Abstract
Most currently recommended therapies for metabolic dysfunction-associated fatty liver disease (MAFLD) involve diet control and exercise therapy. We searched PubMed and compiled the most recent research into possible forms of programmed cell death in MAFLD, including apoptosis, necroptosis, autophagy, pyroptosis and ferroptosis. Here, we summarize the state of knowledge on the signaling mechanisms for each type and, based on their characteristics, discuss how they might be relevant in MAFLD-related pathological mechanisms. Although significant challenges exist in the translation of fundamental science into clinical therapy, this review should provide a theoretical basis for innovative MAFLD clinical treatment plans that target programmed cell death.
Keywords: Metabolic dysfunction-associated fatty liver disease, Apoptosis, Necroptosis, Autophagy, Pyroptosis, Ferroptosis
Introduction
Metabolic dysfunction-associated fatty liver disease (MAFLD; also referred to as metabolic-associated fatty liver disease) has replaced nonalcoholic fatty liver disease (NAFLD) to more accurately reflect the mechanism seen in patients with metabolic dysfunction and fatty livers [1–5]. Therefore, MAFLD is used throughout this article.
MAFLD is a chronic disease that has become one of the most common reasons for liver transplantation, especially in Western countries [6]. The condition is closely related to metabolic disorders, including type 2 diabetes, hypertension, hyperlipidemia, and obesity. The current internationally accepted clinical practice guidelines state that a diagnosis of MAFLD should be based on histological (biopsy), imaging and/or blood biomarker evidence of fat accumulation in the liver (hepatic steatosis) along with one of the following criteria: obesity, confirmed type 2 diabetes mellitus (T2DM), or evidence of metabolic dysregulation [2, 7]. Other pathogenic factors and pathological mechanisms known to be involved in the complex disease progression of MAFLD include exercise, diet, intestinal microflora disorders, genetic susceptibility, fat accumulation, lipotoxicity, oxidative stress, endoplasmic reticulum stress, mitochondrial dysfunction, and intestine–liver axis-related signal transduction, among others [6].
Cell death occurs in various physiological and pathological processes in the body [8]. In specific diseases, it triggers distinct responses that are context-dependent [9]. The death of liver cells can trigger and aggravate chronic inflammation and liver fibrosis, advancing to chronic cirrhosis and liver cancer. While cell death in the liver releases a variety of pro-inflammatory cytokines and triggers the organ's regeneration responses, it also comes with the risk of acute liver failure. Chronic liver disease is indicative of an insufficient response to cell death, leading to ineffective wound healing, which increases the risk of fibrosis and hepatocellular carcinoma (HCC) [10, 11].
In vitro and in vivo experiments have demonstrated the key role of liver cell death in MAFLD. Liver cell damage, liver cell death, inflammation and oxidative stress, the principal pathogenic features of MAFLD, are interrelated [12]. In this review, we discuss possible forms of regulatory cell death (RCD) or programmed cell death (PCD) and their molecular mechanisms in MAFLD. The aim is to provide a theoretical basis for targeting RCD or PCD pathways as a novel treatment for MAFLD.
Apoptosis
The concept of apoptosis was first proposed by Kerr et al.. Although it initially attracted little attention, excellent research progress has been made in the last ten years [13]. Apoptosis is considered a basic biological and physiological process. Its dysregulation is involved in various diseases and pathologies, including damage from drug toxicity, immune responses, infections and tumors, and metabolic disorders [14]. It has highly specific and recognizable morphological features. It is characterized by concentrated chromatin and cell shrinkage caused by DNA breakage. Under normal circumstances, the entire process of apoptosis is controllable, meaning that the integrity of the cell plasma membrane is maintained to prevent the leakage of cell contents, thus preventing inflammation [11].
Mechanisms of apoptosis
Apoptosis primarily occurs the external death receptor pathway or the internal mitochondrial pathway. In addition, it can be triggered by mitochondrial dysfunction, lysosomal permeability, endoplasmic reticulum stress (ERS), and nuclear DNA damage [15] (see Fig. 1 for details).
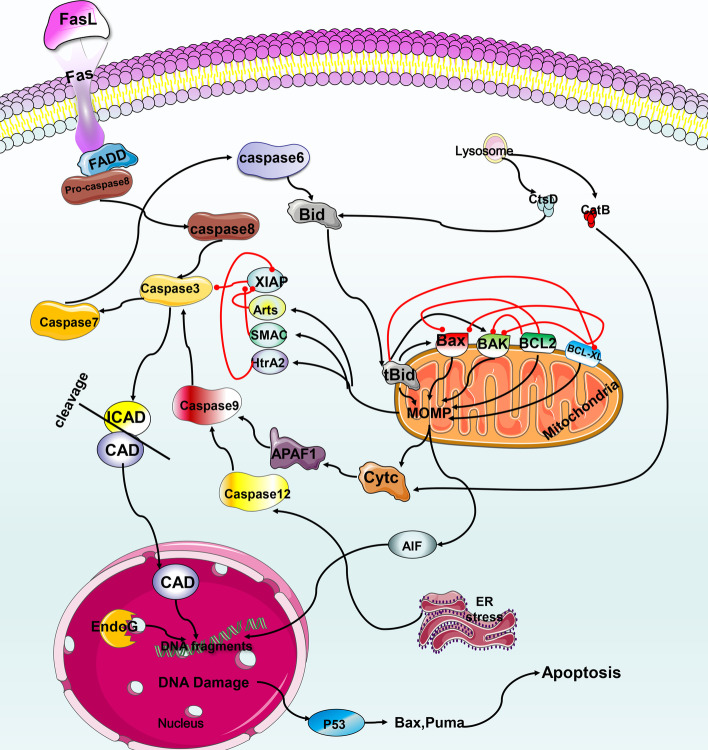
Fig. 1.
Mechanism of apoptosis. (1) After Fas and FasL are combined in the death receptor pathway, they recruit molecules, such as FADD, and activate caspase cascade reactions, including activation of caspase 8, caspase 3 and caspase 7. Caspase 8 activation can be blocked by C-FLIP. Then, caspase 3 and caspase 7 continue to cleave cell substrates, such as ICAD, and trigger apoptosis. (2) Caspase 3 and caspase 7 in the death receptor pathway may activate caspase 6 to cleave Bid into tBid, which is transferred to the mitochondria and induces the release of cytochrome c (cytc), AIF, Smac/DIABLO, Omi/HtrA2, etc. Cytc activates caspase 9, APAF1, etc. to cause apoptosis, and the other three inhibit the XIAP survival protein to promote apoptosis. (3) AIF translocation from the mitochondria to the nucleus may interact with EndoG to trigger DNA degradation and apoptosis. P53 induces apoptosis by transactivating the expression of various proapoptotic proteins. (4) Lysosomal permeabilization induces apoptosis through the release of ctsB and ctsD to activate the mitochondrial pathway. (5) Dysfunction of the endoplasmic reticulum leads to UPR-induced apoptosis by activating caspase 12, 9 and 3
The external death receptor pathway is primarily mediated by members of the tumor necrosis factor (TNF) superfamily, such as Fas and the TNF receptor (TNFR). After activation by an exogenous ligand, a series of reactions lead to apoptosis (Fig. 2). Caspase 9 is the promoter of the intrinsic mitochondrial pathway and the B-cell lymphoma/leukemia-2 (BCL2) protein family mediates it. The BCL2 protein family can be divided into 3 subclasses. The first includes pro-apoptotic proteins, such as Bcl-2–associated X protein (Bax), BCL-2 homologous antagonist/killer (Bak), BCL-2 ovarian killer (Bok), BCL2-associated agonist of cell death (Bad), Noxa, and BCL-2 binding component 3 (PUMA). The second is anti-apoptotic proteins, such as BCl2 and BCL-XL (also known as BCL2L1). The third is the BH3-only protein, BH3-interacting domain death agonist (Bid), which is primarily responsible for crosstalk between the two apoptosis pathways. Bid is activated by caspase 6 to mediate mitochondrial outer membrane permeabilization (MOMP) and apoptosis [16].
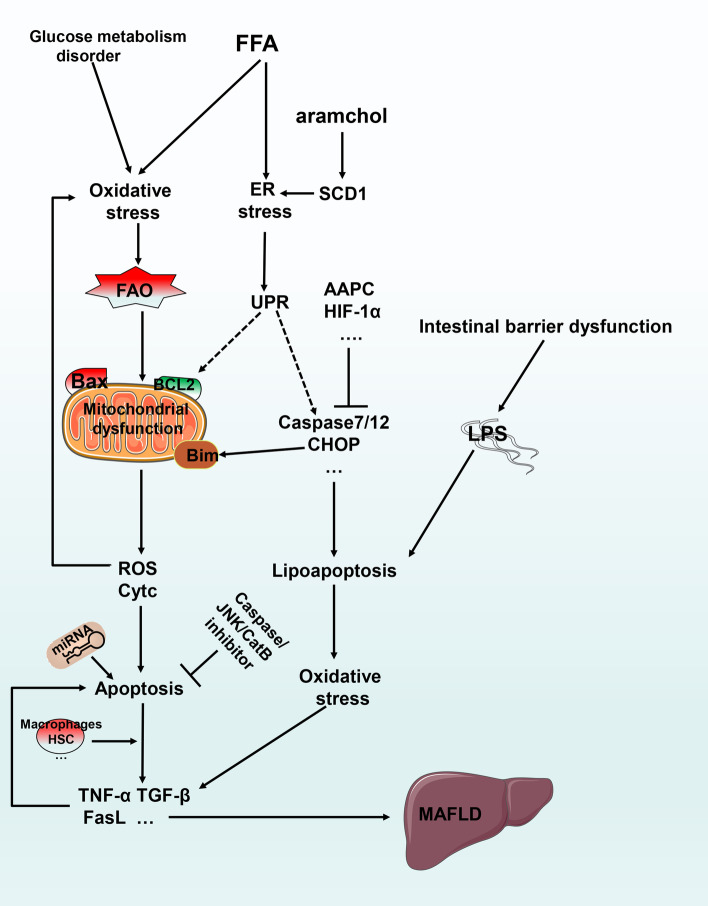
Fig. 2.
The relationship between apoptosis and MAFLD. Excessive FFAs and other compounds causes oxidative stress, ER stress and other causes of mitochondrial dysfunction, resulting in the release of ROS and cytochrome c with subsequent apoptosis. ER stress may cause lipoapoptosis through caspase 7/12 and other mechanisms, eventually leading to the development of MAFLD. MiRNAs can regulate apoptosis through different mechanisms. Intestinal barrier dysfunction causes LPS to activate lipoapoptosis
MAFLD and apoptosis
Liver biopsy remains the standard for MAFLD testing. Studies have shown that the levels of markers of hepatocyte apoptosis in plasma from patients with MAFLD correlate with histological severity. Therefore, the detection of activated caspase or the cytokeratin-18 (CK-18) fragment cleaved by caspase 3 are potentially noninvasive ways to detect human MAFLD and liver fibrosis [17]. However, when CK-18 levels, histology and liver fat contents were studied for a large multi-ethnic group of people, it was found that the degree of MAFLD could not be determined based on CK-18 [18]. Despite this, it remains useful when combined with other noninvasive methods, particularly because its levels are not affected by confounding factors, including age and obesity. Thus, it has the potential for clinical applications: further research is needed on population factors and the severity of fibrosis, among other things, to establish parameters for its use as a biomarker of MAFLD [18].
The accumulation of excess saturated fatty acids leads to apoptosis through oxidative stress and endoplasmic reticulum stress [19]. Hypertriglyceridemia, which results in lipoapoptosis, is found in non-obese MAFLD patients [20]. In fact, lipoapoptosis induced by free fatty acids is the primary form of apoptosis in MAFLD.
Lipoapoptosis enhances oxidative stress, which in turn increases the apoptosis, inflammation and fibrosis, creating a vicious cycle [21]. Egnatchik et al. found that primary hepatocytes and H4IIEC3 cells with palmitate and calcium chelators induced mitochondrial dysfunction by altering calcium homeostasis in the endoplasmic reticulum, which enhanced reactive oxygen species (ROS) production and apoptosis [22].
Lipoapoptosis and ERS are related, with the death receptor DR5 activated by ERS-related protein expression (CHOP) protein or by further binding c-Jun N-terminal kinase (JNK) to activate Bim (also known as BCL2L11), PUMA and other proteins to further activate caspase 3 [23]. Wang et al. experimentally demonstrated that Asiatic acid (AAPC) inhibits the expression of CHOP, caspase 12, JNK and other proteins, thereby attenuating ERS, apoptosis and lipid metabolism disorders [24].
In a recent clinical trial aramchol, a stearoyl-CoA desaturase 1 (SCD1) modulator, was used to treat MAFLD subjects for 3 months. Patient liver fat content was reduced and the treatment was well tolerated. Aramchol may regulate SCD1 fatty acid enzymes, ultimately affecting ERS and apoptosis [25, 26]. Recent studies showed that in a MAFLD mouse model, caspase 2 deletion reduced apoptosis and inhibited the profibrotic pathway, in turn inhibiting MAFLD progression [27]. Ferreira et al. evaluated subjects using the Kleiner-Brunt scale and found that the activation of caspases 3 and 2 and the DNA fragmentation in the liver of patients with severe MAFLD were significantly higher than in steatosis patients [28].
Intestinal barrier dysfunction in MAFLD leads to lipopolysaccharide (LPS) leakage of and disturbance of the intestinal flora, which may lead to excessive fat accumulation and lipoapoptosis [29]. Li et al. used LPS and free fatty acids (FFAs) to induce MALFD in vitro and found that the treatment upregulated Bax, cleaved caspase 3 and 8, and downregulated Bcl-2, leading to apoptosis, which was TNF-α- and caspase-dependent [30] (see Fig. 2 for details).
MAFLD-related external death receptor pathway
Increased Fas expression is observed in patients with MAFLD [31]. Fas and FasL expressions and the rate of apoptosis are also higher in obese children with obstructive sleep apnea (OSA) than in obese children without OSA [32]. A study of MAFLD patients diagnosed by liver biopsy found that their CK-18 and TNF-α levels were higher than those of the control group [33]. The expressions of external death receptor pathway-related proteins are higher in MAFLD patients, and the use of relevant drugs improves disease-related indicators by inhibiting these proteins. Studies have found that transaminase levels, insulin resistance, adiponectin levels, liver fibrosis and steatosis improve in MAFLD patients when TNF-α levels are attenuated [34]. Furthermore, inhibition of Fas reduces hepatocyte apoptosis and reduces associated liver damage [35]. In addition, Kroy et al. found that deletion of c-Met in the liver cells of mice fed a methionine- and choline-deficient (MCD) diet led to upregulation of fatty acid metabolism genes. The increase in TUNEL-positive cells and superoxide anions aggravates the progression of MAFLD, while knockout of caspase 8 inhibits progression [36].
Interestingly, Physi et al. found that gastric bypass surgery (Roux-en-Y gastric bypass, RYGB) yields similar improvements in disease-related indicators as can be achieved with apoptosis-inhibiting drug therapy,and the levels of glucose-regulated protein-78 (Grp78), X-box binding protein-1 (XBP-1), spliced XBP-1, fibroblast growth factor 21 (FGF21), other ERS-related proteins and excessive apoptosis were all reduced compared to the control group [37]. Aerobic exercise improves MAFLD by inhibiting TNF-α and reducing ROS and cytochrome c levels [38].
MAFLD-related internal mitochondrial apoptosis
The internal mitochondrial apoptosis pathway is also activated by the abovementioned factors. Resistance to lipoapoptosis in MAFLD is partly due to the existence of a hedgehog autocrine survival signaling pathway [39]. Geng et al. used Smad4 knockout mice to demonstrate the protective effect of Smad4 deletion on MAFLD: it blocks the mitochondrial apoptotic pathway by inhibiting expression of the pro-apoptotic genes Bax and caspase 3 [40].
Disorders in glucose metabolism in MAFLD can easily lead to hyperinsulinemia and hyperglycemia. Hyperglycemia can cause increased oxidative stress and trigger mitochondrial dysfunction, including mitochondrial depolarization, cytochrome c release, and changes in Bax and Bcl-2 expression. Recent studies have shown that casein kinase 2-interacting protein-1 (CKIP-1) may regulate insulin signaling by inhibiting the phosphorylation of JNK1. The CKIP-1-deficient MAFLD model exhibits more severe fatty liver through an increase in phosphorylated JNK1, which further inhibits insulin receptor substrate-1 (IRS-1) serine phosphorylation and IRS-1 tyrosine phosphorylation, ultimately aggravating insulin resistance, hyperglycemia, apoptosis and MAFLD [41]. Cyanidin-3-O-β-glucoside (C3G) inhibits the caspase 3, caspase 9, Bax and JNK pathways by reducing hyperglycemia-induced oxidative stress and related mitochondrial disorders to improve MAFLD [42]. Other, similar studies indicate that intermittent hyperglycemia (IHG) in the setting of lipotoxicity may lead to oxidative stress and hepatocyte apoptosis by increasing mitochondrial permeability transition (MPT) and mitochondrial dysfunction, which promotes MAFLD [43].
MAFLD-related miRNAs regulate apoptosis
Analysis of various MAFLDs have enabled researchers to determine multiple miRNAs with expression changes related to the condition. It is believed that there may be therapeutic potential in regulating apoptosis through relevant miRNAs [44, 45]. Castro et al. identified a relationship between the miR-34a–SIRT1–p53 pro-apoptotic pathway and hepatocyte apoptosis: miR-34a, apoptosis and acetylated p53 levels increased in MAFLD livers, while the SIRT1 level decreased. This pathway is specifically regulated by ursodeoxycholic acid (UCDA) [46]. One study found that after treatment with creatine, the miR-34a–SIRT1–p66shc anti-apoptotic pathway may reduce high-fat diet or palmitic acid-induced increases in cleaved caspase 3, caspase 3 and caspase 9, thereby improving MAFLD [47]. MiR-296-5p and miR-615-3p regulate lipid-associated apoptosis levels in MAFLD by negatively regulating PUMA, a pro-apoptotic protein, and CHOP [48, 49]. Notably, miR-21 may play a distinct role in MAFLD. Rodrigues et al. found that combined ablation of miR-21 with obeticholic acid improves MAFLD-related pathology, including steatosis, inflammation and lipoapoptosis [50].
Necroptosis
Necroptosis was first observed in experiments by Ray et al. in 1996 [51]. It is characterized by morphological changes similar to those observed in necrosis, but is distinct in that it is a controllable form of death. Its morphological changes include organelle swelling, damage to the plasma membrane and release of cellular contents, which may lead to the occurrence of secondary inflammation [12]. Key molecules for necroptosis include mixed lineage kinase domain-like (MLKL), and RIP protein kinase family members RIPK1 and RIPK3.
TNF is a key cytokine in inflammation and other aspects of biology. TNFR1 has been widely studied in regulating cell survival, apoptosis and necroptosis [52]. TNFR1-mediated signal transduction is an example of the molecular mechanism of necroptosis and the conversion between apoptosis and necroptosis (see Fig. 3 for details).
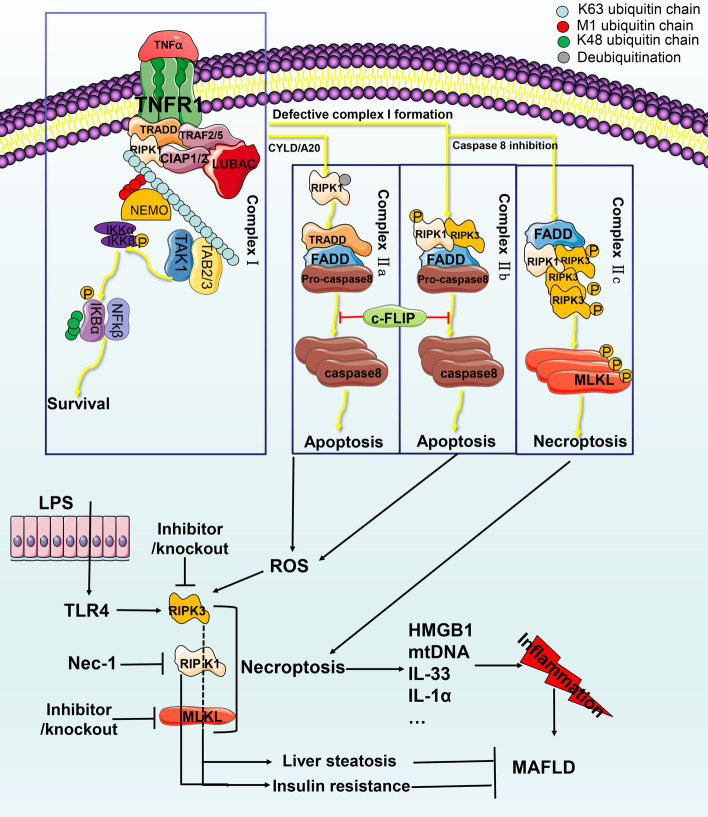
Fig. 3.
Mechanism of necroptosis and its relationship with MAFLD. (1) TNF activates TNFR1 and recruits TRADD, TRAF2/5, clAP1/2 and LUBAC to form complex I. RIPK1, TAB2/3, Clap1/2 and LUBAC undergo M1 and K48 ubiquitination events and connect and recruit NEMO. IKKa, IKKb and RIPK ubiquitination events lead to the formation of the TAK complex, which phosphorylates IKK2 and activates the NF-KB pathway, cFLIP protein formation, K48 phosphorylation events and proteasome degradation. (2) CYLD or A20 deubiquitinates RIPK1 and induces dissociation of TRADD and RIPK1 to form complex IIa, including FADD, pro-caspase 8, and TRADD. Complex IIb includes RIPK1/3, FADD, and caspase 8. cFLIP inhibits complex IIa and IIb-induced apoptosis. (3) When caspase 8 is inhibited, complex IIc (the necrosome) is formed, containing RIPK1, RIPK3 and MLKL. RIPK1 phosphorylates RIPK3 and activates MLKL to cause changes in cell membrane permeability and necroptosis. The released substances cause inflammation. Activated TLR4 and ROS-activated RIPK3 may be related to liver fibrosis and insulin resistance. Inhibition of RIPK1 and MLKL may improve liver steatosis and insulin resistance
MAFLD and necroptosis
The destruction of membrane integrity during necroptosis releases various proinflammatory mediators and promotes the progression of MAFLD [53]. Ding et al. found that AS1842856 improves MAFLD by inhibiting forkhead box protein O1 (FOXO1), ERS and necroptosis [54]. TNF-α-mediated necroptosis is widely recognized as the most classical pathway. Both human MAFLD samples and experimental animal MAFLD livers can show elevated TNF-α [55] and TNFR1 [56] levels. In addition to TNF-α, other pattern recognition receptors, such as toll-like receptor 4 (TLR4), are also involved in the activation of necroptosis. In MAFLD, hepatocytes are exposed to a large number of TLR4 ligands, such as gut-derived LPS [57], which subsequently activate the TLR4 receptor. It activates RIPK3 and MLKL, which trigger necroptosis [58].
RIPK3 in MAFLD
RIPK3 kinase is an indispensable component of necroptosis. Studies have shown that the expression levels of RIPK3 correlate with the sensitivity of cells to necroptosis and are very low in the liver under normal physiological conditions. This indicates that under normal circumstances, necroptosis does not frequently occur in the liver and may be a back-up programmed death regulatory mechanism when apoptosis fails [59–61].
Human MAFLD liver samples show strong upregulation of RIPK3 [62]. Gautheron found that RIPK3 mediates MAFLD development through a positive feedback loop with JNK that includes liver cell death, inflammation, and fibrosis [63]. Based on results for various experimental MAFLD animal models, the role of RIPK3 appears to be context-dependent. The lack of RIPK3 in high-fat diet experiments by Roychowdhury et al. showed that increased liver damage, liver steatosis, ALT/AST, inflammation and apoptosis may occur due to inhibition of necroptosis, which converts the form of programmed cell death to apoptosis, exacerbating damage [64]. Saeed et al. also used a high-fat diet to induce a model of RIPK3 knockout, which aggravated liver steatosis but partially inhibited inflammation [65]. Due to the complexity of RIPK3, it seems that direct targeted inhibition of RIPK3 kinase is not an ideal strategy for MAFLD therapy. Further research is needed to clarify the role of RIPK3, and other key molecules should be examined as well, including RIPK1 and MLKL.
RIPK1 and MLKL in MAFLD
Studies have shown that RIPK3 self-oligomerization is sufficient to induce necroptosis. RIPK1 kinase acts as a positive regulator of RIPK3 by forming a amyloid-like oligomers with RIPK3. It can also act as a negative regulator of RIPK3 in cells to promote cell survival [66]. Studies have shown that using RIAP-56, a potent inhibitor of RIPK1, improves the histological characteristics of MAFLD in HFD mice through MLKL, reducing liver inflammation, fibrosis and liver lipid accumulation. Its mechanism may involve ameliorating mitochondrial dysfunction and promoting β oxidation [67].
In addition, MLKL deficiency may be regulated by phosphatidylinositol (3,4,5)-trisphosphate (PIP3) in liver cells, which reduces insulin resistance and glucose intolerance. Paradoxically, it was found that neither MLKL nor RIPK1 inhibition reduced inflammation [68]. Saeed et al. found that patients with MAFLD exhibited increased MLKL levels compared to the non-MAFLD group and that MLKL−/− mice induced with a high-fat diet showed decreased transferase levels, triglycerides, MAFLD activity scores, steatosis score, inflammation, balloon degeneration, and expression of de novo lipogenesis (DNL) genes in the liver [69]. However, Suda found that RIPK1 antisense knockdown caused α-galactosylceramide-treated C57BL/6 mice to undergo large-scale apoptosis-type immune liver injury. Lethality and was not associated with the inhibition of NF-kB and necroptosis [70].
Most current research involves knockout or inhibition of key molecules of systemic necroptosis in disease models. Therefore, further studies on pure liver-specific knockout and immune system-related connections are needed.
Autophagy
The research history of autophagy spans several decades. It was first officially named autophagy by de Duve, who was building on earlier discoveries [71–73]. The name derives from the Greek roots phagía = eat or consume and auto = self [74]. Cell autophagy involves the formation of autophagosomes (bilayer membrane vesicles that originate as omegasomes on the endoplasmic reticulum) and autophagolysosomes. Their function is to recover and reuse unwanted cellular proteins [75, 76], carbohydrates [77] and lipids [78]. Autophagy is an important form of death that controls the degradation of intracellular waste and efficiently recycles substances.
Molecular mechanisms of autophagy
Selective autophagy can be targeted to degrade functionally damaged or excessive organelles, microorganisms, lipids, etc. [79]. There are two key steps in the metabolic autophagy pathway in the liver: formation and degradation (autophagic flux). Three major forms of autophagy coexist: macroautophagy, chaperone-mediated autophagy and microautophagy. For more detailed molecular mechanisms, see the extensive reviews on autophagy [80, 81]. Herein, we primarily focus on macroautophagy (hereinafter referred to as autophagy) and its selective forms (see Fig. 4 for details).
Fig. 4.
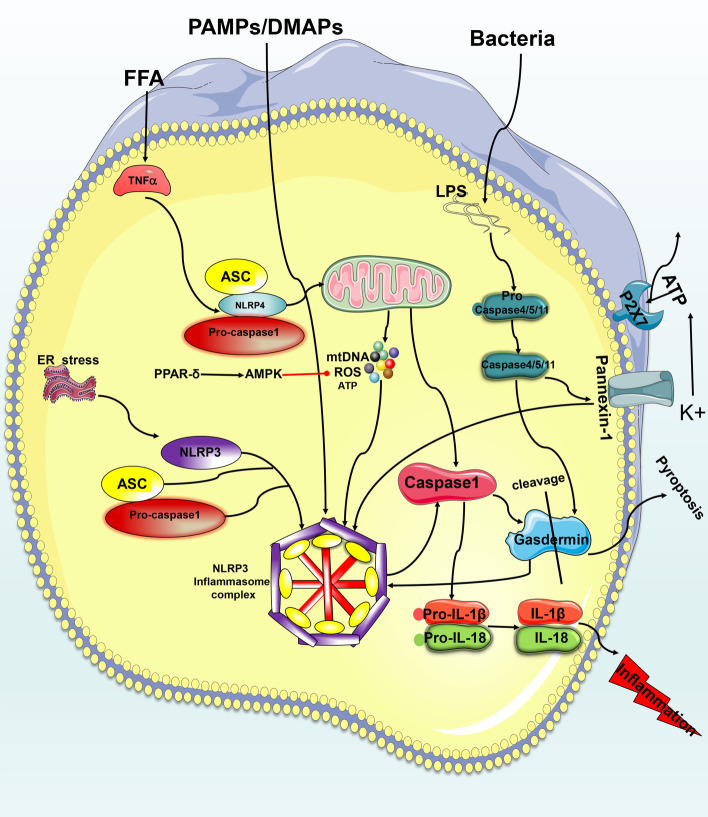
Mechanism of pyroptosis. (1) In the classical pathway, PAMPs/DAMPs activate assembly of the NLRP3 complex and sequentially cleave and activate caspase 1 to cause the maturation and release of IL-1β and IL-18 or to activate gasdermin to form a pore structure that leads to pyroptosis. (2) In the nonclassical pathway, activation of caspase 4, 5 and 11 cleaves GSDMD and causes pyroptosis. The two pathways engage in crosstalk with each other via panxinin-1 and GSDMD
MAFLD and autophagy
Under physiological conditions, autophagy is primarily induced by starvation. It maintains the body's metabolic homeostasis by regulating biochemical reactions, such as gluconeogenesis and fatty acid oxidation [82]. The liver is one of the principal vital organs of metabolism. Studies show that starvation stimuli activate autophagy in the liver peaks in just a few hours [83].
Autophagy is indispensable for liver metabolism and has been considered to have a protective effect on the liver. When mice lack key Atg proteins of autophagy, liver cells are more susceptible to damage [84]. MAFLD patients often have a high-fat diet. Short-term high-fat intake will activate autophagy to prevent the occurrence of lipotoxicity. However, long-term chronic high-fat intake restricts autophagosome and lysosome fusion, increasing the risk for MAFLD development [85].
Autophagy and lipid metabolism in MAFLD
The selective form of autophagy for fat is referred to as lipophagy [86]. Under physiological conditions, it primarily works through the synergistic effect of cytosolic and lysosomal lipases and the lipid droplet transport of RAB7 to engulf lipids and increase free fatty acid content. In pathological conditions, impaired autophagy can lead to fat accumulation. Ubiete-Franco et al. found that the levels of glycine N-methyltransferase (GNMT) in patients with MAFLD were reduced and that GNMT knockout mice exhibited increased levels of methionine and its metabolite S-adenosylmethionine (SAMe) along with inhibited autophagic flux through methylated PP2A, which may be one of the mechanisms leading to increased liver fat production [87]. Byun et al. found that phosphorylation of Jumonji-D3 (JMJD3) at Thr1044 by FGF21 signal-activated PKA increases its nuclear localization and interaction with the nuclear receptor PPARα to transcriptionally activate autophagy genes, such as Tfeb, Atg7 and Atgl, causing lipolysis. It also reduces the liver expression of JMJD3, Atg7, LC3 and ULK1 in MAFLD [88]. Wang et al. found that formononetin causes lipophagy to reduce fat accumulation by activating AMPK and promoting nuclear translocation of TFEB in HFD mice [89]. Tang et al. found that osteopontin (OPN) was elevated in an MAFLD mouse model and that this, combined with integral αVβ3 and αVβ5, can reduce FFA-induced autophagy in HepG2 cells, leading to lipid accumulation, which is reversed by inhibiting OPN [90]. Some traditional Chinese herbal medicine seems to have advantages in treating MAFLD with autophagy as the target [91, 92]. Ren et al. used catalpol, an iridoid glucoside derived from the rehmannia root, to improve hepatic steatosis in ob/ob fatty liver mouse models induced by a high-fat diet, and the authors speculated that catalpol’s anti-fat denaturation may enhance nuclear translocation of TFEB through phosphorylation activation of AMPK [93].
However, there are also studies showing that impaired autophagy reduces fat production. For example, when autophagy in the mouse liver is impaired, triglyceride levels decrease and ketone body production is impaired [94].
AMPK/mTOR-regulated autophagy may represent a target for MAFLD. Tong et al. found that PPARα regulates autophagy through the AMPK/mTOR pathway, reduces intrahepatic fat and stimulates β-oxidation in liver cells [95]. Zeng et al. found that acetylshikonin (AS) improves MAFLD and may increase liver autophagy levels through the AMPK/mTOR pathway [96].
Autophagy, metabolic stress and insulin resistance in MAFLD
Obesity models often exhibit decreased expression of the ATG7 protein. Impaired autophagy is usually accompanied by ER stress and insulin resistance. The latter leads to elevated insulin, which will in turn aggravate autophagy dysfunction [97, 98]. This may be controversial because Yan et al. found that chlorogenic acid inhibits autophagy through the JNK pathway to reduce insulin resistance and improve MAFLD [99].
Zhang et al. found that increased levels of P62 and LC3-II in experimental MAFLD mice indicate the inhibition of autophagy and cause increases in GRP78, PDI, p-PERK, p-eIF2a and eIF2a, indicating the emergence of ER stress [100]. Similarly, Lee et al. found that Eucommia ulmoides leaf extract improves both autophagic flux and HFD-induced steatosis in mice by inhibiting mTOR and the ER stress-related proteins PERK, p-eIF2a, GRP78 and CHOP [101]. Unconventional activation of sterol regulatory element-binding protein 2 (SREBP-2) leads to MAFLD cholesterol accumulation. Deng et al. found that inhibition of SREBP-2 reduces ERS by enhancing the LC3-II-to-LC3-I ratio, autophagic flux and lipolysis, and inhibiting PERK-P-EIF2α signaling [102]. However, Kim et al. reported that administration of lovastatin and ezetimibe increased SREBP-2 in HFD mice and resulted in the interaction of patatin-like phospholipase domain-containing enzyme 8 (PNPLA8) with LC3 to promote autophagy and reduce hepatic steatosis [103].
Additionally, Jiang Zhi granules (JZGs) inhibit palmitate-induced autophagosome flux damage and promote the fusion of autophagosomes and lysosomes to restore autophagy, protecting liver cells from oxidative stress damage and mitochondrial disorders [104]. Irbesartan inhibits PKC and activates AMPK and ULK1, increasing the number of autolysosomes and autophagosomes. Upregulation of the autophagy proteins Atg5 and LC3BII/I reduces lipid deposition, improving mitochondrial function and reducing ROS levels [105].
Autophagy and MAFLD-related liver fibrosis
Lipid bilayer stress can induce ER stress and control autophagy to mediate the steady state of the unfolded protein response (UPR) via the IRE-1–XBP-1 axis in MAFLD [106]. XBP1-mediated UPR activates HSCs to secrete collagen 1-α in a TGF-β-independent manner through autophagy. This effect is disrupted by inhibiting autophagy [107]. Transforming growth factor-β activated kinase-1 (TAK1) can be triggered in response to different cytokines, including IL-1, TNF-α and TGF-β. When TAK1 is enabled, it enhances autophagic activity by inhibiting mTORC1 activity and the AMPK pathway. The authors found that mTORC1 activity was enhanced, while AMPK and autophagy were inhibited, in TAK1-deficient mice, leading to spontaneous liver fibrosis and even cirrhosis. The inhibition of mTORC1 reversed the abovementioned phenotype [108].
It is useful to note that the role of autophagy in MAFLD-related fibrosis may have two sides. Autophagy can engulf lipid droplets in HSCs through lipid phagocytosis, providing energy substrates, such as ATP for HSC trans differentiation, which ultimately ameliorates liver fibrosis. Furthermore, autophagy-deficient cells exhibit lower HSC activation rates [109, 110].
Others
In addition to the three PCDs mentioned above, there may be other PCDs in MAFLD. Because the overall experimental evidence on their potential functions in MAFLD seems not so advanced, we recommend that researchers continue to conduct in-depth investigations of these pathways.
Pyroptosis and MAFLD
Pyroptosis is a newly discovered form of programmed death that is characterized by cellular content release mediated by caspases. It was officially named pyroptosis from the Greek roots pyro and ptosis to reflect its proinflammatory activity [111].
The morphological characteristics of pyroptosis are distinct from other forms of programmed death. It involves rapid rupture of the plasma membrane and the release of proinflammatory intracellular contents. Nuclear DNA lysis also occurs in pyroptosis, similarly to apoptosis [112]. Pyroptosis involves caspases 1, 4, 5 and 11 sensing different pathogen-associated molecular pattern (PAMP) or damage-associated molecular pattern (DAMP) activation and forming a pore-like structure on the cell membrane. Leakage of inflammatory mediators, such as IL-18/1β, leads to cell lysis and death [113]. Pyroptosis may occur through the classic caspase 1-dependent pathway [114] and the nonclassical caspase 4/5 (mouse caspase-11) pathway [115]. Its key molecules include NOD-LRR and pyrin domain-containing 3 (NLRP3); gasdermin D (GSDMD); and caspase 1, 4, 5 or 11 (see Fig. 5 for details).
Fig. 5.
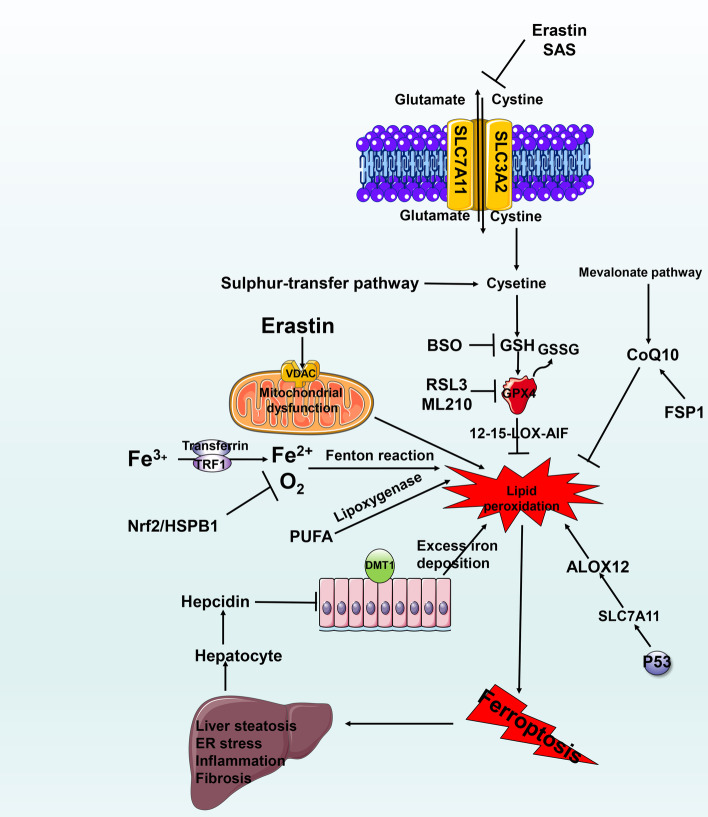
The molecular mechanism of ferroptosis and its relationship with MAFLD. The Fenton reaction and lipoxygenase regulation of ferroptosis via three regulatory pathways. Inhibition of the xc-system leads to depletion of GSH and excessive iron deposition through the Fenton reaction, causing lipid peroxidation and further leading to the progression of MAFLD
Recent research suggests that pyroptosis represents a key link to MAFLD [116]. Zhong et al. found significant pyroptosis in a mouse model of MAFLD induced by HFD and a MAFLD model in liver cells induced by FFA. Genipin(GNP)reduced the expressions of pyroptosis-related genes and release of lactate dehydrogenase by inhibiting uncoupling protein-2 (UCP2).Overexpression of UCP2 upregulated the degree of pyroptosis. Finally, they proved that GNP, a natural water-soluble cross-linking agent, alleviates MAFLD by inhibiting UCP2-mediated pyroptosis [117].
Inhibiting activation of the NLR family to mediate pyroptosis may be a potential therapy for MAFLD. A large induction of NLRP3 activates MAFLD [116]. Studies have shown that berberine has a therapeutic effect on MAFLD through inhibition of the ROS–TXNIP axis, NLRP3, caspase 1 activity, and GSDMD-N expression [118]. In addition to NLRP3, the NLR family members NOD-LRR and pyrin domain-containing 4 (NLRP4) are also related to MAFLD. Recently, Chen et al. constructed a MAFLD cell model in vitro and found that NLRP4 is regulated by TNF-α levels and can be ectopically transferred to the mitochondria after activation by free fatty acids, and the rest of the process was similar to NLRP3.
Caspase 1 activation and lysing induces expression of IL-18 and IL-1β, eventually leading to pyroptosis and the release of proinflammatory cytokines [119]. It is worth noting that the IL-18 and IL-1β released by MAFLD during pyroptosis seem to be a double-edged sword [113]. Studies by Yamanishi et al. have demonstrated that IL-18 is essential for normal lipid metabolism in the liver and that its deficiency causes fat accumulation [120]. However, IL-1β is considered a pro-inflammatory cytokine that promotes the development of MAFLD. Studies have shown that GSDMD and GSDMD-N levels are higher in human MAFLD liver samples and are related to the MAFLD activity score (NAS) and fibrosis, while GSDMD knockout in the MCD diet-induced MAFLD model relieves MAFLD and fibrosis by reducing NF-KB activation and proinflammatory cytokines, such as TNF-α, IL-1 and MCP-1 [116].
Ezquerro et al. found that inhibition of TNF-α-induced apoptosis, autophagy and pyroptosis may exert protective effects against MAFLD [121]. Wang et al. discovered that GSDME converts caspase 3-mediated apoptosis into pyroptosis [122].
Ferroptosis and MAFLD
Ferroptosis was first observed in experiments by Dolma et al. in 2003 [123]. Until 2012, the oncogenic RAS-selective lethal small molecule erastin, which was observed in experiments by Dixon et al., was known to induce this form of death, which is distinct from apoptosis [124].
Morphologically, ferroptosis is characterized by cytological changes that include reduced cell volume and increased mitochondrial membrane density [125]. It is characterized by iron dependence and lipid peroxidation. It involves the pharmacological cross-regulation of l-glutathione (GSH) and glutathione peroxidase 4 (GPX4). The classical pathway primarily includes enzymatic reactions (the lipoxygenase pathway) and nonenzymatic reactions (the Fenton reaction) [126]. System xc-regulated GSH production through glutamate–cysteine transport inside and outside the cell. GPX4 is one of the GPXs with antioxidant activity. It can remove lipid peroxides formed by polyunsaturated fatty acid phospholipids with the help of system xc-regulated glutathione content, thereby reducing lipid peroxidative damage in the cell membrane [127, 128] (see Fig. 6 for details).
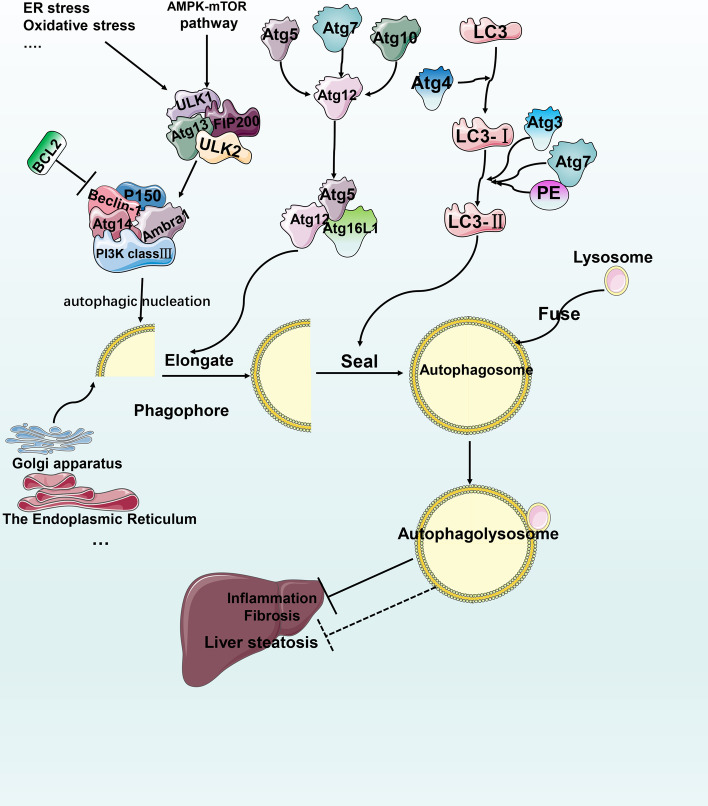
Fig. 6.
The molecular mechanism of autophagy and its relationship with MAFLD. (1) Under normal conditions, mTORC1 phosphorylates and combines with ULK1/2, mAtg13 protein and FIP200 protein to form a functional silencing complex. When ER stress, oxidative stress, etc. activate autophagy, the functional silencing complex dissociates, and autophagosomes begin to recruit Atg protein, ULK1/2, mAtg13 and FIP200, to form a functional complex. (2) The autophagy-inducing complexes PtdIns3K class III, hVps34, Beclin1, and P150 are transported to the ER through microtubules to begin autophagic nucleation to induce the formation of restricted membranes. (3) Two ubiquitin-like conjugation systems, Atg12-Atg5-Atg16 L and PE/LC3, form a complex and participate in membrane elongation and isolate membrane-formation events to finally close into a double-layer membrane vesicle structure. (4) The fusion of autophagosomes and lysosomes leads to cargo degradation. The degraded products (amino acids, etc.) are then released into the cytosol through the lysosomal membrane permease, which produces a subsequent response that may be related to MAFLD-induced lipid accumulation, fibrosis and inflammation
Therefore, classic ferroptosis inducers (FINs) are divided into two categories. Class I inducers are primarily systemic xc inhibitors, which are characterized by reduced synthesis of GSH and exhaustion of GSH content, leading to damage to the antioxidant system, peroxidative damage and ferroptosis [129]. Class I includes erastin and SAS. Class II inducers, such as RSL3 and ML210, directly inhibit GPx4 activity to trigger ferroptosis [130].
The two primary regulatory pathways of ferroptosis are the mevalonate pathway (primarily regulates GPX4 through isopentenyl pyrophosphate (IPP), which stabilizes the selenocysteine-specific tRNA) [131] and the sulfur-transfer pathway (regulates the body’s methionine, and sulfur-containing amino acid levels to ensure conversion to cysteine to synthesize GSH to help GPX4 regulate ferroptosis) [132], although there are others [133]. More detailed molecular mechanisms can be found in reviews on these topics [133].
Whether ferroptosis occurs depends on the balance between the accumulation of iron ROS and the body’s antioxidant system. When hydrogen atoms are extracted from unsaturated fatty acids, lipid peroxidation reactions begin, including a destructive chain reaction that produces heterogeneous groups of lipid peroxides [134], eventually resulting in cell dysfunction and the production of malondialdehyde (MDA) and 4-hydroxy-2,3-nonanal (4-HNE). 4-HNE was noted in the cytoplasm to disrupt liver cells in MAFLD patients through the Fenton reaction [135]. MDA and 4-HNE are elevated in 90% of MAFLD patients [136]. Imai et al. suggested that GPX4 antioxidant properties may play a key role in MAFLD [137]. Carlson et al. found that mice with specific deletion of the GPX4 gene in hepatocytes died during the embryonic stage and had extensive hepatocyte degeneration [138]. Combined with the results of studies by Kim et al., who found high regulation of Gpx4 in the liver [139], these findings suggest a correlation between ferroptosis and MAFLD. In addition, the use of RSL-3, a GPX4 inhibitor, may affect lipid peroxidation in the liver through a 12/15-Lox-AIF-related pathway. 12/15-LOX activation aggravates endoplasmic reticulum stress, inflammation, liver steatosis, and liver damage, and MAFLD is improved by the use of iron chelators [140].
Studies have found that liver iron deposition in MAFLD patients positively correlates with histological severity and can lead to the development of MAFLD [141]. Both lipid peroxidation and excess iron deposition may exacerbate MAFLD through ferroptosis. Fe3+ found in people’s daily diets is reduced to Fe2+ and then absorbed by the divalent metal transporter 1 (DMT-1) protein in the small intestine and stored in the intestinal cells or excreted outside the cell base. It is subsequently transported out through ferroportin and further reoxidized to Fe3+ by hephaestin [142]. In this process, hepatocytes control the production of hepcidin by sensing the iron content in the body. Hepcidin can reduce the expression of DMT-1 and thus reduce the intestinal absorption of iron [143]. In patients with MAFLD, hepcidin levels in the serum and in white fat were accompanied by upregulation of DMT-1 [144]. Upregulation of transferrin receptor 1 (TRE1) was also detected in a fatty liver mouse model [145]. TRE1 is a receptor that binds transferrin and is expressed on activated hepatic stellate cells (HSCs). It aids ferroptosis by increasing iron intake and reducing iron output through transferrin [146]. Excessive iron content deposition promotes liver fibrosis through lipid peroxidation. Ramm et al. identified a relationship between iron load and the activation of HSCs. Iron loading increased the numbers of activated HSCs and collagen deposition [147].
Crosstalk between different PCDs in MAFLD
As mentioned earlier, apoptosis and necroptosis transform each other. Wang et al. discovered that GSDME converts TNFα-induced apoptosis into pyroptosis [122]. Bcl2 anti-apoptotic proteins can inhibit autophagy, whereas other pro-apoptotic proteins can promote autophagy [148]. Mitophagy is a form of autophagy that selectively clears damaged mitochondria. When damaged or inhibited, the failure to clear damaged mitochondria results in mitochondrial dysfunction, which produces excessive ROS and causes NLRP3 activation of pyroptosis. It promotes the production of pro-inflammatory factors to create a pro-inflammatory environment [149]. The activation of autophagy can be considered anti-inflammatory, possibly through inhibition of the activation of NLRP3 [150] and control of mitochondrial homeostasis [151]. Qiu et al. found that arsenic trioxide (AsO) induces MAFLD in mice, accompanied by NLRP3 activation, autophagy and increased lipid accumulation. However, supplementation with taurine (Tau) reduced MAFLD levels, possibly by reducing CTSB-dependent NLRP3 activation and pyroptosis [152]. In addition, lipid peroxidation acts as a bridge between autophagy and ferroptosis [153]. Park et al. found that: autophagy induces ferroptosis by degrading ferritin and inducing TFR1 expression; the ferroptosis inducers eastsin and RSL3 promote the assembly of autophagosomes and autophagy; and the inhibition of autophagy can induce intracellular iron consumption, reducing the occurrence of lipid peroxidation and ferroptosis [154]. However, Takamura et al. used autophagy-deficient mice to demonstrate that direct inhibition of autophagy leads to tumorigenesis [155]. Therefore, it may not be a useful therapeutic approach to directly inhibit autophagy. It may be better to indirectly regulate the related signaling cascade of upstream pathways, such as the AMPK/mTOR-related signaling pathway.
Future directions: drugs targeting PCD to treat MAFLD
The pathogenesis of MAFLD is very complex, and thus far, there are no FDA-approved drugs on the market to treat this condition. Some fundamental molecular experiments have shown different degrees of therapeutic effects in MAFLD by targeting PCD. Further conversion into clinical applications is urgently needed.
The most widely used apoptosis-related pharmacological inhibitors, such as IDN-6556 and GS-9450, are being used in current clinical trials, and exciting results have been obtained (see Table 1 for details). Twenty-eight days of treatment with emricasan significantly reduced levels of ALT and CK-18, showing good safety and tolerance [156]. In addition, patients have been recruited for the phase IIb clinical trial of emricasan for MAFLD, with the primary outcome being improvement in fibrosis without MAFLD worsening (NCT02686762). The latest announcement said there was no statistically significant difference between all treatment groups and the placebo group (Table 2).
Table 1.
The relationship between cell death and MAFLD
| Name | Relationship with MAFLD |
|---|---|
| Apoptosis |
Intestinal barrier dysfunction, oxidative stress, and ER stress lead to lipoapoptosis and activate the external death receptor pathway and internal mitochondrial pathway Glucose metabolism disorder activates the internal mitochondrial pathway and is regulated by several miRNAs |
| Necroptosis |
Oxidative stress and intestinal barrier dysfunction trigger TNF-α-mediated necroptosis The key molecule RIPK1 is involved in the regulation of RIPK3 function and in the mutual transformation with apoptosis The interaction between RIPK3 and JNK is involved in disease progression, although the specific role is not clear Inhibition of MLKL improves insulin resistance, regulates fat metabolism, etc |
| Pyroptosis | Intestinal barrier dysfunction, ER stress, and oxidative stress all activate the assembly of NLRP3, and the secretion of inflammatory factors IL-1β and IL-18 leads to pyroptosis |
| Ferroptosis | Imbalance in the intracellular antioxidant system caused by excessive iron deposition and oxidative stress leads to disorders of the ferroptosis regulatory system and further affects lipid accumulation, inflammation, liver fibrosis, etc |
| Autophagy | Autophagy affects insulin resistance, fat metabolism, inflammation, liver fibrosis, etc. by regulating ER stress, oxidative stress, etc |
Table 2.
MAFLD-related programmed cell death markers and targets
| Name | Contents | Refs. |
|---|---|---|
| Cytokeratin-18 | It alone is not enough as a valuable biomarker, but it may have clinical significance when combined with other non-invasive methods of invasion, such as with serum adiponectin, serum resistin, uric acid (NCT01068444) | [18, 164, 165] |
| Fas/TNF-α | Inhibitor of death receptor-associated protein, including pentoxifylline, YLGA(Try-Leu-Gly-Ala) peptides | [35, 166] |
| Caspase enzymes | Inhibitor of apoptosis-related caspase enzymes, including VX-166, GS-9450 (NCT00740610), PF-03491390 (NCT02077374), and emricasan (NCT02686762, NCT02077374) | [34, 156, 157, 167] |
| R-3032 | A ctsb inhibitor | [168] |
| Aramchol | Aramchol inhibits the liver enzyme stearoyl coenzyme A desaturase (SCD) | [25] |
| Selonsertib | ASK1 inhibitor | [169] |
| The farnesoid X-activated receptor (FXR) | Its agonist (NCT01265498) can improve serum transaminase levels in patients with MAFLD | [170] |
| Thioredoxin (TRX) | Oxidative stress can lead to a variety of PCD. TRX is induced by oxidative stress. Compared with healthy controls, the level of TRX rises significantly, but whether it can be used as a biomarker still needs further research | [171] |
| XIAP | XIAP antisense oligonucleotide (AEG35156) increases progression-free survival and overall survival | [172] |
| RIPK1/3 | Although RIPK1/3 currently has inhibitors (GSKʹ840, GSKʹ843, GSKʹ872), according to different preclinical studies, it seems that directly targeting RIPK1/3 is not a better treatment for MAFLD, and further studies are needed | [173] |
| MLKL | Theoretically, the MLKL inhibitor (necrosulfonamide) may improve MAFLD, but evidence from well-designed clinical studies is still needed | [173] |
| NLRP3 inflammasome | Inhibiting the activation of the MAFLD inflammasome is an innovative treatment method, including an inhibitor of the NALP3 inflammasome (glyburide), a caspase 1 inhibitor (Pralnacasan), and an IL-1β antibody (canakinumab) or endogenous IL-1β inhibitor (anakinra) | [174] |
Although the results of emricasan clinical trials for MAFLD are not ideal, it is undeniable that combined with the cascade reaction of caspase enzymes, the future research direction of direct pharmacological inhibitors targeting apoptosis may be more focused on inhibiting multiple targets or multiple PCD-related targets, due to the interaction between different PCD. A feasible strategy may be the selection of appropriate biomarkers, and then based on these, accurate and specific selection of drugs to achieve personalized treatment. Another effective method may be targeting pathways that regulate PCD, like AMPK/mTOR. In addition, the design of MAFLD clinical trial schemes, with unified diet and exercise management of subjects, accurate selection of clinical endpoints and so on, may improve the success rate of clinical trials.
In the MAFLD models induced by methionine–choline-deficient diets (MCDs) and high-fat diets (HFDs), apoptosis increased significantly, but VX-166 reduced caspase 3- and TUNEL-positive cells and decreased inflammation and other related indicators [157]. The caspase inhibitors IDN6556 and PF-03491390 reduce transaminase activity in patients with HCV [34, 158]. Pan-caspase inhibitors are currently available to study the clinical inhibition of apoptosis, whereas pharmacological targets for necroptosis are needed. Research shows considerable liver protection in MAFLD in the Ripk3−/− genotype or with the use of dabrafenib, which inhibits RIPK3 kinase activity [159]. In addition, most pharmacological inhibitors that target pyroptosis concentrate on NLRP3, which reduces pyrolysis and the release of IL-1β and IL-18 and may have an improved effect on MAFLD. Inhibitory strategies for ferroptosis include directly inhibiting lipid peroxidation. Representative inhibitors of RTAs include α-tocopherol (α-TOH) [160], ferrostatin-1 (fer-1) [161] and liproxstain-1 (lip-1) [161]. The other method is to enhance Gpx4 activity by inhibiting ACSL4 and preventing activation of PUFAs for esterification to lysophospholipids by LPCAT3 [162] or by supplementing with nonoxidizing fatty acids, such as D-PUFA or MUFA [163].
It is worth noting that the role of autophagy in MAFLD seems to require further research. However, most current research drugs have both advantages and limitations for preclinical trial interventions and animal model studies of MAFLD. There are currently MAFLD models including dietary models, like HFD, MCD, choline-deficient l-amino acid-defined (CDAA); genetic models; and chemical models, like CCL4 and tetracycline. These can be easily analyzed to a certain extent and may partially reflect the disease characteristics and drug efficacy for MAFLD. However, due to the multiple complex pathological factors of MAFLD, these confounding factors are difficult to reproduce in animals. At the same time, there are some structural differences between animals and human beings and great differences in their MAFLD characteristics and drug response. Therefore, to assure the feasibility, safety and efficiency of therapy, the development of novel drugs for the treatment of MAFLD to target PCD should be mainly based on clinical experimental studies with better experimental designs as well as disease models for preclinical studies that can truly reflect the characteristics of human MAFLD.
Conclusions
Over the past 10 years, our understanding of various types of programmed cell death has changed dramatically. Under normal physiological conditions, the various forms of programmed death play unique roles in maintaining the steady state of the normal body. However, when one or more of these processes is disrupted, disease may occur.
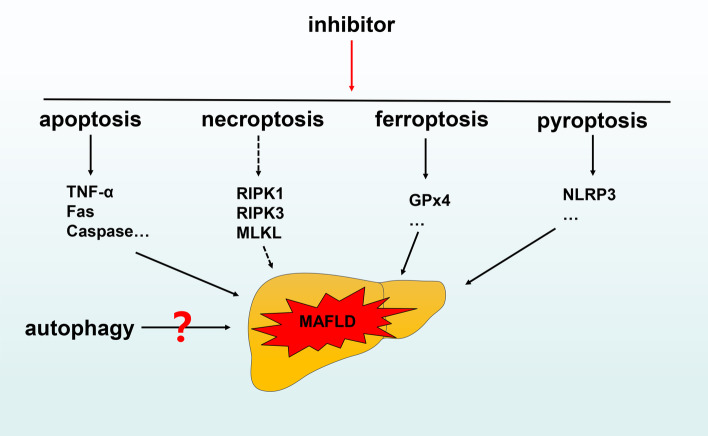
The pathogenesis of MAFLD is very complex. A variety of pathological factors, including oxidative stress and endoplasmic reticulum stress, activate various forms of programmed cell death and play key roles in this process. In other words, various pathogenic factors activate cell death programs. Therefore, the future direction of innovative treatment for MAFLD should include directly targeting activated cell death programs to improve disease, using compounds such as pan-caspase inhibitors (see Fig. 7 for details).
Fig. 7.
The relationship between programmed cell death and MAFLD. Inhibiting relevant targets in the apoptosis, ferroptosis and pyroptosis pathways may have therapeutic effects on MAFLD. In addition, there are various research results on targeting necroptosis and autophagy for treatment of MAFLD. Further research is needed
Here, we primarily reviewed and summarized the possible forms of programmed death in MAFLD, but there are still many issues that need to be addressed: for example, the specific interaction between the various forms of programmed cell death and their impact on disease. More importantly, to more accurately control the input and output of signals and accurately select one or more forms of death, we need a better understanding for targeting programmed death to treat MAFLD. This will provide a theoretical basis and guide future research.
Acknowledgements
Not applicable.
Abbreviations
- NAFLD
Nonalcoholic fatty liver disease
- MAFLD
Metabolic (dysfunction)-associated fatty liver disease
- HCC
Hepatocellular carcinoma
- CVD
Cardiovascular disease
- RCD
Regulatory cell death
- PCD
Programmed cell death
- HCC
Hepatocellular carcinoma
- MOMP
Mediates outer membrane permeabilization
- CK-18
Cytokeratin-18
- MCD
Methionine–choline-deficient diet
- Grp78
Glucose-regulated protein-78
- XBP-1
X-box binding protein-1
- FGF21
Fibroblast growth factor 21
- RYGB
Gastric bypass surgery
- ERS
Endoplasmic reticulum stress
- TNF
Tumor necrosis factor
- TNFR
TNF receptor
- BCL2
B cell lymphoma/leukemia-2
- Bax
Accumulation of Bcl-2–associated X protein
- Bak
BCL-2 homologous antagonist/killer
- Bok
BCL-2 ovarian killer
- Bad
BCL2-associated agonist of cell death
- PUMA
BCL-2-binding component 3
- BCL-XL
Also known as BCL2L1
- BID
BH3-interacting domain death agonist
- ROS
Reactive oxygen species
- CHOP
ERS-related protein expression
- Bim
(Also known as BCL2L11)
- AAPC
Asiatic acid
- JNK
C-Jun N-terminal kinase
- SCD1
Stearoyl-CoA desaturase 1
- LPS
Lipopolysaccharide
- FFA
Free fatty acid
- OSA
Obstructive sleep apnea
- DPP4
Dipeptidyl peptidase 4
- BP
Blueberries and probiotics
- JAK1
Janus kinase 1
- STAT3
Activators of transcription 3
- CKIP-1
Casein kinase 2 interacting protein-1
- IRS-1
Insulin receptor substrate-1
- C3G
Cyanidin-3-O-β-glucoside
- IHG
Intermittent hyperglycemia
- MPT
Mitochondrial permeability transition
- SIRT1
Sirtuin 1
- UCDA
Ursodeoxycholic acid
- CA
Creatine
- PA
Palmitic acid
- HFD
High-fat diet
- DCA
Deoxycholic acid
- PDCD4
Programmed cell death 4
- RIPK1
RIP protein kinase family 1
- RIPK3
RIP protein kinase family 3
- MLKL
Pseudokinase mixed lineage kinase domain-like
- CD95
CD95-type DR group
- TRAIL
TNF-related apoptosis inducing ligand
- FOXO1
Forkhead box protein O1
- TLR4
Toll‐like receptor 4
- TRIF
Toll IL-1 receptor (TIR) domain-containing adaptor-inducing interferon-β
- ELA
Elafibranor
- Nrf-2
Nuclear factor (erythroid-derived 2)-like 2
- HO-1
Heme oxygenase 1
- PIP3
Phosphatidylinositol (3,4,5)-trisphosphate
- DNL
De novo lipogenesis
- αGalCer
α-Galactosylceramide
- PAMPs
Pathogen-associated molecular patterns
- DAMPS
Damage-associated molecular patterns
- NLRP3
NOD-LRR and pyrin domain-containing 3
- ASC
Caspase-recruitment domain
- IL-1β
Interleukin-1β
- IL-18
Interleukin-18
- IL-22
Interleukin-22
- GSDMD
Cleaves gasdermin D
- P2X7
Ligand-gated ion channel 7
- UCP2
Uncoupling protein-2
- GNP
Genipin
- API
Apigenin
- NLRP4
NOD-LRR and pyrin domain-containing 4 arsenic trioxide (AsO)
- Tau
Taurine
- AMPK
AMP-activated protein kinase
- GPX4
Glutathione peroxidase 4
- GSH
L-glutathione
- L-glutathione
Phosphorylase kinase G2
- PUFAs
Polyunsaturated fatty acids
- SLC7A11
Solute carrier family 7 member 7
- SLC3A2
Solute carrier family 3 member 2
- FINs
Ferroptosis inducers
- WA
Withaferin A
- VDACs
Voltage-dependent anion channels
- COA
Coenzyme A
- IPP
Isopentenyl pyrophosphate
- HSPB1
Heat shock factor protein 1 (HSF1)-heat shock protein b 1
- Keap1
Kelch-like ECH-associated protein
- AIFM2
Apoptosis-inducing factor mitochondrial
- 4-HNE
4-Hydroxy-2,3-nonanal
- MDA
Malondialdehyde
- TRF1
Transferrin receptor 1
- Nrf-2
Erythroid-derived 2)-like 2
- DMT-1
Divalent metal transporter-1
- ULK1
Unc-51-like kinase 1
- ULK2
Unc-51-like kinase 2
- mTORC1
Rapamycin complex 1
- PE
Phosphatidylethanolamine
- LC3
The microtubule-associated protein 1 light chain 3
- Atg
Autophagy-related proteins
- GNMT
Glycine N-methyltransferase
- SAMe
S-adenosylmethionine
- JAMD3
Jumonji-D3
- SREBP-2
Sterol regulatory element-binding protein 2
- PNPLA8
Phospholipase domain-containing enzyme 8
- JZG
Jiang Zhi granule
- TAK1
Transforming growth factor-β activated kinase-1
- TRADD
TNFR1-associated death domain protein
- TRAF2
TNF receptor-associated factor 2
- TRAF5
TNF receptor-associated factor 5
- cIAP1
E3 ubiquitin ligases cellular inhibitor of apoptosis 1
- cIAP2
E3 ubiquitin ligases cellular inhibitor of apoptosis 2
- LUBAC
Linear ubiquitin chain assembly complex
- NEMO
NF-κB essential modulator
- IKKβ
IκB kinase 2, also known as IKK2
- IKKα
IκB kinase α
- TAB2/3
TAK1 binding protein 2/3
- HSC
Hepatic stellate cells
- TGF-β
Transforming growth factor beta
- UPR
Unfolded protein response
Authors' contributions
JZ is responsible for the collection, collation and writing of the original manuscript. JP and YH are responsible for the revision and review of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81673750) and the National Natural Science Foundation of China (No. 81830119).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yiyang Hu, Email: yyhuliver@163.com.
Jinghua Peng, Email: pengjinghua2004@163.com.
References
- 1.Eslam M, Sanyal AJ, George J, Sanyal A, Neuschwander-Tetri B, Tiribelli C, et al. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 2.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Shiha G, Korenjak M, Eskridge W, Casanovas T, Velez-Moller P, Högström S, et al. Redefining fatty liver disease: an international patient perspective. Lancet Gastroenterol Hepatol. 2021;6(1):73–79. doi: 10.1016/S2468-1253(20)30294-6. [DOI] [PubMed] [Google Scholar]
- 4.Shiha G, Alswat K, Al Khatry M, Sharara A, Örmeci N, Waked I, et al. Nomenclature and definition of metabolic-associated fatty liver disease: a consensus from the Middle East and north Africa. Lancet Gastroenterol Hepatol. 2021;6(1):57–64. doi: 10.1016/S2468-1253(20)30213-2. [DOI] [PubMed] [Google Scholar]
- 5.Mendez-Sanchez N, Arrese M, Gadano A, Oliveira CP, Fassio E, Arab JP, et al. The Latin American Association for the Study of the Liver (ALEH) position statement on the redefinition of fatty liver disease. Lancet Gastroenterol Hepatol. 2021;6(1):65–72. doi: 10.1016/S2468-1253(20)30340-X. [DOI] [PubMed] [Google Scholar]
- 6.Pierantonelli I, Svegliati-Baroni G. Nonalcoholic Fatty Liver Disease. Transplantation. 2019;103(1):e1–e13. doi: 10.1097/TP.0000000000002480. [DOI] [PubMed] [Google Scholar]
- 7.Eslam M, Sarin SK, Wong VW-S, Fan J-G, Kawaguchi T, Ahn SH, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;8:9. [DOI] [PubMed]
- 8.Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29(5):347–364. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luedde T, Kaplowitz N, Schwabe RF. Cell Death and Cell Death Responses in Liver Disease: Mechanisms and Clinical Relevance. Gastroenterology. 2014;147(4):765–83.e4. doi: 10.1053/j.gastro.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syn W-K, Choi SS, Diehl AM. Apoptosis and Cytokines in Non-Alcoholic Steatohepatitis. Clin Liver Dis. 2009;13(4):565–580. doi: 10.1016/j.cld.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwabe RF, Luedde T. Apoptosis and necroptosis in the liver: a matter of life and death. Nat Rev Gastroenterol Hepatol. 2018;15(12):738–752. doi: 10.1038/s41575-018-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi M, Price D, Ryter S, Choi A. Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight. 2019;4(15):e128834. doi: 10.1172/jci.insight.128834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerr J, Wyllie A, Currie A. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tushar P. Apoptosis in hepatic pathophysiology. Clin Liver Dis. 2000;4(2):295–317. doi: 10.1016/s1089-3261(05)70112-4. [DOI] [PubMed] [Google Scholar]
- 15.Alkhouri N, Carter-Kent C, Feldstein A. Apoptosis in nonalcoholic fatty liver disease: diagnostic and therapeutic implications. Expert Rev Gastroenterol Hepatol. 2011;5(2):201–212. doi: 10.1586/egh.11.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao P, Sun X, Chaggan C, Liao Z, In Wong K, He F, et al. An AMPK-caspase-6 axis controls liver damage in nonalcoholic steatohepatitis. Science. 2020;367(6478):652–660. doi: 10.1126/science.aay0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandelia C, Collyer E, Mansoor S, Lopez R, Lappe S, Nobili V, et al. Plasma Cytokeratin-18 Level As a Novel Biomarker for Liver Fibrosis in Children With Nonalcoholic Fatty Liver Disease. J Pediatr Gastroenterol Nutr. 2016;63(2):181–187. doi: 10.1097/MPG.0000000000001136. [DOI] [PubMed] [Google Scholar]
- 18.Cusi K, Chang Z, Harrison S, Lomonaco R, Bril F, Orsak B, et al. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;60(1):167–174. doi: 10.1016/j.jhep.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 19.de Freitas Carvalho MM, Lage NN, de Souza Paulino AH, Pereira RR, de Almeida LT, da Silva TF, et al. Effects of açai on oxidative stress, ER stress, and inflammation-related parameters in mice with high fat diet-fed induced NAFLD. Sci Rep-UK. 2019;9:1. doi: 10.1038/s41598-019-44563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung JCF, Loong TCW, Wei JL, Wong GLH, Chan AWH, Choi PCL, et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2016;65(1):54–64. doi: 10.1002/hep.28697. [DOI] [PubMed] [Google Scholar]
- 21.Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83(5):655–663. doi: 10.1097/01.lab.0000069036.63405.5c. [DOI] [PubMed] [Google Scholar]
- 22.Egnatchik R, Leamy A, Jacobson D, Shiota M, Young J. ER calcium release promotes mitochondrial dysfunction and hepatic cell lipotoxicity in response to palmitate overload. Mol Metab. 2014;3(5):544–553. doi: 10.1016/j.molmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akazawa Y, Nakao K. Lipotoxicity pathways intersect in hepatocytes: endoplasmic reticulum stress, c-Jun N-terminal kinase-1, and death receptors. Hepatol Res. 2016;46(10):977–984. doi: 10.1111/hepr.12658. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Lao L, Pang X, Qiao Q, Pang L, Feng Z, et al. Asiatic acid from Potentilla chinensis alleviates non-alcoholic fatty liver by regulating endoplasmic reticulum stress and lipid metabolism. Int J Immunopharmaco. 2018;65:256–267. doi: 10.1016/j.intimp.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Safadi R, Konikoff F, Mahamid M, Zelber-Sagi S, Halpern M, Gilat T, et al. The fatty acid-bile acid conjugate Aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12(12):2085–2091. doi: 10.1016/j.cgh.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 26.Alkhouri N, Dixon L, Feldstein A. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroent. 2009;3(4):445–451. doi: 10.1586/egh.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machado M, Michelotti G, Pereira Tde A, Boursier J, Kruger L, Swiderska-Syn M, et al. Reduced lipoapoptosis, hedgehog pathway activation and fibrosis in caspase-2 deficient mice with non-alcoholic steatohepatitis. Gut. 2015;64(7):1148–1157. doi: 10.1136/gutjnl-2014-307362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferreira D, Castro R, Machado M, Evangelista T, Silvestre A, Costa A, et al. Apoptosis and insulin resistance in liver and peripheral tissues of morbidly obese patients is associated with different stages of non-alcoholic fatty liver disease. Diabetologia. 2011;54(7):1788–1798. doi: 10.1007/s00125-011-2130-8. [DOI] [PubMed] [Google Scholar]
- 29.Yeh M, Brunt E. Pathology of nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;128(5):837–847. doi: 10.1309/RTPM1PY6YGBL2G2R. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Wang C, Lu J, Huang K, Han Y, Chen J, et al. PPAR δ inhibition protects against palmitic acid-LPS induced lipidosis and injury in cultured hepatocyte L02 cell. Int J Med Sci. 2019;16(12):1593–1603. doi: 10.7150/ijms.37677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feldstein A, Canbay A, Angulo P, Taniai M, Burgart L, Lindor K, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125(2):437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 32.Alkhouri N, Kheirandish-Gozal L, Matloob A, Alonso-Álvarez M, Khalyfa A, Terán-Santos J, et al. Evaluation of circulating markers of hepatic apoptosis and inflammation in obese children with and without obstructive sleep apnea. Sleep Med. 2015;16(9):1031–1035. doi: 10.1016/j.sleep.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Polyzos S, Kountouras J, Papatheodorou A, Katsiki E, Patsiaoura K, Zafeiriadou E, et al. Adipocytokines and cytokeratin-18 in patients with nonalcoholic fatty liver disease: Introduction of CHA index. Ann Hepatol. 2013;12(5):749–757. [PubMed] [Google Scholar]
- 34.Shiffman M, Pockros P, McHutchison J, Schiff E, Morris M, Burgess G. Clinical trial: the efficacy and safety of oral PF-03491390, a pancaspase inhibitor - a randomized placebo-controlled study in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2010;31(9):969–978. doi: 10.1111/j.1365-2036.2010.04264.x. [DOI] [PubMed] [Google Scholar]
- 35.Zou C, Ma J, Wang X, Guo L, Zhu Z, Stoops J, et al. Lack of Fas antagonism by Met in human fatty liver disease. Nat Med. 2007;13(9):1078–1085. doi: 10.1038/nm1625. [DOI] [PubMed] [Google Scholar]
- 36.Kroy D, Schumacher F, Ramadori P, Hatting M, Bergheim I, Gassler N, et al. Hepatocyte specific deletion of c-Met leads to the development of severe non-alcoholic steatohepatitis in mice. J Hepatol. 2014;61(4):883–890. doi: 10.1016/j.jhep.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 37.Mosinski J, Pagadala M, Mulya A, Huang H, Dan O, Shimizu H, et al. Gastric bypass surgery is protective from high-fat diet-induced non-alcoholic fatty liver disease and hepatic endoplasmic reticulum stress. Acta Physiol (Oxf) 2016;217(2):141–151. doi: 10.1111/apha.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo R, Liong E, So K, Fung M, Tipoe G. Beneficial mechanisms of aerobic exercise on hepatic lipid metabolism in non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2015;14(2):139–144. doi: 10.1016/s1499-3872(15)60355-1. [DOI] [PubMed] [Google Scholar]
- 39.Kakisaka K, Cazanave S, Werneburg N, Razumilava N, Mertens J, Bronk S, et al. A hedgehog survival pathway in 'undead' lipotoxic hepatocytes. J Hepatol. 2012;57(4):844–851. doi: 10.1016/j.jhep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin G, Wang G, Guo D, Bai R, Wang M, Du S. Deletion of Smad4 reduces hepatic inflammation and fibrogenesis during nonalcoholic steatohepatitis progression. J Digest Dis. 2018;19(5):301–313. doi: 10.1111/1751-2980.12599. [DOI] [PubMed] [Google Scholar]
- 41.Zhan Y, Xie P, Li D, Li L, Chen J, An W, et al. Deficiency of CKIP-1 aggravates high-fat diet-induced fatty liver in mice. Exp Cell Res. 2017;355(1):40–46. doi: 10.1016/j.yexcr.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 42.Jiang X, Tang X, Zhang P, Liu G, Guo H. Cyanidin-3-O-β-glucoside protects primary mouse hepatocytes against high glucose-induced apoptosis by modulating mitochondrial dysfunction and the PI3K/Akt pathway. Biochem Pharmacol. 2014;90(2):135–144. doi: 10.1016/j.bcp.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Yin X, Zheng F, Pan Q, Zhang S, Yu D, Xu Z, et al. Glucose fluctuation increased hepatocyte apoptosis under lipotoxicity and the involvement of mitochondrial permeability transition opening. J Mol Endocrinol. 2015;55(3):169–181. doi: 10.1530/JME-15-0101. [DOI] [PubMed] [Google Scholar]
- 44.Alisi A, Da Sacco L, Bruscalupi G, Piemonte F, Panera N, De Vito R, et al. Mirnome analysis reveals novel molecular determinants in the pathogenesis of diet-induced nonalcoholic fatty liver disease. Lab Invest. 2011;91(2):283–293. doi: 10.1038/labinvest.2010.166. [DOI] [PubMed] [Google Scholar]
- 45.Di Mauro S, Ragusa M, Urbano F, Filippello A, Di Pino A, Scamporrino A, et al. Intracellular and extracellular miRNome deregulation in cellular models of NAFLD or NASH: Clinical implications. Nutr Metab Cardiovas. 2016;26(12):1129–1139. doi: 10.1016/j.numecd.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Castro R, Ferreira D, Afonso M, Borralho P, Machado M, Cortez-Pinto H, et al. miR-34a/SIRT1/p53 is suppressed by ursodeoxycholic acid in the rat liver and activated by disease severity in human non-alcoholic fatty liver disease. J Hepatol. 2013;58(1):119–125. doi: 10.1016/j.jhep.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Shan W, Gao L, Zeng W, Hu Y, Wang G, Li M, et al. Activation of the SIRT1/p66shc antiapoptosis pathway via carnosic acid-induced inhibition of miR-34a protects rats against nonalcoholic fatty liver disease. Cell Death Dis. 2015;6:e1833. doi: 10.1038/cddis.2015.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cazanave S, Mott J, Elmi N, Bronk S, Masuoka H, Charlton M, et al. A role for miR-296 in the regulation of lipoapoptosis by targeting PUMA. J Lipid Res. 2011;52(8):1517–1525. doi: 10.1194/jlr.M014654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyamoto Y, Mauer A, Kumar S, Mott J, Malhi H. Mmu-miR-615-3p regulates lipoapoptosis by inhibiting C/EBP homologous protein. PLoS ONE. 2014;9(10):e109637. doi: 10.1371/journal.pone.0109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodrigues P, Afonso M, Simão A, Carvalho C, Trindade A, Duarte A, et al. miR-21 ablation and obeticholic acid ameliorate nonalcoholic steatohepatitis in mice. Cell Death Dis. 2017;8(4):e2748. doi: 10.1038/cddis.2017.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ray C, Pickup D. The mode of death of pig kidney cells infected with cowpox virus is governed by the expression of the crmA gene. Virology. 1996;217(1):384–391. doi: 10.1006/viro.1996.0128. [DOI] [PubMed] [Google Scholar]
- 52.Siegmund D, Lang I, Wajant H. Cell death-independent activities of the death receptors CD95, TRAILR1, and TRAILR2. FEBS J. 2017;284(8):1131–1159. doi: 10.1111/febs.13968. [DOI] [PubMed] [Google Scholar]
- 53.Han J, Zhong C, Zhang D. Programmed necrosis: backup to and competitor with apoptosis in the immune system. Nat Immunol. 2011;12(12):1143–1149. doi: 10.1038/ni.2159. [DOI] [PubMed] [Google Scholar]
- 54.Ding H, Tang Z, Tang N, Zhu Z, Liu H, Pan C, et al. Protective Properties of FOXO1 Inhibition in a Murine Model of Non-alcoholic Fatty Liver Disease Are Associated With Attenuation of ER Stress and Necroptosis. Front Physiol. 2020;11:177. doi: 10.3389/fphys.2020.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roh Y, Seki E. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J Gastroen Hepatol. 2013;1(01):38–42. doi: 10.1111/jgh.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ribeiro P, Cortez-Pinto H, Solá S, Castro R, Ramalho R, Baptista A, et al. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004;99(9):1708–1717. doi: 10.1111/j.1572-0241.2004.40009.x. [DOI] [PubMed] [Google Scholar]
- 57.Nakanishi K, Kaji K, Kitade M, Kubo T, Furukawa M, Saikawa S, et al. Exogenous Administration of Low-Dose Lipopolysaccharide Potentiates Liver Fibrosis in a Choline-Deficient l-Amino-Acid-Defined Diet-Induced Murine Steatohepatitis Model. Int J Mol Sci. 2019;20(11):2724. doi: 10.3390/ijms20112724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaiser W, Sridharan H, Huang C, Mandal P, Upton J, Gough P, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288(43):31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He S, Wang L, Miao L, Wang T, Du F, Zhao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137(6):1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 60.Günther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477(7364):335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vucur M, Reisinger F, Gautheron J, Janssen J, Roderburg C, Cardenas D, et al. RIP3 inhibits inflammatory hepatocarcinogenesis but promotes cholestasis by controlling caspase-8- and JNK-dependent compensatory cell proliferation. Cell Rep. 2013;4(4):776–790. doi: 10.1016/j.celrep.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 62.Afonso M, Rodrigues P, Carvalho T, Caridade M, Borralho P, Cortez-Pinto H, et al. Necroptosis is a key pathogenic event in human and experimental murine models of non-alcoholic steatohepatitis. Clinl Sci. 2015;129(8):721–739. doi: 10.1042/CS20140732. [DOI] [PubMed] [Google Scholar]
- 63.Gautheron J, Vucur M, Reisinger F, Cardenas D, Roderburg C, Koppe C, et al. A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol Med. 2014;6(8):1062–1074. doi: 10.15252/emmm.201403856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roychowdhury S, McCullough R, Sanz-Garcia C, Saikia P, Alkhouri N, Matloob A, et al. Receptor interacting protein 3 protects mice from high-fat diet-induced liver injury. Hepatology. 2016;64(5):1518–1533. doi: 10.1002/hep.28676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saeed W, Jun D, Jang K, Ahn S, Oh J, Chae Y, et al. Mismatched effects of receptor interacting protein kinase-3 on hepatic steatosis and inflammation in non-alcoholic fatty liver disease. World J Gastroenterol. 2018;24(48):5477–5490. doi: 10.3748/wjg.v24.i48.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Orozco S, Yatim N, Werner M, Tran H, Gunja S, Tait S, et al. RIPK1 both positively and negatively regulates RIPK3 oligomerization and necroptosis. Cell Death Differ. 2014;21(10):1511–1521. doi: 10.1038/cdd.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Majdi A, Aoudjehane L, Ratziu V, Islam T, Afonso MB, Conti F, et al. Inhibition of receptor-interacting protein kinase 1 improves experimental non-alcoholic fatty liver disease. J Hepatol. 2020;72(4):627–635. doi: 10.1016/j.jhep.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 68.Xu H, Du X, Liu G, Huang S, Du W, Zou S, et al. The pseudokinase MLKL regulates hepatic insulin sensitivity independently of inflammation. Mol Metab. 2019;23:14–23. doi: 10.1016/j.molmet.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saeed W, Jun D, Jang K, Oh J, Chae Y, Lee J, et al. Decrease in fat de novo synthesis and chemokine ligand expression in non-alcoholic fatty liver disease caused by inhibition of mixed lineage kinase domain-like pseudokinase. J Gastroen Hepatol. 2019;34(12):2206–2218. doi: 10.1111/jgh.14740. [DOI] [PubMed] [Google Scholar]
- 70.Suda J, Dara L, Yang L, Aghajan M, Song Y, Kaplowitz N, et al. Knockdown of RIPK1 Markedly Exacerbates Murine Immune-Mediated Liver Injury through Massive Apoptosis of Hepatocytes, Independent of Necroptosis and Inhibition of NF-κB. J Immunol. 2016;197(8):3120–3129. doi: 10.4049/jimmunol.1600690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clark S. Cellular differentiation in the kidneys of newborn mice studies with the electron microscope. J Biophys Biochem Cytol. 1957;3(3):349–362. doi: 10.1083/jcb.3.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ashford T, Porter K. Cytoplasmic components in hepatic cell lysosomes. Cell Biol. 1962;12:198–202. doi: 10.1083/jcb.12.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Novikoff A. The proximal tubule cell in experimental hydronephrosis. BiophysBiochem Cytol. 1959;6:136–138. doi: 10.1083/jcb.6.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang Z, Klionsky D. Eaten alive: a history of macroautophagy. Nature Cell Biol. 2010;12(9):814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ezaki J, Matsumoto N, Takeda-Ezaki M, Komatsu M, Takahashi K, Hiraoka Y, et al. Liver autophagy contributes to the maintenance of blood glucose and amino acid levels. Autophagy. 2011;7(7):727–736. doi: 10.4161/auto.7.7.15371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432(7020):1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 77.Raben N, Hill V, Shea L, Takikita S, Baum R, Mizushima N, et al. Suppression of autophagy in skeletal muscle uncovers the accumulation of ubiquitinated proteins and their potential role in muscle damage in Pompe disease. Hum Mol Genet. 2008;17(24):3897–3908. doi: 10.1093/hmg/ddn292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saito T, Kuma A, Sugiura Y, Ichimura Y, Obata M, Kitamura H, et al. Autophagy regulates lipid metabolism through selective turnover of NCoR1. Nat Commun. 2019;10(1):1567. doi: 10.1038/s41467-019-08829-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feng Y, He D, Yao Z, Klionsky D. The machinery of macroautophagy. Cell Res. 2014;24(1):24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wen X, Klionsky D. At a Glance: A History of Autophagy and Cancer. Semin Cancer Biol. 2019;66:3–11. doi: 10.1016/j.semcancer.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walker S, Ktistakis N. Autophagosome biogenesis machinery. J Mol Biol. 2019;432(8):2449–2461. doi: 10.1016/j.jmb.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 82.Madrigal-Matute J, Cuervo A. Regulation of Liver Metabolism by Autophagy. Gastroenterology. 2016;150(2):328–339. doi: 10.1053/j.gastro.2015.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deter R, Baudhuin P, De Duve C. Participation of lysosomes in cellular autophagy induced in rat liver by glucagon. J Cell Biol. 1967;35(2):C11–C16. doi: 10.1083/jcb.35.2.c11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Czaja M, Ding W, Donohue T, Friedman S, Kim J, Komatsu M, et al. Functions of autophagy in normal and diseased liver. Autophagy. 2013;9(8):1131–1158. doi: 10.4161/auto.25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodriguez-Navarro J, Kaushik S, Koga H, Dall'Armi C, Shui G, Wenk M, et al. Inhibitory effect of dietary lipids on chaperone-mediated autophagy. Proc Natl Acad Sci U S A. 2012;109(12):E705–E714. doi: 10.1073/pnas.1113036109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, et al. Autophagy regulates lipid metabolism. Nature. 2009;458(7242):1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zubiete-Franco I, García-Rodríguez J, Martínez-Uña M, Martínez-Lopez N, Woodhoo A, Juan V, et al. Methionine and S-adenosylmethionine levels are critical regulators of PP2A activity modulating lipophagy during steatosis. J Hepatol. 2016;64(2):409–418. doi: 10.1016/j.jhep.2015.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Byun S, Seok S, Kim Y, Zhang Y, Yau P, Iwamori N, et al. Fasting-induced FGF21 signaling activates hepatic autophagy and lipid degradation via JMJD3 histone demethylase. Nat Commun. 2020;11(1):807. doi: 10.1038/s41467-020-14384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y, Zhao H, Li X, Wang Q, Yan M, Zhang H, et al. Formononetin alleviates hepatic steatosis by facilitating TFEB-mediated lysosome biogenesis and lipophagy. J Nutr Biochem. 2019;73:108214. doi: 10.1016/j.jnutbio.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 90.Tang M, Jiang Y, Jia H, Patpur B, Yang B, Li J, et al. Osteopontin acts as a negative regulator of autophagy accelerating lipid accumulation during the development of nonalcoholic fatty liver disease. Artif Cells Nanomed Biotechnol. 2020;48(1):159–168. doi: 10.1080/21691401.2019.1699822. [DOI] [PubMed] [Google Scholar]
- 91.Liu C, Liao J, Li P. Traditional Chinese herbal extracts inducing autophagy as a novel approach in therapy of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23(11):1964–1973. doi: 10.3748/wjg.v23.i11.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang L, Yao Z, Ji G. Herbal Extracts and Natural Products in Alleviating Non-alcoholic Fatty Liver Disease via Activating Autophagy. Front Pharmacol. 2018;9:1459. doi: 10.3389/fphar.2018.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ren H, Wang D, Zhang L, Kang X, Li Y, Zhou X, et al. Catalpol induces autophagy and attenuates liver steatosis in ob/ob and high-fat diet-induced obese mice. Aging. 2019;11(21):9461–9477. doi: 10.18632/aging.102396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takagi A, Kume S, Kondo M, Nakazawa J, Chin-Kanasaki M, Araki H, et al. Mammalian autophagy is essential for hepatic and renal ketogenesis during starvation. Sci Rep-UK. 2016;6:18944. doi: 10.1038/srep18944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tong L, Wang L, Yao S, Jin L, Yang J, Zhang Y, et al. PPARδ attenuates hepatic steatosis through autophagy-mediated fatty acid oxidation. Cell Death Dis. 2019;10(3):197. doi: 10.1038/s41419-019-1458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zeng J, Zhu B, Su M. Autophagy is involved in acetylshikonin ameliorating non-alcoholic steatohepatitis through AMPK/mTOR pathway. Biochem Biophys Res Commun. 2018;503(3):1645–1650. doi: 10.1016/j.bbrc.2018.07.094. [DOI] [PubMed] [Google Scholar]
- 97.Yang L, Li P, Fu S, Calay E, Hotamisligil G. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11(6):467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu H, Han J, Cao S, Hong T, Zhuo D, Shi J, et al. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J Biol Chem. 2009;284(45):31484–31492. doi: 10.1074/jbc.M109.033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yan H, Gao Y, Zhang Y, Wang H, Liu G, Lei J. Chlorogenic acid alleviates autophagy and insulin resistance by suppressing JNK pathway in a rat model of nonalcoholic fatty liver disease. J Biosci. 2018;43(2):287–294. [PubMed] [Google Scholar]
- 100.Zhang X, Han J, Man K, Li X, Du J, Chu E, et al. CXC chemokine receptor 3 promotes steatohepatitis in mice through mediating inflammatory cytokines, macrophages and autophagy. J Hepatol. 2016;64(1):160–170. doi: 10.1016/j.jhep.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 101.Lee G, Lee H, Park S, Shin T, Chae H. Eucommia ulmoides Leaf Extract Ameliorates Steatosis Induced by High-fat Diet in Rats by Increasing Lysosomal Function. Nutrients. 2019;11(2):426. doi: 10.3390/nu11020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deng X, Pan X, Cheng C, Liu B, Zhang H, Zhang Y, et al. Regulation of SREBP-2 intracellular trafficking improves impaired autophagic flux and alleviates endoplasmic reticulum stress in NAFLD. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(3):337–350. doi: 10.1016/j.bbalip.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 103.Kim K, Jang H, Yang Y, Park K, Seo J, Shin I, et al. SREBP-2/PNPLA8 axis improves non-alcoholic fatty liver disease through activation of autophagy. Sci Rep-UK. 2016;6:35732. doi: 10.1038/srep35732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng Y, Wang M, Shu X, Zheng P, Ji G. Autophagy activation by Jiang Zhi Granule protects against metabolic stress-induced hepatocyte injury. World J Gastroenterol. 2018;24(9):992–1003. doi: 10.3748/wjg.v24.i9.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.He J, Ding J, Lai Q, Wang X, Li A, Liu S. Irbesartan Ameliorates Lipid Deposition by Enhancing Autophagy via PKC/AMPK/ULK1 Axis in Free Fatty Acid Induced Hepatocytes. Front Physiol. 2019;10:681. doi: 10.3389/fphys.2019.00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koh J, Wang L, Beaudoin-Chabot C, Thibault G. Lipid bilayer stress-activated IRE-1 modulates autophagy during endoplasmic reticulum stress. J Cell Sci. 2018;131(22):217992. doi: 10.1242/jcs.217992. [DOI] [PubMed] [Google Scholar]
- 107.Kim R, Hasegawa D, Goossens N, Tsuchida T, Athwal V, Sun X, et al. The XBP1 arm of the unfolded protein response induces fibrogenic activity in hepatic stellate cells through autophagy. Sci Rep-UK. 2016;6:39342. doi: 10.1038/srep39342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Inokuchi-Shimizu S, Park E, Roh Y, Yang L, Zhang B, Song J, et al. TAK1-mediated autophagy and fatty acid oxidation prevent hepatosteatosis and tumorigenesis. J Clin Invest. 2014;124(8):3566–3578. doi: 10.1172/JCI74068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hernández-Gea V, Ghiassi-Nejad Z, Rozenfeld R, Gordon R, Fiel M, Yue Z, et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology. 2012;142(4):938–946. doi: 10.1053/j.gastro.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thoen L, Guimarães E, Dollé L, Mannaerts I, Najimi M, Sokal E, et al. A role for autophagy during hepatic stellate cell activation. J Hepatol. 2011;55(6):1353–1360. doi: 10.1016/j.jhep.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 111.Cookson B, Brennan M. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9(3):113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 112.Bergsbaken T, Fink S, Cookson B. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7(2):99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhu Y, Velani B, Wan B, Lin S, Wu J. Pyroptosis in Liver Disease: New Insights into Disease Mechanisms. Aging Dis. 2019;10:5. [DOI] [PMC free article] [PubMed]
- 114.Fink S, Cookson B. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8(11):1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 115.Ross C, Chan A, Von Pein J, Boucher D, Schroder K. Dimerization and auto-processing induce caspase-11 protease activation within the non-canonical inflammasome. Life Sci Alliance. 2018;1(6):e201800237. doi: 10.26508/lsa.201800237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Beier JI, Banales JM. Pyroptosis: An inflammatory link between NAFLD and NASH with potential therapeutic implications. J Hepatol. 2018;68(4):643–645. doi: 10.1016/j.jhep.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhong H, Liu M, Ji Y, Ma M, Chen K, Liang T, et al. Genipin Reverses HFD-Induced Liver Damage and Inhibits UCP2-Mediated Pyroptosis in Mice. Cell Physiol Biochem. 2018;49(5):1885–1897. doi: 10.1159/000493651. [DOI] [PubMed] [Google Scholar]
- 118.Mai W, Xu Y, Xu J, Zhao D, Ye L, Yu G, et al. viaBerberine Inhibits Nod-Like Receptor Family Pyrin Domain Containing 3 Inflammasome Activation and Pyroptosis in Nonalcoholic Steatohepatitis the ROS/TXNIP Axis. Front Pharmacol. 2020;11:185. doi: 10.3389/fphar.2020.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen Y, Ma K. NLRC4 inflammasome activation regulated by TNF-α promotes inflammatory responses in nonalcoholic fatty liver disease. Biochem Biophys Res Commun. 2019;511(3):524–530. doi: 10.1016/j.bbrc.2019.02.099. [DOI] [PubMed] [Google Scholar]
- 120.Yamanishi K, Maeda S, Kuwahara-Otani S, Hashimoto T, Ikubo K, Mukai K, et al. Deficiency in interleukin-18 promotes differentiation of brown adipose tissue resulting in fat accumulation despite dyslipidemia. J Transl Med. 2018;16(1):314. doi: 10.1186/s12967-018-1684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ezquerro S, Mocha F, Frühbeck G, Guzmán-Ruiz R, Valentí V, Mugueta C, et al. Ghrelin Reduces TNF-α-Induced Human Hepatocyte Apoptosis, Autophagy, and Pyroptosis: Role in Obesity-Associated NAFLD. J Clin Endocrinol Metab. 2019;104(1):21–37. doi: 10.1210/jc.2018-01171. [DOI] [PubMed] [Google Scholar]
- 122.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 123.Dolma S, Lessnick S, Hahn W, Stockwell B. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3(3):285–296. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 124.Dixon S, Lemberg K, Lamprecht M, Skouta R, Zaitsev E, Gleason C, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yu H, Guo P, Xie X, Wang Y, Chen G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med. 2017;21(4):648–657. doi: 10.1111/jcmm.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kajarabille N, Latunde-Dada G. Programmed Cell-Death by Ferroptosis: Antioxidants as Mitigators. Int J Mol Sci. 2019;20(19):4968. doi: 10.3390/ijms20194968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stockwell B, Friedmann AJ, Bayir H, Bush A, Conrad M, Dixon S, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830(5):3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 129.Yagoda N, von Rechenberg M, Zaganjor E, Bauer A, Yang W, Fridman D, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447(7146):864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Friedmann Angeli J, Schneider M, Proneth B, Tyurina Y, Tyurin V, Hammond V, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nature Cell Biol. 2014;16(12):1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell. 2018;172(3):409–22.e21. doi: 10.1016/j.cell.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 132.McBean G. The transsulfuration pathway: a source of cysteine for glutathione in astrocytes. Amino Acids. 2012;42(1):199–205. doi: 10.1007/s00726-011-0864-8. [DOI] [PubMed] [Google Scholar]
- 133.Conrad M, Pratt DJA. The chemical basis of ferroptosis. Nat Chem Biol. 2019;15(12):1137–47. [DOI] [PubMed]
- 134.Benzie I. Lipid peroxidation: a review of causes, consequences, measurement and dietary influences. Int J Food Sci Nutr. 1996;47(3):233–261. doi: 10.3109/09637489609012586. [DOI] [PubMed] [Google Scholar]
- 135.Pietrangelo A. Iron in NASH, chronic liver diseases and HCC: How much iron is too much? J Hepatol. 2009;50(2):249–251. doi: 10.1016/j.jhep.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 136.Loguercio C, De Girolamo V, de Sio I, Tuccillo C, Ascione A, Baldi F, et al. Non-alcoholic fatty liver disease in an area of southern Italy: main clinical, histological, and pathophysiological aspects. J Hepatol. 2001;35(5):568–574. doi: 10.1016/s0168-8278(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 137.Imai H, Matsuoka M, Kumagai T, Sakamoto T, Koumura T. Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis. Curr Top Microbiol Immunol. 2017;403:143–170. doi: 10.1007/82_2016_508. [DOI] [PubMed] [Google Scholar]
- 138.Carlson B, Tobe R, Yefremova E, Tsuji P, Hoffmann V, Schweizer U, et al. Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Biol. 2016;9:22–31. doi: 10.1016/j.redox.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim J, Kim H, Son C. Tissue-Specific Profiling of Oxidative Stress-Associated Transcriptome in a Healthy Mouse Model. Int J Mol Sci. 2018;19(10):3174. doi: 10.3390/ijms19103174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qi J, Kim J, Zhou Z, Lim C, Kim B. Ferroptosis Affects the Progression of Nonalcoholic Steatohepatitis via the Modulation of Lipid Peroxidation-Mediated Cell Death in Mice. Am J Pathol. 2019;190(1):68–81. doi: 10.1016/j.ajpath.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 141.Atarashi M, Izawa T, Kuwamura M, Yamate J. The role of iron overload in the progression of nonalcoholic steatohepatitis (NASH) Nihon Yakurigaku Zasshi. 2019;154:2. doi: 10.1254/fpj.154.61. [DOI] [PubMed] [Google Scholar]
- 142.Singh B, Arora S, Agrawal P, Gupta S. Hepcidin: a novel peptide hormone regulating iron metabolism. Clin Chim Acta. 2011;412(11–12):823–830. doi: 10.1016/j.cca.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 143.Brasse-Lagnel C, Karim Z, Letteron P, Bekri S, Bado A, Beaumont C. Intestinal DMT1 cotransporter is down-regulated by hepcidin via proteasome internalization and degradation. Gastroenterology. 2011;140(4):1261–71.e1. doi: 10.1053/j.gastro.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 144.Senates E, Yilmaz Y, Colak Y, Ozturk O, Altunoz M, Kurt R, et al. Serum levels of hepcidin in patients with biopsy-proven nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2011;9(4):287–290. doi: 10.1089/met.2010.0121. [DOI] [PubMed] [Google Scholar]
- 145.Britton L, Subramaniam V, Crawford D. Iron and non-alcoholic fatty liver disease. World J Gastroenterol. 2016;22(36):8112–8122. doi: 10.3748/wjg.v22.i36.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang W, Stockwell B. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15(3):234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ramm G, Crawford D, Powell L, Walker N, Fletcher L, Halliday J. Hepatic stellate cell activation in genetic haemochromatosis Lobular distribution, effect of increasing hepatic iron and response to phlebotomy. J Hepatol. 1997;26(3):584–92. [DOI] [PubMed]
- 148.Levine B, Sinha S, Kroemer G. Bcl-2 family members: Dual regulators of apoptosis and autophagy. Autophagy. 2008;4(5):600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.González-Rodríguez A, Mayoral R, Agra N, Valdecantos M, Pardo V, Miquilena-Colina M, et al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 2014;5(4):e1179. doi: 10.1038/cddis.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chuang S, Yang C, Chou C, Chiang Y, Chuang T, Hsu L. TLR-induced PAI-2 expression suppresses IL-1β processing via increasing autophagy and NLRP3 degradation. Proc Natl Acad Sci U S A. 2013;110(40):16079–16084. doi: 10.1073/pnas.1306556110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu H, Chen Q. Hypoxia activation of mitophagy and its role in disease pathogenesis. Antioxid Redox Signal. 2015;22(12):1032–1046. doi: 10.1089/ars.2014.6204. [DOI] [PubMed] [Google Scholar]
- 152.Qiu T, Pei P, Yao X, Jiang L, Wei S, Wang Z, et al. Taurine attenuates arsenic-induced pyroptosis and nonalcoholic steatohepatitis by inhibiting the autophagic-inflammasomal pathway. Cell Death Dis. 2018;9(10):946. doi: 10.1038/s41419-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Su L, Zhang J, Gomez H, Murugan R, Hong X, Xu D, et al. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid Med Cell Longev. 2019;2019:5080843. doi: 10.1155/2019/5080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Park E, Chung S. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019;10(11):822. doi: 10.1038/s41419-019-2064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25(8):795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shiffman M, Freilich B, Vuppalanchi R, Watt K, Chan J, Spada A, et al. Randomised clinical trial: emricasan versus placebo significantly decreases ALT and caspase 3/7 activation in subjects with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2019;49(1):64–73. doi: 10.1111/apt.15030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Anstee Q, Concas D, Kudo H, Levene A, Pollard J, Charlton P, et al. Impact of pan-caspase inhibition in animal models of established steatosis and non-alcoholic steatohepatitis. J Hepatol. 2010;53(3):542–550. doi: 10.1016/j.jhep.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 158.Pockros P, Schiff E, Shiffman M, McHutchison J, Gish R, Afdhal N, et al. Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology. 2007;46(2):324–329. doi: 10.1002/hep.21664. [DOI] [PubMed] [Google Scholar]
- 159.Galluzzi L, Kepp O, Chan FK, Kroemer G. Necroptosis: Mechanisms and Relevance to Disease. Annu Rev Pathol. 2017;12:103–130. doi: 10.1146/annurev-pathol-052016-100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Poon J, Zilka O, Pratt DJAcs. Potent Ferroptosis Inhibitors Can Catalyze the Cross-Dismutation of Phospholipid-Derived Peroxyl Radicals and Hydroperoxyl Radicals. J Am Chem Soc. 2020;142(33):14331–42. [DOI] [PubMed]
- 161.Zilka O, Shah R, Li B, Friedmann Angeli J, Griesser M, Conrad M, et al. On the Mechanism of Cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the Role of Lipid Peroxidation in Ferroptotic Cell Death. ACS Sens. 2017;3(3):232–243. doi: 10.1021/acscentsci.7b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Doll S, Proneth B, Tyurina Y, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem bIol. 2017;13(1):91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shah R, Shchepinov M, Pratt D. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Sens. 2018;4(3):387–396. doi: 10.1021/acscentsci.7b00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Younossi ZM, Jarrar M, Nugent C, Randhawa M, Afendy M, Stepanova M, et al. A Novel Diagnostic Biomarker Panel for Obesity-related Nonalcoholic Steatohepatitis (NASH) Obes Surg. 2008;18(11):1430–1437. doi: 10.1007/s11695-008-9506-y. [DOI] [PubMed] [Google Scholar]
- 165.Huang J, Yeh M, Huang C, Huang C, Tsai P, Tai C, et al. Cytokeratin-18 and uric acid predicts disease severity in Taiwanese nonalcoholic steatohepatitis patients. PLoS ONE. 2017;12(5):e0174394. doi: 10.1371/journal.pone.0174394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sharma B, Kumar A, Garg V, Reddy R, Sakhuja P, Sarin S. A Randomized Controlled Trial Comparing Efficacy of Pentoxifylline and Pioglitazone on Metabolic Factors and Liver Histology in Patients with Non-alcoholic Steatohepatitis. J Clin Exp Hepatol. 2012;2(4):333–337. doi: 10.1016/j.jceh.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ratziu V, Sheikh M, Sanyal A, Lim J, Conjeevaram H, Chalasani N, et al. A phase 2, randomized, double-blind, placebo-controlled study of GS-9450 in subjects with nonalcoholic steatohepatitis. Hepatology. 2012;55(2):419–428. doi: 10.1002/hep.24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Canbay A, Guicciardi M, Higuchi H, Feldstein A, Bronk S, Rydzewski R, et al. Cathepsin B inactivation attenuates hepatic injury and fibrosis during cholestasis. J Clin Invest. 2003;112(2):152–159. doi: 10.1172/JCI17740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol. 2018;53(3):362–376. doi: 10.1007/s00535-017-1415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Neuschwander-Tetri B, Loomba R, Sanyal A, Lavine J, Van Natta M, Abdelmalek M, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nakashima T, Sumida Y, Furutani M, Hirohama A, Okita M, Mitsuyoshi H, et al. Elevation of serum thioredoxin levels in patients with nonalcoholic steatohepatitis. Hepatol Res. 2005;33(2):135–137. doi: 10.1016/j.hepres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 172.Lee F, Zee B, Cheung F, Kwong P, Chiang C, Leung K, et al. Randomized Phase II Study of the X-linked Inhibitor of Apoptosis (XIAP) Antisense AEG35156 in Combination With Sorafenib in Patients With Advanced Hepatocellular Carcinoma (HCC) Am J Clin Oncol. 2016;39(6):609–613. doi: 10.1097/COC.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 173.Kopalli S, Kang T, Koppula S. Necroptosis inhibitors as therapeutic targets in inflammation mediated disorders - a review of the current literature and patents. Expert Opin Ther Pat. 2016;26(11):1239–1256. doi: 10.1080/13543776.2016.1230201. [DOI] [PubMed] [Google Scholar]
- 174.Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol. 2015;12(7):387–400. doi: 10.1038/nrgastro.2015.94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.