Abstract
Healthy brain development takes place within the context of individual experience. Here, we describe how certain early experiences are necessary for typical brain development. We present evidence from multiple studies showing that severe early life neglect leads to alterations in brain development, which compromises emotional, behavioral, and cognitive functioning. We also show how early intervention can reverse some of the deleterious effects of neglect on brain development. We conclude by emphasizing that early interventions that start at the earliest possible point in human development are most likely to support maximal recovery from early adverse experiences.
INTRODUCTION
Beginning from the moment of birth, healthy brain development requires adequate nurturing relationships. Caregivers regulate a baby’s physiology by responding to signals of hunger or sickness, by soothing the baby to sleep, and by insuring proper body temperature through close physical contact. Caregiving relationships also provide a critical foundation for emotional and cognitive development. By providing adequate exposure to language, interactive play, and appropriate emotional feedback, caregivers dynamically support the development of neural circuitry underlying self-regulation and cognition. Put simply, caregiving quality feeds emotional health and intelligence.
Much of our knowledge on this topic comes from research comparing children reared in responsive family environments with children who, unfortunately, are reared in inadequate caregiving environments, such as low-quality institutional settings, which essentially represent an extreme environment. Studies examining children reared in institutional settings show convincingly that sub-par early experiences have direct and profoundly negative consequences for the developing brain. Institutional rearing is often characterized by high child-to-caregiver ratios and unresponsive, overly regimented routines. Children are often forced to eat, sleep, and toilet together, regardless of individual needs. They are deprived of critical opportunities to develop selective attachments with primary caregivers and are exposed to a reduced range of sensory, linguistic, and cognitive input.
Here, we present findings from the Bucharest Early Intervention Project (BEIP), a randomized controlled intervention for institutionally reared children led by Drs Charles A. Nelson, Nathan A. Fox, and Charles H. Zeanah. As part of this study, infants and toddlers living in institutions were randomly placed in foster care. These children were followed through age 12 and then compared with children who remained in the institution as well as with demo- graphically matched, non-neglected children reared in their own families. Prior to the BEIP, our understanding of the impact of institutional care came from children adopted after experiencing institutional neglect. Although these studies suggest that adoption can ameliorate the negative impact of institutional rearing on cognitive functioning,1–3 they are limited by the protocol of adopted children not being randomly selected for adoption. The design of the BEIP helps to overcome this important sampling bias.4–8
In the sections that follow, we provide a general overview of how brain development takes place within the context of individual experience. We describe how certain early experiences are necessary for typical brain development and how structural and functional neural changes arise from different early-life circumstances. In addition to presenting evidence from the BEIP, we highlight findings from other key studies. In the final sections, we demonstrate how early intervention can help to reverse some of the deleterious effects of neglect on brain development (see D’Souza and Karmiloff-Smith, Neurodevelopmental disorders, WIREs Cogn Sci, also in the collection How We Develop). We conclude with an emphasis on the importance of intervention timing.
THE BRAIN DEVELOPS WITHIN THE CONTEXT OF EXPERIENCE
The development of the human brain begins within weeks of conception and continues until late adolescence and early adulthood. It is important to note that the brain continues to adapt and change in response to experience even into adulthood (the ability of the brain to be molded by experience is generally referred to as ‘neural plasticity’; see Power and Schlaggar, Neural plasticity across the lifespan, WIREs Dev Biol, also in the collection How We Develop). Human and animal studies show that brain development results from a complex interaction of biological and environmental influences. Whereas our genes provide essential information for establishing basic patterns of neuronal growth and connectivity, our individual experiences can affect gene expression and the trajectory of brain development.9,10 If exposed to stimulating and responsive environments, neural development is more likely to develop optimally. However, in less ideal environmental conditions, the foundational structure of the brain can be compromised, causing abnormalities in systems sub-serving healthy physical, cognitive, and social development.
One way to appreciate the influence of life experiences on brain development is to differentiate between experience-expectant and experience-dependent development.11 Experience-expectant development refers to development that occurs in response to certain life experiences that are typically shared by all members of a species. For example, starting at or before birth, it is ‘expected’ that humans will be exposed to auditory stimuli, patterned light, and opportunities to move around and manipulate objects. These experiences support the development of neural pathways associated with hearing, speech and language, vision, and locomotion. In addition, humans are routinely exposed to caregiving experiences that support neural circuitry involved in cognitive and emotional development.
Experience-dependent development, on the other hand, refers to development that occurs as a result of experiences that vary across individual members of a species. These experiences also shape development and are part of what makes each individual unique. For example, the learning of certain skills (such as reading or writing) depends on specific experiences that some individuals may have access to, while others may not.
In summary, there are certain experiences that are required for optimal brain development to support typical physical, cognitive, and emotional functioning. Many of these experiences need to occur at specific points in development (called ‘sensitive periods’) for humans to develop optimally (see Power and Schlaggar, Neural plasticity across the lifespan, WIREs Dev Biol, also in the collection How We Develop). Variations in individual experiences across the lifespan can also shape brain development, but normative trajectories of brain development can occur without specific exposure to these experiences (see Brown, Individual differences in human brain development, WIREs Cogn Sci, also in the collection How We Develop).
HOW DO WE STUDY THE EFFECT OF EARLY EXPERIENCE?
Animal research has contributed enormously to our understanding of the impact of early rearing experiences on brain development. There are known similarities in neural circuitry in humans and other mammals, including rodents. Therefore, findings from animal studies are often used to generate and test hypotheses regarding the influence of caregiving on human brain development.
For example, in one study, rat pups exposed to either highly responsive or excessively harsh caregiving during the first days of postnatal life showed dramatically different patterns in gene expression and neural circuitry in brain regions related to stress regulation and memory.12 A critical feature of this study was the ‘cross-fostering’ design in which pups were reassigned at birth to be raised by another mother. Specifically, pups born to unresponsive mothers were reassigned to highly responsive mothers, and those born to responsive mothers were reassigned to unresponsive mothers. As adults, pups reared by responsive mothers showed greater expression of a specific gene involved in stress regulation (the glucocorticoid receptor gene) in the hippocampus in comparison to pups reared by unresponsive mothers. This is note- worthy because the hippocampus is involved in learning, memory, and stress regulation. As a result, it was possible to conclude that the quality of caregiving that pups received early in life, but not the genetic relatedness of the mothers and pups, was responsible for the observed long-term changes in brain development.
Ethical issues preclude us from performing similar experiments in humans. However, studies involving children exposed to adverse conditions have shown patterns of results that are comparable to those found using animals. Retrospective and prospective research has investigated the influence of family violence, maltreatment, and co-occurring risk factors (i.e., parental addiction or severe economic hardship) on brain development. This body of literature has produced convincing evidence that extreme childhood stressors interfere with healthy brain development and lead to deficits in cognitive and emotional functioning.
Child neglect is an equally harmful early rearing condition. In families, this can occur in a variety forms and may include caregivers’ failure to support children’s emotional or cognitive development and/or attend to children’s basic physical, medical, or educational needs; in general, such caregivers threaten their children’s safety, health, and general well-being. Institutional neglect is a more severe form of neglect in which young children are reared in settings with little opportunity to develop a relationship with a stable caregiver, are deprived of normative caregiving input that supports cognitive and emotional development, and may or may not have their physical needs taken care of.9 The BEIP and several related studies have examined the short- and long-term consequences of extreme early psychosocial deprivation on brain and behavioral development.
HOW DO WE ASSESS THE IMPACT OF EARLY EXPERIENCE ON BRAIN DEVELOPMENT?
One of the first studies to investigate the influence of early neglect on brain development utilized positron emission tomography (PET) imaging.13 PET measures glucose metabolism, a marker of functional activity in the brain. In this study, brain activity in institutionally reared children was compared with brain activity in two other groups: the first included non-neglected children with a neurodevelopmental disorder (epilepsy), and the second included healthy adults. The institutionally reared children showed significant reductions in levels of glucose metabolism in prefrontal regions (the orbital frontal gyrus and infralimbic prefrontal cortex), in the medial temporal lobe (amygdala and hippocampus), in the lateral temporal cortex, and the brainstem and showed patterns of neural activation that were more similar to the children with neurodevelopmental problems, when compared with typical adults. Many of these regions that showed reduced activation in the institutionally reared children are critically involved in cognition and emotion regulation; therefore, the authors proposed that these functional alterations underlie common neglect-associated deficits in social–emotional and cognitive functioning.
More recent studies using magnetic resonance imaging (MRI) have shown that institutionally reared children exhibit significant reductions in overall brain volume14 and corresponding decreases in total and cortical ‘gray matter’ (brain tissue composed of neuronal cell bodies, and other cells known as glia) and ‘white matter’ brain tissue composed of myelinated axons, which extend from the cell bodies and support neural transmission across regions of the brain.14–16 Previously institutionalized adopted youth have shown smaller superior and posterior cerebellar lobes, structures known to be involved in motor control and learning.17
In some studies, institutional neglect has also been associated with alterations in the development of the amygdala. This is a brain structure located in the temporal lobe that is involved in emotion, threat detection, and processing of novel stimuli. Both increases and decreases in amygdala volume have been found in children with histories of institutional neglect.18–20 Functional alterations in the amygdala have been observed among previously institutionalized adopted youth in two studies.3,21,22 These structural and functional changes were associated with problems in emotion and behavioral regulation,18,19 direction of eye gaze in social contexts,21 and social behavior.3 In recent work, institutionally reared children showed alterations in patterns of connectivity between the prefrontal cortex and the amygdala when compared with non-neglected children. Interestingly, the neglected children (but not the non-neglected children) showed an inverse pattern of connectivity between these frontal and limbic regions that is not typically observed until humans reach later adolescence or early adulthood. This ‘more mature’ pattern of connectivity was also associated with reduced symptoms of anxiety, suggesting that some neural alterations that arise from exposure to neglect may actually serve a compensatory function.23
Diffusion tensor imaging (DTI), which measures microstructural properties of white matter fiber tracts, has also been used to investigate the effects of institutional rearing. In several investigations, institutionally reared children showed alterations in the organization of white matter tracts connecting the limbic and para-limbic regions,1,15,24,25 language regions,1 fronto-striatal regions,1,26 fronto-temporal regions,15 and the cerebellum.15 Several of these alterations predicted increased risk for behavioral problems,15,26 neurocognitive deficits,15 and language delays.1
Electroencepholograpy (EEG) measures the electrical activity of the brain and has taught us much of what we know regarding the effects of severe neglect on early brain development. EEG is recorded non-invasively with sensors (or electrodes) placed on the scalp, making it well suited for studying brain development in young children. Oscillatory patterns of neural activity have been recorded in response to cognitive and emotional tasks (called event-related potentials, ERPs) and during a ‘resting state’ (i.e., when children are not engaged in specific cognitive activities). Differences in the frequency and timing of EEG patterns can tell us about the effects of the early caregiving environment on neuro- developmental processes.
The first wave of findings from the BEIP revealed that children reared in institutions showed patterns of neural activity characterized by relatively higher levels of low-frequency power (in the theta band) and lower levels of higher-frequency power (in the alpha and beta range) relative to children reared by their birth parents.27 This activity profile is consistent with earlier studies in children with learning and attention difficulties.28,29 Furthermore, such atypical EEG patterns have been associated with the risk for hyperactivity and impulsivity later in development.30
The ERPs of institutionally reared and family-reared children were measured while the children were engaged in one of two tasks. In the first task, children were presented with repeating images of a familiar caregiver’s face and a stranger’s face. In the second task, children viewed faces displaying various positive and negative emotions. Across both of these tasks, institutionally reared children showed lower amplitudes of all ERP components than family-reared children.31,32 Moreover, the blunted brain responses to faces appeared to confer the risk for anxiety and attention problems later in life.33
TIMING AND DURATION OF ADVERSE EXPERIENCES IMPACTS RECOVERY
The BEIP has demonstrated that early intervention improves brain activity in institutionally reared children randomized into foster care. Group differences in EEG patterns were compared relatively soon after the children were placed in foster care (within 8–20 months of removal from the institution) and again when children reached 8 years of age. Although the effects of intervention on resting EEG were modest within the first 2 years after removal,34 the positive effects of the intervention were quite pronounced by 8 years of age. In fact, the institutionally reared children placed into foster care before 2 years of age showed EEG patterns comparable to those of the children raised by their birth parents; those removed from the institution at later ages showed less evidence for recovery (Figure 1).35
FIGURE 1 |.
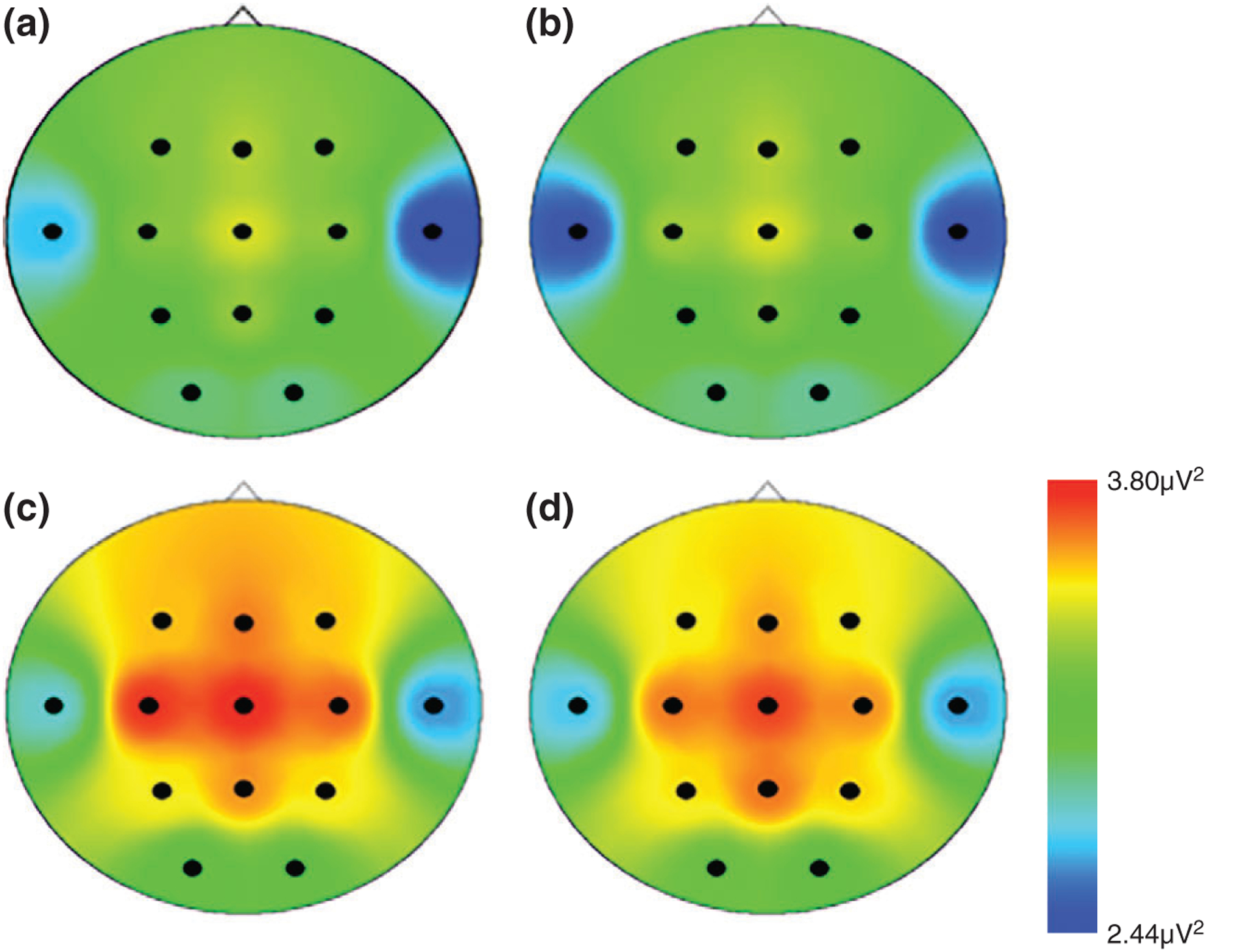
Distribution of alpha power across the scalp for (a) children who remained in the institution (i.e., the care-as-usual group (b) children placed into foster care after 24 months (i.e., the foster care group; FCG > 24 months), (c) children placed into foster care before 24 months (i.e., the foster care group; FCG < 24 months), and (d) children reared with their biological parents, (i.e., the never institutionalized group). (Reprinted with permission Ref 35 in accordance to the Creative Commons Attribution (CC BY) license. Copyright 2010 PLOS)
There was also evidence for intervention- supported recovery in ERP responses to social stimuli. When children reached 42 months of age, those in foster care showed an enhancement in their P1 response (a component associated with early visual processing) relative to children who remained in the institution. This intervention effect was observed for both emotion and face recognition tasks.36,37 At 8 years of age, the children in foster care continued to show evidence of remediation of their P1 response to fearful faces,38 suggesting that these neural changes are stable and long lasting.
Results from an MRI study conducted when children in the BEIP reached 8 years of age further indicated the potential benefits of early intervention. Children in foster care specifically showed improvements in the total amount of white matter in the brain, with levels that were not significantly different from typically reared children (Figure 3). In contrast, children who remained in the institution showed significantly reduced white matter levels. There were no intervention effects on cortical gray matter or overall brain volume (Figure 2).16
FIGURE 3 |.
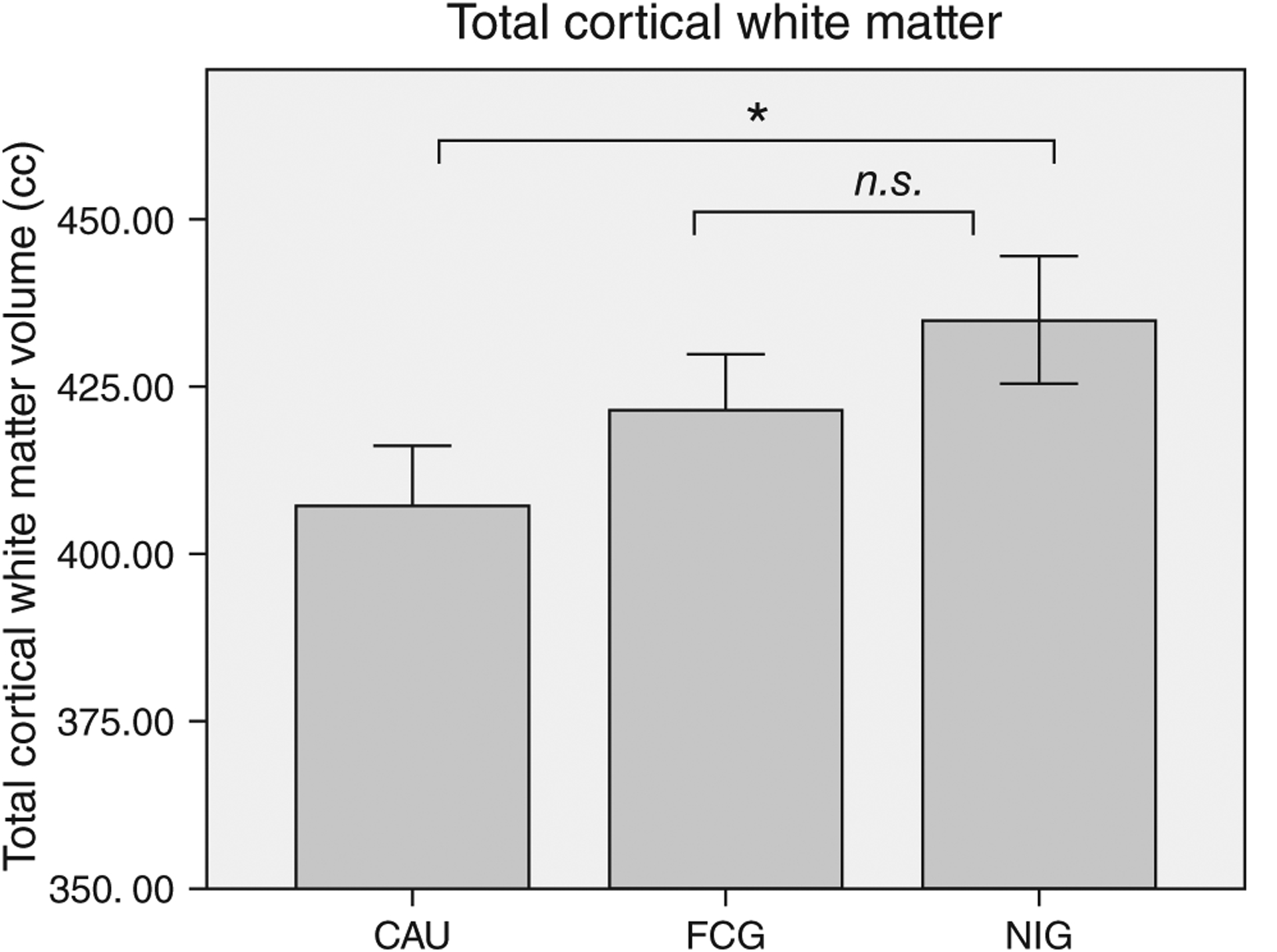
Average total cortical white matter volume in cubic centimeters (cm3) for children who remained in the institution (i.e., the care-as-usual group; CAU), children placed into foster care (i.e., the foster care group; FCG), and children reared by their biological parents (the never institutionalized group; NIG); error bars are ±1 standard error mean (SEM). (Adapted from Ref 16)
FIGURE 2 |.
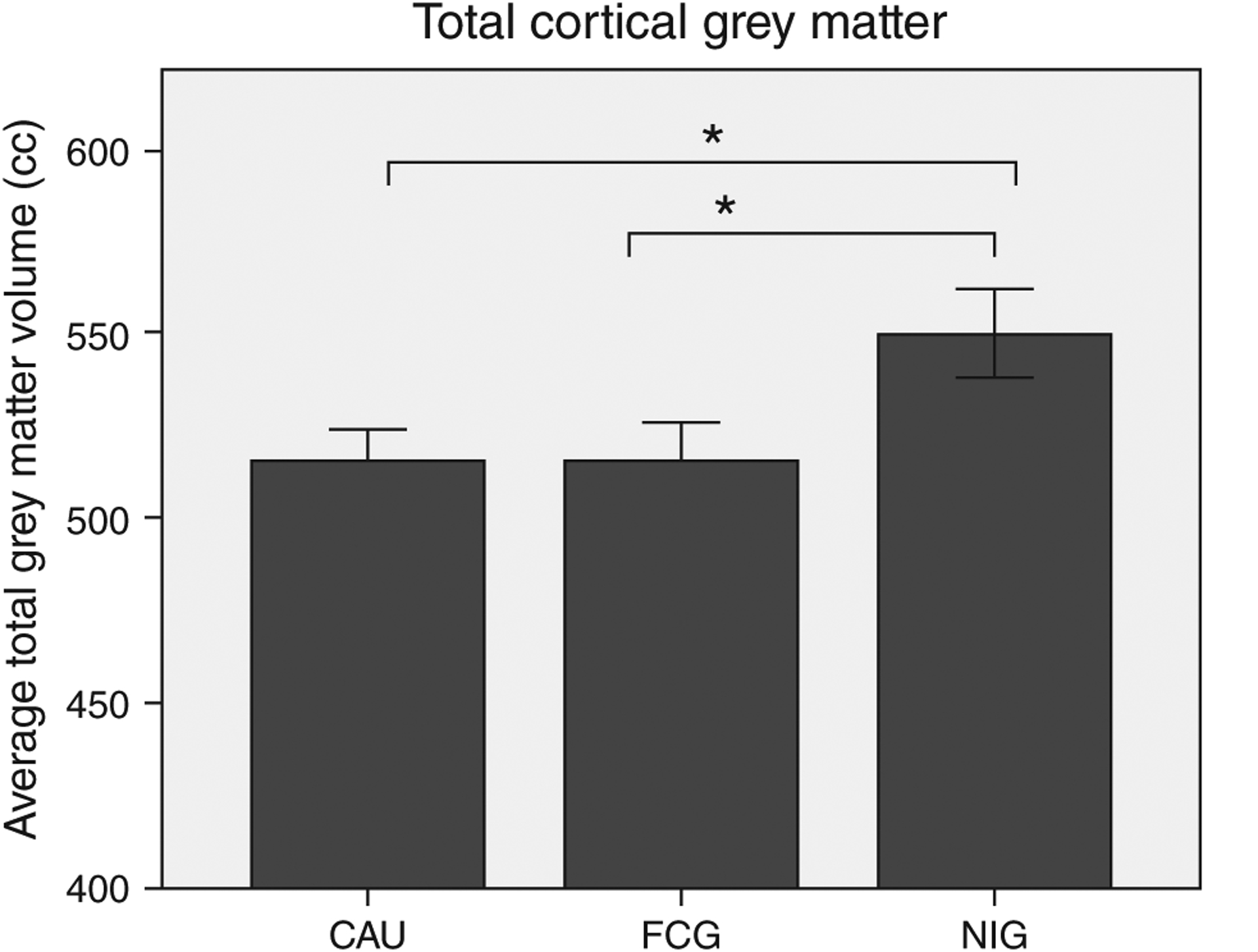
Average total cortical gray matter volume in cubic centimeters (cm3) for children who remained in the institution (i.e., the care-as-usual group; CAU), children placed into foster care (i.e., the foster care group; FCG), and children reared by their biological parents (the never institutionalized group; NIG); error bars are ±1 SEM. (Adapted from Ref 16)
CONCLUSION
Research on institutionally reared children provides clear evidence for the role of early experiences in shaping brain development (see Stiles, Principles of brain development, WIREs Cogn Sci, also in the collection How We Develop). Children who experience substantial neglect, especially during the first few years, exhibit dramatic alterations in brain development. These alterations are observed both structurally and functionally. In general, the longer the brain is deprived of ‘expected’ experiences, the greater the impairment.14,19
Importantly, the brain can recover if children are placed into more nurturing environments, although the patterns of recovery are complex. Some aspects of brain function and structure may be more responsive to environmental enrichment than others. Similarly, the degree to which children show remediation in certain neural processes may depend on the timing of the intervention, with greater improvements observed for children who receive intervention at the earliest ages. Finally, some aspects of neural recovery may be immediate, whereas others may take time to emerge.
This body of research has critical implications for social policy and public health. Institutional neglect is one of many early adverse experiences. Children reared in neglectful or abusive families face deficits in brain, behavioral, and emotional development. Consistent with the objectives of many current child welfare legislative acts (i.e., the Adoption Assistance and Child Welfare Act of 1980, P.L. 96–272, the Adoption and Safe Families Act (ASFA) of 1997, P.L. 105–89, and the Family Preservation and Support Services Program enacted as part of the Omnibus Reconciliation Act of 1993, P.L. 103–66), many at-risk children are likely to benefit if we prioritize policies and programs that increase access to prevention and intervention programs. Also, these children have the greatest chance to benefit from these programs if they begin as early as possible.
Footnotes
Conflict of interest: The authors have declared no conflicts of interest for this article.
FURTHER READING
Readers can refer to the following website for a list of related publications: http://www.bucharestearlyinterventionproject.org.
REFERENCES
- 1.Kumar A, Behen ME, Singsoonsud P, Veenstra AL, Wolfe-Christensen C, Helder E, Chugani HT. Microstructural abnormalities in language and limbic pathways in orphanage-reared children: a diffusion tensor imaging study. J Child Neurol 2014, 29:318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson CA 3rd, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science 2007, 318:1937–1940. [DOI] [PubMed] [Google Scholar]
- 3.Olsavsky AK, Telzer EH, Shapiro M, Humphreys KL, Flannery J, Goff B, Tottenham N. Indiscriminate amygdala response to mothers and strangers after early maternal deprivation. Biol Psychiatry 2013, 74:853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millum J, Emanuel EJ. Ethics. The ethics of international research with abandoned children. Science 2007, 318:1874–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson CA, Fox NA, Zeanah CH. Romania’s Abandoned Children: Deprivation, Brain Development and the Struggle for Recovery. Cambridge, MA: Harvard University Press; 2014. [Google Scholar]
- 6.Wassenaar DR. Commentary: Ethical considerations in international research collaboration: The Bucharest Early Intervention Project. Inf Mental Hlth J 2006, 27:577–580. [DOI] [PubMed] [Google Scholar]
- 7.Zeanah CH, Koga SF, Simion B, Stanescu A, Tabacaru CL, Fox NA, Nelson CA. Ethical considerations in international research collaboration: The Bucharest Early Intervention Project. Inf Mental Hlth J 2006, 27:559–576. [DOI] [PubMed] [Google Scholar]
- 8.Zeanah CH, Nelson CA, Fox NA, Smyke AT, Marshall P, Parker SW, Koga S. Designing research to study the effects of institutionalization on brain and behavioral development: the Bucharest Early Intervention Project. Dev Psychopathol 2003, 15:885–907. [DOI] [PubMed] [Google Scholar]
- 9.National Scientific Council on the Developing Child. The Science of Neglect: The Persistent Absence of Responsive Care Disrupts the Developing Brain: Working Paper No. 12. 2012.
- 10.Fox SE, Levitt P, Nelson CA 3rd. How the timing and quality of early experiences influence the development of brain architecture. Child Dev 2010, 81:28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Dev 1987, 58:539–559. [PubMed] [Google Scholar]
- 12.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 1999, 286:1155–1158. [DOI] [PubMed] [Google Scholar]
- 13.Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage 2001, 14:1290–1301. [DOI] [PubMed] [Google Scholar]
- 14.Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, Rutter M, Sonuga-Barke EJ. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J Child Psychol Psychiatry 2009, 50:943–951. [DOI] [PubMed] [Google Scholar]
- 15.Hanson JL, Adluru N, Chung MK, Alexander AL, Davidson RJ, Pollak SD. Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Dev 2013, 84:1566–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA 3rd. Variation in neural development as a result of exposure to institutionalization early in childhood. Proc Natl Acad Sci USA 2012, 109:12927–12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer PM, Hanson JL, Pierson RK, Davidson RJ, Pollak SD. Cerebellar volume and cognitive functioning in children who experienced early deprivation. Biol Psychiatry 2009, 66:1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, Shirtcliff EA, Pollak SD, Davidson RJ. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry 2015, 77:314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, Eigsti IM, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci 2010, 13:46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, Jenness J, Lau JY, Ackerman JP, Pine DS, et al. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn Affect Behav Neurosci 2010, 10:34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Dev Sci 2011, 14:190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grabe HJ, Schwahn C, Mahler J, Appel K, Schulz A, Spitzer C, Fenske K, Barnow S, Freyberger HJ, Teumer A, et al. Genetic epistasis between the brain- derived neurotrophic factor Val66Met polymorphism and the 5-HTT promoter polymorphism moderates the susceptibility to depressive disorders after childhood abuse. Prog Neuropsychopharmacol Biol Psychiatry 2012, 36:264–270. [DOI] [PubMed] [Google Scholar]
- 23.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA 2013, 110:15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eluvathingal TJ, Chugani HT, Behen ME, Juhasz C, Muzik O, Maqbool M, Chugani DC, Makki M. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics 2006, 117:2093–2100. [DOI] [PubMed] [Google Scholar]
- 25.Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT. Altered water diffusivity in cortical association tracts in children with early deprivation identified with Tract-Based Spatial Statistics (TBSS). Cereb Cortex 2010, 20:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behen ME, Muzik O, Saporta AS, Wilson BJ, Pai D, Hua J, Chugani HT. Abnormal fronto-striatal connectivity in children with histories of early deprivation: a diffusion tensor imaging study. Brain Imaging Behav 2009, 3:292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall PJ, Fox NA, Bucharest Early Intervention Project Core Group. A comparison of the electroencephalogram between institutionalized and community children in Romania. J Cogn Neurosci 2004, 16:1327–1338. [DOI] [PubMed] [Google Scholar]
- 28.Barry RJ, Johnstone SJ, Clarke AR. A review of electrophysiology in attention-deficit/hyperactivity disorder: II. Event-related potentials. Clin Neurophysiol 2003, 114:184–198. [DOI] [PubMed] [Google Scholar]
- 29.Chabot RJ, di Michele F, Prichep L. The role of quantitative electroencephalography in child and adolescent psychiatric disorders. Child Adolesc Psychiatr Clin N Am 2005, 14:21–53, v–vi. [DOI] [PubMed] [Google Scholar]
- 30.McLaughlin KA, Fox NA, Zeanah CH, Sheridan MA, Marshall P, Nelson CA. Delayed maturation in brain electrical activity partially explains the association between early environmental deprivation and symptoms of attention-deficit/hyperactivity disorder. Biol Psychiatry 2010, 68:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker SW, Nelson CA, Bucharest Early Intervention Project Core Group. An event-related potential study of the impact of institutional rearing on face recognition. Dev Psychopathol 2005, 17:621–639. [DOI] [PubMed] [Google Scholar]
- 32.Parker SW, Nelson CA, Bucharest Early Intervention Project Core Group. The impact of early institutional rearing on the ability to discriminate facial expressions of emotion: an event-related potential study. Child Dev 2005, 76:54–72. [DOI] [PubMed] [Google Scholar]
- 33.Slopen N, McLaughlin KA, Fox NA, Zeanah CH, Nelson CA. Alterations in neural processing and psychopathology in children raised in institutions. Arch Gen Psychiatry 2012, 69:1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall PJ, Reeb BC, Fox NA, Nelson CA 3rd, Zeanah CH. Effects of early intervention on EEG power and coherence in previously institutionalized children in Romania. Dev Psychopathol 2008, 20:861–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanderwert RE, Marshall PJ, Nelson CA 3rd, Zeanah CH, Fox NA. Timing of intervention affects brain electrical activity in children exposed to severe psychosocial neglect. PLoS One 2010, 5:e11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moulson MC, Fox NA, Zeanah CH, Nelson CA. Early adverse experiences and the neurobiology of facial emotion processing. Dev Psychol 2009, 45:17–30. [DOI] [PubMed] [Google Scholar]
- 37.Moulson MC, Westerlund A, Fox NA, Zeanah CH, Nelson CA. The effects of early experience on face recognition: an event-related potential study of institutionalized children in Romania. Child Dev 2009, 80:1039–1056. [DOI] [PubMed] [Google Scholar]
- 38.Nelson CA, Westerlund A, McDermott JM, Zeanah CH, Fox NA. Emotion recognition following early psychosocial deprivation. Dev Psychopathol 2013, 25:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]


