Abstract
Objectives
In March 2020, the World Health Organization declared the coronavirus disease 2019 (COVID-19) a pandemic. In absence of official recommendations, implementing daily multidisciplinary team (MDT) COVID-19 meetings was urgently needed. Our aim was to describe our initial institutional standard operating procedures for implementing these meetings, and their impact on daily practice.
Methods
All consecutive patients who were hospitalized in our institution due to COVID 19, from March 31 to April 15, 2020, were included. Criteria to be presented at MDT meetings were defined as a proven COVID-19 by PCR or strongly suspected on CT scan, requiring hospitalization and treatment not included in the standard of care. Three investigators identified the patients who met the predefined criteria and compared the treatment and outcomes of patients with predefined criteria that were presented during MDT meeting with those not presented during MDT meeting. COVID-19 MDT meeting implementation and adhesion were also assessed by a hospital medical staff survey.
Results
In all, 318 patients with confirmed or suspected COVID-19 were examined in our hospital. Of these, 230 (87%) were hospitalized in a COVID-19 unit, 91 (40%) of whom met predefined MDT meeting criteria. Fifty (55%) patients were presented at a MDT meeting versus 41 (45%) were not. Complementary exploration and inclusion in the CorImmuno cohort were higher in MDT meeting group (respectively 35 vs. 15%, P = 0.03 and 80 versus 49%, P = 0.0007). Prescription of hydrocortisone hemisuccinate was higher in group of patients not presented during MDT meeting (24 vs. 51%, P = 0.007). Almost half of the patients fulfilling the inclusion criteria were not presented at MDT meeting, which can be partly explained by technical software issues.
Conclusions
Multidisciplinary COVID-19 meetings helped implementing a single standard of care, avoided using treatments that were untested or currently being tested, and facilitated the inclusion of patients in prospective cohorts and therapeutic trials.
Keywords: COVID-19, Multidisciplinary meetings, Standard of care, Prospective cohorts, Therapeutic trials
1. Introduction
On March 11, 2020, the World Health Organization (WHO) declared the outbreak of severe acute respiratory coronavirus 2 (SARS-CoV-2) a global pandemic. SARS-CoV-2 is responsible for the coronavirus disease 2019 (COVID-19), leading to death in 2.5% of confirmed cases and 10.8% of hospitalized patients in France [1].
In the midst of the pandemic, scientists attempted to better understand COVID-19 pathophysiology in order to develop an effective treatment. However, despite multiple emerging therapies and numerous daily publications, there were not yet any strong international treatment recommendations. Recently, studies revealed that dexamethasone resulted in lower 28-day mortality among patients receiving either invasive mechanical ventilation or oxygen alone [2], that remdesivir was superior to placebo in shortening the recovery time in COVID-19 hospitalized adults [3], and that tocilizumab likely reduced the risk of mechanical ventilation or death by day 14 [4]. In our university hospital, located in Paris, France, several departments were involved in COVID-19 management, including the departments of infectious diseases, virology, pulmonology, internal medicine, nephrology, pharmacy and intensive care.
Due to the lack of official recommendations, an urgent need for implementing daily multidisciplinary COVID-19 meetings was unanimously felt during the first wave. These meetings were aimed to investigate the various disease phenotypes, propose a standard of care (SOC) for COVID-19, and collectively decide on the best possible treatment for each patient based on the available evidence.
Our aim was to describe our initial institutional SOC for the first-wave COVID-19 management, as well as the implementation of a multidisciplinary team (MDT) COVID-19 meeting and its preliminary impact on our daily practice.
2. Methods
2.1. MDT meeting criteria
The setting-up of a MDT meeting in France relies on the recommendations from both the Plan Cancer 2003–2007 and Haute Autorité de Santé française, as defined by the Article D. 6124-131 of the French Public Health Code. These recommendations are similar to many others, as recently described in a systematic review [5].
We have reviewed specifications of MDT meeting: (1) relevance for the institution; (2) affiliation to our institution; (3) name of the meeting; (4) professionals concerned by the meeting; (5) meeting coordinator and (6) medical conclusions (or consensual decision).
The meeting operating procedures were also checked as for the following: (1) use of a secure software; (2) name of the referring physician; (3) date and time of the meetings established at specific time intervals; (4) selection criteria of the patients presented; (5) mandatory items for the file's content; (6) traceability of the questions asked with the answers provided; (7) justified inclusion in therapeutic trials, and justified use of a treatment different to the one recommended by the MDT meeting.
We evaluated the compliance of the MDT meetings with predefined quality criteria and their impact on therapeutic management, to amend the operating procedures as necessary and further improve the quality of subsequent MDT meetings. Data analysis was restricted to the first 15 days because they correspond to the epidemic peak and allowed analysing the data and quickly validating the method.
2.2. Overall population and MDT meeting study population
Overall population was identified by two databases: patients with a positive SARS CoV2 polymerase chain reaction (PCR), and patients with a CT-scan defined as suspected of COVID 19 by radiologists. We included all consecutive patients who were hospitalized in our institution due to COVID-19, during the two first weeks of implementation of the MDT meetings, i.e. between March 31 and April 15, 2020.
MDT study population was patients among the overall population who required a hospitalization but not in ICU and a treatment not included in the standard of care. Three of us (AC, PC and CRD) identified and adjudicated the patients who met the predefined criteria to be presented at MDT meetings. Finally, MDT study population was divided in two groups, patients who were and those who were not presented at a MDT meeting.
2.3. Data collection
Data collections were: demographic data, medical history, clinical and biological data at presentation. Need for oxygen, complementary exploration and treatment during hospitalization were collected. Days 14 and 28 status were also reported.
2.4. Statistical analysis
Statistical analysis was performed using GraphPad® (San Diego, CA, USA). Quantitative data were reported as median (interquartile range [IQR]) and categorical data as number of events (percentages). Differences between groups were assessed with the Mann-Whitney test for quantitative variables. Differences between groups were assessed using the chi-squared test for categorical variables or Fisher's exact test if the expected value was lower than 5.
This work was approved by the “Comité d’évaluation des protocoles de recherche (CEPRO)” from the “Société de Pneumologie de Langue Française (SPLF)” on August 26, 2020.
3. Results
3.1. Institutional SOC for COVID-19 management before MDT meeting implementation
At the beginning of the COVID-19 epidemic, the initial SOC in our conventional unit was based on evidence for treating hypoxemic bilateral pneumonia: (i) dual intravenous antibiotherapy [6]; (ii) oxygenotherapy; (iii) venous thromboembolism prevention [7]. The antibiotherapy combined a third-generation cephalosporin with a macrolide or, in case of potential drug-drug interactions, with a quinolone as proposed in the ATS/IDSA guidelines [8]. The macrolide used was azithromycin, for his anti-infective property (intracellular germ target) and potential antiviral/antiinflammatory effects (“cytokine storm” in COVID-19 pneumonia) [8].
In case of respiratory worsening evolution, an alternative diagnosis to COVID-19 aggravation was searched for, such as pulmonary embolism, pulmonary oedema, or bacterial superinfection. Due to the lack of randomized controlled trials, it was decided to assess disease evolution and discuss additional treatments on a case-by-case scenario, including alternative treatments alone rather than in association, such as oral hydroxychloroquine, oral lopinavir/ritonavir, intravenous hydrocortisone hemisuccinate, or methylprednisolone. Each department decided on their own which drug was likely to be the most appropriate.
3.2. Urgent need for a COVID-19 MDT meeting
In the COVID-19 setting and pending on the results of ongoing and future therapeutic trials, some clinicians wished to prescribe certain medications on a “compassionate” basis. However, such prescriptions can only be deemed acceptable when, according to the practitioner, there are sufficient scientific data to support these treatments’ ability to improve or stabilise the patient's clinical state. For this purpose, our Pharmacy department edited a drug prescription form including reminders of dosage, duration and contra indication for the treatments prescribed off label. The patient was orally informed and gave his/her consent for this prescription, both of which were documented in the patient file.
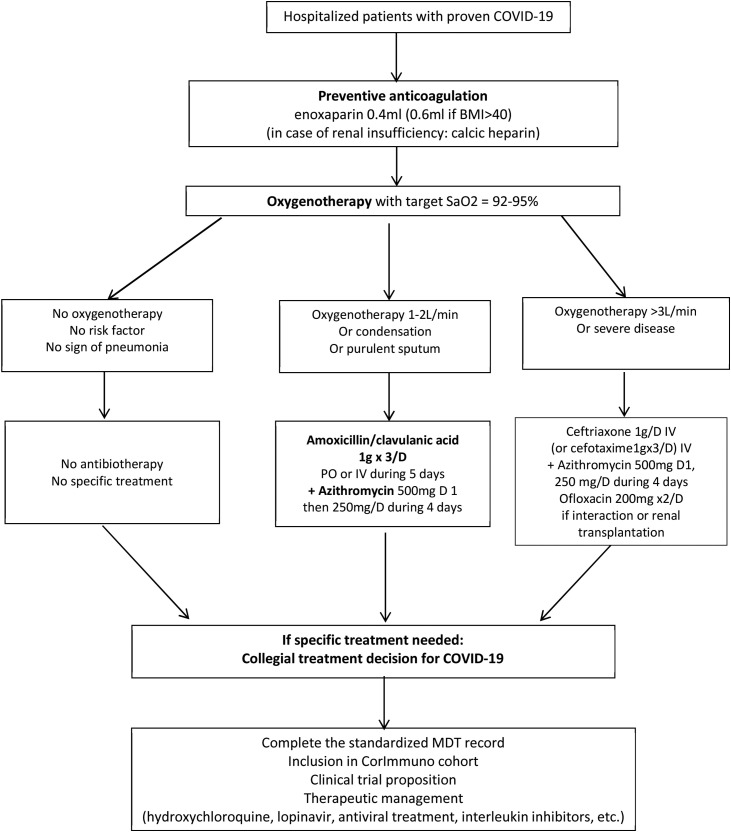
In addition, our institution requested that such prescriptions be made only following a collective assessment that was to be validated by a “compassionate treatment committee.” Given the lack of official recommendations, the medical hospital community decided on March 31, 2020 to create and implement institutional COVID-19 MDT meetings (Fig. 1 ).
Fig. 1.
Local standardised recommendations of the COVID-19 multidisciplinary meeting. COVID-19: coronavirus disease 19; MDT: multidisciplinary team; SaO2: arterial oxygen saturation; BMI: body mass index.
3.3. Daily COVID-19 MDT meeting's operating procedures
MDT meeting characteristics are listed in Table 1 . Due to the number of daily hospitalized patients and the rapid change in their clinical condition, we decided to set up daily afternoon meetings using videoconferencing with the institutional secured software Lync® for social distancing. The institutional software single patient file was employed, enabling us to create a standardized file as shown in Table 2 , following discussions among the experts of each COVID-19-involved specialty.
Table 1.
Characteristics of the MDT meetings’ operating procedures.
| Name | COVID-19 MDT meeting |
|---|---|
| Coordinator | Five COVID-19 expert members, from different specialties, for each of the five working days: two pneumology, one internal medicine, one infectious disease, and one ICU |
| Quorum | Five experts of the main specialty departments (pneumology, ICU, infectious disease, internal medicine) were required to ensure the quorum of the MDT meeting. As thoracic CT-scan suggestive features were essential for COVID-19 diagnosis, the presence of a thoracic radiologist was deemed essential. Because of potential drug-drug interactions, as well as the strain on medications, a pharmacist was also required. In addition, a virologist should also be present to discuss the virologic results |
| Name of referring physician | For follow-up on the decision taken, including explanation to the patient and subsequent organization of care |
| Patients to be presented | With the exception of ICU patients, all COVID-19 patients (confirmed by a positive PCR or a thoracic CT-scan suggestive of COVID-19) who required a therapeutic escalation or inclusion in a clinical trial (defined in Fig. 1) were recommended to be presented All available therapeutic options were similarly discussed |
COVID-19: coronavirus disease 19; MDT: multi-disciplinary team; ICU: intensive care unit; CT: computed tomography; PCR: polymerase chain reaction.
Table 2.
Standardized form for patient presentation at COVID-19 MDT meeting.
| Item | Answer |
|---|---|
| Aggravating factors of COVID-19 | |
| Hypertension | |
| Smoking habits | |
| Diabetes | |
| Comorbidities impacting treatment | |
| Day of first COVID-19 symptoms | |
| Day of first PCR SARS-CoV2 + | |
| Pulmonary involvement | |
| Pulse saturation in oxygen | |
| Need for oxygen support (L/min); high flow | |
| Thoracic CT-scan pattern | |
| WHO scale of COVID-19 used in CORIMUNO trials (4: hospitalized; 5:oxygen; 6: high-flow oxygen; 7: ICU) | |
| Extra-pulmonary involvement | |
| Creatinine level | |
| Potassium level | |
| Transaminase level | |
| Lymphocytes level | |
| Platelets level | |
| Macrophagic activation syndrome (yes/no) | |
| Disseminated intravascular coagulation (yes/no) | |
| Troponin/BNP levels | |
| CRP level | |
| Procalcitonin level | |
| ECG (QTc interval) | |
| Ongoing treatment | |
| Antibacterial | |
| Azithromycin | |
| Others | |
| Withold/withdraw ICU admission | |
| Question and proposition of the physician |
COVID-19: coronavirus disease 19; MDT: multidisciplinary team; PCR: polymerase chain reaction; CT: computed tomography; CRP: C-reactive protein; ICU: intensive care unit; WHO: World Health Organization; BNP: brain natriuretic peptide; ECG: electrocardiogram.
3.4. Report of the first 15 days of COVID-19 MDT meetings
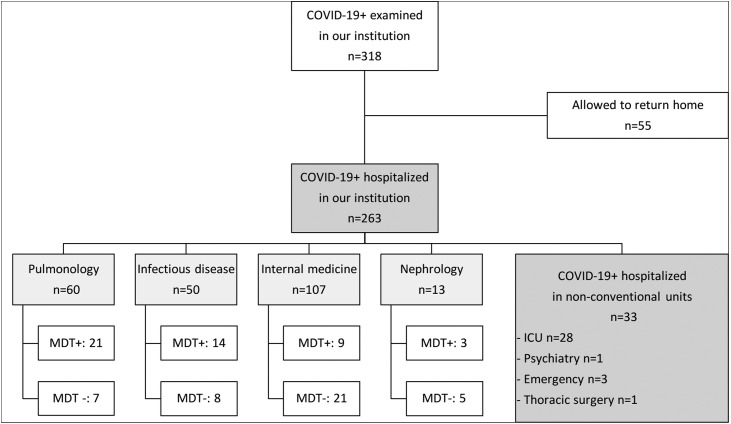
From March 31 to April 15, 2020, 318 patients with COVID-19 symptoms and either a positive PCR for the SARS-CoV-2 or a COVID-19-compatible CT-scan were examined in our institution. Among these 318 patients, 263 (83%) needed hospitalization, and the 55 others were discharged. Among these 263 patients, 230 (87%) required conventional hospitalization in one of the four COVID-19 units (pulmonology, infectious disease, internal medicine, and nephrology), 28 (10.6%) were admitted to the intensive care unit (ICU), three were hospitalized in the emergency department, and one in the psychiatry and thoracic surgery departments, respectively (Fig. 2 ). Among the 230 patients hospitalized in conventional unit, 91 (40%) were retrospectively analysed as fulfilling the MDT meeting criteria, 50 (55%) of whom were presented, whereas the remaining 41 (45%) were not (Fig. 2).
Fig. 2.
Flow chart of COVID-19 patients between March 31 and April 15, 2020. MDT+: Patients fulfilling the MDT meeting criteria that were presented in MDT meeting. MDT-: Patients fulfilling the MDT meeting criteria that were not presented in MDT meeting. COVID-19+: Positive SARS-CoV-2 PCR and/or compatible thoracic CT-scan; COVID-19: coronavirus disease 19; MDT: multidisciplinary team; ICU: intensive care unit.
Fig. 2 illustrates the number of patients fulfilling the MDT meeting criteria and the number of patients discussed during COVID-19 MDT meetings for each of the four COVID-19 units. Among the patients fulfilling the criteria, the proportion of patients actually discussed during COVID-19 MDT meetings was 75% for the pulmonology unit, 64% for the infectious disease unit, 38% for the nephrology unit, and 31% for the internal medicine unit (Table 3 , Fig. 2). Among the patients hospitalized in internal medicine, 16 (57%) were in a specific geriatric unit and 13/16 (81%) were not presented at the MDT meeting. Three ICU patients were presented during a MDT meeting (data not shown).
Table 3.
Baseline patient characteristics.
| MDT meeting (n = 50) | No MDT meeting (n = 41) | P | |
|---|---|---|---|
| Age, years | 66 [53–73] | 70 [57–82] | 0.07 |
| Gender male, n (%) | 36 (72%) | 27 (66%) | 0.65 |
| COVID-19 units, n | |||
| Pulmonology | 21 (75%) | 7 (25%) | 0.0004 |
| Infectious disease | 14 (64%) | 8 (36%) | 0.13 |
| Non-geriatric internal medicine | 6 (43%) | 8 (57%) | 0.71 |
| Geriatric internal medicine | 3 (19%) | 13 (81%) | 0.001 |
| Nephrology | 3 (38%) | 5 (62%) | 0.62 |
| Tobacco status | |||
| Current | 2 (4%) | 3 (8%) | 0.65 |
| Former | 12 (24%) | 8 (19%) | 0.79 |
| Never | 36 (72%) | 30 (73%) | 0.9 |
| Comorbidities | |||
| Diabetes | 19 (38%) | 15 (37%) | 0.89 |
| Arterial hypertension | 25 (50%) | 23 (56%) | 0.56 |
| Other cardiovascular disease | 13 (26%) | 8 (19%) | 0.55 |
| Obesity (BMI > 30) | 17 (34%) | 13 (32%) | 0.81 |
| COPD | 2 (4%) | 1 (2%) | 1 |
| Renal failure chronic | 6 (12%) | 11 (27%) | 0.11 |
| Cancer | 4 (8%) | 2 (5%) | 0.68 |
| Home treatment | |||
| ACE inhibitors/ARBs | 12 (24%) | 15 (36%) | 0.19 |
| Inhaled or oral corticosteroids | 3 (6%) | 4 (10%) | 0.69 |
| Anti-tumoral treatment | 2 (4%) | 0 | 0.49 |
| Days between symptoms and hospitalization | 6 [5–8] | 8 [6–11] | 0.02 |
| Diagnosis | |||
| PCR | 43 (86%) | 40 (97%) | 0.05 |
| CT-scan | 49 (98%) | 38 (93%) | 0.22 |
| Pulse saturation in oxygen, % | 95 [94–96] | 94 [90–96] | 0.08 |
| Worst WHO class during the first 14 days | |||
| Class 4 | 4 (8%) | 2 (5%) | 0.68 |
| Class 5 | 40 (80%) | 36 (88%) | 0.32 |
| Need for oxygen, l.min−1 | 4 [2–6] | 6 [4–9] | 0.008 |
| Class 6 | 2 (4%) | 3 (7%) | 0.65 |
| Class 7 to 9 | 4 (8%) | 0 (0%) | 0.12 |
| Extra-pulmonary involvement | |||
| Lymphocytes, 109.l−1 | 0.81 [0.54-1.11] | 0.79 [0.55-1.04] | 0.91 |
| CRP, mg.l−1 | 154 [75–222] | 153 [67–201] | 0.36 |
| Procalcitonin, μg.l−1 | 0,17 [0.08-1.66] | 0.25 [0.12-1.10] | 0.18 |
| Ongoing treatment | |||
| Antibacterial | 49 (98%) | 41 (100%) | 0.36 |
| Azithromycin | 38 (76%) | 33 (80%) | 0.61 |
| Anticoagulation | 50 (100%) | 41 (100%) | 1 |
Continuous data are reported as median [interquartile range] and categorical data as number of events (percentages). COVID-19: coronavirus disease 19; MDT: multidisciplinary team; BMI: body mass index; COPD: chronic obstructive pulmonary disease; ACE: angiotensin converting enzyme; ARBs: angiotensin II receptor blockers; PCR: polymerase chain reaction; CT: computed tomography; CRP: C-reactive protein; WHO: World Health Organization.
3.5. Baseline patient characteristics
The baseline characteristics of the 91 patients fulfilling the MDT meeting criteria are listed in Table 3. The patients who were presented during MDT meeting were likely to be older than those not presented, the between-group difference being not statistically significant. Furthermore, among the patients hospitalized in geriatric internal medicine unit, there were significantly more patients who were not presented during a MDT meeting.
3.6. Treatment and issues
Table 4 describes the treatment and issues pertaining to the 91 patients fulfilling the MDT meeting. There were significantly more additional investigations, mostly thoracic CT-scans for pulmonary embolism, in patients presented during MDT meeting than in those not presented (P = 0.003). The number of patients included in the CORIMUNO cohort [4] was significantly higher among patients presented during MDT meeting as compared with those not presented (P = 0.0007). Patients not presented in MDT meetings received more frequently corticosteroids (51 versus 24%; P = 0.007) and referring physicians less frequently defined their resuscitation status (37 versus 14%; P = 0.01). However, they were similarly included in described clinical trials (P = 0.54). On Days 14 and 28, no significant differences were observed in terms of hospital discharge, hospitalisation or alive status between patients presented during MDT meetings and those not presented.
Table 4.
Treatment and issues.
| MDT meeting (n = 50) | No MDT meeting (n = 41) | P | |
|---|---|---|---|
| Treatment, n (%) | |||
| Complementary exploration | 18 (35%) | 6 (15%) | 0.03 |
| 48-hours simple surveillance | 9 (18%) | 5 (12%) | 0.56 |
| Inclusion in clinical trial | 20 (40%) | 19 (46%) | 0.54 |
| Inclusion in the CorImuno cohort | 41 (80%) | 20 (49%) | 0.0007 |
| Modification in antibiotherapy | 6 (12%) | 1 (2%) | 0.12 |
| Hydrocortisone hemisuccinate | 12 (24%) | 21 (51%) | 0.007 |
| Corticotherapy by intravenous bolus | 2 (4%) | 2 (5%) | 1 |
| Non clinical trial use of tocilizumab | |||
| Lopinavir-ritonavir, or hydroxychloroquine | 5 (10%) | 0 (0%) | 0.06 |
| Drug sheet completed/compassional drug used | 4/5 | 0/0 | 0.12 |
| Resuscitation status | |||
| No | 9 (18%) | 11 (26%) | 0.31 |
| Unknown | 7 (14%) | 15 (37%) | 0.01 |
| Yes | 34 (68%) | 15 (37%) | 0.005 |
| Day 14 status, n (%) | |||
| Return home | 18 (36%) | 18 (44%) | 0.44 |
| Rehabilitation | 10 (20%) | 5 (12%) | 0.4 |
| Conventional hospitalization | 8 (16%) | 5 (12%) | 0.76 |
| Intensive care unit | 10 (20%) | 5 (12%) | 0.27 |
| Death | 4 (8%) | 8 (20%) | 0.13 |
| Day 28 status, n (%) | |||
| Return home | 32 (64%) | 26 (63%) | 0.98 |
| Rehabilitation | 5 (10%) | 1 (3%) | 0.17 |
| Conventional hospitalization | 3 (6%) | 3 (7%) | 0.81 |
| Intensive care unit | 4 (8%) | 3 (7%) | 0.91 |
| Death | 6 (12%) | 8 (20%) | 0.39 |
Continuous data are reported as median (interquartile range) and categorical data as number of events (percentages). MDT: multidisciplinary team.
3.7. Adhesion to and limitations of MDT meetings for caregivers
Adhesion to and limitations of MDT meetings for caregivers were evaluated in COVID-units (Table 5 ). Overall, 43 (84.3%) out of 51 non-ICU physicians involved in the COVID-19 units answered our survey, including 20 residents (46.5%), six fellows (13.9%), and 18 attending physicians (41.8%). Pulmonology and internal medicine were the most represented departments with 18 (41.8%) and 15 physicians (34.8%), respectively. All participants declared being aware of the MDT meetings, which helped them in their patient care (93.0%). MDT meetings were permitted more often by the titular physicians than by the residents (51.1% versus 13.9%). Creation of a MDT meeting file and access to the standardized COVID-19 MDT record were deemed easy for half of the residents and for over 75% of the titular physicians. The cases were presented by residents (n = 12, 27.9%), fellows, (n = 6, 13.9%), and attending physicians (n = 11, 25.5%), and particularly by pneumologists (n = 17), internal medicine specialists (n = 8), and infectious disease specialists (n = 3). MDT meetings also aimed to promote the clinical trials and help physician (>90%) to include their patients and to remain informed of the progress of the different trials (Table 5).
Table 5.
Survey to evaluate MDT meeting adherence and limitations for caregivers.
| n = 43 (86.2%) | |
|---|---|
| Grade | |
| Resident, n (%) | 20 (45.5%) |
| Fellow, n (%) | 6 (13.6%) |
| Attending, n (%) | 18 (40.9%) |
| Unit | |
| Pulmonology, n (%) | 18 (40.9%) |
| Infectious disease, n (%) | 7 (15.9%) |
| Internal medicine/geriatry, n (%) | 15 (34.1%) |
| Nephrology, n (%) | 3 (6.8%) |
| Aware of a special daily COVID-19 MDT meeting in Tenon hospital, n (%) | 44 (100%) |
| Easy access to the creation of a specific form, n (%) | 30 (68.2%) |
| Easy access to the link to participate remotely, n (%) | 28 (63.6%) |
| Easy access to the standard text, n (%) | 28 (63.6%) |
| One of your patients already been discussed, n (%) | 29 (65.9%) |
| Did this meeting help you in your care, yes, n (%) | 40 (90.9%) |
| Did this meeting help you include patients in clinical trials, yes, n (%) | 29 (65.9%) |
| Does this meeting allow you to learn about clinical trials, yes, n (%) | 38 (86.3%) |
4. Discussion
This study demonstrates the feasibility of rapidly implementing MDT meeting in the context of a pandemic. However, while caregivers recognize the value of MDT meetings, their attendance to such meetings was not optimal. Significant differences in the therapeutic management of patients with similar WHO Group clinical severities were observed, depending on whether their medical file was presented or not at a MDT meeting. Patients not presented at a MDT meeting more often received drugs that were not yet validated as part of a trial.
4.1. MDT meetings help define a SOC in the absence of recommendations
At the beginning of pandemic, physicians treated COVID-19 pneumonia as they would do it in case of severe community-acquired pneumonia [6], provided that the patients were likely immunocompetent and did not require ICU admission. Such treatment strategy was implemented in 92% of patients who were eligible for discussion at a MDT meeting. Arterial oxygen saturation was targeted to ≥ 94% and patients received venous thromboembolic prophylaxis, unless contraindicated. The favourite anti-infective treatment was combination of a beta-lactam with a macrolide, quinolones being reserved for patients with long QT syndrome or potential drug-drug interactions (26%). Physicians preferred macrolides over quinolones because COVID-19 pneumonia resembles atypical pneumonia [9]; most patients are non-smokers [10] and macrolides reduce inflammation [11]. Nevertheless, no therapeutic trials or cohort studies has yet demonstrated the macrolide benefits in this indication, so far [12]. In addition, many studies have reported a risk of cardiac toxicity [13], particularly when macrolides are combined with chloroquine or hydroxychloroquine [14], [15] and, especially, when they are used in hospitalized COVID-19 patients, many of whom display cardiovascular comorbidities [16].
Most teams currently favour discontinuing antibiotic treatment early if PCR testing is positive for COVID-19 pneumonia, and in the absence of evidence of bacterial co-infection, this last being less common in COVID-19 pneumonia (6%, [17]) than in influenza-associated pneumonia (20%, [18]). MDT meeting was not used to discuss treatment or ICU admission limitation, which have to be defined before as stipulated on the form for patient presentation at COVID-19 MDT meeting.
4.2. MDT meetings help reduce the use of non-evaluated therapeutic strategies
Daily COVID-19 MDT meetings were meant for diagnosis, therapeutic discussion, and as an exchange vector of great educational value among professionals, as it enables analysing the treatment's risk, benefit, and impact on the patient's quality of life. MDT meetings can be used in other specialties like non-oncologic services, especially for complex management issues [5]. In such cases, all disciplines that are essential for diagnosis and treatment must be represented.
Corticosteroids were the treatment most frequently administered in combination with antibiotics, with 62% of patients discussed at MDT meetings receiving them (data not shown). The rational for the use of corticosteroids was its anti-inflammatory effect in a context of cytokine storm that appeared secondary to the viral step in rapidly worsening patients. However, there were no data yet on using corticosteroids in COVID-19 patients. Concerning their use in severe acute respiratory syndrome coronavirus-1 and Middle East respiratory syndrome-related coronavirus, most publications did not recommend them [19], while their use in influenza cases still appeared conflicting [20], [21]. They were administered to patients who scored 5 on the WHO 10-point Clinical Progression Scale, who were receiving at least 6L/min of oxygen, or who deteriorated on 3L/min but who did require neither respiratory support (non-invasive ventilation or high flow oxygen) nor ICU admission. Corticosteroids were also deemed warranted if alveolar consolidation mimicking organizing pneumonia was found on chest CT-scan and if no evidence of bacterial superinfection was documented. The corticosteroid regimen was adapted from the one developed by the French GERMOP study group on rare lung diseases for organizing pneumonia [22]. The results of the British Recovery trial mean that dexamethasone is now widely considered as the standard of care for hypoxemic Covid-19 pneumonia [2].
We used hydroxychloroquine (4%) and lopinavir/ritonavir (10%) very little, owing to the scandal in France surrounding the first, while the results with the second have been fairly poor, and we were awaiting the Discovery trial to begin in our institution. The trial results with these two treatments have been broadly negative so far [23]. With no clear guidelines to follow, most patients were included in the prospective CORIMUNO cohort (80%), 42% of whom were randomized into the cohort-embedded CORIMUNO-TOCI 1 trial [4].
4.3. Patients presented to MDT meeting were not representative of the overall population treated in the institution
Although physicians strongly agreed that MDT meetings were useful (Table 5), nearly half of cases eligible for MDT discussion were not presented at meetings for discussing the use of additional treatments besides antibiotics, which particularly applied to internists (31%) that were also those who had the poorer attendance. This can be partly accounted for by that several participants experienced technical issues while trying to follow meetings on Webex. In contrast, pulmonologists showed good attendance (75%) maybe since the meetings were held in the pulmonology department building.
While being able to rely on the MDT meetings provided physicians with reassurance, the meetings were not appropriate to every situation encountered across the departments. Particularly for acute geriatric patients that were older, had more comorbidities, have higher need for oxygen therapy and with limitation for ICU admission. As in all diseases, this demonstrates once more why specific guidelines on diagnosing and treating the geriatric population are urgently needed [24].
The MDT meetings seem less appropriate for intensive care patients and very few were indeed presented. There are several reasons for this: (1) patients were presented during ICU daily meeting; (2) some ICU patients were referred from other hospitals, with their therapeutic strategy discussed before their venue in our institution; (3) during the COVID-19 epidemic peak, some patients from our region were transferred from the ICU to another institution, including those receiving mechanical ventilation in attempt to offer bed admission for outside patients.
To conclude, the sudden arrival of the global COVID-19 pandemic coupled with the discovery of a novel disease made imperative to set up MDT meetings that respected social distancing. Nevertheless, the urgency of the situation did not prevent us from implementing strict MDT best practices. However, this study highlighted that the patients presented at MDT meetings were not representative of the entire population treated in our institution, particularly the elderly and critically ill patients who probably should have specific MTD meetings. These meetings helped us to: (i) implement a single standard of care within our institution; (ii) avoid using treatments that are untested or only just being tested outside a therapeutic trial; (iii) facilitate the inclusion of patients in prospective cohorts and therapeutic trials; iv) share our feelings and experiences among colleagues. Furthermore, these meetings have contributed to collectively disseminate evolving knowledge in the pathophysiology and treatments of the COVID-19 in the face of this unprecedented health crisis. Finally, coming together to face decisions and the difficulty of certain end-of-life situations was also a qualitative benefit on a human level, although not quantifiable by this study.
Author contributions
All authors have approved the manuscript and have significantly contributed to it.
Disclosure of interest
The authors declare that they have no competing interest.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.resmer.2021.100828.
Appendix A. Supplementary data
References
- 1.COVID-19 Map - Johns Hopkins Coronavirus Resource Center [Internet]. [cited 2020 Nov 11]. Available from: https://coronavirus.jhu.edu/map.html.
- 2.RECOVERY Collaborative Group, Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. Dexamethasone in hospitalized patients with Covid-19 - Preliminary Report. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [Epub 2020 Jul 17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermine O., Mariette X., Tharaux P.-L., Resche-Rigon M., Porcher R., Ravaud P., et al. Effect of tocilizumab vs usual care in adults hospitalized with Covid-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Réunion de concertation pluridisciplinaire [Internet]. Haute Autorité de Santé [cited 2020 Jul 19]. Available from: https://www.has-sante.fr/jcms/c_2806878/fr/reunion-de-concertation-pluridisciplinaire.
- 6.Wiersinga W.J., Bonten M.J., Boersma W.G., Jonkers R.E., Aleva R.M., Kullberg B.J., et al. Management of community-acquired pneumonia in adults: 2016 guideline update from the Dutch Working Party on Antibiotic Policy (SWAB) and Dutch Association of Chest Physicians (NVALT) Neth J Med. 2018;76:4–13. [PubMed] [Google Scholar]
- 7.Smith K., Krajewski K.C., Krajewski M.P. Practical considerations in prevention and treatment of venous thromboembolism in hospitalized patients with COVID-19. Am J Health Syst Pharm. 2020;77:1739–1745. doi: 10.1093/ajhp/zxaa245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metlay J.P., Waterer G.W., Long A.C., Anzueto A., Brozek J., Crothers K., et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Revel M.-P., Parkar A.P., Prosch H., Silva M., Sverzellati N., Gleeson F., et al. COVID-19 patients and the radiology department – advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI) Eur Radiol. 2020;30:4903–4909. doi: 10.1007/s00330-020-06865-y. [Epub 2020 Apr 20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q., et al. COVID-19 Task Force of YO-IFOS. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288:335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmermann P., Ziesenitz V.C., Curtis N., Ritz N. The Immunomodulatory effects of macrolides – a systematic review of the underlying mechanisms. Front Immunol. 2018;9:302. doi: 10.3389/fimmu.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molina J.M., Delaugerre C., Le Goff J., Mela-Lima B., Ponscarme D., Goldwirt L., et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi Y., Lim H.-S., Chung D., Choi J.-G., Yoon D. Risk evaluation of azithromycin-induced QT prolongation in real-world practice. Biomed Res Int. 2018;2018:1574806. doi: 10.1155/2018/1574806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chorin E., Wadhwani L., Magnani S., Dai M., Shulman E., Nadeau-Routhier C., et al. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020;17:1425–1433. doi: 10.1016/j.hrthm.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiolet T., Guihur A., Rebeaud M.E., Mulot M., Peiffer-Smadja N., Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:19–27. doi: 10.1016/j.cmi.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aggarwal G., Henry B.M., Aggarwal S., Bangalore S. Cardiovascular safety of potential drugs for the treatment of Coronavirus disease 2019. Am J Cardiol. 2020;128:147–150. doi: 10.1016/j.amjcard.2020.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacIntyre C.R., Chughtai A.A., Barnes M., Ridda I., Seale H., Toms R., et al. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infect Dis. 2018;18:637. doi: 10.1186/s12879-018-3548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 20.Torres A., Loeches I-M-, Sligl W., Lee N. Severe flu management: a point of view. Intensive Care Med. 2020;46:153–162. doi: 10.1007/s00134-019-05868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lansbury L.E., Rodrigo C., Leonardi-Bee J., Nguyen-Van-Tam J., Lim W.S. Corticosteroids as adjunctive therapy in the treatment of influenza: an updated Cochrane Systematic Review and Meta-analysis. Crit Care Med. 2020;48:e98–e106. doi: 10.1097/CCM.0000000000004093. [DOI] [PubMed] [Google Scholar]
- 22.Crestani B., Taillé C., Borie R., Debray M.-P., Danel C., Dombret M.-C., et al. Pneumopathie organisée. Presse Med. 2010;39:126–133. doi: 10.1016/j.lpm.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Ader F., Discovery French Trial Management Team Protocol for the DisCoVeRy trial: multicentre, adaptive, randomised trial of the safety and efficacy of treatments for COVID-19 in hospitalised adults. BMJ Open. 2020;10:e041437. doi: 10.1136/bmjopen-2020-041437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turnheim K. Drug therapy in the elderly. Exp Gerontol. 2004;39:1731–1738. doi: 10.1016/j.exger.2004.05.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.