Abstract
Background
Obesity and inactivity are associated with increased risk of cancer-related and overall mortality in breast cancer, but there are few data in metastatic disease.
Methods
Cancer and Leukemia Group B 40502 was a randomized trial of first-line taxane-based chemotherapy for patients with metastatic breast cancer. Height and weight were collected at enrollment. After 299 patients enrolled, the study was amended to assess recreational physical activity (PA) at enrollment using the Nurses’ Health Study Exercise Questionnaire. Associations with progression-free survival (PFS) and overall survival (OS) were evaluated using stratified Cox modeling (strata included hormone receptor status, prior taxane, bevacizumab use, and treatment arm). All statistical tests were 2-sided.
Results
A total of 799 patients were enrolled, and at the time of data lock, median follow-up was 60 months. At enrollment, median age was 56.7 years, 73.1% of participants had hormone receptor–positive cancers, 42.6% had obesity, and 47.6% engaged in less than 3 metabolic equivalents of task (MET) hours of PA per week (<1 hour of moderate PA). Neither baseline body mass index nor PA was statistically significantly associated with PFS or OS, although there was a marginally statistically significant increase in PFS (hazard ratio = 0.83, 95% confidence interval = 0.79 to 1.02; P = .08) and OS (hazard ratio = 0.81, 95% confidence interval = 0.65 to 1.02; P = .07) in patients who reported PA greater than 9 MET hours per week vs 0-9 MET hours per week.
Conclusions
In a trial of first-line chemotherapy for metastatic breast cancer, rates of obesity and inactivity were high. There was no statistically significant relationship between body mass index and outcomes. More information is needed regarding the relationship between PA and outcomes.
Obesity is a well-established risk factor for poor prognosis in women with early stage breast cancer (1-4). A meta-analysis conducted by the World Cancer Research Fund of 82 reports evaluating the relationship between body mass index (BMI) and mortality in women with stage I-III breast cancer reported a 35% increase in breast cancer–related mortality and a 41% increase in overall mortality in women with obesity at the time of breast cancer diagnosis as compared with women who were of normal weight (4). The relationship between obesity and poor outcomes was seen in both pre- and postmenopausal women. Other meta-analyses have demonstrated that obesity is associated with increased risk of cancer recurrence and mortality in both hormone receptor–positive and hormone receptor–negative breast cancers (5,6).
Although data are more limited, physical activity (PA) is also increasingly being linked to breast cancer outcomes. A meta-analysis of 13 cohort studies evaluating the relationship between PA after breast cancer diagnosis and mortality in individuals with stage I-III breast cancer demonstrated that breast cancer survivors who engaged higher levels of PA after cancer diagnosis had a 37% lower risk of breast cancer–specific mortality (hazard ratio [HR] = 0.63, 95% confidence interval [CI] = 0.50 to 0.78; P < .01) compared with less active breast cancer survivors (7).
Less is known regarding the relationships between obesity and inactivity and outcomes in individuals with metastatic breast cancer (MBC). A few studies have evaluated the relationship between BMI and progression-free survival (PFS) or overall survival (OS) in individuals with MBC (8–12), but most reports have focused specifically on individuals with HER2-positive breast cancer or with brain metastases. Results of these studies have been mixed. There is almost no information about PA patterns among individuals with MBC or about the relationship between PA and cancer outcomes in this population.
We evaluated the relationships between BMI and PA patterns with PFS and OS in patients participating in Cancer and Leukemia Group B (CALGB) 40502 (13), a randomized trial comparing the efficacy of 3 chemotherapy regimens, in combination with bevacizumab, as first-line therapy for patients with MBC or locally recurrent, unresectable breast cancer.
Methods
Study Cohort
The patient cohort for this study was derived from the study population of CALGB 40502 (NCT00785291) (13,14), a phase III randomized trial comparing paclitaxel with nanoparticle albumen bound-(nab-)paclitaxel or ixabepilone +/- bevacizumab as first-line chemotherapy for locally recurrent or MBC. Participants were randomly assigned 1:1:1 to paclitaxel (90 mg/m2), nab-paclitaxel (150 mg/m2), or ixabepilone (16 mg/m2). All chemotherapy was administered 3 weeks on, 1 week off. Participants were also treated with bevacizumab (10 mg/kg every 2 weeks). In March 2011, the protocol was amended to make bevacizumab use optional; nevertheless, 97% of participants received the agent. Eligibility criteria included the presence of metastatic or locally recurrent breast cancer; no prior chemotherapy for advanced disease; being at least 12 months from adjuvant taxane; and having measurable disease, adequate organ function and Eastern Cooperative Oncology Group performance status (ECOG PS) of 1 or less, and no grade 2 or greater peripheral neuropathy (13).
The study was open to enrollment between November 2008 and November 2011. The CALGB, ECOG, Southwest Oncology Group, and North Central Cancer Treatment Group (NCCTG) participated in the study (CALGB and NCCTG are now part of the Alliance for Clinical Trials in Oncology). All participants signed an institutional review board–approved, protocol-specific informed consent document meeting all federal and institutional regulatory requirements. Study results have been published previously (13).
Measures
Height and weight were collected at the time of participant enrollment. BMI was calculated according to the formula: BMI = weight (kg)/height (m) (2). BMI categories were defined according to the World Health Organization as underweight (<18.5 kg/m2), normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), and obese (≥30 kg/m2).
An amendment was added to the protocol after 299 participants had been enrolled requiring that participants complete the Nurses’ Health Study Exercise Questionnaire (NHSEQ) at the time of study enrollment. The NHSEQ captures the frequency, type, and duration of recreational PA. Metabolic equivalents of task (MET) hours of weekly recreational PA were calculated using the Ainsworth Physical Activity Compendium (15).
Statistical Analysis
The analysis cohort is comprised of eligible participants who were classified as normal weight, overweight, or obese at the time of study entry using the World Health Organization classification scheme. Given the small number of participants classified as underweight, these participants (n = 9) were omitted from analyses. PA was categorized as 0-9 MET hours per week and more than 9 MET hours per week, based on guidelines’ recommendation of 150-180 minutes of moderate-intensity PA per week, which is consistent with 9 MET hours per week of PA (16,17).
Chi-square tests were used to assess whether patient or disease characteristics at registration differed with respect to BMI or PA category. PFS was defined as the time from date of randomization until date of first progression or death from any cause. Patients without documentation of a disease event were censored at the date of their last disease evaluation. OS was defined as the time from date of randomization until death due to any cause. Stratified Cox modeling with the stratification factors treatment assignment, hormone receptor status, prior taxane, and use of bevacizumab were used to assess whether PFS or OS differed with respect to BMI or PA in the analysis cohort. For the subgroup of patients with hormone receptor–positive breast cancer and the group of patients with hormone receptor–negative breast cancer, stratified log-rank tests with treatment assignment as the stratification factor were used to assess whether PFS or OS differed with respect to BMI or PA. Adverse event data were collected using Common Terminology Criteria for Adverse Events v4.0 criteria. Chi-square tests were used to assess whether proportion of severe adverse events (grade 3 or higher regardless of attribution) differed by BMI and PA within treatment arms. Analyses were performed using SAS software (version 9.4; SAS Institute Inc) on a database locked on May 3, 2017.
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies.
Results
Participant Characteristics
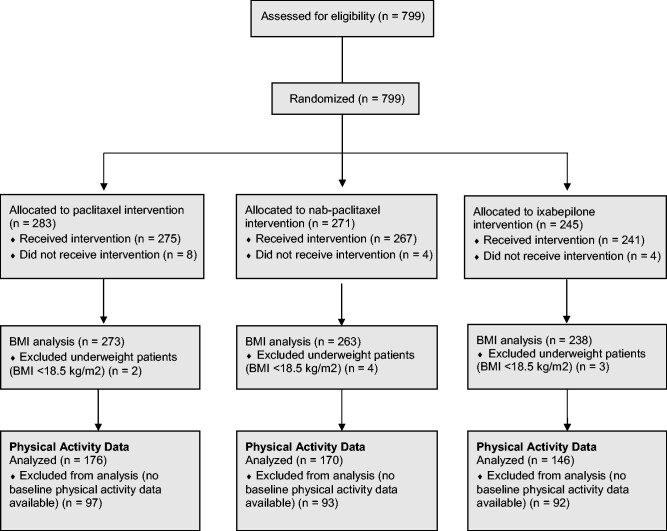
Overall, 799 individuals were enrolled in CALGB 40502, 783 of whom initiated protocol treatment (see Consort Diagram, Figure 1). Of these 783 participants, 9 were found to be underweight and omitted from analyses. Thus, the analytic cohort for this report is comprised of s the 774 eligible participants who initiated protocol treatment and were classified as normal weight, overweight, or obese at the time of study entry. Baseline characteristics are presented in Table 1. Median age was 56.7 years, 73.1% of patients had hormone receptor–positive cancers, 43.8% had received a taxane in the adjuvant setting, 57.5% were diagnosed with MBC at least 2 years after initial diagnosis, and 61.1% had an ECOG score of 0.
Figure 1.
CONSORT diagram. BMI = body mass index.
Table 1.
Patient characteristics by baseline body mass index (n = 774)
| Characteristic | Body mass index |
P | ||
|---|---|---|---|---|
| Normal | Overweight | Obese | ||
| (n = 202) | (n = 242) | (n = 330) | ||
| Median age (range), y | 57 (23-85) | 56 (34-86) | 56 (28-83) | .76a |
| Age, No. (%), y | .86b | |||
| 20-49 | 52 (25.7) | 61 (25.2) | 80 (24.2) | |
| 50-69 | 127 (62.9) | 154 (63.6) | 220 (66.7) | |
| 70 to ≥80 | 23 (11.4) | 27 (11.2) | 30 (9.1) | |
| Sex, No. (%) | .80c | |||
| Male | 2 (1.0) | 3 (1.2) | 6 (1.8) | |
| Female | 200 (99.0) | 239 (98.8) | 324 (98.2) | |
| Race, No. (%) | .02b | |||
| White | 167 (82.7) | 206 (85.1) | 251 (76.1) | |
| Black | 22 (10.9) | 23 (9.5) | 61 (18.5) | |
| Other/unknown | 13 (6.4) | 13 (5.4) | 18 (5.5) | |
| Taxane as adjuvant therapy, No. (%) | .12b | |||
| Yes | 78 (38.6) | 104 (43.0) | 157 (47.6) | |
| No | 124 (61.4) | 138 (57.0) | 173 (52.4) | |
| Visceral metastases, No. (%) | .93b | |||
| Yes | 159 (79.1) | 193 (80.4) | 262 (79.4) | |
| No | 42 (20.9) | 47 (19.6) | 68 (20.6) | |
| Missing | 1 | 2 | 0 | |
| Disease-free interval, No. (%) | .04b | |||
| 0 (de novo) | 18 (8.9) | 28 (11.6) | 46 (13.9) | |
| <1 y | 44 (21.8) | 39 (16.1) | 77 (23.3) | |
| 1 y < disease-free interval < 2 y | 18 (8.9) | 20 (8.3) | 39 (11.8) | |
| >2 y | 122 (60.4) | 155 (64.0) | 168 (50.9) | |
| Clinical stage, No. (%) | .70b | |||
| Not IV | 20 (11.0) | 20 (9.1) | 27 (8.8) | |
| IV | 162 (89.0) | 199 (90.9) | 281 (91.2) | |
| Missing | 20 | 23 | 22 | |
| Tumor subtype, No. (%) | .51b | |||
| ER and/or PR positive | 153 (75.7) | 178 (73.6) | 235 (71.2) | |
| ER and PR negative | 49 (24.3) | 64 (26.4) | 95 (28.8) | |
| ECOG performance, No. (%) | .04b | |||
| 0 | 116 (57.7) | 164 (68.3) | 193 (58.5) | |
| 1 | 78 (38.8) | 71 (29.6) | 126 (38.2) | |
| Missing | 8 | 7 | 11 | |
Two-sided Wilcoxon rank sum test or Kruskal-Wallis test. ECOG = Eastern Cooperative Oncology Group; ER = estrogen receptor; PR = progesterone receptor.
Two-sided χ2 test.
Two-sided Fisher exact test.
BMI and Tumor and Host Characteristics
Distribution of BMI categories was similar across treatment groups (data not shown). Median BMI was 28.6 kg/m2 (interquartile range = 24.7-33.1 kg/m2). Overall, 26.1% of participants were classified as normal weight, 31.2% overweight, and 42.6% obese. BMI was statistically significantly associated with race and performance status. Individuals with obesity were more likely to be Black (18.5% vs 10.9% [normal weight] and 9.5% [overweight]; P = .02) and less likely to have had a disease-free interval of more than 2 years between initial diagnosis and diagnosis of metastatic disease compared with normal weight or overweight individuals (50.9% vs 60.4% [normal weight] and 64.0% [overweight]; P = .04). No statistically significant relationship was found between BMI and tumor hormone receptor status or the presence of visceral metastases.
Baseline BMI and Cancer Outcomes
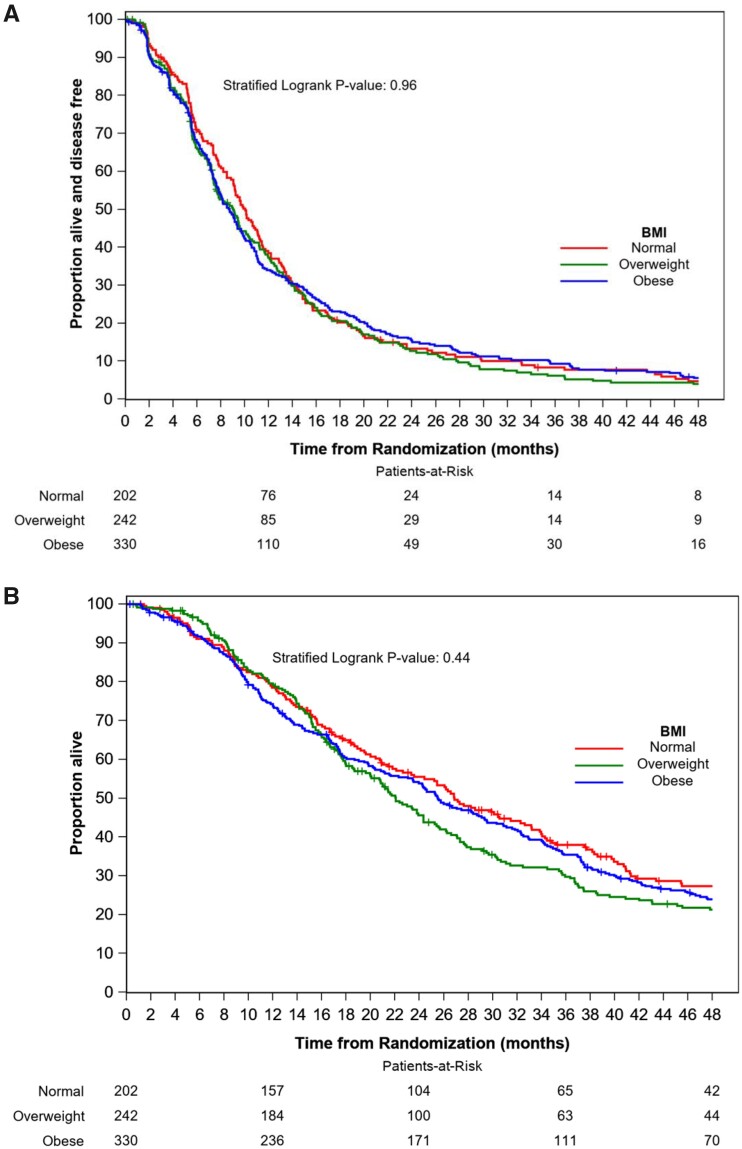
At a median follow-up of 60 months, there were 725 PFS events and 622 deaths among the 774 study participants. No statistically significant difference in PFS was found between those patients classified as obese (HR = 1.01, 95% CI = 0.83 to 1.22; P = .93) or overweight (HR = 1.03, 95% CI = 0.84 to 1.26; P = .77) relative to those classified as having normal weight. Similarly, no statistically significant difference in OS was found between those patients classified as obese (HR = 1.03, 95% CI = 0.84 to 1.26; P = .79) or overweight (HR = 1.14, 95% CI = 0.92 to 1.41; P = .24) relative to those classified as having normal weight. Median PFS and OS were 9.8 months (95% CI = 9.0 to 11.1) and 26.8 months (95% CI = 22.1 to 32.3) in patients classified as normal weight, 9.1 months (95% CI = 7.6 to 10.3) and 22.0 months (95% CI = 19.9 to 25.2) in patients classified as overweight, and 8.7 months (95% CI = 7.8 to 9.7) and 25.8 months (95% CI = 23.5 to 29.5) in patients classified as obese (Figure 2). Subset analyses showed that these results were similar in the subsets of participants with hormone receptor–positive and hormone receptor–negative tumors (data not shown).
Figure 2.
Kaplan-Meier plots of progression-free survival (A) and overall survival (B) by body mass index (BMI). Two-sided stratified log-rank test was used with stratification factors including treatment assignment, hormone receptor status, prior taxane, and use of bevacizumab.
Physical Activity and Tumor and Host Characteristics
Of the 774 patients included in the BMI analyses, 492 enrolled after the activation of the protocol amendment instituting the collection of PA data. Patient and tumor characteristics of these participants are included in Table 2. No statistically significant differences were found with respect to availability of PA data, and there were no differences in PA levels across treatment groups (data not shown). Participants engaged in a median of 3.3 MET hours per week of PA (about 1 hour of moderate intensity activity per week; interquartile range = 0.7-12.7 MET hours per week). Almost 70% of participants engaged in fewer than 9 MET hours per week of PA, with 47.6% of patients engaging in fewer than 3 MET hours per week.
Table 2.
Patient characteristics by baseline physical activity (n = 492)
| Characteristic | Physical activity MET hours per week |
P | |
|---|---|---|---|
| 0-9 (n = 338) | > 9 (n = 154) | ||
| Median age (range), y | 57 (28-86) | 57 (33-83) | .29a |
| Age, No. (%), y | .48b | ||
| 20-49 | 83 (24.6) | 40 (26.0) | |
| 50-69 | 214 (63.3) | 101 (65.6) | |
| 70 to ≥80 | 41 (12.1) | 13 (8.4) | |
| Sex, No. (%) | .05 | ||
| Male | 3 (0.9) | 5 (3.2) | |
| Female | 335 (99.1) | 149 (96.8) | |
| Race, No. (%) | .27b | ||
| White | 263 (77.8) | 127 (82.5) | |
| Black | 56 (16.6) | 17 (11.0) | |
| Other/unknown | 19 (5.6) | 10 (6.5) | |
| Taxane as adjuvant therapy, No. (%) | .26b | ||
| Yes | 159 (47.0) | 64 (41.6) | |
| No | 179 (53.0) | 90 (58.4) | |
| Visceral metastases, No. (%) | .23b | ||
| Yes | 268 (79.3) | 115 (74.7) | |
| No | 69 (20.5) | 39 (25.3) | |
| Missing | 1 | 0 | |
| Disease-free interval, No. (%) | .63b | ||
| 0 (de novo) | 38 (11.2) | 14 (9.1) | |
| <1 y | 68 (20.1) | 38 (24.7) | |
| 1 y < disease-free interval < 2 y | 36 (10.7) | 14 (9.1) | |
| >2 y | 196 (58.0) | 88 (57.1) | |
| Clinical stage, No. (%) | .80b | ||
| Not IV | 27 (8.6) | 13 (9.4) | |
| IV | 286 (91.4) | 126 (90.6) | |
| Missing | 25 | 15 | |
| Tumor subtype, No. (%) | .57b | ||
| ER and/or PR positive | 254 (75.1) | 112 (72.7) | |
| ER and PR negative | 84 (24.9) | 42 (27.3) | |
| ECOG performance, No. (%) | <.001b | ||
| 0 | 187 (57.2) | 112 (75.7) | |
| 1 | 140 (42.8) | 36 (24.3) | |
| Missing | 11 | 6 | |
Two-sided Wilcoxon rank sum test or Kruskal-Wallis test. ECOG = Eastern Cooperative Oncology Group; ER = estrogen receptor; MET = metabolic equivalents of task; PR = progesterone receptor.
Two-sided χ2 test.
Baseline PA was statistically significantly associated with performance status (Table 2). Fewer participants who engaged in fewer than 9 MET hours per week of PA had a performance status of 0 compared with participants who engaged in more than 9 MET hours per week (57.2% vs 75.7%; P < .001). No statistically significant association was found between PA and race, age, tumor hormone receptor status, duration of disease-free interval between diagnosis and development of metastatic disease, or presence of visceral metastases.
Baseline PA and Cancer Outcomes
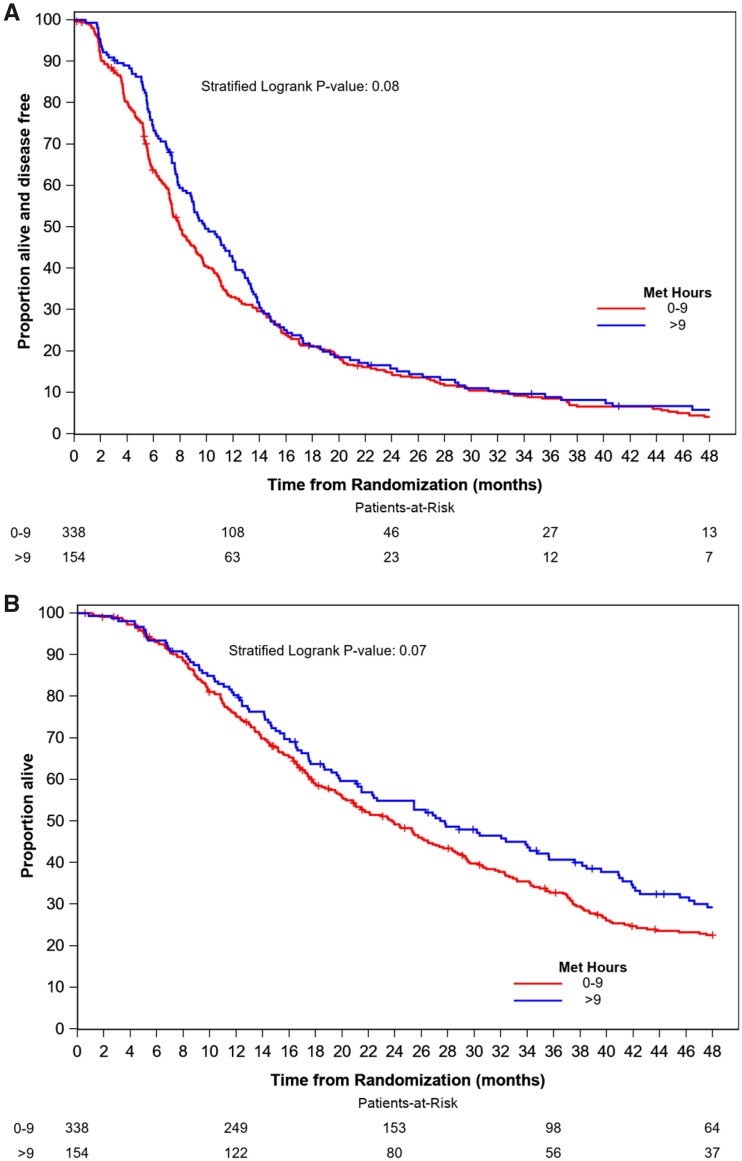
After 60 months of median follow-up, 467 PFS events and 392 deaths had occurred in the 492 participants for whom baseline PA was available. There was a marginally statistically significant increase in PFS (HR = 0.83, 95% CI = 0.79 to 1.02; P = .08) and OS (HR = 0.81, 95% CI = 0.65 to 1.02; P = .07) in participants who completed more than 9 MET hours per week of PA relative to those who completed 0-9 MET hours per week of PA (Figure 3). Median PFS and OS were 8.0 months (95% CI = 7.4 to 9.2) and 23.6 months (95% CI = 20.3 to 26.5), respectively, in participants completing 0-9 MET hours per week of PA and 9.8 months (95% CI = 8.8 to 12.0) and 27.8 months (95% CI = 21.5 to 35.6) in participants completing more than 9 MET hours per week.
Figure 3.
Kaplan-Meier plots of progression-free survival (A) and overall survival (B) by physical activity. Two-sided stratified log-rank test was used with stratification factors including treatment assignment, hormone receptor status, prior taxane, and use of bevacizumab. MET = metabolic equivalents of task.
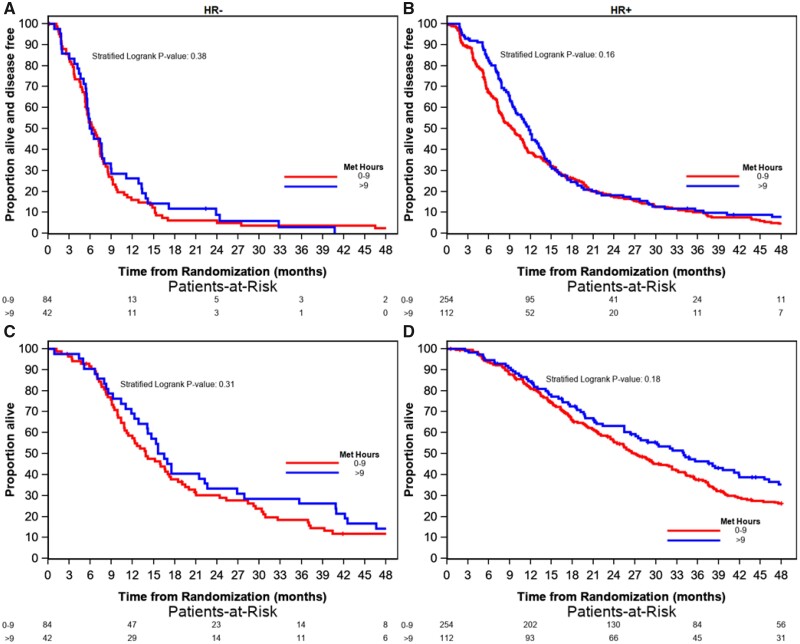
Among the 366 participants with hormone receptor–positive cancers, there was a trend between PA and longer PFS (HR = 0.83, 95% CI = 0.79 to 1.02; P = .16) and OS (HR = 0.81, 95% CI = 0.65 to 1.02; P = .18) for those who completed more than 9 MET hours per week of PA relative to those who completed 0-9 MET hours per week of PA (Figure 4). Median PFS and OS were 9.5 months (95% CI = 8.0 to 10.9) and 27.1 months (95% CI = 24.0 to 32.3), respectively, in participants who completed 0-9 MET hours per week of PA and 11.7 months (95% CI = 9.9 to 13.5) and 34.0 months (95% CI = 27.4 to 42.0) in participants who completed more than 9 MET hours per week of PA. Among the 126 participants with hormone receptor–negative cancers, no statistically significant association was seen between PA and PFS (P = .31) or OS (P = .38). Median PFS and OS were 6.6 months (95% CI = 5.6 to 7.4) and 13.9 months (95% CI = 11.4 to 18.5), respectively, in participants who completed 0-9 MET hours per week of PA and 5.9 months (95% CI = 5.5 to 8.9) and 15.6 months (95% CI = 14.1 to 22.6) in participants who completed more than 9 MET hours per week of PA.
Figure 4.
Kaplan-Meier plots of progression-free survival by physical activity for patients with hormone receptor–negative (a) and hormone receptor–positive disease (b) and overall survival by physical activity for patients with hormone receptor–negative (c) and hormone receptor–positive disease (D). Two-sided stratified log-rank tests were used with treatment assignment as the stratification factor. MET = metabolic equivalents of task.
BMI, PA, and Treatment Toxicity
Toxicity information by treatment arm was available for 792 patients with baseline BMI data and 495 patients with baseline PA data (Table 3). The majority of patients experienced at least one grade 3 or greater hematologic or nonhematologic toxicity. There was no statistically significant difference by BMI in the proportion of patients who reported a grade 3 or higher nonhematologic toxicity in participants treated with paclitaxel (P = .99) or nab-paclitaxel (P = .70). However, among participants treated with ixabepilone, individuals who were overweight or had obesity were more likely to experience a grade 3 or greater nonhematologic toxicity than individuals of normal weight (P = .05). There were no statistically significant differences in rates of hematologic or nonhematologic toxicity by PA.
Table 3.
Proportion of participants with grade 3 or higher toxicity by body mass index and physical activity
| Treatment assignment and toxicity category | Body mass index | Physical activity (MET hours per week) | |||||
|---|---|---|---|---|---|---|---|
| (n = 792) |
(n = 495) |
||||||
| Normal | Overweight | Obese | P | 0-9 | >9 | P a | |
| Paclitaxel, No. | 74 | 79 | 129 | 115 | 64 | ||
| Hematologic, No. (%) | 19 (25.7) | 19 (24.1) | 26 (20.2) | .63 | 27 (23.5) | 13 (20.3) | .63 |
| Nonhematologic, No. (%) | 45 (60.8) | 49 (62.0) | 79 (61.2) | .99 | 73 (63.5) | 40 (62.5) | .90 |
| Nab-paclitaxel, No. | 71 | 93 | 104 | 120 | 50 | ||
| Hematologic, No. (%) | 32 (45.1) | 52 (55.9) | 62 (59.6) | .16 | 69 (57.5) | 28 (56.0) | .86 |
| Nonhematologic, No. (%) | 49 (69.0) | 63 (67.7) | 76 (73.1) | .70 | 87 (72.5) | 34 (68.0) | .56 |
| Ixabepilone, No. | 64 | 76 | 102 | 106 | 40 | ||
| Hematologic, No. (%) | 8 (12.5) | 6 (7.9) | 14 (13.7) | .47 | 12 (11.3) | 5 (12.5) | .84 |
| Nonhematologic, No. (%) | 33 (51.6) | 51 (67.1) | 71 (69.6) | .05 | 65 (61.3) | 23 (57.5) | .67 |
Two-sided χ2 test. MET = metabolic equivalents of task.
Given that chemotherapy-induced peripheral neuropathy is the most prevalent nonhematologic toxicity associated with taxane treatment, the relationship between BMI and PA and the incidence of grade 2 or greater neurotoxicity was explored. Among participants treated with ixabepilone, the incidence of grade 2 or greater neurotoxicity differed with respect to PA, with 67.5% of individuals completing more than 9 MET hours per week experiencing neuropathy vs 43.4% in individuals reporting 0-9 MET hours per week (P = .009). There were no statistically significant differences in rates of neurotoxicity by BMI.
Discussion
In the largest study to date evaluating the relationship between BMI and recreational PA to cancer outcomes in individuals with MBC, there was no statistically significant relationship between either BMI or recreational PA and PFS or OS. Although OS was marginally longer in participants who engaged in higher levels of PA, especially in those with hormone receptor–positive cancers, these relationships were not statistically significant. Notably, in this cohort of individuals initiating first-line chemotherapy for MBC, the prevalence of obesity and inactivity were high, with 42.6% of participants classified as obese and 47.6% engaging in less than an hour of recreational moderate-intensity PA each week. Although the majority of study participants experienced chemotherapy toxicity, BMI and PA were generally not associated with treatment toxicity, with the exception of higher rates of toxicity in participants with obesity who were treated with ixabepilone and higher rates of neurotoxicity in participants treated with ixabepilone who engaged in greater amounts of PA.
Our data regarding the lack of relationship between BMI and PFS and OS are consistent with a pooled analysis looking at the relationship between BMI and cancer outcomes in 489 Italian patients with MBC participating in 3 clinical trials of first-line anthracycline and/or taxane-based chemotherapy (12). In that analysis, the median PFS was 10.9 months in normal weight individuals, 13.0 months in overweight individuals, and 12.2 months in individuals with obesity (P = .17). Median OS was 32.0 months in normal weight individuals, 33.2 months in overweight individuals, and 30.7 months in individuals with obesity (P = .60).
Other reports have evaluated the relationship between BMI and cancer outcomes in individuals with MBC with mixed results (8–11). In patients initiating first-line HER2–directed therapy for MBC, 2 studies did not demonstrate any relationship between BMI and PFS (8,9), but 1 study did find a relationship between obesity and higher risk of overall mortality (HR = 1.29, 95% CI = 1.09 to 1.52; P = .003) in women treated with pertuzumab with or without ado-trastuzumab emtansine (8). Similarly, in patients with MBC and brain metastases, 1 study of 84 individuals with newly diagnosed brain metastases treated with radiation demonstrated a statistically significant shorter OS in patients with a BMI of 25 kg/m2 or more vs less than 25 kg/m2 (13.7 months vs 30.6 months; P < .001) (10), and a second study of 228 individuals with MBC and brain metastases did not find a statistically significant relationship between BMI and OS (10,11). More work is needed to understand the relationship between BMI and outcomes in individuals with advanced cancer in these populations, but our work and the pooled Italian analyses suggest that there is unlikely to be a statistically significant relationship between BMI and outcomes in the broader population of individuals with MBC.
There is less information regarding PA patterns, and the relationship between PA and outcomes, in individuals with MBC. Our study suggested that less than one-third of study participants engaged in recommended levels of weekly recreational PA, and 47.6% engaged in little or no regular PA at study entry. Notably, we found that individuals who reported more PA were more likely to have better performance status. Performance status has been shown to be a predictor of PFS and OS in cancer patients (18–20), and individuals with better performance status are likely to have greater capacity to exercise. However, exercise interventions have been shown to improve functional status in cancer patients (21–23), including patients with advanced disease (24,25), making it difficult to determine the independent effects of PA and performance status on PFS and OS. Randomized trials assessing the impact of exercise interventions on performance status and treatment outcomes in individuals with MBC will be needed to untangle the complex relationship between PA and performance status in this patient population.
Although our data were collected in the setting of a clinical trial, where treatments were standardized and collection of PFS and OS outcome data was robust, a number of limitations of our study should be acknowledged. First, our analyses were based on a single measure of BMI and PA at the time of study entry, and thus, we are not able to evaluate the relationship between weight change or PA performed during trial participation and cancer outcomes. Second, our sample size for PA analyses was relatively small, given that collection of these data was initiated after the trial was underway. Additionally, although these analyses were preplanned and were adjusted for other known factors that could impact outcomes such as performance status and patient age, analyses were not adjusted for multiple comparisons. This is particularly relevant in the exploratory analyses looking at the relationship between BMI and PA and toxicity, given the relatively small sample size and number of comparisons.
In conclusion, BMI and levels of recreational PA at the time of treatment initiation were not statistically significant prognostic factors in a group of individuals with MBC initiating first-line chemotherapy. The lack of a statistically significant relationship between BMI and outcomes in MBC was consistent with other reports of patients initiating chemotherapy in the setting of advanced disease. There was some suggestion of longer OS in individuals who engaged in higher levels of activity, especially in those with hormone receptor–positive cancer. More information about the relationship between PA and outcomes in individuals with advanced disease is needed, especially given the high rates of inactivity and the potential benefits of PA in terms of other health outcomes in individuals with MBC.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), UG1CA233180, UG1CA233253, UG1CA233339, UG1CA233373, and U10CA180820 (ECOG-ACRIN); https://acknowledgments.alliancefound.org. Also supported in part by funds from Abraxis BioScience (Celgene). Bevacizumab was provided by Genentech under agreement with the National Cancer Institute.
Notes
Role of the funder: The funder had no role in the conception, design, or implementation of this project, analysis of the data, or preparation of the manuscript.
Disclosures: Hope S. Rugo reports research support (paid to institution) from Pfizer, Merck, Novartis, Lilly, Genentech, OBI, Odonate, Daiichi, Eisai, Seattle Genetics, Macrogenics, Immunomedics, and Sermonix; and consulting for Puma and Samsung. Debra L. Toppmeyer reports that her spouse is employed at Merck in the CV division in med affairs. Carey Anders reports research funding from Puma, Lilly, Merck, Seattle Genetics, Nektar, Tesaro, G1-Therapeutics, ZION, Novartis, and Pfizer; compensated consultant roles at Genentech (1/2019-), Eisai (1/2019-), IPSEN (2/2019 -), Seattle Genetics (11/15/2019-11/15/2020); Astra Zeneca (3/2020-6/2020), Novartis (5/2020-5/2022), Immunomedics (10/1/2020-9/22/2021), and Elucida (9/2020); and royalties from UpToDate and Jones and Bartlett. Cynthia Ma reports funding from Puma and Pfizer and consulting fees from AstraZeneca, Lilly, Athenex, and Seattle Genetics. All other authors report no conflicts of interest.
Author contributions: Conceptualization- JL. Data curation- LH, VS. Formal analysis- LH, VS. Investigation- JL. Methodology- JL, LH, WTB, VS. Writing—original draft- JL, LH, VS. Writing—review & editing- all authors
Acknowledgements: We would like to thank the patients who participated in CALGB 40502 as well as all the CALGB–NCCTG–Alliance, ECOG-ACRIN, and SWOG investigators and research coordinators for their efforts on behalf of the patients and protocol.
Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Prior Presentations: This work was presented in part as a poster at the San Antonio Breast Cancer Symposium in 2017.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Protani M, Coory M, Martin JH.. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123(3):627–635. [DOI] [PubMed] [Google Scholar]
- 2. Chlebowski R, Aiello E, McTiernan A.. Weight loss in breast cancer patient management. J Clin Oncol. 2002;20(4):1128–1143. [DOI] [PubMed] [Google Scholar]
- 3. Goodwin P. Energy balance and cancer prognosis: breast cancer. In: McTiernan A, ed. Cancer Prevention and Management through Exercise and Weight Control. Boca Raton, FL: Taylor and Francis Group; 2006:405–435. [Google Scholar]
- 4. Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Niraula S, Ocana A, Ennis M, Goodwin PJ.. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat. 2012;134(2):769–781. [DOI] [PubMed] [Google Scholar]
- 6. Fontanella C, Lederer B, Gade S, et al. Impact of body mass index on neoadjuvant treatment outcome: a pooled analysis of eight prospective neoadjuvant breast cancer trials. Breast Cancer Res Treat. 2015;150(1):127–139. [DOI] [PubMed] [Google Scholar]
- 7. Friedenreich CM, Stone CR, Cheung WY, Hayes SC.. Physical activity and mortality in cancer survivors: a systematic review and meta-analysis. JNCI Cancer Spectr. 2020;4(1):pkz080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martel S, Poletto E, Ferreira AR, et al. Impact of body mass index on the clinical outcomes of patients with HER2-positive metastatic breast cancer. Breast. 2018;37:142–147. [DOI] [PubMed] [Google Scholar]
- 9. Krasniqi E, Pizzuti L, Barchiesi G, et al. Impact of BMI on HER2+ metastatic breast cancer patients treated with pertuzumab and/or trastuzumab emtansine. Real-world evidence. J Cell Physiol. 2020;235(11):7900–7910. [DOI] [PubMed] [Google Scholar]
- 10. Cacho-Diaz B, Spinola-Marono H, Reynoso N, Gonzalez-Aguilar A, Mohar-Betancourt A.. Role of overweight, obesity, and comorbidities in the prognosis of patients with breast cancer with brain metastases. Clin Breast Cancer. 2019;19(2):e394–e398. [DOI] [PubMed] [Google Scholar]
- 11. McCall NS, Simone BA, Mehta M, et al. Onco-metabolism: defining the prognostic significance of obesity and diabetes in women with brain metastases from breast cancer. Breast Cancer Res Treat. 2018;172(1):221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gennari A, Nanni O, Puntoni M, et al. Body mass index and prognosis of metastatic breast cancer patients receiving first-line chemotherapy. Cancer Epidemiol Biomarkers Prev. 2013;22(10):1862–1867. [DOI] [PubMed] [Google Scholar]
- 13. Rugo HS, Barry WT, Moreno-Aspitia A, et al. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound nab-paclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N063H (Alliance). J Clin Oncol. 2015;33(21):2361–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clinicaltrials.org. Paclitaxel, nab-paclitaxel, or ixabepilone with or without bevacizumab in treating patients with stage IIIc or stage IV breast cancer. https://clinicaltrials.gov/ct2/show/NCT00785291. Accessed July 27, 2020.
- 15. Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. [DOI] [PubMed] [Google Scholar]
- 16. Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274. [DOI] [PubMed] [Google Scholar]
- 17. U.S. Department of Health and Human Services and U.S. Department of Agriculture. Appendix 1: physical activity guidelines for Americans. In: 2015-2020 Dietary Guidelines for Americans. 8th Edition; 2015. https://health.gov/our-work/food-and-nutrition/2015-2020-dietary-guidelines/.
- 18. Li T, Zhu YH, Zhang J, et al. Objective response of first-line chemotherapy of triple-negative breast cancer translates into survival benefit: an analysis in an independent, prospective clinical trial and a real-world setting. Neoplasma. 2021;67(06):1400–1408. [DOI] [PubMed] [Google Scholar]
- 19. Hopkins AM, Rowland A, Logan JM, Sorich MJ.. Primary predictors of survival outcomes for HER2-positive advanced breast cancer patients initiating ado-trastuzumab emtansine. Breast. 2019;46:90–94. [DOI] [PubMed] [Google Scholar]
- 20. Edwards BJ, Zhang X, Sun M, et al. Overall survival in older patients with cancer. BMJ Support Palliat Care. 2020;10(1):25–35. [DOI] [PubMed] [Google Scholar]
- 21. Montagnese C, Porciello G, Vitale S, et al. Quality of life in women diagnosed with breast cancer after a 12-month treatment of lifestyle modifications. Nutrients. 2020;13(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arrieta H, Astrugue C, Regueme S, et al. Effects of a physical activity programme to prevent physical performance decline in onco-geriatric patients: a randomized multicentre trial. J Cachexia Sarcopenia Muscle. 2019;10(2):287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leclerc AF, Foidart-Dessalle M, Tomasella M, et al. Multidisciplinary rehabilitation program after breast cancer: benefits on physical function, anthropometry and quality of life. Eur J Phys Rehabil Med. 2017;53(5):633–642. [DOI] [PubMed] [Google Scholar]
- 24. Headley JA, Ownby KK, John LD.. The effect of seated exercise on fatigue and quality of life in women with advanced breast cancer. Oncol Nurs Forum. 2004;31(5):977–983. [DOI] [PubMed] [Google Scholar]
- 25. Cormie P, Galvao DA, Spry N, Joseph D, Taaffe DR, Newton RU.. Functional benefits are sustained after a program of supervised resistance exercise in cancer patients with bone metastases: longitudinal results of a pilot study. Support Care Cancer. 2014;22(6):1537–1548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.