Abstract
Background:
Facilitating neural activity using non-invasive brain stimulation may improve extinction-based treatments for posttraumatic stress disorder (PTSD).
Objective/hypothesis:
Here, we examined the feasibility of simultaneous transcranial direct current stimulation (tDCS) application during virtual reality (VR) to reduce psychophysiological arousal and symptoms in Veterans with PTSD.
Methods:
Twelve Veterans with PTSD received six combat-related VR exposure sessions during sham-controlled tDCS targeting ventromedial prefrontal cortex. Primary outcome measures were changes in skin conductance-based arousal and self-reported PTSD symptom severity.
Results:
tDCS + VR components were combined without technical difficulty. We observed a significant interaction between reduction in arousal across sessions and tDCS group (p = .03), indicating that the decrease in physiological arousal was greater in the tDCS + VR versus sham group. We additionally observed a clinically meaningful reduction in PTSD symptom severity.
Conclusions:
This study demonstrates feasibility of applying tDCS during VR. Preliminary data suggest a reduction in psychophysiological arousal and PTSD symptomatology, supporting future studies.
Keywords: Virtual reality, Posttraumatic stress disorder, Brain stimulation, Transcranial direct current stimulation, Treatment, Therapy
Introduction
Posttraumatic stress disorder (PTSD) is a chronic and disabling condition [1]. Unfortunately, up to half of patients who receive evidence-based PTSD psychotherapy have significant residual symptoms [2] with even poorer efficacy in military Veterans [3]. At its core, PTSD reflects a persistent maladaptive fear response, with failure of the prefrontal cortex, particularly ventromedial prefrontal cortex (VMPFC), to modulate fear signals from the amygdala and dorsal anterior cingulate [4–8]. Since trauma processing has been associated with reduced neural activity in medial prefrontal cortex, among other areas, in individuals with PTSD [9], facilitating endogenous VMPFC activity – in the context of feared stimuli – using non-invasive brain stimulation represents a novel and conceptually valid way to improve treatment efficacy.
Initial studies in laboratory models support using VMPFC-targeted stimulation to reduce maladaptive fear responses during extinction of conditioned fear [10–12]. Therefore, the next critical step requires testing brain stimulation plus exposure to a trauma-relevant context. Because in vivo exposure is often not possible (or ethical), virtual reality (VR) can provide an immersive, contextually relevant environment [13–15] to also allow concurrent psychophysiological monitoring. This is important because reductions in PTSD-related arousal are associated with therapeutic response [16,17], and may provide an objective measure of efficacy.
To this end, we piloted VMPFC-targeted transcranial direct current stimulation (tDCS) during exposure to warzone-related VR (tDCS + VR) in Veterans with warzone-related PTSD. tDCS modulates neuronal resting membrane potentials using a weak (subthreshold) constant current [18,19], and therefore may facilitate learning and memory [20]. Since PTSD is a disorder of learning and memory, stimulation during PTSD-relevant habituation may be particularly effective [21,22]. We hypothesized that, compared to sham, tDCS + VR would result in a more rapid decline in psychophysiological arousal and be associated with clinically meaningful effects.
Methods
Twelve male Veterans with warzone-related PTSD (mean age 40.5 years, SD 8.8, range 30–53 years) who served in Iraq and/or Afghanistan completed six VR sessions (Bravemind; Virtually Better Inc.) over two weeks (study design informed by Rothbaum et al. [15]). Participants were randomized to receive 25 min of 2 mA active or sham tDCS delivered using a neuroConn DC Stimulator Plus (neuroCare Group GmbH) in a single-blind design with anode over AF3 and cathode over PO8. Electrode configuration was informed by prior work [10,11] with stimulation designed to target the VMPFC (Fig. 1A). Outcome measures included psychophysiological arousal (skin conductance reactivity [SCR]) during each VR session, and self-reported PTSD symptoms (PTSD checklist for DSM-5; PCL-5) [23] obtained at baseline, after all VR sessions, and one month later. Other treatments (i.e., medications/psychotherapy) were stable for ≥6 weeks prior to participation and remained unchanged throughout. Each VR session included three 8-min driving scenarios with standardized presentation of 12 warzone events (IEDs, ambushes, vehicle accidents, etc.) in each drive. VR included a head-mounted display with integrated head tracking and stereo earphones presenting combat-related multisensory information, including visual (e.g., landscape), auditory (e.g., explosions), olfactory (e.g., weapon fire), and haptic (e.g. driving vibrations) input (Fig. 1B). tDCS was started simultaneously with VR.
Fig. 1.
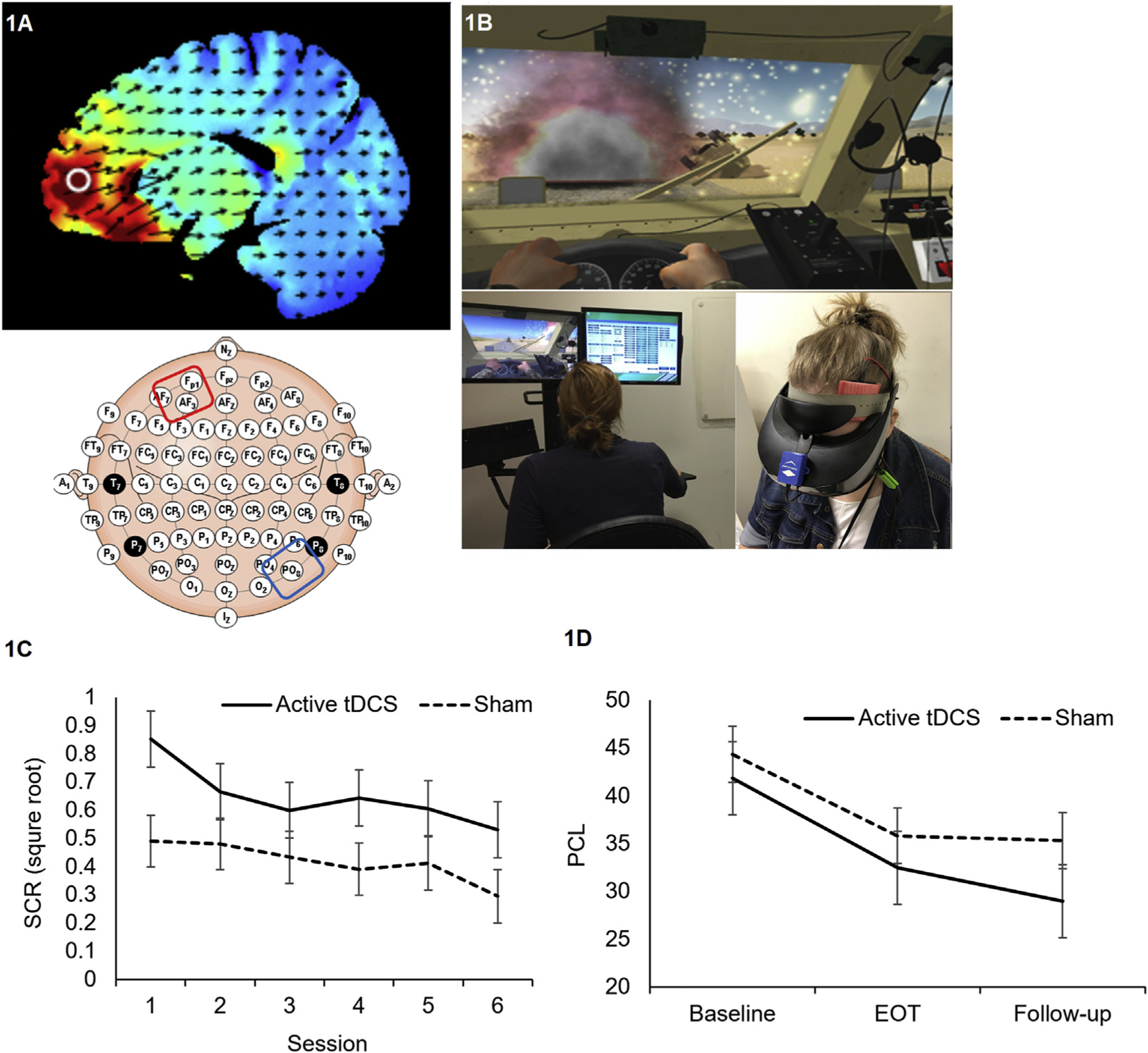
Electrical field modeling of tDCS electrode montage (1A). Example of VR stimulus in driving scenario and tDCS set-up (1B). Average skin conductance reactivity across VR stimuli during each session separated by group (1C). Average self-reported PTSD symptom severity during baseline, end of treatment (EOT), and at one-month follow-up (1D). Bars represent standard error.
Biopac systems (v.4.3, Goleta, CA) were used for data acquisition and preprocessing. We recorded 2 min of baseline SCR before VR for use as a covariate in analyses. SCR to VR events was calculated following procedures used in conditioning [10,24–26]. We used linear mixed models to test the effects of repeated VR sessions, with two-tailed t-tests to compare changes in PCL-5 scores over time, performed in SPSS (v21, Armonk, NY). Stimulation side effects were assessed using a questionnaire based on recommendations by Brunoni et al. [27]. All procedures were approved by the Providence VA Institutional Review Board.
Results
We observed a significant main effect of VR session on SCR for both the active and sham groups combined (F(5,2390.9) = 13.20, p < .001), and a significant VR session by tDCS group interaction (F(5,2391.4) = 2.46, p = .03), favoring active over sham tDCS; SCRs to VR events diminished more quickly over sessions when combined with active tDCS. Although follow up testing revealed that VR led to a decrease in physiological responding across sessions in both groups (all p < .001), the above significant interaction indicates that this decrease was greater in the tDCS + VR group (Fig. 1C). Participants who received active stimulation exhibited a statistical trend towards larger SCRs (F(1,11.1) = 3.24, p = .099). There were no significant SCR changes within VR sessions (all p > .1).
Additional exploratory analyses modeled changes in psychophysiological response to individual VR events. This was informed by observations that SCRs were stronger for certain events (e.g., explosions vs. helicopter flyover). Results were similar, revealing significant main effects of specific events (F(35,1991.1) = 15.44, p < .001) and a significant group by event interaction (F(35,1991.1) = 1.85, p = .002), favoring active tDCS + VR over sham.
Both groups demonstrated clinically meaningful reduction in PTSD symptoms (active: baseline PCL-5 41.83 ± 10.6, endpoint 32.5 ± 16.3; sham: baseline 44.33 ± 15.5, endpoint 35.8 ± 16.2; p = .048 for within-subjects’ comparisons, p > .1 for group contrasts). Participants who received tDCS + VR appeared to continue improving during the 1-month follow-up (active: 29.0 ± 13.4; sham: 35.3 ± 19.6; Cohen’s d = 0.37, active > sham (Fig. 1D)).
Stimulation side effects were mild and consistent with prior reports of tDCS use in psychiatry [28]. Participants could not accurately guess group assignment (χ2(2) = 1.67, p > .1). tDCS did not obstruct head movements during VR; tDCS impedance and electrode location remained stable throughout VR.
Discussion
These preliminary results demonstrate the technical feasibility of tDCS + VR and its potential to improve psychophysiological arousal and clinical symptoms of PTSD. While we approach this pilot data with caution [29], the direction of effects on all measured variables was in the hypothesized direction. Observed reductions in arousal between sessions is consistent with mechanistic models of PTSD treatment [30], and suggests that VMPFC-targeted tDCS might enhance habituation/extinction-based processes underlying exposure to reduce PTSD symptoms. Future work should include measures of state anxiety, and establish whether the observed trend for increased arousal during active stimulation is related to tDCS, or a feature of our sample size.
To our knowledge this is the first use of simultaneous VR and non-invasive brain stimulation for any psychiatric disorder. If replicated, this represents a path for novel treatment development, combining lessons learned from non-invasive brain stimulation, exposure-based psychotherapy, and VR.
Acknowledgments
We thank Causey Dunlap BS for her assistance with data collection. This work was supported in part by a Career Development Award (IK2 CX000724) from the U.S. Department of Veterans Affairs (Clinical Sciences Research and Development), and the Center for Neurorestoration and Neurotechnology (Rehabilitation Research and Development, N9228-C; N2864-C) at the Providence VA Medical Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The authors report no conflict of interest. We thank all the participants.
References
- [1].Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatr 1995;52: 1048–60. [DOI] [PubMed] [Google Scholar]
- [2].Bradley R, Greene J, Russ E, Dutra L, Westen D. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatr 2005;162:214–27. [DOI] [PubMed] [Google Scholar]
- [3].Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatr 2013;74(1):478–550. [DOI] [PubMed] [Google Scholar]
- [4].Koch SB, Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress Anxiety 2016;33:592–605. [DOI] [PubMed] [Google Scholar]
- [5].Etkin A Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatr 2007;164:1476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol 2012;63:129–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Quirk GJ, Garcia R, González-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatr 2006;60:337–43. [DOI] [PubMed] [Google Scholar]
- [8].VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem 2014;113:3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld R, Gati JS, Menon RS. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatr 2001;158: 1920–2. [DOI] [PubMed] [Google Scholar]
- [10].van ‘t Wout M, Longo SM, Reddy MK, Philip NS, Bowker MT, Greenberg BD. Transcranial direct current stimulation may modulate extinction memory in posttraumatic stress disorder. Brain and behavior 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].van ‘t Wout M, Mariano TY, Garnaat SL, Reddy MK, Rasmussen SA, Greenberg BD. Can transcranial direct current stimulation augment extinction of conditioned fear? Brain Stimulation 2016;9:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Raij T, Nummenmaa A, Marin M-F, Porter D, Furtak S, Setsompop K, Milad MR. Prefrontal cortex stimulation enhances fear extinction memory in humans. Biol Psychiatr 2018;84:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wiederhold BK. Virtual reality and applied psychophysiology. Appl Psychophysiol Biofeedback 2005;30:183–5. [DOI] [PubMed] [Google Scholar]
- [14].Opriş D, Pintea S, García-Palacios A, Botella C, Szamosközi Ş, David D. Virtual reality exposure therapy in anxiety disorders: a quantitative meta-analysis. Depress Anxiety 2012;29:85–93. [DOI] [PubMed] [Google Scholar]
- [15].Rothbaum BO, Price M, Jovanovic T, Norrholm SD, Gerardi M, Dunlop B, Davis M, Bradley B, Duncan EJ, Rizzo A. A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. Am J Psychiatr 2014;171:640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Griffin MG, Resick PA, Galovski TE. Does physiologic response to loud tones change following cognitive–behavioral treatment for posttraumatic stress disorder? J Trauma Stress 2012;25:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Blanchard EB, Hickling EJ, Veazey CH, Buckley TC, Freidenberg BM, Walsh JD, Keefer L. Treatment-related changes in cardiovascular reactivity to trauma cues in motor vehicle accident-related PTSD. Behav Ther 2002;33:417–26. [Google Scholar]
- [18].Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F. Transcranial direct current stimulation: state of the art 2008. Brain Stimulation 2008;1:206–23. [DOI] [PubMed] [Google Scholar]
- [19].Huang Y, Liu AA, Lafon B, Friedman D, Dayan M, Wang X, Bikson M, Doyle WK, Devinsky O, Parra LC. Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation. Elife 2017;6, e18834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Coffman BA, Clark VP, Parasuraman R. Battery powered thought: enhancement of attention, learning, and memory in healthy adults using transcranial direct current stimulation. Neuroimage 2014;85:895–908. [DOI] [PubMed] [Google Scholar]
- [21].Baek K, Chae J-H, Jeong J. The effect of repetitive transcranial magnetic stimulation on fear extinction in rats. Neuroscience 2012;200:159–65. [DOI] [PubMed] [Google Scholar]
- [22].Marin M-F, Lonak SF, Milad MR. Augmentation of evidence-based psychotherapy for PTSD with cognitive enhancers. Curr Psychiatr Rep 2015;17:39. [DOI] [PubMed] [Google Scholar]
- [23].Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The ptsd checklist for dsm-5 (pcl-5). Scale available from: the National Center for PTSD at, www.ptsd.va.gov; 2013.
- [24].Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol 2000;109:290. [PubMed] [Google Scholar]
- [25].McLaughlin N, Strong D, Abrantes A, Garnaat S, Cerny A, O’Connell C, Fadok R, Spofford C, Rasmussen S, Milad M. Extinction retention and fear renewal in a lifetime obsessive–compulsive disorder sample. Behav Brain Res 2015;280: 72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pitman RK, Orr SP. Test of the conditioning model of neurosis: differential aversive conditioning of angry and neutral facial expressions in anxiety disorder patients. J Abnorm Psychol 1986;95:208. [DOI] [PubMed] [Google Scholar]
- [27].Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol 2011;14: 1133–45. [DOI] [PubMed] [Google Scholar]
- [28].Philip NS, Nelson BG, Frohlich F, Lim KO, Widge AS, Carpenter LL. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatr 2017;174:628–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatr 2006;59:990–6. [DOI] [PubMed] [Google Scholar]
- [30].Cooper AA, Clifton EG, Feeny NC. An empirical review of potential mediators and mechanisms of prolonged exposure therapy. Clin Psychol Rev 2017;56: 106–21. [DOI] [PMC free article] [PubMed] [Google Scholar]


