Abstract
Transient cardiovascular and cerebrovascular responses within the first minute of active standing provide the means to assess autonomic, cardiovascular and cerebrovascular regulation using a real-world everyday stimulus. Traditionally, these responses have been used to detect autonomic dysfunction, and to identify the hemodynamic correlates of patient symptoms and attributable causes of (pre)syncope and falls.
This review addresses the physiology of systemic and cerebrovascular adjustment within the first 60 s after active standing. Mechanical factors induced by standing up cause a temporal mismatch between cardiac output and vascular conductance which leads to an initial blood pressure drops with a nadir around 10 s. The arterial baroreflex counteracts these initial blood pressure drops, but needs 2–3 s to be initiated with a maximal effect occurring at 10 s after standing while, in parallel, cerebral autoregulation buffers these changes within 10 s to maintain adequate cerebral perfusion. Interestingly, both the magnitude of the initial drop and these compensatory mechanisms are thought to be quite well-preserved in healthy aging.
It is hoped that the present review serves as a reference for future pathophysiological investigations and epidemiological studies. Further experimental research is needed to unravel the causal mechanisms underlying the emergence of symptoms and relationship with aging and adverse outcomes in variants of orthostatic hypotension.
Keywords: Initial orthostatic hypotension, Systemic hemodynamics, Cerebral perfusion, Cerebral autoregulation, Anoxia reserve time, Aging
1. Introduction
Orthostatic stresses are common daily events for humans. An average adult stands approximately 50–60 times per day (Smith et al., 2015). Orthostasis result in a rapid shift of blood away from the chest to the distensible venous capacitance system below the diaphragm and instantaneously places the brain above heart leve. This results in a fall in peripheral and cerebral perfusion pressure with compensatory adjustments promptly instituted to counteract the fall in arterial blood pressure (BP). In healthy individuals almost all hemodynamic events take place within 30 s s after the onset of standing up (Rowell, 1993, Smith et al., 1994, Smit et al., 1999, Hainsworth, 2004, van Lieshout et al., 2003).
In the 1980s, methods became available to monitor rapid arterial BP changes continuously and noninvasively (Imholz et al., 1990a, Westerhof et al., 2015). These extraordinary scientific developments enabled clinicians and researchers to noninvasively study fluctuations in BP during the first 60 s of standing (Wieling et al., 1992, Tanaka et al., 1996, Thomas et al., 2009, Stewart and Clarke, 2011, Finucane et al., 2014, van Wijnen et al., 2017, van Wijnen et al., 2018, Finucane et al., 2019).
Others have also addressed the initial cerebral circulatory adjustments to standing (Harms et al., 2000; van Lieshout et al., 2003; Mol et al., 2019; O’Connor et al., 2020). The active stand test has emerged as an important tool in the clinical assessment of orthostatic hypotension in both younger and older patients (Shen et al., 2017; Brignole and Moya, 2018). It can be used in the identification of the multiple variants of OH including initial OH, delayed BP recovery, and sustained OH (Fig. 1) (van Wijnen et al., 2017; van Wijnen et al., 2018; Finucane et al., 2019; Moloney et al., 2020).
Fig. 1.
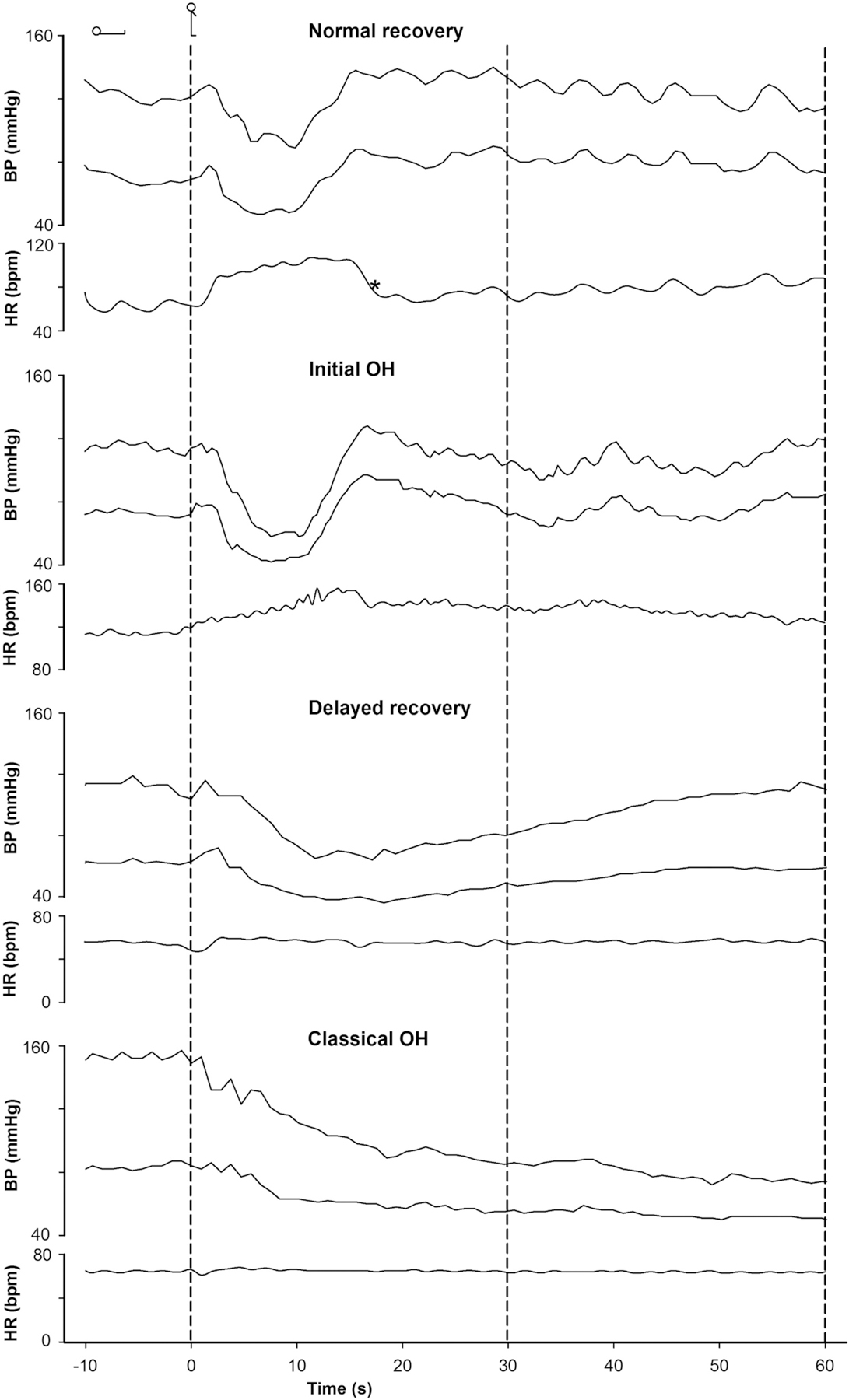
Spectrum of normal and abnormal blood pressure (BP) and heart rate (HR) responses during the first 60 s after active standing. Measurement of orthostatic BP with continuous noninvasive measurement in four subjects. The last 15 s in the supine position and 60 s of standing are illustrated. Dashed vertical lines indicate the onset of the active stand. Normal orthostatic BP recovery in a 26-year old healthy female (A), initial orthostatic hypotension (IOH) in a 14-year old healthy male (transient systolic BP decrease >40 mmHg within 15 s of standing) (B), delayed BP recovery in a 76-year old man with a history of hypertension on 3 vasoactive drugs (systolic BP fall of >20 mmHg at 30 s of standing, but not meeting the criteria of classical orthostatic hypotension) (C) and classical orthostatic hypotension in a 76-year old man with primary autonomic failure (sustained fall in systolic BP of ≥20 mmHg between 60 and 180 s of standing) (D) are illustrated. BP = blood pressure. HR = heart rate. OH = orthostatic hypotension.* = rebound bradycardia.
This review will address the physiology of systemic and cerebrovascular adjustment within the first 60 s after active standing using noninvasive monitoring. The focus is on physiology because we consider it important to ground epidemiological observational associations in biological explanations (Trichopoulos, 1996; Vandenbroucke and de Craen, 2001; Mukherjee, 2015).
The aim of the present review is therefore:
to provide an overview on the initial systemic circulatory adjustments in healthy young and older adults within the first 60 s after active standing
to describe adjustments in the cerebral circulation in the first 60 s after active standing
to address the clinical relevance of knowing the physiology in detail
2. Systemic circulatory adjustments within the first 60 s after standing
In the following section we will discuss the hemodynamic mechanisms underlying the BP response after active standing. We will address the responses in young adults first, given that in this age group the time course has been studied in great detail (Borst et al., 1982, Borst et al., 1984, Sprangers et al., 1991a, Tanaka et al., 1996, Thomas et al., 2009, Stewart and Clarke, 2011, Lewis et al., 2011, Lewis et al., 2013). We then follow with a discussion of the response in older adults.
2.1. Studies in young adults
To understand and disentangle the role of gravity and muscle contraction involved in the circulatory response to active standing a number of key experiments were performed in the 1980s and 1990s (See Figs. 2 and 3) (Borst et al., 1982, Borst et al., 1984, Imholz et al., 1990b, Sprangers et al., 1991b, Wieling et al., 1996).
Fig. 2.
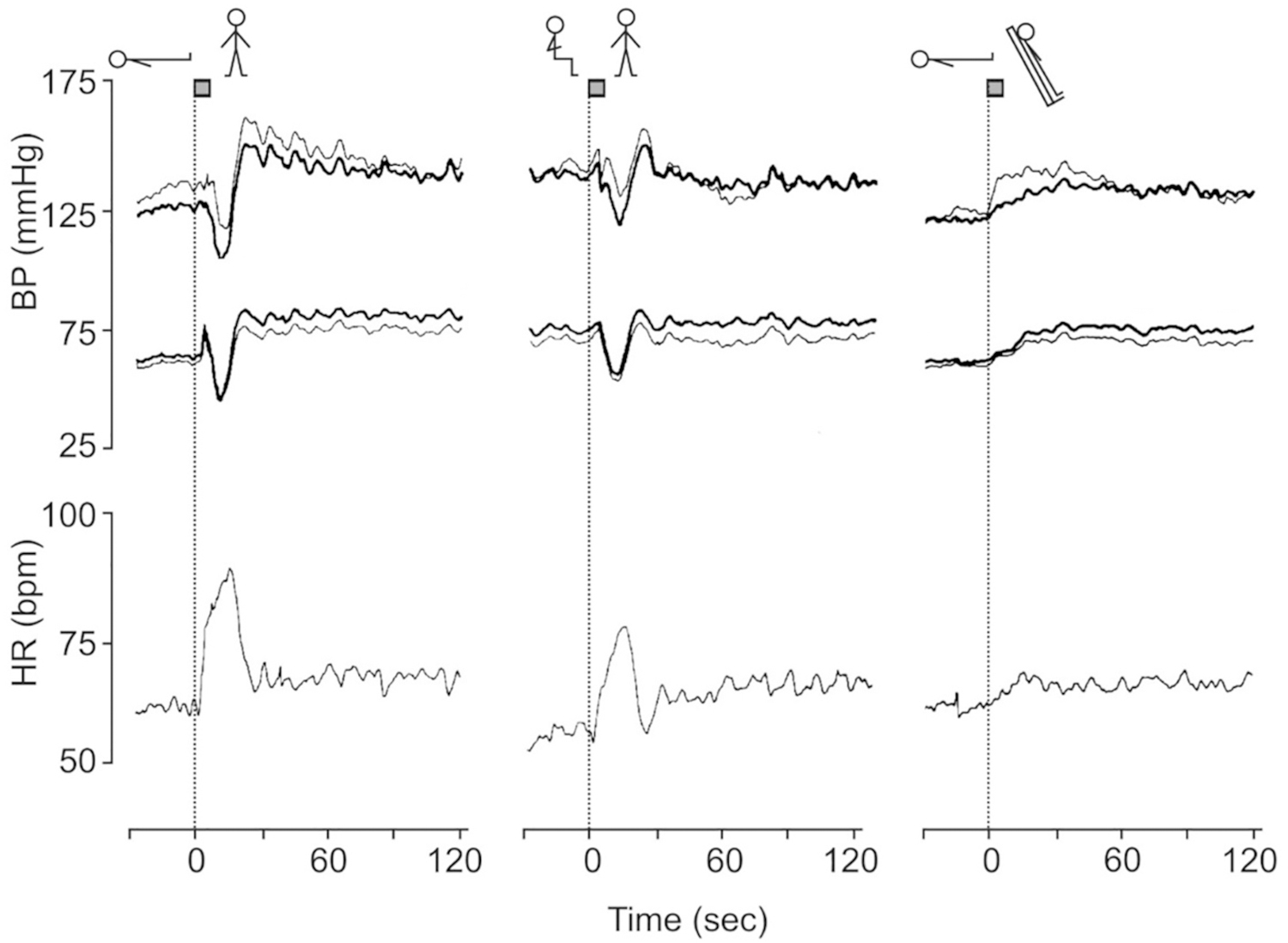
Group average intrabrachial (bold line) and finger (thin lines) blood pressure (BP) and heart rate (HR) responses in 11 male volunteers aged 22–40 years to three orthostatic maneuvers (standing from supine, standing from sitting and head-up tilting) preceded by a period of at least 5 min supine rest. The time needed to change posture amounted to about 3 s as is indicated at the top of the dotted line at T = 0. [From Imholz et al., 1990a with permission].
Fig. 3.
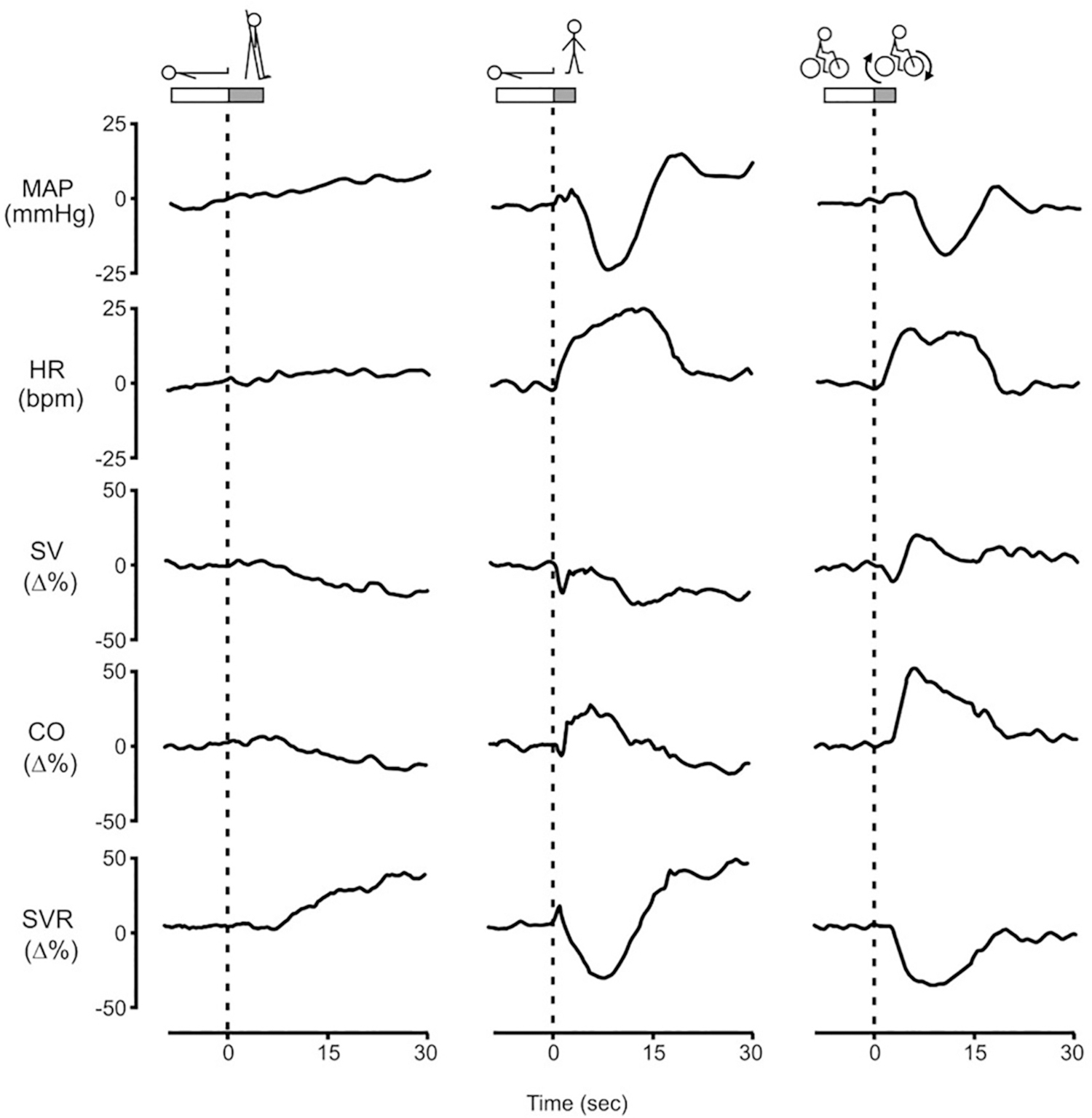
The initial circulatory adjustment induced by head-up tilting, standing from supine and short-lasting bicycle exercise in 8 adult males aged 20–38 years after 5 min of rest. To avoid inadvertent Valsalva straining, the subjects were trained to perform the maneuvers during normal inspiration, facilitated by starting the three stressors at end expiration.
MAP = mean arterial pressure. HR = heart rate. SV = stroke volume. CO = cardiac output. SVR = systemic vascular resistance. The time needed to perform the maneuvers is indicated at the top of the dotted line at T = 0. SV, CO and SVR are computed by pulse wave analysis and expressed as percentage of change. [Revised after Sprangers et al., 1991b with permission].
A key observation in the first experiments was that a typical transient fall in BP upon active standing from supine and sitting is not present or far less prominent during a passive head-up tilt (HUT) (Fig. 1) (Borst et al., 1982, Borst et al., 1984, Imholz et al., 1990a).
The role of muscle contractions was examined next. Fig. 3 shows hemodynamic responses induced by three different stressors, HUT (passive change of posture, in which only gravity varies), standing (active change of posture with contraction of leg and abdominal muscles in addition to the effect of gravity) and a 3 s bout of bicycle exercise in sitting position (contraction of leg and abdominal muscles without change of posture and therefore no gravitational effect) (Sprangers et al., 1991a). They represent an average response obtained in 8 healthy male volunteers aged 20–38 years.
The changes following passive HUT, the classical provocation to study gravitational effects on the circulation (Smith et al., 1994), will be addressed first.
2.1.1. Head-up tilting
Participants were tilted 70 degrees head up within 6 s using an electrically driven tilt table with a foot support. HUT is accompanied by a transfer of 0.5–1.0 L of blood towards the venous capacitance system below the diaphragm. Downward blood pooling with a fall in venous return to the heart results in a diminution of central blood volume and a consequent decrease in cardiac filling pressure and in stroke volume (SV). The bulk of the transfer of blood occurs within 30 s (Smith et al., 1994; Smit et al., 1999). On head-up tilting, SV does not decline until after some six beats of normal stroke output (Fig. 2). Then SV gradually diminishes to reach a new stable level. The delay in the fall in SV in the first 5 s of HUT can be attributed to a discharge of blood from the apical pulmonary veins to the left ventricle (van Heusden et al., 2006; Sheriff et al., 2007; Halliwil, 2007). HR accelerates almost immediately, but because it does not fully compensate for the decrease in SV, cardiac output (CO) decreases by 21 ± 5% after 1 min of standing. However, the simultaneous increase in systemic vascular resistance (SVR) by 40 ± 9% results in a ~10 mmHg net rise in MAP. The rise in MAP is attributed to the hydrostatic position of the carotid baroreceptors which sense a decrease in BP in the upright position (Rowell, 1993; Smit et al., 2002; Hainsworth, 2004).
2.1.2. Response to cycling
The hemodynamic response on active standing in 3 s is conspicuously different from the gradual changes induced by passive tilting (Fig. 3). These marked transient hemodynamic changes are also observed during a 3 s bout of upright cycling on an ergometer. In both maneuvers strong contraction of leg and abdominal muscles occur, as opposed to HUT. The 3 s bout of upright cycling-exercise was designed to investigate these effects. After 2 min sitting on a flywheel bicycle ergometer the subjects were given a verbal command to abruptly start cycling for a 3 s duration and then return to a motionless state. The ergometer was set to 50 W but the considerable initial resistance to bring the flywheel in motion needed a forceful contraction of leg and abdominal muscles. Onset to cycling was accompanied by a marked transient increase in CO with a maximum increase of 50 ± 3% at 5 s. The marked increase in CO can be attributed to the forceful skeletal muscle contractions compressing venous vessels in the legs and abdomen, thereby causing an immediate translocation of blood towards the heart increasing right ventricular filling. The delay of the increase in left ventricular SV and CO of about 3 s (Fig. 2) is due to the transfer time between the right and the left ventricle (Wieling et al., 1996). Simultaneously with the increase in CO, a pronounced fall in SVR (−41 ± 6% at 8 s) that exceeds the rise in CO is found, resulting in a marked drop in MAP (−18 ± 2% at 10 s). Rapid vasodilation in the contracting leg muscles and muscle pump induced widening of the arteriovenous pressure gradient across the muscle with an increase in vascular conductance are the most likely mechanism to explain the large fall in SVR/increase in conductance (Sprangers et al., 1991b, Sheriff et al., 2007, Tschakovsky et al., 2011). Activation of cardiopulmonary receptors by the abrupt large transient increase in right atrial pressure (12 ± 2 mmHg at 3 s) resulting in reflex vasodilatation in skeletal muscles is also considered to contribute (Sprangers et al., 1991a, Callister et al., 1994, Wieling et al., 1996).
2.1.3. Active standing
Active standing increases HR abruptly in the first 3 s with a further rise to a peak at 12 s, declines to a nadir around 20 s, and then stabilises. The primary steep HR increase manifests the reflex inhibition of cardiac vagal tone and can be attributed to a reflex originating in the contracting muscles or to a central command (exercise reflex) (Borst et al., 1982; Wieling et al., 1985). The more gradual secondary HR rise, starting around 5 s, relates to the fall in arterial pressure and diminished arterial baroreceptor activation (Fig. 3) with further reflex inhibition of cardiac vagal tone and increased sympathetic outflow to the sinus node. The subsequent rapid decrease in HR is associated with the recovery of arterial pressure, again mediated through the arterial baroreflex by an increase in vagal outflow to the sinus node (Borst et al., 1982; Borst et al., 1984). Just as in cycling, active standing induces a rise in CO and fall in MAP with a nadir in MAP of −23 ± 2 mmHg at 8 s. The stable SV on standing vs. the marked SV increase observed by cycling is explained by the effect of gravity superimposed on the muscle pumping effect. With the combination of a stable SV and a marked increase in HR, CO rises to a maximum (24 ± 6%) at 6 s following standing up. Comparable to cycling, SVR decreases markedly(−36 ± 3% at 7 s), which exceeds the simultaneous increase in CO, resulting in a decrease in MAP. Within 30 s the transient fall in BP on standing is counteracted. This is attributed to an arterial baroreflex mediated increase in sympathetic outflow to resistance vessels and is supported by different studies. Firstly, the recovery of BP observed after 8 s follows the time course of sympathetically mediated changes in arteriolar vasomotor tone which need 2–3 s to be initiated and have maximal effect at about 10 s (Eckberg and Sleight, 1992). Secondly, it has been shown that in young adults after administration of the a1 receptor blocker prazosin and in patients with neurogenic orthostatic hypotension (OH) BP fails to recover after the nadir (Lewis et al., 2013; Harms et al., 2000). Thirdly, clonidine, which acts centrally to lower BP via a decrease in sympathetic activity, impairs the initial BP recovery upon standing with loss of BP overshoot and delayed recovery (Coupland et al., 1995). Impaired BP recovery is clinically important particularly in older adults and associated with poor health outcomes (See final clinical perspective section).
2.1.4. Role of leg and abdominal muscle contractions during standing
Standing up from the supine position is invariably accompanied by (involuntary) contraction of leg and abdominal muscles with a precipitous transient increase of intra-abdominal pressure (43 ± 22% on average in young adults) (Tanaka et al., 1996) and abrupt rise (about 10 mmHg) of right atrial pressure resulting in an increase in right ventricular filling and therefore CO (Sprangers et al., 1991b). This mechanical effect can be enhanced by forceful voluntary tensing of leg and abdominal muscles during the stand up, which markedly attenuates the initial fall in BP (Krediet et al., 2007; van Wijnen et al., 2016).
As far as engagement of the skeletal muscle pump, subjects with (near)syncope on standing typically experience the (near) faint after having walked about 5 steps (Wieling et al., 2007; Stewart and Clarke, 2011). This implies that starting to walk immediately after standing up may actually increase the initial fall in BP by enhancing vasodilation in leg muscle.
2.2. Studies in older adults
With the above observations in young adults as a background we will now focus on the circulatory responses within the first minute of active standing in older adults in early physiological studies (Imholz et al., 1990b, Wieling et al., 1992) and more recently in epidemiological studies (Finucane et al., 2014).
2.2.1. Early physiological studies
Imholz et al. studied active stands in 40 physically active healthy older adults aged 70–86 years, with no known comorbidities and no prescribed medication using FinAP. The duration of supine rest was 5 min. Stand up time was 3–6 s with a helping hand provided if needed. Before instrumentation of the volunteers a 10–15 min period was used to practice the stand-up maneuver (Imholz et al., 1990a).
Fig. 4 compares the hemodynamic responses in 37 older adults from the Imholz study (Wieling et al., 1992) and 10 young adults (ten Harkel et al., 1990). The response in the 10 young adults is almost identical to that described in Fig. 2.
Fig. 4.
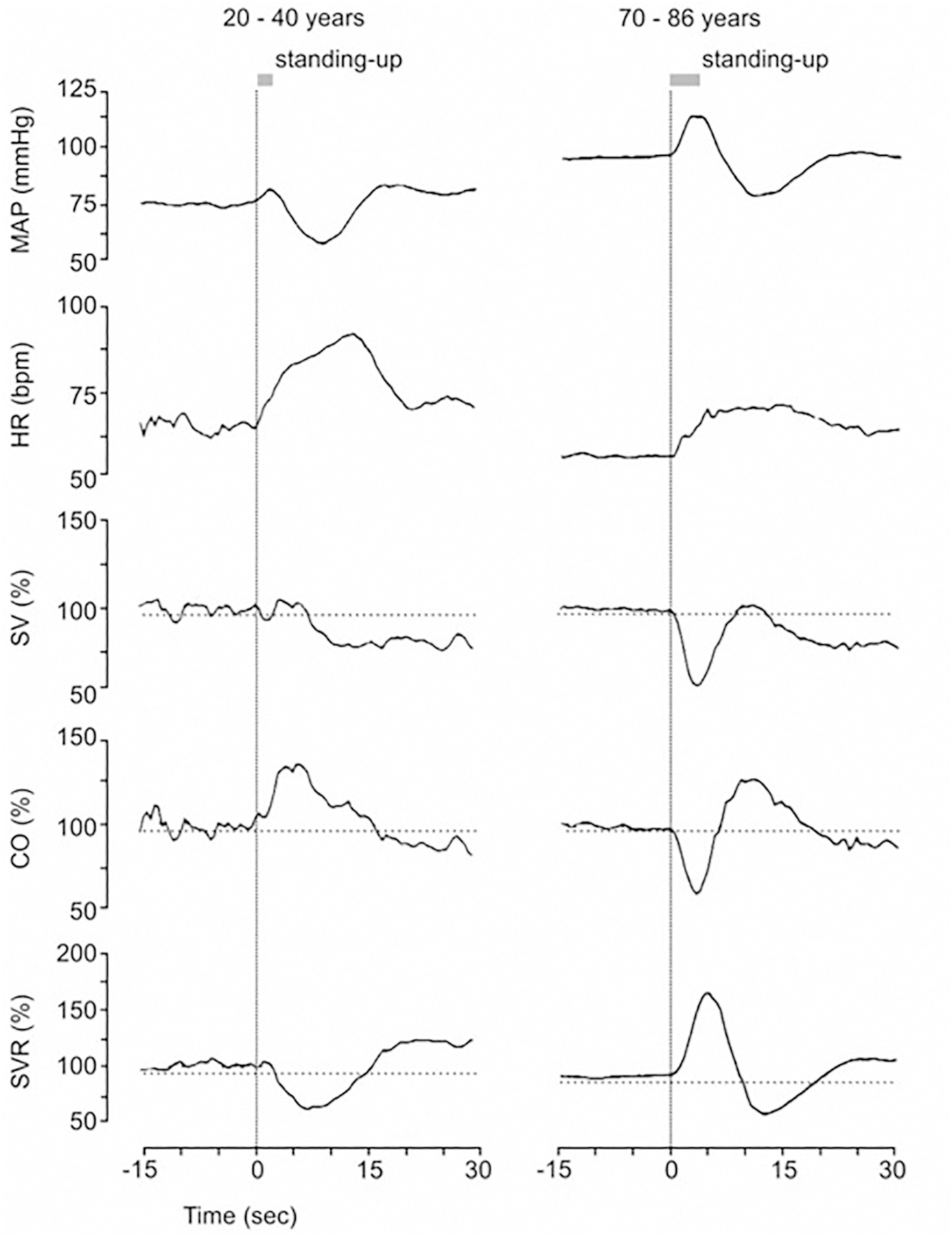
The effect of age on the initial hemodynamic responses to standing. Group mean finger arterial BP (MAP) and relative changes in stroke volume (SV), cardiac output (CO) and systemic vascular resistance (SVR) during the initial response upon standing. Average responses of 10 young participants (aged 22–40 years) (left panel) and 37 elderly participants (aged 70–86 years) (right panel) are shown. [Revised after ten Harkel et al., 1990 and Wieling et al., 1992 with permission].
In older adults the cardiovascular response to active standing differed from the response in young adults [Fig. 4]. The initial HR response was blunted as a sign of diminished vagal withdrawal (Eckberg and Sleight, 1992; Wieling and Karemaker, 2013) and an immediate temporary increase in MAP (17 ± 2 mmHg, p < 001 vs young adults) occurred that lasted for about 5 s. An increase in SVR underlies the immediate BP rise during the act of standing (6 s duration). CO decreased on standing. At 9.5 s, the immediate increase in BP was followed by a transient dip (−17 ± 2 mmHg) from baseline and recovery around 20 s. The magnitude of the drop in MAP was similar for young and older adults (−16 ± 2 mmHg vs −17 ± 2 mmHg) with a smaller transient rise in CO together with a less pronounced drop in SVR in the older adults. Importantly, it must be realized that the stimulus inducing cardiovascular reflex responses must be different across age groups since the older adults standing response was accompanied by the aforementioned pronounced and prolonged increase in BP. These immediate changes in SVR and CO resemble the circulatory response induced by the Valsalva maneuver (Eckberg and Sleight, 1992; Pott et al., 2003). They can be attributed to straining that accompanied the considerable physical activity needed for this age group to stand up quickly (Wieling et al., 1992).
2.2.2. TILDA studies
Recently in the Irish Longitudinal Study on Aging (TILDA) (2009–2019), FinAP active stand responses alongside comprehensive health, social and economic circumstances have been collected for the first time, as part of a large, longitudinal prospective population study of community-dwelling older adults aged 50 years and over. Normative and OH prevalence data for the active stand responses have been characterized in both the overall TILDA community population and its healthy subsample, where ‘healthy’ is defined as physically and socially active, independent, without cardiovascular (excluding hypertension) or other physical or cognitive health conditions and not taking any medications (Finucane et al., 2014; Van Wijnen et al., 2017). In both TILDA populations the active stand response was similar in temporal characteristics to the responses noted above (Fig. 4) although notably the drop in systolic BP was larger in both the overall population (ranging from 34 mmHg (IQR, 25.4–43.5 mmHg) to 45.3 mmHg [IQR, 33.4–59.0 mmHg] depending on age and gender) and the healthy subsample (40 ± 17 mmHg). The prevalence of delayed recovery was also high in both overall and over 70’s healthy TILDA populations (15.6% and 24% respectively) (Finucane et al., 2014).
3. Cerebrovascular adjustments in the first 60s of active standing
3.1. Hydraulic/physical changes on standing
Standing up challenges the cerebral circulation. From supine to upright the adult brain at eye level becomes positioned 25–30 cm above the heart. This simple hydrostatic effect lowers MAP instantaneously at eye level by about 20 mmHg (van Lieshout et al., 1985, Rowell, 1993, Hainsworth, 2004, Bronzwaer et al., 2017). Intracranial pressure does not drop to the same extent as MAP, consequently cerebral perfusion pressure is lower (Rosner and Coley, 1986). In addition to the lowered MAP and perfusion pressure at brain level, a change in cerebral venous outflow (Gisolf et al., 2004a) and lower PaCO2 (Van Lieshout et al., 2003, Gisolf et al., 2004b, Immink et al., 2005) may modify cerebral blood flow (CBF) on standing.
The PaCO2 has a pronounced influence on CBF. Hypocapnia induces cerebral vasoconstriction and can reduce CBF by 2% to 3% per mmHg in end-tidal CO2 (PETCO2) (Van Lieshout et al., 2003; Van Beek et al., 2008). In the initial phase of standing the PETCO2 decreases on average by 2–4 mmHg (Thomas et al., 2009; Lewis et al., 2013), but the changes in PETCO2 are difficult to interpret. This is due to a discrepancy between PaCO2 and PETCO2 arising from a perfusion mismatch with the reduction in PaCO2 being smaller than in PETCO2 in the initial phase of standing (Gisolf et al., 2004a). In addition, a CO2 transfer time of about 5 heart beats from the lungs to the brain (Wieling et al., 1992; Thomas et al., 2009) and a latency of changes in the brain PaCO2 and vasoconstriction of cerebral vessels (Thomas et al., 2009) needs to be considered. It seems reasonable to speculate that the changes in PETCO2 are unlikely to contribute considerably to the fall in CBF in the initial phase of standing.
3.2. Dynamic cerebral autoregulation
The potential role of dynamic cerebral autoregulation as a compensatory mechanism for the sudden fall in systemic BP induced by active standing is important to consider. In the following we will address studies that assessed dynamic cerebral autoregulation during an initial fall in BP induced by standing up from the supine (Van Lieshout et al., 2001; Kim et al., 2011) and sitting position (Sorond et al., 2005).
3.2.1. Cerebral autoregulatory control in young adults
Van Lieshout et al. studied MAP and mean middle cerebral artery velocity (MCA Vmean) responses induced by standing up from supine in 10 young adults aged 21–38 years using transcranial Doppler ultrasound (Fig. 5).
Fig. 5.
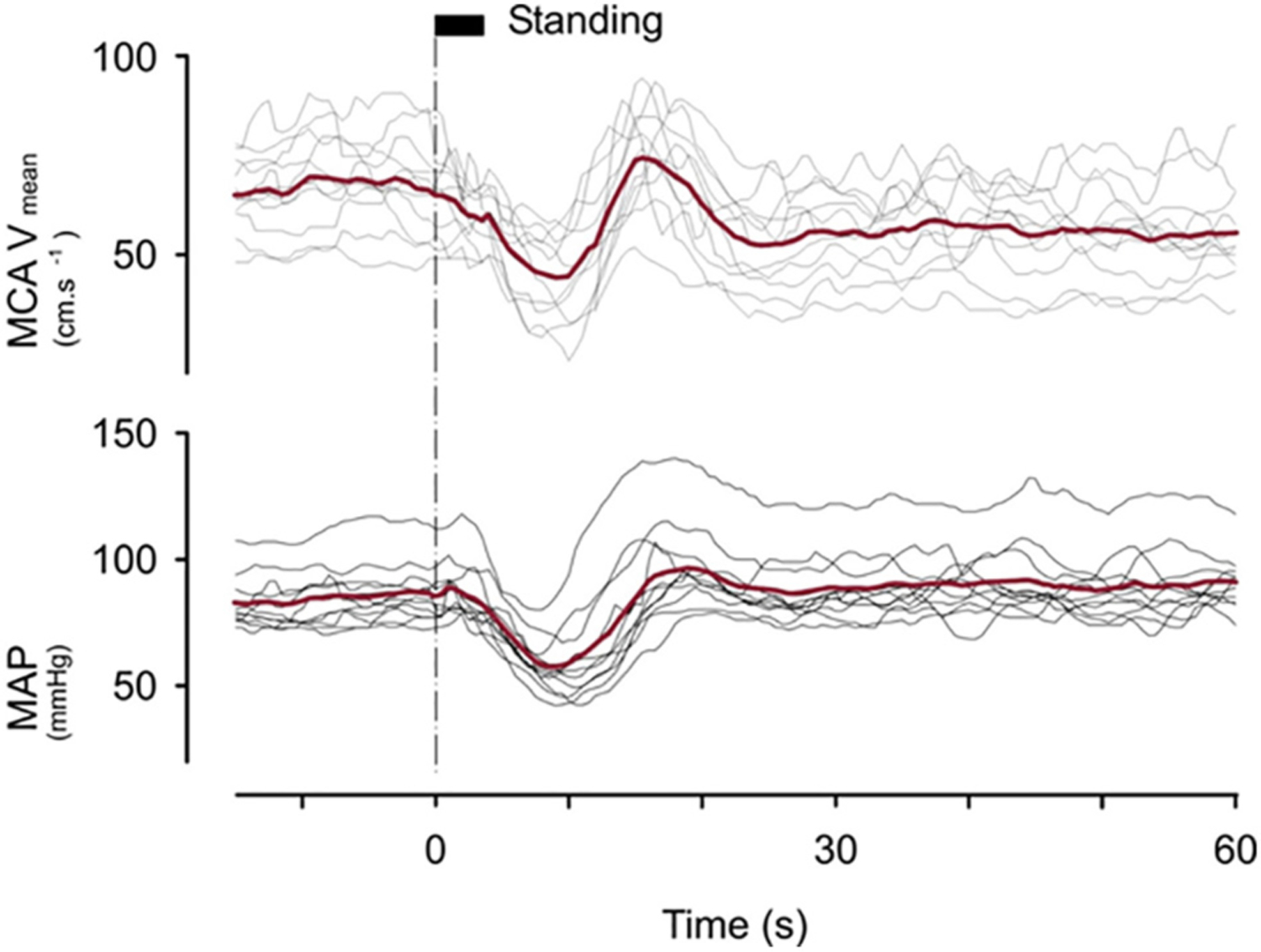
Mean middle cerebral artery blood velocity (MCA Vmean) and mean arterial pressure (MAP) responses induced by standing from supine in 10 young adults aged 21–38 years using transcranial Doppler ultrasound. Supine rest before standing amounted to at least 30 min. The thin lines indicate individual responses; thick line, averaged responses. The time needed to change posture is indicated at the top of the dotted line at T = 0 is rather long (about 5 s) in order not to lose the transcranial Doppler ultrasound signal. [From van Lieshout et al., 2001 with permission].
A large initial fall in MAP (about 25 mmHg) with a nadir of approximately 60 mmHg occurring at 8 s was observed. Cerebral autoregulation did not attenuate a steep initial fall in MCA Vmean [Fig. 5]. Almost identical patterns of mean MAP and MCA Vmean responses were reported by Thomas et al. in 46 young adults with a mean age of 25 years (Thomas et al., 2009). The faster recovery of MCA Vmean compared to MAP after the nadir at 10s manifests the effect of cerebral autoregulation (Fig. 4).
A delay in cerebral autoregulation of 7–9 s is also clearly visible in the studies by Sorond et al., 2005 and Kim et al., 2011 (Figs. 6 and 7). This delay indicates that cerebral autoregulation under these circumstances is rather slow. This is in accordance with data from the literature. The latency of cerebral autoregulation (i.e. vasodilatation) is about 3–5 s and it takes 5–10 s before the full effect of counter-regulation is reached (Aaslid et al., 1989; Immink et al., 2005; Rickards et al., 2007).
Fig. 6.
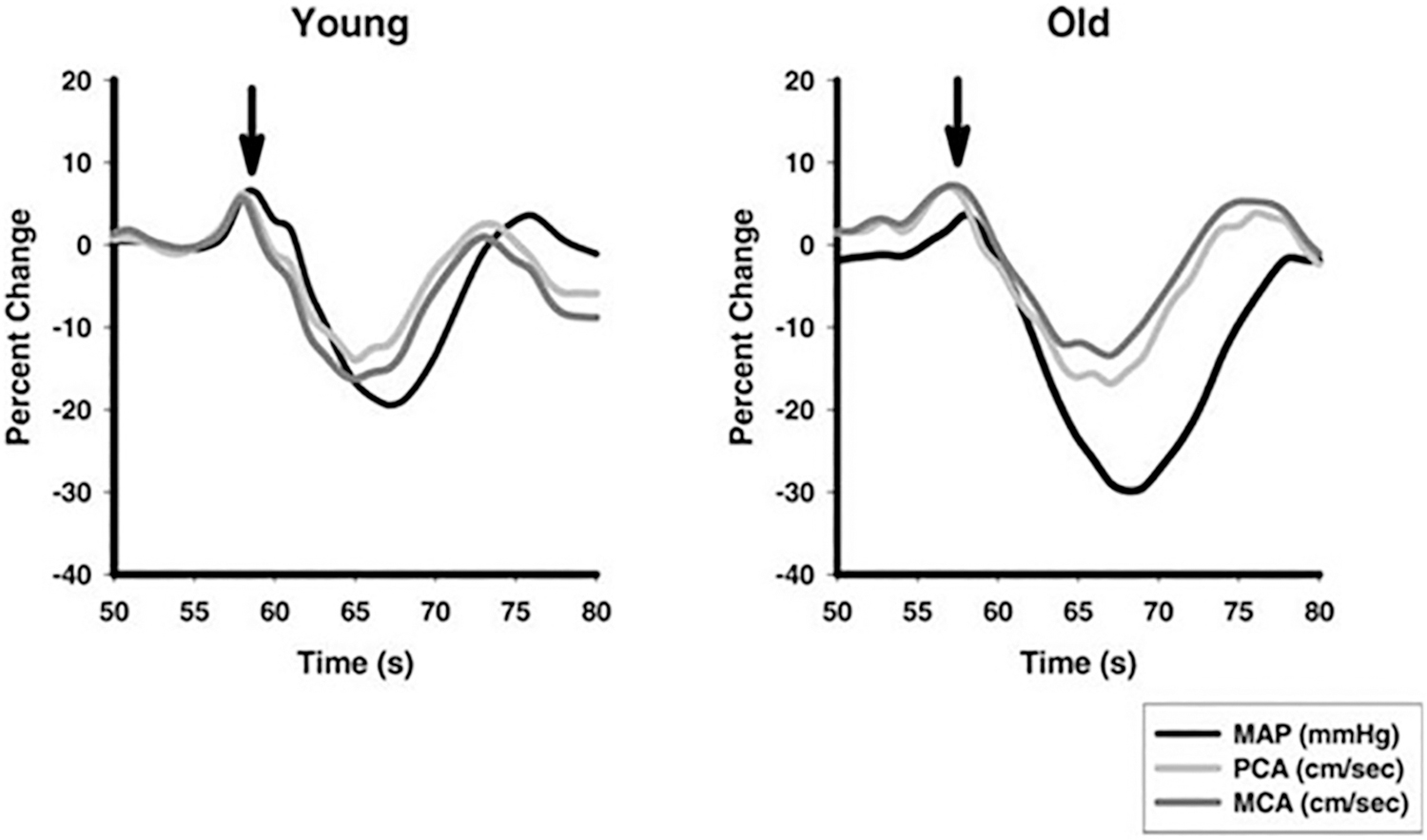
Relative percent changes over time (s) in mean arterial pressure (MAP) black line, middle (MCA, dark gray line), and posterior (PCA, light gray line) cerebral artery blood flow velocities during the sit-to-stand protocol in 13 young (mean age 30 years) and 13 older (mean age 73 years) healthy normotensive volunteers A sit to stand test was used. Subjects stood up after 5 min sitting in a straight-backed chair with their legs elevated at 90 degrees in front of them on a stool. Arrow indicates active standing. [From Sorond et al., 2005 with permission].
Fig. 7.
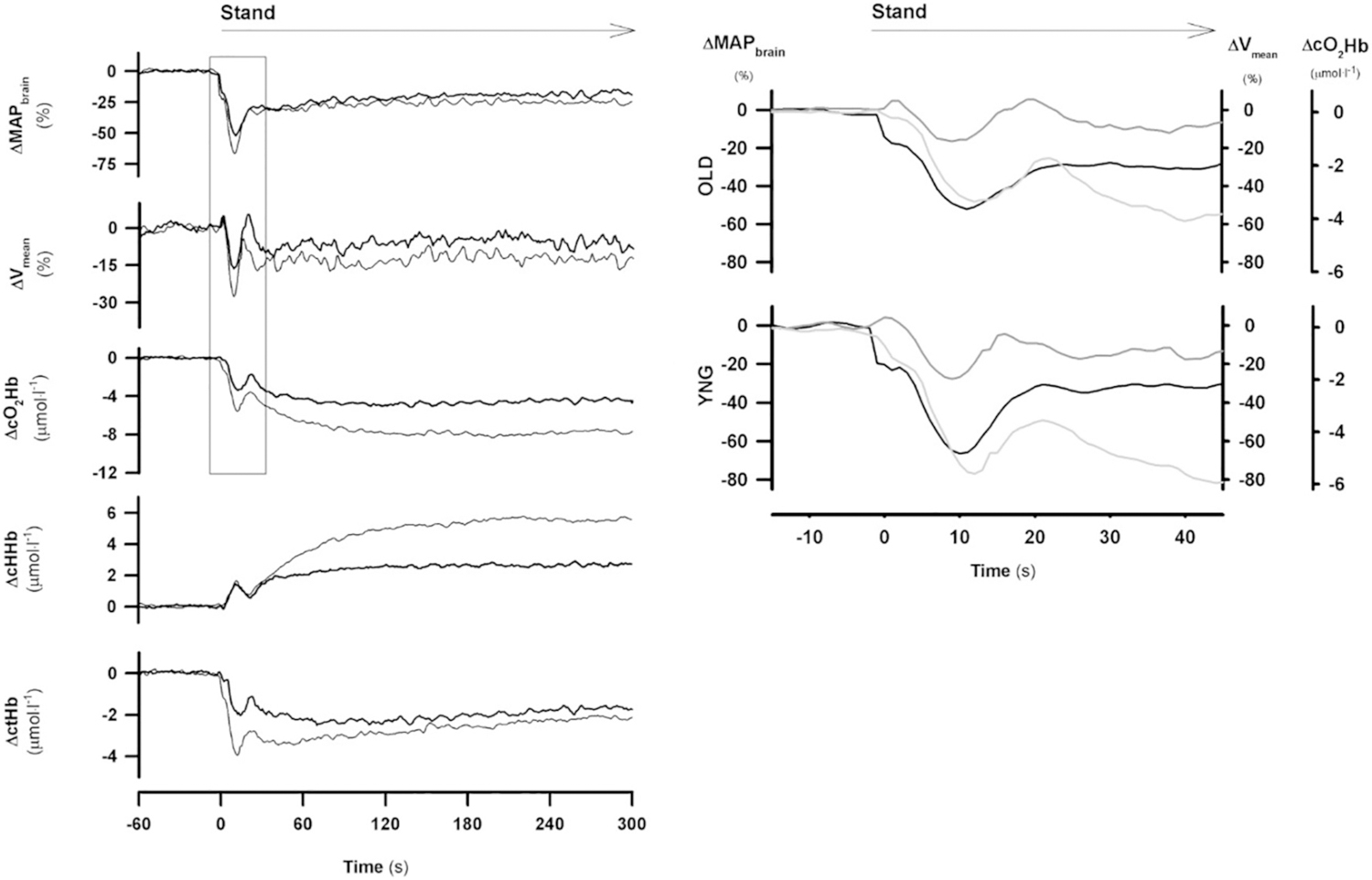
Left panel: postural cerebrovascular response in younger (YNG, n = 15, thin line) vs older group (OLD, n = 15, thick line). Following instrumentation and 5 min supine rest subjects stood up.
Left panel: mean arterial pressure at brain level (MAPbrain), mean middle cerebral artery blood flow velocity (Vmean), changes in oxygenated ∆cO2Hb), deoxygenated (∆cHHb) and total hemoglobin (∆ctHb) concentration. Right panel: initial postural responses of MAPbrain (black line), Vmean (dark gray line) and ∆cO2Hb (light gray line) in OLD (upper panel) vs. YNG (lower panel) [from Kim et al., 2011 with permission].
3.2.2. Cerebral autoregulatory control remains intact during active standing in older adults
Sorond et al. compared middle (MCA) and posterior (PCA) cerebral artery velocities in 13 young (mean age 30 years) and 13 older (mean age 73 years) healthy normotensive volunteers (Fig. 6) (Sorond et al., 2005). A sit to stand test was used. The sit-to-stand test technique is a suitable measure of dynamic cerebral autoregulation (Lipsitz et al., 2000; Sorond et al., 2009).
In young subjects a fall in MAP of −21 ± 2% was observed with a nadir at 9 s after the onset of standing. The recovery of MCA and PCA started at 7 s. In the older subjects the nadir in MAP was larger (−31 ± 3% P < 0.05) and occurred later (at 10 s). The decrease in MCA and PCA in the older subjects responses were similar (around 15%).
Kim et al. studied the effects of aging on cerebrovascular responses. Fifteen young volunteers with a median age of 29 years (interquartile range 27–33) and 15 older volunteers with a median age of 59 years (interquartile range 52–65) were studied (Fig. 7) (Kim et al., 2011). The nadir of MAP occurred at 9 s in both age groups and was, in contrast to the Sorond study larger in the young compared to in the older subjects (−67 ± 3% vs −52 ± 3% at brain level p < 0.05). The fall in MCA Vmean was also larger in the young subjects (−29 ± 3% vs −16 ± 4% p < 0.05).
Sorond et al. and Kim et al. concluded that cerebrovascular autoregulation to orthostatic stress was preserved in older adults.
4. Time course and magnitude of the hemodynamic events on active standing
Table 1 gives an overview of the time of the peaks and nadirs and magnitude of hemodynamic events induced by standing up in young adults in three studies discussed above (Sprangers et al., 1991b, Tanaka et al., 1996, Thomas et al., 2009).
Table 1.
Time of the peaks and nadirs and magnitude of hemodynamic
| Hemodynamic event | Time | Magnitude | Recovery/overshoot BP | Reference |
|---|---|---|---|---|
| MAPmin | 8 s | −23 ± 2 mmHg | Around 20 s | Sprangers et al., 1991b |
| 9.5 ± 0 s | −44 ± 5 mmHg | Around 20 s | Tanaka et al., 1996 | |
| 11±0 s | −36 ± 2 mmHg | Around 20 s | Thomas et al., 2009 | |
| ABDOMPRESmax | 2 s | 43 ± 8 mmHg | Tanaka et al., 1996 | |
| RAP max | 3 s | 10 mmHg | Sprangers et al., 1991b | |
| COmax | 6 s | 24 ± 6% | Sprangers et al., 1991b | |
| 9.5 | 37 ± 9% | Tanaka et al., 1996 | ||
| ~8 s | 49 ± 5% | Thomas et al., 2009 | ||
| SVRmin | 7 (2–13) | −36 ± 3% | Sprangers et al., 1991b | |
| 9.5 s | −58 ± 4% | Tanaka et al., 1996 | ||
| – | −61 ± 2% | Thomas et al., 2009 |
MAPmin = mean arterial pressure nadir; ABDOMPRESmax = peak abdominal pressure increase; RAPmax = peak right atrial pressure increase; COmax = peak cardiac output left ventricle; SVRmin = nadir systemic vascular resistance.
Intraabdominal pressure and right atrial pressure increased instantaneously during the standing up maneuver and peaked at 2–3 s. The peak in CO and nadir in SVR occurred between 6 and 10 s. Nadirs of MAP occurred between 8 and 11 s and a recovery around 20 s. The magnitude of the responses showed a considerable variation (see also MAP changes in Fig. 4).
Further physiological studies are needed to detail the hemodynamic events on standing in older adults.
5. The duration, not the magnitude, of initial reductions in systemic blood pressure predicts symptoms of presyncope
5.1. Studies in healthy recreationally active young adults
Carefully performed studies in Ainslie’s laboratory (Thomas et al., 2009, Lewis et al., 2011, Lewis et al., 2013) provide data on IOH and delayed BP recovery related symptoms within the first 60 s of standing in healthy recreationally active young adults.
Thomas et al. investigated whether the occurrence of IOH and related symptoms are related to the occurrence of presyncope induced by 60 degree HUT combined with lower body negative pressure (LBNP) (Thomas et al., 2009). Presyncope was defined as a drop in systolic BP below 80 mmHg, the manifestation of severe symptoms, or both. Thirty-eight young adults (mean age 25 years) were studied. Beat-to-beat BP, MCA Vmean, PETCO2 and cerebral cortical tissue oxygenation (near-infrared spectroscopy) were measured (Thomas et al., 2009).
A mean fall in systolic BP pressure of −42/3 mmHg with pronounced transient hypotension (nadir of systolic BP on average 80 mmHg and of MAP on average around 50 mmHg) were found around 11 s after the onset of active standing. Recovery of BP occurred in almost all participants within 20 s. Symptoms of hypotension consisted of light-headedness, present in 16/38 subjects. None of the subjects fainted. Presyncope induced by HUT combined with LBNP elicited symptoms of light-headedness, visual disturbances and nausea in 28/38 participants. However, the duration and pattern of hypotension was gradual and prolonged (about 30 s) during HUT with LBNP versus standing. The nadir of MAP during HUT with LBNP (on average around 50 mmHg) was identical to the nadir on standing.
The study by Thomas et al. showed that a transient deep fall of BP on standing with MAP around 50 mmHg at heart level (i.e. a MAP of only 30 mmHg at eye level) is well tolerated in young adults. Since MAP nadirs on standing and HUT combined with LBNP were similar (around 50 mmHg) we may conclude that it is the duration of hypotension and hypoperfusion, rather than the magnitude of the BP level determine whether presyncope occurs. The cardiovascular and cerebrovascular changes during IOH were unrelated to those at presyncope induced by HUT combined with LBNP. Interestingly, there was no relationship between the hemodynamic change and the incidence of subjective symptoms in either scenario suggesting that symptoms are not directly related or predictable from any of the haemodynamic variables measured. Lewis et al. documented in 12 young adults that after alpha-blockade the magnitude of the fall in BP was similar (around 40 mmHg in MAP), but with a lower nadir (39 vs 51 mmHg) and that the recovery of BP was severely delayed and the steady-state value reached 20–30 s after standing was much lower in those with alpha blocker (MAP on average around 60 mmHg vs 95 mmHg). Severe presyncopal symptoms (light-headedness, visual disturbances and nausea) occurred in 8/12 subjects in the first 20 s and only 2/12 could stand for 2 min (Lewis et al., 2013). This relates to the larger associated decline in MCA Vmean. The study by Lewis et al. suggests that in the presence of alpha-blockade sustained hypotension of about 60 mmHg in MAP at heart level may result in near-syncope in young adult subjects but it is difficult to disentangle these effects.
5.2. Studies in patients with sympathetic failure and older adults
In patients with sympathetic failure, the orthostatic reduction in cerebral blood velocity and oxygenation is larger. Nevertheless, orthostatic tolerance may vary considerably between patients with the time tolerated in the upright position ranging between 1 to (at least) 5 min of standing. Patients who become symptomatic within 5 min of standing are characterized by a pronounced orthostatic fall in BP, cerebral blood velocity, and oxygenation manifest within the first 10 s of standing with very low values of MAP at brain level (around 20 mmHg) (Harms et al., 2000).
Data in older adults in the TILDA studies provide some information on OH and related symptoms in the first minute of standing. Briggs et al. documented that symptomatic delayed BP recovery at 30 s after standing (fall in BP > 20 mmHg) was associated with depression. This group of individuals had an average SBP nadir of 80 mmHg at 10 s standing. Briggs et al. noted that those with IOH alone without delayed recovery (fall in systolic BP > 40 mmHg with or without symptoms) did not have poor outcomes (Briggs et al., 2018). These findings suggest that delayed recovery of BP is potentially of more importance than the initial degree of drop in BP when considering old age and tends to agree with Lewis et al., 2013 in young adults (see above).
Other studies from TILDA including Frewen 2014, Hayakawa et al., 2015, Finucane et al., 2017 and McNicholas et al., 2018 come to a similar conclusion that delayed BP recovery tends to be associated with poorer health outcomes (e.g. poor cognitive function, conversion to dementia, rate of cognitive decline, falls) while initial BP drops do not. This was similarly seen in the ARIC cohort study where measurements between 20 and 60 s of standing were most strongly associated with falls and fractures (Juraschek et al., 2017).
According to traditional teaching, symptoms of OH on average occur with sustained systolic BP <80 mmHg and syncope on average with sustained systolic BP of 50–60 mmHg at heart level, but there is significant variation in these values (Giese et al., 2004, Wieling et al., 2009, Jardine et al., 2018, Wieling et al., 2013, Bachus et al., 2018). Thus, the level of BP at which symptoms appear in the studies is still open to debate (Thomas et al., 2009, Lewis et al., 2011, Lewis et al., 2013, Briggs et al., 2017) and is dependent heavily on hypotension duration (see above).
It is important to realize that the level at which hypoperfusion related symptoms or syncope occur is dependent on the lower threshold of cerebral autoregulation, which is characterized by significant inter-individual variability. It has been shown that the autoregulation curve shifts importantly to the right in patients with severe hypertension, such that a decline in cerebral blood flow occurs at higher values in hypertensive individuals. This can explain why people with severe hypertension may develop symptoms of cerebral hypoperfusion at higher BP levels than normotensives (Strandgaard, 1976).
5.3. Cerebral anoxia reserve time
The time span from the start of critical cerebral hypoperfusion to loss of consciousness is known as “cerebral anoxia reserve time”. In young adults cerebral anoxia reserve time amounts on average to 6–8 s (Wood, 2000; Wieling et al., 2009). No data are available comparing the anoxia reserve time in young adults with those in older subjects, but the following observations suggest that the anoxia reserve time in older adults is quite similar to that measured in young adults. First, records of spontaneous episodes of presyncope and syncope in older patients during very prolonged ECG monitoring showed that spontaneous episodes of asystole lasting >6 s elicited symptoms of presyncope or syncope in up to 40% of 23 older patients with recurrent syncope (Menozzi et al., 1993). With shorter asystolic episodes lasting 3–6 s patients were symptomatic in only 0.7%. Second, symptomatic hypotension in patients with cardioinhibitory carotid sinus massage occurs on average after a pause of 7.9 s. The asystolic pause is at least 6 s long in 75% of the patients (Puggioni et al., 2002; Wieling et al., 2013). Accordingly, Maggi et al. demonstrated in 16 patients with predominant cardioinhibitory carotid sinus hypersensitivity that during spontaneous syncope captured with an implantable loop record the average duration of asystole was 9 s (range 8–18 s) (Maggi et al., 2007).
6. Clinical perspective
With the above information about the physiology of systemic and cerebral circulatory adjustment within the first 60 s after active standing as a background we will address the clinical relevance of knowing the physiology in detail.
1). Normal initial BP and HR responses.
A normal initial BP response on standing consists of a transient fall of BP with a nadir around 8 s followed by a rapid recovery/overshoot of BP within 30 s. An accompanying normal initial HR response includes an immediate HR increase, a large HR peak and a subsequent HR recovery (Fig. 1 panel A). For reasons explained above these combined responses indicate intact afferent, central and efferent cardiac vagal and sympathetic vasomotor baroreflex pathways (Wieling and Karemaker, 2013). The relation of the HR decrease to the recovery/overshoot in arterial BP is a measure of arterial baroreceptor sensitivity. Baroreflex sensitivity is central to cardiovascular health (Lauer, 2016). Accordingly, a study by McCrory et al., in 4475 participants of the TILDA cohort has shown that the presence of a HR recovery on standing is an important independent predictor of mortality (McCrory et al., 2016).
2). Initial orthostatic hypotension (IOH).
Most people occasionally experience transient light-headedness and visual disturbances i.e. seeing black spots typically occurring 7–10 s after standing up. Symptoms usually disappear within 20–30 s (Wieling et al., 2007). Such complaints result from temporary cerebral hypoperfusion occurring in the initial phase of standing as explained above. An abnormally large initial fall in BP is defined by a transient BP drop ≥40 mmHg in systolic BP within 15 s after the onset of standing followed by quick recovery (Fig. 1 panel B) (Freeman et al., 2011; Van Wijnen et al., 2017). IOH is a physiological sign, symptoms do not need to be present. A clinical history of IOH is a frequent cause (7–14%) of unexplained syncope in patients referred to tertiary centres (Van Wijnen et al., 2017, Van Twist et al., 2018, de Jong et al., 2020). However in the absence of a clear history, IOH as a physiological sign is a less accurate predictor of outcome. Importantly IOH as a physiological sign has a high prevalence (van Twist et al., 2020) and is not associated with poor health outcomes in older adults (Finucane et al., 2017, Briggs et al., 2018, Van Twist et al., 2018) and even better physical, functional and cognitive performance has been reported in patients with IOH (Saedon et al., 2020).
As far as applying the physiological knowledge described above physical counter maneuvers e.g. buttock clenching are an effective strategy to combat IOH (Krediet et al., 2007).
3). Delayed recovery of BP.
Delayed BP recovery is characterized by a systolic BP fall of >20 mmHg at 30–40 s of standing, but not meeting the criteria of classical orthostatic hypotension (Fig. 1 panel C) (Finucane et al., 2014, Van Wijnen et al., 2017, Briggs et al., 2018, Finucane et al., 2019). Physiological studies indicate that diminished vasoconstrictor capacity is a likely underlying mechanism (see above). However, recent studies document that hemodynamic BP recovery patterns are heterogenous (van Wijnen et al., 2018), apart from diminished vasoconstrictor capacity due to autonomic dysfunction, volume status and medication use should be taken into account (Finucane et al., 2014; van Wijnen et al., 2018).
Epidemiological evidence of studies using continuous BP measurement suggest that delayed BP recovery tends to be associated with poorer health outcomes (e.g. all cause mortality, unexplained falls, poor cognitive function, conversion to dementia, depression, physic, functional and cognitive performance) (Lagro et al., 2014, Hayakawa et al., 2015, Finucane et al., 2017, McNicholas et al., 2018, Briggs et al., 2018, Saedon et al., 2020).
The association of early hypotension on standing with poor health outcomes was similarly seen in studies using oscillometric BP measurements between 20 and 60 s of standing. Early hypotension was associated with falls and fractures (Juraschek et al., 2017) and dementia and cognitive decline (Rawlings et al., 2018). Self-reported dizziness was found to be more consistently associated with neurological outcomes (MRI findings, cognition, dementia or stroke) than OH (fall in systolic/diastolic BP of >20/10 mmHg after 3 min standing) in a recent study by Juraschek (Juraschek et al., 2020).
4). Classical orthostatic hypotension (OH).
OH is defined as a sustained BP drop of >20 mmHg following standing (Fig. 1 panel D) (Freeman et al., 2011). Orthostatic hypotension is an established risk factor for common age-related outcomes e.g. falls, unexplained falls, syncope, cognition, depression, fractures, mortality (Rutan et al., 1992; Tinetti et al., 1994; Gangavati et al., 2011).
7. Conclusion
This review provides an integrative physiological view on the systemic and cerebral circulatory adjustment within the first 60 s after active standing with almost all studies performed in young adults to date. We would recommend that future work should employ carefully performed physiological studies in older adults to disentangle the link between blood pressure characteristics, cerebral blood flow and symptoms.
Grant support
CF holds Science Foundation Ireland and Irish Research Council grants; Grant No: 19/IFA/7409 and Grant No: GOIPG/2018/134.
LP is supported by the Irish Research Council: Government of Ireland Postgraduate Scholarship Programme 2018 (Grant No: GOIPG/2018/134) and the Fundació Universitària Agustí Pedro i Pons, Universitat de Barcelona.
LL holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew Senior Life and reports grants from the National Institute on Aging: R01 AG025037, P30 AG031679, and R01 AG059089.
SJ reports National Institute of Health grant support; NIH/NHLBI: K23HL135273-04.
Footnotes
Declaration of competing interest
There are no conflict of interest.
References
- Aaslid R, Lindegaard KF, Sorteberg W, et al. , 1989. Cerebral autoregulation dynamics in humans. Stroke 20, 45–52. [DOI] [PubMed] [Google Scholar]
- Bachus E, Holm H, Hamrefors V, et al. , 2018. Monitoring of cerebral oximetry during head-up tilt test in adults with history of syncope and orthostatic intolerance. Europace 20, 1535–1542. [DOI] [PubMed] [Google Scholar]
- Borst C, Wieling W, van Brederode JF, et al. , 1982. Mechanisms of initial heart rate response to postural change. Am. J. Phys 243, H676–H681. [DOI] [PubMed] [Google Scholar]
- Borst C, van Brederode JF, Wieling W, et al. , 1984. Mechanisms of initial blood pressure response to postural change. Clin. Sci 67, 321–327. [DOI] [PubMed] [Google Scholar]
- Briggs R, Carey D, Kennelly SP, et al. , 2018. Longitudinal association between symptomatic orthostatic hypotension at 30 seconds post standing and incident late life depression. Hypertension 71, 964–954. [DOI] [PubMed] [Google Scholar]
- Brignole M, Moya A, 2018. de Lange FJ’., et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur. Heart J. 39, 1883–1948. [DOI] [PubMed] [Google Scholar]
- Bronzwaer AGT, Verbree J, Stok WJ, et al. , 2017. The cerebrovascular response to lower-body negative pressure vs. head-up tilt. J. Appl. Physiol 122, 877–883. [DOI] [PubMed] [Google Scholar]
- Callister R, Ng AV, Seals DR, 1994. Arm muscle sympathetic nerve activity during preparation for and initiation of leg-cycling exercise. J. Appl. Physiol 77, 1403–1410. [DOI] [PubMed] [Google Scholar]
- Coupland NJ, Bailey JE, Wilson SJ, et al. , 1995. The effects of clonidine on cardiovascular responses to standing in healthy volunteers. Clin. Auton. Res 5, 171–177. [DOI] [PubMed] [Google Scholar]
- de Jong JSY, Snijders Blok MR, Thijs RD, Harms MPM, Hemels MEW, de Groot RD, et al. , 2020. Diagnostic Yield and Accuracy of Diagnoses in a Tertiary Syncope Unit with Structured Approach: a Prospective Cohort Study. In: Europace in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Sleight P, 1992. Human Baroreflexes in Health and Disease. Clarendon Press, Oxford, Monograph of the Physiological Society. [Google Scholar]
- Finucane C, O’Connell MD, Fan CW, et al. , 2014. Age-related normative changes in phasic orthostatic blood pressure in a large population study: findings from the Irish longitudinal study on ageing (TILDA). Circulation 130, 1780–1789. [DOI] [PubMed] [Google Scholar]
- Finucane C, O’Connell MD, Donoghue O, et al. , 2017. Impaired orthostatic blood pressure recovery is associated with unexplained and injurious falls. J. Am. Geriatr. Soc 65, 474–482. [DOI] [PubMed] [Google Scholar]
- Finucane C, van Wijnen VK, Fan CW, et al. , 2019. A practical guide to active stand testing and analysis using continuous beat-to-beat non-invasive blood pressure monitoring. Clin. Auton. Res 29, 427–441. [DOI] [PubMed] [Google Scholar]
- Freeman R, Wieling W, Axelrod FB, et al. , 2011. April. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res 21 (2), 69–72. [DOI] [PubMed] [Google Scholar]
- Frewen J, Finucane C, 2014. Orthostatic hypotension is associated with lower cognitive performance in adults aged 50 plus with supine hypertension. J Gerontol Series A: Biomedical Sciences and Medical Sciences 69, 878–885. [DOI] [PubMed] [Google Scholar]
- Gangavati A, Hajjar I, Quach L, et al. , 2011. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J. Am. Geriatr. Soc 59, 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese AE, Li V, McKnite S, et al. , 2004. Impact of age and blood pressure on the lower arterial pressure limit for maintenance of consciousness during passive upright posture in healthy vasovagal fainters: preliminary observations. Europace 6, 457–462. [DOI] [PubMed] [Google Scholar]
- Gisolf J, van Lieshout JJ, van Heusden K, et al. , 2004a. Human cerebral venous outflow pathway depends on posture and central venous pressure. J. Physiol. 560, 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisolf J, Wilders R, Immink RV, et al. , 2004b. Tidal volume, cardiac output and functional residual capacity determine end-tidal CO2 transient during standing up in humans. J. Physiol 554, 579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth R, 2004. Pathophysiology of syncope. Clin. Auton. Res 14 (Suppl. 1), 1/18–1/19. [DOI] [PubMed] [Google Scholar]
- Halliwil JR, 2007. Virtual conductance, real hypotension: what happens when we stand up too fast ? J. Appl. Physiol 103, 421–422. [DOI] [PubMed] [Google Scholar]
- Harms MPM, Colier WNJM, Wieling W, et al. , 2000. Orthostatic tolerance, cerebral oxygenation and blood velocity in humans with sympathetic failure. Stroke 31, 1608–1614. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, McGarrigle CA, Coen RF, et al. , 2015. Orthostatic blood pressure behavior in people with mild cognitive impairment predicts conversion to dementia. J AmGeriatr Soc 63, 1868–1873. [DOI] [PubMed] [Google Scholar]
- Imholz BP, Dambrink JH, Karemaker JM, et al. , 1990a. Orthostatic circulatory control in the elderly evaluated by non-invasive continuous blood pressure measurement. Cardioscular. Res 79, 73–79. [DOI] [PubMed] [Google Scholar]
- Imholz BP, Settels JJ, van der Meiracker AH, et al. , 1990b. Non-invasive continuous finger blood pressure measurement during orthostatic stress compared to intra-arterial pressure. Cardiovasc. Res 24, 214–221. [DOI] [PubMed] [Google Scholar]
- Immink R, van Montfrans GA, Stam J, et al. , 2005. Dynamic cerebral autoregulation in acute lacunar and middle artery territory stroke. Stroke 36, 2595–2560. [DOI] [PubMed] [Google Scholar]
- Jardine DL, Wieling W, Brignole M, et al. , 2018. The pathophysiology of the vasovagal response. Heart Rhythm 15, 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraschek SP, Daya N, Rawlings AM, et al. , 2017. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Intern. Med 177, 1316–1323. Heart Rhythm 2018;15:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraschek SP, Longstreth WT Jr., Lopez OL, et al. , 2020. October 6. Orthostatic hypotension, dizziness, neurology outcomes, and death in older adults. Neurology 95 (14), e1941–e1950. 10.1212/WNL.0000000000010456. Epub 2020 Jul 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Bogert WJL, Immink RV, et al. , 2011. Effects of aging on the orthostatic cerebrovascular response. Neurobiol. Aging 32, 344–353. [DOI] [PubMed] [Google Scholar]
- Krediet CTP, Go-Schön IK, Kim YS, et al. , 2007. Management of initial orthostatic hypotension: lower body muscle tensing attenuates the transient arterial blood pressure decrease upon standing from squatting. Clin Sci(Lond) 113, 401–407. [DOI] [PubMed] [Google Scholar]
- Lagro J, Schoon Y, Heerts I, et al. , 2014. Impaired systolic blood pressure recovery directly after standing predicts mortality in older falls clinic patients. J GerontolA Biol medical SciMed Sci 69, 471–478. [DOI] [PubMed] [Google Scholar]
- Lauer MS, 2016. Heart rate recovery coming back full-circle to the baroreflex. Circ. Res 119, 582–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis NCS, Atkinson G, Lucas SJE, et al. , 2011. Is there diurnal variation in initial and delayed orthostatic hypotension during standing and head-up tilt. Chronobiol. Int 28, 135–145. [DOI] [PubMed] [Google Scholar]
- Lewis NC, Ainslie PN, Atkinson G, et al. , 2013. Initial orthostatic hypotension and cerebral blood flow regulation: effect of alpha1-adrenoreceptor activity. Am J Physiol Regul Integr Comp Physiol 304, R147–R154. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Mukai S, Hamner J, et al. , 2000. Dynamic regulation of middle cerebral artery blood flow velocity in aging and hypertension. Stroke 31, 1879–1903. [DOI] [PubMed] [Google Scholar]
- Maggi R, Menozzi C, Brignole M, et al. , 2007. Cardioinhibitory carotid sinus hypersensitivity predicts an asystolic mechanism of spontaneous neurally mediated syncope. Europace 9, 563–567. [DOI] [PubMed] [Google Scholar]
- McCrory C, Berkman L, Nolan H, et al. , 2016. Speed of heart rate recovery in response to orthostatic challenge. Circ. Res 119, 666–675. [DOI] [PubMed] [Google Scholar]
- McNicholas T, Tobin K, Carey D, et al. , 2018. Is baseline orthostatic hypotension associated with a decline in global cognitive performance at 4-year follow-up? Data from TILDA (the Irish longitudinal study on ageing). J Am Heart Association 7 (19), e008976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menozzi C, Brignole M, Lolli G, et al. , 1993. Follow-up of asystolic episodes in patients with cardioinhibitory, neurally mediated syncope and VVI pacemaker. Am. J. Cardiol 72, 1152–1155. [DOI] [PubMed] [Google Scholar]
- Mol A, Woltering JHH, Collier WNJM, et al. , 2019. Sensitivity and reliability of cerebral oxygenation responses to postural changes measured with near-infrared spectroscopy. Europ J Appl Physiol 119, 1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney D, O’Connor J, Newman L, et al. , 2020. Clinical clustering of eight orthostatic haemodynamic patterns in The Irish Longitudinal Study on Ageing (TILDA). Age Ageing afaa174. 10.1093/ageing/afaa174. Online ahead of print. [DOI] [PMC free article] [PubMed]
- Mukherjee S, 2015. The Laws of Medicine; Field Notes from an Uncertain Science. Simon & Schuster UK Ltd, London, Great Britain. [Google Scholar]
- O’Connor JD, Matthew DL, O’Connell DLO, et al. , 2020. Impact of standing speed on the peripheral and central hemodynamic response to orthostasis. Evidence from the Irish longitudinal study on aging. Hypertension 75, 524–531. [DOI] [PubMed] [Google Scholar]
- Pott F, van Lieshout JJ, Ide K, et al. , 2003. Middle cerebral artery blood velocity during intense static exercise is dominated by a Valsalva maneuver. J. Appl. Physiol 94, 1335–1344. [DOI] [PubMed] [Google Scholar]
- Rawlings AA, Juraschek SP, Heiss G, et al. , 2018. Associaton of orthostatic hypotensin with incident dementia, stroke, and cognitive decline. Neurology 91, e759–e768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puggioni EGV, Brignole M, Menozzi C, et al. , 2002. Results an complications of the carotid sinus massage performed according to the “methods of symptoms”. Am. J. Cardiol 89, 599–601. [DOI] [PubMed] [Google Scholar]
- Rickards CA, Cohen KD, Bergeron LL, et al. , 2007. Cerebral blood flow response and its association with symptoms during orthostatic hypotension. Aviat. Space Environ. Med 78, 653–658. [PubMed] [Google Scholar]
- Rosner MJ, Coley IB, 1986. Cerebral perfusion pressure, intracranial pressure, and head elevation. J. Neurosurg 65, 636–641. [DOI] [PubMed] [Google Scholar]
- Rowell LB, 1993. Human Cardiovascular Control. Oxford University Press, Oxford. [Google Scholar]
- Rutan GH, Hermanson B, Bild DE, et al. , 1992. Orthostatic hypotension in older adults. The cardiovascular health study. CHS collaborative research group. Hypertension 19, 508–519. [DOI] [PubMed] [Google Scholar]
- Saedon NI, Frith J, Goh CH, et al. , 2020. Orthostatic blood pressure changes and physic, functional and cognitive performance; the MEL0R study. Clin Auton Res 30, 129–1378. [DOI] [PubMed] [Google Scholar]
- Shen WK, Sheldon RS, Benditt DG, et al. , 2017. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. Heart Rhythm 14, e155–e217. [DOI] [PubMed] [Google Scholar]
- Sheriff DD, Nadland IH, Toska K, 2007. Hemodynamic consequences of rapid changes in posture in humans. J. Appl. Physiol 103, 452–458. [DOI] [PubMed] [Google Scholar]
- Smit AA, Halliwill JR, Low PA, et al. , 1999. Pathophysiological basis of orthostatic hypotension in autonomic failure. J. Physiol 519, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AJJ, Timmers HJLM, Wieling W, et al. , 2002. Long-term effects of carotid sinus denervation on arterial blood pressure in humans. Circulation 105, 1329–1335. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Porth CM, Erickson M, 1994. Hemodynamic response to the upright posture. J. Clin. Pharmacol 34, 375–386. [DOI] [PubMed] [Google Scholar]
- Smith L, Hamer M, Ucci M, et al. , 2015. Weekday and weekend patterns of objectively measured sitting, standing, and stepping in a sample of office-based workers: the active buildings study. BMC Public Health 17 (15), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Khavari S, Serrador JM, et al. , 2005. Regional cerebral autoregulation during orthostatic stress: age-related differences. J. Gerontol 60A, 1484–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Khavari S, Serrador JM, et al. , 2009. The sit-to-stand technique for the measurement of dynamic cerebral autoregulation. Ultrasound in Med & Biol 365, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprangers RLH, van Lieshout JJ, Karemaker JM, et al. , 1991a. Circulatory responses to stand up: discrimination between the effects of respiration, orthostasis and exercise. Clin. Physiol 11, 221–230. [DOI] [PubMed] [Google Scholar]
- Sprangers RLH, Wesseling KH, Imholz AL, et al. , 1991b. Initial blood pressure fall on stand up and exercise explained by changes in total peripheral resistance. J. Appl. Physiol 70, 523–530. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Clarke D, 2011. He’s dizzy when he stands up. An Introduction to Initial Orthostatic Hypotension. J Pediatric 158, 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandgaard S, 1976. Autoregulation of cerebral blood flow in hypertensive patients. The modifying influence of prolonged antihypertensive treatment on the tolerance to acute, drug-induced hypotension. Circulation 53, 720–727. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Sjoberg BJ, Thulesius O, 1996. Cardiac output and blood pressure during active and passive standing. Clin. Physiol 16, 157–170. [DOI] [PubMed] [Google Scholar]
- Ten Harkel AD, van Lieshout JJ, Van Lieshout EJ, et al. , 1990. Assessment of cardiovascular reflexes: influence of posture and period of preceding rest. J. Appl. Physiol 68, 147–153. [DOI] [PubMed] [Google Scholar]
- Thomas KN, Cotter JD, Williams MJA, et al. , 2009. Initial orthostatic hypotension is unrelated to orthostatic tolerance in healthy young subjects. J. Appl. Physiol 107, 506–517. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Baker DI, McAvay G, et al. , 1994. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N. Engl. J. Med 331, 821–827. [DOI] [PubMed] [Google Scholar]
- Trichopoulos D, 1996. The future of epidemiology. BMJ 313, 436–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschakovsky ME, Matusiak K, Vipond C, et al. , 2011. Lower limb-localized vascular phenomena explain initial orthostatic hypotension upon standing from squat. Am. J. Physiol. Heart Circ. Physiol 301, H2102–H2112. [DOI] [PubMed] [Google Scholar]
- Van Beek AHEA, Claassen JAHR, Olde Rikkert MGM, et al. , 2008. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cerebral Blood Flow & Metabolism 28, 1071–1085. [DOI] [PubMed] [Google Scholar]
- Van Heusden K, Gisolf J, Stok WJ, et al. , 2006. Mathematical modeling of gravitational effects on the circulation: importance of the time course of venous pooling and blood volume changes in the lungs. Am. J. Physiol. Heart Circ. Physiol 291, H2152–H2165. [DOI] [PubMed] [Google Scholar]
- Van Lieshout JJ, Pott F, Madsen PL, et al. , 2001. Muscle tensing during standing. Effects on cerebral tissue oxygenation and cerebral artery blood velocity. Stroke 32, 1546–1551. [DOI] [PubMed] [Google Scholar]
- Van Lieshout JJ, Wieling W, Karemaker JM, et al. , 2003. Syncope, cerebral perfusion, and oxygenation. J. Appl. Physiol 94, 833–848. [DOI] [PubMed] [Google Scholar]
- Van Twist DJL, Dinh H, Bouwmans EME, et al. , 2018. Initial orthostatic hypotension among patients with unexplained syncope: an overlooked diagnosis ? Intern J Cardiol 271, 269–273. [DOI] [PubMed] [Google Scholar]
- van Twist DJL, Mostard GJM, Sipers WMWH, 2020. Delayed recovery from initial orthostatic hypotension: an expression of frailty in the elderly. Clin Autom Res 30, 105–106. [DOI] [PubMed] [Google Scholar]
- Van Wijnen VK, Harms MP, Go-Schon IK, et al. , 2016. Initial orthostatic hypotension in teenagers and young adults. Clin. Auton. Res 26, 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wijnen VK, Finucane C, Harms MPM, et al. , 2017. Noninvasive beat-to-beat finger arterial pressure monitoring during orthostasis: a comprehensive review of normal and abnormal responses at different ages. J. Intern. Med 282, 468–483. [DOI] [PubMed] [Google Scholar]
- van Wijnen VK, Harms MPM, Wieling W, 2018. Orthostatic hypotension in the first minute after standing up: what is the clinical relevance and do symptoms matter? Hypertension 71, 816–818. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke JP, de Craen AJ, 2001. Alternative medicine: a “mirror image” for scientific reasoning in conventional medicine. Ann. Intern. Med 135, 507–513. [DOI] [PubMed] [Google Scholar]
- Westerhof BE, Settels JJ, Bos WJ, et al. , 2015. Bridging cardiovascular physics, physiology, and clinical practice: Karel H. Wesseling, pioneer of continuous noninvasive hemodynamic monitoring. Am. J. Physiol. Heart Circ. Physiol 308, H153–H156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieling W, Karemaker JM, 2013. Measurement of heart rate and blood pressure to evaluate disturbances in neurocardiovascular control. In: Mathias CJ, Bannister R, editors. Autonomic Failure. A Textbook of Clinical Disorders of the Autonomic Nervous System Fifth Edition ed. Oxford University Press; 290–306. [Google Scholar]
- Wieling W, Borst C, Van Lieshout JJ, et al. , 1985. Assessment of methods to estimate impairment of vagal and sympathetic innervation of the heart in diabetic autonomic neuropathy. Neth J Med 28, 383–392. [PubMed] [Google Scholar]
- Wieling W, Veerman DP, Dambrink JH, et al. , 1992. Disparities in circulatory adjustment to standing between young and elderly subjects explained by pulse contour analysis. Clin. Sci 83, 149–155. [DOI] [PubMed] [Google Scholar]
- Wieling W, Harms MP, ten Harkel AD, et al. , 1996. Circulatory response evoked by a 3 s bout of dynamic leg exercise in humans. J. Physiol 494, 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieling W, Krediet CT, van Dijk N, et al. , 2007. Initial orthostatic hypotension: review of a forgotten condition. Clin. Sci 112, 157–165. [DOI] [PubMed] [Google Scholar]
- Wieling W, Thijs RD, van Dijk N, et al. , 2009. Symptoms and signs of syncope: a review of the link between physiology and clinical clues. Brain 132, 2630–2642. [DOI] [PubMed] [Google Scholar]
- Wieling W, Krediet CTP, Solari D, et al. , 2013. At the heart of the arterial baroreflex: a physiological basis for a new classification of carotid sinus hypersensitivity. J. Intern. Med 273, 345–358. [DOI] [PubMed] [Google Scholar]
- Wood EH, 2000. Prevention of G-LOC by beat-to-beat detection of zero pressure at brain level. Aviat. Space Environ. Med 71, 1167–1168. [PubMed] [Google Scholar]


