Highlights
-
•
Co-infection with COVID-19 and HIV should be considered in people with risk factors.
-
•
People with AIDS can have both COVID-19 and opportunistic infections.
-
•
People with AIDS and COVID-19 may be at risk for immune reconstitution syndrome.
-
•
Further study of COVID-19 in people with AIDS is needed.
Keywords: COVID-19, HIV, AIDS, PJP, IRIS
Abstract
Background
Case reports, case series and cohort studies have been published describing the clinical course and outcomes of people living with human immunodeficiency virus (PLWH) who contract coronavirus disease 2019 (COVID-19) pneumonia. However, the majority of the published work focuses on patients with well-controlled human immunodeficiency virus (HIV) on antiretroviral therapy (ART).
Case presentation
We present a case of a new diagnosis of HIV with Acquired Immune Deficiency Syndrome (AIDS) made simultaneously to diagnosis of COVID-19, with co-infection with pneumocystis jirovecii pneumonia (PJP) and possible cytomegalovirus (CMV) pneumonitis. The patient decompensated following initiation of ART, suggestive of possible immune reconstitution inflammatory syndrome (IRIS).
Conclusions
This case illustrates the importance of maintaining a high suspicion for HIV/AIDS in patients with risk factors. Additionally, this case raises the possibility that IRIS may develop in the setting of ART initiation in patients with COVID-19.
Background
Co-infection with human immunodeficiency virus (HIV) and coronavirus disease 2019 (COVID-19) remains incompletely understood [1,2]. Poorly controlled HIV or acquired immune deficiency syndrome (AIDS) is a risk factor for many opportunistic infections, however it is unclear if AIDS increases risk of COVID-19 acquisition or severity. Alternatively, over-exuberant immune response can be a feature of severe COVID-19 infections, against which HIV-associated immune dysfunction has been hypothesized to be protective, and it has been suggested that some antiretroviral therapy (ART) may have activity against SARS-COV-2 [3].
The majority of published data is in patients with well-controlled HIV although the literature is growing around persons living with HIV (PLWH) complicated by AIDS and COVID-19 [1,2]. There have also been reports of opportunistic infections in the subset of PLWH who have AIDS and COVID-19, including prior reports of COVID-19 and pneumocystis jirovecii pneumonia (PJP) [4]. We present a patient simultaneously diagnosed with COVID-19 and HIV/AIDS complicated by both PJP and possible CMV pneumonitis, who decompensated after initiation of ART, suggestive of immune reconstitution inflammatory syndrome (IRIS).
Case presentation
A 38-year-old previously healthy cis-gender man who has sex with men presented with two weeks of exertional dyspnea and dry cough. He noted significant weight loss and anorexia over the preceding 6 months, however denied additional symptoms. He had no known exposures to persons with COVID-19 or HIV.
On presentation, he was febrile to 101.3 °F, tachycardic with heart rate 121 beats/min, tachypneic with respiratory rate of 26 breaths/min and hypoxic (oxygen saturation 93 % on room air). Exam was notable for fine pulmonary rales. Labs are summarized in Table 1. Additionally, his COVID-19 nasopharyngeal polymerase chain reaction (PCR) swab was positive, as was HIV testing. Computed tomography (CT) of the chest showed bilateral diffuse ground glass opacification (GGO) with areas of crazy paving in the lower lobes and subpleural reticular opacities.
Table 1.
Laboratory values at admission and on hospital day 13.
| Laboratory | |||
|---|---|---|---|
| Admission | Hospital day 13 | Normal Range | |
| Hematology | |||
| White blood cell count (K/μL) | 4.9 | 10.2 | 4.0–10.0 |
| Hemoglobin (g/dL) | 10.3 | 11.4 | 13.7–17.5 |
| Hematocrit (%) | 31.1 | 33.2 | 40–51 |
| Platelet count (K/μL) | 271 | 262 | 150–400 |
| Absolute neutrophil count (K/μL) | 3.7 | 9.56 | 1.6–6.1 |
| Absolute lymphocyte count (K/μL) | 0.86 | 0.50 | 1.2–3.7 |
| Absolute CD4 lymphocyte count (cells/μL) | 51 | -- | 350−1100 |
| CD4 Cells, Percent | 6% | -- | |
| Absolute CD8 lymphocyte count (cells/μL) | 568 | -- | 193–685 |
| CD8 Cells, Percent | 68 % | -- | |
| CD4/CD8 ratio | 0.09 | -- | 0.84–3.0 |
| Chemistry | |||
| Sodium (mEq/L) | 137 | 128 | 135–147 |
| Potassium (mEq/L) | 3.9 | 4.7 | 3.5–5.4 |
| Chloride (mEq/L) | 105 | 98 | 96–108 |
| Bicarbonate (mEq/L) | 18 | 18 | 22–32 |
| Urea Nitrogen (mg/dL) | 10 | 30 | 6–20 |
| Creatinine (mg/dL) | 0.8 | 1.0 | 0.5–1.2 |
| Glucose (mg/dL) | 107 | 103 | 70–100 |
| Aspartate Aminotransferase (IU/L) | 34 | 63 | 0–40 |
| Alanine Aminotransferase (IU/L) | 21 | 99 | 0–40 |
| Alkaline Phosphatase (IU/L) | 70 | 89 | 40–130 |
| Total Bilirubin (mg/dL) | 1.1 | 0.3 | 0–1.5 |
| Lactate Dehydrogenase (IU/dL) | 522 | 349 (on 7/1/20) | 94–250 |
| Troponin T (ng/mL) | <0.01 | <0.01 (on 7/1/20) | 0–0.01 |
| Viral Load | |||
| HIV-1 Viral load (log10 copies/mL) | 5.6 | 3.1 | |
| Inflammatory Markers | |||
| C-Reactive Protein (mg/L) | 8.2 | 119.1 (on 7/7/20) | 0–5.0 |
| Ferritin (ng/mL) | 864 | 924 | 30–400 |
| Fibrinogen (mg/dL) | -- | 693 | 180−400 |
| D-Dimer (ng/mL) | 3695 | 887 | 0–500 |
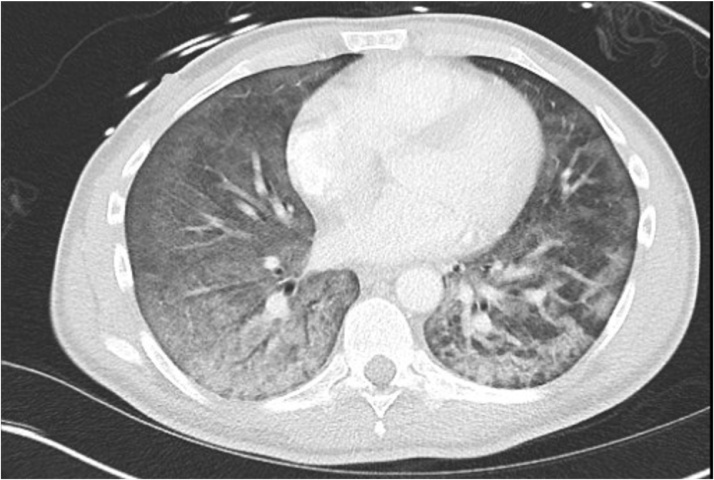
On hospital day (HD) 1, he was noted to have worsening hypoxemia, with oxygen saturation 77 % on room air, which improved with nasal cannula oxygen. He received remdesivir on HD 2 through 6, and was started on empiric oral trimethoprim/sulfamethoxazole (TMP/SMX) and prednisone 40 mg every 12 h for presumed PJP. On HD 2, he had improving clinical status with resolution of fevers and hypoxemia. He was initiated on ART (bictegravir, emtricitabine, and tenofovir alafenamide). On HD 6 he re-developed an oxygen requirement. On HD 9 steroids were tapered to 40 mg daily, and further decreased to 20 mg daily on HD 11. On HD 13 had a fever of 101.8 °F with laboratory changes as summarized in Table 1. Repeat chest CT showed worsening diffuse bilateral GGO (Fig. 1). Prednisone dose was increased to 60 mg daily on HD 13. He required intubation for hypoxemia on HD 15. He was found to have positive serum CMV PCR (4.06 log IU/mL) and was started on IV ganciclovir. After intubation, a bronchoalveolar lavage (BAL) was positive for PJP by immunofluorescence (IF) and for CMV by PCR (4.7 log IU/mL). He was noted to have increasing inflammatory markers, so was given pulse-dose methylprednisolone 500 mg daily for possible IRIS on HD 17 through HD 19, with temporary improvement in inflammatory markers and ventilator requirements. ART was continued throughout hospitalization. On HD 20 his steroids were transitioned to prednisone 60 mg daily. He worsened again, requiring extracorporal membrane oxygenation (ECMO) initiation on HD 22. He had refractory hypoxemia despite ECMO, and was ultimately transitioned to comfort measures and passed away.
Fig. 1.
CT chest with diffuse bilateral ground glass opacification with areas of crazy paving and subpleural reticular opacities.
Discussion
The majority of the descriptions of HIV and COVID-19 co-infection to date are in persons with well-controlled HIV on ART [1,2]. In these studies, additional risk factors for COVID-19 infection (eg. hypertension, hyperlipidemia, diabetes, etc) were frequently reported, similar to the general population [1,2]. The described clinical presentation of PLWH with COVID-19 has also been similar to the general population, with the majority presenting with cough, fevers, arthralgias/myalgias, headache, or sore throat [1,2].
A recent systematic review of the literature through October 2020 included 82 studies and a total of 643,018 PLWH, including 19 patients not on ART. COVID-19 outcomes in PLWH with well-controlled HIV were similar to those in the general population with a similar distribution of patients fully recovering, requiring ICU levels of care, or dying. No difference was detected in clinical features, laboratory findings or severity of illness between those on ART and those not on ART, although this number was too small to provide definite conclusions. The systematic review also identified patients with co-diagnoses of new HIV and COVID-19, which together with the case we present, highlights the importance of maintaining a high suspicion for HIV infection in patients who are not improving as expected [2].
While there is increasing data surrounding poorly-controlled HIV and opportunistic infections in patients with COVID-19, there have not yet been reports of immune reconstitution inflammatory syndrome (IRIS) in PLWH with COVID-19. There is one case report of a patient who did not have HIV but who had pancytopenia from chemotherapy and presented with COVID-19 pneumonia, who had an IRIS-like reaction after receiving G-CSF [5]. IRIS can either present as unmasking IRIS, in which an underlying undiagnosed opportunistic infection is unmasked, or as paradoxical IRIS, in which symptoms from an infection appear to worsen after starting ART [6]. The opportunistic infections most commonly associated with IRIS include CMV retinitis, cryptococcal meningitis, and tuberculosis [7]. Other diseases associated with IRIS include Kaposi’s sarcoma, Mycobacterium avium complex, Herpes Simplex Virus, Varicella Zoster Virus, Hepatitis B and C, and Toxoplasma gondii [6,7]. IRIS is relatively rare in PJP, seen in only 4 % of patients with HIV-associated PJP who are initiated on ART [8]. IRIS with CMV pneumonia is even more rare, with one case report in the literature [9]. Despite the risk of IRIS, current guidelines recommend initiation of treatment within two weeks of diagnosis of HIV even in the presence of opportunistic infections, with the exception of acute cryptococcal meningitis, because of the benefits associated with early initiation of ART [10].
Given the multiple, co-occurring infections, it is difficult to know exactly which diagnosis (or combination of diagnoses) drove our patient's clinical course. A diagnosis of IRIS is supported by worsening hypoxia four days after starting ART, while on the appropriate steroid dose for PJP (prednisone 40 mg every 12 h), a higher dose than is recommended for COVID-19 pneumonia (40 mg prednisone daily, equivalent to 6 mg dexamethasone daily) [11,12]. He developed fevers, rising inflammatory markers and worsening radiographic findings two days after this prednisone dose was decreased to 40 mg daily, with subsequent improvement on pulse dose methylprednisolone. This correlation between clinical status and steroid dose could be consistent with IRIS possibly related to COVID-19 given the rarity of IRIS with PJP or CMV pneumonitis. However, as steroids are also mainstays of treatment in both PJP and COVID-19 pneumonia, either of these etiologies could have contributed to this correlation. Additionally, COVID-19 pneumonia alone can present with a delayed pro-inflammatory respiratory decompensation, although this has been described typically between days 4 and 10 of illness, while our patient decompensated at HD 13 (approximately one month after symptom onset) [13,14]. It is also possible that more than one of his pro-inflammatory diagnoses contributed to his respiratory failure and ultimate demise.
Conclusions
This case report demonstrates the potential for multiple concurrent infections in patients with either COVID-19 or HIV. It highlights the importance of considering HIV in patients with risk factors and consistent presentations. Despite social isolation measures, new cases of both acute and chronic HIV continue to be diagnosed, in patients with COVID-19 and those undergoing routine screening [15]. Furthermore, this case raises the concern that in patients with AIDS and COVID-19, initiation of ART could cause IRIS and lead to worsened outcomes. More data is needed to investigate this possibility, and the potential risks of ART initiation in similar clinical scenarios.
Most current evidence suggests PLWH with well-controlled HIV have similar outcomes to the general population. However, of the few described cases of PLWH with AIDS or poorly controlled HIV, there have been multiple cases with poor outcomes, including our patient. This supports the possibility that poorly-controlled HIV increases risk of severe COVID-19. These findings are significantly limited by the paucity of data, highlighting the need for further investigation into the effects of COVID-19 among PLWH, particularly those with poorly-controlled disease.
Funding
This work was not supported by external funding.
Consent
Verbal consent was obtained from the patient shortly before he was intubated. However, we were unable to obtain written consent prior to intubation and subsequent expiration of the patient. Consent not obtained from next-of-kin as it was unknown if he had disclosed his diagnosis of HIV to his next-of-kin.
Ethical approval
None.
Author statement
Elisabeth Merchant, MD and Kristin Flint, MD: Writing – Original Draft, Review and Editing.
Barbra Blair, MD and Dan Barouch, MD: Writing – Review and editing; Supervision.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Costenaro P., Minotti C., Barbieri E., Giaquinto C., Donà D. SARS-CoV-2 infection in people living with HIV: a systematic review. Rev Med Virol. 2020 doi: 10.1002/rmv.2155. [DOI] [PubMed] [Google Scholar]
- 2.Lee K.W., Yap S.F., Ngeow Y.F., Lye M.S. COVID-19 in people living with HIV: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18:3554. doi: 10.3390/ijerph18073554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fung M., Babik J.M. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa863. Oxford Academic. Clinical Infectious Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broadhurst A.G.B., Lalla U., Taljaard J.J., Louw E.H., Koegelenberg C.F.N., Allwood B.W. The diagnostic challenge of pneumocystis pneumonia and COVID-19 co-infection in HIV. Respirol Case Rep. 2021;9 doi: 10.1002/rcr2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mertens J., Laghrib Y., Kenyon C. A case of steroid-responsive, COVID-19 immune reconstitution inflammatory syndrome following the use of granulocyte colony-stimulating factor. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huis in’ t Veld D., Sun H.Y., Hung C.C., Colebunders R. The immune reconstitution inflammatory syndrome related to HIV co-infections: a review. Eur J Clin Microbiol Infect Dis. 2012;31:919–927. doi: 10.1007/s10096-011-1413-9. [DOI] [PubMed] [Google Scholar]
- 7.Müller M., Wandel S., Colebunders R., Attia S., Furrer H., Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Achenbach C.J., Harrington R.D., Dhanireddy S., Crane H.M., Casper C., Kitahata M.M. Paradoxical immune reconstitution inflammatory syndrome in HIV-infected patients treated with combination antiretroviral therapy after AIDS-defining opportunistic infection. Clin Infect Dis. 2012;54:424–433. doi: 10.1093/cid/cir802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petarra-Del Río S., Rodriguez-Hernandez A., Anguiano-Landa L., Aguilar-Portillo G., Zavala-Trujillo I., Nava-Zavala A.H. Immune reconstitution inflammatory syndrome and cytomegalovirus pneumonia case report: highlights and missing links in classification criteria and standardized treatment. Case Rep Infect Dis. 2017;2017:1–7. doi: 10.1155/2017/9314580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Günthard H.F., Saag M.S., Benson C.A., del Rio C., Eron J.J., Gallant J.E. Antiretroviral drugs for treatment and prevention of HIV infection in Adults: 2016 recommendations of the international antiviral society-USA Panel. JAMA - J Am Med Assoc. 2016;(316):191–210. doi: 10.1001/jama.2016.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panel on Opportunistic Infections in Adults and Adolescents with HIV . 2021. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health and the HIV Medicine Association of the Infectious Diseases Society of America. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. [Accessed 4/21/2021 [W4-W8]] [Google Scholar]
- 12.COVID-19 Treatment Guidelines Panel . National Institutes of Health; 2021. Coronavirus disease 2019 (COVID-19) treatment guidelines. Available at https://www.covid19treatmentguidelines.nih.gov/. [Accessed 4/21/2021] [PubMed] [Google Scholar]
- 13.Wang F., Qu M., Zhou X., Zhao K., Lai C., Tang Q. The timeline and risk factors of clinical progression of COVID-19 in Shenzhen, China. J Transl Med. 2020;18:270. doi: 10.1186/s12967-020-02423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen P.A., Hall L.E., John J.N., Rapoport A.B. The early natural history of SARS-CoV-2 infection: clinical observations from an urban, ambulatory COVID-19 clinic. Mayo Clin Proc. 2020;95:1124–1126. doi: 10.1016/j.mayocp.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanford K.A., Friedman E.E., Schmitt J., Spiegel T., Ridgway J.P., Moore M. Routine screening for HIV in an urban emergency department during the COVID-19 pandemic. AIDS Behav. 2020;1:3. doi: 10.1007/s10461-020-02899-x. [DOI] [PMC free article] [PubMed] [Google Scholar]