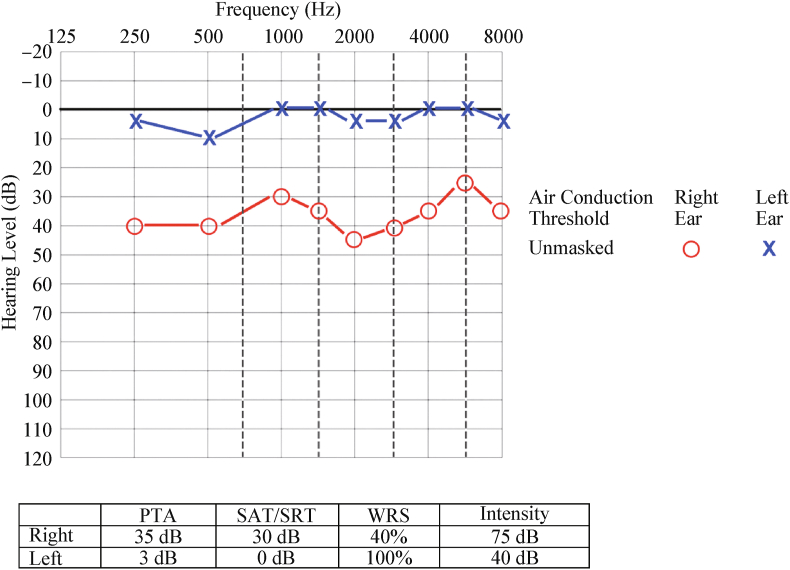
An otherwise healthy 12 year-old male (BMI 16.6) presented for evaluation of a complaint of right hearing loss and failed hearing screenings after passing a newborn hearing screen. Tympanic membranes showed normal compliance and middle ear pressure and distortion product otoacoustic emissions were normal bilaterally. Audiometry revealed mild to moderate sensorineural right hearing loss with disproportionately poor speech discrimination on the right and normal hearing on the left (Fig. 2). Initial magnetic resonance imaging (MRI) of the brain and skull base demonstrated, in the right geniculate ganglion region, a cystic lesion overlying the cochlea and extending into the middle ear. This lesion was T1 hypointense, T2 hyperintense, without diffusion restriction, and non-enhancing with administration of Gadolinium contrast (Fig. 3). Subsequent non-contrast temporal bone CT confirmed a right-sided expansile lesion at the right geniculate fossa with expansion of the tympanic and labyrinthine segments of the fallopian canal (Fig. 3). There was also dehiscence of the anterior crus of the superior semicircular canal (SCC) and basal turn of the cochlea.
Fig. 2.
Audiogram. Twelve year-old girl presented with right sided asymmetric sensorineural hearing loss over several years, with speech discrimination of 40% and 100% in the right and left ears, respectively.
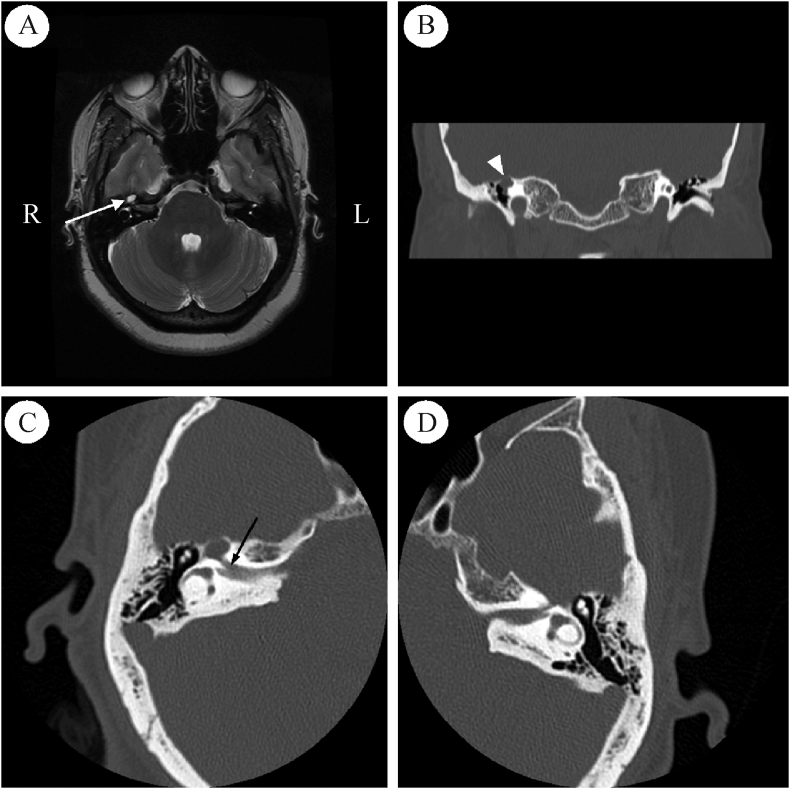
Fig. 3.
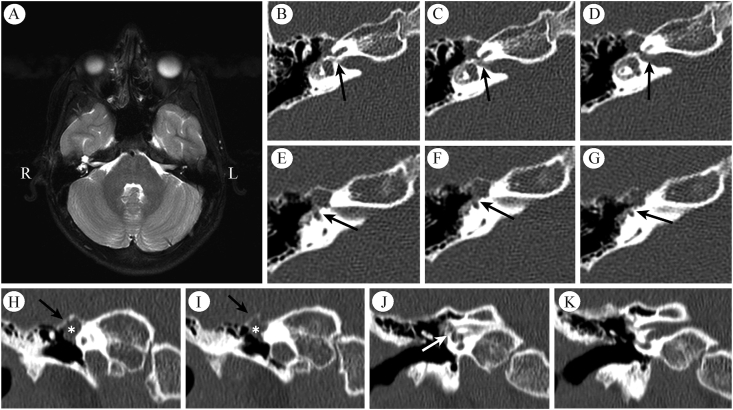
Case 1 preoperative imaging. A: Magnetic resonance imaging demonstrates a T2-hyperintense, T1-hypointense, and non-contrast enhancing cystic lesion of the right geniculate ganglion. B-D: Serial axial temporal bone computed tomography views demonstrate expanded labyrinthine segment of the fallopian canal (arrows) with cochlear dehiscence and (E-G) dehiscence of the ampullated end of the superior semicircular canal. H–K: Coronal slices demonstrate intact middle fossa floor (black arrows) and an expansile lesion intrinsic to the geniculate ganglion (∗) with possible cochlear dehiscence (white arrow).
The patient underwent transcanal middle ear exploration which revealed a fluid-filled cyst (Fig. 4). Intraoperative aspiration of the cystic fluid demonstrated beta-2 transferrin positivity. The middle ear component of the cyst was excised and facial function remained intact post-operatively. Due to ongoing risk of meningitis and a desire to restore hearing, the patient underwent right partial labyrinthectomy, CSF leak closure and cochlear implantation. Intraoperatively, a recurrence of clear fluid emanating from the region of the geniculate ganglion and fallopian canal was noted, as was dehiscence of the bone overlying the tympanic segment of the facial nerve and destruction of portions of the superior and lateral semicircular canals. The otic capsule was sealed with bone dust/Tisseel, the contents of the middle ear and canal skin removed, and a right cochlear implant placed. Using abdominal fat, the middle ear and external auditory canal were obliterated and the meatus was closed. The patient has since (10 months prior at the time of writing) recovered without complications.
Fig. 4.
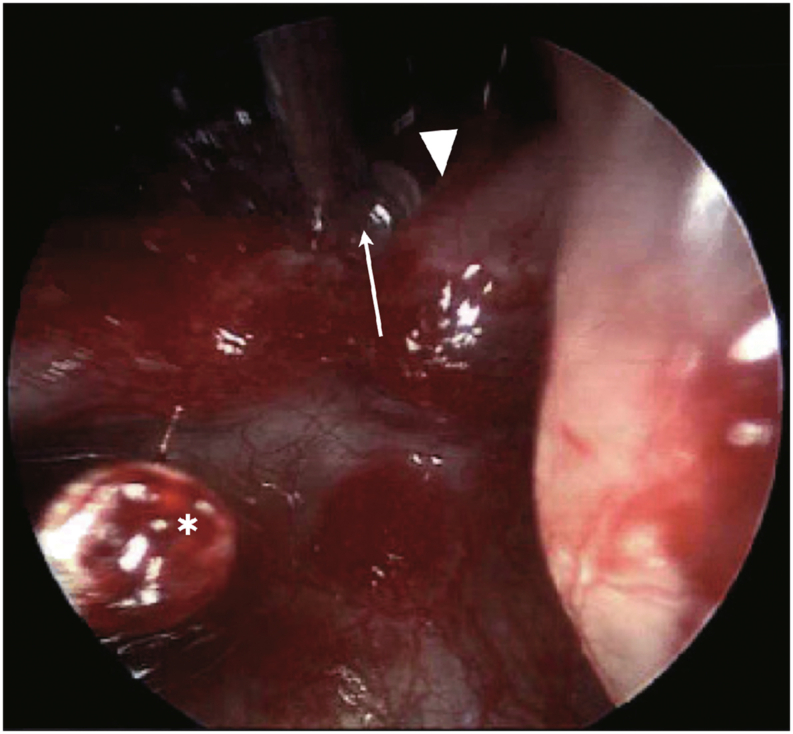
Case 1 intraoperative view. Intraoperative image of transcanal approach to perigeniculate arachnoid cyst following removal of the incus, demonstrating the malleus, stapes capitulum (∗), cochleariform process (arrowhead), and a clear fluid-filled cyst (arrow) in the regions just distal to the geniculate ganglion.
Case 2
A six year-old female with a history of Kawasaki’s disease originally presented with two prior episodes of meningitis due to Streptococcus pneumonia. Temporal bone CT demonstrated an abnormally enlarged geniculate fossa on the right without evidence of bony erosion. Gradient echo MR sequence demonstrated T2 hyperintense fluid signal in the geniculate ganglion consistent with CSF. This was favored to represent an extension of the subarachnoid space resulting in an arachnoid cyst of the geniculate ganglion (Fig. 5). Audiometry revealed a moderate sensorineural hearing loss in the right ear. Due to the risk of recurrent meningitis, the patient underwent a subtotal petrosectomy with a blind sac closure of the EAC (Fig. 6). Intraoperatively, geniculate expansion was noted to cause a cochlear dehiscence. Since then (15 months prior at the time of writing), the patient has done well, with no further episodes of meningitis nor CSF leakage.
Fig. 5.
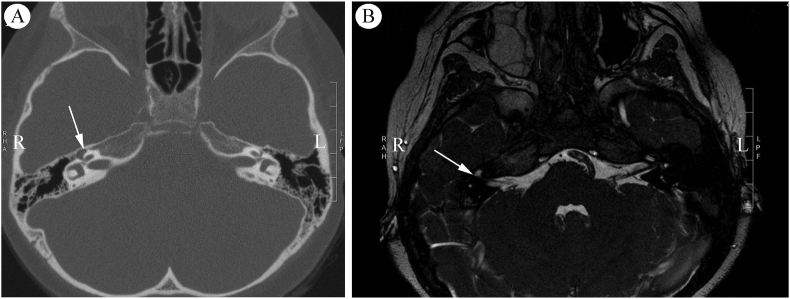
Case 2 pre-operative Imaging. A: Axial temporal bone computed tomography demonstrates a smoothly dilated right geniculate fossa (arrow). B: Magnetic resonance imaging shows a fluid signal at the geniculate ganglion suspicious for CSF accumulation (arrow).
Fig. 6.
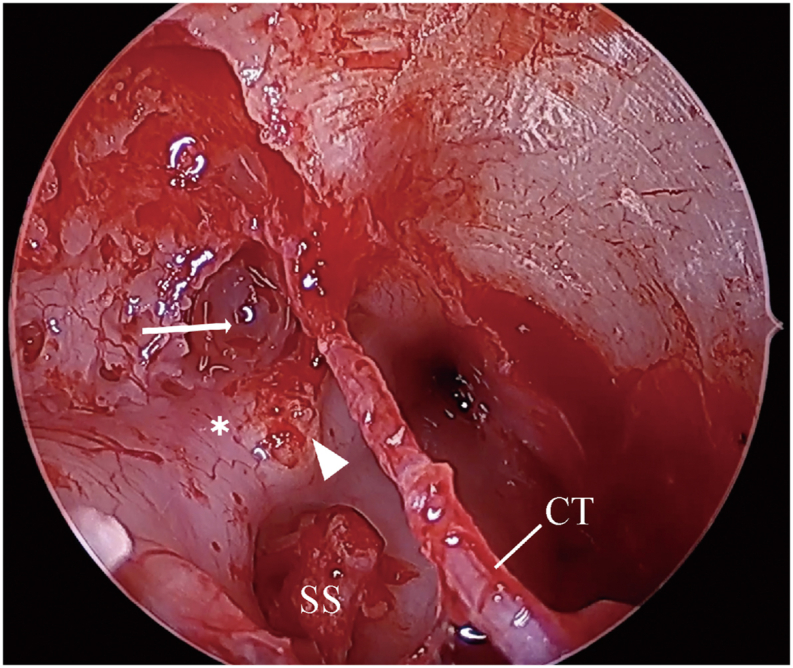
Case 2 intraoperative view. Intraoperative image demonstrating CSF accumulation at the geniculate region (arrow), tympanic segment of facial nerve (∗), cochleariform process (arrowhead), chorda tympani (CT), and stapes superstructure (SS).
Case 3
A 45 year-old woman (BMI 27) presented with dizziness of one-year duration. MRI of the brain and skull base revealed a 5 × 3 mm cystic lesion corresponding to the location of the right geniculate ganglion. The lesion was hypointense on T1, hyperintense on T2, without diffusion restriction, and non-enhancing with administration of Gadolinium contrast. Labyrinthine and IAC fluid spaces appeared grossly intact. Notably, MRI also demonstrated signs of intracranial hypertension, including flattened pituitary gland along the floor of sella turcica and prominent CSF signal within the optic sheath. Subsequent temporal bone CT without contrast revealed a hypodense lesion located along the superior aspect of the right geniculate ganglion with scalloped bone (Fig. 7). Her physical exam revealed normal facial function but was notable for a few beats of nystagmus on positional testing with Dix-Hallpike maneuver to each side, consistent with bilateral posterior semicircular canal BPPV. There was no evidence of CSF leak or meningitis, and audiometry revealed thresholds within normal limits. The patient was treated with vestibular physical therapy for positional vertigo (10 months prior at the time of writing) and is undergoing monitoring of the cystic geniculate lesion with serial MRIs without clinical evidence of CSF leak or meningitis.
Fig. 7.
Case 3 imaging. A: Magnetic resonance imaging shows T2-hyperintensity at the right geniculate ganglion indicative of a cystic lesion (arrow). Fluid in the optic nerve sheath complex and empty sella (not pictured) is suggestive of intracranial hypertension. B: Coronal temporal bone computed tomography shows erosion (arrowhead) of the middle fossa floor overlying the geniculate ganglion. C: Right axial CT is notable for an enlarged labyrinthine segment of the fallopian canal (arrow) without involvement of the cochlea. D: Contralateral left-sided axial CT with uninvolved labyrinthine segment for comparison.
Temporal bone histology
Eleven temporal bones from 11 unique individuals were identified on previous review of 300 specimens,2 as having atypical findings in the geniculate ganglion region, and were further specifically examined for patterns of arachnoid extension for this study. Table 1 shows demographic data, where available, of these donors, recorded cause of death on autopsy, and corresponding histopathologic findings in the geniculate region. Four specimens did not have demographic or autopsy data. Type I and II arachnoid patterns were identified in 3 (27%) and 8 (73%) temporal bones, respectively. No type III arachnoid patterns were identified in our study.
Table 1.
Demographic characteristics of temporal bone donors.
| Patient No. | Specimen No. | Age of death, (yr) | Gender | Race | Laterality | GG dehiscent (Y/N) | Arachnoid pattern (I/II/III) | Cause of death |
|---|---|---|---|---|---|---|---|---|
| 1 | 4748 | 66 | M | White | R | Y | I | Cardiac failure |
| 2 | 10727 | 37 | M | White | L | N | II | Acute hemorrhagic nephritis |
| 3 | 4532 | 47 | M | White | R | Y | II | Meningitis |
| 4 | 4872 | 56 | M | White | R | Y | II | Ruptured aortic aneurysm |
| 5 | 4894 | 69 | M | White | L | Y | II | Metastatic rectal carcinoma |
| 6 | 11095 | 47 | M | White | L | N | I | Spongio-blastoma |
| 7 | 12019 | 55 | M | White | L | Y | II | Operative removal of brain tumor |
| 8 | 7827 | – | – | – | L | Y | II | – |
| 9 | 11731 | – | – | – | L | Y | II | – |
| 10 | 11901 | – | – | – | R | Y | II | – |
| 11 | 11999 | – | – | – | L | Y | I/II | – |
–: Demographic information unavailable.
Arachnoid tissue in the geniculate fossa may be influenced by extension from the IAC (intrinsic) or infiltration of temporal dura from a dehiscent middle fossa floor (extrinsic). Varying degrees of bony dehiscence of the middle fossa floor overlying the geniculate ganglion were found in 9/11 (82%) of temporal bone specimens. Fig. 8 shows representative specimens and the spectrum of bony dehiscence observed. In all cases, we found that intrinsic extension of the arachnoid mater into the fallopian canal could be distinguished from extrinsic infiltration from the middle fossa, by contiguity of the subarachnoid space and/or dissection and displacement of neuronal fibers within the substance of the geniculate ganglion.
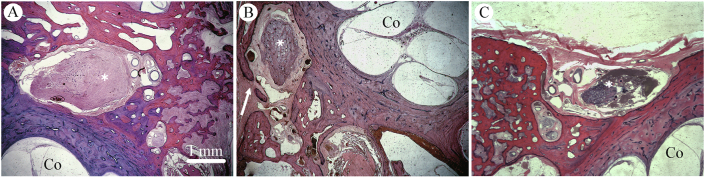
Fig. 8.
Spectrum of bony dehiscence of middle fossa floor overlying the geniculate fossa. Photomicrographs at the level of the geniculate ganglion (∗) demonstrating spectrum of middle fossa architecture in the geniculate region. A: Intact middle fossa floor overlying geniculate fossa. Specimen 11095 L. B: Focal dehiscence (arrow) containing fibromyxoid tissue contiguous with temporal lobe dura. Specimen 4748 R. C: Complete bony dehiscence with intact dura mater. Specimen 7827 L. Original magnification 2.5×. Co, cochlea.
Where available, the contralateral ears served as controls, which uniformly contained type I arachnoid patterns. Fig. 9 shows a representative control ear (specimen 11095 R), demonstrating the lateral extent of the subarachnoid space terminating at approximately the labyrinthine–geniculate junction. The geniculate fossa is occupied by tightly-packed and well-organized neuronal bodies and axons in an epineural sheath without arachnoid infiltration.
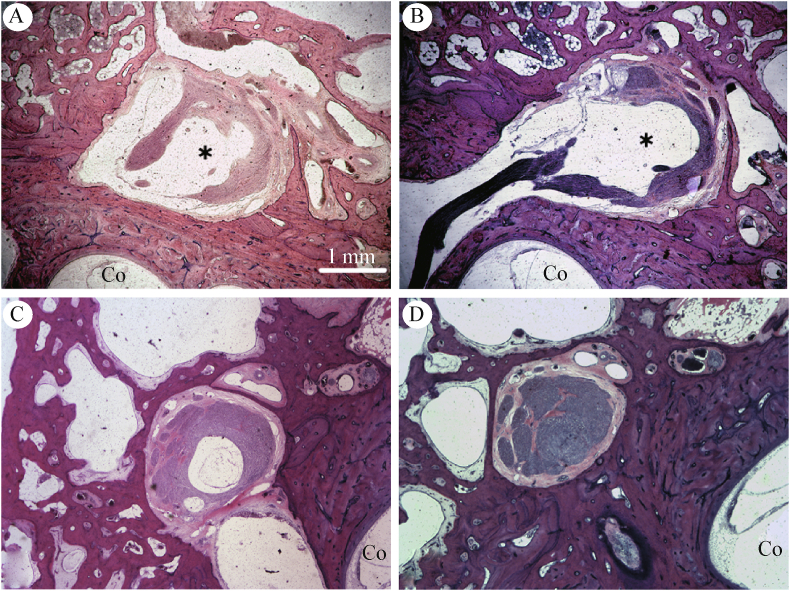
Fig. 9.
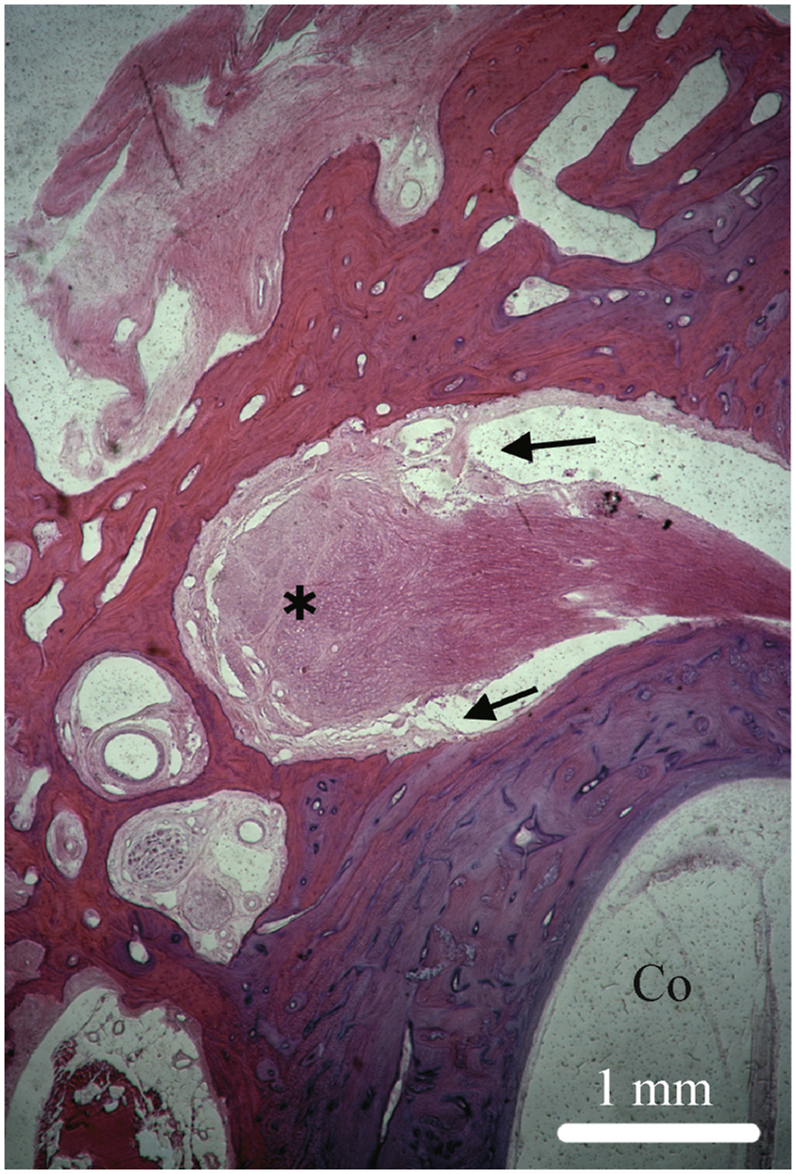
Type I pattern. HE stain of a vertically sectioned temporal bone specimen. Representative photomicrographs depict a type 1 arachnoid pattern over the facial nerve and geniculate ganglion (∗). The subarachnoid space is contiguous from the fallopian canal in the labyrinthine segment to its termination (arrows) at the geniculate ganglion. Note the normal interface between the geniculate, labyrinthine segment of the fallopian canal, and basal turn of the cochlea (Co). Original magnification 2.5×. Specimen 11095 R.
Fig. 10 shows images from a specimen (4532 R) representative of the type II arachnoid extension into the geniculate ganglion. Notably, there is lateral extension of the subarachnoid space into the substance of the geniculate ganglion and dissection of the nerve fascicles (Fig. 10A and B). The ganglion appears displaced by the subarachnoid space. The axonal bundle is reconstituted however in the tympanic segment, without evidence of further subarachnoid extension (Fig. 10C and D). The middle fossa floor is well pneumatized without evidence of bony dehiscence or dural disruption. Interestingly, autopsy records (Table 1) indicate that the patient from which this specimen was obtained suffered from meningitis at time of death. Unfortunately, more detailed clinical data were not available regarding the etiology or clinical presentation of meningitis. Histopathologic examination of the temporal bone specimen however, does not indicate increased cellular infiltration of the fallopian canal, temporal bone, or middle ear to specifically suggest otogenic meningitis.
Fig. 10.
Type II arachnoid pattern. Sequential photomicrographs of HE stained vertical temporal bone sections along the course of the fallopian canal demonstrating a representative type II pattern. A-C: The subarachnoid space does not terminate at the distal extent of the labyrinthine fallopian canal but extends into the substance of the geniculate ganglion (∗) with dissection of nerve fascicles and displacement of the ganglion. D: However, tympanic facial nerve bundle is intact without subarachnoid space extension. Original magnification 2.5×. Co, cochlea. Specimen 4532 R.
Fig. 11 shows representative images from other specimens (4872 R and 11095 L) with type II subarachnoid space extension. In contrast to Fig. 10, specimen 4872 R demonstrates a well-defined fibrous ring at the terminus of the labyrinthine fallopian canal (Fig. 11A). On subsequent slices however, dehiscence in the fibrous ring becomes evident through which the subarachnoid space extends distally into the geniculate ganglion from the IAC (Fig. 11B). Specimen 11095 L not only demonstrates a type II pattern but also osseous communication with adjacent air cells in the geniculate fossa (Fig. 11D). Together, arachnoid extension and consequent CSF ingress into neighboring cell tracts may serve as a putative mechanism by which CSF leaks may occur from the fallopian canal in the geniculate region.
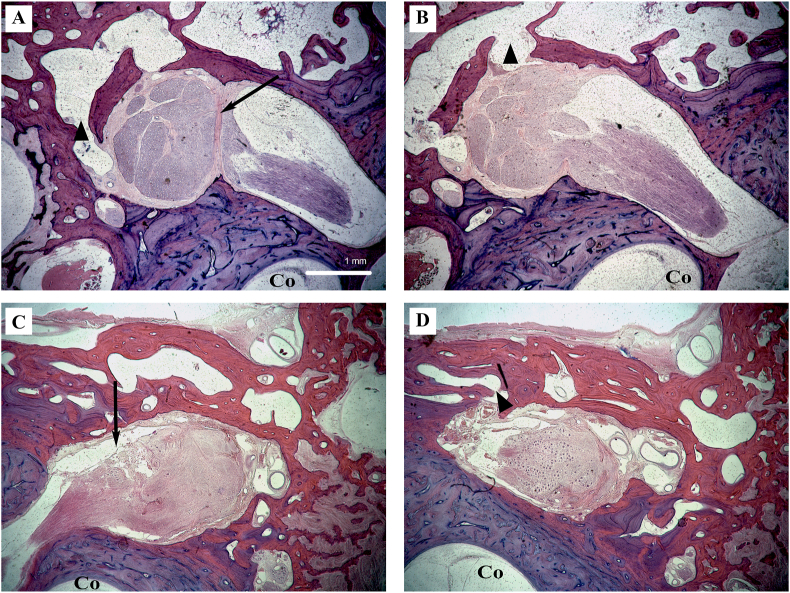
Fig. 11.
Type II arachnoid pattern and fistulae with adjoining air cells. A: Representative photomicrographs of vertical temporal bone section demonstrating a fibrous ring (arrow) at the terminus of the labyrinthine fallopian canal that typically blocks intrusion of the subarachnoid space from the IAC into the geniculate ganglion. B: On subsequent sections however, a dehiscence in the fibrous ring allows subarachnoid space extension into the geniculate ganglion, forming a type II pattern. Osseous communication with neighboring air cells (arrowheads) then presents a pathway for CSF egress into the temporal bone and leading to clinical CSF leak. Specimen 4872 R. C: Similarly in a different specimen, arachnoid space extension from the IAC into the geniculate fossa and (D) osseous communication with adjacent air cells again suggests a path for CSF fistulization. Specimen 11095 L. Original magnification 2.5×. Co, cochlea.
Discussion
We present rarely seen clinical cases of expansile arachnoid cysts in the geniculate region with the potential of CSF leak, and histopathologic correlates and precursors from a human temporal bone collection. Extensions of the subarachnoid space beyond the labyrinthine segment of the facial nerve into the substance of the geniculate ganglion or more distally is estimated to occur in up to 12% of the population.7 Most do not however result in clinical CSF leak given how rarely the fallopian canal is reported to be the site of origin. It is apparent from the presented cases and histopathologic observations that these presumed arachnoid cysts within the geniculate fossa can enlarge to produce erosion and osseous remodeling of the labyrinth, as well as the formation of CSF fistulae into adjacent air cell tracts or the middle ear (Fig. 11D). Reports of CSF leaks originating from the fallopian canal has been limited to case reports and small case series. Published reports of 10 adult and 7 children patients with this entity were identified. These reports described CSF leaks emanating from sites both proximal10, 11, 12,14, 15, 16, 17,19,20,24 and distal to the geniculate ganglion, including some from the tympanic segment8,11,13,18,25 (Table 2).
Table 2.
Summary of published cases of CSF otorrhea originating from the fallopian canala.
| Reference | Age | Gender | Laterality | Site of fallopian canal dehiscence | Additional clinical sequelae |
|---|---|---|---|---|---|
| Dey et al, 201910 | 53 | F | R | Geniculate ganglion | |
| Dhanasekar et al, 201011 | 9 | M | R | Geniculate ganglion and proximal tympanic segment | Meningitis |
| Foyt and Brackmann, 200012 | 34 | M | R | Geniculate ganglion | Meningitis, recurrent rhinorrhea |
| 5 | M | L | Geniculate ganglion | Chronic OM | |
| Harrington and Birck, 196714 | 9 | F | L | Geniculate ganglion | Meningitis, postoperative rhinorrhea |
| Markou et al, 201115 | 43 | F | L | Geniculate ganglion | Meningitis |
| Mong et al, 200916 | 25 | F | R | Geniculate ganglion | Chronic OM, Persistent postoperative rhinorrhea |
| 7 | M | L | Geniculate ganglion | Meningitis, postoperative partial facial palsy | |
| Petrus and Lo, 199917 | 34 | M | R | Geniculate ganglion | Meningitis, CSF rhinorrhea |
| 5 | M | L | Geniculate ganglion | Chronic OM | |
| Sagardoy et al, 201619 | NR | F | NR | Geniculate ganglion | Meningitis |
| Teufert and Slattery, 201320 | 45 | F | R | Geniculate ganglion | Rhinorrhea |
| Legent et al, 198924 | 7 | M | NR | Geniculate ganglion | Meningitis |
| Isaacson et al, 20028 | 37 | M | L | Tympanic segment | Meningitis, facial palsy |
| Gacek and Leipzig, 197913 | 2 | M | R | Tympanic segment | Meningitis |
| Piane et al, 200118 | 64 | F | L | Tympanic segment | |
| Barcz et al, 198525 | 14 | M | L | Tympanic segment | Meningitis |
NR, not reported; OM, otitis media.
In case series, only appropriate individual case(s) shown.
While observation is a viable treatment strategy for asymptomatic geniculate cysts (Case 3), those presenting with concern for CSF leak require definitive treatment due to the risk of meningitis (Cases 1 and 2). Cryptic meningitis should warrant a detailed work-up in search of an etiology. Cystic CSF lesions of the geniculate ganglion are rare but important findings that may be particularly difficult for clinicians and radiologists to identify due to their peripheral location and small size, which may only be captured on fine-slice images. Undetected and untreated, they may lead to occult CSF leak and recurrent meningitis with serious health consequences as illustrated in the case presentations here. Interestingly, meningitis was an autopsy finding in one temporal bone specimen in the present study (specimen 4532 R) that also exhibited type II subarachnoid space extension in the geniculate ganglion (Fig. 10A and B). Lack of further clinical data regarding meningitis etiology or clinical presentation preclude determination of whether atypical arachnoid extension in this specimen was coincidental or causal.
While the mechanism leading to atypical subarachnoid space extension into the geniculate ganglion or beyond remains unknown, the clinical cases presented here, in both children and adults, offer clues regarding potential underlying pathophysiology. Increased intracranial pressure, such as due to idiopathic intracranial hypertension (IIH), is strongly associated with spontaneous CSF leak from the temporal bone7,9,10,26; however, the relationship between elevated ICP and subarachnoid extension distal to the labyrinthine segment has not been studied. Locations at which cranial nerves pierce the meninges to exit the skull base, such as V3 at foramen ovale, are well-recognized sites of meningocele formation due to elevated ICP.27,28 The formation of arachnoid cysts in the geniculate fossa may follow a similar pathophysiologic process, leading to increased risk of spontaneous CSF leak from the fallopian canal in some individuals.7,10,12 Although the patient in Case 3 did not suffer from CSF leak, elevated ICP due to IIH could be surmised as the etiology of her arachnoid cyst within the geniculate ganglion. The patient had an elevated BMI and exhibited radiographic signs consistent with increased ICP, including prominent CSF signals in the sella and optic sheath. However, the lack of corresponding clinical data for temporal bone specimens examined in this study preclude us from drawing inferences regarding the extent to which patterns of arachnoid extension are a primary process or secondary to elevated ICP.
Dehiscences in the skull base are unlikely to occur solely due to increased intracranial pressure and appear to be facilitated by the ectopic placement of arachnoid granulations along the skull base.2 Yew et al2 observed arachnoid granulations penetrating dura mater to make direct contact with cortical surfaces of the temporal bone in 12.7% of donors to the Johns Hopkins Temporal Bone Collection. Furthermore, incomplete ossification of the temporal bone occurs in up to 30% of temporal bones,1 and failure of postnatal temporal bone ossification may be the primary mechanism underlying a number of otologic pathologies including superior canal dehiscence29,30 and labyrinthine and cochlear-facial dehiscence.31 A congenital etiology of some arachnoid cysts is suggested by their presentation in two otherwise healthy children in this report and seven pediatric cases in the published literature (Table 2). A congenital or developmental basis is also consistent with the relatively high number of incidental type II and III arachnoid patterns observed by Gacek.7
A broader survey of temporal bones is needed to determine the overall prevalence of atypical arachnoid patterning in the geniculate ganglion. Evaluation of specimens from neonates and children may help to understand how arachnoid extension patterns vary with age or when normal development is disturbed. This study is further limited by the lack of detailed clinical histories of many temporal bone donors making it difficult to ascertain the clinical significance of observed histopathologic findings. Additionally, dural infiltration from the temporal lobe could confound the interpretation of arachnoid patterns in the geniculate ganglion in specimens where geniculate dehiscence is present. We sought to mitigate this problem by following the facial nerve through consecutive sections from the labyrinthine to tympanic segments and evaluating the integrity of the overlying middle fossa floor (Fig. 10). Many previous temporal bone studies have featured horizontal sections. Our histologic survey, which comprises vertical sections, allows for visualization of the confluence of the labyrinthine segment and the geniculate ganglion in the same section, which is advantageous for the purposes of this study.
Conclusion
We highlight three unique cases of arachnoid cysts in the geniculate region as a rare etiology of CSF fistulae of the temporal bone, and dehiscence of the labyrinth. These cases may illustrate distinct mechanisms of arachnoid extension or subsequent expansion. A survey of temporal bone specimens for histologic correlates confirms previously reported patterns of subarachnoid extension along the fallopian canal. Extension into the substance of the geniculate ganglion and more distally, is potentially a precursor to expansile cysts such as those appearing in 20 published cases including three presented in this report.
Author contributions
Emerson E. Lee: Methodology, Formal analysis, Investigation, Resources, Data Curation, Writing- Original Draft, Writing- Review & Editing, Visualization. Nicholas S. Andresen: Formal analysis, Investigation, Resources, Data Curation, Writing- Review & Editing. Bryan McKenzie: Conceptualization, Methodology, Investigation, Resources, Data Curation, Writing- Review & Editing. Jeffrey D. Sharon: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data Curation, Writing- Original Draft, Writing- Review & Editing, Visualization. Howard W. Francis: Methodology, Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data Curation, Writing- Original Draft, Writing- Review & Editing, Visualization, Supervision, Project administration. Daniel Q. Sun: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data Curation, Writing- Original Draft, Writing- Review & Editing, Visualization, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Dr. Mohamed Lehar for his assistance with the Johns Hopkins Temporal Bone Collection and Dr. Amanda Lauer for her assistance with microscopy.
Edited by Yu-Xin Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Proctor B., Nielsen E., Proctor C. Petrosquamosal suture and lamina. Otolaryngol Head Neck Surg. 1981;89:482–495. doi: 10.1177/019459988108900325. [DOI] [PubMed] [Google Scholar]
- 2.Yew M., Dubbs B., Tong O. Arachnoid granulations of the temporal bone: a histologic study of dural and osseous penetration. Otol Neurotol. 2011;32:602–609. doi: 10.1097/MAO.0b013e3182129026. [DOI] [PubMed] [Google Scholar]
- 3.Gacek R.R. Arachnoid granulation cerebrospinal fluid otorrhea. Ann Otol Rhinol Laryngol. 1990;99:854–862. doi: 10.1177/000348949009901102. [DOI] [PubMed] [Google Scholar]
- 4.Åhrén C., Thulin C.A. Lethal intracranial complications following inflation in the external auditory canal in treatment of serous otitis media and due to defects in the petrous bone. Acta Otolaryngol. 1965;60:407–421. [Google Scholar]
- 5.Lang D.V. Macroscopic bony deficiency of the tegmen tympani in adult temporal bones. J Laryngol Otol. 1983;97:685–688. doi: 10.1017/s0022215100094834. [DOI] [PubMed] [Google Scholar]
- 6.Merchant S.N., McKenna M.J. Neurotologic manifestations and treatment of multiple spontaneous tegmental defects. Am J Otol. 2000;21:234–239. doi: 10.1016/s0196-0709(00)80015-0. [DOI] [PubMed] [Google Scholar]
- 7.Gacek R.R. Anatomy and significance of the subarachnoid space in the fallopian canal. Am J Otol. 1998;19:358–365. [PubMed] [Google Scholar]
- 8.Isaacson J.E., Linder T.E., Fisch U. Arachnoid cyst of the fallopian canal: a surgical challenge. Otol Neurotol. 2002;23:589–593. doi: 10.1097/00129492-200207000-00031. [DOI] [PubMed] [Google Scholar]
- 9.Brainard L., Chen D.A., Aziz K.M., Hillman T.A. Association of benign intracranial hypertension and spontaneous encephalocele with cerebrospinal fluid leak. Otol Neurotol. 2012;33:1621–1624. doi: 10.1097/MAO.0b013e318271c312. [DOI] [PubMed] [Google Scholar]
- 10.Dey J.K., Van Gompel J.J., Lane J.I., Carlson M.L. Fallopian canal meningocele with spontaneous cerebrospinal fluid otorrhea: case report and systematic review of the literature. World Neurosurg. 2019;122:e285–e290. doi: 10.1016/j.wneu.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Dhanasekar G., Pearman K., Irving R. Meningocoele of fallopian canal causing recurrent meningitis. J Laryngol Otol. 2010;124:460–462. doi: 10.1017/S0022215109991599. [DOI] [PubMed] [Google Scholar]
- 12.Foyt D., Brackmann D.E. Cerebrospinal fluid otorrhea through a congenitally patent fallopian canal. Arch Otolaryngol Head Neck Surg. 2000;126:540–542. doi: 10.1001/archotol.126.4.540. [DOI] [PubMed] [Google Scholar]
- 13.Gacek R.R., Leipzig B. Congenital cerebrospinal otorrhea. Ann Otol Rhinol Laryngol. 1979;88:358–365. doi: 10.1177/000348947908800311. [DOI] [PubMed] [Google Scholar]
- 14.Harrington J.W., Jr., Birck H.G. Recurrent meningitis due to congenital petrous fistula. A case report. Arch Otolaryngol. 1967;85:572–575. doi: 10.1001/archotol.1967.00760040574021. [DOI] [PubMed] [Google Scholar]
- 15.Markou K., Goudakos J., Franco-Vidal V., Vergnolles V., Vignes J.R., Darrouzet V. Spontaneous osteodural defects of the temporal bone: diagnosis and management of 12 cases. Am J Otolaryngol. 2011;32:135–140. doi: 10.1016/j.amjoto.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Mong S., Goldberg A.N., Lustig L.R. Fallopian canal meningocele: report of two cases. Otol Neurotol. 2009;30:525–528. doi: 10.1097/MAO.0b013e3181a66f16. [DOI] [PubMed] [Google Scholar]
- 17.Petrus L.V., Lo W.W. Spontaneous CSF otorrhea caused by abnormal development of the facial nerve canal. AJNR Am J Neuroradiol. 1999;20:275–277. [PMC free article] [PubMed] [Google Scholar]
- 18.Piane R., Cerase A., Mezzedimi C., Bellussi L. Spontaneous onset of CSF otorrhea from a facial canal fistula in an adult: case report. Acta Otorhinolaryngol Belg. 2001;55:279–284. [PubMed] [Google Scholar]
- 19.Sagardoy T., de Mones E., Bonnard D., Darrouzet V., Franco-Vidal V. Arachnoid cyst of the fallopian canal and geniculate ganglion area: our experience of 9 cases. Clin Otolaryngol. 2017;42:461–466. doi: 10.1111/coa.12629. [DOI] [PubMed] [Google Scholar]
- 20.Teufert K.B., Slattery W.H. Cerebrospinal fluid leak of the fallopian canal. Ear Nose Throat J. 2013;92:E20–E23. [PubMed] [Google Scholar]
- 21.Crowe S.J., Guild S.R., Polvogt L.M. Observations on the pathology of high-tone deafness. J Nervous Ment Dis. 1934;80:480. [Google Scholar]
- 22.Nager G.T., Nager G. Waverly Press Inc.; Baltimore: 1993. Pathology of the Ear and Temporal Bone. [Google Scholar]
- 23.Sun D.Q., Zhou X., Lin F.R., Francis H.W., Carey J.P., Chien W.W. Racial difference in cochlear pigmentation is associated with hearing loss risk. Otol Neurotol. 2014;35:1509–1514. doi: 10.1097/MAO.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 24.Legent F., Gayet M., Albisetti J., Korb G., Beauvillain C., Bordure P. Malformation of the facial canal, meningitis and cerebrospinal otorrhea. Ann Otolaryngol Chir Cervicofac. 1989;106:197–200. [PubMed] [Google Scholar]
- 25.Barcz D.V., Wood R.P., 2nd, Stears J., Jafek B.W., Shields M. Subarachnoid space: middle ear pathways and recurrent meningitis. Am J Otol. 1985;6:157–163. [PubMed] [Google Scholar]
- 26.Stucken E.Z., Selesnick S.H., Brown K.D. The role of obesity in spontaneous temporal bone encephaloceles and CSF leak. Otol Neurotol. 2012;33:1412–1417. doi: 10.1097/MAO.0b013e318268d350. [DOI] [PubMed] [Google Scholar]
- 27.Bialer O.Y., Rueda M.P., Bruce B.B., Newman N.J., Biousse V., Saindane A.M. Meningoceles in idiopathic intracranial hypertension. AJR Am J Roentgenol. 2014;202:608–613. doi: 10.2214/AJR.13.10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobre M.C., Fischbein N. Do not touch’ lesions of the skull base. J Med Imaging Radiat Oncol. 2014;58:458–463. doi: 10.1111/1754-9485.12195. [DOI] [PubMed] [Google Scholar]
- 29.Carey J.P., Minor L.B., Nager G.T. Dehiscence or thinning of bone overlying the superior semicircular canal in a temporal bone survey. Arch Otolaryngol Head Neck Surg. 2000;126:137–147. doi: 10.1001/archotol.126.2.137. [DOI] [PubMed] [Google Scholar]
- 30.Minor L.B., Solomon D., Zinreich J.S., Zee D.S. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998;124:249–258. doi: 10.1001/archotol.124.3.249. [DOI] [PubMed] [Google Scholar]
- 31.Fang C.H., Chung S.Y., Blake D.M. Prevalence of cochlear-facial dehiscence in a study of 1,020 temporal bone specimens. Otol Neurotol. 2016;37:967–972. doi: 10.1097/MAO.0000000000001057. [DOI] [PubMed] [Google Scholar]