Abstract
Background
Definitive radiation therapy (dRT) is an effective initial treatment of intermediate-risk (IR) and high-risk (HR) prostate cancer (PCa). PSMA PET/CT is superior to standard of care imaging (CT, MRI, bone scan) for detecting regional and distant metastatic PCa. PSMA PET/CT thus has the potential to guide patient selection and the planning for dRT and improve patient outcomes.
Methods
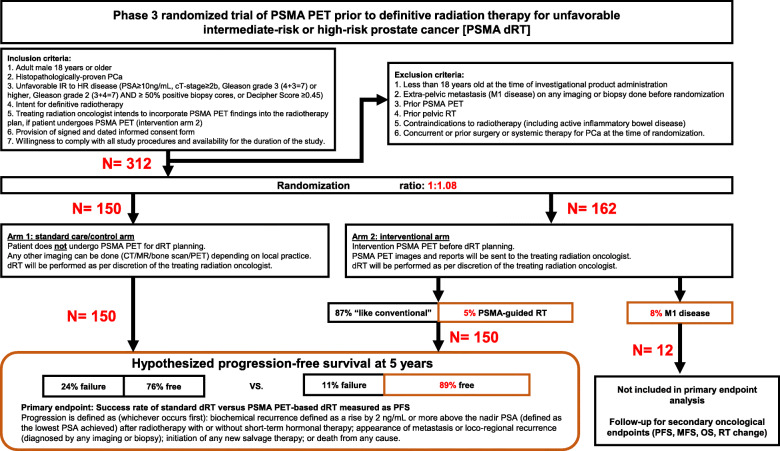
This is a multicenter randomized phase 3 trial (NCT04457245). We will randomize 312 patients to proceed with standard dRT (control Arm, n = 150), or undergo a PSMA PET/CT scan at the study site (both 18F-DCFPyL and 68Ga-PSMA-11 can be used) prior to dRT planning (intervention arm, n = 162). dRT will be performed at the treating radiation oncologist facility. In the control arm, dRT will be performed as routinely planned. In the intervention arm, the treating radiation oncologist can incorporate PSMA PET/CT findings into the RT planning. Androgen deprivation therapy (ADT) is administered per discretion of the treating radiation oncologist and may be modified as a result of the PSMA PET/CT results. We assume that approximately 8% of subjects randomized to the PSMA PET arm will be found to have M1 disease and thus will be more appropriate candidates for long-term systemic or multimodal therapy, rather than curative intent dRT. PET M1 patients will thus not be included in the primary endpoint analysis. The primary endpoint is the success rate of patients with unfavorable IR and HR PCa after standard dRT versus PSMA PET-based dRT. Secondary Endpoints (whole cohort) include progression free survival (PFS), metastasis-free survival after initiation of RT, overall survival (OS), % of change in initial treatment intent and Safety.
Discussion
This is the first randomized phase 3 prospective trial designed to determine whether PSMA PET/CT molecular imaging can improve outcomes in patients with PCa who receive dRT. In this trial the incorporation of PSMA PET/CT may improve the success rate of curative intent radiotherapy in two ways: to optimize patient selection as a biomarker and to personalizes the radiotherapy plan.
Clinical trial registration
UCLA
- IND#147591
- ○ Submission: 02.27.2020
- ○ Safe-to-proceed letter issued by FDA: 04.01.2020
UCLA IRB #20–000378
ClinicalTrials.gov Identifier NCT04457245. Date of Registry: 07.07.2020.
Essen
EudraCT 2020–003526-23
Keywords: Prostate cancer, PSMA, PET/CT, Randomized phase 3 trial, Definitive radiation therapy
Background
Definitive Radiation Therapy for clinically localized prostate cancer
Standard options for the initial management of men with clinically localized prostate cancer (PCa) include radiation therapy (RT; external beam and/or brachytherapy, with or without androgen deprivation therapy [ADT]), radical prostatectomy, or active surveillance in carefully selected patients. The choice of treatment is determined by a variety of factors, including risk stratification, patient preference, clinician judgment, and resource availability. Although there are few randomized trials comparing RT with radical prostatectomy, the trials completed to date and observational data suggest that outcomes with either external beam RT or brachytherapy (using adequate dosing schedules and contemporary treatment techniques) are similar to those with radical prostatectomy when men with clinically localized PCa are stratified based on clinical tumor (T) stage, pretreatment serum prostate-specific antigen (PSA), and Gleason score [1–4]. Several studies have evaluated treatment outcomes for definitive radiation therapy (dRT) for PCa in patients with low-, intermediate- (IR) and high-risk (HR) disease. Progression-free survival (PFS) for patients with IR and HR PCa following dRT treatment ranges from 53 to 97% and 42–86%, respectively (Table 1) [5].
Table 1.
Studies evaluating several treatment regimens of RT on patients with low-, intermediate- and high-risk PCa
| Author, year | Ref | RT type | n | total dose (Gy) | total fractions | Gy/ fraction | PFS def. | Risk group n | 5-year PFS (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LR | IR | HR | LR | IR | HR | ||||||||
| Kuban, 2008 | [6] | 3D | 151 | 78 | 39 | 2 | P | 30 | 68 | 53 | 100 | 86 | 69 |
| 3D | 150 | 70 | 35 | 2 | P | 31 | 71 | 48 | 88 | 83 | 54 | ||
| Al-Mamgani, 2008 | [7] | 3D | 333 | 78 | 39 | 2 | P | 63 | 90 | 180 | N/A | 70 | N/A |
| 3D | 331 | 68 | 34 | 2 | P | 56 | 89 | 185 | N/A | 60 | N/A | ||
| Zietman, 2010 | [8] | 3D | 195 | 79 | 44 | 1.8 | P | 116 | 72 | 7 | 95 | 79 | N/A |
| 3D | 197 | 70 | 39 | 1.8 | P | 111 | 76 | 10 | 75 | 68 | N/A | ||
| Dearnaley, 2007 | [9] | 3D | 422 | 74 | 37 | 2 | P | 99 | 127 | 184 | 71 | 71 | N/A |
| 3D | 421 | 64 | 32 | 2 | P | 95 | 137 | 175 | 60 | 60 | N/A | ||
| Michalski, 2010, 2012 | [10, 11] | 3D | 108 | 68 | 38 | 1.8 | P | 55 | 37 | 69 | 68 | 70 | 42 |
| 3D | 300 | 74 | 41 | 1.8 | P | 91 | 75 | 39 | 73 | 62 | 62 | ||
| 3D | 167 | 79 | 44 | 1.8 | P | 85 | 54 | 36 | 67 | 70 | 70 | ||
| 3D | 256 | 74 | 37 | 2 | P | 92 | 109 | 40 | 84 | 74 | 54 | ||
| 3D | 220 | 78 | 39 | 2 | P | 80 | 109 | 32 | 80 | 69 | 67 | ||
| Beckendorf, 2011 | [12] | 3D | 153 | 70 | 35 | 2 | P | – | 153 | – | – | 68 | – |
| 3D | 153 | 80 | 40 | 2 | P | – | 153 | – | – | 74 | – | ||
| Michalski, 2014 | [13] | 3D+ IMRT | 748 | 79 | 44 | 1.8 | P | – | 748 | – | – | 75 | – |
| 3D+ IMRT | 751 | 70 | 39 | 1.8 | P | – | 751 | – | – | 60 | – | ||
| 3D | (491) | N/A | N/A | N/A | P | 30 | 68 | 53 | N/A | N/A | N/A | ||
| IMRT | (257) | N/A | N/A | N/A | P | 31 | 71 | 48 | N/A | N/A | N/A | ||
| Lukka, 2005 | [14] | 3D | 470 | 66 | 33 | 2 | A | – | 470 | – | N/A | 53 | N/A |
| 3D | 466 | 52 | 20 | 2.63 | A | – | 466 | – | N/A | 60 | N/A | ||
| Yeoh, 2011 | [15] | 3D | 109 | 64 | 32 | 2 | P | – | 109 | – | N/A | 58 | N/A |
| 3D | 108 | 55 | 20 | 2.75 | P | – | 108 | – | N/A | 69 | N/A | ||
| Arcangeli, 2012 | [16] | 3D | 85 | 80 | 40 | 2 | P | – | – | 85 | N/A | N/A | 79 |
| 3D | 83 | 62 | 20 | 3.1 | P | – | – | 83 | N/A | N/A | 85 | ||
| Pollack, 2013 | [17] | IMRT | 152 | 76 | 38 | 2 | P | – | 101 | 51 | N/A | 86 | 86 |
| IMRT | 151 | 70 | 26 | 2.7 | P | – | 98 | 53 | N/A | 86 | 86 | ||
| Kuban, 2010 | [18] | IMRT | 102 | 76 | 42 | 1.8 | P | 30 | 1 | 1 | 96 | 96 | N/A |
| IMRT | 102 | 72 | 30 | 2.4 | P | 30 | 1 | 1 | 97 | 97 | N/A | ||
| Mantz, 2014 | [19] | Gantry | 102 | 40 | 5 | 8.0 | P | 40 | – | – | 100 | N/A | N/A |
| Katz, 2010, 2011 | [20, 21] | RA | 304 | 35 | 5 | 7.0 | P | 211 | 81 | 12 | 99 | 93 | 75 |
| 36 | 5 | 7.3 | P | ||||||||||
| Fuller, 2014 | [22] | RA | 60 | 38 | 4 | 9.5 | P | 40 | 39 | – | 100 | 92 | N/A |
| Dubray, 2016 | [23] | 3D without ADT | 191 | 80 | 40 | 2 | – | – | 191 | – | – | 76 | – |
| with ADT | 179 | – | – | 179 | – | – | 84 | – | |||||
| Dong, 2017 | [24] | 3D/IMRT without ADT | 979 | 74–80 or 70.2 | 40 or 26 | 1.8–2.7 | – | – | 979 | – | – | 87.3 | – |
| with ADT | 155 | – | – | 155 | – | – | 84 | – | |||||
3D-CRT 3 dimensional conformal radiation therapy, PFS progression-free survival, HR high risk, IMRT intensity modulated radiation therapy, IR intermediate risk, LR low risk, N/A not applicable. The definition of PFS (Phoenix, P; or ASTRO A) is listed
Conventional Imaging studies (radionuclide bone scan, computed tomography [CT] of the abdomen and pelvis, multiparametric magnetic resonance imaging [MRI]) are used selectively to assess for extraprostatic extension, regional lymphadenopathy, or distant metastases, for patients with IR and HR disease [25]. MRI is used for early detection of cancer and also often used for the purpose of assisting with RT planning and contouring..
Positron emission tomography (PET) using small molecule probes targeting prostate-specific membrane antigen (PSMA PET/CT) is superior to standard of care imaging for detecting regional and distant metastatic recurrent PCa at low PSA levels [26–30], highly specific [30] and reproducible [31]. Studies have demonstrated clear diagnostic accuracy superiority in large numbers of patients with biochemical recurrent PCa after curative treatment compared to conventional imaging for detection of locoregional recurrence and/or metastases [29, 32]. PSMA PET/CT outperformed planar bone scan for detection of osseous metastases in large retrospective analyses [33, 34]. The detection rate of PSMA PET/CT for recurrent PCa exceeds that of choline PET [35, 36], and of 18F-Fluciclovine PET [29, 37, 38]. PSMA PET/CT can improve RT planning and patient selection for salvage radiation therapy (SRT) thus may potentially improve its outcome [39–41]. Randomized trials investigating the outcome of SRT based on PSMA PET/CT and conventional imaging are now ongoing (NCT03525288, NCT03762759, NCT03582774) [42].
Impact of PSMA PET/CT on primary staging of prostate Cancer
The potential role of PSMA PET/CT in primary staging of patients with IR and HR PCa has been explored in only a small number of studies outlined below (Table 2).
Table 2.
Literature review of the impact of PSMA PET on primary staging of patients with prostate cancer
| Author and year | Study Design | Location | N | Population | Median PSA ng/mL (range) | Improvement with PSMA PET |
|---|---|---|---|---|---|---|
| Budäus et al. 2016 [48] | R | Hamburg, Germany | 30 | HR PCa prior to RP | 8.8 (1.4–376) | Se 33%, spec 100%, PPV 100%, NPV 69% |
| Calais et al. 2018 [45] | P | Los Angeles, USA | 73 | IR/HR PCa prior to RT planning | 13.9 (0.22–909) | 9.5% uptaged to M1 |
| Demirkol et al. 2015 [49] | R | Istanbul, Turkey | 8 | HR PCa for staging | 15 (0.3–20) | N/A |
| Fendler et al. 2016 [50] | R | Munich, Germany | 21 | PCa for staging | N/A | Se 67%, spec 92%, PPV 97%, NPV 42% Acc 72% |
| Frenzel et al. 2018 [51] | R | Hamburg, Germany | 20 | PCa prior to RT planning | 7.1 (0.48–137) | N/A |
| Herlemann et al. 2016 [52] | R | Munich, Germany | 20 | HR PCa prior to RP | a56 (3.3–363) | Se 84%, spec 82%, PPV 84%, NPV 82% |
| Hijazi et al. 2015 [53] | R | Göttingen, Germany | 12 | PCa for staging | 48 (6–90) | Se 94%, spec 99%, PPV 89%, NPV 99.5% |
| Hirmas et al. 2018 [54] | R | Amman, Jordan | 21 | HR PCa for staging | 38 (0.6- > 100) | Se 85% Acc 85.7%, PPV 100% |
| Hruby et al. 2018 [55] | R | NSW, Australia | 109 | IR/HR PCa prior to EBRT | 9.9 (1.23–240) | 21% upstaged, 3% downstaged |
| Kabasakal et al. 2015 [56] | R | Istanbul, Turkey | 15 | PCa for staging | 37.78 (5.12–70.47) | N/A |
| Maurer et al. 2015 [57] | R | Munich, Germany | 130 | HR PCa prior to RP | 11.6 (0.57–244) | Se 68%, spec 99%, PPV 95%, NPV 94% |
| Rahbar et al. 2015 [58] | P | Münster, Germany | 6 | HR PCa prior to RP | 52.7 (5.7–111.1) | Se 92%, spec 92%, PPV 96%, NPV 85% |
| Rhee et al. 2016 [59] | P | Queensland, Australia | 20 | PCa prior to RP | 6.1 (3.5–45) | Se 49%, spec 95%, PPV 85% NPV 88% |
| Roach et al. 2017 [44] | P | Sydney, Australia | 108 | IR/HR PCa for staging | 8.6 (0.18–120) | 20% upstaged, 1% downstaged |
| Sachpekidis et al. 2016 [60] | P | Heidelberg, Germany | 24 | HR PCa | 24.1 (3.2–200) | N/A |
| Schwenck et al. 2016 [61] | P | Tübingen, Germany | 20 | HR PCa for staging, PSMA vs choline | 26 (N/A) | N/A |
| Sterzing et al. 2016 [62] | R | Heidelberg, Germany | 15 | HR PCa for staging | 7 (0.28–45) | 13.7% changed their TNM staging |
| Uprimny et al. 2017 [63] | R | Innsbruck, Austria | 90 | PCa, other analysis | 9.7 (2.2–188.4) | N/A |
| Van Leeuwen et al. 2017 [64] | P | Sydney, Australia | 30 | IR/HR PCa prior to RP | 8.1 (5.2–10.1) | Se 58%, spec 100%, PPV 94%, NPV 98% |
| Zamboglou et al. 2015 [65] | R | Freiburg, Germany | 22 | PCa prior to RT planning | 20.4 (1.22–66.9) | GTV-PET larger than GTV-MRI |
aValue for mean reported, not median. GTV gross tumor volume, HR High-risk, IR intermediate-risk, LND lymph node dissection, N/A not applicable, NPV negative predictive value, P prospective study, PPV positive predictive value, R retrospective study, RP radical prostatectomy, RT radiotherapy, Se Sensitivity, spec specificity
Despite the clear diagnostic superiority of PSMA PET/CT in initial staging of PCa [43], its impact on outcome of patients with IR and HR PCa has not been assessed prospectively. At the time of study design, several mostly retrospective studies reported the accuracy of PSMA PET/CT at initial staging (Table 2). Six studies (highlighted in bold) evaluated the impact of PSMA PET/CT (whether exclusively or not) on dRT planning of patients. Four of these studies are retrospective. One prospective study is a multicenter Australian study with 420 patients (108 patients for primary staging of IR and HR disease and 312 patients for restaging/biochemical recurrence) [44]. Comparison was made between both groups and the impact was shown to be greater in the group of patients with biochemical failure after definitive surgery or dRT than in patients undergoing primary staging. The other prospective study is a US post-hoc analysis of an intention-to-treat population of 73 patients with localized PCa without prior local therapy who underwent PSMA PET/CT for initial staging [45]. The scan had a major impact on intended definitive PCa dRT planning in 16.5% of patients when RT fields were intended to cover the prostate, seminal vesicles and the pelvic LNs, and in 37% when RT fields were intended to cover only the prostate and seminal vesicles. Recent studies from Koerber et al. detail aspects of nodal involvement and highlight impact in a mixed primary/recurrence population [46, 47].
PSMA PET/CT thus has the potential to guide dRT planning in patients and improve outcomes. Five studies have shown that dRT planning based on PSMA PET/CT results can change local plan in 13–19.5% and detect extra-pelvic disease in 6.4–9.5% of patients (Table 3).
Table 3.
Changes in RT plan in studies assessing effect of PSMA PET results on treatment plan
| Author and year | N | Inclusion criteria | Median PSA level, ng/mL (range) | Change in planned pelvic RT | ||
|---|---|---|---|---|---|---|
| % local plan change | % extra-pelvic disease | local plan change details | ||||
| Calais et al. 2018 [54] | 73 | IR/HR PCa prior to RT planning | 13.9 (0.22–909) | 7–19.5%a | 9.5% | covered pelvic LNs detected |
| Frenzel et al. 2018 [51] | 20 | PCa prior to RT planning | 7.1 (0.48–137) | 15% | N/A | shifted from IR to HR, one patient had boost to distant PET findings |
| Hruby et al. 2018 [55] | 109 | IR/HR PCa prior to RT planning | 9.9 (1.23–240) | 14.7% | 6.4% | covered pelvic LNs detected |
| Roach et al. 2017 [44] | 108 | IR/HR PCa prior to RP/ EBRT/ systemic treatment | 8.6 (0.18–120) | 15% | 9% | higher dose and volume |
| Sterzing et al. 2016 [62] | 15 | HR PCa for staging | 7 (0.28–45) | 13% | N/A | covered pelvic LNs detected |
aDepending on initial intent to include elective pelvic nodal RT (change: 7%) or not (change: 19.5%). +Mean value reported, not median. EBRT external-beam radiotherapy
Treatment outcomes of dRT for PCa
Several studies have evaluated treatment outcomes of dRT for PCa in patients with low-, IR and HR disease. PFS for patients with IR and HR PCa following dRT ranges from 53 to 97% and 42–86%, respectively (Table 3) [5].
Current prospective trials evaluating the impact of PSMA PET/CT on prostate RT planning
A prospective, randomized and multicenter study demonstrated superior accuracy of PSMA PET/CT along with impact on first- and second-line management of PCa patients with high-risk features (proPSMA study) [43]. Among the 339 recruited men, PSMA PET/CT-CT had a 27% greater accuracy than that of conventional imaging (92% vs 65%). There was higher rate of management change associated with PSMA PET/CT vs. conventional imaging (41 vs. 23% men).
Trial NCT03525288 currently taking place at Centre hospitalier de l’Université de Montréal (CHUM), Canada, aims to compare second generation 18F-DCFPyL PET with conventional imaging prior to RT planning in patients with HR, recurrent or oligometastatic PCa. Patients included will be randomized to either 18F-DCFPyL PET or conventional imaging prior to treatment planning, which will depend on imaging results of each arm. PFS will be assessed as primary endpoint with aims of showing that PSMA PET/CT findings will lead to improved cancer control outcomes compared to RT guided by conventional staging only.
Trial NCT03344822 taking place in Central Hospital in Nancy, France, aims to evaluate the difference of management intent after the initial staging of patients with HR PCa with PSMA PET/CT in comparison of 18F-Choline PET results. Each patient will receive a 18F-Choline PET followed by PSMA PET/CT.
It is unclear if incorporation of PSMA PET/CT imaging into the planning of dRT could improve its likelihood of success. There is no current randomized prospective trial designed to determine whether PSMA PET/CT can improve outcomes in patients with primary IR or HR PCa undergoing dRT.
The purpose of the present trial is to evaluate the success rate of is to compare the success rate of patients with unfavorable IR and HR PCa after standard dRT versus PSMA PET-based dRT.
Rationale for study design and hypothesis
The overall study design is shown in Fig. 1
Fig. 1.
Flowchart of literature search and selection of studies
From our comprehensive literature review and subsequent meta-analysis, we have found that the 5-year PFS with dRT given to patients with primary PCa (IR and HR combined), with or without ADT, reaches 76% based on data from the meta-analysis of Table 1 studies (random effect-model). In addition, PSMA PET/CT changes the management plan prior to RT for patients with primary PCa in at least 13% of cases using the same model (meta-analysis of Table 3 studies). Therefore, we hypothesized that the incorporation of PSMA PET/CT to RT planning would improve the 5-year PFS of dRT in patients with unfavorable IR or HR PCa by 13% to reach 89% (intervention group) versus 76% without PSMA PET/CT (control group).
In this study, patients will be randomized into two arms:
standard RT arm 1: Patient does not undergo PSMA PET/CT for RT planning. RT will be performed as routinely planned in accordance to initial stratification. Any other imaging is allowed if done per routine care. If a control group patient undergoes a PSMA PET/CT scan at another institution he will be discontinued from the study.
PSMA PET/CT-based RT arm 2: Patient undergoes PSMA PET/CT for RT planning. Treating radiation oncologist will incorporate PSMA PET/CT findings into the RT planning and in accordance to initial stratification. Conventional imaging for staging purposes is not mandatory.
The 5-year PFS is expected to be 76% in Arm 1 (standard RT) and 89% in arm 2 (PSMA PET/CT-based RT), and since 8% will be found ineligible to continue this study post PSMA PET/CT, patients will be randomized in a 1:1.08 ratio. The primary endpoint of the trial is PFS. We will compare PFS between the two randomized treatment arms, stratified by very high risk (T3b or primary ISUP grade 5 pattern or ≥ 5 cores with International Society of Urological Pathologists (ISUP) grade group 4–5 or higher)) versus less than very high risk. We assumed, based on data from the meta-analyzed proportion for extra-pelvic disease (random effect model), that approximately 8% (n = 12) of subjects randomized to arm 2 will be found to be ineligible for dRT due to the presence of extra-pelvic metastatic (M1) detected by PSMA PET/CT (meta-analysis of Table 3 studies). These patients will not be included in analysis of the primary endpoint but will still be followed for other secondary endpoints.
The overall approach is to use PSMA PET not as a diagnostic tool but rather as biomarker for patient selection. The primary objective of the trial is not to assess whether curative RT will improve the oncological outcome of patients after PSMA-based staging. Rather, it is to determine if integration of PSMA PET/CT at the time of RT planning increases success rate of curative intent RT. We will compare the success rate of patients with unfavorable IR and HR PCa who actually underwent dRT: standard dRT versus PSMA PET-based dRT.
Objective of the trial
The aim of the study is to compare the outcome of patients with unfavorable IR and HR PCa after standard dRT versus PSMA PET/CT-based dRT. Outcome will be assessed in parallel trials at UCLA (18F-DCFPyL PET) and Essen (68Ga-PSMA-11 PET).
Trial design
This is a prospective multicenter interventional controlled randomized open-label phase 3 clinical trial with parallel assignment. The intervention is one PSMA PET/CT scan (both 18F-DCFPyL and 68Ga-PSMA-11 can be used) performed at the nuclear medicine department of the study site. DRT is performed at the treating radiation oncologist facility. Patients are followed remotely by the nuclear medicine clinical research team. The aim of the study is to compare the outcome of patients with unfavorable IR and HR PCa after standard dRT versus PSMA PET/CT-based dRT. Final analysis will be conducted on joint datasets.
A total of 312 patients will be randomized into two arms (randomization ratio 1:1.08): control arm 1 (n = 150, without PSMA PET/CT scan) and intervention arm 2 (n = 162 with PSMA PET/CT scan). Screening, randomization, follow-up and data management will be performed centrally. RT can be done anywhere, at the institution of choice of the referring radiation oncologist and/or the patient. The investigators will rely on the medical records obtained from the treating physicians as the primary source of outcome data.
The study is powered for 5-year PFS. If a patient assigned to the control arm undergo a PSMA PET/CT scan at another institution prior to dRT, he will be discontinued from the study. Trials at UCLA and Essen are independent. Final analysis may be conducted on joint datasets from multiple sites, if acquired under similar PSMA-targeted PET dRT randomized study protocols.
Methods
Study population
In order to be eligible to participate in this study, an individual must meet all of the following criteria:
Adult male 18 years or older.
Histopathologically-proven PCa
- Unfavorable IR to HR disease
- PSA ≥ 10 ng/mL
- or cT-stage≥2b
- or ISUP grade 3 (Gleason 4 + 3 = 7) or higher
- or ISUP grade 2 (Gleason 3 + 4 = 7) AND ≥ 50% positive biopsy cores
- or Decipher Score ≥ 0.45
Intent for definitive radiotherapy
Treating radiation oncologist intends to incorporate PSMA PET/CT findings into the radiotherapy plan, if patient undergoes PSMA PET/CT (intervention arm 2)
Provision of signed and dated informed consent form
Stated willingness to comply with all study procedures and availability for the duration of the study.
An individual who meets any of the following criteria will be excluded from participation in this study:
Less than 18 years old at the time of investigational product administration
Extra-pelvic metastasis (M1 disease) on any imaging or biopsy done before randomization
Prior PSMA PET/CT
Prior pelvic RT
Contraindications to radiotherapy (including active inflammatory bowel disease)
Concurrent or prior surgery or systemic therapy for PCa at the time of randomization.
Intervention
Study procedure
Patients allocated to the PSMA dRT arm (arm 2) will undergo one PSMA PET/CT scan at the nuclear medicine department of the study site before dRT planning.
Investigational PET imaging drug
Small radiolabeled ligands 18F-DCFPyL (UCLA) or 68GaPSMA11 (Essen) can be used as the PET radiopharmaceutical.
PET/CT imaging protocol specifics
Oral hydration is recommended immediately after injection the radiotracer before start of the scan.
PET/CT images will be acquired at 50–100 min (target 60 min) after intravenous injection of the radiotracer.
PET/CT scan coverage will extend from mid-thigh to the vertex.
A diagnostic CT will be acquired just before the PET imaging acquisition for attenuation correction.
CT-Contrast may be administered if requested by the referring clinician or the attending nuclear medicine physician. Details are outside the scope of this study protocol.
PET images will be acquired in 3D mode with a weight-based time per-bed-position
The PET emission scan will be corrected for decay, dead-time, random events, and scatter. PET images will be corrected for attenuation using segmented attenuation data of the low-dose CT scan. PET images will be reconstructed using ordered subset expectation maximization (OSEM) and filtered to a spatial resolution of 5 mm (isotropic) with a Gauss-filter.
PET/CT imaging analysis and image transfer
PET/CT Images will be reviewed and analyzed using dedicated workstations by a board certified nuclear medicine physician and a board certified radiologist during consensus clinical readouts in the Nuclear Medicine Departments using recent reporting guidelines (PROMISE criteria, miTNM standardized framework) with access to all medical records [66]. No blinded independent central readers will be used.
CD/DVD containing the PSMA PET/CT DICOM images and PET/CT report will be systematically delivered to the treating radiation oncologist.
Radiation therapy (RT) management
DRT is performed at the treating radiation oncologist facility. The modality, dose, fractionation, and target volumes of the RT are at the discretion of the treating radiation oncologist. EBRT regimens including conventional, hypofractionated, and extremely hypofractionated (SBRT) are allowable. Brachytherapy (LDR or HDR), alone or in combination with EBRT, is also allowable. The use of neoadjuvant, concurrent, or adjuvant ADT is also at the discretion of the treating physician.
Routine care dRT for intermediate or HR localized PCa typically involves either radiotherapy directed to the prostate and seminal vesicles alone, or the prostate, seminal vesicles, and pelvic lymph nodes. In addition, the RT can be delivered either with or without concurrent ADT. The choice of treating the prostate and seminal vesicles alone with or without ADT vs including also pelvic lymph nodes with ADT, is based upon clinico-pathologic features and practice patterns of the treating physician. The treating physician will be asked to describe their general treatment plan prior to randomization.
In some cases, due to specific anatomical features of the patient (for example, location of small bowel), or patient’s preference (for example, patient may decline ADT), the general treatment plan may be modified during the radiotherapy planning process. The treating physician is encouraged not to de-escalate therapy based on results of the PSMA PET/CT. For example, a PSMA PET/CT with no evidence of disease outside the prostate does not exclude the possibility of microscopic disease outside the prostate (for example, in pelvic lymph nodes).
Patients randomized to arm 1 do not undergo PSMA PET/CT and dRT will be performed as routinely planned per discretion of the treating radiation oncologist in accordance with the initial general treatment plan whenever possible. Any other imaging is allowed for RT planning if done per routine care. Systemic therapy, if needed, will be performed as per discretion of the treating radiation oncologist or other physician. If a control group patient undergoes a PSMA PET/CT scan at another institution prior to dRT, he will be discontinued from the study.
Patients randomized to arm 2 that have a negative PSMA PET/CT scan will undergo RT as routinely planned by the treating radiation oncologist in accordance with the initial general treatment plan whenever possible. Concurrent systemic therapy, if needed, will be performed as per discretion of the treating radiation oncologist or physician.
Patients randomized to arm 2 where PSMA PET/CT detects PSMA-positive lesions within the pelvis (prostate, seminal vesicles, extraprostatic extension, lymph nodes) will undergo RT performed by the treating radiation oncologist in accordance with the new findings. For example, the plan may include adapted/extended target volumes to cover all pelvic PSMA-positive lesions within the irradiated volumes. Additionally, the RT may incorporate focal dose escalation to the PSMA-positive lesions. Concurrent systemic therapy, if indicated, will be performed as per discretion of the treating radiation oncologist or other physician, and may be escalated in intensity or duration as a result of the PSMA PET/CT findings (for example, due to discovery of N1 disease).
Patients randomized to arm 2 that have a PSMA PET/CT showing PSMA-positive lesions outside the pelvis or osseous structures within the pelvis (i.e., M1 disease) will undergo treatment as per discretion of the treating radiation oncologist or other physician. However, the patient will not be included in analysis of the primary endpoint, because curative intent dRT is not an option for de novo M1 patients. We assume that approximately 8% of subjects randomized to arm 2 will be found to be ineligible for RT due to PSMA detected M1, and thus will not be included for primary endpoint analysis.
Outcome measures
Primary Endpoint Measures
Success rate of patients with unfavorable IR and HR PCa after standard dRT versus PSMA PET-based dRT measured as PFS after initiation of dRT [Time Frame: from date of randomization to first occurrence of progression, assessed up to 5 years]. Patients who do not underdo dRT are not included in the primary endpoint analysis.
Progression is defined as (whichever occurs first):
A biochemical recurrence defined as a rise by 2 ng/mL or more above the nadir PSA (defined as the lowest PSA achieved) after radiotherapy with or without short-term hormonal therapy [67].
Appearance of metastasis or loco-regional recurrence (diagnosed by any imaging or biopsy).
Initiation of any new salvage therapy
Death from any cause.
The investigators will rely on the medical records obtained from the treating physicians as the primary source of outcome data.
Secondary endpoints measures
Loco-regional progression free survival (PFS)
Diagnosis of local recurrence or pelvic nodal disease (N1) can be obtained by any imaging or biopsy.
Death from any cause
-
2)
Metastasis-free survival after initiation of RT
Diagnosis of extra-pelvic metastatic (M1) disease can be obtained by any imaging or biopsy.
Death from any cause
-
3)
Overall survival (OS)
-
4)
Change in initial treatment intent (%) as assessed by the comparison of the intended RT plan collected on questionnaires before randomization to the delivered RT plan.
-
5)
PSMA PET/CT derived predictors of PFS and OS.
-
6)
Safety
The investigators will rely on questionnaire, study and medical records obtained from the treating physicians as the primary source of outcome data.
Timeline
Screening and enrollment
Patients seen in consultation in a radiation oncology, urology/uro-oncology, or nuclear medicine clinic who are being evaluated for potential RT for PCa will be informed of this clinical study if eligible. Referring physicians will be educated on the study goals and logistics in order to recruit potential eligible and interested patients. The decision to participate will be entirely voluntary. Eligible patients who decide not to participate will be offered all other standard of care approaches. Treating radiation oncologist must intend to incorporate PSMA PET/CT findings into the radiotherapy plan if patient undergoes PSMA PET/CT (as per stratification). Patients will be consented either in person or over the phone (Signed consent form will be obtained by fax or email in the latter case).
Randomization and intervention
All the data management such as the randomization allocation will be performed by the lead institution in the online clinical trial database. This is an open label study. Trial participants, care providers, outcome assessors, and data analysts will be aware of the assignment after enrollment is completed. The randomization number and assignment will be communicated by phone or email to the treating physician. Patients will be informed by phone or email of the randomization assignment. If patient is randomized to investigational arm 2, he will be contacted and scheduled for a PSMA PET/CT scan at participating sites.
Outcome follow-up
Clinical, PSA and imaging follow-up will be conducted as per standard of care. Current standard of care usually includes weekly on treatment visits during radiotherapy followed by follow-up visits with radiation oncologist at least every 3 to 4 months for the first year and every 6 months for the next 5 years and annually thereafter (NCCN and ASTRO clinical guidelines). Imaging follow-up can be ordered when disease progression is suspected. We recommend restaging be initiated after PSA biochemical failure (Phoenix criteria). NCCN clinical guidelines recommend that work-up for progression may include prostate MRI, TRUS biopsy, abdominal CT, MRI, bone scan and/or PET. Interpretation of follow-up imaging will be performed by local reads.
Research investigators or their staff will conduct telephone and secure electronic messaging follow-ups with treating physicians at 3-month intervals for the first year following completion of radiotherapy, and then at 6 month intervals. Through these telephone and secure electronic messaging follow-ups with treating physicians, the study team can access PSA measurements and required follow-up information.
Study duration
We expect to enroll 312 patients within 2 years of study initiation. Patients will be followed (phone calls/ secure emails) until either one of the following conditions occur:
Five [5] years after the date of randomization.
Biochemical progression.
Diagnostic of metastatic disease.
Initiation of any additional salvage therapy.
Patient randomized to control arm 1 (control) undergo a PSMA PET/CT scan at another institution
Death.
Sample size determination
From our comprehensive literature review and subsequent meta-analysis, we found that 13% of patients would have had at least one lesion detected by PSMA PET/CT that would otherwise not be covered by the standard radiation fields covering both the prostate and pelvic lymph nodes (RTOG consensus delineations, meta-analysis of 1 studies). We therefore hypothesize that the incorporation of PSMA PET/CT to RT planning will improve 5-year PFS by 13%. Based on our meta-analysis of Table 3. Studies (random effects model), we estimate that the 5-year PFS of patients with PCa (IR and HR combined), with or without ADT, after standard dRT would be 76%. Therefore, we assume the 5-year PFS to be 76% in arm 1 (standard dRT) and 89% in arm 2 (PSMA PET/CT-based RT). We also assume that approximately 8% of subjects randomized to arm 2 will have extra-pelvic metastasis detected by PSMA PET/CT, and therefore are not curable by dRT. These patients will therefore not be included in the analysis of the primary endpoint but will still be followed for other secondary endpoints. We will compare the PFS between the two randomized treatment arms, stratified by very high risk (T3b or primary ISUP grade 5 pattern or ≥ 5 cores with ISUP grade group 4–5 vs less than very high risk. Final analysis may be conducted on joint datasets from multiple sites, if acquired under similar PSMA-targeted PET dRT randomized study protocols.
When the sample size in each group is 138, with a total number of events required of 43, a 0.050 level two-sided log-rank test for equality of survival curves will have 80% power to detect the difference between a PSMA group freedom from failure rate at 5-years of 89% and a control group freedom from failure rate at 5-years of 76% (a constant hazard ratio of 2.35). To account for an approximate 5–10% drop-out rate, we will plan for 150 patients per group, requiring 312 patients randomized as we expect that 12 in the PSMA group will have M1 disease and not be evaluable for the primary endpoint. Therefore, we will study 312 patients that are planned for dRT.
In case the above estimated total number of events is not reached at the end of the prespecified follow-up period, extension of this period with respective additional funding will be requested.
Allocation sequence generation, concealment mechanism and implementation
The randomization will be processed via Research Electronic Data Capture (REDCap) code for all subjects from all participating sites. The participating site(s) will receive the randomization assignment by the leading institution after enrollment. The list will only be accessible for researchers or study personnel not actively involved in the recruitment process. We will use one stratification factor: risk level (very high risk vs less than very high risk).
This is an open label study. Trial participants, care providers, outcome assessors, and data analysts will be aware of the assignment after enrollment is completed. The randomization number and assignment will be communicated to the treating physician and patient.
Data collection, management and monitoring
The REDCap® study database will have validated range checks for data entry fields, branching logic, and rigorous pre-testing to make sure the data are appropriately capture. The nuclear medicine research team will enter all data of each patient into REDCap® database. The nuclear medicine research team will have full access to all interim and final results of the study through the REDCap® database and is responsible for the final decision to terminate the trial. There is no planned interim analysis. All the data management will be performed by the nuclear medicine research team in the REDCap® online database. During the clinical investigation, the UCLA nuclear medicine research team will evaluate the progress of the trial, including periodic assessments of data quality and timeliness, participant recruitment, accrual and retention, participant risk versus benefit, and other factors that can affect study outcome. All the datasets generated during the current study will be stored and managed on the REDCap® database. All data generated and/or analyzed during this study will be publicly available (own DOI) after completion of the study and the publication of the article of the final analysis of study. Even if the required number of patients to reach statistical power is not met, patients already enrolled in the trial will still be followed for 5 years as this would remain highly valuable and unique data.
Statistical methods
Per-Protocol Analysis Dataset: n = 300 (sample size in each group is n = 150)
The primary endpoint (5-year PFS) will be analyzed in patients who actually underwent dRT. We assumed that approximately 8% of subjects randomized to arm 2 will have extra-pelvic metastasis detected by PSMA PET/CT, and therefore will not be treated with dRT. These patients will not be included in the analysis of the primary endpoint but will still be followed for other secondary endpoints.
Intention-to-Treat (ITT) Analysis Dataset: n = 312 (all randomized participants)
The secondary endpoints will be analyzed in all randomized participants.
Safety Analysis Dataset: n = 162 (intervention arm 2, PSMA PET/CT scan)
Analysis of the primary endpoint will be conducted upon reaching a sample size in each group of at least 150 and total number of 43 events. Survival curves will be constructed using the Kaplan-Meier method. We will use a stratified log rank test in our primary analysis to compare the PFS between the two randomized treatment arms, stratified by very high risk (T3b or primary ISUP grade 5 pattern or ≥ 5 cores with ISUP grade group 4–5 vs less than very high risk. Secondary analyses will utilize Cox-proportional hazards regression models. These models will include covariates pelvic LN RT, ISUP grade, T stage, initial PSA, and PSMA PET/CT nodal stage. Residual analyses will be performed to evaluate the proportional hazards assumptions of the Cox model. We will estimate the 95% confidence interval for the restricted mean PFS (over the planned 5-year follow-up duration) in each treatment group as well as the 95% confidence interval for the difference in mean PFS between the groups.
Final analysis may be conducted on joint datasets from multiple sites, when acquired under similar PSMA-targeted PET dRT randomized study protocols.
Similar to the primary endpoint, secondary survival endpoints (loco-regional PFS, metastasis free survival after RT, OS) will be compared between groups with stratified log rank tests and then with Cox proportional hazards regression models to control for above baseline subject characteristics.
Discussion
PSMA PET/CT is a highly sensitive imaging test to localize PCa. However, it is unclear if incorporation of PSMA PET/CT imaging into the planning of dRT could improve its likelihood of success. No randomized prospective trial has been designed to determine whether PSMA PET/CT can improve 5-year outcomes in patients with IR and HR PCa. The purpose of this trial is to compare the success rate of patients with unfavorable IR and HR PCa after standard dRT versus PSMA PET-based dRT.
The planned exclusion of patients with M1 disease identified by PET from the interventional arm for the primary endpoint may generate some confusion. The role of PSMA PET/CT as localization and stratification tool has been discussed extensively when building the study design by the investigators.
The primary endpoint is not PFS after randomization, rather, the primary endpoint is the success rate of curative intent radiotherapy (measured as PFS) in patients who undergo curative intent radiotherapy. To undergo curative intent radiotherapy, a patient must be eligible for it, and this eligibility is contingent upon having no M1 lesions on diagnostic imaging. We contend that it is ethically questionable to ignore PSMA PET/CT findings for choice of primary treatment, even if performed on trial.
In this trial the incorporation of PSMA PET/CT may improve the success rate of curative intent radiotherapy in two ways. First, PSMA PET/CT may identify patients who are found to have distant metastatic disease for whom curative intend radical radiotherapy may not be appropriate (other strategies would be more appropriate). Here, the PSMA PET/CT is used as a biomarker to optimize patient selection. A similar approach would be HER2 and Trastuzumab in breast cancer. The HER2 testing does not improve the outcome of all patients but identify patients HER2+ who will benefit from Trastuzumab. Second, in patients without distant metastatic disease, PSMA PET/CT may improve the efficacy of radiotherapy directed to local and regional disease (i.e., through more accurate target delineation and higher radiation dose delivered to gross disease). Here, PSMA PET/CT personalizes the radiotherapy plan for the patient based on the specific extent of their disease, rather than applying an essentially standardized set of treatment volumes and prescription doses.
The estimated 8% of patients randomized to arm 2 will be patients who are not eligible to receive curative intent radiotherapy. Our trial tests the clinical impact of individualized therapy provided by PSMA PET/CT. This can be considered within the broader framework of precision oncology. More commonly in the context of precision oncology, a genomic biomarker is used to guide patient selection for a systemic therapy appropriate only for biomarker selected patients. In our trial, a functional imaging biomarker is used to select patients for an anatomically defined local therapy. Genomic analyses and imaging represent two distinct sources of information. This biologic and anatomic data can be both prognostic of outcome and predictive for treatment selection. The common objective for both genomics-guided precision oncology and functional imaging-guided precision radiotherapy is the same: to match the right treatments to the right patients to improve efficacy of treatment and to avoid futile care.
The risk level of a PSMA PET imaging scan is insignificant, especially in patients who receive RT (at least 10,000 times the amount of radiation). However, the radiation oncologists may use the PSMA PET imaging scan to direct radiotherapy with the potential to modify RT parameters or exclude patients from RT. Thus, the study is considered “greater than minimal risk”. Even if the positive predictive value of PSMA PET is high (> 85%) [32, 68] we cannot formally rule-out treatment changes induced by false-positive findings. We also believe that it is important to know wether incorporating PSMA PET info does not improve cure rate because of false positives.
Potential pitfalls in study design include i) drop-out of patients randomized to the control arm as patients may be able to undergo PSMA PET/CT scans in other institutions; iv) potential FDA approval and incorporation into guidelines of PSMA PET/CT imaging probes (68Ga-PSMA-11 or 18F-DCFPyL) in the near future, which would in essence lead to termination of the outlined enrollment design. As PSMA PET/CT imaging may become standard of care, randomizing patients to the control arm would no longer be feasible. Therefore, the time period for patient recruitment may be limited. Even if the required number of patients to reach statistical power (n = 312) is not met, patients already enrolled in the trial will still be followed for 5 years as this would remain highly valuable and unique data.
This is the first prospective multicenter randomized phase 3 trial designed to determine whether PSMA PET/CT molecular imaging can improve outcome of dRT in patients with IR and HR PCa. Positive outcome would enable better patient selection, an important step towards individualized medicine.
Acknowledgements
We thank Jessica Jensen (Progenics) for her collaboration and support. We thank all the patients and their referring physicians whose willingness to participate make this study possible. We thank the whole staff team of the UCLA Nuclear Medicine and Theranostics Division whose hard work make this study possible.
Abbreviations
- 68Ga-
Gallium-68
- ADT
Androgen deprivation therapy
- ASTRO
American Society for Radiation Oncology
- CT
Computed tomography
- DKTA
University of Duisburg-Essen and German Cancer Consortium
- dRT
Definitive Radiation Therapy
- FDA
Food and Drug Administration
- GCP
Good Clinical Practice
- HIPAA
Health Information Portability and Accountability Act
- HR
High Risk
- IND
Investigational New Drug
- IR
Intermediate Risk
- IRB
Institutional Review Board
- ITT
Intention-To-Treat
- MBq
MegaBequerel
- LN
Lymph Node
- mCi
millicurie
- MRI
Magnetic resonance imaging
- NCCN
National Comprehensive Cancer Network
- OS
Overall Survival
- OSEM
Ordered Subset Expectation Maximization
- PCa
Prostate cancer
- PET/CT
Positron Emission Tomography/Computed Tomography
- PFS
Progression-free survival
- PSA
Prostate-specific antigen
- PSMA
Prostate-specific membrane antigen
- REDCap
Research Electronic Data Capture
- RT
Radiation Therapy
- RTOG
Radiation Therapy Oncology Group
- SBRT
Stereotactic Body Radiation Therapy
- TRUS
Transrectal Ultrasound
- UCLA
University of California, Los Angeles
- US
United States
Authors’ contributions
All authors read and approved the final manuscript. SZ and JCa conducted the manuscript writing. WF, NH, ME, AK, NN and JCa are the main study designers. BH, JCz, KH, DE contributed to the study design. JCa is the IND holder and principal investigator at UCLA. WF is the sponsor representative and principal investigator at the University Hospital Essen. NN and AK are the co-principal investigators at UCLA. All Authors read and approved the manuscript.
Funding
UCLA: This is an investigator-initiated trial with institutional funding (UCLA Ahmanson Translational Theranostics Division). This study is supported by Progenics Pharmaceuticals Inc. which supplies study drug (18F-DCFPyL) and financial support for the PET/CT technical costs associated with the study under the PyL™ Research Access Program.
Essen: This is an investigator-initiated trial with institutional and public funding.
Investigators:
JCa is the recipient of grants from the ERF-SNMMI (2019–2021 Molecular Imaging Research Grant for Junior Academic Faculty), the Prostate Cancer foundation (2020 Young investigator award), the Philippe Foundation Inc. (NY, USA) and the ARC Foundation (France) (International Mobility Award SAE20160604150).
BH received funding from the German Research Foundation (Deutsche Forschungsgemeinschaft grant HA 5160/5–1).
KH received funding from the German Research Foundation (Deutsche Forschungsgemeinschaft grant HE 5247/4–1).
WF received financial support from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG, grants FE1573/1–1 / 807122 and FE1573/3–1 / 659216), Mercator Research Center Ruhr (MERCUR, An-2019-0001), IFORES (D/107–81260, D/107–30240), Doktor Robert Pfleger-Stiftung, and Wiedenfeld-Stiftung/Stiftung Krebsforschung Duisburg.
NN is a Prostate Cancer Foundation Young Investigator, received a VA CDA2 (5IK2BX002520), VA CSR&D Merit, a STOP Cancer Foundation Career Development Award.
JCz is the recipient of a grant from the Prostate Cancer Foundation (2019 Challenge Award, 19CHAL02) and from the Johnson Comprehensive Cancer Center NIH-NCI Cancer Center Support Grant (P30 CA016042).
Availability of data and materials
The nuclear medicine research team will have full access to all interim and final results of the study through the REDCap® database. There is no planned interim analysis. All data generated and/or analyzed during this study will be publicly available (own DOI) after completion of the study and the publication of the article of the final analysis of study. The datasets generated and/or analyzed during the trail will not be publicly available before completion of the study but can be available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The protocol, the informed consent from (ICF) and all forms of participant information related to the study have been reviewed and approved by the UCLA institutional review board (UCLA IRB#20–000378). Any changes made to the protocol will be submitted as a modification and will be approved by the IRB prior to implementation.
All subjects must sign and personally date the IRB approved ICF after receiving detailed written and verbal information about the reason, the nature and the details of the trial prior to the initiation of any study-related procedures. Patients will be informed of the extent to which their confidential health information generated from this study may be used for research and publication purposes. This will be done according to the guidelines provided in the Declaration of Helsinki, ICH E6 Guideline for Good Clinical Practice (GCP) and government regulations, including (as applicable) the US Code of Federal Regulations Title 21 CFR 50.20 through 50.27. The protocol, the ICF and all forms of participant information related to the study are currently being reviewed by the Essen institutional review board.
Consent for publication
Patients will be informed of the extent to which their confidential health information generated from this study may be used for research and publication purposes.
Competing interests
JCa reports prior consulting activities outside of the submitted work for Advanced Accelerator Applications, Blue Earth Diagnostics, Curium Pharma, GE Healthcare, Janssen, Progenics, Radiomedix and Telix pharmaceuticals.
SZ was a consultant for ABX and Sofie Biosciences, outside of the submitted work.
ME was a consultant for ABX, Blue Earth Diagnostics and Progenics and has patent rights on rhPSMA, outside of the submitted work.
BH reports personal fees from ABX, Bayer, Lightpoint Medical, Inc., Janssen R&D, Bristol-Myers-Squibb and Astellas and travel from AstraZeneca, Janssen R&D and Astellas.
KH is a board member, and holds equity in Sofie Biosciences. Intellectual property is patented by the University of California and licensed to Sofie Biosciences. KHe was a consultant for Advanced Accelerator Applications, Amgen, Bayer, Curium Pharma, GE Healthcare, IPSEN, Janssen Pharmaceuticals, BTG, Sirtex, Novartis, ROTOP, Bain Capital outside of the submitted work.
JCz is a founder, board member, and holds equity in Sofie biosciences and Trethera Therapeutics. Intellectual property is patented by the University of California and licensed to Sofie Biosciences and Trethera Therapeutics. No other potential conflict of interest relevant to this article was reported.
WF was a consultant for Endocyte and BTG, and he received fees from RadioMedix and Bayer outside of the submitted work.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shaojun Zhu and Nader Hirmas contributed equally to this work.
Contributor Information
Jeremie Calais, Email: jcalais@mednet.ucla.edu.
Wolfgang P. Fendler, Email: wolfgang.fendler@uk-essen.de
References
- 1.Roach M, Ceron Lizarraga TL, Lazar AA. Radical prostatectomy versus radiation and androgen deprivation therapy for clinically localized prostate Cancer: how good is the evidence? Int J Radiat Oncol Biol Phys. 2015;93(5):1064–1070. doi: 10.1016/j.ijrobp.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375(15):1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 3.Bryant RJ, Oxley J, Young GJ, Lane JA, Metcalfe C, Davis M, Turner EL, Martin RM, Goepel JR, Varma M, Griffiths DF, Grigor K, Mayer N, Warren AY, Bhattarai S, Dormer J, Mason M, Staffurth J, Walsh E, Rosario DJ, Catto JWF, Neal DE, Donovan JL, Hamdy FC, for the ProtecT Study Group The ProtecT trial: analysis of the patient cohort, baseline risk stratification and disease progression. BJU Int. 2020;125(4):506–514. doi: 10.1111/bju.14987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neal DE, Metcalfe C, Donovan JL, Lane JA, Davis M, Young GJ, et al. Ten-year mortality, disease progression, and treatment-related side effects in men with localised prostate Cancer from the ProtecT randomised controlled trial according to treatment received. Eur Urol. 2020 Mar 1;77(3):320–30. [DOI] [PubMed]
- 5.Zaorsky NG, Shaikh T, Murphy CT, Hallman MA, Hayes SB, Sobczak ML, Horwitz EM. Comparison of outcomes and toxicities among radiation therapy treatment options for prostate cancer. Cancer Treat Rev. 2016;48:50–60. doi: 10.1016/j.ctrv.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuban DA, Tucker SL, Dong L, Starkschall G, Huang EH,Cheung MR, et al. Long-term results of the M. D. Anderson randomizeddose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys.2008;70:67–74. [DOI] [PubMed]
- 7.Al-MamganiA, van Putten WLJ, Heemsbergen WD, van Leenders GJLH, Slot A, Dielwart MFH, etal. Update of Dutch multicenter dose-escalation trial of radiotherapy forlocalized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72:980–8. [DOI] [PubMed]
- 8.ZietmanAL, Bae K, Slater JD, Shipley WU, Efstathiou JA, Coen JJ, et al. Randomizedtrial comparing conventional-dose with high-dose conformal radiation therapy inearly stage adenocarcinoma of the prostate: long-term results from protonradiation oncology group/american college of radiology 95-09. J Clin Oncol OffJ Am Soc Clin Oncol. 2010;28:1106–11. [DOI] [PMC free article] [PubMed]
- 9.DearnaleyDP, Sydes MR, Graham JD, Aird EG, Bottomley D, Cowan RA, et al. Escalated-doseversus standard-dose conformal radiotherapy in prostate cancer: first resultsfrom the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8:475–87. [DOI] [PubMed]
- 10.MichalskiJ, Winter K, Roach M, Markoe A, Sandler HM, Ryu J, et al. Clinical outcome ofpatients treated with 3D conformal radiation therapy (3D-CRT) for prostate cancer on RTOG 9406. Int J Radiat Oncol Biol Phys. 2012;83:e363-370. [DOI] [PMC free article] [PubMed]
- 11.Michalski JM,Bae K, Roach M, Markoe AM, Sandler HM, Ryu J, et al. Long-term toxicityfollowing 3D conformal radiation therapy for prostate cancer from the RTOG 9406phase I/II dose escalation study. Int J Radiat Oncol Biol Phys. 2010;76:14–22. [DOI] [PMC free article] [PubMed]
- 12.BeckendorfV, Guerif S, Le Prisé E, Cosset J-M, Bougnoux A, Chauvet B, et al. 70 Gy versus80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys. 2011;80:1056–63. [DOI] [PubMed]
- 13.Michalski JM,Moughan J, Purdy JA, Bosch WR, Bahary J, Lau H, et al. Initial Results of a Phase 3 Randomized Study of High Dose 3DCRT/IMRT versus Standard Dose3D-CRT/IMRT in Patients Treated for Localized Prostate Cancer (RTOG 0126). IntJ Radiat Oncol Biol Phys. 2014;90:1263.
- 14.Lukka H, Hayter C, Julian JA, Warde P, MorrisWJ, Gospodarowicz M, et al. Randomized trial comparing two fractionationschedules for patients with localized prostate cancer. J Clin Oncol Off J AmSoc Clin Oncol. 2005;23:6132–8. [DOI] [PubMed]
- 15.Yeoh EE, Botten RJ, Butters J, Di Matteo AC,Holloway RH, Fowler J. Hypofractionated versus conventionally fractionatedradiotherapy for prostate carcinoma: final results of phase III randomizedtrial. Int J Radiat Oncol Biol Phys. 2011;81:1271–8. [DOI] [PubMed]
- 16.ArcangeliS, Strigari L, Gomellini S, Saracino B, Petrongari MG, Pinnarò P, et al. Updated results and patterns of failure in a randomized hypofractionation trialfor high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:1172–8. [DOI] [PubMed]
- 17.Pollack A, Walker G, Horwitz EM, Price R, FeigenbergS, Konski AA, et al. Randomized trial of hypofractionated external-beamradiotherapy for prostate cancer. J Clin Oncol Off J Am Soc Clin Oncol.2013;31:3860–8. [DOI] [PMC free article] [PubMed]
- 18.Kuban DA, Nogueras-Gonzalez GM, Hamblin L, LeeAK, Choi S, Frank SJ, et al. Preliminary Report of a Randomized Dose EscalationTrial for Prostate Cancer using Hypofractionation. Int J Radiat Oncol Biol Phys. 2010;78:S58–9.
- 19.MantzC. A Phase II Trial of Stereotactic Ablative Body Radiotherapy for Low-Risk Prostate Cancer Using a Non-Robotic Linear Accelerator and Real-Time Target Tracking: Report of Toxicity, Quality of Life, and Disease Control Outcomeswith 5-Year Minimum Follow-Up. Front Oncol. 2014;4.doi:10.3389/fonc.2014.00279. [DOI] [PMC free article] [PubMed]
- 20.Katz AJ, Santoro M, DiBlasio F, Ashley R. StereotacticBody Radiation Therapy for Low, Intermediate, and High-risk Prostate Cancer:Disease Control and Quality of Life. Int J Radiat Oncol Biol Phys.2011;81:S100.
- 21.KatzAJ, Santoro M, Ashley R, Diblasio F, Witten M. Stereotactic body radiotherapyas boost for organ-confined prostate cancer. Technol Cancer Res Treat.2010;9:575–82. [DOI] [PubMed]
- 22.FullerDB, Naitoh J, Mardirossian G. Virtual HDR CyberKnife SBRT for Localized Prostatic Carcinoma: 5-Year Disease-Free Survival and Toxicity Observations.Front Oncol. 2014;4. doi:10.3389/fonc.2014.00321. [DOI] [PMC free article] [PubMed]
- 23.Dubray BM, Salleron J, Guerif SG, Le Prise E,Reynaud-Bougnoux A, Hannoun-Levi J-M, et al. Does short-term androgen depletionadd to high dose radiotherapy (80 Gy) in localized intermediate risk prostatecancer? Final analysis of GETUG 14 randomized trial (EU-20503/NCT00104741). JClin Oncol. 2016;34 15_suppBudäusl:5021–5021.
- 24.Dong Y, Ruth KJ, Churilla TM, Viterbo R,Sobczak ML, Smaldone MC, et al. The need for androgen deprivation therapy inpatients with intermediate-risk prostate cancer treated with dose-escalatedexternal beam radiation therapy. Can J Urol. 2017;24:8656–62. [PubMed]
- 25.Bekelman JE, Rumble RB, Chen RC, Pisansky TM, Finelli A, Feifer A, et al. Clinically localized prostate Cancer: ASCO clinical practice guideline endorsement of an American urological association/American Society for Radiation Oncology/Society of Urologic Oncology Guideline. J Clin Oncol Off J Am Soc Clin Oncol. 2018:JCO1800606. [DOI] [PubMed]
- 26.Morigi JJ, Stricker PD, van Leeuwen PJ, Tang R, Ho B, Nguyen Q, et al. Prospective comparison of 18F-Fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate Cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med Off Publ Soc Nucl Med. 2015;56(8):1185–1190. doi: 10.2967/jnumed.115.160382. [DOI] [PubMed] [Google Scholar]
- 27.Rauscher I, Maurer T, Beer AJ, Graner F-P, Haller B, Weirich G, et al. Value of 68Ga-PSMA HBED-CC PET for the assessment of lymph node metastases in prostate Cancer patients with biochemical recurrence: comparison with histopathology after salvage lymphadenectomy. J Nucl Med Off Publ Soc Nucl Med. 2016;57(11):1713–1719. doi: 10.2967/jnumed.116.173492. [DOI] [PubMed] [Google Scholar]
- 28.Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med Off Publ Soc Nucl Med. 2015;56(5):668–674. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]
- 29.Calais J, Ceci F, Eiber M, Hope TA, Hofman MS, Rischpler C, Bach-Gansmo T, Nanni C, Savir-Baruch B, Elashoff D, Grogan T, Dahlbom M, Slavik R, Gartmann J, Nguyen K, Lok V, Jadvar H, Kishan AU, Rettig MB, Reiter RE, Fendler WP, Czernin J. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-Centre, single-arm, comparative imaging trial. Lancet Oncol. 2019;20(9):1286–1294. doi: 10.1016/S1470-2045(19)30415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, Christidis D, Bolton D, Hofman MS, Lawrentschuk N, Murphy DG. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate Cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. 2020;77(4):403–417. doi: 10.1016/j.eururo.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 31.Fendler WP, Calais J, Allen-Auerbach M, Bluemel C, Eberhardt N, Emmett L, et al. 68Ga-PSMA-11 PET/CT interobserver agreement for prostate cancer assessments: an international multicenter prospective study. 2017. [DOI] [PubMed] [Google Scholar]
- 32.Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, Nguyen HG, Reiter RE, Rettig MB, Okamoto S, Emmett L, Zacho HD, Ilhan H, Wetter A, Rischpler C, Schoder H, Burger IA, Gartmann J, Smith R, Small EJ, Slavik R, Carroll PR, Herrmann K, Czernin J, Hope TA. Assessment of 68 Ga-PSMA-11 PET accuracy in localizing recurrent prostate Cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5(6):856–863. doi: 10.1001/jamaoncol.2019.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pyka T, Okamoto S, Dahlbender M, Tauber R, Retz M, Heck M, Tamaki N, Schwaiger M, Maurer T, Eiber M. Comparison of bone scintigraphy and (68) Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43(12):2114–2121. doi: 10.1007/s00259-016-3435-0. [DOI] [PubMed] [Google Scholar]
- 34.Thomas L, Balmus C, Ahmadzadehfar H, Essler M, Strunk H, Bundschuh RA. Assessment of bone metastases in patients with prostate Cancer-a comparison between (99m)Tc-bone-Scintigraphy and [(68) Ga]Ga-PSMA PET/CT. Pharm Basel Switz. 2017;10(3). [DOI] [PMC free article] [PubMed]
- 35.Bluemel C, Krebs M, Polat B, Linke F, Eiber M, Samnick S, Lapa C, Lassmann M, Riedmiller H, Czernin J, Rubello D, Bley T, Kropf S, Wester HJ, Buck AK, Herrmann K. 68Ga-PSMA-PET/CT in patients with biochemical prostate Cancer recurrence and negative 18F-choline-PET/CT. Clin Nucl Med. 2016;41(7):515–521. doi: 10.1097/RLU.0000000000001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwenck J, Rempp H, Reischl G, Kruck S, Stenzl A, Nikolaou K, Pfannenberg C, la Fougère C. Comparison of (68) Ga-labelled PSMA-11 and (11) C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging. 2017;44(1):92–101. doi: 10.1007/s00259-016-3490-6. [DOI] [PubMed] [Google Scholar]
- 37.Calais J, Fendler WP, Herrmann K, Eiber M, Ceci F. Comparison of 68Ga-PSMA-11 and 18F-Fluciclovine PET/CT in a case series of 10 patients with prostate Cancer recurrence. J Nucl Med Off Publ Soc Nucl Med. 2018;59(5):789–794. doi: 10.2967/jnumed.117.203257. [DOI] [PubMed] [Google Scholar]
- 38.Calais J, Ceci F, Eiber M, Hope TA, Hofman MS, Rischpler C, Bach-Gansmo T, Fendler WP, Czernin J. What is the best PET target for early biochemical recurrence of prostate cancer?–authors’ reply. Lancet Oncol. 2019;20(11):e609–e610. doi: 10.1016/S1470-2045(19)30654-0. [DOI] [PubMed] [Google Scholar]
- 39.Calais J, Cao M, Nickols NG. The utility of PET/CT in the planning of external radiation therapy for prostate Cancer. J Nucl Med. 2018;59(4):557–567. doi: 10.2967/jnumed.117.196444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calais J, Czernin J, Cao M, Kishan AU, Hegde JV, Shaverdian N, Sandler K, Chu FI, King CR, Steinberg ML, Rauscher I, Schmidt-Hegemann NS, Poeppel T, Hetkamp P, Ceci F, Herrmann K, Fendler WP, Eiber M, Nickols NG. 68 Ga-PSMA-11 PET/CT mapping of prostate Cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. J Nucl Med. 2018;59(2):230–237. doi: 10.2967/jnumed.117.201749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emmett L, Tang R, Nandurkar RH, Hruby G, Roach PJ, Watts JA, et al. 3-year freedom from progression following 68 GaPSMA PET CT triaged management in men with biochemical recurrence post radical prostatectomy. Results of a prospective multi-center trial. J Nucl Med. 2019:jnumed.119.235028. [DOI] [PubMed]
- 42.Calais J, Czernin J, Fendler WP, Elashoff D, Nickols NG. Randomized prospective phase III trial of 68Ga-PSMA-11 PET/CT molecular imaging for prostate cancer salvage radiotherapy planning [PSMA-SRT] BMC Cancer. 2019;19(1):18. doi: 10.1186/s12885-018-5200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multi-Centre study. Lancet. 2020:S0140673620303147. [DOI] [PubMed]
- 44.Roach PJ, Francis R, Emmett L, Hsiao E, Kneebone A, Hruby G, et al. The impact of 68 Ga-PSMA PET/CT on management intent in prostate cancer: results of an Australian prospective multicenter study. J Nucl Med. 2017:jnumed.117.197160. [DOI] [PubMed]
- 45.Calais J, Kishan AU, Cao M, Fendler WP, Eiber M, Herrmann K, et al. Potential impact of 68Ga-PSMA-11 PET/CT on the planning of definitive radiation therapy for prostate Cancer. J Nucl Med Off Publ Soc Nucl Med. 2018;59(11):1714–1721. doi: 10.2967/jnumed.118.209387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koerber SA, Will L, Kratochwil C, Haefner MF, Rathke H, Kremer C, et al. 68Ga-PSMA-11 PET/CT in primary and recurrent prostate carcinoma: implications for Radiotherapeutic management in 121 patients. J Nucl Med Off Publ Soc Nucl Med. 2018. [DOI] [PMC free article] [PubMed]
- 47.Koerber SA, Stach G, Kratochwil C, Haefner MF, Rathke H, Herfarth K, et al. Lymph node involvement in treatment-naïve prostate cancer patients – correlation of PSMA-PET/CT imaging and Roach formula in 280 men in the Radiotherapeutic management. J Nucl Med. 2019:jnumed.119.227637. [DOI] [PMC free article] [PubMed]
- 48.BudäusL, Leyh-Bannurah S-R, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial Experience of (68)Ga-PSMA PET/CT Imaging in High-risk Prostate Cancer Patients Prior to Radical Prostatectomy. Eur Urol. 2016;69:393–6. [DOI] [PubMed]
- 49.DemirkolMO, Acar Ö, Uçar B, Ramazanoğlu SR, Sağlıcan Y, Esen T. Prostate-specific membrane antigen-based imaging in prostate cancer: impact on clinical decision making process. The Prostate. 2015;75:748–57. [DOI] [PubMed]
- 50.Fendler WP, Schmidt DF, Wenter V, ThierfelderKM, Zach C, Stief C, et al. 68Ga-PSMA PET/CT Detects the Location and Extent ofPrimary Prostate Cancer. J Nucl Med Off Publ Soc Nucl Med. 2016;57:1720–5. [DOI] [PubMed]
- 51.FrenzelT, Tienken M, Abel M, Berliner C, Klutmann S, Beyersdorff D, et al. The impact of [68Ga]PSMA I&T PET/CT on radiotherapy planning in patients with prostate cancer. Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al. 2018;194:646–54. [DOI] [PubMed]
- 52.HerlemannA, Wenter V, Kretschmer A, Thierfelder KM, Bartenstein P, Faber C, et al. 68Ga-PSMA Positron Emission Tomography/Computed Tomography Provides AccurateStaging of Lymph Node Regions Prior to Lymph Node Dissection in Patients withProstate Cancer. Eur Urol. 2016;70:553–7. [DOI] [PubMed]
- 53.HijaziS, Meller B, Leitsmann C, Strauss A, Meller J, Ritter CO, et al. Pelvic lymphnode dissection for nodal oligometastatic prostate cancer detected by68Ga-PSMA-positron emission tomography/computerized tomography. The Prostate.2015;75:1934–40. [DOI] [PubMed]
- 54.Hirmas N, Al-Ibraheem A, Herrmann K, Alsharif A, Muhsin H, Khader J, et al. [68Ga]PSMA PET/CT Improves Initial Staging and Management Plan of Patients with High-Risk Prostate Cancer. Mol Imaging Biol. 2019;21:574–81. [DOI] [PubMed]
- 55.Hruby G, Eade T, Emmett L, Ho B, Hsiao E, Schembri G, et al. 68 Ga-PSMA-PET/CT staging prior to definitive radiationtreatment for prostate cancer. Asia Pac J Clin Oncol. 2018;14:343–6. [DOI] [PubMed]
- 56.Kabasakal L, Demirci E, Ocak M, Akyel R, Nematyazar J,Aygun A, et al. Evaluation of PSMA PET/CT imaging using a 68Ga-HBED-CC ligandin patients with prostate cancer and the value of early pelvic imaging. NuclMed Commun. 2015;36:582–7. [DOI] [PubMed]
- 57.Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M,Haller B, Weirich G, et al. Diagnostic Efficacy of (68)Gallium-PSMA PositronEmission Tomography Compared to Conventional Imaging for Lymph Node Staging of130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. JUrol. 2016;195:1436–43. [DOI] [PubMed]
- 58.Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U,Schäfers M, Essler M, et al. German Multicenter Study Investigating177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J NuclMed. 2017;58:85–90. [DOI] [PubMed]
- 59.RheeH, Ng KL, Tse BW-C, Yeh M-C, Russell PJ, Nelson C, et al. Using prostatespecific membrane antigen (PSMA) expression in clear cell renal cell carcinomafor imaging advanced disease. Pathology (Phila). 2016;48:613–6. [DOI] [PubMed]
- 60.SachpekidisC, Kopka K, Eder M, Hadaschik BA, Freitag MT, Pan L, et al. 68Ga-PSMA 11Dynamic PET/CT Imaging in Primary Prostate Cancer. Clin Nucl Med. 2016;41:e473–9. [DOI] [PubMed]
- 61.SchwenckJ, Rempp H, Reischl G, Kruck S, Stenzl A, Nikolaou K, et al. Comparison of(68)Ga-labelled PSMA-11 and (11)C-choline in the detection of prostate cancermetastases by PET/CT. Eur J Nucl Med Mol Imaging. 2017;44:92–101. [DOI] [PubMed]
- 62.Sterzing F, Kratochwil C, Fiedler H, Katayama S, HablG, Kopka K, et al. 68Ga-PSMA-11 PET/CT: a new technique with high potential forthe radiotherapeutic management of prostate cancer patients. Eur J Nucl Med MolImaging. 2016;43:34–41. [DOI] [PMC free article] [PubMed]
- 63.Uprimny C, Kroiss AS, Decristoforo C, Fritz J,von Guggenberg E, Kendler D, et al. 68Ga-PSMA-11 PET/CT in primary staging ofprostate cancer: PSA and Gleason score predict the intensity of traceraccumulation in the primary tumour. Eur J Nucl Med Mol Imaging. 2017;44:941–9. [DOI] [PubMed]
- 64.van Leeuwen PJ, Emmett L, Ho B, Delprado W,Ting F, Nguyen Q, et al. Prospective evaluation of 68Gallium-prostate-specificmembrane antigen positron emission tomography/computed tomography forpreoperative lymph node staging in prostate cancer. BJU Int. 2017;119:209–15. [DOI] [PubMed]
- 65.ZamboglouC, Wieser G, Hennies S, Rempel I, Kirste S, Soschynski M, et al. MRI versus 68Ga-PSMAPET/CT for gross tumour volume delineation in radiation treatment planning ofprimary prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:889–97. [DOI] [PubMed]
- 66.Eiber M, Herrmann K, Calais J, Hadaschik B,Giesel FL, Hartenbach M, et al. Prostate Cancer Molecular Imaging StandardizedEvaluation (PROMISE): Proposed miTNM Classification for the Interpretation ofPSMA-Ligand PET/CT. J Nucl Med Off Publ Soc Nucl Med. 2018;59:469–78. [DOI] [PubMed]
- 67.Roach M, HanksG, Thames H, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemicalfailure following radiotherapy with or without hormonal therapy in men withclinically localized prostate cancer: recommendations of the RTOG-ASTRO PhoenixConsensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–74. [DOI] [PubMed]
- 68.Hope TA, Goodman JZ, Allen IE, Calais J, Fendler WP, Carroll PR. Meta-analysis of 68Ga-PSMA-11 PET accuracy for the detection of prostate Cancer validated by histopathology. J Nucl Med Off Publ Soc Nucl Med. 2018. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The nuclear medicine research team will have full access to all interim and final results of the study through the REDCap® database. There is no planned interim analysis. All data generated and/or analyzed during this study will be publicly available (own DOI) after completion of the study and the publication of the article of the final analysis of study. The datasets generated and/or analyzed during the trail will not be publicly available before completion of the study but can be available from the corresponding author on reasonable request.