Abstract
The sow at parturition is challenged with respect to energy status due to increases in energetic expenses associated with 1) nest building 2) uterine contractions, and 3) colostrum production. A previous study indicated that sows were depleted of glucogenic energy around farrowing. The aim was to investigate whether intravenous infusion of glucose from observed nest-building behavior to 24 h postpartum affected the farrowing kinetics and colostrum production in sows. Ten multiparous sows (DanBred landrace × DanBred Yorkshire) were fitted with a jugular vein catheter on each side (one for infusion and the other one for blood sampling). Sows were infused with either 0.9% saline (CON; n = 5) or 10% glucose (GLU; n = 5) solution at a constant rate of 125 mL/h. From day 108 of gestation, sows were fed once daily with 3.33 kg of a standard lactation diet. During farrowing, sows were monitored to register the onset of farrowing, time of birth, birth status (live or dead), sex, stillbirth rate (SR), and weight of newborn piglets. Farrowing assistance (FA) was provided when the birth interval exceeded 60 min. In late gestation, 1 mL of blood was collected every third hour for blood gas analysis and every sixth hour for harvesting plasma. During farrowing, 1 mL (for blood gas) and 9 mL of blood were collected at 0, 3, 6, 9, 12, 15, 18, 21, and 24 h in milk (HIM). Colostrum and milk samples were collected at 0, 6, 12, 18, 24, and 36 HIM and also at 3, 10, 17, and 24 d in milk. Compared with CON sows, GLU infusion decreased the SR (16.1% vs. 7.4%; P = 0.03), FA (21% vs. 9.0%; P = 0.01), and surprisingly also blood glucose at the onset of farrowing (5.53 vs. 5.09 mmol/L; P = 0.03), respectively. A tendency to higher plasma lactate at the onset of farrowing (P = 0.05) but decreased piglet survival from 0 to 24 h (P = 0.06) was also found for GLU sows. No effects of treatment on farrowing duration or mean birth intervals were found. Lactate in whole blood (P = 0.003) and plasma (P = 0.002) was increased for GLU sows as compared with CON sows during the colostrum period. No effect of GLU infusion was seen on colostrum and milk composition and yield. The increase in lactate was most likely due to a shift toward a greater proportion of glucose oxidation and insufficient O2 supply to fuel uterine contractions. In conclusion, infusion of glucose reduced the frequency of SR and FA, and improved energy status of the sow which seems to be crucial to enhance total piglet survival.
Keywords: colostrum production, energy supply, farrowing kinetics, glucose homeostasis, stillbirth, transition sow
Introduction
A key factor for maximizing sow productivity and realizing the potential profit of large litter sizes is a reduction in stillborn piglets. The majority of stillborn piglets die during the birth process (Friendship et al., 1990) because of successive uterine contractions and ruptured umbilical cord (Alonso-Spilsbury et al., 2005). Stillbirth rate (SR) and farrowing duration (FD) are important because FD is a key factor influencing SR (van Dijk et al., 2005; Oliviero et al., 2010), and recently it was found that the FD was affected by the level of plasma glucose at the onset of farrowing (Feyera et al., 2018). The latter study reported that the sow plasma glucose 1 h after the onset of farrowing dropped from above 6 to as low as 2 mmol/L if the time from the last meal until onset of farrowing (TLMUOF) increased from 2 to 9 h, which indicated that sows most likely became depleted of energy during the nest-building process. That study also showed that the probability of stillbirth and farrowing assistance (FA) increased greatly when TLMUOF exceeded 3 h. The concentration of arterial plasma glucose measured 1 h after the birth of the first piglet was negatively correlated with FD and TLMUOF (Feyera et al., 2018). The concentration of glucose is important for the farrowing process because glucose and triglycerides are the key energy metabolites extracted by the uterus during farrowing (Feyera et al., 2018), indicating that a sufficient supply of glucogenic energy (and glucose homeostasis) is important to achieve a rapid farrowing with no or minor complications. Prior to the onset of farrowing, glucose is used as fuel for physical activity associated with nest-building behavior (Theil, 2002), and, during farrowing, glucose is used as fuel for uterine contractions (Feyera et al., 2018) and as a precursor for colostral lactose and fat synthesis (Feyera et al., 2019). Therefore, insufficient glucose may most likely compromise either uterine contractions or colostrum production, because nest building occurs earlier relative to farrowing than the other two traits, and all seems to be fueled by the very last meal prior to parturition.
For the sow, most of the dietary energy is absorbed as glucose within 4 to 6 h following a meal (Serena et al., 2009) meaning that sows that start to farrow many hours after the last meal must rely on stored energy to complete the farrowing. It was hypothesized that a constant intravenous infusion of glucose from the first sign of parturition (nest building or milk in the teats) until 24 h after farrowing will ensure proper energy in sows where glucose homeostasis is challenged due to only one daily meal. The aim was to demonstrate and investigate the importance of high-energy status for achieving a successful farrowing.
Materials and Methods
The present experiment complied with the Danish Ministry of Justice Law number 382 (June 10, 1987) and Act number 726 (September 9, 1993, as amended by act number 1081 on December 20, 1995), concerning experiments with the care of animals.
Experimental design and animals
Ten fourth parity sows (DanBred Landrace × DanBred Yorkshire) were stratified for body weight and allocated to one of two treatments. Sows were fitted with two catheters, one in the right jugular vein (for infusion) and the other one in the left jugular vein (for blood sampling). Catheters were inserted 3 to 4 d before expected farrowing, while sows were kept in nose restraint. The infusion rate was constant at 125 mL/h with saline (CON) or 10% glucose (GLU) solution, and on a daily basis, GLU sows received 300 g glucose via the infusion, and this amount of energy was similar to extra 0.4 kg of feed. Infusion was initiated when nest-building behavior (i.e., restless or stereotypic behavior) or milk in the mammary glands was observed and continued until 24 h after the firstborn piglet.
Management, housing, and feeding
Approximately, 1 wk before expected farrowing, sows were moved to the farrowing unit, housed individually in pens with partly slatted floor, and fed a standard lactation diet based on wheat, barley, and soybean meal (Table 1) until weaning on day 28 of lactation. From day 108 of gestation until day 2 of lactation, sows were fed 3.33 kg/d. From day 2 of lactation, feed allowance increased from 3.81 to 8.57 kg/d on day 14 and then kept constant until weaning. If litter size was 13, 12, 11, 10, or ≤9 piglets, the feed allowance was reduced to 97%, 94%, 91%, 88%, or 85%, respectively, of planned daily feed supply. From day 108 of gestation until day 1 of lactation, sows were fed only one daily meal, whereas from day 2 to 28 of lactation, sows were fed three equal meals in 8 h intervals. Feed residues were collected daily and weighed daily pre-farrowing and weekly post-farrowing (days 7, 14, 21, and 28 of lactation) to assess the realized feed intake.
Table 1.
Dietary ingredients and calculated chemical composition
| Lactation diet | |
|---|---|
| Ingredients, g/kg, as-fed basis | |
| Barley | 445 |
| Wheat | 262 |
| Toasted dehulled soybean meal | 125 |
| Sunflower meal, dehulled | 40.0 |
| Wheat bran | 21.0 |
| Oat | 20.0 |
| Soya hulls | 20.0 |
| Sugar beet pulp | 20.0 |
| Palm oil | 9.00 |
| Calcium carbonate | 8.00 |
| Mono-calcium phosphate | 7.30 |
| Vitamin premix1 | 5.50 |
| Sodium chloride | 5.20 |
| l-Lysine | 4.40 |
| Axtra XB2 | 2.00 |
| Vitamin E | 1.80 |
| Threonine | 1.00 |
| Phytase | 0.60 |
| dl-methionine | 0.40 |
| Analyzed chemical composition, g/kg as-fed basis | |
| FUsow3/kg | 1.07 |
| MJ ME/kg | 13.4 |
| Dry matter | 881 |
| Crude protein | 170 |
| Fat | 43 |
| Ash | 51 |
| Starch | 460 |
1Supplied per kilogram of diet: 140 mg iron, 15.0 mg Cu, 51 mg Mn, 111 mg Zn, 0.80 mg iodine, 0.30 mg Se, and 22 mcg cholecalciferol.
2Beta-xylanase (12,200 U/g) and beta-glucanase (1,520 U/g).
31 FUsow is the potential physiological energy equivalent to 7.70 MJ netto energy for sows.
Sows were weighed and back fat scanned on day 108 of gestation and on day 2 of lactation. Birth of the first piglet within a litter was considered the onset of farrowing. Time from the onset of farrowing until the birth of the last piglet in the litter was defined as FD. Immediately after birth, the umbilical cord was closed with a plastic strip to prevent bleeding and shortened to 10 to 15 cm, and birth weight of the piglet was recorded. Time of birth and birth status (live or dead) were recorded. Mummified piglets were excluded. When the birth interval exceeded 60 min, FA was provided. The weight of piglets was registered at 12, 24, and 36 h in milk (HIM). On day 2, litters were standardized to 14 piglets or the number of functional teats by moving the smallest piglets away. Dead piglets were weighed and registered to assess the daily litter gain and average litter size.
Sampling
Blood
Collection of blood was initiated after either nest-building behavior or milk in the mammary gland was observed in late gestation. Thus, sampling of 1-mL blood was collected every third hour for immediate analysis for blood gas and whole blood metabolites, and 9-mL blood sample was collected every sixth hour for harvesting plasma. In the colostrum period (i.e., following the birth of the first piglet), 1 mL and 9 mL of blood were sampled at 0, 3, 6, 9, 12, 15, 18, 21, and 24 HIM. Before collecting blood, 3 mL was discarded due to the risk of pollution with saline in the catheter. Hereafter, 1 mL of blood was collected in heparinized syringes (Rapidlyte, Siemens Healthcare Diagnostics Inc. Tarrytown, NY). The 9-mL blood samples were collected by 10 mL syringes and transferred to heparinized vacutainer tubes (Grein Bio-One, GmbH, Kremsmünster, Austria) and stored on ice until centrifuged for 10 min at 1,558 × g at 4 °C. Plasma was harvested in tubes and stored at −20 °C until analysis. On days 3 and 28 of lactation, sows were administered with 0.0425 g/kg live weight of deuterium solution (40% deuterium oxide [SIGMA-ALDRICH, MO] and 60% saline [9 mg/mL NaCl/mL, B. Braun, Melsungen AG, Germany]) intravenously through the jugular vein catheter or intramuscular, respectively. Shortly prior to the administration of deuterium, a background sample was drawn to measure background D2O level. Another blood sample was drawn 5 h after injection to estimate the total pool size of protein and fat on days 3 and 28 of lactation.
Colostrum and milk
Colostrum from 0 and 6 HIM was collected without injection of oxytocin. Colostrum from 12, 18, 24, and 36 HIM and milk from days 3, 10, 17, and 24 of lactation were collected after injection of oxytocin (10 IU/mL; MSD Animal Health A/S, Copenhagen, Denmark) either intravenous (0.3 mL) or intramuscular (2.0 mL). Colostrum and milk samples were stored at −20 °C until analysis.
Chemical analysis
Colostrum and milk were analyzed for fat, lactose, protein, and DM content with infrared spectroscopy (Milkoscan 4000, FOSS MilkoScan, Hillerød, Denmark). Energy content in colostrum and milk was calculated based on the energy values of 39.8, 23.8, and 16.3 kJ/g for fat, protein, and lactose, respectively.
Whole blood collected in heparinized syringes was analyzed for O2, CO2, glucose, lactate, pH, Na+, K+, and Cl− immediately after collection using a Rapid point 500 blood gas analyzer (Siemens Healthcare Diagnostics Inc. Tarrytown, NY). Plasma glucose, lactate, and triglycerides were measured according to the standard procedures (Siemens Diagnostics Clinical Methods for ADVIA 1800) using an auto-analyzer (ADVIA 1800 Chemistry System, Siemens Medical Solution, Tarrytown, NY). The nonesterified fatty acids (NEFA) content in plasma was determined using the Wako, NEFA C ACS-ACOD assay method (Wako Chemicals GmbH, Neuss, Germany) using an auto-analyzer (ADVIA 1800 Chemistry System, Siemens Medical Solution, Tarrytown, NY). The Porcine Insulin Enzyme-Linked Immunosorbent Assay kit (Mercodia AB, Uppsala, Sweden) was used to determine plasma insulin, and intra- and inter-assay variation was 2.9% and 5.1%, respectively, for the low control (0.03 ng/mL) and 4.9% and 23.9% for the high control (0.5 ng/mL). The dietary content of dry matter (DM), crude protein, fat, ash, and starch was analyzed and calculated as described by Krogh et al. (2017).
Calculations and statistical analysis
Piglet colostrum intake was calculated according to Theil et al. (2014) based on weight gain in the colostral period (0 to 24 HIM). Colostrum yield (CY) of sows was calculated by summing the colostrum intake of individual piglets within each litter. The sow milk yield was calculated according to Hansen et al. (2012) based on the average daily litter gain and the average litter size within each week of lactation (i.e., days 2 to 7, 7 to 14, 14 to 21, and 21 to 28). Survival rate 0 to 24 h and survival rate until 24 h were defined as the percentage of live-born piglets and total born piglets, respectively, surviving until 24 h after the firstborn piglet. The trait FA was defined as the percentage of piglets being born by assistance. Dietary metabolizable energy (ME) intake was calculated according to Theil et al. (2020) based on the intake of energy (in feed units [FU] per day) and calculated ME concentration in the diet (mega joule (MJ) ME/kg DM = 4.121 + 9.096 MJ ME/FU × FUsow/kg DM). The FUsow/kg DM is the energy concentration in FU per kilogram DM and amounted to 1.22 FU/kg DM. Body protein and fat mobilization were calculated as the difference of body pools of sows on days 3 and 28. The body pools were calculated based on D2O space, body weight, and back fat thickness according to Pedersen et al. (2019).
The sow was regarded being the experimental unit with respect to infusion of glucose or saline. All data were analyzed using SAS version 9.4 (SAS Inst. Inc., Cary, NC). Sow live weight, back fat, feed intake, FD, TLMUOF, CY, total born, live born, birth weight, weaning weight, piglet colostrum intake, piglet gain colostrum composition, milk composition, milk yield, water intake, and piglet average daily gain (ADG) were analyzed using the GLM procedure in SAS with treatment (CON and GLU) as fixed effect. Sow was included as a random effect to account for repeated measurements when analyzing piglet birth weight, birth interval, colostrum intake, FA, and survival rate 0 to 24 h (with and without stillborn) and analyzed with the MIXED procedure in SAS (Littell et al., 1996), and likewise, blood gases, minerals, and plasma metabolites were analyzed with treatment (CON and GLU), HIM, and their interactions (treatment × HIM) as fixed effects and sow as a random effect. The covariance related to HIM was described using an autoregressive process of order 1. FA, survival rate 0 to 24 h, and survival rate until 24 h were analyzed using logistic regression, that is, a binomial generalized linear mixed-effects model with LOGIT as link function. Effects were analyzed using odds ratios and back-transformed to percentage of piglets surviving or being born with FA, and repeated measurements within sows (piglets within a litter) were taken into account. Birth interval and insulin concentration were log-transformed before performing statistical analysis to stabilize the residual variance and reported as the back-transformed mean value. For FA, SR, survival rate, birth interval, and insulin concentration, the 95% confidence intervals are presented. All other data are presented as least square means with SEM as a measure of variance. Results were considered statistically significant when P < 0.05 and considered a trend when P ≤ 0.10.
Results
Farrowing performance
The total born piglets per litter ranged from 22 to 29 (24.6 ± 2.68; Table 2) where the total live-born piglets/litter ranged from 17 to 25 (21.7 ± 2.75). Infusion of glucose decreased the mean SR from 16.1% to 7.4% (P = 0.03), the FA from 21% to 9% (P = 0.01), and the blood glucose concentration at onset from 5.53 to 5.09 mmol/L (P = 0.03). Further, a tendency to greater blood lactate at the onset of farrowing from 1.45 to 2.25 mmol/L (P = 0.05) and a decreased survival rate of live-born piglets from 99.0% to 94.7% (P = 0.06) was found for sows receiving GLU infusion. The average CY increased numerically from 6.71 to 7.06 kg for GLU sows compared with CON sows while the overall mean CY was 6.88 ± 0.86 kg. The survival rate until 24 h and weaning weight/litter were numerically greater for GLU sows compared with CON (87.7% vs. 83.1% and 105 vs. 97 kg, respectively), although these changes were not statistically significant. The average FD was 8.1 ± 4.3 h, and the average TLMUOF was 11.5 ± 5.5 h. These traits were not affected by the infusion treatment (P > 0.10).
Table 2.
Characteristics and performance of sows and piglets
| Treatment (Trt) | P-value | |||
|---|---|---|---|---|
| Item | CON | GLU | SEM | Trt |
| Sows | ||||
| No. of sows | 5 | 5 | ||
| Mean parity | 4.0 | 4.0 | ||
| Sow weight day 108, kg | 323 | 325 | 15.45 | 0.92 |
| Sow weight day 2, kg | 303 | 298 | 14.86 | 0.81 |
| Back fat day 108, mm | 14.8 | 15.0 | 1.89 | 0.92 |
| Back fat day 2, mm | 13.8 | 15.0 | 1.85 | 0.66 |
| Back fat loss day 2 to 28, mm/d | 0.06 | 0.12 | 0.02 | 0.05 |
| Protein mobilization day 3 to 28, kg/d | 0.12 | 0.02 | 0.04 | 0.11 |
| Fat mobilization day 3 to 28, kg/d | 0.23 | 1.21 | 0.36 | 0.09 |
| Feed intake last week pre-farrow, kg/d | 3.33 | 3.33 | 0.00 | 1.00 |
| Feed intake last day pre-farrow, kg/d | 3.14 | 3.05 | 0.12 | 0.58 |
| Farrowing | ||||
| FD, h | 8.17 | 8.09 | 2.02 | 0.98 |
| TLMUOF, h | 12.6 | 10.5 | 2.57 | 0.58 |
| CY (0 to 24 h), kg | 6.71 | 7.06 | 0.40 | 0.56 |
| Plasma glucose at time 01, mmol/L | 5.53a | 5.09b | 0.12 | 0.03 |
| Plasma lactate at time 01, mmol/L | 1.45 | 2.25 | 0.25 | 0.05 |
| FA2, % | 21.0a | 9.0b | 0.01 | |
| [14.69;29.02] | [5.06;15.55] | |||
| Piglets | ||||
| Total born, n | 24.8 | 24.4 | 1.26 | 0.83 |
| Live-born piglets, n | 20.8 | 22.6 | 1.22 | 0.33 |
| SR, % | 16.1a | 7.4b | 0.03 | |
| [10.65;23.68] | [3.88;13.57] | |||
| Birth weight, kg | 1.19 | 1.24 | 0.06 | 0.52 |
| Weaning weight/piglet, kg | 8.09 | 8.44 | 0.40 | 0.56 |
| Weaning weight/litter, kg | 97.0 | 105 | 7.52 | 0.49 |
| Number of piglets weaned/litter6, n | 12.0 | 12.4 | 0.76 | 0.72 |
| Birth interval3, min | 14.3 | 12.6 | — | 0.75 |
| [8.56;23.87] | [7.59;21.01] | |||
| Piglet colostrum intake 0 to 24 h, g | 321 | 324 | 25.0 | 0.94 |
| Piglet gain 0 to 24 h, g | 42 | 44 | 18.5 | 0.94 |
| Survival rate 0 to 24 h4, % | 99.0 | 94.7 | — | 0.06 |
| [93.49;99.86] | [88.68;97.60] | |||
| Survival rate until 24 h5, % | 83.1 | 87.7 | — | 0.30 |
| [75.42;88.69] | [80.60;92.45] | |||
1Time 0 is when the first piglet was born.
2% of total born.
3Log-transformed when analyzed and reported after transformed back.
4% of live born surviving until 24 h after the birth of firstborn piglet.
5% of total born surviving until 24 h after the birth of firstborn piglet.
6Litters were equalized to 14 piglets on day 2.
a,bLeast squares treatment means with different superscript letters differ significantly (P < 0.05).
Plasma concentration of blood gases, minerals, energy metabolites, and insulin
The plasma concentration of O2 decreased from −15 to −3 HIM (P = 0.02) and Ca2+ decreased from −21 to −3 HIM (P < 0.001; Table 3). In contrast, the concentration of lactate measured in whole blood (P < 0.001) and in plasma (P < 0.01) increased as the sow approached the onset of farrowing and concomitantly plasma NEFA also tended to increase (P = 0.05). Other measured blood gases, metabolites, or minerals were not affected by glucose infusion or HIM before the onset of farrowing.
Table 3.
Venous concentration of blood gases, minerals, and plasma metabolites before farrowing, from −33 to −3 HIM
| Treatment (Trt) | HIM1 | P-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | GLU | SEM | −33 | −27 | −21 | −15 | −9 | −3 | SEM | Trt | HIM | Trt × HIM | |
| Whole blood | |||||||||||||
| O2 mmol/L | 4.42 | 4.49 | 0.18 | 4.40ab | 4.41ab | 4.50ab | 4.79a | 4.52ab | 4.11b | 0.22 | 0.78 | 0.02 | 0.49 |
| CO2 mmol/L | 29.4 | 28.9 | 0.87 | 30.2 | 29.1 | 28.9 | 28.8 | 28.8 | 29.1 | 0.87 | 0.68 | 0.59 | 0.86 |
| pH | 7.47 | 7.47 | 0.01 | 7.47 | 7.48 | 7.47 | 7.46 | 7.47 | 7.48 | 0.01 | 0.97 | 0.31 | 0.43 |
| Na+, mmol/L | 138 | 138 | 0.59 | 139 | 138 | 138 | 138 | 138 | 139 | 0.57 | 0.81 | 0.07 | 0.46 |
| K+, mmol/L | 3.91 | 4.15 | 0.10 | 3.93 | 3.98 | 4.02 | 4.10 | 4.07 | 4.08 | 0.09 | 0.13 | 0.18 | 0.74 |
| Ca++, mmol/L | 1.22 | 1.21 | 0.02 | 1.23ab | 1.23ab | 1.24a | 1.22ab | 1.20b | 1.20b | 0.01 | 0.66 | <0.001 | 0.16 |
| Cl−, mmol/L | 103 | 103 | 1.14 | 103 | 103 | 104 | 104 | 103 | 103 | 0.88 | 0.80 | 0.08 | 0.13 |
| Glucose, mmol/L | 4.83 | 4.75 | 0.15 | 4.78 | 4.67 | 4.82 | 4.83 | 4.95 | 4.69 | 0.22 | 0.68 | 0.80 | 0.48 |
| l-Lactate, mmol/L | 1.41 | 1.74 | 0.17 | 1.32b | 1.26b | 1.41ab | 1.54ab | 1.97a | 1.94a | 0.19 | 0.20 | <0.001 | 0.64 |
| Plasma | |||||||||||||
| Glucose, mmol/L | 5.37 | 5.53 | 0.14 | 5.44 | 5.45 | 5.21 | 5.38 | 5.99 | 5.21 | 0.27 | 0.41 | 0.09 | 0.08 |
| l-Lactate, mmol/L | 1.45 | 1.78 | 0.15 | 1.40b | 1.38b | 1.40b | 1.42b | 1.95ab | 2.15a | 0.24 | 0.17 | <0.01 | 0.97 |
| Urea, mmol/L | 3.07 | 3.36 | 0.27 | 3.32 | 3.43 | 3.21 | 3.17 | 3.03 | 3.12 | 0.25 | 0.46 | 0.45 | 0.89 |
| TG2, umol/L | 0.34 | 0.39 | 0.05 | 0.33 | 0.28 | 0.40 | 0.43 | 0.36 | 0.37 | 0.06 | 0.53 | 0.41 | 0.51 |
| NEFA, µekv/L | 585 | 370 | 88.8 | 313 | 242 | 554 | 462 | 536 | 759 | 153 | 0.12 | 0.05 | 0.92 |
| Insulin, ng/L | 49.5 | 90.4 | — | 71.3 | 96.8 | 56.6 | 49.8 | 105.4 | 43.6 | — | 0.11 | 0.44 | 0.35 |
| [9.66;253] | [17.7;463] | [13.9;365] | [18.9;496) | [11.1;290] | [9.73;255} | [20.6;540} | [8.52;223] | ||||||
1Samples timepoints are grouped in 6 h intervals from −36 to −31, −30 to −25, −24 to −19, −18 to −13, −12 to −7, and −6 to 0.
2 Triglycerides.
a,bLeast squares treatment means with different superscript letters differ significantly (P < 0.05).
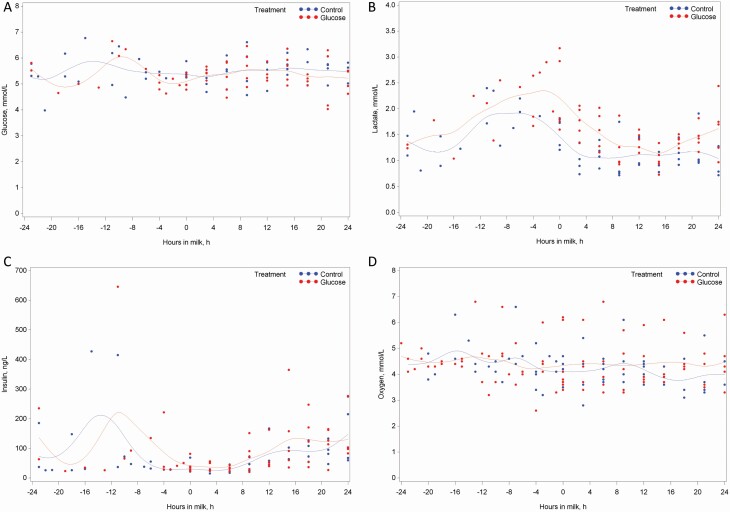
An interaction between HIM and treatment was found for O2 (P = 0.02; Table 4), where GLU sows had increased O2 concentration after the onset of farrowing (Figure 1). Infusion of glucose increased the concentration of lactate measured in whole blood (P = 0.003; 1.48 vs. 1.14 mmol/L) and in plasma (P = 0.002; 1.53 vs. 1.15 mmol/L) during the colostrum period (i.e., 0 to 24 h postpartum) as compared with control sows. The concentration of plasma lactate was elevated at the onset of farrowing irrespective of treatments (P = 0.002), and plasma NEFA was increased at 6 HIM as compared with 15, 18, and 24 HIM (P = 0.02). The plasma concentration of insulin increased from 0 to 24 HIM (P < 0.001), and blood pH was increased at 9 and 12 HIM as compared with 3 and 18 HIM (P = 0.04). Tendencies to elevated concentrations of K+ in whole blood at 0, 3, and 6 HIM (P = 0.08) and in GLU sows (P = 0.08) were found. Other measured blood gasses, minerals, and metabolites were not affected by treatment or HIM (P > 0.10).
Table 4.
Venous concentration of blood gases, minerals, and plasma metabolites during colostrum period from 0 to 24 HIM
| Treatment (Trt) | HIM | P-value | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | GLU | SEM | 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | SEM | Trt | HIM | Trt × HIM | |
| Whole blood | ||||||||||||||||
| O2, mmol/L | 4.30 | 4.23 | 0.23 | 4.30 | 4.15 | 4.14 | 4.23 | 4.26 | 4.40 | 4.36 | 4.12 | 4.02 | 0.28 | 0.72 | 0.22 | 0.02 |
| CO2, mmol/L | 31.3 | 30.6 | 0.69 | 30.0 | 30.1 | 29.1 | 29.9 | 29.0 | 29.8 | 30.0 | 30.5 | 30.8 | 0.65 | 0.87 | 0.19 | 0.13 |
| pH | 7.51 | 7.49 | 0.01 | 7.50ab | 7.48b | 7.50ab | 7.52a | 7.52a | 7.51ab | 7.48b | 7.51ab | 7.50ab | 0.01 | 0.30 | 0.04 | 0.51 |
| Na+, mmol/L | 140 | 140 | 0.51 | 139 | 140 | 141 | 140 | 141 | 140 | 139 | 140 | 140 | 0.58 | 0.44 | 0.25 | 0.58 |
| K+, mmol/L | 3.90 | 4.06 | 0.06 | 4.07 | 4.05 | 4.19 | 3.95 | 3.89 | 3.89 | 3.95 | 3.94 | 3.90 | 0.08 | 0.08 | 0.08 | 0.18 |
| Ca++, mmol/L | 1.18 | 1.21 | 0.03 | 1.17 | 1.18 | 1.18 | 1.18 | 1.20 | 1.21 | 1.21 | 1.21 | 1.21 | 0.02 | 0.39 | 0.18 | 0.70 |
| Cl−, mmol/L | 103 | 103 | 0.66 | 103 | 103 | 103 | 103 | 103 | 102 | 102 | 102 | 102 | 0.58 | 0.68 | 0.18 | 0.85 |
| Glucose, mmol/L | 4.84 | 4.88 | 0.12 | 4.57 | 4.68 | 4.76 | 4.95 | 4.78 | 5.14 | 4.92 | 5.11 | 4.84 | 0.18 | 0.84 | 0.16 | 0.56 |
| l-Lactate, mmol/L | 1.14b | 1.48a | 0.07 | 1.74a | 1.39b | 1.29bc | 1.11bc | 1.26bc | 0.99c | 1.29bc | 1.39b | 1.34b | 0.11 | 0.003 | 0.001 | 0.64 |
| Plasma | ||||||||||||||||
| Glucose, mmol/L | 5.52 | 5.40 | 0.14 | 5.30 | 5.36 | 5.30 | 5.65 | 5.41 | 5.81 | 5.48 | 5.45 | 5.40 | 0.21 | 0.58 | 0.37 | 0.81 |
| l-Lactate, mmol/L | 1.15b | 1.53a | 0.07 | 1.85a | 1.43ab | 1.35bc | 1.11bc | 1.29bc | 1.02c | 1.31bc | 1.34bc | 1.37b | 0.12 | 0.002 | 0.002 | 0.43 |
| Urea, umol/L | 3.32 | 3.47 | 0.34 | 3.18 | 3.21 | 3.22 | 3.30 | 3.42 | 3.65 | 3.63 | 3.52 | 3.41 | 0.27 | 0.77 | 0.49 | 0.94 |
| TG, umol/L | 0.24 | 0.25 | 0.02 | 0.30 | 0.28 | 0.25 | 0.21 | 0.21 | 0.21 | 0.24 | 0.25 | 0.27 | 0.03 | 0.82 | 0.32 | 0.15 |
| NEFA, µekv/L | 574 | 524 | 75.2 | 560abc | 690ab | 741a | 592abc | 492abc | 438bc | 427c | 492abc | 470bc | 86.8 | 0.66 | 0.02 | 0.65 |
| Insulin, ng/L | 56.2 | 65.1 | — | 34.4cd | 30.1d | 27.4d | 51.3c | 66.3bc | 101ab | 103ab | 92.3ab | 118a | — | 0.47 | <0.001 | 0.97 |
a,bLeast squares treatment means with different superscript letters differ significantly (P < 0.05).
Figure 1.
The ontogeny of venous plasma glucose (A), plasma lactate (B), insulin (C), and O2 (D) in sows infused with saline (blue) or 10% glucose (red) solution from when the sign of nest-building behavior observed until 24 h after the onset of farrowing. The line represents the mean value of each sampling time.
The ontogeny of plasma concentrations of glucose, lactate, insulin, and O2 relative to the onset of farrowing is plotted in Figure 1. Glucose infusion did neither increase plasma glucose nor insulin but increased the concentration of plasma lactate prior to and after the onset of farrowing. Glucose infusion increased the blood O2 as compared with control sows after the onset of farrowing.
Sow performance
Infusion of glucose did not affect colostrum composition (Table 5). Colostrum content of DM (P < 0.001), protein (P < 0.001), casein (P < 0.001), and energy (P < 0.001) decreased from 0 to 36 HIM, while fat (P < 0.001) and lactose (P < 0.001) concomitantly increased.
Table 5.
Colostrum composition at 0, 12, 24, and 36 HIM of CON sows and GLU sows
| Treatment (Trt) | HIM | P-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | GLU | SEM | 0 | 6 | 12 | 18 | 24 | 36 | SEM | Trt | HIM | Trt × HIM | |
| DM, % | 20.9 | 20.9 | 0.60 | 25.9a | 23.2b | 20.5c | 17.1d | 18.6cd | 20.1c | 0.81 | 0.93 | <0.001 | 0.47 |
| Fat, % | 5.24 | 5.13 | 0.30 | 3.97c | 3.87c | 5.05bc | 4.80bc | 6.14ab | 7.27a | 0.50 | 0.79 | <0.001 | 0.49 |
| Lactose, % | 3.99 | 3.93 | 0.06 | 3.30c | 3.40c | 3.90b | 4.41a | 4.34a | 4.40a | 0.08 | 0.54 | <0.001 | 0.24 |
| Protein, % | 11.0 | 10.9 | 0.58 | 17.3a | 14.9b | 10.7c | 7.62d | 7.50d | 7.92d | 0.72 | 0.94 | <0.001 | 0.12 |
| Casein, % | 9.51 | 9.50 | 0.52 | 15.4a | 13.1b | 9.17c | 6.53d | 6.27d | 6.63d | 0.64 | 0.98 | <0.001 | 0.12 |
| Energy, MJ/kg | 5.33 | 5.29 | 0.18 | 6.23a | 5.63ab | 5.63ab | 5.19b | 4.94b | 5.49b | 0.24 | 0.87 | <0.001 | 0.52 |
a,bLeast squares treatment means with different superscript letters differ significantly (P < 0.05).
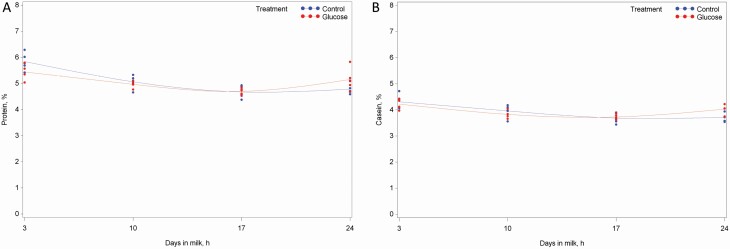
For the milk composition, an interaction between treatment and days in milk (DIM) was found for protein (P = 0.03), and a tendency was found for casein (P = 0.05; Table 6). The ontogeny of protein and casein from 3 to 24 DIM is plotted in Figure 2. Protein content was decreased at 3 DIM and increased at 24 DIM for GLU sows as compared with CON sows, and casein was increased on 24 DIM. Milk content of lactose (P < 0.001) increased from 3 to 24 DIM, and milk protein (P < 0.001) and casein (P < 0.001) decreased concomitantly. Milk yield (P < 0.001), feed intake (P < 0.001), and piglet ADG (P < 0.001) increased from 3 to 24 DIM, and water intake increased from 3 to17 DIM. The piglet ADG was numerically greater for GLU sows as compared with CON sows (22 g/d on day 3 and 27 g/d on day 24).
Table 6.
Milk composition on 3, 10, 17, and 24 DIM and average daily milk yield, water intake, feed intake, and piglet ADG of CON sows and GLU sows
| Treatment (Trt) | DIM | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | GLU | SEM | 3 | 10 | 17 | 24 | SEM | Trt | DIM | Trt × DIM | |
| DM, % | 18.6 | 18.5 | 0.33 | 19.2 | 18.4 | 17.7 | 18.7 | 0.43 | 0.51 | 0.14 | 0.33 |
| Fat, % | 7.72 | 7.68 | 0.42 | 8.35 | 7.62 | 6.95 | 7.88 | 0.46 | 0.94 | 0.12 | 0.46 |
| Lactose, % | 5.11 | 5.05 | 0.09 | 4.78b | 5.04a | 5.32a | 5.19a | 0.09 | 0.66 | <0.001 | 0.57 |
| Protein, % | 5.09 | 5.06 | 0.08 | 5.64a | 5.02b | 4.69b | 4.97b | 0.09 | 0.82 | <0.001 | 0.03 |
| Casein, % | 3.91 | 3.94 | 0.06 | 4.25a | 3.89ab | 3.71b | 3.87b | 0.07 | 0.72 | <0.001 | 0.05 |
| Energy, MJ/kg | 5.12 | 5.08 | 0.15 | 5.43 | 5.05 | 4.75 | 5.17 | 0.18 | 0.85 | 0.05 | 0.36 |
| Milk yield2, kg/d1 | 12.8 | 13.4 | 0.88 | 8.93c | 12.7b | 15.3a | 15.2a | 0.73 | 0.64 | <0.001 | 0.43 |
| Water intake, L/d1 | 47.6 | 42.7 | 8.69 | 30.5b | 49.2ab | 51.2a | 49.7ab | 6.9 | 0.70 | 0.003 | 0.71 |
| Feed intake, kg/d1 | 7.24 | 7.22 | 0.15 | 5.17c | 7.54b | 8.03a | 8.17a | 0.14 | 0.93 | <0.001 | 0.94 |
| Piglet ADG, g/d1 | 240 | 251 | 14.0 | 166c | 233b | 287a | 295a | 12.7 | 0.61 | <0.001 | 0.19 |
1Average daily of day 2 to 7, 8 to 14, 15 to 21, and 22 to 28.
2Calculated according to Hansen et al. (2012).
a,bLeast squares treatment means with different superscript letters differ significantly (P < 0.05).
Figure 2.
The ontogeny of milk protein (A) and casein (B) in sows infused with saline (blue) or 10% glucose (red) solution from when the sign of nest-building behavior was observed until 24 h after the onset of farrowing. The line represents the mean value of each sampling time.
Discussion
Sows approaching farrowing spend extra energy on nest-building behavior, and during farrowing, substantial amounts of energy are required for uterine contractions (Feyera et al., 2018) and colostrum production (Feyera et al., 2019). For all these three biological processes, glucose is a central energy substrate for muscles (standing activity, posture shifts, and uterine contractions), and it is used as a precursor for the synthesis of lactose and fat for colostrum within the mammary gland. The partitioning of glucose among organs (e.g., locomotory muscles, uterus, and mammary gland) is partly controlled by the presence of different types of glucose transporters with different affinities. Normally, the mammary gland has the highest priority due to the presence of glucose transporter-1, whereas locomotory muscles have lower priority due to the high abundance of glucose transporter-4, which are dependent on insulin (Theil et al., 2012). However, for the sow approaching farrowing, substantial amounts of glucose originating from starch in the last meal ingested before farrowing are utilized for physical activity during nest building and hence it is being utilized as fuel during nest building and unintendedly “prioritized” above the mammary gland and the uterus. Most likely, the high level of physical activity during nest building immediately prior to farrowing helps to explain why sows may become depleted of energy prior to or during farrowing.
Energy status during nest building
The intense nest-building behavior occurring 6 to 12 h prior to farrowing (Wischner et al., 2009) is energy demanding because of high locomotory activity where the energy is being oxidized and CO2 released. Heat production is roughly doubled when sows are in standing posture as compared with lying (Theil, 2002), and Noblet et al. (1993) reported that the energetic costs associated with standing activity amount to 0.37 MJ per kg0.75 per day or almost as high as the maintenance requirement (0.46 MJ per kg0.75 per day). Assuming that sows on average perform 6 h of extra standing activity, this amounts to approximately 5 MJ of additional heat or 16% greater heat production than late gestating sows without nest-building behavior (Theil et al., 2002). These findings emphasize that increased physical activity during nest building most likely has a considerable impact on the energy status of the sow at the onset of farrowing. Feyera et al. (2018) reported that the sow plasma glucose 1 h after the onset of farrowing dropped from above 6 to as low as 2 mmol/L if the TLMUOF increased from 2 to 9 h, which indicated that sows most likely became depleted of energy during the nest-building process, especially if the onset of farrowing occurred several hours after the last meal was supplied. In the present study, the mean plasma glucose concentration at the onset of farrowing was above 5 mmol/L for all sows, indicating that sows were not at all depleted of energy during nest building, even though the sows were fed only one meal a day. This meal pattern was chosen to increase the likelihood of having sows with the onset of farrowing many hours after the last meal was served to challenge their energy status. Most likely, the reason why sows were not depleted of energy around the onset of farrowing is that sows in the present study consumed 3.33 kg/d of feed during the last week of gestation (and slightly less at the day of farrowing; 3.05 and 3.14 kg/d), whereas sows in the studies reported by Feyera et al. (2018) were fed approximately 2.6 to 2.9 kg/d the last 3 d before expected farrowing. We speculate whether the lower energy status reported in some sows by Feyera et al. (2018) reflects that the glycogen depot in the liver was depleted and that could explain why some sows were not able to maintain a stable plasma glucose 8 to 10 h after the last meal in that study.
Farrowing dynamics
The farrowing performance was challenged by the experimental design due to the supply of only one meal daily. As a consequence, net absorption of glucose from digested starch is high in the first few hours after feeding, but then it declines and the net absorption of glucose becomes very low 5 to 6 h after feeding and onwards (Serena et al., 2009). Our previous study showed that FD, SR, and the need for FA increased when the time since the last meal exceeded 3 h, indicating that sows may be exhausted (Feyera et al., 2018). During farrowing, Feyera et al. (2018) showed that the uterus extracts glucose and triglycerides as key energy metabolites. Extraction of triglycerides by the uterus in that study was very surprising because NEFA from maternal adipose tissues and not triglycerides are normally mobilized when sows are in negative energy balance, but the uptake of triglycerides to the uterus strongly suggested that the sows (and hence the uterus) lack sufficient glucogenic energy. Most likely, the uterus extracted triglycerides to use glycerol as glucogenic substrate and the three NEFA attached in the triglyceride molecule as ketogenic energy. The intravenous infusion of glucose adopted in the present study was, therefore, carried out to understand whether the uterus indeed lacks glucogenic energy during the farrowing process, and the increased blood O2 after farrowing and reduced incidence of stillborn piglets and FA in sows infused with glucose supported our hypothesis. Surprisingly, the glucose infusion did not translate into higher plasma glucose concentration around farrowing, suggesting that more glucose was utilized, for example, for synthesis of colostrum components (GLU sows produced 5% more colostrum than CON sows, although it was not statistically significant). Similar results were found by Gourley et al. (2020) where blood glucose was lower at the onset of farrowing, 2 h after the onset, and at the end of farrowing for sows fed four daily meals as compared with sows fed only one daily meal. This was especially surprising since the TLMUOF was shorter for the sows having lower blood glucose. The present study and the study by Gourley et al. (2020) suggest that glucose was rapidly utilized for other purposes, and most likely a great part was used for colostrum production.
Energy supply for uterine contractions
The smooth muscles surrounding the uterus contract during farrowing to expel fetuses through the birth canal. The increasing litter sizes in Danish modern prolific sows (Hansen, 2020) increase the timespan of which the uterine smooth muscles are working hard to expel fetuses. For intense and extended work, muscles should ideally perform aerobic metabolism, but when the workload increases, the demand for oxygen exceeds the supply from arterial blood and, therefore, the metabolism shift to anaerobic metabolism. Under aerobic metabolism, lactate is used as an energy source in the muscle, but during anaerobic work, lactate is produced by the muscles and released into the blood circulation. Our previous study showed that the uterus extracted lactate prior to farrowing but released lactate during farrowing and after, suggesting inadequate O2 supply to meet the demand for aerobic metabolism during the expulsive phase (Feyera et al., 2018). Most likely, the sows in the present study had low oxygenation of smooth muscles surrounding the uterus potentially already before the onset of farrowing when the uterus prepares for delivery. This is seen as the level of lactate increased prior to farrowing, and the GLU sows had a tendency to increased plasma lactate at the onset of farrowing as compared with the CON sows (2.25 vs. 1.45 mmol/L, respectively). It is most likely that the control sows had to cover a greater proportion of the metabolic activity using fat as a fuel, whereas sows with glucose infusion could rely more on oxidation of glucogenic energy, and carbohydrates are indeed the preferred substrates for oxidation (Theil et al., 2002). As a consequence, the frequency of FA was lower in GLU than in CON sows (9% vs. 21%, respectively), and for the GLU sows, only one out of the five sows was assisted, while four out of the five CON sows were assisted, although it should be emphasized that the number of sows is fairly low.
CY and composition
CY is highly variable among sows (Quesnel et al., 2015) but limited by the sow capacity when the litter size exceeds approximately 15 live-born piglets. For colostrogenesis, Feyera et al. (2019) demonstrated that the net mammary uptake of O2, lactate, triglycerides, NEFA, and SCFA increased from the onset of farrowing as the farrowing progressed and as the number of suckling piglets increased. Furthermore, the mammary uptake of carbon was comparable to the output of carbon in fat, lactose, and CO2, meaning that almost all colostral fat and lactose were synthesized after the onset of farrowing. Nutritional means to alter colostrum composition have been seen, with colostral fat being the most variable component (Farmer and Quesnel, 2009), but this was not seen in the present study. Instead, GLU sows produced 5% more colostrum than the CON sows, and even though this difference was not statistically different, it may explain why the plasma glucose was not elevated in sows receiving glucose infusion because the mammary glands then drained more glucose from the blood.
Piglet mortality and colostrum intake
The overall aim of improving farrowing kinetics is to reduce the frequency of stillborn piglets that most commonly die of asphyxia due to weak uterine contractions, which in turn causes the fetuses to stay too long in the birth canal (Alonso-Spilsbury et al., 2005). With increasing litter sizes, as in the modern Danish hyperprolific sow, the FD increases (Fahmy and Friend, 1981), which increases the risk of asphyxia for the piglet (Herpin et al., 1996). A previous study found an average SR as the mean across seven different trials being 9.4% with an average litter size of 18.6 piglets (Feyera et al., 2019). Compared with the present study with litter sizes ranging from 22 to 29 piglets with an average litter size of 24.6, the SR was lower for GLU sows as compared with CON sows (7.4% vs. 16.1%, respectively) and even lower than the mean across the seven experiments. This clearly indicates that the glucose infusion was able to counteract the unfavorable energy supply originating from only one daily meal. Because of the very large litter size and the low SR, the sow CY was very high as compared with previous study (Vadmand et al., 2015), whereas the mean piglet intake of colostrum (323 g/piglet) was lower than reported previously (389 g/piglet; Feyera et al., 2019). The CY is limited by the capacity of the sow in litters with more than 15 piglets (Krogh, 2017); therefore, the average colostrum intake is lowered in large litters and most likely this challenges weak piglets, also because high litter size increases the within-litter competition. This is in line with the findings by Decaluwe et al. (2014) where the average colostrum intake decreases 20 g for each additional live-born piglet within the litter. Colostrum intake and hence sow CY are important for piglet survival in the first 24 h postpartum (Le Dividich et al., 2005; Quesnel et al., 2012) because of a high energy requirement of newborn piglets and substantial competition among littermates (Le Dividich et al., 2005). In the present study, a tendency to lower the survival rate of piglets during the colostrum period was observed for sows infused with glucose as compared with control sows (94.7% vs. 99%; P = 0.06). It may be speculated that some of the piglets that previously died during the farrowing process due to energy depletion of the sow now instead die in the colostrum period. However, the survival rate until 24 h of total born piglets was numerically greater in sows infused with glucose as compared with control sows (87.7% vs. 83.1%) indicating that improved energy status and in turn improved farrowing kinetics will improve the overall piglet survival.
Performance until weaning
At weaning, it is desired to wean large, healthy, and robust pigs that are ready for the weaning and fattening periods, and, to reach that, a high milk production is crucial. The milk production was really high in both treatment groups, as indicated by the mean litter weaning weight of 101 kg and mean piglet weight at weaning above 8 kg. Interestingly, piglets weighed approximately 0.3 kg extra at weaning and sows appeared to mobilize more body fat when infused with GLU as compared with CON sows. This supports that the colostrum period is important for the piglet growth rate throughout lactation as reported by Krogh et al. (2016), although neither the CY nor the weaning weight was significantly affected by the infusion treatments.
Conclusions
Intravenous infusion of glucose in the transition period improved farrowing kinetics by reducing SR and FA indicating that adequate energy supply and GLU homeostasis are important factors for a successful farrowing. The plasma concentration of glucose at the onset of farrowing was lower for GLU sows as compared with CON sows indicating that sows infused with glucose utilized the glucose as rapidly as it was infused. During farrowing, GLU sows had increased levels of lactate indicating that the O2 supply was inadequate to meet the demand for aerobic metabolism presumably in the smooth muscles surrounding the uterus. The CY of all sows was very high compared with previous studies, but due to a very high litter size and low SR, the average colostrum intake was clearly lower when compared with previous studies. A tendency to lower survival rate from 0 to 24 h of GLU sows as compared with CON sows are speculated to be an indirect consequence of reduced SR and increased within-litter competition. However, a numerical increase of total survival rate until 24 h suggests that improved farrowing kinetics did indeed improve the total piglet mortality until the end of the colostrum period. The improvements achieved using glucose infusion should be studied further to implement the findings in a more practical, applicable, and consumer acceptable way.
Acknowledgments
We would like to thank the staff personnel in the pig herd for their assistance during the experimental period and the technicians in the Department of Animal Science, Aarhus University, for the analyses of plasma and feed samples.
Glossary
Abbreviations
- ADG
average daily gain
- CON
sows infused with 0.9% saline
- CY
colostrum yield
- DIM
days in milk
- FA
farrowing assistance
- FD
farrowing duration
- FU
feed units
- GLU
sows infused 10% glucose
- HIM
hours in milk
- NEFA
nonesterified fatty acids
- SR
stillbirth rate
- TLMUOF
time from last meal until the onset of farrowing
Conflict of interest statement
The authors declare no conflict of interest.
Literature Cited
- Alonso-Spilsbury, M., Mota-Rojas D., Villanueva-García D., Martínez-Burnes J., Orozco H., Ramírez-Necoechea R., Mayagoitia A. L., and Trujillo M. E.. . 2005. Perinatal asphyxia pathophysiology in pig and human: a review. Anim. Reprod. Sci. 90:1–30. doi: 10.1016/j.anireprosci.2005.01.007 [DOI] [PubMed] [Google Scholar]
- Decaluwe, R., Maes D., Wuyts B., Cools A., Piepers S., and Janssens G. P. J.. . 2014. Piglets’ colostrum intake associates with daily weight gain and survival until weaning. Livest. Sci. 162:185–192. doi: 10.1016/j.livsci.2014.01.024. [DOI] [Google Scholar]
- van Dijk, A. J., van Rens B. T., van der Lende T., and Taverne M. A.. . 2005. Factors affecting duration of the expulsive stage of parturition and piglet birth intervals in sows with uncomplicated, spontaneous farrowings. Theriogenology 64:1573–1590. doi: 10.1016/j.theriogenology.2005.03.017 [DOI] [PubMed] [Google Scholar]
- Fahmy, M. H., and Friend D. W.. . 1981. Factors influencing, and repeatability of the duration of farrowing in Yorkshire sows. Can. J. Anim. Sci. 61:17–22. doi: 10.4141/cjas81-003 [DOI] [Google Scholar]
- Farmer, C., and Quesnel H.. . 2009. Nutritional, hormonal, and environmental effects on colostrum in sows. J. Anim. Sci. 87((13 Suppl):):56–64. doi: 10.2527/jas.2008-1203 [DOI] [PubMed] [Google Scholar]
- Feyera, T., Pedersen T. F., Krogh U., Foldager L., and Theil P. K.. . 2018. Impact of sow energy status during farrowing on farrowing kinetics, frequency of stillborn piglets, and farrowing assistance. J. Anim. Sci. 96:2320–2331. doi: 10.1093/jas/sky141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyera, T., Zhou P., Nuntapaitoon M., Sørensen K. U., Krogh U., Bruun T. S., Purup S., Jørgensen H., Poulsen H. D., and Theil P. K.. . 2019. Mammary metabolism and colostrogenesis in sows during late gestation and the colostral period. J. Anim. Sci. 97:231–245. doi: 10.1093/jas/sky395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friendship, R. M., Metzger K. R., Robinson N. P., and Doig G. S.. . 1990. Cesarean section in the sow: a retrospective analysis of litter size and stillbirth rate. Can. Vet. J. 31:697–699. [PMC free article] [PubMed] [Google Scholar]
- Gourley, K. M., Swanson A. J., Royall R. Q., DeRouchey J. M., Tokach M. D., Dritz S. S., Goodband R. D., Hastad C. W., and Woodworth J. C.. . 2020. Effects of timing and size of meals prior to farrowing on sow and litter performance. Transl. Anim. Sci. 4:724–736. doi: 10.1093/tas/txaa066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, C. 2020. National average in Danish pig production [In Danish: Landsgennemsnit for produktivitet i produktion af grise i 2019] SEGES Svineproduktion. Available from https://svineproduktion.dk/publikationer/kilder/notater/2020/2014.Accessed October 13, 2020.
- Hansen, A. V., Strathe A. B., Kebreab E., France J., and Theil P. K.. . 2012. Predicting milk yield and composition in lactating sows: a Bayesian approach. J. Anim. Sci. 90:2285–2298. doi: 10.2527/jas.2011-4788 [DOI] [PubMed] [Google Scholar]
- Herpin, P., Le Dividich J., Hulin J. C., Fillaut M., De Marco F., and Bertin R.. . 1996. Effects of the level of asphyxia during delivery on viability at birth and early postnatal vitality of newborn pigs. J. Anim. Sci. 74:2067–2075. doi: 10.2527/1996.7492067x [DOI] [PubMed] [Google Scholar]
- Krogh, U. 2017. Mammary plasma flow, mammary nutrient uptake and the production of colostrum and milk in high-prolific sows-impact of dietary arginine, fiber and fat [PhD thesis]. Denmark: Aarhus University. [Google Scholar]
- Krogh, U., Bruun T. S., Poulsen J., and Theil P. K.. . 2017. Impact of fat source and dietary fibers on feed intake, plasma metabolites, litter gain and the yield and composition of milk in sows. Animal 11:975–983. doi: 10.1017/S1751731116002585 [DOI] [PubMed] [Google Scholar]
- Krogh, U., N. Oksbjerg, P. Ramaekers, and P. K. Theil. 2016. Long-term effects of maternal arginine supplementation and colostrum intake on pre- and postweaning growth in pigs. J. Anim. Sci. 94:117–120. doi: 10.2527/jas.2015-9492 [DOI] [Google Scholar]
- Le Dividich, J., Rooke J. A., and Herpin P.. . 2005. Nutritional and immunological importance of colostrum for the new-born pig. J. Agric. Sci. 143:469–485. doi: 10.1017/s0021859605005642 [DOI] [Google Scholar]
- Littell, R. C., Milliken G. A., Stroup W. W., and Wolfinger R. D.. . 1996. SAS system for mixed models. Cary (NC): SAS Institute Inc. [Google Scholar]
- Noblet, J., Shi X. S., and Dubois S.. . 1993. Energy-cost of standing activity in sows. Livest. Prod. Sci. 34:127–136. doi: 10.1016/0301-6226(93)90041-F [DOI] [Google Scholar]
- Oliviero, C., Heinonen M., Valros A., and Peltoniemi O.. . 2010. Environmental and sow-related factors affecting the duration of farrowing. Anim. Reprod. Sci. 119:85–91. doi: 10.1016/j.anireprosci.2009.12.009 [DOI] [PubMed] [Google Scholar]
- Pedersen, T. F., Bruun T. S., Trottier N. L., and Theil P. K.. . 2019. Nitrogen utilization of lactating sows fed increasing dietary protein1. J. Anim. Sci. 97:3472–3486. doi: 10.1093/jas/skz213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesnel, H., Farmer C., and Devillers N.. . 2012. Colostrum intake: influence on piglet performance and factors of variation. Livest. Sci. 146:105–114. doi: 10.1016/j.livsci.2012.03.010 [DOI] [Google Scholar]
- Quesnel, H., Farmer C., and Theil P. K.. . 2015. Colostrum and milk production. In: Farmer, C., editor. The gestating and lactating sow. Wageningen, The Netherlands: Wageningen Academic Pulishers; p. 173–192. [Google Scholar]
- Serena, A., Jørgensen H., and Bach Knudsen K. E.. . 2009. Absorption of carbohydrate-derived nutrients in sows as influenced by types and contents of dietary fiber. J. Anim. Sci. 87:136–147. doi: 10.2527/jas.2007-0714 [DOI] [PubMed] [Google Scholar]
- Theil, P. K. 2002. The consequence of activity on heatproduction in sows [In Danish “Aktivitetens betydning for søers varmeproduktion”]. In: Pedersen, S., editor. Thematic meeting: stable environment at different production strategies [In Danish “Temamøde: Staldklimaet ved forskellige produktionsstrategier”]. Vol. 153. Intern DJF Rapport, Foulum, Tjele; p. 61– 69. [Google Scholar]
- Theil, P. K., Chwalibog A., and Jørgensen H.. . 2020. Energy for pigs: metabolism, requirement, utilisation and prediction of dietary content. In: Back Knudsen, K. E., Kjeldsen H. D., Poulsen H. D., and Jensen B. B., editors. , editors. Nutritional physiology of pigs. Copenhagen (Denmark): Danish Pig Research Centre. [Google Scholar]
- Theil, P. K., Flummer C., Hurley W. L., Kristensen N. B., Labouriau R. L., and Sørensen M. T.. . 2014. Mechanistic model to predict colostrum intake based on deuterium oxide dilution technique data and impact of gestation and prefarrowing diets on piglet intake and sow yield of colostrum. J. Anim. Sci. 92:5507–5519. doi: 10.2527/jas.2014-7841 [DOI] [PubMed] [Google Scholar]
- Theil, P. K., Jørgensen H., and Jakobsen K.. . 2002. Energy and protein metabolism in pregnant sows fed two levels of dietary protein. J. Anim. Physiol. Anim. Nutr. (Berlin). 86:399–413. doi: 10.1046/j.1439-0396.2002.00404.x [DOI] [PubMed] [Google Scholar]
- Theil, P. K., Nielsen M. O., Sørensen M. T., and Lauridsen C.. . 2012. Lactation, milk and suckling. In: Bach Knudsen, K. E., Kjeldsen N. J., Poulsen H. D., and Jensen B. B., editors., editors. Nutritional physiology of pigs. Copenhagen (Denmark): Danish Pig Research Centre; pp. 1–49. [Google Scholar]
- Vadmand, C. N., Krogh U., Hansen C. F., and Theil P. K.. . 2015. Impact of sow and litter characteristics on colostrum yield, time for onset of lactation, and milk yield of sows. J. Anim. Sci. 93:2488–2500. doi: 10.2527/jas.2014-8659 [DOI] [PubMed] [Google Scholar]
- Wischner, D., Kemper N., and Krieter J.. . 2009. Nest-building behaviour in sows and consequences for pig husbandry. Livest. Sci. 124:1–8. doi: 10.1016/j.livsci.2009.01.015 [DOI] [Google Scholar]