To develop a vaccine or immunotherapy that would cure the HIV-1 infection, it is important to identify helper T cells able to mount an efficient antibody response. Here, we demonstrate that the generation of HIV-1 Env-specific antibodies facilitating antibody-dependent innate immune responses likely depends on Env-specific IL-21-secreting CD4+ T and peripheral T follicular helper cells.
KEYWORDS: ADCD, ADNP, CD4+ T cells, Env, HIV-1, IL-21, NK cell activation, TLM, antibody, pTfh
ABSTRACT
Antibodies with a functional Fc region were previously associated with protection from HIV-1 acquisition and spontaneous suppression of viral replication. Unlike broadly neutralizing antibodies, they are not restricted to neutralizing epitopes and do not require unconventional structural traits to exert their antiviral activity. They therefore develop earlier after infection and can be detected in the majority of cases. The conditions under which these antibodies are generated, however, remain largely unknown. Here, we demonstrate that the generation of HIV-1 Env-specific antibodies facilitating Fc-dependent innate immune responses, including neutrophil phagocytosis (ADNP), complement deposition (ADCD), and NK cell activation, likely depends on help provided by CD4+ T and peripheral T follicular helper (pTfh) cells secreting interleukin-21 (IL-21). Other proteins, including CD40L, gamma interferon (IFN-γ), and IL-4/13, involved in cross talk between B and T cells were linked to the production of antibodies with a functional Fc region but only when coexpressed with IL-21. As a potential source of these antibodies, we identified a subset of Env-specific memory B cells known to be expanded in chronic HIV-1 infection. The frequency and level of Blimp-1 expression in Env-specific tissue-like memory B cells (TLM) correlated with the functional CD4+ T cell subsets associated with robust antibody-dependent innate responses. Thus, our data suggest a mechanism responsible for the generation of antibodies with functional Fc region in chronically HIV-1-infected individuals that is based on CD4+ T cell-induced activation of memory B cells.
IMPORTANCE To develop a vaccine or immunotherapy that would cure the HIV-1 infection, it is important to identify helper T cells able to mount an efficient antibody response. Here, we demonstrate that the generation of HIV-1 Env-specific antibodies facilitating antibody-dependent innate immune responses likely depends on Env-specific IL-21-secreting CD4+ T and peripheral T follicular helper cells.
INTRODUCTION
The immunopathology of human immunodeficiency virus type 1 (HIV-1) infection is characterized by the depletion of CD4+ T cells in all body compartments, disrupting important immune functions. In particular, T follicular helper (Tfh) cells are preferentially infected by HIV-1 and represent a major reservoir (1–3). Interestingly, however, persistent antigenemia leads to Tfh cell expansion during chronic HIV-1 infection (1, 4, 5). Despite being a preferred target of HIV-1, Tfh cells and their peripheral counterpart (pTfh) cells seem to play a vital role in the establishment of an adaptive immune response against the virus. Their foremost role is to provide helper signals to antigen-specific B cells that are delivered through surface signaling molecules like CD40L, OX40, and ICOS and cytokines, including gamma interferon (IFN-γ), interleukin-21 (IL-21), and IL-4 (6, 7). Previous studies have linked pTfh cells expressing CD40L, IL-21, or IL-4 with the frequency of HIV-1/simian immunodeficiency virus type 1 (SIV-1)-specific B cells and broadly neutralizing antibodies (bNAbs) (8–10). Moreover, preserved pTfh cell responses were linked to spontaneous control over HIV-1 replication and controller status (11, 12). However, pTfh cells of chronically HIV-1-infected individuals show numerous anomalies. Apart from their high susceptibility to infection and virus-driven expansion, HIV-1 interferes with their ability to express important effector molecules (6). It has been demonstrated that pTfh cells of HIV-1+ individuals at the late stages of infection show reduced levels of CD40L (13) and IL-4 (14) but increased ICOS expression compared to that in healthy individuals. Alteration of IL-21 expression has also been shown; however, the direction of change depends on the immune compartment and stage of the disease (14).
The distorted functional profile of pTfh cells in combination with antigenemia leads to a skewing of the B cell compartment, including anomalies such as decreased responsiveness to B cell receptor (BCR) stimulation and proliferation potential, reduced complement receptor (CD21) expression, and high frequency of circulating non-HIV-1-specific plasmablasts in chronically infected individuals (15, 16). Particularly affected are memory B cells that become dominated by unconventional memory subsets like activated (AM) and tissue-like memory (TLM) cells showing markers of exhaustion and activation (17). Aberrations in the memory compartment result in the poor response to vaccination and inability to develop protective secondary B cell responses that were observed among HIV-1+ individuals (18).
Detrimental effects of HIV-1 replication on T and B cells are also reflected in the humoral response. Although HIV-1-specific antibodies develop already during the early phase of the disease, they do not exhibit neutralizing activity. First neutralizing antibodies appear only 3 to 13 weeks postinfection but soon become obsolete due to the rapid evolution of the virus (19, 20). Only a small fraction of infected individuals develop potent neutralizing antibodies able to neutralize a broad spectrum of viral quasispecies (21). These antibodies appear at the late stages of the infection and show uncommon modifications of the immunoglobulin structure as a result of the prolonged affinity maturation process (21, 22). Designing immunogens that induce bNAbs has proven difficult, and optimizing Fc effector functions might be an alternative and/or complementary strategy to enhance humoral immunity against HIV-1. Relying on their Fc domain immunoglobulins can trigger activation of innate immune responses such as complement deposition (ADCD), monocyte phagocytosis (ADCP), neutrophil phagocytosis (ADNP), and cell killing by NK cells (ADCC). These functions act against free virions as well as infected cells and have been found to play an important role in HIV-1 infection (23–25). For example, ADCC and ADCP correlated with protection from HIV-1 acquisition in the RV144 vaccine trial (26, 27) and experiments conducted on nonhuman primates (28, 29). ADNP is negatively associated with viral load (VL) in untreated HIV-1+ individuals (30). The importance of ADCD was demonstrated by studies showing that inactivation of serum complement in humanized mice abrogates antibody-mediated protection from infection (31). Apart from having potent antiviral activity, antibodies that do not rely solely on their neutralizing ability lack unusual structural traits observed among bNAbs and are therefore readily generated by infected subjects. Collectively, these arguments make antibodies with a functional Fc portion (referred to as functional antibodies throughout this article, although we recognize that neutralization is also a function) a promising target for vaccine design or development of immunotherapies that would cure the HIV-1 infection through the enhancement of innate immune responses.
Despite the well-documented beneficial role of functional antibodies in HIV-1 disease, factors underlying their generation remain largely unknown. Here, we demonstrate that the generation of HIV-1 Env-specific antibodies with functional properties is linked to CD4+ T cell help. The helper signals are transduced through the typical molecules involved in B-T cell cross talk, the most prominent of which proved to be IL-21.
RESULTS
Env-specific pTfh cells have a distinct functional profile compared to Env-specific memory CD4+ T cells.
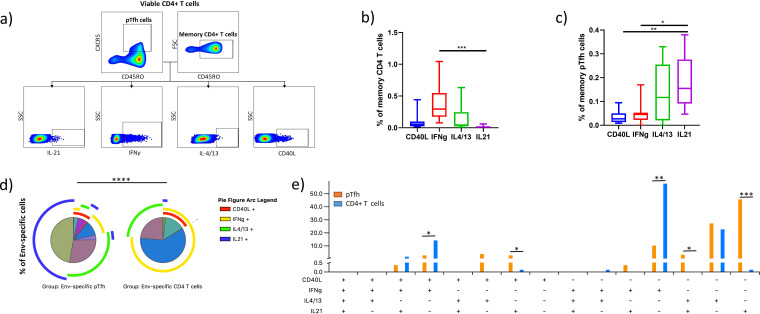
Efficient antibody response against a protein target normally depends on cognate CD4+ T cell help in particular provided by Tfh and pTfh cells. In the context of HIV-1/SIV-1 infection, generation of neutralizing antibodies depends on Env-specific pTfh cells secreting IL-21, IL-4, and CD40L (9, 10). To determine Env-specific CD4+ T and Env-specific pTfh cell functions that are involved in shaping antibody responses, we stimulated peripheral blood mononuclear cells (PBMCs) of chronically HIV-1-infected, treatment-naive individuals with a pool of overlapping peptides based on a sequence of the Env protein and assessed the expression of CD40L, IFN-γ, IL-4/13, and IL-21 by flow cytometry (Fig. 1a). The average frequency of all Env-specific cells was 0.54% (range, 0.22 to 1.06%) within the memory CD4+ T cell population defined as CD45RO+ CD4+ T cells. In comparison, the frequency of Env-specific cells within the pTfh cell population, defined as CXCR5+ CD45RO+ CD4+ T cells, was 0.36% (range, 0.07% to 0.78%). The most frequent Env-specific memory CD4+ T cells were IFN-γ+, followed by IL-4/13+ and CD40L+, while IL-21-secreting cells were comparably rare (Fig. 1b). Conversely, Env-specific pTfh cells primarily secreted IL-21, followed by IL-4/13, while IFN-γ and CD40L expression remained significantly lower (Fig. 1c).
FIG 1.
Functional composition of Env-specific CD4+ T and pTfh cell responses present in the periphery of chronically HIV-1-infected untreated individuals. (a) Gating strategy used to determine functions of memory CD4+ T cells and pTfh cells. (b and c) Frequencies of CD45RO+ CD4+ T cells (b) and CD45RO+ CXCR5+ CD4+ T cells (c) producing CD40L, IFN-γ, IL-4/13, or IL-21 in response to overlapping peptides spanning the Env protein. The frequencies are expressed as a percentage of the parent population. Asterisks indicate the level of significance according to Friedman’s test with Dunn’s post hoc test for multiple comparisons. (d) Pie charts demonstrate the composition of Env-responding pTfh and CD4+ T cells based on the cosecretion of the four measured proteins. Each slice represents a distinct subset, while arcs surrounding the pie chart indicate which subsets express a certain protein. Differences between the pie charts were assessed by the permutation test. (e) Shown is the mean frequency of subsets based on (co)expression of CD40L, IFN-γ, IL-4/13, and IL-21 in the two cell populations; Env-specific pTfh cells and Env-specific memory CD4+ T cells. The frequency is expressed as a percentage of all cells responding to the Env protein. Statistical significance was assessed by the Wilcoxon rank sum test. Shown is the average of eight independent experiments.
To assess the polyfunctionality of the Env-specific bulk CD4+ T cells and pTfh cells, we performed combinatorial expression analysis of flow cytometric data that were graphed and statistically analyzed using SPICE software (32). Comparing pTfh and CD4+ T cells, we observed significantly different functional profiles (P < 0.0001), with pTfh cells showing a more diverse spectrum of polyfunctional subsets than CD4+ T cells. Env-specific pTfh cells were divided into 7 subsets with an average frequency greater than 1%, while only 4 subsets were identified in the case of bulk CD4+ T cells. The most frequently represented subsets within the responding pTfh cell population were those expressing exclusively IL-21 (mean, 46%; range, 21% to 64%), followed by the cells secreting only IL-4/13 (mean, 28%; range, 0% to 64%) and cells secreting only IFN-γ (mean, 11%; range, 0% to 24%). Somewhat less frequent were subsets expressing IL-4/13 in combination with CD40L (mean, 4%; range, 0% to 24%), IL-21 in combination with IL-4/13 (mean, 4%; range, 0% to 18%), IL-21 in combination with CD40L (mean, 3%; range, 0% to 7%), and IFN-γ in combination with CD40L (mean, 3%; range, 0% to 7%). In contrast, Env-specific CD4+ T cells were dominated by the IFN-γ single-positive cells (mean, 59%; range, 8% to 91%), followed by IL-4/13-secreting cells (mean, 23%; range, 1% to 87%) and cells secreting IFN-γ in combination with CD40L expression (mean, 14%; range, 2% to 36%). Another subset that was present at a frequency greater than 1% was the IL-21, IFN-γ, and CD40L triple-positive subset (mean, 2%; range, 1% to 8%). Apart from those, four additional CD4+ T cell subsets were observed in at least 50% of cases at lower frequencies: IL-4/13+ CD40L+ only, IL-21+ CD40L+ only, IFN-γ+ IL-21+ only, and IL-21+ only cells (Fig. 1d). When we compared the prevalence of individual polyfunctional subsets between Env-specific pTfh and bulk CD4+ T cells, we found that the following subsets were significantly more prevalent in the pTfh cell population: Il-21+ only (P = 0.0008), IL-21+ IL-4/13+ only (P = 0.012), and IL-21+ CD40L+ only (P = 0.018). Two of the subsets were more frequently found among the bulk memory CD4+ T cell population: IFN-γ + only (P = 0.0046) and IFN-γ+ CD40L+ only (P = 0.024) (Fig. 1e).
Taken together, our results confirm that we detected Env-specific memory CD4+ T cells and pTfh cells in the periphery of chronically HIV-1-infected individuals. Our data suggest that Env-specific memory CD4+ T cells predominantly secrete IFN-γ but rarely IL-21, while pTfh cells mostly secrete IL-21 but rarely express IFN-γ or CD40L. Moreover, we observed significant differences in the polyfunctionality of the two cell populations.
Production of functional antibodies binding to the Env protein is associated with the magnitude of Env-specific memory CD4+ T cell and pTfh cell responses.
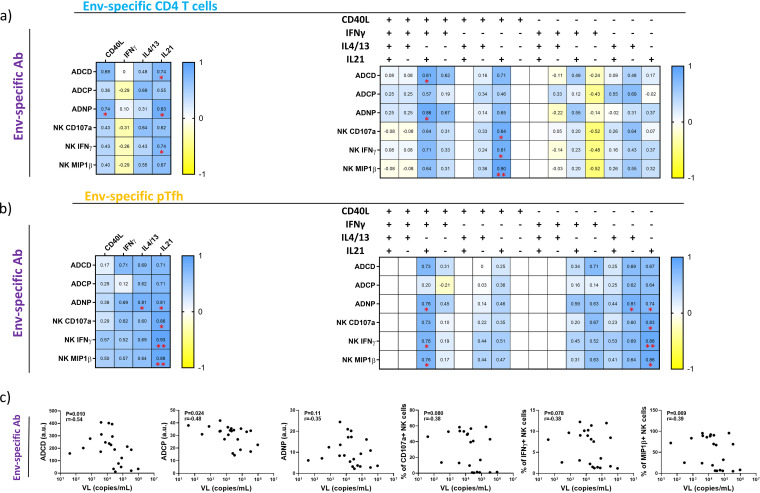
To investigate the role of CD4+ T cell help in the context of Env-specific antibody generation, we next compared frequencies of memory CD4+ T cells expressing CD40L, IFN-γ, IL-4/13, or IL-21 with the magnitude of Fc-dependent antibody effector functions measured in their respective plasma. The correlation analysis showed significant positive associations between the frequency of memory CD4+ T cells expressing IL-21 and the following antibody functions: ADCD (r = 0.74 and P = 0.046), ADNP (r = 0.83 and P = 0.015), and induction of IFN-γ production by NK cells (r = 0.74 and P = 0.046). Memory CD4+ T cells expressing CD40L were positively associated with ADNP (r = 0.74 and P = 0.046) (Fig. 2a). Next, we investigated whether there is an association between the frequency of polyfunctional subsets and the magnitude of functional antibody responses. We found that the frequency of a rare population of Env-specific CD4+ T cells expressing CD40L and IL-21, but not IFN-γ or IL-4/13, positively correlated with plasma levels of antibodies activating NK cells (r = 0.84 and P = 0.013, r = 0.81 and P = 0.019, and r = 0.90 and P = 0.0053 for CD107a, IFN-γ, and MIP1β expression, respectively). Another memory CD4+ T cell subset coexpressing CD40L, IFN-γ, and IL-21 but not IL-4/13 significantly correlated with ADCD and ADNP (r = 0.81 and P = 0.022 and r = 0.86 and P = 0.011, respectively) (Fig. 2a), indicating that memory CD4+ T cells expressing IL-21 and CD40L in combination or without IFN-γ likely support the production of functional antibodies targeting the Env protein.
FIG 2.
Env-specific memory CD4+ T cell and pTfh cell responses but not VL are positively associated with the development of antibody-dependent innate responses targeting Env protein. (a and b) The heat map illustrates correlation strength between the capacity of plasma to evoke antibody-mediated innate responses (left) and frequency of CD4+ T cells (a) or pTfh cells (b) expressing CD40L, IFN-γ, IL-4/13, IL-21, or different combinations of those (top). Each cell is color-coded with respect to Spearman’s r value of the corresponding correlation. Asterisks indicate the level of significance. (c) Graphs show associations between the VL and plasma levels of Env-specific antibodies that induce Fc-dependent effector functions. The strength of correlations was assessed by Spearman’s correlation test.
We next examined the prevalence of Env-specific pTfh cells and their association with the quality of antibody responses directed toward the Env protein. In particular, the frequency of pTfh cells responding to stimulation with an Env peptide pool by the production of IL-21 positively correlated with the level of ADNP-inducing (r = 0.081 and P = 0.022) and NK cell-activating antibodies. In the case of NK cell-activating antibodies, there was a significant correlation for all three measured responses, namely, CD107a, IFN-γ, and MIP1β expression (r = 0.86 and P = 0.011, r = 0.93 and P = 0.0022, and r = 0.88 and P = 0.0072, respectively). Another positive correlation we observed was between the frequency of IL-4/13-secreting pTfh cells and antibodies inducing ADNP (r = 0.081 and P = 0.022) (Fig. 2b). To better understand the role of pTfh cells in the production of Env-specific antibodies, we checked for correlations between the frequency of polyfunctional pTfh cell subsets present in the periphery of chronic HIV-1-infected individuals and the magnitude of antibody-mediated responses. Our analysis revealed that the pTfh cell subset expressing exclusively IL-21 positively correlated with ADNP (r = 0.074 and P = 0.046) and NK cell activation (r = 0.83 and P = 0.015, r = 0.88 and P = 0.0072, and r = 0.86 and P = 0.011 for CD107a, IFN-γ, and MIP1β expression, respectively), while the subset secreting only IL-4/13 showed a positive association with ADNP (r = 0.81 and P = 0.022). Apart from the two single-positive subsets, a subset expressing CD40L, IFN-γ, and IL-21 but not IL-4/13 was linked to the production of antibodies capable of mediating ADNP (r = 0.76 and P = 0.036) and inducing secretion of IFN-γ and MIP1β in NK cells (r = 0.76 and P = 0.036 and r = 0.76 and P = 0.036, respectively) (Fig. 2b). Our data therefore suggest that Env-specific pTfh cells are associated with the production of functional antibodies by secretion of IL-21 or, to a lesser extent, IL-4/13. Coexpression of CD40L and IFN-γ seems to be optional.
Another possible driving force of HIV-1-specific antibody production might be the level of antigen present in plasma as estimated by viral load analysis. Indeed, studies have demonstrated that HIV-1-specific antibody levels dramatically decline with viremia following the onset of antiretroviral therapy (ART) (33) and that the frequency of plasmablasts positively correlates with the viral load in HIV-1-infected viremic individuals (15). We therefore compared HIV-1 viral loads with the degrees of Env-specific antibody responses. Surprisingly, we observed a negative correlation between the VL and ADCD (r = −0.054 and P = 0.010) as well as ADCP (r = −0.48 and P = 0.024) (Fig. 2c).
Taken together, our results demonstrate that different subpopulations of Env-specific CD4+ T cells expressing IL-21 alone or in combination with other proteins are associated with the production of functional antibodies binding to the Env protein. Moreover, the level of these antibodies in plasma was inversely correlated with the viral load, suggesting their capability to limit HIV-1 replication.
Activated Env-specific memory B cells are a likely source of antibodies triggering innate immune responses.
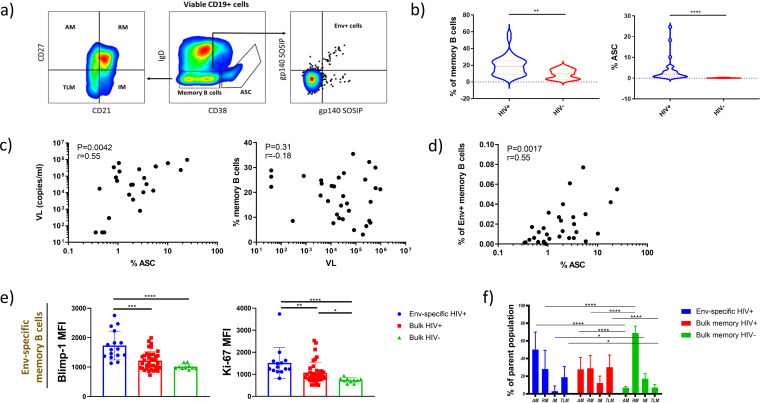
It has been previously shown that memory B cells become activated and differentiate into antigen-secreting cells (ASC) during chronic HIV-1 infection (34). It is, however, not clear whether activated memory B cells produce HIV-1-specific antibodies triggering innate immune responses. To investigate this, we performed a flow cytometric analysis of B cells present in peripheral blood of chronically HIV-1-infected and healthy individuals (Fig. 3a). Similar to previous reports, we observed increased frequencies of memory B cells (IgD− CD38-low B cells) and ASC (IgD− CD38-high B cells) in chronically HIV-1-infected individuals compared to healthy controls (2.1-fold [P = 0.0067] and 26.3-fold [P < 0.0001], respectively) (Fig. 3b). The expansion of ASC was likely driven by viral replication, since we found a positive association between the frequency of ASC and viral load (r = 0.55 and P = 0042). Conversely, the frequency of memory B cells was not linked to the viral load (Fig. 3c). Given the positive association of viral load and the frequency of ASC but not memory B cells, we assumed that the presence of viral antigens leads to activation of HIV-1-specific memory B cells and their differentiation into ASC. To test this, we identified Env-specific memory B cells using two fluorescently labeled trimeric Env proteins based on sequences of A and B clade isolates (BG505 SOSIP.664 [35] and AMC011 SOSIP.v4.2 [36], respectively). The average frequency of Env-specific memory B cells was 0.023% of all B cells, with values ranging from 0 to 0.115%. When we compared levels of Env-specific memory B cells with those of ASC, we found a significant positive correlation between these parameters (r = 0.55 and P = 0.0017), supporting our hypothesis (Fig. 3d). Furthermore, we analyzed the propensity of Env-specific memory B cells to differentiate into ASC. We measured levels of two key proteins involved in this process, Blimp-1 and Ki-67. Blimp-1 is a master regulator of plasma cell differentiation, while Ki-67 serves as an indicator of cell cycling. Both were significantly increased in Env-specific memory B cells compared to bulk memory B cells of HIV-1-infected individuals (1.4-fold increase [P = 0.0006] for Blimp-1 and 1.4-fold increase [P = 0.0068] for Ki-67) and healthy controls (2.1-fold increase [P < 0.0001] for Blimp-1 and 1.7-fold increase [P < 0.0001] for Ki-67). This indicates that Env-specific cells become activated by high antigenemia, clonally expand, and differentiate into ASC (Fig. 3e).
FIG 3.
Env-specific memory B cells potentially differentiate into ASC during chronic HIV-1 infection. (a) Gating strategy used to identify Env-specific memory B cells and their subsets. (b) Frequencies of memory B cells and ASC as a percentage of all B cells. Compared are chronically HIV-1-infected and healthy donors. Statistical significance was assessed by the Mann-Whitney test. (c) Correlations between the viral load and frequency of memory B cells and ASC. (d) Correlation between the frequency of Env-specific memory B cells and ASC. The strength of correlations was assessed by Spearman’s correlation test. (e) Blimp-1 and Ki-67 expression levels in memory B cells from healthy donors and HIV-1-infected individuals. For Env-specific cells, only cases with detectable levels of antigen-specific cells were included. Asterisks indicate statistical significance according to the Kruskal-Wallis test with Dunn’s post hoc test for multiple comparisons. (f) Frequency of the four memory B cell subsets among the Env-specific memory B cells, bulk memory B cells, and memory B cells of healthy individuals. Statistical significance was assessed by two-way ANOVA with Dunnett’s post hoc test for multiple comparisons.
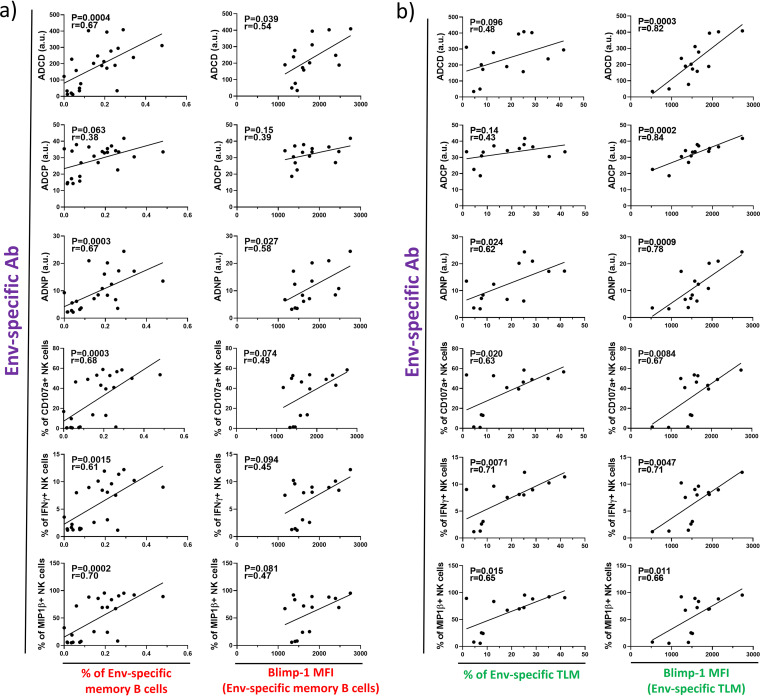
We have shown that ongoing viral replication causes activation of Env-specific memory B cells during chronic HIV-1 infection. To check whether this results in the production of functional antibodies, we compared levels of antibody-mediated innate immune responses measured in plasma of infected individuals with the frequency of memory B cells. Indeed, the frequency of Env-specific memory B cells positively correlated with the majority of the measured antibody functions (Fig. 4a). To find further support for the idea, we compared levels of functional antibodies and the propensity of Env-specific memory B cells to differentiate into ASC. Our data revealed that Blimp-1 levels of Env-specific memory B cells positively correlate with two of the measured antibody functions. In particular, Blimp-1 expression significantly correlated with ADCD and ADNP but also showed a trend toward a positive correlation with the rest of the antibody functions (Fig. 4a).
FIG 4.
Env-specific memory B cells are associated with the production of antibodies triggering innate immune responses. (a) Graphs show correlations between the frequency of Blimp-1 expression levels of Env-specific memory B cells and plasma levels of Env-specific antibodies that induce Fc-dependent effector functions. (b) The same correlative relationships are shown for the tissue-like memory B cells (TLM). The strength of correlations was assessed by Spearman’s correlation test.
It has been previously demonstrated that during chronic HIV-1 there is an increase in the proportion of activated and exhausted memory subsets such as tissue-like memory (TLM), while numbers of resting memory (RM) cells decline (17). To determine whether individual memory subsets are particularly associated with the production of antibodies triggering innate immune responses, we identified the four most common memory subsets in peripheral blood of HIV-1-infected individuals. The distinctions between activated memory (AM), RM, TLM, and intermediate memory (IM) were made based on the expression of CD21 and CD27 (AM, CD27+ CD21−; RM, CD27+ CD21+; TLM, CD27− CD21−; and IM, CD27− CD21+). Similar to previous findings, we observed elevated frequencies of TLM and AM cells and decreased RM cell levels in HIV-1+ individuals compared to healthy controls. This was true for bulk memory B cells as well as Env-specific memory B cells (Fig. 3f). A comparison of memory subset frequencies with levels of functional antibodies revealed TLM as the most likely source of these antibodies. Its frequency significantly correlated with ADNP and NK cell activation and showed a trend toward a positive correlation with ADCP and ADCD (Fig. 4b). None of the other three subsets showed an association with any of the antibody functions. To consolidate the theory of TLM as the memory subset mainly contributing to antibody production, we correlated intracellular Blimp-1 expression levels in TLM cells with antibody-mediated responses. Strikingly, we found strong associations with all of the measured antibody activities (Fig. 4b).
Taken together, our results demonstrated that activation of Env-specific memory B cells is linked to the production of antibodies triggering innate immune responses. In particular, the TLM subset was strongly associated with the antibody functions.
Env-specific pTfh cells possibly shift the subset distribution of cognate memory B cells toward TLM and are associated with their differentiation into ASC.
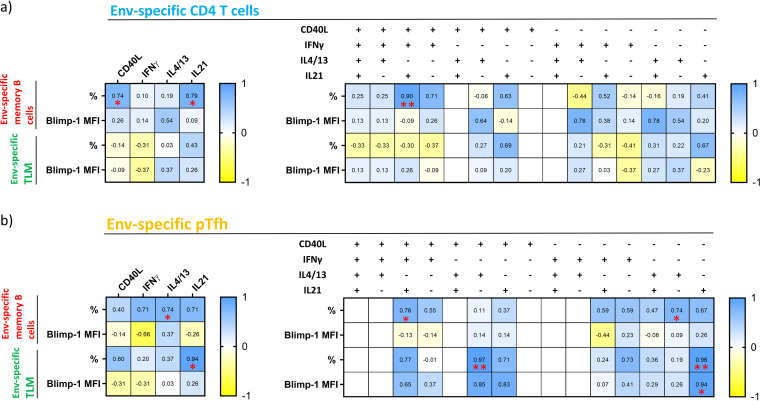
Our findings suggest that the generation of Env-specific antibodies mediating innate immune responses depends on certain CD4+ T cell subsets. To investigate whether Env-specific CD4+ T cells influence the antibody production by providing signals to cognate memory B cells, we first compared their frequencies and Blimp-1 expression level with the frequencies of memory CD4+ T cells expressing CD40L, IFN-γ, IL-4/13, or IL-21. We found that the bulk memory CD4+ T cells expressing CD40L or IL-21 positively correlated with the levels of Env-specific memory B cells (r = 0.74 and P = 0.046 and r = 0.79 and P = 0.028, respectively). No associations were found with the frequency of TLM cells or the propensity of memory B cells to differentiate into ASC (Fig. 5a). To obtain a deeper insight, we next assessed possible associations between the prevalence of polyfunctional Env-specific CD4+ T cells and memory B cells. We observed that the frequency of bulk Env-specific memory B cells increases with the frequency of CD4+ T cells expressing CD40L, IL-21, and IFN-γ but not IL-4/13 (r = 0.90 and P = 0.0046). The same subset frequency was significantly correlated with high levels of Env-specific antibodies evoking innate immune responses, suggesting its prominent role in providing signals to cognate B cells. Interestingly, no associations were found between Env-specific memory CD4+ T cells and TLM, suggesting that bulk memory CD4+ T cells contribute to antibody production exclusively by the generation or sustaining of cognate memory B cells (Fig. 5a).
FIG 5.
Frequency and Blimp-1 levels of Env-specific memory B cells positively correlate with the same functional CD4+ T and pTfh cell subsets as antibody-mediated functions. (a and b) The heat map illustrates correlation strength between the B cell parameters (left) and CD4+ T cell (a) or pTfh cell (b) functions (top). Each cell is color-coded with respect to Spearman’s r value of the corresponding correlation. Asterisks indicate the level of significance.
Next, we investigated whether pTfh cells influence the frequency and activation of Env-specific memory B cells. Indeed, our data showed that the prevalence of Env-specific pTfh cells secreting IL-4/13 significantly correlated with the memory B cell frequency (r = 0.074 and P = 0.046). Furthermore, the frequency of pTfh cells expressing IL-21 positively correlated with the percentage of TLM (r = 0.94 and P = 0.017). This supports the observation that Env-specific pTfh cells expressing IL-21 play a key role in the production of antibodies triggering innate effector functions (Fig. 5b). To get a more detailed picture of the interplay between Env-specific pTfh cells and memory B cells, we compared the frequency and Blimp-1 levels of the latter with frequencies of different polyfunctional pTfh cell subsets. In line with our previous findings, we observed that the frequency of Env-specific memory B cells increased with that of pTfh cells secreting exclusively IL-4/13 (r = 0.74 and P = 0.046) and the subset coexpressing CD40L, IL-21, and IFN-γ (r = 0.76 and P = 0.036). Our data also revealed an association between the frequency of TLM and pTfh cells secreting exclusively IL-21 (r = 0.96 and P = 0.0023). Moreover, the TLM cell frequency positively correlated with the frequency of cells expressing CD40L and IL-4/13 but not IL-21 or IFN-γ, revealing another subset that contributes to the skewing of memory B cells toward TLM (r = 0.97 and P = 0.0015). Considering the tendency of TLM to differentiate into ASC, we found that pTfh cells expressing IL-21 are the main subset responsible for Blimp-1 upregulation (r = 0.94 and P = 0.017) (Fig. 5b). Our results therefore suggest that pTfh cells expressing IL-4/13 or IL-21 in combination with CD40L and IFN-γ are linked to high frequencies of Env-specific memory B cells. pTfh cells secreting IL-21 alone are associated with the expansion and differentiation of TLM cells into ASC. Moreover, the pTfh cells expressing IL-4/13 possibly direct memory B cells toward the TLM phenotype, but only in combination with CD40L expression.
Collectively, our data indicate that Env-specific CD4+ T and pTfh cells expressing IL-4/13, or a combination of CD40L, IL-21, and IFN-γ, are associated with high levels of cognate memory B cells. Meanwhile, the frequency of TLM cells and their tendency to differentiate into ASC correlates with the frequency of pTfh cells expressing IL-21.
DISCUSSION
HIV-1 readily infects virus-specific CD4+ T cells and affects their functional composition. The outcome is a desynchronized helper activity that causes anomalies in the B cell compartment and hinders the formation of an effective humoral response. Nevertheless, CD4+ T cells are vital for the formation of an adaptive immune response against HIV-1. In particular, HIV-1-specific pTfh cells were associated with the production of protective broadly neutralizing antibodies and control over viremia (8–12). However, broadly neutralizing antibodies require an extensive maturation process and generally emerge not earlier than 2 to 3 years after the infection in a fraction of cases (37). Conversely, antibodies whose antiviral function relies on the Fc portion can be detected already during the early stages of infection (25, 38). Furthermore, it is well documented that antibody-dependent innate immune responses correlate with protection from HIV-1 acquisition in vaccine trials (26, 28) and that patients who spontaneously suppress HIV-1 infection exhibit potentiated Fc-dependent antibody activities (23, 39–41). It is, therefore, of great importance to elucidate CD4+ T cell functions driving the production of such antibodies. Here, we demonstrate that the production of HIV-1 Env-specific antibodies with functions beyond neutralization likely depends on cognate CD4+ T and pTfh cells secreting IL-21 alone or in combination with CD40L, IFN-γ, or IL-4/13.
CD4+ T cells are an indispensable component of our immune system orchestrating functions of innate and adaptive immune cells (42); by providing survival and differentiation signals to B cells, they support multiple steps of antibody production. During HIV-1 infection, selective depletion of HIV-1-specific CD4+ T and pTfh cells takes place along with the alteration of their functional composition (1, 6). It has been previously demonstrated that CD4+ T cells of chronically HIV-1-infected individuals predominantly express IFN-γ but rarely IL-21, while the pTfh cell population produces larger amounts of IL-21 than of IFN-γ (9, 14). Moreover, both populations have decreased capacity to express CD40L in settings of HIV-1 infection (13, 14). In line with these findings, we demonstrated that the Env-specific CD4+ T cell population produces large amounts of IFN-γ, intermediate levels of IL-4/13 and CD40L, and low levels of IL-21. Conversely, the Env-specific pTfh cell population responded to stimulation with Env-based peptides predominantly by expression of IL-21 and IL-4/13, while the frequency of pTfh cells expressing CD40L and IFN-γ remained low. Further differences in the expression profiles of these two subsets were revealed by the analysis of polyfunctionality, where the pTfh cell population proved to have a more diverse array of subsets.
Next, we assessed the role of Env-specific CD4+ T and pTfh cell responses in the production of antibodies triggering innate immune responses. For both populations, IL-21 secretion was the best correlate of functional antibody production. This is in concordance with previous studies highlighting the association of IL-21 with controller status (12) and broadly neutralizing antibody responses in HIV-1/SIV-1 infection (10, 43). Further helper functions linked to antibody-dependent innate responses included CD40L and IL-4/13 expression. Both were previously associated with the magnitude of B cells and neutralizing antibody responses directed against SIV (10). Interestingly, bulk CD4+ T cells only correlated with antibody-mediated innate functions when they expressed IL-21 in combination with other proteins, while in the pTfh cell population, IL-21 expression alone was sufficient. This suggests a superior role of Env-specific pTfh cells in the production of antibodies with a functional Fc portion. Another molecule that might influence the production of functional antibodies is PD-1, whose expression on pTfh cells was previously associated with HIV-1 neutralization (8). We did not observe any correlations between the Fc-dependent antibody functions and PD-1 expression on pTfh cells, suggesting that PD-1 is not a critical factor for the production of antibodies with functional a Fc domain yet may be a factor for neutralization capacity. Interestingly, ADCP was the only function that did not significantly correlate with any of the measured CD4+ T cell or pTfh cell functions. However, we observed a clear positive trend with IL-4/13- and IL-21-secreting memory CD4+ T and pTfh cells and a significant correlation between the ADCP and Blimp-1 expression level of TLM cells that was, in turn, positively associated with IL-21-secreting pTfh. Collectively, these arguments make the conclusion that ADCP does not depend on CD4+ T cell help premature. Apart from T cell help, antibody response depends on the presence of antigen in the circulation. Surprisingly, however, we observed a negative relationship between innate functions measured in plasma and viral load, indicating that these antibodies might limit viral replication during the chronic stage of the disease.
Memory B cells of chronically HIV-1-infected individuals are prone to differentiate into antibody-secreting cells (16, 34) and are associated with the production of HIV-1-specific antibodies (44, 45). In accordance with these findings, we observed increased levels of Blimp-1 and Ki-67 in memory B cells of HIV-1+ individuals, indicating their increased propensity to proliferate and differentiate into ASC. The highest levels of the two proteins harbored Env-specific memory B cells, suggesting antigen-dependent activation. The frequency of Env-specific memory B cells and their Blimp-1 levels were positively associated with most of the measured antibody functions. These associations became even more obvious when we compared levels of antibody effector functions with the frequency of different Env-specific memory B cell subsets and their Blimp-1 levels. An unusual memory subset known to be expanded in HIV-1 infection (17) and harbor a large proportion of HIV-1-specific memory B cells (46) was highly associated with levels of antibodies triggering innate immune responses. Our findings, therefore, propose tissue-like memory B cells as a likely source of functional antibodies targeting Env protein.
HIV-1-specific pTfh cells were previously shown to promote antibody secretion by memory B cell (47, 48). We therefore checked whether Env-specific CD4+ T cells and pTfh cells influence the frequency of cognate memory B cells and their Blimp-1 levels in chronic individuals. Indeed, the same functional subsets that were associated with the production of functional antibodies showed positive correlations with memory B cell levels and their differentiation potential. While Env-specific CD4+ T cells only correlated with the frequency of bulk Env-specific memory B cells, pTfh cells also correlated with the frequency of TLM and their Blimp-1 levels. That confirms the prominent role of pTfh cells in the induction of antibody response triggering innate immunity.
Taken together, our results show that HIV-1-specific CD4+ T cells and pTfh cells secreting IL-21 alone or together with other stimulatory proteins are linked to the formation of cognate memory B cells and possibly contribute to their differentiation into ASC. This might lead to the production of antibodies mediating innate immune functions associated with control of viral replication.
MATERIALS AND METHODS
Study participants.
Peripheral blood samples from 39 chronically HIV-1 infected individuals were used for this study. All participants were untreated and had a viral load ranging from 955,100 to below 40 copies per milliliter of blood. The average age in the cohort was 42 ± 11 (mean ± standard deviation [SD]) years, and 23% of the participants were females. Samples were collected at the University Hospital Essen in Germany as a part of the SCABIO clinical study aimed at understanding immunological changes in HIV-1 infection to define strategies for HIV-1 treatment, cure, and vaccination. Measurements of HIV-1 viral load were performed by the central diagnostics laboratory of University Hospital Essen using the standard clinical procedure.
Ethics statement.
All individuals participating in this study provided written informed consent. The SCABIO study was approved by the Ethics Committee of the Medical Faculty of the University of Duisburg-Essen, Germany (approval number 17-7846-BO). The local institutional review board (IRB) of the University Duisburg-Essen approved the performed laboratory testing and in vitro studies.
Enrichment of B cells by magnetic separation.
Cryopreserved PBMCs were thawed in R10 medium (RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, penicillin [100 U/ml], and streptomycin [100g/ml]) and rested at 37°C and 5% CO2 overnight. The next morning, cells were counted and subjected to magnetic cell separation using the EasySep human pan-B cell enrichment kit. The separation was performed according to the manufacturer’s protocol. Briefly, the antibody cocktail recognizing antigens on the surface of non-B cells was added to the cells, followed by the addition of magnetic particle suspension. Tubes containing cells were then inserted into a magnet, allowing immobilization of all cells present in peripheral blood except B cells. Untouched B cells were then separated from the immobilized cells by decanting. The purity of isolated B cells was greater than 90%.
Phenotypic analysis of B cells by flow cytometry.
Enriched B cells were resuspended in staining buffer (phosphate-buffered saline [PBS] supplemented with 2% heat-inactivated fetal calf serum) with added anti-CD4-BV510 (clone RPA-T4) antibody and incubated for 10 min at 4°C. After a washing step, a mixture of recombinant, biotinylated HIV-1 Env proteins (BG505 SOSIP.664 [35] and AMC011 SOSIP.v4.2 [36]) was added to the cells at an equimolar ratio. Binding of Env probes was carried out at 4°C for 1 h, after which cells were fixed in 2% formaldehyde. Before proceeding with the staining process, each sample was split into two equal portions for two different antibody panels. The first portion was stained with streptavidin-allophycocyanin (APC) and streptavidin-BV421 premixed at a 1:2 molar ratio, while the second with streptavidin-phycoerythrin (PE)-Cy7 and streptavidin-BV421 premixed at an equimolar ratio. The ratio of streptavidin conjugates was pretitrated to achieve comparable fluorescence intensities of the two fluorochromes used together. Cells were then washed in a serum-free medium and stained with Zombie Aqua viability dye. Before the surface marker staining, cells were incubated in a solution of antibody binding to human Fc receptors (FcR blocking reagent; Miltenyi Biotec) to reduce unspecific antibody binding. Two different panels of antibodies were used, the first including anti-CD3-BV510 (clone UCHT1), anti-CD19-APC-Cy7 (clone HIB19), anti-CD21-fluorescein isothiocyanate (FITC) (clone Bu32), anti-CD27-AF700 (clone O323), anti-CD38-BV605 (clone HIT2), anti-IgD-BV786 (clone IA6-2), anti-CD279-PE-Cy7 (clone EH12.2H7) and the second including anti-CD3-BV510 (clone UCHT1), anti-CD19-APC-Cy7 (clone HIB19), anti-CD21-FITC (clone Bu32), anti-CD27-AF700 (clone O323), anti-CD38-BV605 (clone HIT2), and anti-IgD-BV786 (clone IA6-2). Surface staining was performed at 4°C for 15 min. Next, cells were fixed and permeabilized (Foxp3/transcription factor staining buffer set; eBioscience) for 45 min and stained for intracellular antigens using anti-Ki-67-PE-Dazzle (clone Ki-67) and anti-AhR-PE (clone T49.550) for the first panel and anti-Blimp1-PE-Dazzle (clone 6D3), anti-AID-APC (EK2-5G9), and anti-AhR-PE (clone T49.550) for the second panel. Cells were incubated at 4°C for 30 min and subsequently washed with PBS. All used antibodies and probes were used in pretitrated concentrations. Labeled cells were analyzed with a BD FACS Celesta flow cytometer. Compensation was performed with single-stained capture beads (CompBeads). Possible fluctuations in laser intensity were measured every time before the experiment, using multifluorescence calibration beads (Rainbow calibration particles). If needed, detection voltages were adjusted to maintain equivalent fluorescence readings throughout the experiment. Data were analyzed with FlowJo v9.4.1.
Assessment of CD4+ T cell functional profile by flow cytometry.
Cryopreserved PBMCs were thawed in R10 medium and rested at 37°C and 5% CO2 overnight. Cells were counted the next morning and plated onto 24-well plates. To achieve stimulation of Env-specific CD4+ T cells, two different pools of overlapping peptides spanning the entire HIV-1 Env proteome were added to each specimen. The first pool of peptides was based on a consensus sequence of clade B HIV-1 isolates and the second on clade A HIV-1 isolates. The final concentration of each peptide present in the two pools was 1 μg/ml. A mixture of anti-CD28 and anti-CD49d antibodies was added for costimulation. For a negative control, cells were treated equally without the addition of peptides; for a positive control, cells were stimulated with phorbol myristate acetate (PMA) and ionomycin. After 1 h of incubation, Golgi Stop and Golgi Plug protein trafficking inhibitors were added and stimulation was continued for 5 more hours. Stimulated cells were then washed with PBS and subsequently stained with Zombie Aqua viability dye. Next, cells were washed with staining buffer, and a cocktail of antibodies against surface antigens was added: anti-CD8-AF488 (clone RPAT8), anti-CD45RO-BV605 (clone UCHL1), anti-CXCR5-BV785 (clone J252D4), and anti-CD279-PE-Cy7 (clone EH12.2H7). After surface staining, cells were fixed and permeabilized (Fix/Perm kit) to allow staining of intracellular antigens. The mixture of antibodies for intracellular staining was composed of anti-CD3-AF700 (clone OKT3), anti-CD4-APC-Cy7 (clone RPA-T4), anti-CD40L-PE-Dazzle (clone TRAP1), anti-IFN-γ-PE (clone B27), anti-IL-21-APC (clone 3A3-N2), anti-IL-4-BV421 (clone MP4-25D2), and anti-IL-13-BV421 (clone JES10-5A2). Finally, cells were washed in PBS and acquired on a BD FACS Celesta flow cytometer. Incubation steps required for staining and fixation of cells were carried out at 4°C for 15 min. All antibodies and staining reagents were used in pretitrated amounts. Compensation was performed with single-stained capture beads (CompBeads), and fluctuations in laser intensity were measured every time before the experiment, using multifluorescence calibration beads (Rainbow calibration particles). Detection voltages were adjusted accordingly to maintain equivalent fluorescence readings throughout the experiment. Data were analyzed with FlowJo v9.4.1.
Measurement of ADCP, ADNP, and ADCD.
Antibody-dependent cellular phagocytosis (ADCP), antibody-dependent neutrophil phagocytosis (ADNP), and antibody-dependent complement deposition (ADCD) assays were performed as previously described (49–51). In brief, a mixture of biotinylated BG505 SOSIP.664 and AMC011 SOSIP.v4.2 proteins was coupled to 1-μm yellow (ADCP and ADNP) and red (ADCD) fluorescent neutravidin beads for 2 h at 37°C. Excess antigen was removed with three washes with 0.1% bovine serum albumin in PBS. Next, 1.82 × 108 antigen-coated beads were added to each well of a 96-well round-bottom plate and incubated with various dilutions of plasma samples in PBS (ADCP, 1:100, 1:200, and 1:800; ADNP, 1:50, 1:100, and 1:200; and ADCD, 1:10, 1:40, and 1:80) at 37°C for 2 h. After the formation of immune complexes, beads were washed once with PBS. For ADCP, 2.5 × 104 THP-1 cells were seeded into each well of a microtiter plate and rested for 16 h at 37°C. The cells were then combined with fluorescent beads carrying immune complexes. To assess neutrophil-mediated phagocytosis, blood from healthy donors was treated with ammonium-chloride-potassium (ACK) lysis buffer. A total of 5 × 104 erythrocyte-free cells were added per well and incubated for 1 h at 37°C. Subsequently, cells were stained with an anti-Cd66b-Pacific Blue (clone G10F5) antibody to allow the detection of neutrophils. For the ADCD assay measuring antibody-dependent complement deposition of C3, lyophilized guinea pig complement was first reconstituted with deionized water and further diluted in gelatin Veronal buffer containing Mg2+ and Ca2+. The complement solution was then combined with immune complexes for 20 min at 37°C. After washing twice with 15 mM EDTA in PBS, immune complexes were stained using fluorescein-conjugated goat IgG fraction to guinea pig complement C3. Finally, all samples were fixed in 4% paraformaldehyde and acquired on an Intellicyt iQue Screener Plus flow cytometer equipped with a robotic plate-loading arm. All events were gated on single cells and bead positive events. For ADCP and ADNP, a phagocytosis score was calculated as the percentage of bead positive cells × geometric mean fluorescence intensity (GMFI)/1,000. For ADCD, the median of C3 positive events is reported. All samples were run in duplicate on different days. Area under the curve (AUC) was calculated based on three different plasma dilutions using GraphPad Prism.
Measurement of antibody-dependent NK cell activation.
For analysis of NK cell-related responses, 96-well enzyme-linked immunosorbent assay (ELISA) plates were coated with a 1:1 mix of BG505 SOSIP.664 and AMC011 SOSIP.v4.2 at 3 μg/ml in PBS at 37°C for 2 h. The plates were then washed and blocked with 5% bovine serum albumin (BSA) in PBS overnight at 4°C. NK cells were isolated from buffy coats from healthy donors (Massachusetts General Hospital [MGH] blood donor center) using the RosetteSep NK enrichment kit. Isolated NK cells were then rested overnight in the presence of IL-15 (1 ng/ml). Plasma samples were diluted 1:20, 1:40, and 1:80 in PBS and added to antigen-coated wells to allow immune complex formation. A staining cocktail of anti-CD107a-PE-Cy5 (clone H4A3), brefeldin A, and monensin was added to NK cells before they were transferred to plates with immune complexes. A total of 5 × 104 NK cells were incubated in each well for 5 h at 37°C. Afterward, cells were fixed and stained for surface markers with anti-CD16-APC-Cy7 (clone 3G8), anti-CD56-PE-Cy7 (clone B159), and anti-CD3-Pacific Blue antibodies (clone UCHT1). Subsequently, cells were permeabilized using Perm buffer and intracellular staining with anti-IFN-γ-FITC (clone 25723.11) and anti-MIP-1β-PE (clone D21-1351) was performed. Samples were acquired on Intellicyt iQue Screener Plus flow cytometer equipped with a robotic plate-loading arm. NK cells were defined as CD3−, CD16+, and CD56+. The assay was performed in duplicate across two blood donors, and AUC was calculated based on three different plasma dilutions using GraphPad Prism.
Statistical analysis.
Statistical analysis and graphing of the data were performed using GraphPad Prism and SPICE software. Statistical significance of differences between two groups was analyzed with the Wilcoxon rank sum or Mann-Whitney test, and differences between the pie charts were assessed by permutation test. Comparisons between three or more groups were assessed by one-way analysis of variance (ANOVA) (Friedman’s test for matched data and the Kruskal-Wallis test for the unmatched data) in case of one independent variable and two-way ANOVA for two independent variables. Corrections for multiple comparisons were performed by Dunn’s test for one-way ANOVA and Dunnett’s test for two-way ANOVA. The strength of correlations was assessed by Spearman’s correlation test. Statistical significance is indicated by the following annotations in figures: *, P < 0.05; **, P < 0.01; ***, P < 0.001; and ****, P < 0.0001.
ACKNOWLEDGMENTS
We thank volunteers that participated in the SCABIO study and the study team of HPSTD-Ambulanz at University Hospital Essen.
The following reagents were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 consensus subtype B Env peptide pool (catalog no. 12540) and HIV-1 consensus A Env peptide pool.
Financial support for this study was provided by the German research association Deutsche Forschungsgemeinschaft (DFG) through the research training group Graduiertenkolleg 1949/2, entitled Immunantwort in Infektionskrankheiten—Regulation zwischen angeborener und erworbener Immunität.
We declare no competing financial interests.
REFERENCES
- 1.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. 2013. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med 210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia M, Gorgolas M, Cabello A, Estrada V, Ligos JM, Fernandez-Guerrero M, Barros C, Lopez-Bernaldo JC, De La Hera FJ, Montoya M, Benito JM, Rallon N. 2017. Peripheral T follicular helper cells make a difference in HIV reservoir size between elite controllers and patients on successful cART. Sci Rep 7:16799. doi: 10.1038/s41598-017-17057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pallikkuth S, Sharkey M, Babic DZ, Gupta S, Stone GW, Fischl MA, Stevenson M, Pahwa S. 2015. Peripheral T follicular helper cells are the major HIV reservoir within central memory CD4 T cells in peripheral blood from chronically HIV-infected individuals on combination antiretroviral therapy. J Virol 90:2718–2728. doi: 10.1128/JVI.02883-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pissani F, Streeck H. 2014. Emerging concepts on T follicular helper cell dynamics in HIV infection. Trends Immunol 35:278–286. doi: 10.1016/j.it.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, Davis I, Farber D, Hartjen P, Haag F, Alter G, Schulze Zur Wiesch J, Streeck H. 2012. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest 122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graff-Dubois S, Rouers A, Moris A. 2016. Impact of chronic HIV/SIV infection on T follicular helper cell subsets and germinal center homeostasis. Front Immunol 7:501. doi: 10.3389/fimmu.2016.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crotty S. 2011. Follicular helper CD4 T cells (TFH). Annu Rev Immunol 29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 8.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, Haddad EK, International AIDS Vaccine Initiative Protocol C Principal Investigators, Poignard P, Crotty S. 2013. Human circulating PD-1+CXCR3−CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz BT, Teigler JE, Pissani F, Oster AF, Kranias G, Alter G, Marovich M, Eller MA, Dittmer U, Robb ML, Kim JH, Michael NL, Bolton D, Streeck H. 2016. Circulating HIV-specific interleukin-21(+)CD4(+) T cells represent peripheral Tfh cells with antigen-dependent helper functions. Immunity 44:167–178. doi: 10.1016/j.immuni.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto T, Lynch RM, Gautam R, Matus-Nicodemos R, Schmidt SD, Boswell KL, Darko S, Wong P, Sheng Z, Petrovas C, McDermott AB, Seder RA, Keele BF, Shapiro L, Douek DC, Nishimura Y, Mascola JR, Martin MA, Koup RA. 2015. Quality and quantity of TFH cells are critical for broad antibody development in SHIVAD8 infection. Sci Transl Med 7:298ra120. doi: 10.1126/scitranslmed.aab3964. [DOI] [PubMed] [Google Scholar]
- 11.Buranapraditkun S, Pissani F, Teigler JE, Schultz BT, Alter G, Marovich M, Robb ML, Eller MA, Martin J, Deeks S, Michael NL, Streeck H. 2017. Preservation of peripheral T follicular helper cell function in HIV controllers. J Virol 91:e00497-17. doi: 10.1128/JVI.00497-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cubas R, van Grevenynghe J, Wills S, Kardava L, Santich BH, Buckner CM, Muir R, Tardif V, Nichols C, Procopio F, He Z, Metcalf T, Ghneim K, Locci M, Ancuta P, Routy JP, Trautmann L, Li Y, McDermott AB, Koup RA, Petrovas C, Migueles SA, Connors M, Tomaras GD, Moir S, Crotty S, Haddad EK. 2015. Reversible reprogramming of circulating memory T follicular helper cell function during chronic HIV infection. J Immunol 195:5625–5636. doi: 10.4049/jimmunol.1501524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanham G, Penne L, Devalck J, Kestens L, Colebunders R, Bosmans E, Thielemans K, Ceuppens JL. 1999. Decreased CD40 ligand induction in CD4 T cells and dysregulated IL-12 production during HIV infection. Clin Exp Immunol 117:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colineau L, Rouers A, Yamamoto T, Xu Y, Urrutia A, Pham HP, Cardinaud S, Samri A, Dorgham K, Coulon PG, Cheynier R, Hosmalin A, Oksenhendler E, Six A, Kelleher AD, Zaunders J, Koup RA, Autran B, Moris A, Graff-Dubois S. 2015. HIV-infected spleens present altered follicular helper T cell (Tfh) subsets and skewed B cell maturation. PLoS One 10:e0140978. doi: 10.1371/journal.pone.0140978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckner CM, Moir S, Ho J, Wang W, Posada JG, Kardava L, Funk EK, Nelson AK, Li Y, Chun TW, Fauci AS. 2013. Characterization of plasmablasts in the blood of HIV-infected viremic individuals: evidence for nonspecific immune activation. J Virol 87:5800–5811. doi: 10.1128/JVI.00094-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moir S, Fauci AS. 2017. B-cell responses to HIV infection. Immunol Rev 275:33–48. doi: 10.1111/imr.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kardava L, Moir S, Shah N, Wang W, Wilson R, Buckner CM, Santich BH, Kim LJ, Spurlin EE, Nelson AK, Wheatley AK, Harvey CJ, McDermott AB, Wucherpfennig KW, Chun TW, Tsang JS, Li Y, Fauci AS. 2014. Abnormal B cell memory subsets dominate HIV-specific responses in infected individuals. J Clin Invest 124:3252–3262. doi: 10.1172/JCI74351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerneis S, Launay O, Turbelin C, Batteux F, Hanslik T, Boelle PY. 2014. Long-term immune responses to vaccination in HIV-infected patients: a systematic review and meta-analysis. Clin Infect Dis 58:1130–1139. doi: 10.1093/cid/cit937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 20.Tomaras GD, Haynes BF. 2009. HIV-1-specific antibody responses during acute and chronic HIV-1 infection. Curr Opin HIV AIDS 4:373–379. doi: 10.1097/COH.0b013e32832f00c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landais E, Moore PL. 2018. Development of broadly neutralizing antibodies in HIV-1 infected elite neutralizers. Retrovirology 15:61. doi: 10.1186/s12977-018-0443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mascola JR, Haynes BF. 2013. HIV-1 neutralizing antibodies: understanding nature’s pathways. Immunol Rev 254:225–244. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 24.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. 2014. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell 158:1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ackerman ME, Mikhailova A, Brown EP, Dowell KG, Walker BD, Bailey-Kellogg C, Suscovich TJ, Alter G. 2016. Polyfunctional HIV-specific antibody responses are associated with spontaneous HIV control. PLoS Pathog 12:e1005315. doi: 10.1371/journal.ppat.1005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast AS, Schoen MK, Rolland M, Suscovich TJ, Mahan AE, Liao L, Streeck H, Andrews C, Rerks-Ngarm S, Nitayaphan S, de Souza MS, Kaewkungwal J, Pitisuttithum P, Francis D, Michael NL, Kim JH, Bailey-Kellogg C, Ackerman ME, Alter G. 2014. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med 6:228ra38. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Roman VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, Florese R, Robert-Guroff M. 2005. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol 174:2185–2189. doi: 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- 29.Florese RH, Van Rompay KK, Aldrich K, Forthal DN, Landucci G, Mahalanabis M, Haigwood N, Venzon D, Kalyanaraman VS, Marthas ML, Robert-Guroff M. 2006. Evaluation of passively transferred, nonneutralizing antibody-dependent cellular cytotoxicity-mediating IgG in protection of neonatal rhesus macaques against oral SIVmac251 challenge. J Immunol 177:4028–4036. doi: 10.4049/jimmunol.177.6.4028. [DOI] [PubMed] [Google Scholar]
- 30.Worley MJ, Fei K, Lopez-Denman AJ, Kelleher AD, Kent SJ, Chung AW. 2018. Neutrophils mediate HIV-specific antibody-dependent phagocytosis and ADCC. J Immunol Methods 457:41–52. doi: 10.1016/j.jim.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Gauduin MC, Weir R, Fung MS, Koup RA. 1998. Involvement of the complement system in antibody-mediated post-exposure protection against human immunodeficiency virus type 1. AIDS Res Hum Retroviruses 14:205–211. doi: 10.1089/aid.1998.14.205. [DOI] [PubMed] [Google Scholar]
- 32.Roederer M, Nozzi JL, Nason MC. 2011. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keating SM, Pilcher CD, Jain V, Lebedeva M, Hampton D, Abdel-Mohsen M, Deng X, Murphy G, Welte A, Facente SN, Hecht F, Deeks SG, Pillai SK, Busch MP. 2017. HIV antibody level as a marker of HIV persistence and low-level viral replication. J Infect Dis 216:72–81. doi: 10.1093/infdis/jix225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Z, Luo Z, Wan Z, Wu H, Li W, Zhang T, Jiang W. 2015. HIV-associated memory B cell perturbations. Vaccine 33:2524–2529. doi: 10.1016/j.vaccine.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanders RW, Derking R, Cupo A, Julien JP, Yasmeen A, de Val N, Kim HJ, Blattner C, de la Pena AT, Korzun J, Golabek M, de Los Reyes K, Ketas TJ, van Gils MJ, King CR, Wilson IA, Ward AB, Klasse PJ, Moore JP. 2013. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog 9:e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Gils MJ, van den Kerkhof TL, Ozorowski G, Cottrell CA, Sok D, Pauthner M, Pallesen J, de Val N, Yasmeen A, de Taeye SW, Schorcht A, Gumbs S, Johanna I, Saye-Francisco K, Liang CH, Landais E, Nie X, Pritchard LK, Crispin M, Kelsoe G, Wilson IA, Schuitemaker H, Klasse PJ, Moore JP, Burton DR, Ward AB, Sanders RW. 2016. An HIV-1 antibody from an elite neutralizer implicates the fusion peptide as a site of vulnerability. Nat Microbiol 2:16199. doi: 10.1038/nmicrobiol.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, Werner L, Mlisana K, Sibeko S, Williamson C, Abdool Karim SS, Morris L, Team CS, CAPRISA002 Study Team. 2011. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J Virol 85:4828–4840. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ackerman ME, Dugast AS, McAndrew EG, Tsoukas S, Licht AF, Irvine DJ, Alter G. 2013. Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcgammaR2a and FcgammaR2b. J Virol 87:5468–5476. doi: 10.1128/JVI.03403-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ljunggren K, Moschese V, Broliden PA, Giaquinto C, Quinti I, Fenyo EM, Wahren B, Rossi P, Jondal M. 1990. Antibodies mediating cellular cytotoxicity and neutralization correlate with a better clinical stage in children born to human immunodeficiency virus-infected mothers. J Infect Dis 161:198–202. doi: 10.1093/infdis/161.2.198. [DOI] [PubMed] [Google Scholar]
- 40.Forthal DN, Landucci G, Haubrich R, Keenan B, Kuppermann BD, Tilles JG, Kaplan J. 1999. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J Infect Dis 180:1338–1341. doi: 10.1086/314988. [DOI] [PubMed] [Google Scholar]
- 41.Forthal DN, Landucci G, Keenan B. 2001. Relationship between antibody-dependent cellular cytotoxicity, plasma HIV type 1 RNA, and CD4+ lymphocyte count. AIDS Res Hum Retroviruses 17:553–561. doi: 10.1089/08892220151126661. [DOI] [PubMed] [Google Scholar]
- 42.Luckheeram RV, Zhou R, Verma AD, Xia B. 2012. CD4(+)T cells: differentiation and functions. Clin Dev Immunol 2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pallikkuth S, Parmigiani A, Pahwa S. 2012. The role of interleukin-21 in HIV infection. Cytokine Growth Factor Rev 23:173–180. doi: 10.1016/j.cytogfr.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonsignori M, Moody MA, Parks RJ, Holl TM, Kelsoe G, Hicks CB, Vandergrift N, Tomaras GD, Haynes BF. 2009. HIV-1 envelope induces memory B cell responses that correlate with plasma antibody levels after envelope gp120 protein vaccination or HIV-1 infection. J Immunol 183:2708–2717. doi: 10.4049/jimmunol.0901068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rouers A, Klingler J, Su B, Samri A, Laumond G, Even S, Avettand-Fenoel V, Richetta C, Paul N, Boufassa F, Hocqueloux L, Mouquet H, Rouzioux C, Lambotte O, Autran B, Graff-Dubois S, Moog C, Moris A, ANRS CO21 Cohort. 2017. HIV-specific B cell frequency correlates with neutralization breadth in patients naturally controlling HIV-infection. EBioMedicine 21:158–169. doi: 10.1016/j.ebiom.2017.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS. 2008. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med 205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Claireaux M, Galperin M, Benati D, Nouel A, Mukhopadhyay M, Klingler J, de Truchis P, Zucman D, Hendou S, Boufassa F, Moog C, Lambotte O, Chakrabarti LA. 2018. A high frequency of HIV-specific circulating follicular helper T cells is associated with preserved memory B cell responses in HIV controllers. mBio 9:e00317-18. doi: 10.1128/mBio.00317-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thornhill JP, Fidler S, Klenerman P, Frater J, Phetsouphanh C. 2017. The role of CD4+ T follicular helper cells in HIV infection: from the germinal center to the periphery. Front Immunol 8:46. doi: 10.3389/fimmu.2017.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischinger S, Fallon JK, Michell AR, Broge T, Suscovich TJ, Streeck H, Alter G. 2019. A high-throughput, bead-based, antigen-specific assay to assess the ability of antibodies to induce complement activation. J Immunol Methods 473:112630. doi: 10.1016/j.jim.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, Schoen MK, Tafesse F, Martin C, Leung V, Mahan AE, Sips M, Kumar MP, Tedesco J, Robinson H, Tkachenko E, Draghi M, Freedberg KJ, Streeck H, Suscovich TJ, Lauffenburger DA, Restrepo BI, Day C, Fortune SM, Alter G. 2016. A functional role for antibodies in tuberculosis. Cell 167:433–443.e14. doi: 10.1016/j.cell.2016.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ackerman ME, Moldt B, Wyatt RT, Dugast AS, McAndrew E, Tsoukas S, Jost S, Berger CT, Sciaranghella G, Liu Q, Irvine DJ, Burton DR, Alter G. 2011. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods 366:8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]