Abstract
Primary progressive aphasia (PPA) is a neurodegenerative dementia syndrome that can be associated with multiple neuropathologic entities, including Alzheimer’s disease (AD) and all major forms of frontotemporal lobar degeneration (FTLD). It is classified into several subtypes, each defined by the nature of the language domain that is most impaired. The asymmetric neurodegeneration of the language dominant (usually left) hemisphere is the one consistent feature of all PPA variants. This feature offers unique opportunities for exploring mechanisms of selective vulnerability in neurodegenerative diseases. The focal cortical degeneration in PPA has also led to new insights on the neuroanatomy of language. This chapter reviews some of the current trends in PPA research as well as the challenges that remain to be addressed on the nosology, clinicopathologic correlations, and therapy of this syndrome.
INTRODUCTION
Primary progressive aphasia (PPA) is a major syndrome of frontotemporal lobar degeneration (FTLD) and accounts for nearly 25% of all cases (1). Approximately 60% of PPA is associated with FTLD and the remaining 40% with Alzheimer’s disease (AD) neuropathology. Information on PPA prevalence is very limited. One study from the UK suggests an approximate prevalence of 3–4/100,000, a level comparable to what has been reported for ALS (1). The one common denominator for all PPA, whether caused by FTLD or AD, is the preferential degeneration of the language network, usually located in the left hemisphere of the brain. Current research on primary progressive aphasia (PPA) is pursuing multiple directions. For one, there is lively interest in formulating personalized interventions aimed not only at the nature of the language disturbance but also at the biology of the underlying disease condition. Secondly, the asymmetric involvement of the left hemisphere and the multiplicity of the underlying degenerative diseases are generating new insights on the heterogeneity of dementias, the probabilistic relationship of syndrome to pathology, and the mechanisms of selective vulnerability. Thirdly, the slow dissolution of language on a background of focal cortical dysfunction is offering new paradigms for exploring the anatomy of the language network, a pursuit that has already prompted modifications of classic models. These are the three current trends that will be reviewed in this chapter. Given the constraints of space and the vast literature on PPA, the account will be selective and based predominantly on the PPA research programs at Northwestern University where a cohort of 235 PPA patients have been enrolled, 97 of whom have come to brain autopsy.
The diagnosis of primary progressive aphasia (PPA) is made when a neurodegenerative disease causes a progressive and initially isolated dissolution of language (not just speech) and where comportment, personality, explicit memory, and visuospatial cognition remain relatively intact for at least the first year or two. The impairment can be expressed as deficits in word retrieval, object naming, sentence construction, or language comprehension, either singly or in combination. If the language disturbance were to be reversed, at least within the first few years, could the patient have resumed most customary professional, social and recreational activities? If this question can be answered in the affirmative, the diagnosis of PPA is solidified.
The existence of progressive aphasias had been known for more than 100 years. Pick, Sérieux, Dejerine, Franceschi, and Rosenfeld were among the first to report such patients (2–6). Few of these cases would have qualified for a rigorous PPA diagnosis because of additional cognitive and behavioral impairments early in the course of illness. The one exception is the patient reported in 1897 by Dejerine and Sérieux, who appears to have had a progressive form of semantic aphasia without additional impairments (7). Progressive aphasias did not attract much, if any, attention during most of the 20th century. The current resurgence of interest in this condition can be traced to the 1982 report of six patients who experienced a slowly progressive aphasia in the absence of other cognitive or behavioral impairments (8). The syndrome was subsequently named ‘primary progressive aphasia,’ and diagnostic criteria were established (9, 10). The following decades witnessed a rapidly expanding literature on PPA and on overlapping entities designated progressive nonfluent aphasia (PNFA) and semantic dementia (SD) (11). For a number of years research on PNFA and SD developed in parallel to research on PPA. In 2011 an international group of investigators presented classification guidelines that incorporated PNFA and SD under the PPA umbrella (12). This unitary approach stimulated rapid progress in this field.
DIAGNOSIS, NOMENCLATURE, AND SUBTYPING
Three critical features define PPA: 1) Adult-onset and progressive language impairment; 2) absence of other salient behavioral or cognitive deficits for approximately the first two years; 3) neurodegenerative disease as the only cause of impairment (10). These criteria help to filter out patients where progressive aphasias arise in conjunction with equally prominent speech apraxia, behavioral disturbance, amnesia, associative agnosia, or visuospatial impairment. However, in the course of initial quantitative neuropsychological assessment patients may show subtle impairments in non-language tasks, especially those related to executive function. Such abnormalities do not by themselves preclude a PPA diagnosis unless they are associated with consequential functional abnormalities in the corresponding non-language domains.
Many neuropsychological tests require verbal responses and verbal instructions. The clinician needs to consider the influence of the aphasia on these aspects of performance. For example, a patient with PPA who cannot name a famous face is not necessarily prosopagnosic, a patient who cannot verbalize the nature of an object does not necessarily lack knowledge of the object, a patient who cannot learn a word list is not necessarily amnestic. Conversely, patients who cannot produce words because of articulation deficits, those who cannot repeat language because of general working memory limitations, those who misname objects or faces they do not recognize, or those who have impoverished speech because of abulia or impaired executive function are not necessarily aphasic. As in the case of many other syndromes, the diagnosis of PPA relies on the judgment and experience of the clinician. While clear-cut cases do exist, there are also cases where the salience and primacy of the aphasia will generate debate especially if the patient is examined a few years after symptom onset. In some patients the aphasia will remain the only salient feature for over a decade (13). Other patients, however, may first come to a specialty clinic at a time when the disease has progressed to encompass other cognitive domains. The term ‘PPA plus’ (PPA+) can be used to designate such patients, based on the assumption that the disease had started as PPA but that it had since spread beyond the language network (14).
In contrast to many other dementias, where the patient has little insight into the predicament, patients with PPA are usually the first to notice and report the difficulty. At those stages of the disease, neurological evaluations, MRI, and metabolic PET scans may be negative. This combined with lack of recognition of these symptoms in general practice, may lead to referrals to otolaryngologists or psychiatrists (15). Patients and families often ask whether the diagnosis is PPA or AD. When AD biomarkers are positive, the clinician will have to explain that the patient has both PPA and AD, that PPA refers to the symptoms that bring the patient to the clinic, and that AD refers to the abnormal amyloid and tau proteins in the brain that attack the language centers. There was a time when PPA was under-diagnosed. There are now instances where it seems to be over-diagnosed, probably because language impairments can be so prominent during the office evaluation that other equally substantial cognitive and behavioral impairments become overlooked. This issue comes up most commonly in patients with prominent apraxia of speech or executive dysfunction who are also aphasic. We give these patients descriptive diagnoses such as ‘apraxia of speech with aphasia’ or ‘aphasic frontal syndrome.’
Once the PPA diagnosis is established, the subtyping exercise can be initiated. At the time of writing, the 2011 guidelines dominate this process (12). They help to classify PPA into nonfluent/agrammatic, logopenic and semantic variants. Although this system has been immensely influential, and is even frequently mandated during the review of manuscripts submitted for publication, it has widely recognized shortcomings (16–18). For one, a strict adherence to the 2011 guidelines entails arduous assessment of nearly a dozen separate aspects of language. Secondly, even if the guidelines are strictly applied, approximately a third of the patients will fail to be classified into any of the three variants. Thirdly, there are certain feature clusters that allow the same patient to simultaneously fit the designation of both nonfluent/agrammatic and logopenic PPA. Yet another challenge is posed by the evolution over time so that a patient who fits one subtype initially may best fit another as the disease progresses.
The following modifications have helped us address these concerns (16). 1) The ‘absence of definite grammar and comprehension impairment’ is made to be a core feature of the logopenic variant. This prevents the double-assignment problem. 2) In contrast to the 2011 guidelines, repetition impairment is not considered an obligatory core feature of the logopenic variant. This practice reduces the number of unclassifiable patients since repetition failure is common is many forms of aphasia. 3) Patients with combined impairments of grammar and word comprehension even early in the disease, and who would therefore remain unclassifiable by the 2011 guidelines, make up a fourth variant of ‘mixed’ PPA. 4) The semantic variant is diagnosed only when poor word comprehension is the principal feature. When additional and equally prominent impairments of object or face recognition (not just naming) are detected, a diagnosis of semantic dementia (SD) is made (11). This recommendation is at odds with the 2011 guidelines, which would diagnose semantic PPA even in patients with significant face and object recognition impairment (i.e., visual associative agnosia).
The differentiation of semantic PPA from SD revolves on the observation that semantic PPA is a selective aphasic syndrome of the left anterior temporal lobe whereas the SD syndrome reflects a wider deficit with a more bilateral anatomical substrate (19–22). It should be pointed out, however, that many semantic PPA patients may also have lesser atrophy in the right anterior temporal lobe and that further spread of neurodegeneration within the right hemisphere may lead some, but not all, to eventually develop the additional face and object recognition deficits of SD (Fig. 1). It is not surprising, therefore, that some authors have considered semantic PPA and SD to be the two sides of the same coin (23, 24). The question is whether syndromic designations should be based on clinical presentation at disease onset, as we advocate, or based on possible progression trajectories.
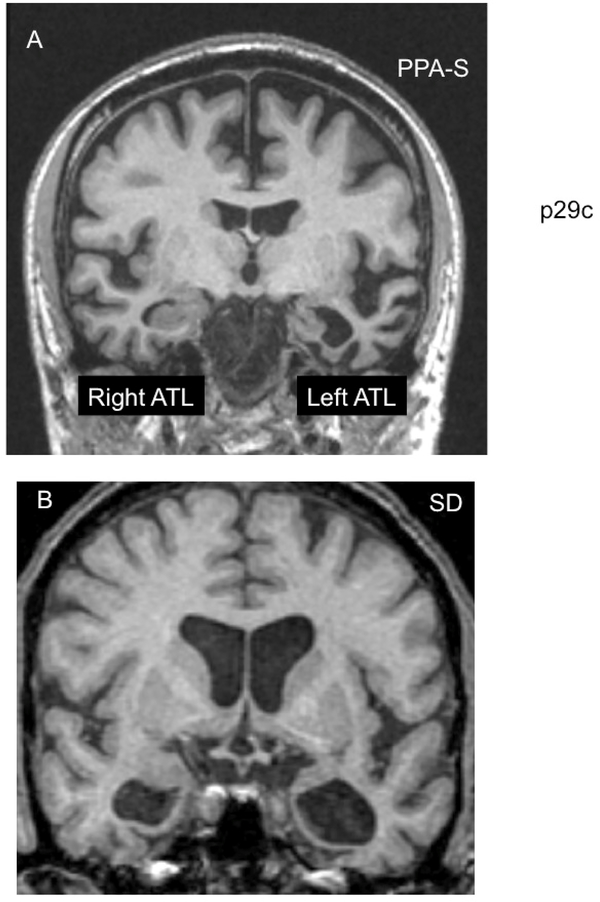
Figure 1: PPA-S versus SD-.
Figure 1A shows the MRI scan of a right-handed man with symptom onset at the age of 59. On examination seven years later the clinical patterns was PPA-S and atrophy was much more prominent in the left anterior temporal lobe (ATL). At that time he had severe word comprehension impairments but no difficulty with non-verbal object recognition either in testing or in everyday life. In comparison, Figure 1B shows the MRI scan of a right-handed man with symptom onset at the age of 65. Three years later, at his initial visit, ATL atrophy was bilateral. He had prominent word comprehension and object recognition impairments. This combination led to a subsequent diagnosis of semantic dementia (SD).
The modifications listed above lead to a classification method based on a template where the Y-axis represents worsening impairment in grammatical processing and the X-axis represents worsening impairment in single word comprehension (15). Each of the 4 PPA subtypes will cluster within a different quadrant of this template. The nonfluent/agrammatic PPA patients, for example will cluster in the upper left quadrant (impaired grammar but spared comprehension); the semantic PPA patients will cluster in the lower right quadrant (impaired comprehension but spared grammar); the mixed PPA patients will cluster in the lower left quadrant (combined impairments of grammar and comprehension); and the logopenic PPA patients will cluster in the upper right quadrant (relatively spared grammar and comprehension). The logopenic group would have met the PPA criteria through impairments of word retrieval, naming, and spelling. Specific tests for assessing grammar and comprehension and their normative values have been reported (15). As patterns of agrammatism vary greatly from language to language, considerable attention is being directed to the adaptation of grammar tests for languages other than English (25).
Some logopenic patients maintain fluency as they circumvent word-finding failures through circumlocution; others pause after word retrieval failures and produce halting nonfluent speech that appears similar to what is seen in patients with nonfluent/agrammatic PPA. Word finding impairments and paraphasias may lead to sentences that appear to have abnormalities of grammar but, in fact, are grammatically intact, albeit circumlocutious. The delineation of logopenic from agrammatic PPA can thus be quite challenging (17). Quantitative analyses of speech samples show that the nonfluent/agrammatic patients make word-finding pauses that are longer before verbs whereas logopenic patients make pauses that are longer before nouns (26). Furthermore, patients with nonfluent/agrammatic PPA display a preferential impairment of verb rather than object naming whereas the converse may be seen in logopenic PPA (27). When research objectives necessitate such distinctions, these features may help to establish a quantitative differentiation of nonfluent/agrammatic from logopenic forms of PPA.
The 2011 guidelines did not prescribe acronyms for the three variants. At the present, nfvPPA, lvPPA, svPPA are the most popular choices. Alternative acronyms such as naPPA, agPPA, PPA-NFV, LPA, PPA-SV have also been used, albeit more rarely (28–31). The ‘nfv’ prefix is particularly problematic because it appears to overlook grammar, which is the single most characteristic impairment of this subtype. The choice of ‘nfv’ was probably based on experience derived from stroke aphasia where low fluency can be used as a proxy for agrammatism. In PPA, grammar and fluency can be dissociated, especially in logopenic patients, as noted above, where long word finding pauses diminish fluency but without grammatical impairment (32). Based on these considerations and also in order to underscore the primacy of the PPA diagnosis, we have used the alternative acronyms of PPA-G, PPA-L, PPA-S and PPA-M for the nonfluent/agrammatic, logopenic, semantic and mixed variants, respectively. It may take another collective effort to determine whether the 2011 criteria should be modified along the lines listed above and whether the acronyms can be harmonized.
Clinical progression patterns vary by subtype and are likely to reflect the differential anatomical trajectories of disease spread. In PPA-S, the spread of atrophy from the anterior temporal lobe to orbitofrontal, insular or contralateral temporal lobe can lead to the additional face and object recognition impairments of SD, and to the behavioral abnormalities seen in bvFTD. In PPA-G, spread of atrophy from the inferior frontal gyrus to other premotor and frontal cortices can lead to the abnormalities seen in apraxia of speech, corticobasal syndrome, supranuclear ophthalmoplegia and frontal-type amotivational executive dysfunction. In PPA-L spread of atrophy from the temporoparietal junction to surrounding cortices can lead to additional impairments of explicit memory and constructions. For all subtypes, the spread of atrophy tends to be more pronounced in the left hemisphere and there are substantial interindividual differences in the speed and trajectory of progression (33).
ASYMMETRY OF NEUROPATHOLOGY AND GENETICS
In our group of 97 consecutive autopsies, the primary neuropathology was FTLD with tauopathy (FTLD-tau) in 29%, FTLD with transactive response DNA binding protein 43 (FTLD-TDP) in 25%, and AD in 44%. All 3 major neuropathologic forms of FTLD-tau (Pick’s disease, corticobasal degeneration (CBD), progressive supranuclear palsy (PSP), and all 3 major forms of FTLD-TDP (types A, B and C) were represented. However, there were also disease-specific preferential patterns of atrophy. For example, AD almost always led to peak atrophy that included the temporoparietal junction; TDP-C almost always led to severe anterior temporal atrophy; Pick’s disease routinely caused combined atrophy of anterior temporal and prefrontal cortex; PSP and CBD tended to be associated with surprisingly modest cortical atrophy, usually in dorsal premotor or inferior frontal cortex. The one common denominator of nearly all cases is the leftward asymmetry of the atrophy (Figs 2, 3). What is surprising is that the asymmetry is almost always maintained up to the time of death (Fig. 2). The initial predilection of the language-dominant left hemisphere is therefore not a random event at disease onset but a core biological feature of the syndrome.
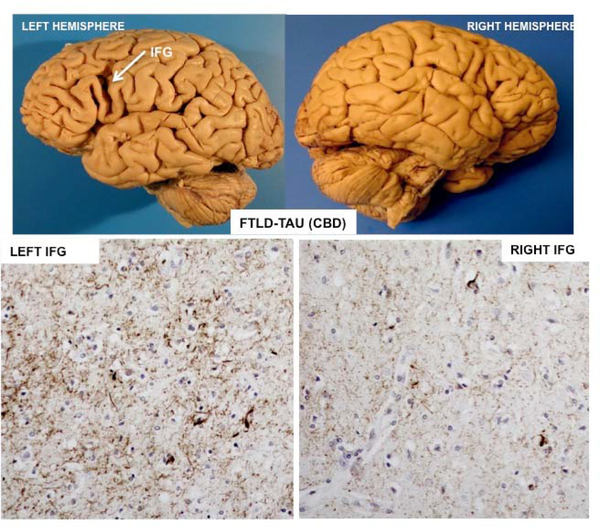
Figure 2: Asymmetry of neurodegeneration-.
Post-mortem examination of a right-handed woman with symptom onset at the age of 72 and findings of agrammatic PPA with prominent word finding impairments. Death occurred 6 years later. The primary neuropathology was found to be FTLD-tau of the CBD type. The top figures show the profound asymmetry of atrophy. There is an almost cystic area of atrophy around the left inferior frontal gyrus (IFG) but no comparable atrophy of the right. The photomicrographs in the bottom, based on phospho-tau immunostaining in the same patient, show the tauopathy to be more intense in the left IFG than in the right.
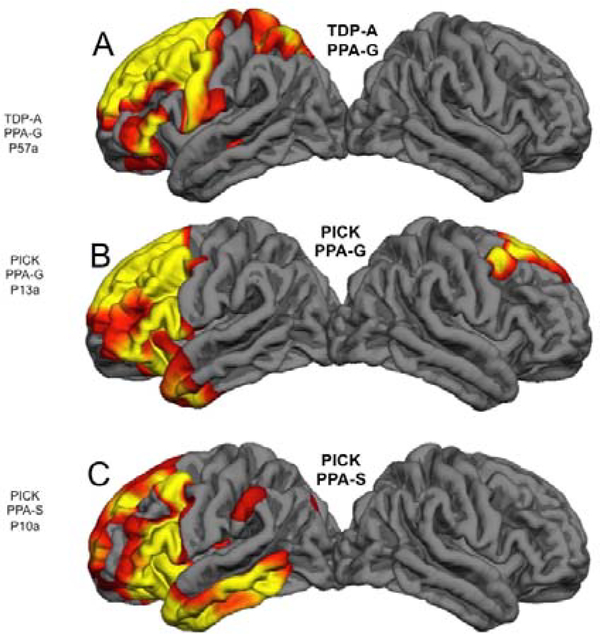
Figure 3: Correspondences of Pathology, Atrophy and Syndrome-.
Quantitative MRI morphometry in 3 right-handed patients who had come to post mortem brain autopsy. Areas of significant cortical thinning compared to controls are shown in red and yellow. A- Onset of PPA-G was at the age of 65. The scan was obtained 2 years after onset. B- Onset of PP-G was at the age of 57. The scan was obtained 5 years after onset. C- Onset of PPA-S was at the age of 62. The scan was obtained 5 years after onset. Despite the differences in neuropathology and clinical syndrome, the one common denominator is the profound leftward asymmetry of atrophy.
There was nearly equal representation of males and females in our autopsy cohort. Age of onset varied from 41 to 80 with a mean of 61±8 years. Survival from symptom onset to death varied from 2 to 23 years with a mean of 9.69±3.93. Survival tended to be the longest for those with AD (10.8±4.4) and FTLD-TDP type C (12.4±2.6) and shortest for those with FTLD-TDP types A and B (5.8±2.2).
The relationship of PPA variants to the underlying neuropathologic entity is probabilistic rather than absolute (34). Autopsy data show that the vast majority of PPA-S cases have had TDP-C pathology but approximately 20% have had Pick’s disease; the majority of PPA-G cases have had FTLD-tau (all types) but approximately 30% have had FTLD-TDP or AD; the majority of PPA-L cases have had AD but 30% have shown FTLD-tau or FTLD-TDP. Figure 3 illustrates the clinicopathologic heterogeneity of PPA, namely, that the same neuropathologic entity can cause more than one aphasic variant and that the same PPA variant may be caused by more than one neuropathologic entity. In the case of Fig. 3 A and B, FTLD-TDP, type A and Pick’s disease cause nearly identical atrophy patterns favoring the frontal components of the language network and each give rise to PPA-G, a clinical syndrome that fits the presence of frontal lobe atrophy encompassing the left inferior frontal gyrus. Figure 3 B and C raise challenging questions. They show atrophy patterns in 2 different patients with Pick’s disease at autopsy, one with PPA-G (Fig. 3B), the other with PPA-S at the time of imaging (Fig. 3C). Although the semantic aphasia in Fig. 3C could be attributed to the greater atrophy of the anterior and lateral temporal lobe compared to Fig. 3B, it is difficult to understand why this patient was not also agrammatic since the frontal atrophy is nearly as extensive as in the other two cases with PPA-G. Perhaps this discrepancy can be blamed on vagaries of cortical morphometry performed on single subjects or, alternatively, on individual variations in the functional anatomy of the language network.
During life, cortical thinning (i. e., atrophy) and hypometabolism are the two most conspicuous markers of asymmetric neurodegeneration. Considerable progress has been made in exploring the potential cellular substrates of the asymmetrical atrophy (Fig. 2). For example, the NFT (but not the amyloid plaques) of AD, the tauopathy of CBD/PSP, Pick bodies, and the abnormal TDP-43 deposits of FTLD-TDP generally display greater densities in the left hemisphere than in the right hemisphere and also greater densities in language-related than other cortical areas of the left hemisphere (35–40). Focal atrophy patterns may therefore reflect the density of these histopathologic disease markers. In at least some PPA patients with AD neuropathology, the NFT may also be more numerous in language related cortices of the left hemisphere than in medial temporal areas, a distribution that deviates from the Braak and Braak pattern of neuropathology and that underlies the atypical preservation of episodic memory in these patients (38, 40).
Quantitative investigations have looked into the concordance between PPA subtypes and neurodegeneration markers. A study of 4 right-handed PPA patients with FTLD-TDP (type A) neuropathology showed that the densities of TDP-43 inclusions and activated microglia were higher in the left hemisphere than in the right (36). Within the left hemisphere, the density of TDP-43 proteinopathy was higher in language cortices than in memory-related limbic areas. Neuronal densities in the 4 TDP-43 cases were also lower in the left hemisphere than in the right and mirrored the asymmetric distribution of microglia. Two of the patients had PPA-G and displayed the highest density of TDP-43 precipitates in the frontal components of the language network whereas two others had PPA-L and displayed the highest density of precipitates in the temporoparietal components of the language network (36). Furthermore, in one left-handed logopenic PPA patient with documented right hemisphere language dominance and FTLD-TDP, the atrophy and microscopic neurodegeneration was also asymmetric but favored the right hemisphere (41). The cellular pathology in FTLD-TDP therefore asymmetrically targets the language-dominant hemisphere and mirrors the anatomical predilection patterns that characterize individual PPA variants. Similar patterns may also exist in the tauopathies that cause PPA (39). Some patients, especially those with PPA-G and FTLD-tau, may have no detectable cortical atrophy in the initial years of disease. These patients display abnormalities of functional connectivity, suggesting that physiological perturbations of the language network may precede atrophy (42). In this group of patients, the neurodegeneration may be particularly prominent in subcortical white matter (43).
In our autopsy cohort of 97 cases, a third of TDP-A cases had granulin (GRN) mutations. No other disease-causing mutations were encountered. Mutations in the GRN gene constitute the most common genetic correlate of familial PPA (44). In such families some members may have PPA and others bvFTD (45, 46). Rarely, all affected members of a GRN family will have PPA (47). Even then, the type of aphasia may differ from one sibling to another (48). The literature also contains rare associations of PPA with mutations in the presenilin (PS1), tau (MAPT) and C9orf72 genes (49–51).
The heterogeneity of phenotypes encountered within GRN families show that molecular underpinnings alone are not sufficient to account for the patterns of selective vulnerability and their clinical manifestation. The biological mechanisms underlying the selective and asymmetric involvement of the language-dominant hemisphere in PPA remain to be elucidated. One line of investigation has focused on the significantly higher frequency of learning disabilities, including dyslexia, in PPA patients and their first-degree relatives compared to control populations and patients with other dementias (52–54). Some families of PPA probands had strikingly high prevalence of developmental dyslexia in siblings or children (52). Follow-up research has replicated this association and raised the possibility that it may be peculiar to PPA-L (55). These observations led to the speculation that at least some cases of PPA could be arising on a developmentally or genetically based vulnerability of the left hemisphere language network. In some family members this vulnerability would interfere with the acquisition of language and lead to dyslexia while in others it would make the language network a locus of least resistance for the effects of an independently arising neurodegenerative process, leading to PPA (56). So far, linkage studies addressing this hypothesis have not detected an association between PPA and known dyslexia genes (44). Given the polygenic nature of dyslexia, negative results may reflect an insufficient number of cases.
CONTRIBUTIONS TO THE ANATOMY OF LANGUAGE
The classic Wernicke-Lichtheim-Geschwind model of language revolved around two epicenters, namely Broca’s area in the inferior frontal gyrus (IFG) and Wernicke’s area in the temporoparietal junction (TPJ). The former has been linked to fluency and grammar and the latter to language comprehension. The literature of the past 100 years displays greater agreement on the location and function of Broca’s area than of Wernicke’s area (57). These two epicenters are connected through the arcuate fasciculus, which is thought to play a critical role in enabling language repetition by linking phonological encoding to articulatory programs. This basic model has undergone major revisions through investigations with functional imaging, event-related potentials, and sophisticated neuropsychological assessments (58–60).
Each of these approaches has advantages and disadvantages. Cerebrovascular lesions cause sudden and irreversible destruction of the core lesion site. However, the damage usually extends into deep white matter. The exact contribution of the damaged cortical region to the ensuing language impairment is therefore difficult to specify. Functional mapping approaches based on MRI and electrical recordings, on the other hand, can reveal activity confined to the cerebral cortex but cannot differentiate areas that are critical for a function from those that have collateral participatory roles.
Investigations based on focal cortical atrophy can circumvent some of these shortcomings. Regions where the magnitude of cortical thinning correlates with the magnitude of impairment can be said to have critical (rather than participatory) roles in maintaining the integrity of that function. Consequently, PPA has offered new tools for investigating the cortical anatomy of the language network without the deep white matter problem of stroke or the collateral activation dilemma of functional brain mapping. Nonetheless, clinicoanatomical correlations in PPA are not without caveats. For one, the slow evolution of the lesion is likely to trigger compensatory plasticity that may complicate the interpretation of correlations. Secondly, even areas of peak atrophy may contain residual neurons that could sustain some functionality of that region (56). Thirdly, each neuropathologic entity may trigger a different pattern of cortical injury. For example, the neurofibrillary tangles of AD have a predilection for deep cortical layers whereas the opposite is the case for Pick’s disease.
Despite these potential complications, clinicoanatomical investigations on PPA have generated new insights into the functional anatomy of language. Each PPA variant is associated with a characteristic location of peak atrophy, Broca’s area (IFG) in PPA-G, Wernicke’s area (TPJ) in PPA-L, and the anterior half of the temporal lobe (ATL) in PPA-S (61–63). The anatomical correlate of PPA-G is in keeping with prevailing models of language, which give Broca’s area a critical role in the maintenance of fluency and grammar (64). The relationships in PPA-L and PPA-S, however, are in conflict with classic aphasiology and also with most contemporary models of language. For one, traditional models of language exclude the ATL. For example, an influential review published at the height of 20th century aphasiology states that the probability that a lesion would impair comprehension is “very high in or near the first temporal gyrus, and fades out with different gradients (varying among individuals) toward the poles. And by the time it gets to any pole (occipital, temporal, or frontal) the probability is essentially zero.” (65). Research on PPA-S has contradicted this statement by showing that damage to the left ATL, including the temporal pole, causes severe impairments of word comprehension. Based on this finding, the proposal has been made that this region should be considered a core component of the language network.
This proposal has generated considerable debate. The disagreement revolves around an alternative interpretation of ATL damage, namely that it causes more than a language deficit (i.e., aphasia), and that it triggers a universal loss of semantic knowledge not only for words but also for faces and objects (66). Based on this point of view, the syndrome of ATL damage was designated semantic dementia (SD), a syndrome defined by the combination of semantic aphasia (word comprehension deficit) with visual associative agnosia (loss of face and object recognition) (11, 66). Such patients would not fit the diagnostic criteria for PPA since the aphasia would no longer constitute the only dominant feature. This potential dilemma can be resolved by considering the differential impact of left versus right ATL damage (23, 24, 57). We find that atrophy located predominantly in the left ATL leads to preferential word but not object recognition impairments. The patients may not be able to name objects or faces but are fully cognizant of their identity and nature. Such patients would fit the criteria for a PPA diagnosis (67).
Resting state functional imaging experiments also show that the left ATL has asymmetric functional connectivity patterns that support its inclusion within the language network (68). We have never encountered a right-handed patient with severe word comprehension impairment who has not also had substantial left ATL atrophy extending all the way into the pole. However, we have seen patients with such a location of atrophy with severe anomia but no word comprehension impairment. In these patients, the comprehension impairment of PPA-S emerges as the atrophy extends posteriorly from the anterior tip of the left temporal lobe into its middle third (57). In fact, functional MRI studies in PPA are showing that the connectivity of the middle temporal gyrus with the ATL may be essential for the integrity of word comprehension (69). Damage to the anterior tip of the left ATL may therefore be necessary but not sufficient for the emergence of severe word comprehension impairments (see also Fig. 2 B versus C). In our experience, and as shown in Figure 1, the amodal cluster of equally prominent word, face and object recognition impairments characteristic of SD reflects bilateral ATL atrophy (21, 22). This observation provides the anatomical justification for distinguishing PPA-S from SD. When ATL atrophy is predominantly right-sided, the patient may present either with SD or with associative agnosia and prosopagnosia in the absence of aphasia (70, 71).
Patients with PPA-S have severe naming impairments principally because they do not understand the meaning of the word that denotes the object they are asked to name (67). The impairment initially undermines the comprehension of a word at its specific level of meaning (does the word denote a strawberry or a cherry) but later generalizes to the generic meaning of the word (does the word denote a fruit or an animal) (72). Based on these observations in PPA-S, the left ATL can be conceptualized as a transmodal region of cortex where sensory word form information is linked to the multimodal associations that collectively encode the meaning of the word (57). Word recognition at a specific level of meaning requires more extensive associative elaboration and would therefore be more vulnerable to early stages of neurodegeneration.
In contrast to the predictions of classic aphasiology, patients with PPA-L have normal single word comprehension despite peak atrophy sites that encompass the traditional boundaries of Wernicke’s area. For example, regression analyses in 73 PPA patients showed no correlation between atrophy in Wernicke’s area and impairment of word comprehension (57, 73). Instead, atrophy in Wernicke’s area was strongly correlated with impaired language repetition (73). The latter results are in keeping with observations on PPA and stroke aphasia, which give the temporoparietal region a critical role in phonological loop functionality (74–76).
In summary, investigations on PPA-L show that severe degeneration of Wernicke’s area does not impair single word comprehension whereas investigations on PPA-S show that an intact Wernicke’s area is not sufficient to sustain word comprehension if the ATL is damaged. The body of work on PPA therefore leads to the conclusion that Wernicke’s area is neither necessary nor sufficient for word comprehension. This conclusion can be reconciled with classic aphasiology by keeping in mind that nearly all reports linking Wernicke’s area to word comprehension are based on cerebrovascular lesions. Such lesions include not only the cortex of Wernicke’s area but also deep white matter axons that are likely to carry projections of otherwise intact distal posterior and contralateral cortices. The resultant additional cortical disconnections may explain why stroke in Wernicke’s region impairs comprehension while neurodegeneration in Wernicke’s cortex does not (77).
An additional contribution of PPA to the anatomy of language comes through the discovery of the aslant tract, a pathway that connects the core language network with dorsal premotor cortex and that appears to play a major role in sustaining fluency (78). Patients with PPA may also show patterns of aphasia that have not been observed in other settings. For example, some patients may show a preferential inability to name objects orally but not in writing and fail to understand words they hear but not those they read (79). These patients do not fit the pattern seen in pure word deafness because they are anomic and they do not fit the pattern of auditory agnosia because they can match objects to their characteristic sounds. Investigations on this small group of patients has helped to explore the functionality of a putative ‘auditory word form area’ that sits at the confluence of modality-specific pathways for word comprehension and language repetition.
The totality of these investigations on PPA support an anatomical organization that resolves the large-scale language network into dorsal and ventral (rather than anterior and posterior) streams of processing (59). The dorsal route mediates phonological encoding, articulatory programming and fluency and also enables the sequencing of morphemes and words into grammatically correct sentences. The ventral route mediates the lexicosemantic processes of object naming and word comprehension. Word finding is a joint function of both routes and therefore the most common presenting complaint in PPA.
THERAPEUTIC INTERVENTIONS
The heterogeneity of PPA highlights the need to individualize therapeutic approaches. Interventions in individual patients should target the underlying disease as well as the symptom complex. The former step requires the use of in vivo biomarkers. There are excellent CSF and PET biomarkers for detecting PPA patients with AD neuropathology and blood-based biomarkers may be on the horizon. However, current tau ligands for PET do not yet offer reliable identification of non-AD tauopathies associated with CBD, PSP and Pick’s disease (80). When such biomarkers become available, they will enable the identification of PPA patients with FTLD-tau and, by exclusion, those with FTLD-TDP. The goal of these diagnostic investigations is to prescribe approved medications (e.g. cholinesterase inhibitors if AD) or to channel the patient to relevant disease-specific clinical trials. Although clinical examination is rarely sufficient to specify the underlying disease entity, we have found that single word comprehension deficits that arise as the most salient feature of PPA, are never associated with AD. The presence of this feature may therefore be used to forego AD biomarker testing.
The non-pharmacologic interventions aimed at the language impairment include speech therapy and brain stimulation modalities such as transcranial magnetic stimulation (TMS) or transcranial direct current stimulation (tDCS) (81). Promising effects have been reported following left hemisphere tDTS in PPA-S (82). If confirmed, this may well be the first time that brain stimulation will be shown to have therapeutic effects in an FTLD syndrome.
Evidence for the effectiveness of speech-language therapy in PPA is encouraging but not definitive (83, 84). Utilization of this intervention modality is low in part due to the misconception that speech-language therapy is not appropriate for neurodegenerative syndromes where worsening is inevitable (85, 86). An additional barrier is the lack of familiarity of speech-language pathologists with neurodegenerative conditions. Speech-language therapy in PPA requires personalization to fit the pattern of impairment and its evolution over time including adapting communication strategies and modalities as the illness progresses. Additional questions to be resolved include the relative usefulness of multicomponent, impairment-based, or compensatory approaches and the comparative benefits of dyadic versus patient-only approaches. In each case, ecologically meaningful and statistically robust outcome measures will need to be devised.
Recent developments in telemedicine raise the possibility of delivering speech-language therapy in the home of the individual living with PPA (87, 88). Communication Bridge, for example, is a two-arm, randomized control trial of speech-language intervention delivered through video chat for individuals with PPA (88). The experimental arm uses a client-informed, dyadic approach for individuals with PPA and their communication partner. Impairment-based exercises using personalized stimuli and compensatory strategies are utilized to address real-world communication difficulties. The trial includes an individually tailored web-application with native practice exercises and education materials that participants rehearse between treatment sessions. To evaluate whether treatment gains are relevant to the daily functions of the participant, outcomes are measured using a communication confidence rating scale and goal attainment scores. This method allows the targeting of individualized goals of high relevance to participants. In the future, transcranial stimulation could be combined with speech-language therapy to attain even more effective benefits (81).
CONCLUSIONS
Despite its relative rarity, PPA has led to major conceptual advances in understanding the heterogeneity of dementia, the principles of selective brain vulnerability, and the neuroanatomy of the language network. PPA was arguably the first entity to show that there is more to dementia than memory loss, that the same clinical syndrome can be caused by multiple neuropathologies, that the same neuropathology can cause multiple syndromes, and that the relationship of syndrome to neuropathology is probabilistic rather than deterministic. Future work on PPA is likely to shed new light on the anatomical tropisms of neurodegenerative diseases and on the internal architecture of the language network.
Acknowledgments
SUPPORT: R01 DCOO8552 and K23 DC014303 from the National Institute of Deafness and Communication Disorders; P30 AG013854 and AG056258 from the National Institute on Aging; NS085770 from the National Institute of Neurological Disorders and Stroke.
REFERENCES
- 1.Coyle-Gilchrist TS, Dick KM, Patterson K, Rodriquez PV, Wehmann E, Wilcox A, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86:1736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenfeld M Die partielle Grosshirnatrophie. Journal of Psychology and Neurology. 1909;14:115–30. [Google Scholar]
- 3.Pick A Ueber die Beziehungen der senilen Hirnatrophie zur Aphasie. Prager Medizinische Wochenschrift. 1892;17:165–7. [Google Scholar]
- 4.Pick A Zur Symptomatologie der linksseitigen Schlaffenlappenatrophie. Monatsschrift für Psychiatrie und Neurologie. 1904;16:378–88. [Google Scholar]
- 5.Franceschi F Gliosi perivasculare in un caso de demenza afasica. Annali di Neurologia. 1908;26:281–90. [Google Scholar]
- 6.Sérieux P Sur un cas de surdité verbale pure. Revue de Medecine. 1893;13:733–50. [Google Scholar]
- 7.Dejerine J, Sérieux P. Un cas de surdité verbale pure terminée par aphasie sensorielle, suivie d’autopsie. Comptes Rendues des Séances de la Société de Biologie (Paris). 1897;49:1074–7. [Google Scholar]
- 8.Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11(6):592–8. [DOI] [PubMed] [Google Scholar]
- 9.Mesulam MM. Primary progressive aphasia--differentiation from Alzheimer’s disease [editorial]. Ann Neurol. 1987;22(4):533–4. [DOI] [PubMed] [Google Scholar]
- 10.Mesulam M-M. Primary Progressive Aphasia. Ann Neurol. 2001;49:425–32. [PubMed] [Google Scholar]
- 11.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration. A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. [DOI] [PubMed] [Google Scholar]
- 12.Gorno-Tempini ML, Hillis A, Weintraub S, Kertesz A, Mendez MF, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weintraub S, Rubin NP, Mesulam MM. Primary progressive aphasia. Longitudinal course, neuropsychological profile, and language features. Arch Neurol. 1990;47(12):1329–35. [DOI] [PubMed] [Google Scholar]
- 14.Mesulam M-M, Weintraub S. Primary progressive aphasia and kindred disorders. In: Duyckaerts C, Litvan I, editors. Handbook of Clinical Neurology. New York: Elsevier; 2008. p. 573–87. [DOI] [PubMed] [Google Scholar]
- 15.Mesulam M-M, Wieneke C, Thompson C, Rogalski E, Weintraub S. Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain. 2012;135:1537–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mesulam M, Weintraub S. Is it time to revisit the classification of primary progressive aphasia? Neurology. 2014;82:1108–9. [DOI] [PubMed] [Google Scholar]
- 17.Sajjadi SA, Patterson K, Arnold RJ, Watson PC, Nestor PJ. Primary progressive aphasia: a tale of two syndromes and the rest. Neurology. 2012;78:1670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wicklund MR, Duffy JR, Strand EA, Machulda MM, Whitwell JL, A. J. Quantitative application of the primary progressive aphasia consensus criteria. Neurology. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambon Ralph MA, Cipolotti L, Manes F, Patterson K. Taking both sides: do unilateral anterior temporal lobe lesions disrupt semantic memory? Brain. 2010;133:3243–55. [DOI] [PubMed] [Google Scholar]
- 20.Mesulam M-M, Rogalski E, Wieneke C, Cobia D, Rademaker A, Thompson C, et al. Neurology of anomia in the semantic subtype of primary progressive aphasia. Brain. 2009;132:2553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gefen T, Wieneke C, Martersteck AC, Whitney K, Weintraub S, Mesulam M-M, et al. Naming vs knowing faces in primary progressive aphasia. A tale of two hemispheres. Neurology. 2013;81:658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurley RS, Mesulam M-M, Sridhar J, Rogalski E, Thompson CK. A nonverbal route to conceptual knowledge involving the right anterior temporal lobe. Neuropsychologia. 2018;117:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adlam A-L, R., Patterson K, Rogers TT, Nestor PJ, Salmond CH, Acosta-Cabronero J, et al. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain. 2006;129:3066–80. [DOI] [PubMed] [Google Scholar]
- 24.Bright P, Moss ME, Stamatakis EA, Tyler LK. Longitudinal studies of semantic dementia: the relationship between structural and functional changes over time. Neuropsychologia. 2008;46:2177–88. [DOI] [PubMed] [Google Scholar]
- 25.Canu E, Agosta F, Imperiale F, Ferraro PM, Fontana A, Magnani G, et al. Northwestern anagram test-Italian (NAT-I) for primary progressive aphasia. Cortex. 2019;119:497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mack JE, Chandler SD, Meltzer-Asscher A, Rogalski E, Weintraub S, Mesulam M-M, et al. What do pauses in narrative production reveal about the nature of word retrieval deficits in PPA. Neuropsychologia. 2015;77:211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hillis AE, Tuffiash E, Caramazza A. Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. J Cog Neurosci. 2002;14:1099–108. [DOI] [PubMed] [Google Scholar]
- 28.Mendez MF, Sabadash V. Clinical amyloid imaging in logopenic progressive aphasia. Alzheimer’s Disease and Associated Disorders. 2015;29:94–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tree J, Kay J. Longitudinal assessment of short-term memory deterioration in logopenic variant primary progressive aphasia with post-mortem confirmed Alzheimer’s disease pathology. Journal of Neuropsychology. 2014;9:184–202. [DOI] [PubMed] [Google Scholar]
- 30.Josephs KA, Duffy J, Strand EA, Machulda MM, Senjem ML, Lowe VJ, et al. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology. 2013;81:337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grossman M The non-fluent/agrammatic variant of primary progressive aphasia. Lancet Neurology. 2012;11:545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson CK, Cho S, Hsu C-J, Wieneke C, Rademaker A, Weitner BB, et al. Dissociations between fluency and agrammatism in primary progressive aphasia. Aphasiology. 2012;26:20–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogalski E, Cobia D, Martersteck AC, Rademaker A, Wieneke CA, Weintraub S, et al. Asymmetry of cortical decline in subtypes of Primary Progressive Aphasia. Neurology. 2014;83:1184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mesulam M-M, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer and frontotemporal pathology in primary progressive aphasia. Brain. 2014;137:1176–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gliebus G, Bigio E, Gasho K, Mishra M, Caplan D, Mesulam M-M, et al. Asymmetric TDP-43 distribution in primary progressive aphasia with progranulin mutation. Neurology. 2010;74:1607–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim G, Ahmadian SS, Peterson M, Parton Z, Memon R, Weintraub S, et al. Asymmetric pathology in primary progressive aphasia with progranulin mutations and TDP inclusions. Neurology. 2016;86:627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim G, Bolbolan K, Gefen T, Weintraub S, Bigio E, Rogalski E, et al. Atrophy and microglial distribution in primary progressive aphasia with transactive response DNA-binding protein-43 kDa. Ann Neurol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gefen T, Gasho K, Rademaker A, Lalehzari M, Weintraub S, Rogalski E, et al. Clinically concordant variations of Alzheimer pathology in aphasic versus amnestic dementia. Brain. 2012;135:1554–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giannini LAA, Xie SX, McMillan CT, Liang M, Williams A, Jester C, et al. Divergent patterns of TDP-43 and tau pathologies in primary progressive aphasia. Ann Neurol. 2019;85:630–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohm DT, Fought AJ, Rademaker A, Kim G, Sridhar J, Coventry C, et al. Neuropathologic basis of in vivo crtical atrophy in the aphasic variant of Alzheimer’s disease. Brain Pathology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim G, S. V, Gefen T, Weintraub S, Bigio E, Mesulam M-M, et al. Asymmetric TDP pathology in primary progressive aphasia with right hemisphere language dominance. Neurology. 2018;90:e396–e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonakdarpour B, Rogalski E, Wang A, Sridhar J, Mesulam M-M, Hurley RS. Functional connectivity is reduced in early-stage primary progressive aphasia when atrophy is not prominent. Alzheimer’s Disease and Associated Disorders. 2017;31:101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caso F, Mandelli ML, Henry ML, Gesierich B, Bettcher BM, Ogar J, et al. In vivo signatures of nonfluent/agrammatic primary progressive aphasia caused by FTLD pathology. Neurology. 2014;82:239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramos EM, Dokuru ER, Van Berlo V, Wojta K, Wang Q, Huang AY, et al. Genetic screen in a large series of patients with primary progressive aphasia. Alzheimer’s & Dementia. 2019;15:553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–9. [DOI] [PubMed] [Google Scholar]
- 46.Gass J, Cannon A, Mackenzie I, Boeve B, Baker M, Adamson J, et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Human Molecular Genetics. 2006. [DOI] [PubMed] [Google Scholar]
- 47.Mesulam M, Johnson N, Krefft TA, Gass JM, Cannon AD, Adamson JL, et al. Progranulin mutations in primary progressive aphasia. Arch Neurol. 2007;64:43–7. [DOI] [PubMed] [Google Scholar]
- 48.Coppola C, Oliva M, Saracino D, Pappata S, Zampella E, Cimini S, et al. One novel GRN null mutation, two different aphasia phenotypes. Neurobiol Age. 2019. [DOI] [PubMed] [Google Scholar]
- 49.Simón-Sánchez J, Dopper EGP, Cohn-Hokke PE, Hukema RK, Nicolau N, Seelar H, et al. The clinical and pathological phenotype of C9ORF72 hexanucleotide repeat expansions. Brain. 2012;135:723–35. [DOI] [PubMed] [Google Scholar]
- 50.Munoz DG, Ros R, Fatas M, Bermejo F, Yebenes JGd. Progressive nonfluent aphasia associated with a new mutation V363I in tau gene. American Journal of Alzheimer’s Disease and Other Dementias. 2007;22:294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Godbolt AK, Beck JA, Collinge J, Garrard P, Warren JD, Fox NC, et al. A presenilin 1 R278I mutation presenting with language impairment. Neurology. 2004;63:1702–4. [DOI] [PubMed] [Google Scholar]
- 52.Rogalski E, Johnson N, Weintraub S, Mesulam M-M. Increased frequency of learning disability in patients with primary progressive aphasia and their first degree relatives. Arch Neurol. 2008;65:244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mesulam M-M, Weintraub S. Primary progressive aphasia: Sharpening the focus on a clinical syndrome. In: Boller F, Forette F, Khachaturian Z, Poncet M, Christen Y, editors. Heterogeneity of Alzheimer’s Disease. Berlin: Springer-Verlag; 1992. p. 43–66. [Google Scholar]
- 54.Rogalski EJ, Rademaker A, Wieneke C, Bigio EH, Weintraub S, Mesulam M-M. Association between the prevalance of learning disabilities and primary progressive aphasia. JAMA Neurology. 2014;71:1576–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller ZA, Mandelli MA, Rankin KP, Henry ML, Babiak MC, Frazier DT, et al. Handedness and language learning disability differentially distribute in progressive aphasia variants. 2013. [DOI] [PMC free article] [PubMed]
- 56.Mesulam M-M, Rogalski E, Wieneke C, Hurley RS, Geula C, Bigio E, et al. Primary progressive aphasia and the evolving neurology of the language network. Nature Reviews Neurology. 2014;10:554–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mesulam M-M, Thompson CK, Weintraub S, Rogalski EJ. The Wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain. 2015;138:2423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friederici AD. The brain basis of language processing: from structure to function. Physiological Reviews. 2011;91:1357–92. [DOI] [PubMed] [Google Scholar]
- 59.Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8:293–402. [DOI] [PubMed] [Google Scholar]
- 60.Hagoort P MUC (Memory, Unification, Control) and beyond. Frontiers in Psychology. 2013;4:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–806. [DOI] [PubMed] [Google Scholar]
- 62.Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, Mesulam M-M. Progression of language impairments and cortical atrophy in subtypes of primary progressive aphasia. Neurology. 2011;76:1804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hagoort P Nodes and networks in the neural architecture for language: Broca’s region and beyond. Current Opin Neurobiol. 2014;28:136–41. [DOI] [PubMed] [Google Scholar]
- 65.Bogen JE, Bogen GM. Wernicke’s region-where is it? Ann N Y Acad Sci. 1976;280:834–43. [DOI] [PubMed] [Google Scholar]
- 66.Patterson K, Nestor P, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience. 2007;8:976–88. [DOI] [PubMed] [Google Scholar]
- 67.Mesulam M-M, Wieneke C, Hurley RS, Rademaker A, Thompson CK, Weintraub S, et al. Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain. 2013;136:601–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hurley RS, Bonakdarpour B, Wang X, Mesulam M-M. Asymmetric connectivity between the anterior temporal lobe and the language network. J Cog Neurosci. 2015;27:464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonakdarpour B, Hurley RS, Wang A, Fereira HR, Basu A, A. C, et al. Perturbations of language network connectivity in primary progressive aphasia. Cortex. 2019;121:468–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakachi R, Muramatsu T, Kato M, Akiyama T, Saito F, Yoshino F, et al. Progressive prosopagnosia at a very early stage of frontotemporal lobar degeneration. Psychogeriatrics. 2007;7:155–62. [Google Scholar]
- 71.Snowden J, Harris JM, Thompson JC, Kobylecki C, Jones M, Richardson AMT, et al. Semantic dementia and the left and right temporal lobes. Cortex. 2018;107:188–203. [DOI] [PubMed] [Google Scholar]
- 72.Hurley RS, Paller K, Rogalski E, Mesulam M-M. Neural mechanisms of object naming and word comprehension in primary progressive aphasia. J Neurosci. 2012;32:4848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mesulam M-M, Rader B, Sridhar J, Nelson MJ, Hyun J, Rademaker A, et al. Word comprehension in temporal cortex and Wernicke area: A PPA perspective. Neurology. 2019;92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Binder JR. The Wernicke area. Neurology. 2015;85:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Binder J Current controversies on Wernicke’s area and its role in language. Current Neurology and Neuroscience Reports. 2017;17:1–10. [DOI] [PubMed] [Google Scholar]
- 77.Mesulam M-M, Rader B, Sridhar J, Nelson MJ, Hyun J, Rademaker A, et al. Word comprehension in temporal cortex and Wernicke area: A PPA perspective. Neurology. 2019;92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Catani M, Mesulam M-M, Jacobsen E, Malik F, Martersteck A, Wieneke C, et al. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain. 2013;136:2619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mesulam M-M, Nelson MJ, Hyun J, Rader B, Hurley RS, Rademakers R, et al. Preferential disruption of auditory word representations in primary progressive aphasia with the neuropathology of FTLD-TDP type A. Cognitive and Behavioral Neurology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marquié M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78:787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cotelli M, Manenti R, Ferrari C, Gobbi E, Macis A, Cappa SF. Effectiveness of language training and non-invasive brain stimulation on oral and written naming performance in primary progressive aphasia: a meta-analysis and systematic review. Neuroscience and Biobehavioral Reviews. 2020;108:498–525. [DOI] [PubMed] [Google Scholar]
- 82.Teichmann M, Lesoil C, Godard J, Vernet M, Bertrand A, Levy R, et al. Direct current stimulation over the anterior temporal areas boosts semantic processing in primary progressive aphasia. Ann Neurol. 2016;80:693–707. [DOI] [PubMed] [Google Scholar]
- 83.Carthery-Goulart MT, da Silveira AC, Machado TH, Mansur LL, Parente MM, Senaha MLH, et al. Interventions for cognitive impairments following primary progressive aphasia. Dementia & Neuropsychologia. 2013;7:121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Volkmer A, Spector A, Meitanis V, Warren JD, Beeke S. Effects of functional communication interventions for people with primary progressive aphasia and their caregivers: a systematic review. Aging and Mental Health. 2019. [DOI] [PubMed] [Google Scholar]
- 85.Riedl L, Last D, Danek A, Diehl-Schmid J. Long-term follow-up in primary progressive aphasia: Clinical course and health care utilization. Aphasiology. 2014;28:981–92. [Google Scholar]
- 86.Taylor C, Kingma RM, Croot K, Nickels L. Speech pathology services for primary progressive aphasia: Exploring an emerging area of preactice. Aphasiology. 2009;23:161–74. [Google Scholar]
- 87.Meyer AM, Getz HR, Brennan DM, Hu TM, Friedman RB. Telerehabilitation of anomia in primary progressive aphasia. Aphasiology. 2016;30:483–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rogalski E, Saxon M, McKenna H, Wieneke C, Rademaker A, Corden M, et al. Communication Bridge: A pilot feasibility study of internet-based speech-language therapy for individuals with primary progressive aphasia. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2016;2:213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]