Abstract
Background and Aims
The long-term conservation of seeds of plant genetic resources is of key importance for food security and preservation of agrobiodiversity. Nevertheless, there is scarce information available about seed longevity of many crops under germplasm bank conditions.
Methods
Through germination experiments as well as the analysis of historical monitoring data, we studied the decline in viability manifested by 1000 maize (Zea mays subsp. mays) seed accessions conserved for an average of 48 years at the CIMMYT germplasm bank, the largest maize seedbank in the world, under two cold storage conditions: an active (–3 °C; intended for seed distribution) and a base conservation chamber (–15 °C; for long-term conservation).
Key Results
Seed lots stored in the active chamber had a significantly lower and more variable seed germination, averaging 81.4 %, as compared with the seed lots conserved in the base chamber, averaging 92.1 %. The average seed viability detected in this study was higher in comparison with that found in other seed longevity studies on maize conserved under similar conditions. A significant difference was detected in seed germination and longevity estimates (e.g. p85 and p50) among accessions. Correlating seed longevity with seed traits and passport data, grain type showed the strongest correlation, with flint varieties being longer lived than floury and dent types.
Conclusions
The more rapid loss of seed viability detected in the active chamber suggests that the seed conservation approach, based on the storage of the same seed accessions in two chambers with different temperatures, might be counterproductive for overall long-term conservation and that base conditions should be applied in both. The significant differences detected in seed longevity among accessions underscores that different viability monitoring and regeneration intervals should be applied to groups of accessions showing different longevity profiles.
Keywords: Corn, ex situ conservation, germination, grain type, maize, plant genetic resources, seedbank, seed viability, Zea mays
INTRODUCTION
The genetic diversity intrinsic to plant genetic resources (PGRs), especially in crop landraces, is fundamental for the selection of the high-yielding, improved cultivars of the future, able to cope with climatic changes and pests, in order to increase agricultural production and sustainability (Guarino and Lobell, 2011; Vincent et al., 2013; Warschefsky et al., 2014). In this scenario, it is of key importance to conserve PGRs for the long term, as well as to keep them readily available for users worldwide (McCouch et al., 2013). Conservation of PGRs is also important to prevent genetic erosion (i.e. the loss of genetic diversity in the form of alleles and genotypes) that has been observed at very high percentages in the last decades in several areas of the world (Hammer et al., 1996; Veteläinen et al., 2009; van de Wouw et al., 2010).
Ex situ seed storage in seedbanks is considered to be one of the most effective strategies for ensuring the conservation and availability of plant species with orthodox seeds (i.e. seeds that can tolerate drying to low moisture content and subsequent freezing; Roberts et al., 1973). Collections of thousands of seed accessions can be stored in relatively small spaces, providing adequate samples of the genetic diversity within plant populations and species. They will remain viable for the long term, but only if properly conserved (Li and Pritchard, 2009; Riviere and Müller, 2017). Worldwide there are >1750 genebanks, conserving >7.4 million accessions (FAO, 2010; Colville and Pritchard, 2019). There have been several incidents of less than expected longevity at conventional seed bank storage conditions (Li and Pritchard, 2009; Colville and Pritchard, 2019). This raises the question of how well seed banks are carrying out their mission of conserving plant genetic resources for the long term.
The CIMMYT (International Maize and Wheat Improvement Center) Germplasm Bank (located at CIMMYT’s headquarters in Texcoco, Mexico) is the largest seedbank in the world, dedicated to conserving genetic resources of maize (Zea mays subsp. mays) focusing on the tropical and sub-tropical areas of the world. The CIMMYT Germplasm Bank stores for the long term >28 000 maize seed accessions, mainly landraces, but also the genetic legacy of CIMMYT’s maize breeding programme, from the diverse pools and populations to the inbred lines (known as CMLs or CIMMYT maize lines). It also conserves maize wild relatives: the ‘teosintes’ that include all undomesticated taxa in the genus Zea, and species from the sister genus, Tripsacum. More than 14 000 samples of maize seeds conserved at CIMMYT are shipped yearly to breeders, researchers and farmers worldwide. As in many international seedbanks, seeds of the same accessions are conserved in two chambers under different temperature regimes, called ‘active’ (–3 °C) and ‘base’ (–15 °C). In the base chamber, seeds are conserved for the long term (several decades), while in the active chamber, seed samples are used for regeneration, distribution and characterization, to avoid using the stocks conserved in the base, which is considered to be the first level of safety backup for the collection (FAO, 2014).
The Genebank Standards for Plant Genetic Resources for Food and Agriculture recommends that the regeneration or recollection of a seed accession should occur when seed viability drops below 85 % of the initial viability. Intervals of viability monitoring need to be calculated according to the decline of seed viability in the target species (FAO, 2014). Within this framework, it emerges that understanding seed longevity differences among accessions (see, for example, Walters et al., 2005; Probert et al., 2009; Mondoni et al., 2011; Guzzon et al., 2018) is crucial for the management of ex situ seed collections, as these data inform the planning of re-collection or regeneration intervals (Walters, 2003). This is particularly relevant for a crop such as maize that shows, in its diverse landraces, several adaptations to local environments and climatic conditions (Huang et al., 2018). It has been demonstrated that seed longevity varies significantly across different populations of the same species, influenced by their climate of origin (Mondoni et al., 2011, 2018; Zani and Müller, 2017). Moreover, this high degree of local adaptations makes the regeneration of seed accessions of maize landraces challenging, because each accession should be repropagated in sites with environmental conditions that are optimal for its growth and seed production. Therefore, understanding seed longevity of the conserved maize accessions is crucial not only to carefully plan their regeneration intervals and allocate adequate resources to the repropagation activities, but also to assess if the achieved repropagation success (i.e. the percentage of accessions with low viability that are successfully repropagated in the field every year) is sufficient to ensure the long-term conservation of high-quality seeds in the entire collection.
Seed longevity is a complex trait, which is influenced by several factors such as taxonomy, seed structure, germination phenology and environmental factors, including climatic conditions experienced by the seeds during the post-zygotic phase (Zani and Müller, 2017), as well as temperature and relative humidity during seed storage (Guzzon et al., 2020). In addition, various molecular and physiological aspects controlling protection and repair mechanisms are important (Walters et al., 2005; Probert et al., 2009; Mondoni et al., 2011; Gianella et al., 2020). Most of the available publications on seed longevity are comparative studies among different species (e.g. Merritt et al., 2014; Zani and Müller, 2017; Solberg et al., 2020), while only a few have addressed differences in seed longevity among accessions of a single plant species, such as Hay et al. (2013) on rice (Oryza sativa); Mondoni et al. (2018) on Silene suecica; and Van Treuren et al. (2018) on wheat (Triticum aestivum) and barley (Hordeum vulgare). These latter studies revealed that within the same taxon, different populations can show considerable differences in seed longevity. Additionally, only a few seed longevity studies were performed on seed material under long-term conservation (cold storage between –15 and –20 °C; Walters et al., 2005; Hay et al., 2013; Desheva, 2016). Since most seedbanks have relatively short histories, declines in seed viability of the conserved accessions are unlikely to be detected (Van Treuren et al., 2018). For this reason, few empirical data on seed longevity under germplasm bank conditions are available for many important crop species. Interestingly, Van Treuren et al. (2018) found that barley and wheat accessions stored in active conditions (4 °C) for 23–33 years showed a noticeable decline in seed viability in terms of seed germination (–30 %), compared with base conditions (−20 °C), where the same accessions maintained very high germination (95 %). This underscores the need to assess seed longevity under the different storage conditions used in any germplasm bank.
In the case of maize, the data on seed longevity under germplasm bank conditions and intraspecific differences in seed longevity are few and contradictory. Roos and Davidson (1992) estimated an average p50 for maize (the time for viability to fall by 50 %) of 65 years, considering the loss of viability in five maize accessions under long-term storage at –18 °C in the USDA’s National Laboratory for Genetic Resource Preservation in Fort Collins, Colorado, USA. Walters et al. (2005) estimated an average p50 of 49 years for >2000 maize accessions also conserved at the USDA genebank. Deseheva (2016) found a very small (<5 %) loss of seed viability in 364 seed accessions stored for 23 years at –18 °C in the National Genebank of Bulgaria. Yamasaki et al. (2020) recently calculated a mean survival time (MST; the estimated time for half of the seed lots to fall below 85 % of the initial germination) of 21.2 years for 3953 maize accessions, conserved at −1 °C and 30 % relative humidity at NARO genebank (Japan) for up to 29 years.
The objective of this study was to fill a tremendous gap in our understanding of seed longevity in maize ex situ collections under long-term conservation. The current study is, to the best of our knowledge, one of the largest seed longevity experiments, using original data (together with historical data), ever performed. We aimed to (1) evaluate the viability of 1000 maize seed accessions conserved under germplasm bank conditions for up to 60 years; (2) compare seed life span of the same accessions conserved in the active and base chambers of CIMMYT; and (3) investigate the relationship between maize seed traits and seed longevity under germplasm bank conditions.
MATERIALS AND METHODS
Conservation conditions
Maize caryopses (hereafter referred to as ‘seeds’) were originally conserved in a seedbank located at the National School of Agriculture at Chapingo (Texcoco, Mexico) since 1943, in what can be considered the first germplasm bank of Latin America. After that, from the 1960s to 1971, the accessions included in this study were conserved in a temporary refrigerated seed storage facility (0 ± 5 °C) located in the basement of the National School of Agriculture at Chapingo University (Texcoco, Mexico), when they were moved to the first CIMMYT germplasm bank at its recently built headquarters in El Batán, Texcoco, Mexico. Here they were conserved in metallic cans with pressure lids at 0 °C and 45 % relative humidity, with the seeds dried below 10 % of moisture content prior to storage (CIMMYT, 1974, 1988). Starting in 1984, each seed collection was divided, and equal halves were moved to a base chamber (–15 °C) and an active chamber (0 °C) in metal cans. The two seed lots of the same accession conserved in the two chambers will be hereafter identified as: the ‘active’ seed lot and ‘base’ seed lot. In the second half of the 1980s, accessions in the base chamber were transferred to sealed aluminium envelopes. In 1996, the whole collection was moved to the current CIMMYT Germplasm Bank inside the Wellhausen-Anderson Plant Genetic Resources Center, where seeds are conserved in hermetic plastic flasks at –3 ± 2 °C (active chamber) and in hermetically sealed aluminium envelopes at –15 ± 3 °C (base chamber).
Study accessions
Germination tests of 987 seed lots from the base chamber were carried out for this study. Of these same accessions, 835 from the active chamber were also tested for germination. The number of seed lots tested for the active chamber is lower than that for the base chamber because some accessions in the active chamber did not have enough seeds to perform the germination test. Nineteen accessions were tested only in the active chamber since data from recent germination tests (carried out from 2016 to 2019) were available for the base chamber. Overall, a total of 1006 accessions from the base chamber were included in this study. One accession was tested only in the active chamber, due to lack of seeds in the base chamber. Overall, 855 accessions from the active chamber were tested in this experiment. The study accessions were chosen according to the following criteria: (1) having complete passport data (including storage date, collecting site geographical information and information on the repropagation site); (2) having both initial and monitoring germination data; (3) being representative of the different grain types of maize (dent, floury, flint, popcorn and sweet) conserved in the CIMMYT collection; (4) being among the oldest collections for each grain type; and (5) maximizing the environmental range (in terms of latitude and elevation of the collecting sites) among the study accessions. The study accessions originated from seed collections made in 33 different countries, covering an altitudinal range from 2 to 3919 m above sea level. Considering the grain type of the study accessions: 363 were dent, 361 flint, 218 floury, 11 popcorn and 54 sweet. Popcorn and sweet accessions were less represented in the experiment in concordance with their overall lower representation in the collection. The ranges of storage dates for each grain type were as follows: dent (1965–1971), flint (1960–1974), floury (1969–1978), popcorn (1970–1996) and sweet (1968–2002). The average age of the accessions tested for this study was 48 years. More recent accessions of popcorn and sweet maize were used in the study due to the scarcity of older accessions of these grain types in the collection. The study seed lots are identified in the CIMMYT GRIN-Global database as well as marked with a special label (Longevity project) in the conservation chambers; those seed lots will not be substituted with new regenerations, with the hope that researchers can continue, in the next decades, to study the viability of this set. Only one accession was represented by seeds from its original collection. All other accessions had been regenerated in a Mexican or US location prior to conservation (11 regeneration locations in total). Historical initial germination data collected prior to storage were available for the study accessions, and the average initial germination was 99 %. Moreover, results of an additional germination monitoring test were available, but only for the active chamber, since systematic germination monitoring of seeds conserved in the CIMMYT base chamber only began in 2012. Prior to the germination tests carried out for this study, seed mass and moisture content of the accessions (from both the active and base chambers) were measured. Seed mass was determined by weighing three replicates of 20 seeds, kept in one of the dry rooms of the CIMMYT Germplasm Bank at 9–15 °C and 10–20 % relative humidity, randomly sampled from each seed lot, using an analytical balance (Adventurer Pro AV 8101, OHAUS, Parsippany, NJ, USA). The seed moisture content (MC) of the accessions was tested using a moisture meter (SL95, Steinlite, Atchison, KS, USA). The accession passport data considered in this study, besides the historical germination data, were: grain type and colour; regeneration site; and co-ordinates (latitude and longitude), elevation and climatic zone of the original collection site. The most relevant passport data and information (accession ID number, grain type, country of collection, final germination in the active and base chamber, seed mass and ageing rate) for all of the study accessions tested can be found in Supplementary data Table S1. Climatic zones of the collecting sites were based on the Köppen–Geiger climatic classification system (Bryant et al., 2017).
Germination tests
Three replicates of 20 seeds of each accession were sown in rolled filter paper (16.6 × 16.6 cm) moistened with distilled water. It was not possible to use a higher number of seeds or replicates in the germination tests due to the low seed number of some accessions. Filter paper rolls were inserted in transparent plastic trays, and the trays were randomly dispersed in an incubator (Biotronette plant growth chamber 844, Lab-Line) at a constant temperature of 25 °C and a 12 h photoperiod. Distilled water was added to the trays as needed, to avoid desiccation. Germination scoring was performed 1 week after sowing. A seed was considered to be germinated if it had developed into a normal seedling, according to ISTA (2018) criteria. A label with a unique, identifier QR code was attached to each paper roll. The germination scoring was performed through the app GB zone, that is connected to the GRIN-Global genebank database, by means of QR code scanners (two models: CS 4070, Symbol, Zebra, USA; and S740 2D, Socketmobile, USA) connected to a laptop or tablet device. The germination experiments were performed in March and April 2020 in the Seed Viability Laboratory of the CIMMYT Germplasm Bank (Texcoco, Mexico). Germination data obtained in this experiment are available in Supplementary data Table S1.
Statistical analysis
Data analysis and graphic representation were carried out in the R software environment for statistical computing and graphics (v. 3.6.2). The R packages used for the analyses are: ‘corrplot’ (Taiyun and Viliam, 2017), ‘dplyr’ (Wickham et al., 2020), ‘ecotox’, ‘fmsb’ (Nakazawa, 2019), ‘ggplot2’ (Wickham, 2016), ‘ggpubr’ (Kassambara, 2020), ‘lsmeans’ (Lenth, 2016), ‘kgc’ (Bryant et al., 2017), ‘multcom’ (Hothorn et al. 2008), ‘psych’ (Revelle, 2019) and ‘statmod’ (Giner and Smyth, 2016). Before analyses, data were checked for normality and homoscedasticity (Shapiro–Wilk’s and Levene’s tests, respectively).
Two Scheirer–Ray–Hare tests were applied to determine if mass and moisture content differed among accessions and conservation chambers (active and base). A Kruskal–Wallis test was used to determine if there were differences among grain types in terms of seed mass. Pairwise comparisons were carried out with the Bonferroni test.
Multiple parameters were used to characterize seed longevity: final germination (germinated/sown seeds at the end of the germination test); and p50 and p85, corresponding to the time for viability to fall to 50 % and 85 % of the initial value (retrieved from historical, pre-storage data), respectively, estimated or predicted by logit analyses. Generalized linear models (GLMs) with binomial distributions, with logit as the link function, were carried out for p50 and p85 predictions of the accessions conserved in the active chamber. Logit was preferred over probit as link function, since logit showed a higher goodness of fit, compared by means of analysis of variance (ANOVA; χ 2 test) and AIC (Akaike information criterion) scores, when compared with probit analysis (d.f. 0, deviance = –462.23, P < 0.001; AIC logit—probit: –460). Moreover, logit function, as previously observed by dos Santos et al. (2019) and de Faria et al. (2020), allows for a more reliable estimate/prediction of viability loss when compared with probit, especially in the tails of the distribution (i.e. < 10 % or >80 % germination), thus making it more suitable for the p85 estimation. Smaller differences between logit and probit models are observed in the central points of the function (50 % germination, p50). Longevity estimates were filtered for those accessions that showed a final germination lower than the initial and a p85 lower than the years of storage. This was done to estimate the p85 based only on observed data and not predictions, in order to avoid unrealistic values driven by the absence of viability loss in a significant percentage of the seed lots. Yamasaki et al. (2020) also highlighted this issue, suggesting that only longevity estimates within the storage period should be considered, while the reliability of a prediction extrapolated beyond the observation period needs further data accumulation and verification, especially considering that viability loss does not follow a linear pattern, but rather a sigmoidal one (Walters et al., 2005).
Another parameter used to characterize seed longevity was the ageing rate (named L), calculated using the following formula:
Where Gi is the initial germination in percentage, tested before storage; Gf is the most recent germination result in percentage; and Y corresponds to the storage time experienced by the seed accession, expressed in years.
A GLM, with a binomial distribution and logit as link function, was applied to determine the effect of the conservation in the two different chambers (active or base) on final germination, and a post-hoc Bonferroni test was used for pairwise comparisons of the same seed accession in the two conservation conditions. A Kendall’s correlation test was performed on germination in the active and base chambers, in order to assess if there is a correlation between the decline in viability in the two chambers, and, in particular, whether the decline in seed viability of any accession in the active chamber is similar to its decline in the base chamber.
Monitoring intervals were calculated as one third of the p85, with an upper limit set to 40 years as suggested by the Genebank Standards for Plant Genetic Resources for Food and Agriculture (FAO, 2014). A Kruskal–Wallis test was used to determine if there were differences among grain types in terms of monitoring intervals. Pairwise comparisons were carried out with the Wilcoxon rank sum test.
A correlation plot was built based on a mixed correlation matrix for both active- and base-conserved seed lots, considering the following variables: ageing rate (L), seed mass and moisture content, grain type and colour, and regeneration site, as well as latitude, longitude, elevation and climatic zone (Köppen–Geiger) of the original collection sites. Moreover, a partial correlation analysis, using Kendall’s test, was performed, for both the chambers, in order to evaluate the relationship of each single variable with the ageing rate (L), adjusting for the effect of the other variables. A GLM with a compound Poisson-gamma tweedie distribution was applied to assess the effect of grain type on the ageing rate (L).
RESULTS
Seed mass and moisture content
Seed mass differed among accessions (d.f. 841, H = 4844.662, P < 0.001), ranging from 1.43 ± 0.05 to 14.53 ± 0.15 g in the different study accessions (see Supplementary data Table S1). Seed mass was not significantly different between the seed lots of the same accession conserved in the two chambers (d.f. 841, H = 99.64, P = 1). Seed mass was different among grain types (d.f. 4, H = 1091.7, P < 0.001); the highest values of seed mass were detected in floury accessions (mean seed mass 7.3 ± 1.2 g), followed by dent (6.6 ± 1.5 g), flint (4.7 ± 1.2 g), sweet (4.7 ± 0.9 g) and popcorn (2 ± 0.4 g).
Moisture content differed among accessions and between chambers (respectively d.f. 841, H = 3110.262, P < 0.001; d.f. 1, H = 982.336, P < 0.001), ranging from 4.98 to 15.16 % (average = 9.42 ± 1.95 %) in the base chamber and from 5.15 to 15.48 % (average = 11.00 ± 1.46 %) in the active chamber.
Final germination: active and base chambers
The final germination data obtained from the experiment showed significant differences among accessions, the two conservation chambers (active and base) and their interaction (see Table 1).
Table 1.
Analysis of deviance using a generalized linear model (GLM) with binomial distribution and the logit link function: model effects on germination percentage of 835 maize accessions conserved in the two different chambers (active and base)
| d.f. | Deviance | Residual d.f. | Residual deviance | P-value | |
|---|---|---|---|---|---|
| Accession | 834 | 10 003.77 | 4173 | 9868.27 | <0.001 |
| Chamber | 1 | 3261.02 | 4172 | 6607.25 | <0.001 |
| Accession × Chamber | 834 | 2923.84 | 3337 | 3678.53 | <0.001 |
Among the 835 accessions tested from both active and base chambers in this experiment, 284 showed a significant difference in germination between the two chambers (34.01 % of total).
Of the 284 statistically significant pairwise comparisons, 275 accessions showed a higher germination in the base chamber (96.83 % of the comparisons). The remaining nine accessions, with a better performance in the active chamber, accounted for only 3.17 % of comparisons.
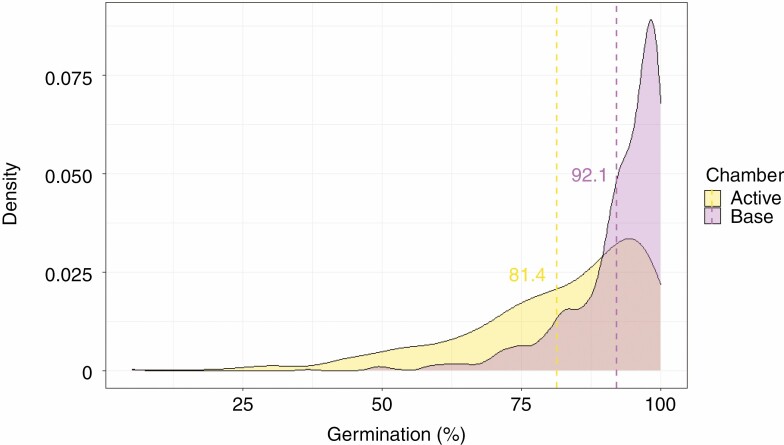
Considering all accessions tested in this experiment from both chambers, the average germination was 92.1 ± 9.1 % for the seed lots conserved in the base chamber and 81.4 ± 16.3 % for the seed lots conserved in the active chamber (Fig. 1). In the base chamber, 84.5 % of the seed lots showed a final germination percentage above the threshold of 85 % of the initial germination value (FAO, 2014), while in the active chamber the final germination of 53 % of the seed lots was above this threshold. Moreover, in the active chamber, we detected twice the number of seed lots in the final germination range between 70 and 85 % of the initial compared with the base chamber, and about seven times the number in the base chamber between 50 and 70 %. Only four seed lots below 50 % of the initial germination were found in the base chamber (accounting for 0.4 % of the total), while 44 seed lots were found in the active chamber (5.2 %, Table 2). Considering the 835 accessions tested for both the active and base chambers, 47.8 and 13.9 % of the seed lots in the active and base chambers, respectively, showed a final germination below the 85 % threshold of the initial germination and therefore need regeneration (FAO, 2014).
Fig. 1.
Density plot representing the frequency of seed lots showing different values of germination percentage on a continuous scale, divided by conservation chamber (active and base). Dashed lines represent the average germination percentage for the two chambers (intercept).
Table 2.
Frequencies of the tested seed lots in active and base chambers divided into four classes based on germination percentage range
| Germination % | % of active accessions | % of base accessions |
|---|---|---|
| >85 % | 53 | 84.5 |
| 70–85 % | 27.3 | 12.8 |
| 50–70 % | 14.6 | 2.2 |
| <50 % | 5.2 | 0.4 |
| Total | 100 | 100 |
Based on a Kendall’s test, a positive correlation was found between the germination of the seed lots of the same accession in the active and base chambers (Tau b = 0.35, P < 0.01, Supplementary data Fig. S1), indicating a similar behaviour, in terms of viability loss, of the same accession conserved in the two different chambers.
p50 and p85
The p50 and p85 predictions were performed only for the active chamber for which historical data on seed germination were available. Three viability data points were available and used in the analysis: initial germination, an intermediate point (corresponding to a viability monitoring test performed between 1985 and 2011, depending on the accession) and the germination data obtained from the current experiment.
Of the total 855 active accessions, logit analysis successfully predicted p85 and p50 for 400 accessions (46.78 % of the total). p85 spanned between 4.2 and 54.4 years, with an average of 37.6 years; p50 values of the same accessions were between 16.5 and 91 years, with an average of 60 years. The observed differences among accessions in longevity estimates were statistically significant (d.f. = 854, residual deviance = 8245.112, P < 0.001).
Due to the fact that reliable longevity estimates (p85 and p50) could not be calculated for all of the accessions, the ageing rate (L) was selected as the longevity indicator for subsequent analyses, making it possible to also include accessions that did not show a decrease in terms of germination across time (L = 0).
Correlations
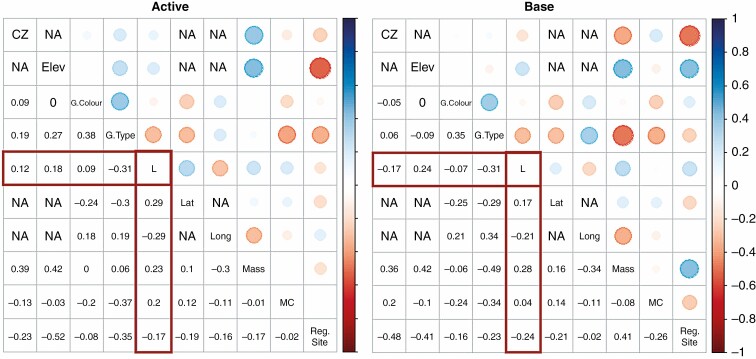
Correlation plots were made for both ‘active’ and ‘base’ seed lots, based on mixed correlation matrices (Fig. 2). Correlations between L and all the other variables were statistically significant (P < 0.5), except for moisture content in the base chamber (P = 0.23).
Fig. 2.
Correlation plots of active and base chambers. Coefficients of correlation are represented by numbers in the lower part of the graph, and by colours in the upper part. Continuous variables: elevation (Elev), ageing rate (L), latitude (Lat), longitude (Long), moisture content (MC) and mass. Polytomous variables: Köppen–Geiger climatic zone (CZ), grain colour (G.Colour), type (G.Type) and regeneration site (Reg.Site). Correlations among geographical variables are indicated as NA.
The ageing rate L showed the strongest correlation with the polytomous variable ‘grain type’ consistently in both conservation chambers (Fig. 2). The partial correlation analysis (Supplementary data Table S2) confirmed that the variable with the strongest correlation with L was grain type, followed by seed mass.
Effect of grain type
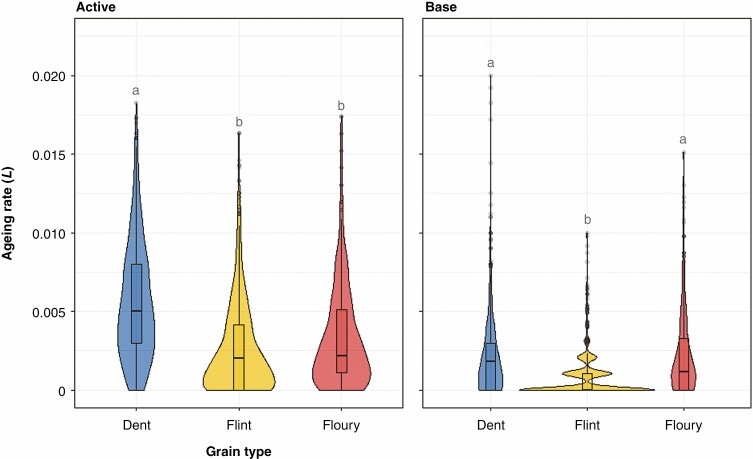
Since grain type showed the strongest correlation with the ageing rate (L), a GLM was performed to understand how L differed among the grain types. Grain type showed a significant effect on the ageing rate L both in the active (d.f. 4, residual deviance = 162.7146, P < 0.001) and in the base chamber (d.f. 4, residual deviance = 223.7943, P < 0.001). In particular, in the active chamber, flint and floury seeds, without significant differences between them (P = 0.22), showed the lowest L, and therefore the lowest loss of seed viability across time, when compared with dent seeds (P < 0.001). In the base chamber, flint seeds showed the lowest L (P < 0.001), while floury and dent seeds did not show differences (P = 0.32). Overall, flint seeds showed a lower ageing rate when compared with the other grain types in terms of both the average and mode of the ageing rate L (Fig. 3). In this latter analysis, only the three major grain types, floury, flint and dent, were considered since the sample sizes were much lower for sweet and popcorn in the set of accession used in this study (see ‘Study accessions’ in the Materials and Methods).
Fig. 3.
Violin and box plots representing the ageing rate (L) in the three most represented grain types, in both the active and base chambers. Letters above violins represent statistically significant differences.
Monitoring intervals
Viability monitoring intervals were calculated as one-third of the p85, with a maximum interval of 40 years between monitoring points, for all the active seed lots, as suggested by FAO (2014). For this calculation, no filter on the p85 was applied; all the p85s, extrapolated from the logit model, were employed in this analysis, since, following FAO (2014) a maximum interval of 40 years is adopted, even when p85 is >120 years.
The average viability monitoring interval for the accessions conserved in the active chamber is 17.5 ± 7.7 years. Monitoring intervals (measured in years) were significantly different among the three main grain types, flint, dent and floury (d.f. = 2, Kruskal–Wallis χ 2 = 80.30, P < 0.001, see Supplementary data Fig. S2), as follows: flint accessions (20.3 ± 9 years, P < 0.001), significantly longer than dent (14.6 ± 4.9 years) and floury (18.0 ± 7.6 years; P < 0.001). The difference in monitoring intervals between dent and floury was also significant (P < 0.01).
DISCUSSION
This set of >1000 maize accessions, stored for as long as 60 years in the CIMMYT germplasm bank, the largest and most diverse collection of maize genetic resources worldwide, provided a unique source of data to study seed longevity. Given the current lack of knowledge on seed longevity of maize accessions conserved under germplasm bank conditions, the data here presented, results of one of the largest seed longevity experiments ever performed, are of great importance to guide ex situ seed conservation of one of the crop species that plays the greatest role in the food security of humanity. Moreover, our results provide new data on seed traits connected to longevity under long-term storage, which merit further investigation.
The results obtained from this study, in terms of both final germination and longevity estimates (p50 and p85), confirmed that maize seeds conserved under germplasm bank conditions are long lived (Walters et al., 2005; Nagel and Börner 2010; Yamasaki et al., 2020; Solberg et al., 2020), showing p50 values of >50 years under long-term storage (Roos and Davidson, 1992; Solberg et al., 2020). Nevertheless, we obtained different longevity values from those reported by previous studies of maize seeds stored under similar conditions (see, for example, Roos and Davidson, 1992; Walters et al., 2005; Deseheva, 2016). Using historical germination data from >2000 maize accessions, Walters et al., (2005) estimated a p50 for maize seeds of 49 years under long-term conservation. The germination results as well as the longevity estimates of the current study indicate that maize accessions conserved at CIMMYT showed a higher seed longevity. The average age of the maize seed accessions considered herein was 48 years, and their average germination was 92.1 % in the base chamber (–15 °C) and 81.4 % in the active chamber (–3 °C; Fig. 1). The average p50 of the seed lots conserved in the active chamber was 60 years; this estimate was calculated only for those seed lots (400 out of 855) showing a decline of viability, thus the overall p50 is likely to be higher, considering the seed longevity of all accessions in both active and base chambers. Moreover, other studies evaluating maize seed longevity under long-term storage conditions similar to those used at the CIMMYT germplasm bank provided different p50 values, e.g. Deseheva (2016) 141 years, Roos and Davidson (1992) 65 years. Future research should aim at understanding those differences in seed longevity found among different conservation facilities to determine whether they are due to different seed regeneration, processing, conservation or data analysis methodologies, or seed-related traits, all of which can influence seed longevity estimates. Many of the seed accessions used in this experiment have been subjected to several changes of locations, containers and conservation conditions in their history (see ‘Conservation conditions’ in the Materials and Methods). Moreover, the MC detected in the study seed lots (9.42 and 11.00 % for the base and active chambers, respectively) was higher than the current standards of conservation (5–8 %), meaning that current seed lots stored at CIMMYT might show even higher longevity than those considered in this experiment. Remedial drying in the drying rooms of the CIMMYT germplasm bank will be performed for those seed lots with MC higher than the current standards of conservation.
Active chambers conserve seed lots that are going to be used and/or distributed so as to not affect the conservation (due to frequent opening of the containers and extraction of the seeds from the chambers) of the seeds stored for the long term in the base chamber (FAO, 2014). Hay et al. (2013) and Van Treuren et al. (2018) observed a significant decline in the germination of barley, rice and wheat seeds conserved in the active chamber (4 °C) compared with the base chamber (–20 °C) after up to 30 years of storage. In the current study, we also detected a significant difference in the final germination of the same accessions in the active vs. the base chamber: 34 % of the seed lots stored in the active chamber had a lower viability than those of the same accessions stored in the base chamber. Moreover, we detected more variable germination percentages among seed lots in the active chamber (Fig. 1; Table 2). These differences in seed viability between active and base conservation conditions may be explained by the fact that seed longevity increases as the storage temperature decreases (Ellis et al., 1992). Additionally, seed lots conserved in the active chamber showed on average a higher moisture content, probably due to more frequent opening of the hermetic containers. This is confirmed by the distribution data of the CIMMYT Germplasm Bank from 1987 until 2020. Considering the study accessions, ‘active’ seed lots were retrieved from the chamber for distribution from three up to 84 times (on average 14 times). On the other hand, ‘base’ seed lots are not used for distribution and are retrieved from the chamber only for viability monitoring. Together with temperature, seed moisture content is the major factor contributing to seed ageing (Zinsmeister et al., 2020)
The more rapid loss of seed viability in the active when compared with the base chamber questions the effectiveness of a seed conservation strategy based on two different thermal storage conditions for the same accessions. Almost half of the seed lots tested in the active chamber will need to be regenerated, being below the 85 % threshold of their initial viability (FAO, 2014), while only 14 % of those same accessions conserved in the base chamber would require regeneration.
The conservation of the same accessions in two separate chambers (active and base) certainly has some advantages; the ‘active’ seed lots are likely to have a higher moisture content, as detected in this study, and could be exposed to frequent changes in temperatures due to more frequent retrieval from the conservation chamber; both factors could affect seed viability in the long term and would occur to a lesser extent in the ‘base’ seed lots. In order to minimize the changes in temperature and relative humidity experienced by ‘active’ seed lots every time an accession is ordered, a few replicates of seeds intended for distribution, both internal and external, could be pre-packed in hermetically sealed containers (e.g. aluminium pouches), avoiding the retrieval of the entire seed lot. Nevertheless, if the ‘active’ seed lots had been stored at the lower temperature conditions of the base chamber, it is safe to assume that the number of ‘active’ seed lots needing regeneration would be significantly lower, improving the overall conservation status of the entire collection and reducing the investments made for monitoring and regeneration (Singh et al., 2012).
We detected a positive correlation between the germination of the same accession from both the active and the base chamber, highlighting an accession-specific behaviour in terms of viability loss. This means that seed accessions with lower viability after storage in the active chamber are likely to also have lower viability after storage in the base chamber (see Supplementary data Fig. S1), as previously observed by Hay et al., (2013) in rice accessions. This can be very important for seedbank management, since the seed lots conserved in the active chamber, i.e. those intended for characterization, distribution, multiplication and research, are more often tested for their viability. Therefore, when a seed lot in the active chamber shows a low viability, the ‘base’ seed lot of the same accession should also be tested to ensure the conservation of high-quality seeds and avoid the loss of genetic resources of inestimable value.
The seed viability and longevity estimates were significantly different among accessions considered in this study; for example, the p50 varied from 16.5 to 91 years. This underscores that within the same plant species, large differences in the longevity profile can be found in different populations, as already observed in several other domesticated and wild species (e.g. Hay et al., 2013; Mondoni et al., 2018; Van Trueren et al., 2018). Due to the increasing age of many historical seedbanks (such as CIMMYT) and therefore the age in storage of many seed accessions, the number of accessions that will need to be regenerated is only going to increase. For this reason, it is important to understand those within-species differences in seed longevity by finding groups of accessions characterized by similar longevity profiles and groups that are particularly affected by seed ageing, in order to plan viability monitoring intervals and regeneration efforts (Hay et al., 2013, Guzzon et al., 2018).
The ageing rate (L) of the study accessions was found to be correlated with seed-related traits, namely seed mass and grain type. The ageing rate was positively correlated with seed mass, which means that larger seeds aged faster than smaller ones (Fig. 2; Supplementary data Table S2). This has been observed in other cereal crop genepools, such as rice (Rao and Jackson, 1996) and wheat wild relatives (Guzzon et al., 2018; Gianella et al., 2020). While there is considerable evidence that seed size has an influence on seed longevity in soil seed banks and in controlled ageing experiments (see, for example, Shutte et al., 2008; Guzzon et al., 2018), the relationship between seed size and longevity under germplasm bank conditions is less understood (Probert et al., 2009). Further research is needed to shed light on this correlation under long-term cold storage.
Among seed-related traits, grain type showed the highest correlation with the ageing rate (Fig. 2; Supplementary data Table S2). Flint accessions are the longest lived among the three main grain types (dent, flint and floury; Fig. 3). This confirmed the observation of Bewley and Black (1994) that seeds of flint varieties are longer lived when compared with other grain types. A similar observation was made by Ortega Paczka (1971), who tested > 4500 maize accessions, conserved at the INIA genebank (Mexico). Grains of varieties of flint maize have mostly hard, glassy endosperm compared with the softer and starchier endosperms typical of dent and floury varieties (Zilic et al., 2011). A glassy endosperm reduces molecular mobility and is directly involved in slowing seed ageing (Buitink and Leprince, 2008; Ballesteros and Walters, 2011). Additionally, we detected that flint seeds have a significantly lower seed mass than dent and floury seeds. We hypothesize that the higher seed longevity detected in flint varieties is due to structural (i.e. glassy endosperm, seed coat and seed mass) or physiological (e.g. antioxidant capacity) traits, but further investigations, e.g genome-wide association studies (GWAS), antioxidant profiling and analyses of viscoelastic properties of the endosperm, need to be carried out, to clarify the biological bases of the differences in seed longevities detected among grain types.
Yamasaki et al. (2020), following the Genebank Standards of the FAO (2014), calculated an average monitoring interval of 7 years for 3953 maize accessions conserved at the NARO genebank in Japan at − 1 °C and 30 % relative humidity. In the current experiment, we calculated an average monitoring interval of 17 years from our study seed lots conserved in the active chamber (–3 °C), within the interval currently used at the CIMMYT Germplasm Bank where ‘active’ seed lots are tested for their viability after 15–20 years of storage. Moreover, significantly different monitoring intervals were obtained for the main grain types in the active chamber. i.e. dent, 15 years; flint, 20 years; floury, 18 years (see Supplementary data Fig. S2). This suggests that different grain types should have different monitoring intervals. Grain type is a qualitative trait based mainly on the seed coat morphology and endosperm texture. Maize landrace kernels can show intermediate or multiple phenotypes between two different grain types. Therefore, more quantitative measures will be needed to study the effect of the grain type on seed longevity and to fine-tune viability monitoring intervals of accessions of different grain types showing different longevity estimates.
Significant correlations were also found between the ageing rate (L) and geographical data of the original collecting sites of the accessions: co-ordinates and elevation data (Fig. 2). It is known that the seed longevity of different populations of the same species can be influenced by the provenance of the populations, as described by latitude and elevation, probably due to adaptive responses to different environmental conditions; see, for example, Probert et al. (2009), Mondoni et al. (2011) and Zani and Müller (2017). It is interesting to see how maize accessions from high altitudes showed on average lower seed longevity, a pattern also observed in other plant species (Fig. 2; Mondoni et al., 2011). These correlations were only partly confirmed by the partial correlation coefficients (Supplementary data Table S2), and further research is needed to understand if those differences are really due to environmental adaptations or to other factors (e.g. differences in moisture content or the geographical distribution of the different grain types; see Supplementary data Table S2). To clarify these possible environmental adaptations, the longevity of similar genotypes collected at different latitudes and altitudes should be tested after the same seed processing (see, for example, Mondoni et al., 2011, 2014).
In conclusion, maize seed accessions conserved at the CIMMYT germplasm bank for up to 60 years showed a high viability (on average >80 %), confirming that maize can be considered a long-lived species under germplasm bank conditions. There have been several debates about the effectiveness of ex situ conservation of large germplasm collections. Some experts raised the concern that large plant germplasm collections cannot be characterized and regenerated fast enough and therefore are merely ‘seed morgues’ (CIMMYT, 1988; Goodman, 1990). We have demonstrated that maize seeds, if properly conserved, can maintain a high viability for decades, providing sufficient time to seed bank curators and researchers for the characterization and regeneration of the accessions, as well as the organization of the composition of the collections, based on genetic diversity, cultural significance and agronomic considerations. Nevertheless, the germination of the seed lots conserved in the active chamber was significantly lower and more variable than in the base chamber. This raises doubts about the current strategy, employed by several international seedbanks, of conserving the same accessions in two chambers with different temperature conditions. Our data indicate that base chamber conditions (cold storage between –15 and –20 °C) should be preferred for the conservation of long-term collections of maize seeds, also for ‘active’ seed lots (intended for regeneration, distribution and characterization) to decrease their loss of viability and, therefore, the frequency of their regeneration. Future revisions of manuals on genebank standards (e.g. FAO, 2014) should consider these results, and similar ones obtained in other crop species (see, for example, Hay et al., 2013, Van Treuren et al., 2018), and direct seedbanks towards the preference of all seed conservation in base chamber conditions. Significant differences were detected in seed longevity among accessions; in particular, flint varieties were longer lived than dent and floury varieties. This underscores the importance of further study of within-crop differences in seed longevity to provide a sound, scientific basis for both viability monitoring and regeneration intervals, on a crop-by-crop basis.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: principal passport data and ageing rate of the accessions tested in the experiment. Table S2: results of the partial correlation analysis for the variables included in the correlation plot. Figure S1: scatterplot of germination percentage in the active and base chambers of the same accessions with regression line. Figure S2: monitoring intervals calculated as one-third of the p85 for the three main grain types, dent, flint and floury.
ACKNOWLEDGMENTS
We are indebted to the maize germplasm bank team for their work on this experiment, especially the members of the Viability Unit: Ángel Jesús Limón Gallegos, Bryan Sánchez Cordero, Irene Nallely Benítez Hernández and Valeria González Meraz. We are indebted to Suketoshi Taba, Jean Hanson, Rafael Ortega Paczka and Garrison Wilkes for the precious information on the history of the CIMMYT maize collection. We thank Antonio da Costa Teixeira (University of Minho, Portugal), Eduardo Fernandez Pascual (University of Oviedo, Spain) and Juan Burgueño (CIMMYT) for fruitful discussions on the analysis of seed longevity data. We thank Dr Susanne Barth and two anonymous reviewers for their invaluable comments on an earlier version of the manuscript. We are extremely grateful to all of the past and present staff members of the CIMMYT Germplasm Bank for the excellent job they have done in conserving the CIMMYT maize collection over all these years. We dedicate this paper to Edwin Wellhausen, the man who started the CIMMYT maize germplasm collection, as the Director of the ‘Races of Maize’ Project, the first Head of the CIMMYT Germplasm Bank, and the first Director General of CIMMYT. The authors have no conflict of interest.
Author contributions: F.G. designed the experiment, carried out the lab activities, analysed the data and wrote the manuscript; M.G. carried out lab activities, analysed the data and wrote the manuscript; J.A.V.J. organized the database and retrieved historical data, organized the data capturing and approved the final version of the manuscript; C.S.C. co-ordinated and carried out the lab activities and approved the final version of the manuscript; D.E.C. designed the experiment, wrote the manuscript and performed the linguistic revision.
FUNDING
This work of the CIMMYT Germplasm Bank is supported by the CGIAR Genebank Platform. M.G. was supported by a mobility grant for PhD students of the University of Pavia for carrying out research activities at CIMMYT.
LITERATURE CITED
- Ballesteros D, Walters C. 2011. Detailed characterization of mechanical properties and molecular mobility within dry seed glasses: relevance to the physiology of dry biological systems. The Plant Journal 68: 607–619. [DOI] [PubMed] [Google Scholar]
- Bewley JD, Black M. 1994. Seeds: physiology of development and germination. Berlin/Heidelberg: Springer Science & Business Media. [Google Scholar]
- Bryant C, Wheeler NR, Rubel F, French RH. 2017. kgc: Koeppen–Geiger climatic zones. R package version 1.0.0.2. https://CRAN.R-project.org/package=kgc. (15 August 2020). [Google Scholar]
- Buitink J, Leprince O. 2008. Intracellular glasses and seed survival in the dry state. Comptes Rendus Biologies 331: 788–795. [DOI] [PubMed] [Google Scholar]
- CIMMYT . 1974. Proceedings: World-wide maize improvement in the 70's and the role for CIMMYT. Texcoco: International Maize and Wheat Improvement Center (CIMMYT). [Google Scholar]
- CIMMYT . 1988. Recent advances in the conservation and utilization of genetic resources. Proceedings of the Global Maize Germplasm Workshop. Texcoco: International Maize and Wheat Improvement Center (CIMMYT). [Google Scholar]
- Colville L, Pritchard HW. 2019. Seed life span and food security. New Phytologist 224: 557–562. [DOI] [PubMed] [Google Scholar]
- Desheva G. 2016. The longevity of crop seeds stored under long-term condition in the National Gene Bank of Bulgaria. Agriculture (Pol’nohospodárstvo) 62: 90–100. [Google Scholar]
- Ellis RH, Hong TD, Roberts EH. 1992. The low-moisture content limit to the negative logarithmic relation between seed longevity and moisture content in three subspecies of rice. Annals of Botany 69: 53–58. [Google Scholar]
- FAO . 2010. The second report on the state of the world’s plant genetic resources for food and agriculture. Rome: FAO. [Google Scholar]
- FAO . 2014. Genebank standards for plant genetic resources for food and agriculture. Rome: FAO. [Google Scholar]
- de Faria RQ, dos Santos ARP, Amorim DJ, Cantão RF, da Silvia EAA, Sartori MMP. 2020. Probit or logit? Which is the better model to predict the longevity of seeds? Seed Science Research 30: 49–58. [Google Scholar]
- Gianella M, Balestrazzi A, Pagano A, et al. 2020. Heteromorphic seeds of wheat wild relatives show germination niche differentiation. Plant Biology (Stuttgart, Germany) 22: 191–202. [DOI] [PubMed] [Google Scholar]
- Giner G, Smyth GK. 2016. statmod: probability calculations for the inverse Gaussian distribution. R Journal 8: 339–351. [Google Scholar]
- Goodman MM. 1990. What genetic and germplasm stocks are worth conserving? In: McGuire PE, Qualset CO, eds. Genetic resources at risk: scientific issues, technologies, and funding policies. Davis, CA: University of California, Davis, 1–9. [Google Scholar]
- Guarino L, Lobell DB. 2011. A walk on the wild side. Nature Climate Change 1: 374–375. [Google Scholar]
- Guzzon F, Bello P, Bradford KJ, Mérida Guzman MA, Costich DE. 2020. Enhancing seed conservation in rural communities of Guatemala by implementing the dry chain concept. Biodiversity and Conservation 29: 3997–4017. [Google Scholar]
- Guzzon F, Orsenigo S, Gianella M, et al. 2018. Seed heteromorphy influences seed longevity in Aegilops. Seed Science Research 28: 277–285. [Google Scholar]
- Hammer K, Knüpffer H, Xhuveli L, Perrino P. 1996. Estimating genetic erosion in landraces – two case studies. Genetic Resources and Crop Evolution 43: 329–336. [Google Scholar]
- Hay FR, de Guzman F, Ellis D, Makahiya H, Borromeo T, Sackville Hamilton NR. 2013. Viability of Oryza sativa L. seeds stored under genebank conditions for up to 30 years. Genetic Resources and Crop Evolution 60: 275–296. [Google Scholar]
- Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biometrical Journal 50: 346–363. [DOI] [PubMed] [Google Scholar]
- Huang C, Sun H, Xu D, et al. 2018. ZmCCT9 regulates maize flowering time. Proceedings of the National Academy of Sciences, USA 115: 334–341. [Google Scholar]
- ISTA . 2018. ISTA handbook on seedling evaluation, 4th edn. Bassersdorf, Switzerland: International Seed Testing Association. [Google Scholar]
- Kassambara A. 2020. ggpubr: ‘ggplot2’ based publication ready plots. R package version 0.4.0. https://CRAN.R-project.org/package=ggpubr. (8 December 2020). [Google Scholar]
- Lenth RV. 2016. Least-squares means: the R package lsmeans. Journal of Statistical Software 69: 1–33. [Google Scholar]
- Li DZ, Pritchard HW. 2009. The science and economics of ex situ plant conservation. Trends in Plant Science 14: 614–621. [DOI] [PubMed] [Google Scholar]
- McCouch S, Baute GJ, Bradeen J, et al. 2013. Feeding the future. Nature 499: 23–24. [DOI] [PubMed] [Google Scholar]
- Merritt DJ, Martyn AJ, Ainsley P, et al. 2014. A continental-scale study of seed lifespan in experimental storage examining seed, plant, and environmental traits associated with longevity. Biodiversity and Conservation 23: 1081–1104. [Google Scholar]
- Mondoni A, Probert RJ, Rossi G, Vegini E, Hay FR. 2011. Seeds of alpine plants are short lived: implications for long-term conservation. Annals of Botany 107: 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondoni A, Orsenigo S, Donà M, et al. 2014. Environmentally induced transgenerational changes in seed longevity: maternal and genetic influence. Annals of Botany 113: 1257–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondoni A, Orsenigo S, Müller JV, Carlsson-Graner U, Jiménez Alfaro B, Abeli T. 2018. Seed dormancy and longevity in subarctic and alpine populations of Silene suecica. Alpine Botany 128: 71–81 [Google Scholar]
- Nagel M, Börner A. 2010. The longevity of crop seeds stored under ambient conditions. Seed Science Research 20: 1–20. [Google Scholar]
- Nakazawa M. 2019. fmsb: functions for medical statistics book with some demographic data. R package version 0.7.0. https://CRAN.R-project.org/package=fmsb. (8 December 2020). [Google Scholar]
- Ortega Paczka RA. 1971. Estudio de algunos factores que afectan el poder germinativo de las semillas de maíz del banco de germoplasma del INIA. Thesis, Chapingo Autonomous University, Mexico. [Google Scholar]
- Probert RJ, Daws MI, Hay FR. 2009. Ecological correlates of ex situ seed longevity: a comparative study on 195 species. Annals of Botany 104: 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao NK, Jackson MT. 1996. Seed longevity of rice cultivars and strategies for their conservation in genebanks. Annals of Botany 77: 251–260. [Google Scholar]
- Revelle W. 2019. psych: procedures for personality and psychological research, Northwestern University, Evanston, Illinois, USA. https://CRAN.R-project.org/package=psychVersion=1.9.12. (8 December 2020). [Google Scholar]
- Rivière S, Müller JV. 2017. Contribution of seed banks across Europe towards the 2020 Global Strategy for plant conservation targets, assessed through the ENSCONET database. Oryx 52: 464–470. [Google Scholar]
- Roberts EH. 1973. Predicting the storage life of seeds. Seed Science and Technology 1: 499–514. [Google Scholar]
- Roos EE, Davidson DA. 1992. Record longevities of vegetable seeds in storage. HortScience 27: 393–396. [Google Scholar]
- dos Santos ARP, de Faria RQ, Amorim DJ, Giandoni VCR, da Silva EAA, Sartori MMP. 2019. Cauchy, Cauchy–Santos–Sartori–Faria, logit, and probit functions for estimating seed longevity in soybean. Agronomy Journal 111: 2929–2939. [Google Scholar]
- Shutte BJ, Regnier EE, Harrison SK. 2008. The association between seed size and seed longevity among maternal families in Ambrosia trifida L. populations. Seed Science Research 18: 201–211. [Google Scholar]
- Singh AK, Varaprasad KS, Venkateswaran K. 2012. Conservation costs of plant genetic resources for food and agriculture: seed genebanks. Agricultural Research 1: 223–239. [Google Scholar]
- Solberg SØ, Yndgaard F, Andreasen C, von Bothmer R, Loskutov IG, Asdal Å. 2020. Long-term storage and longevity of orthodox seeds: a systematic review. Frontiers in Plant Science 11: 1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiyun W, Viliam S. 2017. R package ‘corrplot’: visualization of a correlation matrix. https://github.com/taiyun/corrplot. (8 December 2020). [Google Scholar]
- van Treuren R, Bas N, Kodde J, Groot SPC, Kik C. 2018. Rapid loss of seed viability in ex situ conserved wheat and barley at 4°C as compared to –20°C storage. Conservation Physiology 6: coy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Wouw M, Kik C, Van Hintum T, Van Treuren R, Visser B. 2010. Genetic erosion in crops: concept, research results and challenges. Plant Genetic Resources 8: 1–15. [Google Scholar]
- Veteläinen M, Negri V, Maxted N. 2009. European landraces: on-farm conservation management and use. Bioversity technical bulletin No. 15. Rome: Bioversity International. [Google Scholar]
- Vincent H, Wiersema J, Kell SP, et al. 2013. A prioritised crop wild relative inventory as a first step to help underpin global food security. Biological Conservation 167: 265–275. [Google Scholar]
- Walters C. 2003. Optimising seed banking procedures. In: Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ, Seed conservation: turning science into practice. Kew, London: Royal Botanic Gardens, 723–743. [Google Scholar]
- Walters C, Wheeler LM, Grothenius JM. 2005. Longevity of seeds stored in a genebank: species characteristics. Seed Science Research 15: 1–20. [Google Scholar]
- Warschefsky E, Varma Penmetsa R, Cook DR, von Wettberg EJB. 2014. Back to the wild: tapping evolutionary adaptations for resilient crops through systematic hybridization with crop wild relatives. America Journal of Botany 101: 1791–1800. [DOI] [PubMed] [Google Scholar]
- Wickham H. 2016. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag. [Google Scholar]
- Wickham H, François R, Henry L, Müller K. 2020. dplyr: a grammar of data manipulation. R package version 0.8.5. https://CRAN.R-project.org/package=dplyr. (8 December 2020). [Google Scholar]
- Yamasaki F, Domon E, Tomooka N, Baba-Kasai A, Nemoto H, Ebana K. 2020. Thirty-year monitoring and statistical analysis of 50 species’ germinability in genebank medium-term storage suggest specific characteristics in seed longevity. Seed Science and Technology 48: 269–287. [Google Scholar]
- Zani D, Müller JV. 2017. Climatic control of seed longevity of Silene during the post-zygotic phase: do seeds from warm, dry climates possess higher maturity and desiccation tolerance than seeds from cold, wet climates? Ecological Research 32: 983–994. [Google Scholar]
- Zilic S, Milasinovic M, Terzic D, Barac M, Ignjatovic-Micic D. 2011. Grain characteristics and composition of maize specialty hybrids. Spanish Journal of Agricultural Research 9: 230–241 [Google Scholar]
- Zinsmeister J, Leprince O, Buitink J. 2020. Molecular and environmental factors regulating seed longevity. The Biochemical Journal 477: 305–323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.