Abstract
Background and Aims
Although the plant economic spectrum seeks to explain resource allocation strategies, carbohydrate storage is often omitted. Belowground storage organs are the centre of herb perennation, yet little is known about the role of their turnover, anatomy and carbohydrate storage in relation to the aboveground economic spectrum.
Methods
We collected aboveground traits associated with the economic spectrum, storage organ turnover traits, storage organ inner structure traits and storage carbohydrate concentrations for ~80 temperate meadow species.
Key Results
The suites of belowground traits were largely independent of one another, but there was significant correlation of the aboveground traits with both inner structure and storage carbohydrates. Anatomical traits diverged according to leaf nitrogen concentration on the one hand and vessel area and dry matter content on the other; carbohydrates separated along gradients of leaf nitrogen concentration and plant height.
Conclusions
Contrary to our expectations, aboveground traits and not storage organ turnover were correlated with anatomy and storage carbohydrates. Belowground traits associated with the aboveground economic spectrum also did not fall clearly within the fast–slow economic continuum, thus indicating the presence of a more complicated economic space. Our study implies that the generally overlooked role of storage within the plant economic spectrum represents an important dimension of plant strategy.
Keywords: Anatomy, belowground, herbaceous plant, lignin, non-structural carbohydrates, plant economic spectrum, storage, persistence
INTRODUCTION
The examination of an economic spectrum within plants began with leaves, describing plants towards the fast and slow ends of the continuum favouring an acquisitive strategy and a conservative strategy, respectively (Wright et al., 2004). It was later proposed that an economic spectrum exists within all plant organs and these organs work in tandem (Reich, 2014). For example, plants in a resource-abundant habitat (moist and nutrient-rich) would have a highly acquisitive strategy with short-lived structures and structural investment in organs would be lower, with low leaf dry matter content (LDMC), low wood density, and roots with greater length compared with root mass (Freschet et al., 2010a; Reich, 2014). In contrast, plants in a more stressful habitat would conserve resources by forming long-lived, tougher structures with contrasting characteristics.
Although leaf traits can form a convenient spectrum across different types of plant, there is some dispute regarding the strength of the relationship with other structures (especially root traits; e.g. Valverde-Barrantes et al., 2017), and the characteristics of organs and economic strategy can also vary greatly between woody and herbaceous plants (Klimešová and Herben, 2014). When a plant stem is considered in the plant economic spectrum, it is usually the woody trunk of trees that combines the functions of height growth and competitive ability, vascular connection between roots and leaves, and storage that enables the annual growth of new leaves in temperate zones (Reich, 2014). However, the tree trunk is not completely functionally analogous to the aboveground annual stems of herbaceous perennials (Klimešová et al., 2015). Although both form a connection between belowground organs and leaves, the storage necessary for the regrowth of herbs in seasonal climates is deposited in belowground organs (e.g. rhizomes, bulbs and tubers) (Raunkiær, 1934; Asaeda et al., 2008). This storage represents an important economic allocation of plant resources, yet its role in the plant economic spectrum is largely unknown (Klimešová et al., 2018).
How the inclusion of belowground storage organs would change our perception of the plant economic spectrum could be illustrated by the difference in organ longevity between aboveground stems and belowground storage organs in herbs. Organ longevity is the most important trait in organ economy because plants generally invest less in structures of short-lived organs than in those of long-lived organs; only plants growing in less stressful conditions may afford to produce new organs instead of investing in the maintenance of old organs (Chapin et al., 1990). Although the aboveground stems of herbs are seasonal structures, belowground storage organs persist from 1 year to several decades (Klimešová and Klimeš, 2008). We can further expect that short-lived storage organs differ in their anatomical structure and storage from long-lived structures (Schweingruber and Poschlod, 2005). Thus, we argue that the consideration of storage organ traits (turnover, inner structure and carbohydrate concentration) in herbs will provide a picture of their economy wholly different from that obtained using the traits of aboveground organs.
The turnover of belowground storage organs such as rhizomes (the most common storage organ in the flora of temperate Europe; Klimešová and Klimeš, 2008) was described as falling into two contrasting categories – splitters and integrators – representing the competitive and conservative strategies, respectively (Jónsdóttir and Watson, 1997). Splitters, characterized by low longevity and fast turnover, have a higher lateral spread (horizontal rhizome increment) and multiplication rate (number of ramets) than integrators, which have greater longevity and slower turnover. These strategies correspond to environmental conditions; longevity decreases while lateral spread and multiplication rate increase with greater moisture and nutrient availability (van Groenendael et al., 1996; Klimeš, 2008; Klimešová et al., 2011, 2015; Klimešová and Herben, 2014). The relationship of the morphological characteristics describing the turnover of belowground storage organs to the aboveground traits of the economic spectrum was evaluated for the flora of central Europe; as expected, there was a negative correlation between specific leaf area (SLA) and rhizome persistence (Klimešová et al., 2015).
The persistence and eventual disintegration of a storage organ is a programmed event within plant ontogeny and thus this trait is likely conditioned by organ inner structure, for example by secondary thickening and the abundant presence of lignin (Hay and Kelly, 2008; Watson, 2008). Lignin is an important structural polymer, especially for forming and reinforcing the vascular conduits that connect the roots to the aboveground plant parts (Bazzaz et al., 2000; Klimešová et al., 2018). The content of lignin in storage organs is between that of aboveground stems and leaves (Steen and Larsson, 1986; Freschet et al., 2010a, b; Amougou et al., 2011). Storage organs also contain parenchyma, which is primarily storage tissue (Esau, 1977). It could be expected that the lignin content within storage organs increases with a more conservative strategy (Lens et al., 2016), while the parenchyma content increases with a more acquisitive strategy because greater storage may align with presence in a resource-rich environment. However, greater storage may also be necessary for surviving harsh conditions, and storage organ anatomy has not been studied within the context of the economic spectrum. Additionally, greater vessel diameter has been linked to an increase in hydraulic efficiency but also vulnerability to drought- and frost-induced embolism, and thus it too may be part of the more acquisitive strategy in belowground storage organs (Tyree et al., 1994; Doležal et al., 2019a).
The main function of storage organs is to provide space for the temporary storage of non-structural carbohydrates until later mobilization for use (Chapin et al., 1990). Starch is the most common non-structural carbohydrate in plants but also a large and immobile polysaccharide; thus, many plants also utilize water-soluble molecules that can function as storage or mediate the response to stressors such as drought and frost (Dias-Tagliacozzo et al., 2004; Hisano et al., 2004; Patton et al., 2007; Janeček et al., 2011). These include poly- and oligosaccharides (including raffinose family oligosaccharides and fructans) and the mono- and disaccharides (and small sugar alcohols, e.g. sorbitol; Lewis, 1984). The smallest carbohydrates (mono- and disaccharides) are always present and serve as transport molecules (Liu et al., 2012; Jensen et al., 2016). Carbohydrate type can be phylogenetically constrained, with the notable cases of raffinose family oligosaccharides in the Lamiales (Lewis, 1984) and fructans within the families Asteraceae, Boraginaceae, Campanulaceae and Amaryllidaceae and the Pooidae subfamily of Poaceae (Hendry, 1987). The composition of carbohydrate storage types is known for numerous plant species (e.g. Hendry, 1987; Gomes de Moraes et al., 2016) and it is expected that osmotically active water-soluble carbohydrates are typical of stressful conditions where the growth of plants is constrained by drought or cold. We can therefore expect that because of the role of these carbohydrates in stress response and avoidance, species storing water-soluble poly- and oligosaccharides as the main carbon storage compound will be aligned to the slow end of the economic spectrum.
Changes in carbohydrate storage are usually studied on an intraspecific level (e.g. seasonal refilling and consumption during resprouting) (Lee and Dunton, 1996; McLaurin et al., 1999; Asaeda et al., 2008), while we have no explanation for interspecific differences because comparative studies of the carbohydrate storage strategies of perennial herbs over numerous species and in relation to specific ecological factors are extremely rare (Palacio et al., 2007; Janeček et al., 2011). The extensive storage of carbohydrates is generally interpreted as an adaptation for surviving stressful conditions and damage (Klimeš et al., 1993; Asaeda et al., 2006; Martínez-Vilalta et al., 2016), thus aligning with a more conservative strategy. However, it is important to note that the carbohydrate pool (the amount of carbohydrates in storage organs per plant) is seldom studied because of methodological difficulties (Klimešová et al., 2019). Carbohydrate concentration (the mass of carbohydrates per unit of storage organ dry mass) is easier to study and its relationship to the plant economic spectrum may or may not be the same as the expected relationship of plant economy to the carbohydrate pool.
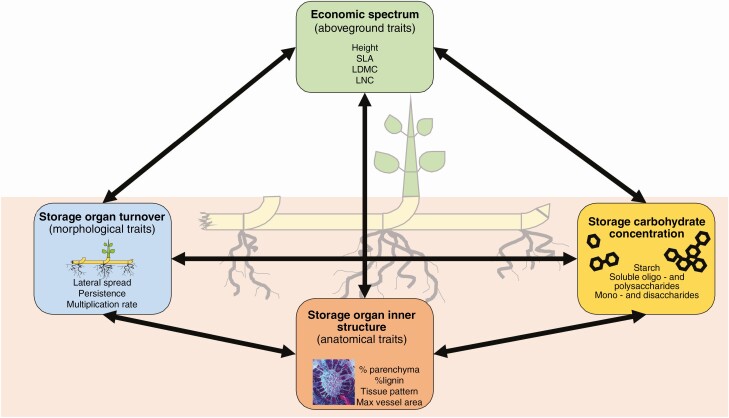
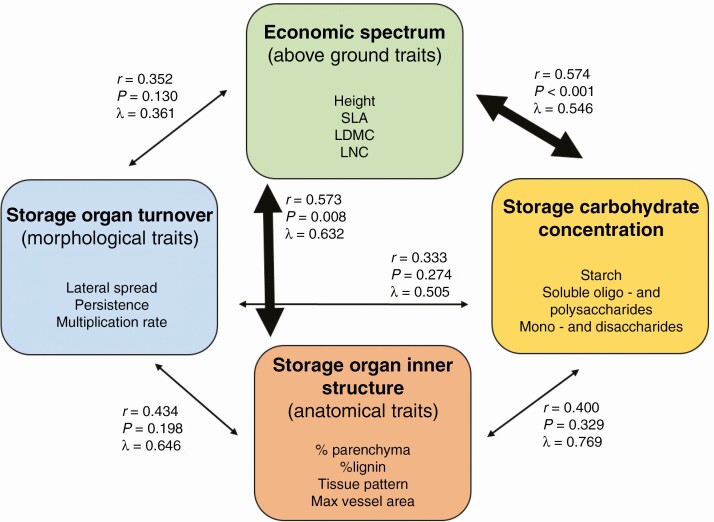
In this study, we are interested in whether there is a united economic spectrum that encompasses both the aboveground acquisitive organs and belowground storage organs (morphological, anatomical and storage traits) of perennial herbs (Fig. 1). We hypothesize that plants on the fast end of the economic spectrum [characterized by high SLA, high leaf nitrogen concentration (LNC), low LDMC and greater height] have faster turnover of storage organs (characterized by large lateral spread, low persistence and high multiplication rate) and high percentages of parenchyma, large conduits, and high total non-structural carbohydrate concentration, formed preferentially by starch. In contrast, plants on the slow end of the economic spectrum are characterized by traits of low turnover (e.g. low lateral spread, high persistence), high percentages of lignified tissue, thin conduits, and low carbohydrate concentration formed by water-soluble sugars in the storage organs. We further hypothesized that storage turnover and storage carbohydrate concentration are interconnected through the inner structure of storage organs. Storage organs with high turnover will be characterized by high carbohydrate concentration because of a higher percentage of parenchyma, and storage organs with slow turnover will be characterized by low carbohydrate concentrations and a high percentage of lignified tissue forming a solid cylinder.
Fig. 1.
Conceptual structure of trait groups and the relationships between aboveground traits and belowground storage traits.
MATERIALS AND METHODS
Study site
We sampled plants on two grasslands (meadows mown once annually for hay without grazing or burning) with different moisture levels. The dry meadow [Čertoryje, in the Bílé Karpaty Mountains, south-east Czech Republic (48°54′ N, 17°25′ E; 440 m a.s.l.)] has a mean annual precipitation around 670 mm, mean annual temperature of 9.1 °C and deep calcium-rich soils that dry out in summer. For a more detailed description see Klimeš (1995) and Doležal et al. (2016). The wet meadow [Ohrazení, near the town of České Budějovice (48°57′ N, 14°36′ E; 500 m a.s.l.)] has a mean annual precipitation around 620 mm, mean annual temperature of 7.8 °C and acidic soil that remains moist throughout the year. For a more detailed description see Lepš (1999) and Doležal et al. (2019b).
Plant collection
Overall, we collected 78 perennial herbs (49 dicots and 29 monocots). Our first sampling yielded 22 species in the dry meadow and 19 species in the wet meadow. We sampled plants in 2006 and 2008 in June (before mowing) and in October (before cold winter temperatures), in five or six replications per species during each sampling. During our second sampling, we collected an additional 36 species in June 2010 in the dry meadow in five replicates. The aboveground biomass was taken into the laboratory and used for measuring aboveground traits (plant height, SLA, LDMC and LNC). The belowground biomass was washed immediately after harvesting, divided into roots, rhizomes and stem bases (if present), and frozen in liquid nitrogen. Because rhizomes and roots of meadow species are intermingled in a dense net, it is virtually impossible to harvest the entire belowground biomass of a plant and thus only fragments of roots and rhizomes were collected. Consequently, only carbohydrate concentrations (and not carbohydrate pool) could be assessed for the harvested plants (see also Klimešová et al., 2017a). Carbohydrates were analysed both in belowground organs of stem origin (stem bases and rhizomes) and in roots; only bulky organs were considered as storage organs for the purpose of this study, even though concentrations in relatively fine roots may be higher than in bulky storage organs (Janeček and Klimešová, 2014).
Storage organ samples for anatomy were not collected during sampling in 2006, 2008 and 2010; additional samples for these species were collected in the same meadows in 2016 and 2019, or data were supplemented with existing cross-sections from Schweingruber et al. (2020). We cut 2-cm-long fragments from the root collar (the area between the root and stem) in non-clonal forbs, 2-cm-long fragments from the most distal part of the rhizomes in clonal forbs, and 2-cm-long fragments from the culm in graminoids (grasses, sedges) at internodes above the base of the culm (for details see Schweingruber and Poschlod, 2005; Klimešová et al., 2019). Fragments were stored in 50 % ethanol to avoid drying and decomposition of samples until processing in the anatomical laboratory of the Department of Functional Ecology, Institute of Botany, Třeboň, Czech Republic.
Trait assessment
Aboveground economic spectrum (leaf traits and plant height)
In the field, we removed one undamaged fully expanded leaf per individual from the stem and weighed it fresh. The maximum height of each plant was measured in the laboratory; this measure is included to indicate plant size because size in herbs can be affected by productivity (Klimešová et al., 2015). For the leafless Juncus effusus, part of a young photosynthetic stem (length >4 cm) was considered to be a leaf (Perez-Harguindeguy et al., 2016). In the laboratory, we measured the one-side projected leaf area for each sample with an AM200 leaf area meter (ADC BioScientific, Hoddesdon, UK) or by scanning it and analysing the image. Leaves were dried at 80 °C (minimum 24 h) and weighed. From the leaf measurements we calculated SLA (the ratio of leaf area to dry weight, mm2 mg−1) and LDMC (the ratio of leaf dry mass divided by fresh mass, %). Dry leaves were ground to a powder using an MM 200 mixer mill (Retsch, Haan, Germany) and after samples had undergone Kjeldahl digestion the nitrogen concentration was measured using a flow injection analysis system (FIA QC8500, Lachat Instruments, USA) (mg g−1).
Storage organ turnover traits
From the CLO-PLA3 database (the database of clonal traits for the flora of Central Europe; Klimešová et al., 2017b), we assessed three clonal traits: rhizome persistence (longevity of connection between ramets, in years); lateral spread (rhizome increment in horizontal direction grown per year); and multiplication rate (number of ramets per parental ramet per year). Rhizome persistence is difficult to measure because of limitations within both anatomical and morphological techniques (Klimešová et al., 2019), and thus it was included as a categorical variable (less than or more than 4 years, according to the CLO-PLA3 database) in the full dataset. Persistence of non-clonal species was set as more than 4 years. For the analysis of relationships between anatomy and persistence, we used a subset of 59 species with more detailed data available, with a close approximation of the number of years of persistence measured by anatomical or morphological techniques for both clonal and non-clonal species. For non-clonal species, lateral spread and multiplication rate were set to zero.
Storage organ inner structure traits
Plant material was sectioned using a sledge microtome (modified Reichert type; © H. Gartner/F.H. Schweingruber, Birmensdorf, Switzerland). Cross-section thickness was between 15 and 40 µm (Gärtner and Schweingruber, 2013). Cross-sections were double-stained using a 1:1 mixture of blue and red dyes (Astra Blue and safranin, respectively). Double staining provided differentiation of lignified xylem cells (fibres, lignified vessels and lignified cells) and cellulose (parenchyma cells and non-lignified cells). The stained cross-sections were dehydrated with a series of ethanol solutions (75 %, 96 % and absolute), washed with xylene and fixed with Canada balsam. The final slides were examined using an Olympus BX53 microscope, an Olympus DP73 camera and cellSense Entry 1.9 software.
To determine the proportion of the three basic tissue types with different functions, a single polygon was drawn over each cross-section to contain all tissue types identified by their colour after staining: blue, parenchyma (storage); white, conduits (water conductive/transport); and red, lignin (mechanical/support). Damaged parts were avoided (Crivellaro et al., 2012). Tissue percentages were quantified using the software ImageJ (Schneider et al., 2012): in the selected polygon 100 randomly placed probes were analysed and the average percentage of the tissue types was calculated.
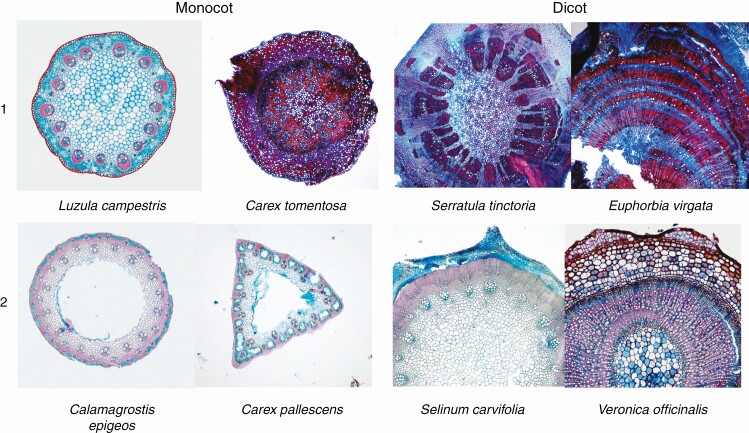
Additionally, cross-sections were divided into four categories based on the anatomical structure and tissue distribution: monocotyledonous herbs with belowground storage stem without hollow pith (Monocot 1) and with hollow pith (Monocot 2); eudicotyledonous herbs with belowground storage stem with parenchyma and rays separating regions of lignified secondary tissue (Dicot 1); and eudicotyledonous herbs with belowground storage stem with solid region of lignified secondary tissue (Dicot 2), (Fig. 2).
Fig. 2.
Examples of the anatomical categories found among the species collected. In monocots, categories are based on the presence or absence of hollow pith, while in dicots they are based on the presence or absence of parenchyma rays separating regions of lignified secondary tissue.
Storage carbohydrate concentrations
Frozen samples of storage organs were freeze-dried, weighed and ground in the laboratory. Glucose, fructose and sucrose were extracted in hot ethanol (Klimešová et al., 2019). In Plantago lanceolata we analysed the content of sorbitol because it is the common sugar alcohol in this species.
Glucose, fructose, sucrose and sorbitol contents were assessed using high-performance anion exchange chromatography with a pulsed amperometric detector (HPAE-PAD; Dionex ISC-3000). Separation was performed using a CarboPac PA1 analytical column (Dionex, Prague, Czech Republic). The content of raffinose family oligosaccharides was calculated as the difference in ethanol-soluble carbohydrates (galactose, glucose, fructose and sucrose) before and after the addition of α-galactosidase (Aspergillus niger; Megazyme, 2020) to the ethanol extract.
Fructan content was analysed by the fructan assay procedure developed by Megazyme International Ireland Ltd (Co. Wicklow, Ireland). This method includes the specific hydrolysis of fructans by fructanase. After hydrolysis, the reducing sugars were measured with the PAHBAH reducing sugar method. The starch content was determined by a total starch assay procedure also developed by Megazyme International. This method uses starch hydrolysis with thermostable α-amylase and amyloglucosidase. The product of these hydrolyses (glucose) is then stained colorimetrically with the glucose-determination reagent GOPOD (glucose oxidase/peroxidase).
All storage carbohydrates were merged to three groups based on size and function (mono- and disaccharides, oligo- and polysaccharides, starch) and expressed as percentages of storage organ dry mass. Mono- and disaccharides (including sorbitol, the small sugar alcohol common in P. lanceolata) are small and have additional transport function, whereas our category of oligo- and polysaccharides (including fructans and raffinose family oligosaccharides) contains all other water-soluble carbohydrates, which are typically of an intermediate size between the first category and starch. Total non-structural carbohydrates were obtained by summing all carbohydrate types found in one sample and were also expressed as the percentage of storage organ dry mass.
Statistical analysis
We used canonical correlation analysis (Hotelling, 1936) to evaluate the links between groups of plant aboveground and belowground traits for 78 of the collected species (49 dicots and 29 monocots). This technique finds the linear combination of two multivariate datasets that maximizes their correlation and thus is suitable for exploration of symmetrical relationships among groups of traits. We assessed the relationships between aboveground economic spectrum traits, belowground storage organ turnover traits, storage organ inner structure traits and storage carbohydrate concentrations using only the summer storage organ values from 2006, 2008 and 2010 (for traits used see Fig. 1). Trait values for each species are the averages of all individuals assessed. To account for variation in evolutionary relatedness between species (Felsenstein, 1985), we used phylogenetic analysis (for description see Revell and Harrison, 2008) with simultaneous estimation of phylogenetic signal (Pagel’s λ; Pagel, 1999), which accounts for the relatedness to the appropriate degree (Freckleton et al., 2002; Revell, 2010).
Because growth is mainly a multiplicative process, many plant characteristics show highly skewed distributions and multiplicative effects are of greater interest than additive ones. We transformed such traits in our dataset (height, lateral spread, multiplication rate, maximum vessel area and carbohydrate concentrations) prior to analyses using natural logarithms (when traits had zero values, we replaced these values by half of the minimum of the rest of the values). Our traits differed in their units, and thus we standardized them to 0 mean and standard deviation 1 prior to canonical correlation analyses.
For two traits (persistence and total carbohydrate concentration) we had more detailed data for a subset of our species (for 59 and 38 species, respectively). To provide better insight into their relationship with storage organ inner structure traits (tissue pattern and parenchyma and lignin percentages), we used phylogenetic linear regressions with simultaneous estimation of phylogenetic signal. Specifically, we used the logarithm of persistence (in years) as the response variable and parenchyma and lignin percentages and their interaction as predictors for the first model. To explore the second relationship, we ran models with the logarithm of total carbohydrate concentration as the response variable and parenchyma as the predictor. We used total carbohydrate concentration in summer and compared the results for the same model with total carbohydrate concentration in autumn, about which we had data for a subset of our species. We also explored if tissue patterns differed in parenchyma and lignin percentages and in storage carbohydrate concentrations (for all 78 species) to get better insight into the effects of this anatomical trait.
To visually assess the interplay of traits both among each other and with greater environmental context, we conducted an exploratory principal components analysis (PCA) with the species optima for moisture and nitrogen using Ellenberg indicator values. Ellenberg indicator values represent the environmental preferences of the species of Central Europe, circumscribing their optima along these gradients (Ellenberg et al., 1992; Dieckmann, 2003). Although primarily experimental observations, their effectiveness in ecological studies has been extensively tested (e.g. Schaffers and Sýkora, 2000; Wamelink et al., 2003). The first two axes of the phylogenetic PCA were fitted using the package phytools version 0.7-20 (Revell, 2012). Traits were standardized to 0 mean and standard deviation 1 prior to the analysis. Ellenberg indicator values (Chytry et al., 2018) and types of anatomy (Monocot 1, 2 and Dicot 1, 2; centroids) were passively projected using the function envfit from package vegan (version 2.5-6; Oksanen et al., 2018).
Analyses were done in R (version 4.0.0; R Core Team, 2020) using the package phytools for canonical correlation analysis (version 0.7-47; Revell, 2012) and package caper for phylogenetic regression (version 1.0.1; Orme et al., 2013). Phylogenetic information about our species was taken from Durka and Michalski (2012).
RESULTS
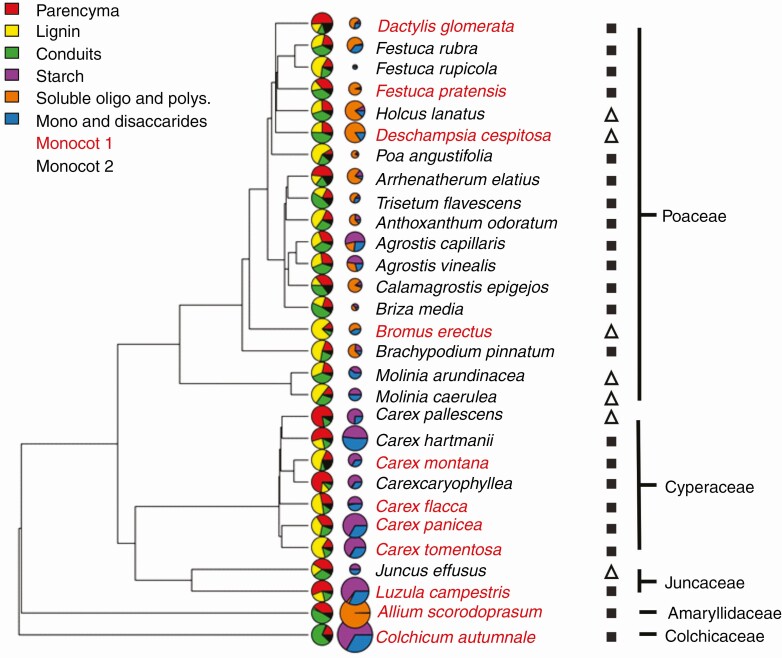
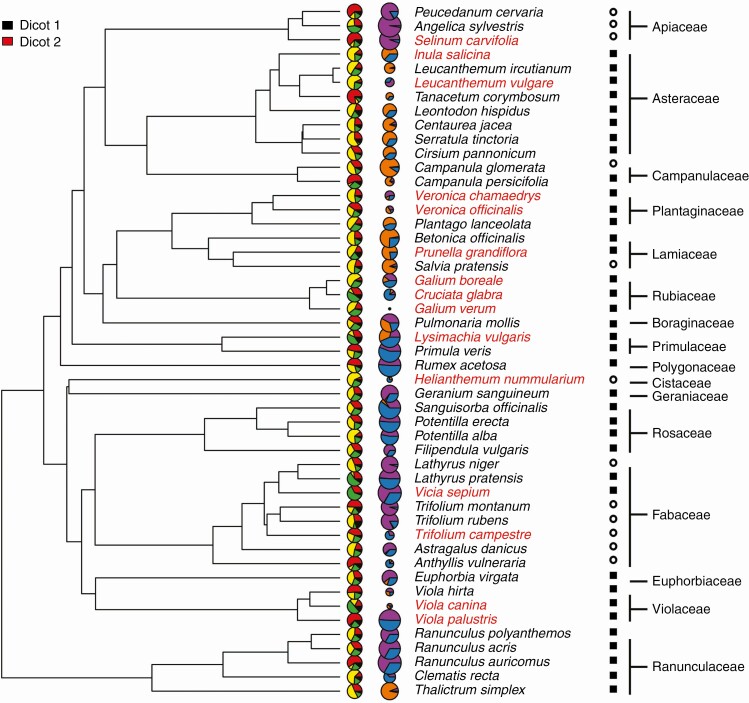
Within our dataset, storage organ lignin percentage varied from <1 % in Vicia sepium to over 76 % in Bromus erectus and parenchyma was from 7 % in Leucanthemum vulgare to 63 % in Selinum carvifolia (Figs 3 and 4). Starch and oligo- and polysaccharides ranged from near total absence in many species to almost 100 % of dry mass of storage organs in Allium scorodoprasum (oligo- and polysaccharides) and Angelica sylvestris (starch). Mono- and disaccharides were similarly variable but never composed >56 % of dry mass of storage organs (Primula veris).
Fig. 3.
Phylogenetic tree of studied monocots with visualization of anatomy and storage proportions, anatomical type and family. The size of the pie charts for carbohydrate concentrations corresponds to the logarithm of total storage carbohydrate concentration and species name colour denotes anatomical category. Storage organ type is denoted by a black square for rhizomes and a white triangle for stem bases.
Fig. 4.
Phylogenetic tree of studied dicots with visualization of anatomy and storage proportions, anatomical type and family. The size of pie charts for carbohydrates corresponds to the logarithm of total storage (for key see Fig. 3) and species name colour denotes anatomical category. Storage organ type is denoted by a black square for rhizomes and a white circle for roots.
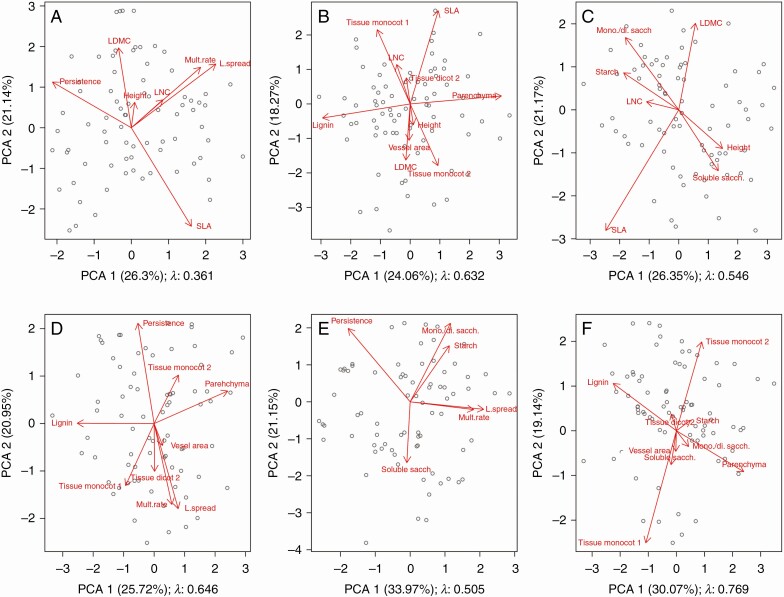
We evaluated links between the four groups of traits and resource acquisition and storage (economic spectrum traits, storage organ turnover traits, storage organ inner structure traits and storage carbohydrate concentrations). There were no significant links between the three belowground storage organ trait groups. However, we found strong links between economic spectrum traits and two of the belowground groups – storage organ inner structure and storage carbohydrate concentrations (Figs 5 and 6). Relationships among aboveground economic traits and storage organ inner structure (Fig. 6B) were driven mainly by high correlations of anatomical types and vessel area with economic traits, whereas percentages of parenchyma and lignin were independent of other compared traits. The relationship between aboveground economic traits and carbohydrate storage concentration was mainly a result of the correlation of plant height and, to a lesser degree, leaf nitrogen with carbohydrate types, while SLA and LDMC were mostly independent (Fig. 6C).
Fig. 5.
Relationships between groups of traits. For each pair the figure shows the correlation among first canonical axes (r), the P-value of the first and all other correlations being zero, and λ (phylogenetic signal). All groups contained 78 species except for storage organ turnover, with only 76 species. Wide arrows denote significant relationships. Details of multivariate comparisons are depicted in Fig. 6.
Fig. 6.
Ordinations of paired groups of traits for the economic spectrum traits and storage organ (SO): turnover, inner structure and storage carbohydrates. (A) Economic spectrum and SO turnover. (B) Economic spectrum and SO inner structure. (C) Economic spectrum and storage carbohydrates. (D) SO turnover and SO inner structure. (E) SO turnover and storage carbohydrates. (F) SO inner structure and storage carbohydrates. Visualized are the first two axes of the phylogenetic PCAs with estimated strength of phylogenetic signal. In (B) and (C) there is significant correlation of canonical axes between groups. Mult. rate, multiplication rate; L. spread, lateral spread; sacch., saccharides
In the analyses of more detailed data for subsets of our species, parenchyma and lignin were negatively correlated (Pearson correlation coefficient, −0.728), and thus their effects on persistence were not fully separable. They had a weak positive effect (P-value of the model, 0.070, λ = 0.42) on persistence, explaining 7.12 % of the variability (adjusted R2).
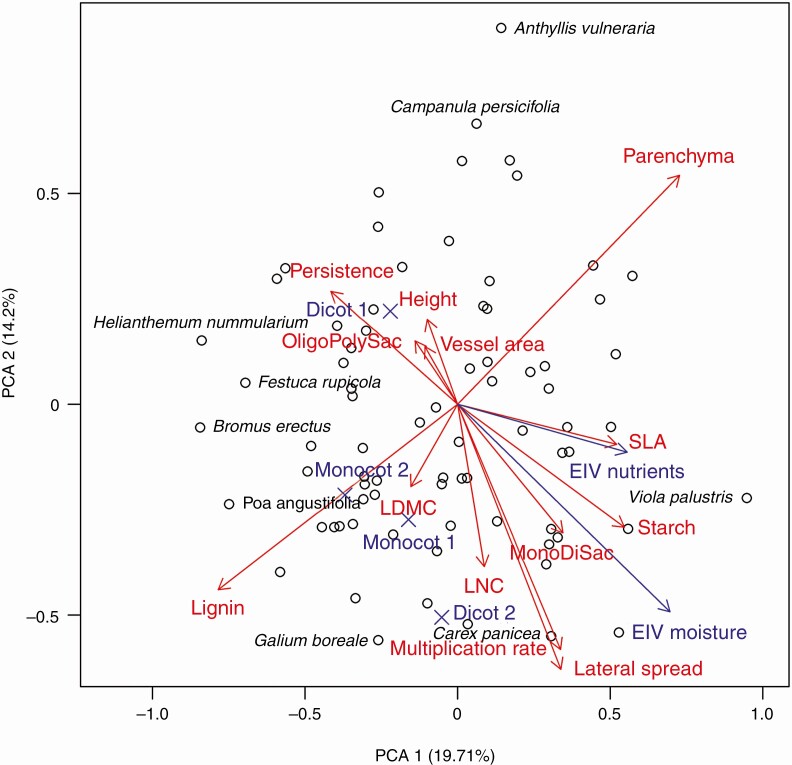
In our ordination combining all traits with Ellenberg indicator values, the first axis of the PCA explained 21.3 % and the second 15.95 % of the variability (Fig. 7). The estimated Pagel’s λ was 0.40. The main traits representing the first axis were SLA and persistence of the storage organ, while the second axis was best represented by plant height, lateral spread and multiplication rate. Passively projected environmental preferences of species were correlated with the first axis.
Fig. 7.
Ordination of traits (aboveground economic spectrum traits, morphological traits, anatomical traits and storage carbohydrates). The first two axes of the phylogenetic PCA (fitted using package phytools version 0.7-20; Revell, 2012) are visualized. Names of species with high scores on the first or second axis are displayed. Shortcuts were used for some variables: SLA, LDMC, LNC, Ellenberg indicator value (EIV), mono- and disaccharides (MonoDiSac) and oligo- and polysaccharides (OligoPolySac). Traits were standardized to mean 0 and standard deviation 1 prior to the analysis. Estimated Pagel’s λ was 0.44. Percentages denote how much variability is captured by each axis. EIVs (Chytry et al., 2018) and types of anatomy (Monocot 1, 2 and Dicot 1, 2; centroids denoted by an X) are passively projected using the envfit function from the vegan package (version 2.5-6; Oksanen et al., 2018). The relative length of red to blue arrows is arbitrary.
Total carbohydrate concentrations in summer and autumn were highly correlated (Pearson correlation coefficient after logarithmic transformation, 0.819). Parenchyma had no effect on summer concentration (adjusted R2 = 1.94 %, P = 0.116, λ = 0.40) or on autumn concentration (adjusted R2 = 3.28 %, P = 0.142, λ = 0.06). However, imperfect stain absorption in some species (possibly Carex pallescens and Tanacetum corymbosum) could have weakened the relationship.
Anatomical types defined by tissue patterns in storage organ cross-sections did not differ in parenchyma (adjusted R2 = −1.66 %, P = 0.630, λ = 0.00) or lignin percentage (adjusted R2 = −2.87 %, P = 0.836, λ = 0.00). Tissue patterns differed in the logarithm of total storage carbohydrate concentration (adjusted R2 = 14.81 %, P = 0.001, λ = 0.00), with higher values for Monocot 1 and Dicot 1 and lower values for Monocot 2 and Dicot 2. On the other hand, tissue patterns did not differ in the concentration of any type of storage carbohydrate (starch, adjusted R2 = −1.51 %, P = 0.605; soluble oligo- and polysaccharides, adjusted R2 = 2.88 %, P = 0.162; mono- and disaccharides, adjusted R2 = −2.75 %, P = 0.815). Estimated phylogenetic signal (λ) was high for models with the individual types of storage carbohydrates (starch, 0.958; soluble oligo- and polysaccharides, 0.878; mono- and disaccharides, 0.390).
DISCUSSION
The traits of belowground storage organs for 78 species from temperate grasslands were, contrary to expectations, largely independent of one another. However, there were correlations of aboveground economic traits with storage organ inner structure, maximum vessel size, and the concentrations of individual types of storage carbohydrate. Although different organ inner structure arrangements did not vary significantly in the concentrations of individual carbohydrate types, they differed in total carbohydrate concentration, indicating an effect of anatomy on carbohydrate storage.
Storage organs and anatomical constraints of their function
Temperate grasslands are a rich mixture of different taxonomical lineages, with 21 families represented in our dataset (five monocotyledonous and 16 eudicotyledonous). This taxonomic diversity enhances richness in anatomical and carbohydrate types (Esau, 1977; Lewis, 1984; Hendry, 1987). The monocots studied are incapable of secondary thickening and have scattered vascular bundles in stem cross-sections; additionally, some species have specialized storage organs like tubers and bulbs. The eudicots have or do not have secondary thickening and have a variety of different patterns of vascular tissue in their stems. In this study, we primarily distinguished between the presence of regions of parenchyma separating those of secondarily thickened lignified tissues (Fig. 2).
In the correlation of aboveground economic traits with organ inner structure, monocot anatomical types formed divergent strategies. Monocot 1 correlated positively with LNC and Monocot 2 with LDMC. Maximum vessel area was also correlated with LDMC and plant height, showing a pattern similar to that found for graminoids in the dry environment of the Western Himalayas, where water-stressed low-altitude plants were characterized by high stature and large vessels (Doležal et al., 2019a) in comparison with plants from wetter and colder high altitudes. Large vessels are usually reported as a component of a faster and more competitive strategy in trees that are not subjected to drought (Reich, 2014; Olson et al., 2018), but their relationship to drought in herbs seems not to mirror that of trees (Dória et al., 2019; Doležal et al., 2019a).
We found that the percentage of storage organ cross-section consisting of parenchyma was largely independent of the concentration of individual types of non-structural carbohydrates. This might be because we used only a single anatomical cross-section for storage organ characterization and thus did not describe possible variability along the length of the organ. On the other hand, we found a relationship between inner structure (rough anatomical types) of the storage organs and total non-structural carbohydrates (i.e. when all carbohydrate types were summed). Their concentrations were higher in species containing additional regions of parenchyma (the pith in Monocot 1 or rays and other regions between areas of secondary thickening in Dicot 1), indicating that anatomical structure is an important clue to interspecific differences in the amount of carbohydrate storage. In trees, the ray parenchyma fraction from species across a broad elevation gradient is correlated with environmental factors, but varies considerably across different clades (Plavcová et al., 2016; Godfrey et al., 2020). Increased parenchyma, and hence storage carbohydrate concentration, in tree species is linked to cold or dry conditions and interpreted as a means of ensuring greater capacity to rescue vascular tissue from hydraulic embolism caused by freezing and drought stress (Morris et al., 2016; Trifilò et al., 2019). The quantity and arrangement of parenchyma may have similar advantages against stress for herbs.
Contrary to our expectation, lignin percentage was not correlated with the persistence of storage organs and therefore lignification is probably not a major cause of organ persistence, even though it does play a role in decomposition after the life of the organ (Freschet et al., 2012). Similarly, the secondary thickening that is generally considered responsible for the persistence of belowground storage organs (Hay and Kelly, 2008; Watson, 2008) did not show the expected pattern. Although the categories of anatomical type did not differ in morphological traits or the concentrations of different storage carbohydrates, they were correlated with traits of the aboveground economic spectrum. Storage organ anatomy deserves further study using experiments and longer environmental gradients. Additionally, more studies should include characterization of more cross-sections along storage organs and with larger regions of the cross-section to better describe a large diversity of anatomical types.
Storage carbohydrates
Although the main storage carbohydrate type can be constrained within certain taxonomical groups, there is still variability, as illustrated by the high concentration of water-soluble oligo- and polysaccharides in Thalictrum simplex in the otherwise starch-rich family of Ranunculaceae (Figs 3 and 4). The identified correlation of carbohydrate type concentration with traits of the aboveground economic spectrum is not easy to explain because of a scarcity of comparative data. The aboveground economic traits and storage carbohydrate concentrations appear to diverge into two strategies, but with different traits within the fast end of the economic spectrum. Small soluble molecules (mono- and disaccharides) and starch aligned with the fast-strategy trait of LNC, while the medium-sized molecules aligned with greater plant height. The main leaf traits, SLA and LDMC, however, remained independent of carbohydrate storage traits. This relationship does not follow the fast–slow dichotomy and hints at an economic space with greater variation and complexity.
The smallest storage molecules are found in all plants (Lewis, 1984), and their use increases when plants that primarily invest in starch are under stress (Alves Vieira et al., 2017). Greater water-soluble sugar concentration is linked to recovery from drought-triggered embolism in trees (Savi et al., 2016) and protection against drought (Küchenmeister et al., 2013; Du et al., 2020), frost (Hisano et al., 2004; Patton et al., 2007) and fire (Gomes de Moraes et al., 2016) in herbaceous plants. However, comparative studies testing these relationships using representative sets of species are rare, and further comparative studies are necessary.
It has also been observed that many species rich in fructans (a common group of oligosaccharides) have fast early-spring growth (Brocklebank and Hendry, 1989), but the cited study compared only 20 species. Additionally, some of the fructan-bearing taxa are incredibly speciose (e.g. Asteraceae, Amaryllidaceae, Pooidae subfamily of Poaceae), feature numerous strategies, and include many species with comparatively late phenology or long growing seasons. The tall meadow plants in our dataset that store oligo- and polysaccharides are usually of late phenology and may be subjected to summer drought. In contrast, many starch-storing small plants in meadows are usually early-flowering species that might be subjected to early-spring frosts and then shading from dominants later in the season. The relationship of storage carbohydrate type to economic strategy in terms of stress response and mobilization needs to be further studied.
Contrary to the lack of relationship of the inner anatomical structure and concentrations of carbohydrate types, we found differences in total non-structural carbohydrate concentration among storage organ anatomical types. This implies that we are on the right track to understanding the relationship between carbohydrate concentrations and carbohydrate pools. However, we must remember that we need to know the storage organ biomass in order to calculate the carbohydrate pool from data about concentration. This parameter is difficult to study, especially in rhizomatous plants (Klimešová et al., 2017a). We can expect that ecosystems other than temperate grasslands, where plants are more compact belowground and not so intermingled with each other (e.g. tropical grasslands), would probably be more suitable for addressing questions regarding the relationship between carbohydrate pool and the aboveground economic spectrum.
Environment
The meadow species we collected on two grasslands represent plants specialized to a gradient of nutrient and water availability, albeit not to its extreme values. In our canonical correlation analysis, we did not confirm the trend found in a previous study surveying clonal and bud bank traits in Central Europe, wherein aboveground traits and storage organ turnover traits were correlated (Klimešová et al., 2015), specifically with a positive relationship between height and lateral spread and a negative relationship between SLA and persistence. This lack of confirmation is probably a result of the shorter environmental gradient and lower number of species than in the previous study. Nevertheless, we do still recognize two main gradients of trait specialization: resource-demanding species (according to preferences for water and nutrients evaluated using Ellenberg indicator values) with high SLA, starch, and mono- and disaccharide concentrations separate from species with high lignin percentages and water-soluble oligo- and polysaccharides, and long persistence of storage organs. In the second gradient, tall plants separate from those with extensive lateral spread and a high multiplication rate. While the first axis combines two well-known relationships within the leaf economic spectrum (Wright et al., 2004) and the splitter–integrator continuum (van Groenendael et al., 1996; Jónsdóttir and Watson, 1997; Klimeš, 2008; Klimešová et al., 2011, 2015), the second seems to reflect the gradient of demographic strategies from tall species with low lateral spread and multiplication rate to small species that quickly spread using short-lived rhizomes (van der Maarel and Sykes, 1993; Herben et al., 2019). The demographic specialization was at least partly connected with the moisture gradient, indicating that stationary persistence is the primary strategy for species adapted to dry and less dense meadows, while mobile individuals (by clonal growth in the horizontal direction) are adapted to the competitive environment of wet meadows. Although the trait groups may have few connections to one another, there may be alignments of these traits at different levels.
To accumulate this dataset of a broad range of traits for a large number of species, we have pooled data collected in different years and from a variety of different specimens. Although the strength of our study would be greater if all measurements had been conducted on all the same individuals and consistently studied the same way and repeated during multiple years, this would have been a massive undertaking. Although there is great need for careful and precise study of the variability and response of these traits under specific conditions, it is also our hope that the belowground traits studied here will be a valuable tool in studying plant ecology across broader scales or when it is not possible to acquire values for all the destructive measures we have used here.
Conclusions
This study is an unprecedented assessment of the relationships between plant traits of the leaf economic spectrum and storage organs of perennial herbs. Although the associations between belowground trait groups within this study are generally weak, we have identified previously unknown links between the aboveground economic spectrum traits and those of storage organ inner structure and storage carbohydrate concentration. Because our study is the first attempt to broaden the understanding of the plant economic spectrum with the inclusion of storage traits, we hope that it opens our vision to new axes of plant specialization and points out that the economic strategies of plants are not a simple spectrum but rather a complicated economic space that should be further explored in its full complexity. Future studies should examine these traits on broader environmental gradients, in different community types and in floras with other evolutionary histories.
ACKNOWLEDGEMENTS
We thank Tomáš Herben for his advice on how to best tell our story.
FUNDING
This work was supported by the Grant Agency of the Czech Republic (grant numbers GA19-13103S and GA19-13231S), a long-term research development project of the Czech Academy of Sciences (number RVO 67985939), and the Ministry of Education, Youth, and Sports of the Czech Republic (number LTT20003).
LITERATURE CITED
- Alves Vieira E, da Cruz Centeno D, Freschi L, Alves da Silva E, Regina Braga M. 2017. The dual strategy of the bromeliad Pitcairnia burchellii Mez to cope with desiccation. Environmental and Experimental Botany 143: 135–148. [Google Scholar]
- Amougou N, Bertrand I, Machet J-M, Recous S. 2011. Quality and decomposition in soil of rhizome, root and senescent leaf from Miscanthus x giganteus, as affected by harvest date and N fertilization. Plant and Soil 338: 83–97. [Google Scholar]
- Asaeda T, Rajapakse L, Manatunge J, Sahara N. 2006. The effect of summer harvesting of Phragmites australis on growth characteristics and rhizome resource storage. Hydrobiologia 553: 327–335. [Google Scholar]
- Asaeda T, Sharma P, Rajapakse L. 2008. Seasonal patterns of carbohydrate translocation and synthesis of structural carbon components in Typha angustifolia. Hydrobiologia 607: 87–101. [Google Scholar]
- Bazzaz FA, Ackerly DD, Reekie EG. 2000. Reproductive allocation in plants. In: Fenner M, ed. Seeds: the ecology of regeneration in plant communities. Wallingford: CABI Publishing, 1–29. [Google Scholar]
- Brocklebank J, Hendry F. 1989. Characteristics of plant species which store different types of reserve carbohydrates. New Phytologist 112: 255–260. [Google Scholar]
- Chapin FS III, Schulze E-D, Mooney HA. 1990. The ecology and economics of storage in plants. Annual Review of Ecology and Systematics 21: 423–447. [Google Scholar]
- Chytry M, Tichy L, Drevojan P, Sádlo J, Zeleny D. 2018. Ellenberg-type indicator values for the Czech flora. Preslia 90: 83–103. [Google Scholar]
- Crivellaro A, McCulloh K, Jokes FA, Lachenbruch B. 2012. Anatomy and mechanical and hydraulic needs of woody climbers contrasted with subshrubs on the island of Cyprus. IAWA Journal 33: 355–373. [Google Scholar]
- Dias-Tagliacozzo GM, Itaya NM, de Carvalho MAM, Figueiredo-Ribeiro RDL, Dietrich SMC. 2004. Fructans and water suppression on intact and fragmented rhizophores of Vernonia herbacea. Brazilian Archives of Biology and Technology 47: 363–373. [Google Scholar]
- Dieckmann M. 2003. Species indicator values as an important tool in applied plant ecology—a review. Basic and Applied Ecology 4: 493–506. [Google Scholar]
- Doležal J, Lehečková E, Sohar K, Altman J. 2016. Oak decline induced by mistletoe, competition and climate change: a case study from central Europe. Preslia 88: 323–346. [Google Scholar]
- Doležal J, Klimeš A, Dvorsky M, Riha P, Klimešová J, Schweingruber F. 2019a. Disentangling evolutionary, environmental and morphological drivers of plant anatomical adaptations to drought and cold in Himalayan graminoids. Oikos 128: 1576–1587. [Google Scholar]
- Doležal J, Lanta V, Mudrák O, Lepš J. 2019b. Seasonality promotes grassland diversity: interactions with mowing, fertilization and removal of dominant species. Journal of Ecology 107: 203–215. [Google Scholar]
- Dória LC, Meijs C, Podadera DS, et al. 2019. Embolism resistance in stems of herbaceous Brassicaceae and Asteraceae is linked to differences in woodiness and precipitation. Annals of Botany 124: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Zhao Q, Chen L, et al. 2020. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiology and Biochemistry 146: 1–12. [DOI] [PubMed] [Google Scholar]
- Durka W, Michalski SG. 2012. Daphne: a dated phylogeny of a large European flora for phylogenetically informed ecological analyses. Ecology 93: 2297–2297. [Google Scholar]
- Ellenberg H, Weber HE, Düll R, Wirth V, Werner W, Paulißen D. 1992. Zeigerwerte von Pflanzen in Mittel-europa, 2nd edn. Göttingen: Goltze. [Google Scholar]
- Esau K. 1977. Anatomy of seed plants. New York: John Wiley & Sons. [Google Scholar]
- Felsenstein J. 1985. Phylogenies and the comparative method. American Naturalist 125: 1–15. [DOI] [PubMed] [Google Scholar]
- Freckleton RP, Harvey PH, Pagel M. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. American Naturalist 160: 712–726. [DOI] [PubMed] [Google Scholar]
- Freschet GT, Cornelissen JHC, van Logtestijn RSP, Aerts R. 2010a. Evidence of the ‘plant economics spectrum’ in a subarctic flora. Journal of Ecology 98: 362–373. [Google Scholar]
- Freschet GT, Cornelissen JH, van Logtestijn RS, Aerts R. 2010b. Substantial nutrient resorption from leaves, stems and roots in a subarctic flora: what is the link with other resource economics traits? New Phytologist 186: 879–889. [DOI] [PubMed] [Google Scholar]
- Freschet GT, Aerts R, Cornelissen JHC. 2012. A plant economics spectrum of litter decomposability. Functional Ecology 26: 56–65. [Google Scholar]
- Gärtner H, Schweingruber FH. 2013. Microscopic preparation techniques for plant stem analysis. Remagen: Kessel. [Google Scholar]
- Godfrey JM, Riggio J, Orozco J, Guzmán-Delgado P, Chin ARO, Zwieniecki MA. 2020. Ray fractions and carbohydrate dynamics of tree species along a 2750 m elevation gradient indicate climate response, not spatial storage limitation. New Phytologist 225: 2314–2330. [DOI] [PubMed] [Google Scholar]
- Gomes de Moraes MG, Carvalho MAM, Franco AC, Pollock CJ, Figueiredo-Ribeiro RCL. 2016. Fire and drought: soluble carbohydrate storage and survival mechanisms in herbaceous plants from the Cerrado. Bioscience 66: 107–117. [Google Scholar]
- van Groenendael JM, Klimeš L, Klimešová J, Hendriks RJJ. 1996. Comparative ecology of clonal plants. In: Silvertown J, Franco M, Harper JL, eds. Plant life histories: ecology, phylogeny, and evolution. Cambridge: Cambridge University Press, 1333–1339. [Google Scholar]
- Hay MJM, Kelly CK. 2008. Have clonal plant biologists got it wrong? The case for changing the emphasis to disintegration. Evolutionary Ecology 22: 461–465. [Google Scholar]
- Hendry G. 1987. The ecological significance of fructan in a contemporary flora. New Phytologist 106: 201–16. [Google Scholar]
- Herben T, Hadincová V, Krahulec F, Pecháčková S, Skálová H. 2019. Two dimensions of demographic differentiation of species in a mountain grassland community: an experimental test. Functional Ecology 33: 1514–1523. [Google Scholar]
- Hisano H, Kanazawa A, Kawakami A, Yoshida M, Shimamoto Y, Yamada T. 2004. Transgenic perennial ryegrass plants expressing wheat fructosyltransferase genes accumulate increased amounts of fructan and acquire increased tolerance on a cellular level to freezing. Plant Science 167: 861–868. [Google Scholar]
- Hotelling H. 1936. Relations between two sets of variates. Biometrika 28: 321–377. [Google Scholar]
- Janeček S, Klimešová J. 2014. Carbohydrate storage in meadow plants and its depletion after disturbance: do roots and stem-derived organs differ in their roles? Oecologia 175: 51–61. [DOI] [PubMed] [Google Scholar]
- Janeček Š, Lanta V, Klimešová J, Doležal J. 2011. Effect of abandonment and plant classification on carbohydrate reserves of meadow plants: carbohydrate reserves of meadow plants. Plant Biology 13: 243–251. [DOI] [PubMed] [Google Scholar]
- Jensen KH, Berg-Sørensen K, Bruus H, et al. 2016. Sap flow and sugar transport in plants. Reviews of Modern Physics 88: 035007. [Google Scholar]
- Jónsdóttir IS, Watson MA. 1997. Extensive physiological integration: an adaptive trait in resource-poor environments? In: de Kroon H, van Groenendael JM, eds. The ecology and evolution of clonal plants. Leiden: Backhuys, 109–136. [Google Scholar]
- Klimeš L. 1995. Small-scale distribution of species richness in a grassland (Bílé Karpaty Mts, Czech Republic). Folia Geobotanica 30: 499–510. [Google Scholar]
- Klimeš L. 2008. Clonal splitters and integrators in harsh environments of the Trans-Himalaya. Evolutionary Ecology 22: 351–367. [Google Scholar]
- Klimeš L, Klimešová J, Osbornová J. 1993. Regeneration capacity and carbohydrate reserves in a clonal plant Rumex alpinus: effect of burial. Vegetatio 109: 153–160. [Google Scholar]
- Klimešová J, Herben T. 2014. Clonal and bud bank traits: patterns across temperate plant communities. Journal of Vegetation Science 26: 243–253. [Google Scholar]
- Klimešová J, Klimeš L. 2008. Clonal growth diversity and bud banks of plants in the Czech flora: an evaluation using the CLO-PLA3 database. Preslia 80: 255–275. [Google Scholar]
- Klimešová J, Doležal J, Sammul M. 2011. Evolutionary and organismic constraints on the relationship between spacer length and environmental conditions in clonal plants. Oikos 120: 1110–1120. [Google Scholar]
- Klimešová J, Tackenberg O, Herben T. 2015. Herbs are different: clonal and bud bank traits can matter more than leaf-height-seed traits. New Phytologist 210: 13–17. [DOI] [PubMed] [Google Scholar]
- Klimešová J, Janecek Š, Bartušková A, et al. 2017. a. Is the scaling relationship between carbohydrate storage and leaf biomass in meadow plants affected by the disturbance regime? Annals of Botany 120: 979–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimešová J, Danihelka J, Chrtek J, de Bello F, Herben T. 2017b. CLO-PLA: a database of clonal and bud-bank traits of the Central European flora. Ecology 98: 1179. [DOI] [PubMed] [Google Scholar]
- Klimešová J, Martínková J, Ottaviani G. 2018. Belowground plant functional ecology: towards an integrated perspective. Functional Ecology 32: 2115–2126. [Google Scholar]
- Klimešová J, Martínková J, Pausas JG, et al. 2019. Handbook of standardized protocols for collecting plant modularity traits. Perspectives in Plant Ecology, Evolution and Systematics 40: 125485. [Google Scholar]
- Küchenmeister K, Küchenmeister F, Kayser M, Wrage-Mönnig N, Isselstein J. 2013. Influence of drought stress on nutritive value of perennial forage legumes. International Journal of Plant Production 18: 1735–8043. [Google Scholar]
- Lee K, Dunton K. 1996. Production and carbon reserve dynamics of the seagrass Thalassia testudinum in Corpus Christi Bay, Texas, USA. Marine Ecology Progress Series 143: 201–210. [Google Scholar]
- Lens F, Picon-Cochard C, Delmas CE, et al. 2016. Herbaceous angiosperms are not more vulnerable to drought-induced embolism than angiosperm trees. Plant Physiology 172: 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepš J. 1999. Nutrient status, disturbance and competition: an experimental test of relationships in a wet meadow. Journal of Vegetation Science 10: 219–230. [Google Scholar]
- Lewis DH. 1984. Occurrence and distribution of storage carbohydrates in vascular plants. In: Lewis DH, ed. Storage carbohydrates in vascular plants: distribution, physiology and metabolism. Cambridge: Cambridge University Press, 1–52. [Google Scholar]
- Liu DD, Chao WM, Turgeon R. 2012. Transport of sucrose, not hexose, in the phloem. Journal of Experimental Botany 63: 4315–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Vilalta J, Sala A, Asensio D, et al. 2016. Dynamics of non-structural carbohydrates in terrestrial plants: a global synthesis. Ecological Monographs 86: 495–516. [Google Scholar]
- van der Maarel E, Sykes MT. 1993. Small-scale plant species turnover in a limestone grassland: the carousel model and some comments on the niche concept. Journal of Vegetation Science 4: 179–188. [Google Scholar]
- McLaurin WJ, Somda ZC, Kays SJ. 1999. Jerusalem artichoke growth, development, and field storage. I. Numerical assessment of plant part development and dry matter acquisition and allocation. Journal of Plant Nutrition 22: 1303–1313. [Google Scholar]
- Megazyme Ltd . 2020. http://www.megazyme.com (18 May 2020).
- Morris H, Plavcová L, Cvecko P, et al. 2016. A global analysis of parenchyma tissue fractions in secondary xylem of seed plants. New Phytologist 209: 1553–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Friendly M, et al. 2018. vegan: community ecology package. R package. https://cran.r-project.org/web/packages/vegan/index.html (12 September 2020). [Google Scholar]
- Olson ME, Soriano D, Rosell JA, et al. 2018. Plant height and hydraulic vulnerability to drought and cold. Proceedings of the National Academy of Sciences of the USA 115: 7551–7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme D, Freckleton R, Thomas G, et al. 2013. caper: comparative analyses of phylogenetics and evolution in R. http://caper.r-forge.r-project.org (12 September 2020). [Google Scholar]
- Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401: 877–884. [DOI] [PubMed] [Google Scholar]
- Palacio S, Maestro M, Montserrat-Martí G. 2007. Relationship between shoot-rooting and root-sprouting abilities and the carbohydrate and nitrogen reserves of Mediterranean dwarf shrubs. Annals of Botany 100: 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton AJ, Cunningham SM, Volenec JJ, Reicher ZJ. 2007. Differences in freeze tolerance of zoysiagrasses: II. Carbohydrate and proline accumulation. Crop Science 47: 2170–2181. [Google Scholar]
- Pérez-Harguindeguy N, Díaz S, Garnier E, et al. 2016. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 64: 715–716. [Google Scholar]
- Plavcová L, Hoch G, Morris H, Ghiasi S, Jansen S. 2016. The amount of parenchyma and living fibers affects storage of nonstructural carbohydrates in young stems and roots of temperate trees. American Journal of Botany 103: 603–612. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2020. R: a language and environment for statistical computing. http://www.rproject.org (15 July 2020). [Google Scholar]
- Raunkiær C. 1934. The life forms of plants and statistical plant geography. Oxford: Oxford University Press. [Google Scholar]
- Reich PB. 2014. The world-wide ‘fast-slow’ plant economics spectrum: a traits manifesto. Journal of Ecology 102: 275–301. [Google Scholar]
- Revell LJ. 2010. Phylogenetic signal and linear regression on species data. Methods in Ecology and Evolution 1: 319–329. [Google Scholar]
- Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Revell LJ, Harrison AS. 2008. PCCA: a program for phylogenetic canonical correlation analysis. Bioinformatics 24: 1018–1020. [DOI] [PubMed] [Google Scholar]
- Savi T, Casolo V, Luglio J, et al. 2016. Species-specific reversal of stem xylem embolism after a prolonged drought correlates to endpoint concentration of soluble sugars. Plant Physiology and Biochemistry 106: 198–207. [DOI] [PubMed] [Google Scholar]
- Schaffers A, Sýkora KV. 2000. Reliability of Ellenberg indicator values for moisture, nitrogen and soil reaction. Journal of Vegetation Science 11: 225–244. [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweingruber FH, Poschlod P. 2005. Growth rings in herbs and shrubs: life span, age determination and stem anatomy. Forest Snow and Landscape Research 79: 195–415. [Google Scholar]
- Schweingruber FH, Kučerová A, Adamec L, Doležal J. 2020. Anatomic atlas of aquatic and wetland plant stems. Cham: Springer International. [Google Scholar]
- Steen E, Larsson K. 1986. Carbohydrates in roots and rhizomes of perennial grasses. New Phytologist 104: 339–346. [Google Scholar]
- Trifilò P, Kiorapostolou N, Petruzzellis F, et al. 2019. Hydraulic recovery from xylem embolism in excised branches of twelve woody species: relationships with parenchyma cells and non-structural carbohydrates. Plant Physiology and Biochemistry 139: 513–520. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Davis SD, Cochard H. 1994. Biophysical perspectives of xylem evolution: is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction? IAWA Journal 159: 335–360. [Google Scholar]
- Valverde-Barrantes OJ, Freschet GT, Roumet C, Blackwood CB. 2017. A worldview of root traits: the influence of ancestry, growth form, climate and mycorrhizal association on the functional trait variation of fine-root tissues in seed plants. New Phytologist 215: 1562–1573. [DOI] [PubMed] [Google Scholar]
- Wamelink GWW, Dobben VJ, Berendse F. 2003. Validity of Ellenberg indicator values from physico-chemical field measurements. Journal of Vegetation Science 14: 619–620. [Google Scholar]
- Watson MA. 2008. Resource storage and the expression of clonal plant life histories. Evolutionary Ecology 22: 471–475. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]