Abstract
In this special issue, we present the main highlights of the first weeks of pharmacovigilance monitoring of coronavirus disease 2019 (COVID-19) vaccines in this unprecedented situation in France: the deployment of a vaccination during an epidemic period with the aim of vaccinating the entire population and the intense pharmacovigilance and surveillance of these vaccines still under conditional marketing authorizations. In this unprecedented situation, the cross approach and interaction between the French pharmacovigilance network and French National Agency for the Safety of Medicines and Health Products (ANSM) has been optimized to provide a real-time safety related to COVID-19 vaccines. Every week, pair of regional pharmacovigilance centers gathered safety data from the French pharmacovigilance network, to acutely expertise all the adverse drug reactions (ADRs) reported with each COVID-19 vaccine within a direct circuit with ANSM. Results of this expertise are presented and discussed with ANSM in order to raise safety signals and take appropriate measures if necessary. These reports are then published online. At the 25th of March 2021, more than 9 815 000 doses were injected and 20,265 ADRs were reported, mostly non-serious (76%). Several potential or confirmed signals were raised at the european level for those vaccines and others ADRs are under special attentions. This underlines the adaptiveness of the French pharmacovigilance system to both the identification of new patient profiles experiencing ADRs and the evolution of the vaccine strategy. Such an efficiency is necessary to manage a careful and acute surveillance of these new COVID-19 vaccines for and to face the pandemic at the same time.
Keywords: Pharmacovigilance, Vaccines, COVID-19, Organization, Adverse drug reactions
Abbreviations
- ADR
adverse drug reaction
- ANSM
Agence nationale de sécurité du médicament et des produits de santé (French National Agency for the Safety of Medicines and Health Products)
- COVID
coronavirus disease
- EMA
European Medicines Agency
- PEG
polyethylene glycol
- RPVC
Regional Pharmacovigilance Center
- SOC
system organ class
Introduction
This is an unprecedented period in many ways… through the emergence of a global pandemic never seen before, along with the development of vaccines in less than 12 months. This has made possible thanks to the research and scientific knowledge acquired with two previous coronaviruses, and the identification of the S protein as the antigen of choice, thanks to long-term performed research on vaccines and mRNA vaccines, thanks to the exceptional mobilization of public and private research, and the involvement of volunteers to rapidly carry out clinical trials, computerized platforms for multicenter clinical trials (real-time sharing of all information, monitoring, results of the primary endpoint, logistics of visits, collection of adverse events) and large investigation teams. This was also possible thanks to the anticipation of firms and governments for the industrial development of production, supported by the continuous evaluation processes driven by the regulatory evaluation agencies [1], [2]. In this unprecedented situation, the commitment of the French Network of 31 Regional Pharmacovigilance Centers (RPVCs) even before the marketed authorizations had allowed to ensure safely use of vaccines. What are the objectives of our network?
-
i)
fast detection of signals;
-
ii)
transparency on the safety profiles of coronavirus disease 2019 (COVID-19) vaccines;
-
iii)
weekly production of expert reports made publicly available on the French National Agency for Medicines and Health Products Safety (ANSM) website;
-
iv)
responsiveness to patients and healthcare professionals questioning about potential adverse effects of these vaccines or symptoms linked to vaccination;
-
v)
responsiveness within the territories in relation with the general public, healthcare professionals, hospital and medical-social establishments.
Unprecedented communication to better and more explain that pharmacovigilance is an opportunity and the essential prerequisite of a policy on medicines including vaccines. This is the French pharmacovigilance style: both haute couture and holistic, allowing to go from patients to signal and from signal to personalized patients and clinicians advising! So scrutinized internationally, concrete and operational with its 2 complementary skills: pharmacological and medical expertise associated with its 2 inseparable approaches (regional and national) in a short and direct circuit with the national institution “Agence nationale de sécurité du médicament et des produits de santé” (French National Agency for the Safety of Medicines and Health Products, ANSM [3], already massively mobilized in 2009 for the H1N1 vaccine [4], [5], [6] and more recently in 2019 with the close pharmacosurveillance of candidates treatments used to fight against COVID-19 [7]. In this special issue, we present the main highlights of the first weeks of pharmacovigilance monitoring of COVID-19 vaccines in this unprecedented situation in France: the deployment of a vaccination during an epidemic period with the aim of vaccinating the entire population and the intense pharmacovigilance and surveillance of these vaccines still under conditional marketing authorizations.
Why vaccines are special drugs?
Vaccination is one of the most successful and cost-effective public health interventions. Nowadays, vaccines are considered as the best response against this pandemic crisis. This can be explained by the eight specificities of vaccines, listed in Table 1 [8].
Table 1.
Particularities of vaccines.
| Particularities | Comments |
|---|---|
| Mechanism of action | Long-term and persistent immunological effect in one or few doses |
| Manufacture | High technology requiring strict specifications, batch releases for reproducibility |
| Indications for use | Mostly in prevention in unaffected subjects according to the official recommendations of national health authorities |
| Impact of use | At the individual and population level in modifying the pathogen epidemiology |
| Unusual characteristic | Phase III clinical trials in more important population size than for classic drugs |
| Unusual population | Administration of vaccine candidates in healthy volunteers (target population) in phase III clinical trials |
| Post-authorization pharmacovigilance | Larger exposed population of healthy subjects implying an extremely low risk to keep a favorable benefit/harm balance |
| Unusual benefit-risk ratio | Extremely low risk |
Vaccines are special drugs because of their mechanism of action and because of their context of use. Vaccines allow preventing healthy people from getting infected by a disease by a mechanism which differs from the classical pharmacological effect and allows providing a persistent immunological protection, mediated by a complex cascades formed of multiple and heterogeneous targets. To resume, a vaccine does not need to be administered every day to produce its long-term pharmacological effect.
Vaccines are special drugs because their manufacture is very specific using high technology, requiring reproducibility. That is why specifications and batch releases are very complicated to set up. As well as the manufacture, vaccines are special drugs because of their indications of use. A vaccine is administered to prevent a disease, and according to official recommendations of national health authorities. Also important to note, the vaccination campaign can be different depending on the country. Vaccines are special drugs as far as their impact can be observed both at the individual level and at the population level against a pathogen, by modifying the disease epidemiology. Vaccines are special drugs because the exposed population is larger, post-authorization pharmacovigilance data collection is therefore greatly increased and it is necessary to quickly assess the risk by taking into account epidemiological and clinical characteristics of the disease and of the exposed population. Interestingly, clinical trials are managed on an important population size compared to other drugs (∼1000–5000 vs. ∼30,000 participants for candidates vaccines) allowing to detect numerous adverse reactions. More precisely phase III clinical trial of NT162b2 vaccine candidate (BioNTech-Pfizer) enrolled 43,548 participants with 2 subgroups of age (16–55 years old and > 55 years old) [9]. In the same way, clinical trial of mRNA-1273 vaccine candidate (Moderna), 30,420 participants were enrolled in the phase III with 2 subgroups of age (18–65 years old and > 65 years old) [10]. Noticeably, exposed population is composed of healthy subjects, and vaccines are thus administered to prevent a potential disease. Therefore, risks have to be very low to ensure a favorable benefit/harm balance. Regarding adverse drug reactions (ADRs), in the phase III clinical trial of NT162b2 vaccine candidate, systemic reactogenicity was more frequently observed after second injection (70% vs. 59% in placebo group), and more severely in 16–55 years old subgroup [9]. Results for mRNA-1273 vaccine candidate also showed systemic reactogenicity more frequently after second injection and more severely in 18–65 years old subgroup [10]. Interestingly, these relevant information are thus reported in the “adverse effects” section of the summaries of the products characteristics [11], [12]. Another relevant example concerns Bell's palsy. Indeed the frequency of Bell's palsy observed in phase III clinical trials of Pfizer COVID-19 vaccine Comirnaty® (4 reports in the vaccine group versus 0 report in the placebo group) and COVID-19 vaccine Moderna® (3 reports in the vaccine group versus 1 in the placebo group) was considered consistent with the expected rate in the general population, its imbalance between groups is puzzling. As far as early transversal pharmacological analysis enabled to propose type I interferons as the potential mechanism, and beside idiopathic causes and viral infections including COVID-19 itself, mRNA vaccines could be a possible cause of Bell's palsy [13]. Thus, these arguments led the European Medicines Agency (EMA) to add these information in the summaries of the products characteristics [11], [12] after the conditional authorization.
The extraordinarily rapid development of these very effective vaccines allowed disposing from determinant new tools in the fight against the COVID-19 pandemic. Because vaccines are special drugs and because the situation is unprecedented, ANSM and the French Pharmacovigilance network strengthen their cross-disciplinary approach and their close collaboration to allow the best safety and transparency in a French quality process.
Which organization for the pharmacovigilance of COVID-19 vaccines?
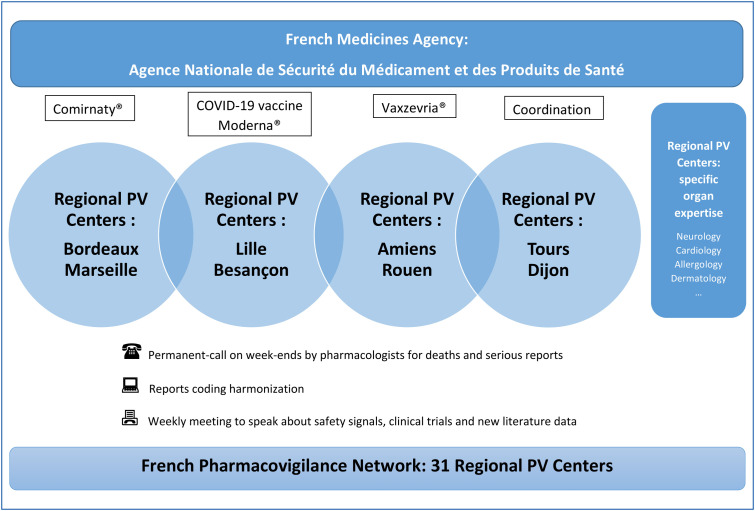
To assess acute evaluation and raise signals, a cross-disciplinary approach between the ANSM and the French pharmacovigilance network is required and thus the circuit has been optimized to answer as efficient as possible to this unprecedented situation. This intensifying of the pre-existing organization and close collaboration is driven by ANSM. Similarly to the impact of the vaccine, the organization for COVID-19 vaccines pharmacovigilance is based on two dimensions. An individual dimension represented by the real-time analysis of all ADRs notifications by each of the 31 Regional RPVCs [14], [15], [16] and a collective dimension represented by a global and scientific analysis of pharmacovigilance experts from RPVCs and ANSM. This is the French pharmacovigilance task-force. This organization allows an analysis and an evaluation in real-time. Beyond real-time ADRs assessments, one pair of RPVCs per vaccine marketed in France everyday colligate and deeply analyze all ADRs data from the vaccine they are in charge of. To complete accurately the assessment of ADRs, several RPVCs also provide a specific organ expertise (like in neurology, cardiology, allergy, dermatology). Every Tuesday, a report of expertise achieved by each of the pairs of RPVCs for each vaccine is transmitted to ANSM. These reports of expertise gather data concerning exposed population, and quantitative and qualitative analysis of ADRs, characterized by age, severity and type of ADRs. Qualitative, quantitative and medical analysis are performed for adverse events of interest (anaphylaxis, zona, Bell's palsy…) in order to describe them according to several characteristics (age, gender, time to onset…) and also to identify atypical and/or serious patterns leading to potential safety signals. Weekly and cumulative data are presented. Then, every Thursday, a meeting takes place to discuss the expert pharmacovigilance reports, including in details significant case reports, potential safety signals, and new literature data, in order to confirm safety signals. If a safety signal has been validated, appropriate risk minimization measures would be issued in relation with the European Medicines Agency (EMA). National safety signals are raised at the EMA. Complete weekly reports including the synthesis of significant case reports are available on the ANSM website [17]. A permanent-call on week-ends ensured by the head of pharmacovigilance centers pharmacologists is also set up to register and evaluate death and serious reports 7-days-a-week. Fig. 1 illustrates all this complete organization.
Figure 1.
French Pharmacovigilance organization for COVID-19 vaccines surveillance. PV: pharmacovigilance.
All these collaborations are precious tools to improve patient's security in front of pandemic worries.
To date, what are the specific attentions?
Close monitoring in real time of ADRs related to COVID-19 vaccines is even more of a challenge as far as this is an unpreceded situation because:
-
i)
people are more vulnerable;
-
ii)
the aim is to vaccinate the entire population;
-
iii)
the monitoring is carried out during epidemic period.
To date, three vaccines have been authorized in Europe, after being authorized in UK and US. Comirnaty® was the first COVID-19 vaccine available in France in December 2020, followed by COVID-19 vaccine Moderna® in January 2021 and Vaxzevria® in February 2021. For all three vaccines, at the 25th of March 2021, more than 9,815,000 doses were injected and 20,265 ADRs were reported, mostly non-serious (76%). Thanks to the French pharmacovigilance task-force, several special attentions and signals were highlighted concerning security vaccines use, gathered in Table 2 .
Table 2.
Special attentions concerning COVID-19 vaccines security pharmacovigilance (data at the 03/25/2021).
| Vaccine and exposition | Number of ADR reports | ADRs under monitoring, according to SOCs | Potential or confirmed signals |
|---|---|---|---|
| Cominarty® BioNTech-Pfizer-m-RNA 1st dose: 4,807,987 2nd dose: 2,443,424 |
12,249 | –Nervous system disorders: convulsions, facial paralysis, anosmia, ageusia –Infections: COVID-19 infections –Cardiac disorders: pericarditis, –Immune system disorders: hypersensitivity/anaphylaxis and asthma –Ear and labyrinth disorders: hearing disorders, vestibular disorders –Vascular disorders: aortic dissection, vasculitis |
–Arterial hypertension –Cardiac arrhythmias –Herpes zoster infection –Thrombocytopenia/spontaneous hematomas –Diabetes imbalances |
| COVID-19 vaccine Moderna®-m-RNA 1st dose: 616,626 2nd dose: 173,223 |
577 | –Vascular disorders: arterial hypertension –Cardiac disorders: cardiac arrhythmias –Infections: herpes zoster infection –General disorders and administration site conditions: reactogenicity |
–Local delayed reactions |
| COVID-19 vaccine AstraZeneca®-Non-replicating viral vector 1st dose: 1,923,580 |
7439 | –Vascular disorders: hypertension, hypotension, epistaxis –Cardiac disorders: myopericarditis/pericarditis, rhythm and conduction disorders –Respiratory, thoracic and mediastinal disorders: exacerbations of dyspnea and asthma –Nervous system disorder: non-infectious encephalitis –Immune system disorders: anaphylactic reactions –General disorders and administration site conditions: hypothermia –Blood and lymphatic system disorders: lymphopenia –Metabolism and nutrition disorders: diabetes imbalances |
–Influenza-like symptoms –Large venous thrombosis (cerebral, digestive), with thrombocytopenia or coagulation disorders |
ADR: adverse drug reaction; COVID: coronavirus disease; SOC: system organ class.
For Comirnaty® vaccine, ADRs concerned mainly women (74%), and persons of 50–64 years-old (43%). A total of five safety signals related to Comirnaty® have been detected thanks to this pharmacovigilance survey: arterial hypertension, cardiac arrhythmias, Herpes zoster infection, thrombocytopenia, spontaneous hematomas, diabetes imbalances. Reports of immediate severe hypersensitivity reactions are rare (n = 51). Mechanisms are not totally elucidated, but polyethylene glycol (PEG) contained in lipid nanoparticles could be involved. This was the first safety signal leading to specific measures for people with a history of PEG hypersensitivity.
Fortunately, these ADRs remain rare. ADRs related to reactogenicity (fatigue, headaches, fever, nausea and vomiting…) are mostly related to the second injection, in particular in young people. These data are consistent with data from clinical trials [9]. ADRs related to severe arterial hypertension (including grade III) occurred immediately or within few hours or days after vaccine injection, in patients with or without a history of hypertension (on an equilibrated treatment or not). Beyond the vaccine context which conducts to noradrenergic discharge, the duration of these hypertensive attacks at very high values or their delayed onset in relation to the vaccine act suggest more specific mechanisms of action related to the vaccine itself (potency of immune stimulation induced by the mRNA vaccine via cytokines, spike protein, and angiotensin 2 converting enzyme) [18], [19], [20]. Noticeably, 313 cases of severe arterial hypertension have been shared with EMA. All of these had a medical impact with at least one or more of the following elements: result in a close medical surveillance, a visit to the emergency room, result in a hospitalization, a consultation (or consultations) with a clinician, associated with other effects (malaise, headache, nausea, epistaxis,..), led to the initiation of anti-hypertensive treatment or the increase of the dosage of pre-existing anti-hypertensive treatment or the addition of another class of anti-hypertensive medication. It is also important to highlight that these ADRs have not been reported in clinical trials and these new information generate new practical consequences.
For COVID-19 Moderna® vaccine, ADRs concerned mainly women (74%), and persons of 75–84 years-old (49%). To date, we almost observed the same safety profile as for Comirnaty® vaccine. Indeed, reports concerned mainly reactogenicity ADRs: reactions at the vaccination site (pain, inflammation, cutaneous eruption), influenza-like syndrome (fever, chills, myalgia, arthralgia, asthenia), lymphadenopathy, digestive disorders and hypersensitivity reactions. All of these ADRs quickly resolved. Only one specific safety signal has been detected: delayed local reactions occurring 5 to 10 days post-vaccination [21].
For Vaxzevria®, ADRs concerned mainly women (74%), and persons of 16–49 years-old (72%). Influenza-like syndrome ADRs were mostly reported, sometimes associated with exacerbations of dyspnea and asthma. These ADRs mostly occurred within 24 hours after the first injection. 12 cases of thrombosis of large veins atypical by their localization (mainly cerebral veinous sinus thrombosis, but also digestive–mesenteric or splanchnic vein thrombosis) that may be associated with thrombocytopenia or Disseminated intravascular coagulation disorders were reported. These cases, describing a new symptomatology, occurred mostly in women with a median delay of 9 days after vaccination in patients (9 patients under 55 years of age, 3 patients over 55 years of age) with no specific history identified to date, apart from oral contraception in 4 cases, associated with a C/S protein deficiency in a fifth and obesity in 1 case. During the pharmacovigilance assessment on April 8, 9 new cases were analyzed, concerning people with a different profile than in the previous periods. They are 4 women and 5 men, older (average age 62 years) who presented more digestive thrombosis. Thromboembolic events are being reviewed by EMA to better understand the mechanism of action, the possible underlying risk factors and any other data needed to understand the phenomenon and guarantee the security of use.
Conclusion
The success of a vaccination campaign depends on three inseparable aspects: safety, transparency and effectiveness. The multiple approach associated with a close collaboration of all entities involved and the optimization of the pre-existing pharmacovigilance organization allows this complete analysis to the French and the authorities in real time. This also highlights, once again, that the French pharmacovigilance system can adapt to identify new patient profiles with adverse events in near real time, but also adapt to complex vaccine strategies. The knowledge provided by the French Pharmacovigilance Network thus enables the vaccination campaign to be managed efficiently and safely, in order to maintain or restore the climate of confidence that is essential for its success. Indeed, such efficiency is necessary to manage a careful and acute surveillance of these new COVID-19 vaccines for and against the pandemic at the same time.
Disclosure of interest
The authors declare that they have no competing interests.
References
- 1.2021. European Medicines Agency (EMA)–Science Medicines Health. https://www.ema.europa.eu/en [Accessed 22 April 2021] [Google Scholar]
- 2.2021. U.S Food and Drug Administration (FDA) https://www.fda.gov/ [Accessed 22 April 2021] [Google Scholar]
- 3.2021. Uppsala Monitoring Centre–VigiBa. https://www.who-umc.org/vigibase/vigibase/ [Accessed 22 April 2021] [Google Scholar]
- 4.Dauvilliers Y., Arnulf I., Lecendreux M., Monaca Charley C., Franco P., Drouot X., et al. Increased risk of narcolepsy in children and adults after pandemic H1N1 vaccination in France. Brain. 2013;136:2486–2496. doi: 10.1093/brain/awt187. [DOI] [PubMed] [Google Scholar]
- 5.Haut Conseil de la Santé Publique . 2013. Avis relatif aux caractéristiques des futurs vaccins pandémiques. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=368 [Accessed 22 April 2021] [Google Scholar]
- 6.Lacroix C., Mallaret M., Jonville-Bera A.P. Pharmacovigilance and drug-induced rare diseases: strengths of the French Network of Regional Pharmacovigilance Centres. Therapie. 2020;75:207–213. doi: 10.1016/j.therap.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 7.The French Pharmacovigilance Network, Grandvuillemin A., Drici M.D., Jonville-Bera A.P., Micallef J., Montastruc J.L. French pharmacovigilance public system and COVID-19 pandemic. Drug Saf. 2021;44:405–408. doi: 10.1007/s40264-020-01034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Louët H., Loupi E., Haramburu F., Abelin A., Guillemot D., Haramburu F., et al. Which pharmacovigilance for vaccines? Therapie. 2007;62:245–247. doi: 10.2515/therapie:2007039. [DOI] [PubMed] [Google Scholar]
- 9.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Résumé des caractéristiques du produit - Comirnaty dispersion à diluer pour solution injectable - Vaccin à ARNm (à nucléoside modifié) contre la COVID-19. n.d. https://ec.europa.eu/health/documents/community-register/2021/20210223151110/anx_151110_fr.pdf.[Accessed 22 April 2021 (34 pp.)].
- 12.Résumé des caractéristiques du produit - COVID-19 Vaccine Moderna, dispersion injectable - Vaccin à ARNm (à nucléoside modifié) contre la COVID-19. n.d. https://ec.europa.eu/health/documents/community-register/2021/20210106150575/anx_150575_fr.pdf.[Accessed 22 April 2021 (31 pp.)].
- 13.Soeiro T., Salvo F., Pariente A., Grandvuillemin A., Jonville-Béra A.P., Micallef J. Type I interferons as the potential mechanism linking mRNA COVID-19 vaccines to Bell's palsy. Therapies. 2021 doi: 10.1016/j.therap.2021.03.005. [S0040595721000962] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olivier P., Montastruc J.L. The nature of the scientific evidence leading to drug withdrawals for pharmacovigilance reasons in France. Pharmacoepidemiol Drug Saf. 2006;15:808–812. doi: 10.1002/pds.1248. [DOI] [PubMed] [Google Scholar]
- 15.Abou Taam M., Jacquot B., Ferard C., Thery A.C., Mounier C., Grandvuillemin A., et al. The French pharmacovigilance surveys: A French distinctiveness, a real input. Therapie. 2020 doi: 10.1016/j.therap.2020.05.011. [S0040-5957(20)30101-3] [DOI] [PubMed] [Google Scholar]
- 16.Soeiro T., Lacroix C., Micallef J. Adverse drug reaction monitoring: Doing it the French way–Act II. Therapie. 2020 doi: 10.1016/j.therap.2020.11.002. [S0040-5957(20)30195-5] [DOI] [PubMed] [Google Scholar]
- 17.Agence Nationale de Sécurité du Médicament et des Produits de Santé. 2021. https://ansm.sante.fr/. [Accessed 22 April 2021].
- 18.Zhang J., Crowley S.D. Role of T lymphocytes in hypertension. Curr Opin Pharmacol. 2015;21:14–19. doi: 10.1016/j.coph.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreira N.S., Tostes R.C., Paradis P., Schiffrin E.L. Aldosterone, inflammation, immune system, and hypertension. Am J Hypertens. 2021;34(1):15–27. doi: 10.1093/ajh/hpaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa L.B., Perez L.G., Palmeira V.A., Macedo e Cordeiro T., Ribeiro V.T., Lanza K., et al. Insights on SARS-CoV-2 molecular interactions with the renin-angiotensin system. Front Cell Dev Biol. 2020;8:559841. doi: 10.3389/fcell.2020.559841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMahon D.E., Amerson E., Rosenbach M., Lipoff J.B., Moustafa D., Tyagi A., et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021 doi: 10.1016/j.jaad.2021.03.092. [S0190-9622(21)00658-7] [DOI] [PMC free article] [PubMed] [Google Scholar]