Abstract
Background and Aims
Mammals and molluscs (MaM) are abundant herbivores of tree seeds and seedlings, but how the trees and their environment affect MaM herbivory has been little studied. MaM tend to move much larger distances during the feeding stage than the more frequently studied insect herbivores. We hypothesize that MaM (1) select and stay within the patches that promise to be relatively the richest in seeds and seedlings, i.e. patches around adult trees that are old and within a distantly related, less productive neighborhood; and (2) try to remain sheltered from predators while foraging, i.e. mammals remain close to adult trees or to cover by herbs while foraging, and might force their mollusc prey to show the opposite distribution.
Methods
We exposed oak acorns and seedlings in a temperate forest along transects from adult conspecifics in different neighbourhoods. We followed acorn removal and leaf herbivory. We used exclusion experiments to separate acorn removal by ungulates vs. rodents and leaf herbivory by insects vs. molluscs. We measured the size of the closest conspecific adult tree, its phylogenetic isolation from the neighbourhood and the herbaceous ground cover.
Key Results
Consistent with our hypothesis, rodents removed seeds around adult trees surrounded by phylogenetically distant trees and by a dense herb cover. Molluscs grazed seedlings surrounding large conspecific adults and where herb cover is scarce. Contrary to our hypothesis, the impact of MaM did not change from 1 to 5 m distance from adult trees.
Conclusions
We suggest that foraging decisions of MaM repulse seedlings from old adults, and mediate the negative effects of herbaceous vegetation on tree recruitment. Also, an increase in mammalian seed predation might prevent trees from establishing in the niches of phylogenetically distantly related species, contrary to what is known from insect enemies.
Keywords: Forest ecology, insects, molluscs and rodents, landscape of fear, leaf herbivory, optimal foraging, phylogenetic Janzen–Connell hypothesis, pathogens, phylogenetic diversity, Quercus, seed and seedling predation
Introduction
The seed and seedling phase is essential for successful tree recruitment, and seed and seedling herbivory by insects has been suggested to control the establishment and coexistence of tree species (Hanley and Sykes, 2009; Terborgh, 2012; Bagchi et al., 2014; Garzon-Lopez et al., 2015). Insect herbivory on seeds and seedlings may be strongly influenced by the spatial distance to the adult tree or the phylogenetic distance of the neighbourhood, according to the Janzen–Connell hypothesis (Janzen, 1970; Connell, 1971) and the phylogenetic Janzen–Connell hypothesis (Liu et al., 2012), respectively: pressure by such insect herbivores on seeds/seedlings decreases with spatial distance to a conspecific adult tree or with phylogenetic distance to an adult tree (Schupp, 1988; Deniau et al., 2018). Seeds are also often consumed by rodents and ungulates (Gonzalez-Rodriguez and Villar, 2012; Schnurr et al., 2004), and seedlings are consumed by molluscs (Jennings and Barkham, 1975; Pigot and Leather, 2008). Slugs, in particular, may be major causes of mortality of seedlings (Pigot and Leather, 2008). Despite this established importance of herbivory by mammals and molluscs (MaM) on seeds and seedlings, little is known about how this phenomenon is affected by trees and their environment.
Mammals and molluscs differ from insect herbivores by the fact that they travel across dozens to thousands of host seeds/plants while feeding (Grimm and Paill, 2001), whereas most insect herbivores stay on a single or a few host plants during their feeding stage (van Asch and Visser, 2007). Foraging across such an amount of host seeds/plants may have two consequences. First, MaM must frequently select and leave patches of seeds and seedlings depending on the perceived quality of these patches relative to the matrix. The numbers of seeds and seedlings within a patch or matrix may be difficult to perceive while being inside the matrix or the patch, but adult trees might be used as proxy: as seed production of adult trees increases with adult age, the patch surrounding an old adult might be richer in seeds and seedlings than a patch surrounding a young adult. Adult age, in turn, can be assessed from trunk size. Even terrestrial molluscs might be able to perceive trunk size as they orient themselves towards larger dark zones (Zieger et al., 2009), i.e. should approach an older tree. Also, some plant lineages produce more and larger and more nutritious seeds and seedlings than others. A patch surrounding an adult of the lineage preferred by given MaM is likely to be particularly rich in preferred seeds and seedlings, and might attract MaM foraging across a matrix of adults belonging to phylogenetically distant lineages. Once MaM reach such a patch of the preferred lineage in a non-preferred matrix, the MaM will probably exploit it down to a low giving-up density of resources (Charnov, 1976, Kotler and Brown, 2003). In contrast, in patches in a matrix composed of adults of the same, preferred lineage, MaM might be less attracted to an individual patch, be readier to leave it and might become temporally satiated during peak periods of seed availability (see Silvertown, 1980 for satiation mechanisms in general).
The second consequence of MaM herbivores being mobile and travelling long distances while feeding is that predators might spot and attack them. MaM need shelter where predators cannot see or attack them. Small mammals are major herbivores of seeds, and are attacked by aerial predators, notably owls. Visual or acoustic orientation of such an aerial predator, as well as attempts at attack, can be obstructed by a dense herb layer hiding the small mammal (Gill and Marks, 1991; Ostfeld and Canham, 1993; George and Bazzaz, 1999). Even trunks of standing trees may provide partial shelter to small mammals up to a few metres, leading to higher seed-removal activity of small mammals close to the trunks (Iida, 2006; Ribeiro and Vieira, 2016). In a ‘landscape of fear’ (Bleicher, 2017), such small-scale shelters are essential. Molluscs are major herbivores of seedlings, and are exposed to a diverse range of predators, including small mammals. It can be speculated that molluscs are sheltered from their predators where these predators are not, i.e. where herbaceous cover is sparse or tree trunks relatively far away. Alternatively, climbing tree trunks might be a strategy to escape ground-dwelling predators such as carabid beetles. We hence hypothesize that mammals prefer to choose to forage on seeds in the direct vicinity of a tree trunk and where cover by herbaceous vegetation is most dense. We hypothesize that molluscs might either take the opposite foraging choices to avoid predation by mammals, or similarly prefer proximity to adult trees to hide close to their trunks.
In most late-successional temperate forests, oaks (Quercus sp.) are abundant and constitute one of the main food sources for herbivores (Gurnell, 1993; den Ouden et al., 2005): seeds (i.e. acorns) are very nutritious and often produced in large quantities, and are preferred by the dominant rodent species such as Apodemus sylvaticus over other tree species, in particular those of gymnosperm trees (Jennings, 1976). Also, leaves of oak seedlings are frequently consumed by herbivores (Deniau et al., 2017). Many temperate forests are highly spatiotemporally heterogeneous where the home range of a given rodent or ungulate acorn predator probably spans zones of different tree species composition (Abramson et al., 2006), providing seeds during different seasons. Within zones dominated by lineages distantly related to oaks, rodents might concentrate foraging activity on patches surrounding adult oaks.
The above hypotheses on choices made by foraging MaM predict under which conditions MaM herbivory on seeds or seedlings should be highest: around adults with a large trunk being surrounded by phylogenetically distant adults and by a dense (mammals) or sparse (molluscs) herbaceous layer, and in the direct vicinity of these trees. We tested these predictions for oaks (Quercus petraea, Q. robur or their hybrids) by comparing acorn and seedling herbivory with each of these conditions. We followed acorn removal, separating ungulates and rodents by exclusion experiments. We followed seedling herbivory, separating herbivory by vertebrates, insects and molluscs also using exclusion experiments. Additionally we discriminated plant attacks by different insect feeding guilds or airborne pathogens by observation. In a phylogenetically proximate neighbourhood, oaks are often separated by ≤10 m, which is why we restricted the spatial distances to a maximum of 5 m. Moreover, the possible shelter effect of a trunk from attack by aerial predators is likely to be only operating across shortest distances (Iida, 2006).
MATERIALS AND METHODS
Study site
We carried out the study in the forest of Rennes, Brittany, France (48°12′N, 1°33′W; approx. 90 m altitude; 3000 ha) between 2013 and 2015. This area is characterized by an oceanic climate, a mean annual temperature of 11.3 °C and a cumulative annual rainfall of 836 mm. The forest is composed of two parcel types dominated by either oak (Quercus petraea, Q. robur or their hybrids) and beech (Fagus sylvatica), or by Scots pine (Pinus sylvestris) interspersed with oaks and other Angiosperms, all typical for European temperate oceanic lowland forests (Yguel et al., 2011; Deniau et al., 2017). In total, ten tree species were found as neighbours of our adult trees (details in Yguel et al., 2011). Understorey vegetation is mostly composed of a fern species (Pteridium aquilinum), a grass species (Molinia caerulea) and some shrubs of common holly (Ilex aquifolium). This forest is inhabited by typical enemies of tree seeds and seedlings occurring in western European temperate forests: vertebrates, notably ungulates (wild boar Sus scrofa and roe deer Capreolus capreolus) and rodents (Apodemus sylvaticus; see Supplementary data Appendix S1); invertebrates, notably insects (see Supplementary data Appendix S2 for Lepidopteran species occurring on adult oak trees) and molluscs (notably Arion sp. and Limax sp.; see Supplementary data Appendix S3); and airborne pathogens such as the mildew fungus Erysiphe sp. Note that the red deer Cervus elaphus is absent from this forest (Office National des Forêts, pers. comm.) and that bird predators of acorns were never observed on the ground.
Within the forest, we selected ten pairs of adult oak trees (Quercus petraea, Q. robur or their hybrids, used by Yguel et al., 2011; see Supplementary data Appendix S4 for geographic distribution of pairs and for distances among pairs, ranging from 0.2 to 5 km, with an average of 2.1 km). In each pair, oaks belonged to the same species. Each pair had one tree in a more oak-dominated zone and one in an adjacent more pine-dominated zone. Diameters at breast height of focal trees ranged from 14 to 32 cm (mean 22 cm, s.d. 5.8 cm), a range corresponding to adult age (e.g. Steele et al., 2007). Larger trees were absent from the more pine-dominated zones and were hence not considered. Selecting adult oak trees in both types of zone ensured a large range of phylogenetic distances between adult oaks and their neighbours (precise quantification of phylogenetic distances is explained below). This spatially paired design was essential to control for variation among pairs due to different macroenvironments (Legendre et al., 2004). Trees within a pair were separated by distances of <150 m. Such distances are within the range covered by rodents (Abramson et al., 2006), and they hence have a chance of also entering patches dominated by trees that are phylogenetically distant from oaks (confirmed by our observations). Within their respective patch, rodents then might take foraging decisions based on the local environment as outlined in the Introduction. Note that while within a pair of focal oaks the mean phylogenetic distances from the respective neighbours were contrasting, these mean phylogenetic distances from neighbours covered a continuous gradient across all focal oaks.
Experimental design: acorn removal
Acorn harvesting.
In late October 2013, we selected five mature oak trees as sources (Q. robur; note that acorn removal was not oak species dependent, Supplementary data Appendix S5) outside the forest on a grassland (as they produce more acorns than trees inside forests; Jones, 1959). We harvested acorns still attached in trees by shaking branches. Twenty-one per cent of the harvested acorns were infested by insects (e.g. weevil larvae), which we identified as those that floated in water or showed a covered hole in their seed coat, a mark left by female insects during oviposition (see Perea et al., 2012). We excluded these infested acorns as they are less preferred by rodents than viable acorns and generally more predated, and hence less likely to survive (de Ouden et al., 2005; Perea et al., 2012). Finally, for each of the five acorn sources, we selected viable acorns approximately similar in size and shape to avoid differences in defence/tolerance or attractiveness (Bogdziewicz et al., 2019; Moreira et al., 2020). We used the same acorn source tree for the two trees of each pair, and across pairs we verified that source provenance did not relate to removal rate [analysis of variance (ANOVA), P > 0.4].
Exposure of acorns in the field and identification of acorn removers.
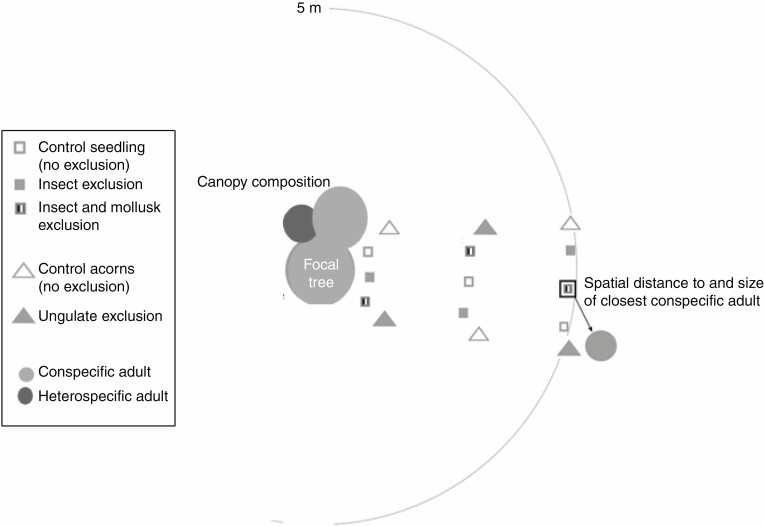
We exposed 2000 viable acorns in the field in late November 2013. We established a transect of 5 m length, starting from the trunk of each of the 20 focal adult oak trees, and avoiding approaching other trees (Fig. 1). Starting from 1 m, at every metre we installed a tray (20 × 20 × 2 cm, wood-coloured, hardly extending above the litter surface) and fixed it in the soil with a staple. We placed ten acorns on each tray that were hence accessible to all removers (i.e. control).
Fig. 1.
Design of the study of acorn removal and seedling herbivory, established for 20 focal oaks. See the Materials and Methods for details
To identify whether ungulates or rodents were the major predators responsible for acorn removal, we chose to exclude one type of enemy. We established a second transect, identical to the first one but oriented in another direction to avoid an unnaturally high density of acorns and hence attraction of removers. Every metre along this transect, we again exposed acorns, but now with a cage made of a 25 mm metal mesh (height 5 cm) fixed with a staple, excluding the mouthparts of ungulates but not of rodents. We placed ten acorns in each cage.
Measurement of acorn removal.
We followed acorn removal every week from the day of exposure in the field in late November 2013. We counted the number of remaining acorns on each tray and cage until all acorns had disappeared (after a maximum of 91 d). We also noted if acorns presented evidence of in situ predation. Then, for each tray and cage, we calculated the delay before acorn removal by averaging the number of days before removal of the ten acorns. We use the term ‘removal’ instead of ‘predation’ as the fate of the removed acorns is unknown.
Seedling herbivory
Seedling harvesting.
In early March 2015, we harvested 18 oak seedlings (Quercus petraea, Q. robur or their hybrids) for each pair of adult oak trees. We harvested seedlings in the more oak-dominated zone of a given parcel, as seedlings were too rare in pine-dominated parcels. We chose seedlings carefully to be approximately of the same height/age (approx. 20 cm, approx. 1 year old) and morphology, to be no longer dependent upon stored reserves (i.e. no acorn attached), to have no visible signs of pathologies or damage and to have intact buds. Seedlings were gently washed to eliminate possible infestation by eggs of herbivores.
Exposure of seedlings in the field and identification of seedling attackers.
Immediately after harvesting, we planted seedlings along a transect of 5 m length, perpendicular to the trunk of each focal adult oak (Fig. 1). We transplanted seedlings at three distances along this transect: 0.5, 2.5 and 5 m. Per distance, three seedlings were placed, spaced by approx. 30 cm. We identified seedling attackers by traces of consumption as follows: airborne pathogens were recognized by the white powdery mantle which covers leaves. Vertebrates kill seedlings completely by cutting stems and uprooting seedlings. Invertebrates induce different leaf damage that is specific to a feeding guild (Giffard et al., 2012; Castagneyrol et al., 2013; see Supplementary data Appendix S6 for pictures): ‘entire-leaf chewers’ eat entire parts of leaves; ‘leaf skeletonizers’ are partial-leaf chewers and leave the veins; ‘leaf rollers’ roll parts of leaves; ‘leaf suckers’ pierce very small holes in leaves; ‘leaf miners’ consume leaves internally and ‘gall makers’ induce new structures on leaves. Chewers may be mollusc or insect chewers.
Feeding traces of mollusc and insect chewers are visually indistinguishable without large magnification (pers. obs.). To separate the respective effects of insect and mollusc chewers, we applied an exclusion experiment. We established three treatments for each position along the transects: (1) control; (2) exclusion of insects, using a synthetic pyrethroid insecticide that kills insects by contact and if it is ingested (Mandarin® Pro 50 g L–1 esfenvalerate, PHILAGRO, France) used at a concentration of 12.5 mg L–1, as recommended by the manufacturer. We sprayed the insecticide on seedling leaves every 2 weeks (note that control seedlings were sprayed with water at the same time, to avoid any potential bias due to watering). Esfenvalerate has no attractive or repulsive effect on slugs (comparisons with controls in Piechowicz et al., 2012) and only minor effects on seedling functions or survival (Root, 1996; Carson and Root, 2000; Mitchell, 2003). (3) Exclusion of both insects (using insecticide as described above) and molluscs. To exclude molluscs, we placed a PVC pipe (10 cm diameter, 15 cm height) around each seedling and inserted this pipe into the soil on 5 cm, hence preventing molluscs from passing underneath. We covered the upper extremity of the PVC pipe by a 5 cm wide strip of copper tape, as it repulses molluscs by contact (Lankau, 2007). Seedling leaves were above the PVC pipe, hence limiting the impact of the PVC pipe on seedling microenvironment.
Measurement of seedling leaf herbivory.
As leaf herbivory may accumulate with time, we began by recording the date of seedling budburst (i.e. leaves totally deployed) based on controls at 3 d intervals. Most seedlings developed only one bud. We thus considered only the first bud that burst for each seedling. As leaf herbivory may accumulate with time, we quantified the age of the seedling leaves on which herbivory was measured. We calculated the leaf age of each seedling at the end of July 2015, when we measured herbivory.
We followed seedling herbivory weekly from the day of transplantation to the end of July 2015, when most damage by herbivores had occurred. We noted the number of leaves per seedling weekly from the end of May to the end of July (30 d). Such a high temporal resolution of the screening allowed us to identify cases where an entire leaf had disappeared due to chewer consumption. In late July, we noted the presence/absence of consumption by vertebrates. We also estimated for each seedling the percentage of leaf area removed (LAR) by chewers, skeletonizers and suckers, and attained by airborne pathogens, using a grid of points (0.25 cm2) (Yguel et al., 2011). The percentage of LAR corresponds to the ratio between the number of points falling on the area consumed by each herbivore, and the number of points forming on the whole surface (including the area consumed by chewers), multiplied by 100. Leaves which have been totally consumed had %LAR = 100, and we calculated the average value across all leaves for each plant. We also counted the number of rollers, miners and gall makers per leaf and then averaged them for each seedling. If a seedling died (as happened in 8.9 % of the seedlings developing leaves, 6.1 % overall) it did not receive a LAR score.
Characterization of conspecific adults and ground cover
First, we evaluated the spatial distance of seedlings to their closest conspecific adult (Fig. 1). Inevitably, when oaks were the dominant species, the focal adult oak was not always the closest conspecific; we thus measured the spatial distance of each transplanted seedling to the stem position of the closest conspecific adult (>2 m height).
Second, we measured the circumference at breast height of the closest conspecific adult and used this measure as a proxy of tree size. We took the average distance and circumference (‘size’ from hereon) when two conspecific adults were equally close to a seedling. For acorns, these measures of closest conspecific adults were not available and we instead used distance to and size of the focal adult tree as proxies. These proxies might be poor far from the focal adult tree, but conclusions did not change when including only acorns at 1 m from the focal adult conspecific (Table 1).
Table 1.
Phylogenetic isolation affecting delay of removal of acorns (left) and of leaf area of seedlings (right), accounting for ground cover and treatment
| Acorn removal delay | Leaf area removed | |||
|---|---|---|---|---|
| t | P | t | P | |
| Phylogen, isolation of focal conspecific adult | –2.84 | 0.012 | 2.73 | 0.014 |
| Treatment | NA | NA | F = 1.79 | 0.196 |
| Size of conspecific adult | Excl | Excl | Excl | Excl |
| Seedling density | –2.69 | 0.017 | 3.65 | 0.002 |
| Moss cover | Excl | Excl | Excl | Excl |
| Tree cover | Excl | Excl | –2.98 | 0.008 |
| Shrub cover | –1.94 | 0.072 | 1.31 | 0.207 |
| Herb cover | –2.35 | 0.033 | 0.88 | 0.392 |
| Treatment × seedling density | NA | NA | F = 8.77 | 0.002 |
| Exclusion all × seedling density | NA | NA | -4.16 | 0.001 |
| Exclusion insects × seedling density | NA | NA | 2.67 | 0.016 |
| Treatment × herb cover | NA | NA | F = 2.58 | 0.105 |
| Exclusion all × herb cover | NA | NA | –2.18 | 0.044 |
| Exclusion insects × herb cover | NA | NA | –1.54 | 0.142 |
| Adjusted R2 | 0.45 | 0.48 | ||
| d.f. for error | 15 | 17 |
Treatment is the exclusion of particular groups of enemies. Treatment was dropped from analyses of acorn removal (see the Materials and Methods) but significantly affected seedling herbivory (Fig. 2). Analyses were limited to acorns and seedlings closest to the focal conspecific adult. Covariates were the random effect tree pair, and (in models of seedling herbivory) the age of leaves. Covariates are not shown. Best sub-set search was applied, and excluded variables are noted ‘Excl’.
Third, we quantified the degree to which the canopy was dominated by species distantly related to oaks rendering the focal oak relatively more attractive for oak enemies. For a given focal adult oak, we determined phylogenetic isolation from the neighbouring canopy, using average phylogenetic distances to each of its adult neighbours with which their crown was in contact, established by Vialatte et al. (2010) and Yguel et al. (2011) (ranging from 0 to 140 million years before present, MYBP; see Supplementary data Appendix S7 for an explanation of the procedure). Finally, we quantified the ground cover around the seedlings in a 1 m2 plot, noting the number of oak seedlings, and the percentage coverage by deadwood, mosses, ‘herbs’ (the fern Pteridium aquilinum and the grass Molinea caerulea) and shrubs (Rubus sp., Lonicera sp. and Ilex aquifolium). The canopy cover was taken from Deniau et al. (2017) and measured at 0.5, 2.5 and 5 m distance from the focal oak, by taking hemispherical photographs prior to canopy closure, just before seedling bud burst. Photos were taken approx. 1 m above planted seedlings, using a Canon Eos 7D (Canon, Tokyo, Japan) with a circular fisheye lens (SIGMA 4.5 mm F 2.8 DC Circular Fisheye 180°; SIGMA, Kawasaki, Japan). All photographs were taken on a uniformly overcast day to ensure the best contrast between tree branches and the sky and homogeneous lighting of the canopy (Rich, 1990). Photos were then traced with Gap Light Analyzer software (Frazer et al., 1999) to extract the percentage of canopy openness.
Data analysis
For all analyses focused on LAR, we used a logit transformation of LAR to enhance the fit of the models to data (Warton and Hui, 2011). Logit of 0 is not defined, hence we added 0.01 to all data points.
We determined which enemies induce the most important damage on acorns and seedlings. In order to identify if acorn removal was determined by ungulates or rodents, we tested the effect of cage (control, ungulate exclusion) on the delay before acorn removal, using an ANOVA. Similarly, we tested the effect of treatment (control, insect exclusion, and insect and mollusc exclusion) on LAR by chewers, using an ANOVA, followed by a Tukey HSD post-hoc. In both cases, we included tree nested in pair as random effect to account for the nested structure of the design.
We then evaluated how acorn removal and seedling herbivory were determined by the different characteristics of conspecific adult and ground cover. Each analysis used multiple explanatory variables. Overall, correlation coefficients among explanatory variables were low, on average 0.14, and a maximum of 0.55 (between phylogenetic isolation and tree cover). One variable could thus not entirely replace another one and we entered them all into a single model explaining either acorn removal or seedling herbivory, partly followed by later variable selection.
We first tested the effects of distance to and size of the conspecific adult, treatment, and the interactions between size and distance, treatment and size, and treatment and distance. Tree pair was included as a random factor to account for the nested structure of the design. We tested the effects of these variables first on delay before acorn removal. In these tests, the conspecific adults were the focal trees, and hence focal tree could not be entered as an additional random factor given that the size of the focal tree did not vary within the focal tree. P-values might hence be inflated, but they were non-significant anyway. Neither treatment nor interactions with treatment was significant, and we hence excluded them from the model and reduced the analysis to the control treatment to avoid pseudoreplication. Then, we tested the effects of distance, size, treatment and their interactions on LAR. In these tests, the conspecific adults were the closest adults including cases of non-focal trees (which could not always be avoided when planting seedlings). We could hence additionally account for the random factor focal tree (nested in tree pair). We also accounted for the leaf age, density of oak seedlings, tree cover, shrub cover, herb cover and moss cover which had been recorded for all seedlings (but not for the seed trays at >1 m from the focal adult tree).
We then tested the effects of phylogenetic isolation of the focal adult tree. As phylogenetic isolation was a single piece of information per focal tree, we restricted the analysis to a single replicate of a treatment per focal tree, the one that is closest to, and hence most representative of, the focal tree. We accounted for the size of the focal tree, density of oak seedlings, tree cover, shrub cover, herb cover, moss cover and treatment. Tree pair was included as a random factor. To account for possible changes in the effects of each explanatory variable with treatment, we fitted separate models including one of the possible interaction terms, retained the significant ones and included them together in a final model (including all interaction terms together would have led to excessive multicollinearity). The final model was reduced by best sub-set selection to avoid overparameterization, using adjusted R2 as the selection criterion. We first tested the effects of these variables on delay before acorn removal. We found that neither treatment nor interactions with treatment was significant and we hence excluded them from the model and reduced the data set to control treatment to avoid pseudoreplication. We then tested the effects of these variables on LAR, including leaf age as covariate.
We always checked the adequacy of models to data with a residuals vs. fitted values plot and a QQ-plot of the residuals. A maximum of 4 out of 179 data points were excluded (for acorn removal across all trays), and results of analyses with and without outlier exclusion are reported throughout (at most, results differed between relationships being marginally significant at P < 0.1 rather than significant at P < 0.05; Supplementary data Appendix S8). Analyses were done using Statistica version 13 [TIBCO Software Inc. (2017, https://www.statsoft.de/en/software/tibco-statisticatm], and R version 3.6.1 (R Core Team, 2019) packages: lme4 (Bates et al., 2015), nlme (Pinheiro et al., 2020), multcomp (Hothorn et al., 2008) and MuMin (Barton, 2015).
RESULTS
Which enemies remove acorns or leaf surface?
All the acorns exposed were removed within 91 d. Mean removal times per tray varied between 7 and 66 d. During the survey, we found no trace of predation on the exposed acorns. Delay before acorn removal was not affected by the exclusion of ungulates (F = 2.17; P = 0.143, d.f. for error = 179, four outliers excluded F = 0.01; P = 0.923, d.f. for error= 175); rodents were hence the main removers.
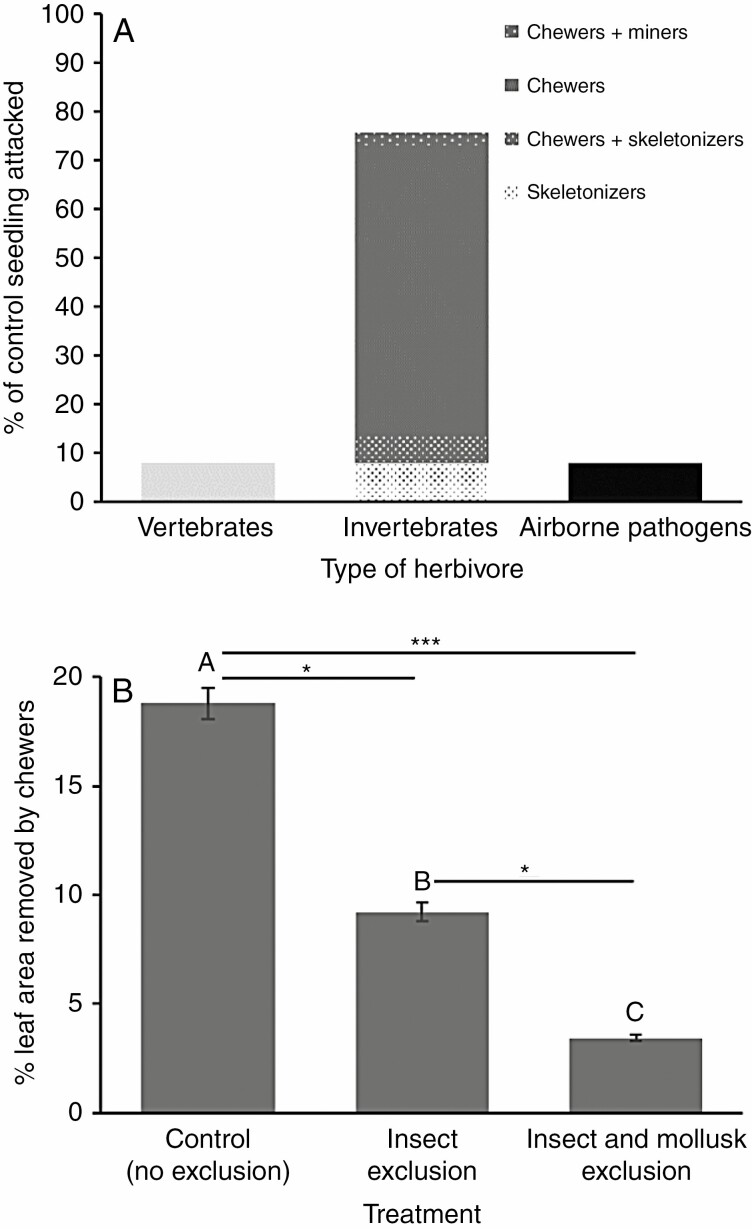
Out of the 180 planted seedlings, 123 had developed leaves and were thus susceptible to attacks by herbivores (37 control, 41 insect exclusion and 45 insect and mollusc exclusion). Herbivory on control seedlings was largely the result of invertebrates (Fig. 2A); 76 % of the seedlings were attacked by invertebrates, against 8 % by vertebrates and 8 % by airborne pathogens. Among seedlings attacked by invertebrates, 89 % were damaged by entire-leaf chewers (i.e. insects or molluscs), 18 % by skeletonizers and 4 % by miners. We observed no damage due to suckers, rollers and gall makers. Damage by leaf chewers was mostly done by insects and molluscs. Excluding insects significantly reduced LAR by half, and excluding both insects and molluscs significantly reduced LAR by a further two-thirds (ANOVA across all treatments, F = 14.04; P < 0.001, d.f. for error = 95, one outlier excluded F = 13.60; P < 0.001, d.f. for error = 94; Fig. 2B).
Fig. 2.
Identification of the main seedling herbivores. (A) Control oak seedlings were mostly attacked by invertebrates, notably chewers. (B) Leaf area removed (LAR, means ± s.e.) was significantly reduced by exclusion of insect chewers and exclusion of both insect and mollusc chewers. See text for statistical tests.
What affects removal of acorns and of leaf surface?
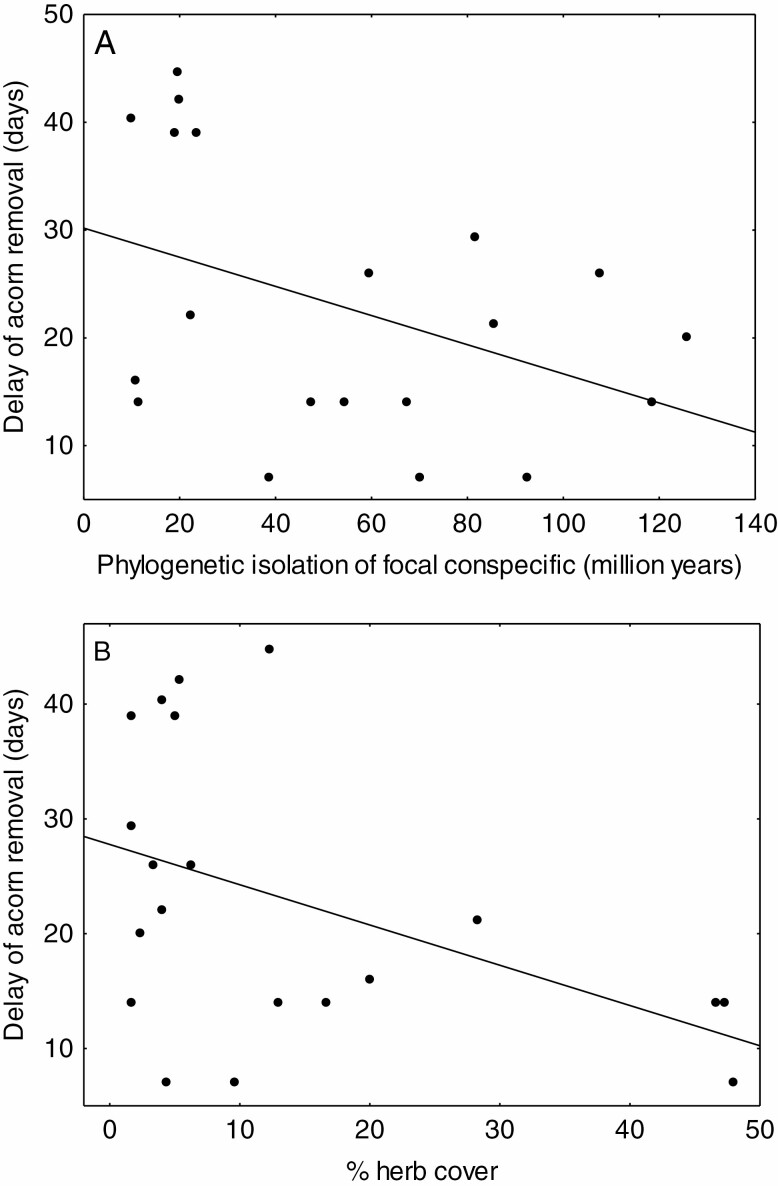
Delay of acorn removal was not affected by spatial distance to a focal conspecific adult tree or its size (t = 0.38, d.f. for error= 85, P = 0.703, t = 1.50, d.f. for error = 85, P = 0.139; Supplementary data Appendix S8). In contrast, acorns were removed faster next to focal conspecific adults that are phylogenetically isolated and that are surrounded by dense herb cover, many seedlings and with a tendency for a dense shrub cover (Table 1). Univariate graphic exploration in Fig. 3 shows that the significant effects of phylogenetic isolation and herb cover in Table 1 correspond to an acceleration of seed removal by approx. 19 and 15 d, respectively. As phylogenetic isolation decreases with the relative abundance of oaks, we verified whether the effect of phylogenetic isolation on acorn removal reflects nothing more than an effect of relative abundance of oaks. We found that this is not the case: if both phylogenetic isolation and relative abundance of oaks are included in the model, they both score as significant (t = –2.92, P = 0.043, and t = –2.82, P = 0.048).
Fig. 3.
Delay of removal of acorns decreases with phylogenetic isolation of the focal conspecific adult from its neighborhood (A) and with herb cover (B). See Table 1 for statistical analysis accounting for multiple explanatory variables.
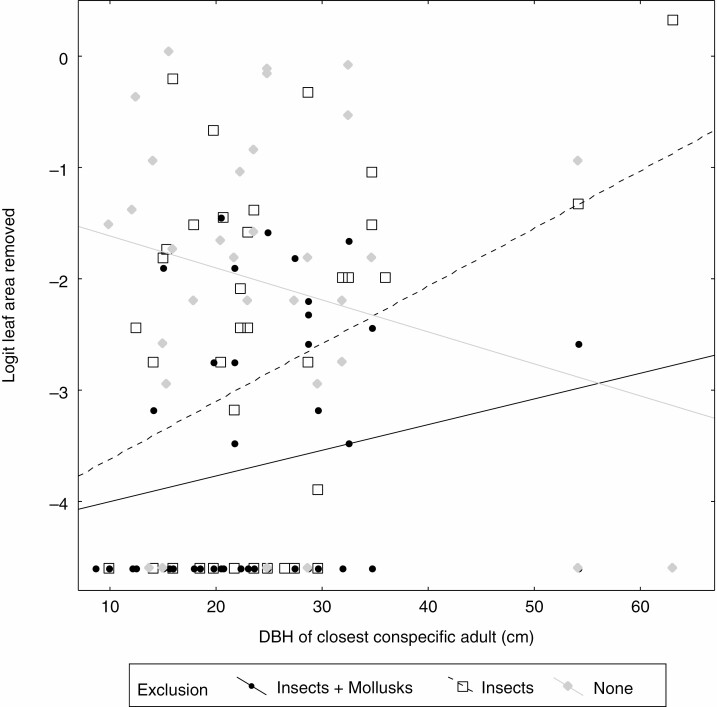
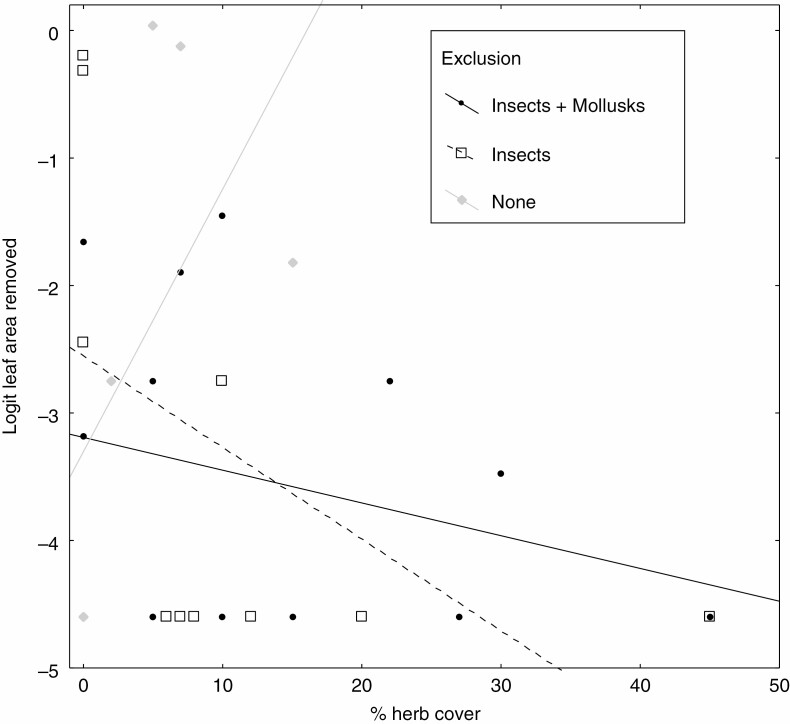
Leaf herbivory was higher on seedlings close to large trees (t = –1.90, d.f. for error= 80, P = 0.062; Supplementary data Appendix S8), in particular when insects had been excluded from seedlings (t = 2.25, d.f. for error = 80, P = 0.027; Supplementary data Appendix S8). Univariate graphic exploration of this result in Fig. 4 shows that leaf herbivory increased most strongly with tree size when only insects and not molluscs were excluded. Also, leaf herbivory was higher on seedlings next to focal adults that are phylogenetically isolated, and in the absence of herb cover (in the ‘all exclusion’ treatment) (Table 1). Univariate graphic exploration of this result in Fig. 5 shows that herbivory decreased most strongly with herb cover when only insects and not molluscs were excluded. Finally, leaf herbivory was significantly increased with seedling density, especially under insect exclusion (Table 1).
Fig. 4.
Leaf herbivory on seedlings depending on the size of the closest conspecific adult for different herbivore exclusion treatments. DBH, diameter at breast height. Full analyses (Supplementary data Appendix S8) accounting for multiple explanatory variables show that under exclusion of insects (or insects and molluscs), herbivory is largest closest to large conspecifics.
Fig. 5.
Leaf herbivory on seedlings depending on herb cover for different herbivore exclusion treatments. Full analyses (Table 1) accounting for multiple explanatory variables show that under exclusion of herbivores, herbivory is greatest in the absence of herb cover.
Discussion
In our study system, MaM play an important role in acorn/seedling predation: rodents removed 100 % of the acorns within at most 91d, and molluscs were responsible for roughly half of the leaf herbivory. We found no effect of spatial distance to adult conspecifics on removal of acorns or leaf area. We found, however, that acorn removal by rodents was faster around conspecific adults surrounded by phylogenetically distant neighbours, where the focal adult tree might appear comparatively more attractive for enemies. Moreover, acorn removal by rodents was faster around adult trees with a high ground cover where acorn predators are sheltered from their own enemies. Seedling herbivory by molluscs increased with size of the conspecific adult and tended to decrease with herb cover. These results are consistent with predictions of our hypothesis that enemy pressure on acorns/seedlings increases with the attractiveness of conspecific adult neighbours to foraging highly mobile enemies such as MaM.
Observed variation in acorn removal may have major consequences
Low phylogenetic isolation and scarcity of herbaceous cover delayed acorn removal by, on average, 2 weeks, with an even stronger effect after accounting for covariables. Across the thousands of acorns produced by a single oak in many years, an average tendency of 2 weeks delay probably corresponds to dozens of additional acorns surviving for several months. Moreover, even a removal delay of only 2 weeks may be essential for acorn survival, especially if acorns lie on the ground, instead of on our wooden acorn trays. With time, acorns on the ground are covered by a litter layer and become less detectable by and accessible to rodents, strongly reducing acorn removal (Crawley and Long, 1995). Moreover, acorns lying on the ground rather than on a tray germinate within 2 weeks (Finch-Savage and Clay, 1994) so that rodents can no longer remove them. Also, during 2 weeks the acorn stocks of rodents might become saturated.
Mammal acorn predators might induce niche conservatism
In our study system, acorn removal by mammals was faster close to adult trees in a phylogenetically distant neighbourhood. Our result is inconsistent with van Ginkel et al. (2013) who found that acorn removal was higher in a deciduous forest (i.e. closely related species) than in a coniferous forest (i.e. distantly related species), due to a higher activity of wild boars in deciduous forests. Our result is also inconsistent with the phylogenetic Janzen–Connell effect (Liu et al., 2012) suggesting that under a phylogenetically distant adult tree, acorns/seedlings suffer little predation (a form of herd immunity effect; Webb et al., 2006). In our case, acorn removal was mostly due to rodents, as exclusion of ungulates had no effect on acorn removal (Fig. 2A). We suggest that a faster acorn removal below an oak surrounded by distant relatives may be due to a concentration of attacks by enemies: rodents who forage in a neighbourhood dominated by distantly related species are attracted by the few oaks available in this environment, and may be reluctant to leave them (see Jennings, 1976 for preference for oak acorns). This mechanism, however, requires that rodents enter the patches dominated by trees phylogenetically distant from oaks. This is likely to be the case in our study, conducted after a mast year and with resource patches being small and proximate (see the Materials and Methods). The observed high acorn removal by rodents in a phylogenetically distant neighbourhood suggests high acorn predation, which in turn may prevent oaks from spreading into such neighbourhoods, thus restricting the access to novel abiotic and biotic niches [in contrast to the adults which benefit from released enemy pressure, and increased support by mutualists (Yguel et al., 2011, 2014a) albeit with decreased support from parasitoids (Yguel et al., 2014b)]. On the contrary, under a closely related canopy, predation pressure by a given number of available rodents is diluted among abundant resources, and acorn predators might leave the patch around one adult already after a slight decrease in patch quality given the high quality of the surrounding patches. Overall, a neighbourhood of closely related species of trees might provide the protection of focal acorns from rodent removal, hence possibly favouring the aggregation of closely related species in the same habitat patch and thereby the conservatism of the habitat niche. Possibly the opposite may be true for the more specialized, less mobile larval weevil pre-dispersal predators of acorns, but this has, to our knowledge, not been tested.
Mammal acorn predators might allow seedling establishment in suitable environments
Acorn removal by rodents also accelerated with cover by a herbaceous layer, i.e. the fern Pteridium aquilinum and the grass Molinia caerulea. Increased acorn predation under fern cover has already been described by George and Bazzaz (1999). Ferns constitute a shelter under which rodents are protected from their predators. Hence, rodents forage frequently under cover (den Ouden et al., 2005), hidden from predators. Grasses such as M. caerulea have the same protecting effect as ferns (Gill and Marks, 1991) as they produce dense tufts of vegetation (Taylor et al., 2001), providing many hideouts for rodents on the ground. Conversely, a reduced acorn removal in understorey-free areas may contribute to the establishment of seedlings. The observed reduction of acorn removal in open conditions suggests reduced predation – in precisely those conditions that are required for seedling growth; seedlings tolerate only little shade (Ellenberg et al., 1992) and suffer from M. caerulea neighbours (M. Deniau et al., unpubl. res.). Overall, understorey-free areas protect acorns from removal by rodents, hence optimizing the living conditions of the forthcoming seedlings. These very seedlings, however, might suffer from increased herbivory by a potential prey of rodents, i.e. molluscs, as suggested by one of our analyses.
Herbivorous molluscs repulse seedlings from large adults
Seedling herbivory by chewers increased with the size of conspecific adult trees, an effect that appeared only when insects were excluded, reflecting the activity of molluscs. Molluscs are generalists (Hunter, 1978), can consume green leaves of trees (Jennings and Barkham, 1975) and may be the main enemies of tree seedlings in temperate forests (see Pigot and Leather, 2008 for Acer pseudoplatanus). Such an accumulation of molluscs around large adult trees has not been demonstrated before, but is plausible because larger trees are more productive and more seedlings might be available in its proximity. We note that the insecticide application and the copper tubes might not have permanently excluded insects and molluscs. Instead, these treatments might have triggered recolonizations by insects and molluscs from the adjacent conspecific adult tree, in particular if that tree is large. In that case, the explanation of the observed patterns would not be foraging mechanisms, but mechanisms of a micro-island biogeography – but the implications would be the same: overall, large adults might prevent conspecific seedlings from establishing in their surrounding due to the impact of mollusc herbivores.
Limitations of the study
First, we considered a very limited gradient of spatial distances of only 5 m. Earlier studies have demonstrated changes in enemy pressure by specialist enemies along such gradients (Schupp, 1988; Deniau et al., 2018), albeit here we did not find such changes. Theoretically, enemy pressure might change across larger distances, although such larger distances may be absent in many temperate forest stands in which conspecific adults are <10 m apart (Ellenberg, 1992). Also, larger distances might not provide shelter from attack by aerial predators of oak enemies.
Second, rodents might have been satiated and forgotten acorns in caches, so that removal did not equal predation. This scenario is unlikely given that acorn production was extremely low in the year of study and, after a year of masting, rodent density was likely to have been very high, a situation in which rodents are predators rather than dispersers (Silvertown, 1980; Koenig and Knops, 2000; Clotfelter et al., 2007; Nopp-Mayr et al., 2012). Even if some acorns are forgotten, this might not change our conclusions, as forgetting might happen equally frequently in phylogenetically proximate and distant neighbourhoods. Also, acorns that are forgotten might eventually die as they have been caged too deep or as they do not stand the competition among the many seedlings emerging from a forgotten cache.
Third, we did not account for the density of autochthonous acorns or for soil pathogens, which may influence foraging decisions. We did, however, account for the density of autochthonous seedlings, which may be representative of densities of acorns. Moreover, above-ground pathogens were rare on leaves (Fig. 2), and below-ground pathogens do not induce increased herbivory close to large adults (Deniau et al., 2018). Additionally, we studied only a single year, one of low acorn production. The observed decline of acorn removal in phylogenetically proximate neighbourhoods might be even more pronounced in high acorn production years when such neighbourhoods are swamped with acorns.
Finally, our interpretation centres around mechanisms related to the movement and patch use of MaM since they utilize many more seeds or seedlings during their life than do insect herbivores. However, MaM may also be more generalist in their host range than many insect herbivores (e.g. Hunter, 1978). The mechanisms we suggest actually imply an intermediate degree of specialization, where MaM may prefer using oak acorns or seedlings, but may survive on other food while traversing a matrix dominated by distantly related species.
Conclusion
We found that mammal and mollusc enemies of acorns and seedlings, respectively, respond to properties of individual adult trees and thereby potentially contribute to structuring forest communities at a very fine scale. Rodents are relatively mobile acorn predators which seem to attack acorns mainly where seedlings would have difficulties in establishing, and acorn predators might contribute to niche conservatism by selecting against acorns in a distantly related neighbourhood. Seedling herbivores operate around large adult trees and might prevent recruitment of seedlings as large adults accumulate generalist mollusc herbivores. These results may have additional implications for forest management. Management practices often recommend keeping old and large trees, and to mix tree species, but without considering the effect on acorn/seedling predators. The present study shows that a mixture of distantly related tree species might be disadvantageous for acorn survival. Moreover, our results suggest that controlling understorey vegetation may help to reduce acorn predation, and that regeneration should already start when adults are still small and hence attract few seedling herbivores.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Appendix S1: identification of rodent species in the field. Appendix S2: Lepidopteran species found on adult oak trees. Appendix S3: identification of mollusc species. Appendix S4: geographic distribution of the ten tree pairs in the forest of Rennes. Appendix S5: removal of Q. petraea vs. Q. robur acorns in the field. Appendix S6: identification of the trophic guilds on seedling leaves. Appendix S7: procedure for calculation of phylogenetic isolation from the canopy. Appendix S8: effect of the size of, and distance from, adult oak on removal of acorns and herbivory of seedlings.
ACKNOWLEDGEMENTS
We thank Stéphanie Llopis for help with field work, the Office National des Forêts for logistic support, Grégoire Pérez, Alain Butet and Jean-Pierre Caudal for help with rodent capture, and Maryvonne Charrier for providing advice on molluscs. Zuzana Münzbergová and two anonymous referees improved the manuscript. M.D., V.J. and A.P. conceived the ideas and methods; M.D. led the field work, with the main support of V.G., M.B. and B.B.; M.D., M.P., A.P., M.B. and V.J. analysed the data and wrote the manuscript, with the support of M.P.
Funding
M.D. was funded by PhD fellowship of the French Ministry of Education.
LITERATURE CITED
- Abramson G, Giuggioli, L, Kenkre VM, et al. . 2006. Diffusion and home range parameters for rodents: Peromyscus maniculatus in New Mexico. Ecological Complexity 3: 64–70. [Google Scholar]
- van Asch M, Visser ME. 2007. Phenology of forest caterpillars and their host trees: the importance of synchrony. Annual Review of Entomology 52: 37–55. [DOI] [PubMed] [Google Scholar]
- Bagchi R, Gallery RE, Gripenberg S, et al. . 2014. Pathogens and insect herbivores drive rainforest plant diversity and composition. Nature 506: 85–88. [DOI] [PubMed] [Google Scholar]
- Barton K. 2015. MuMIn: multi-model inference. R Package version 1.15.1.http://CRAN.R-project.org/package=MuMIn/ (30 October 2015).
- Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48. [Google Scholar]
- Bleicher SS. 2017. The landscape of fear conceptual framework: definition and review of current applications and misuses. PeerJ 5: e3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdziewicz M, Espelta JM, Bonal R. 2019. Tolerance to seed predation mediated by seed size increases at lower latitudes in a Mediterranean oak. Annals of Botany 123: 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson WP, Root RB. 2000. Herbivory and plant species coexistence: community regulation by an outbreaking phytophagous insect. Ecological Monographs 70: 73–99. [Google Scholar]
- Castagneyrol B, Giffard B, Péré C, Jactel H. 2013. Plant apparency, an overlooked driver of associational resistance to insect herbivory. Journal of Ecology 101: 418–429. [Google Scholar]
- Charnov EL. 1976. Optimal foraging, the marginal value theorem. Theoretical Population Biology 9: 129–136. [DOI] [PubMed] [Google Scholar]
- Clotfelter ED, Pedersen AB, Cranford JA, et al. . 2007. Acorn mast drives long-term dynamics of rodent and songbird populations. Oecologia 154: 493–503. [DOI] [PubMed] [Google Scholar]
- Connell JH. 1971. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In: den Boer PJ, Gradwell GR, eds. Dynamics of populations. Wageningen: PUDOC, 298–312. [Google Scholar]
- Crawley MJ, Long CR. 1995. Alternate bearing, predator satiation and seedling recruitment in Quercus robur L. Journal of Ecology 83: 683–696 [Google Scholar]
- Deniau M, Jung V, Le Lann C, Morra T, Murray P, Prinzing A. 2017. Janzen–Connell patterns are not the result of Janzen–Connell process: oak recruitment in temperate forests. Perspectives in Plant Ecology, Evolution and Systematics 24: 72–79. [Google Scholar]
- Deniau M, Vincent J, Le Lann C, et al. . 2018. Janzen–Connell patterns can be induced by fungal-driven decomposition and compensated by ectomycorrhizal fungi accumulated under a closely related canopy. Functional Ecology 32: 785–798. [Google Scholar]
- Ellenberg H, Weber H, Düll R, Wirth V, Werner W, Pauliben D. 1992. Zeigerwerte von Pflanzen in Mitteleuropa. Scripta Geobotanica 18: 1–248. [Google Scholar]
- Finch-Savage LWE, Clay HA. 1994. Water relations of germination in the recalcitrant seeds of Quercus robur L. Seed Science Research 4: 315–322. [Google Scholar]
- Frazer GW, Canham CD, Lertzman KP. 1999. Gap Light Analyzer (GLA), Version 2.0: imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, users manual and program documentation. Burnaby, Canada: Simon Fraser University. [Google Scholar]
- Garzon-Lopez CX, Ballesteros-Mejia L, Ordoñez A, Bohlman SA, Olff H, Jansen PA. 2015. Indirect interactions among tropical tree species through shared rodent seed predators: a novel mechanism of tree species coexistence. Ecology Letters 18: 752–760. [DOI] [PubMed] [Google Scholar]
- George LO, Bazzaz FA. 1999. The fern understory as an ecological filter: emergence and establishment of canopy-tree seedlings. Ecology 80: 833–845. [Google Scholar]
- Giffard B, Jactel H, Corcket E, Barbaro L. 2012. Influence of surrounding vegetation on insect herbivory: a matter of spatial scale and herbivore specialization. Basic and Applied Ecology 13: 458–465. [Google Scholar]
- Gill DS, Marks L. 1991. Tree and shrub seedling colonization of old fields in central New York. Ecological Monographs 61: 183–205. [Google Scholar]
- van Ginkel HAL, Kuijper DPJ, Churski M, Zub K, Szafranska P, Smit C. 2013. Safe for saplings not safe for seeds: Quercus robur recruitment in relation to coarse woody debris in Bialowieza Primeval Forest, Poland. Forest Ecology and Management 304: 73–79. [Google Scholar]
- Gonzalez-Rodriguez V, Villar R. 2012. Post-dispersal seed removal in four Mediterranean oaks: species and microhabitat selection differ depending on large herbivore activity. Ecological Research 27: 587–594. [Google Scholar]
- Grimm B, Paill W. 2001. Spatial distribution and home-range of the pest slug Arion lusitanicus (Mollusca: Pulmonata). Acta Oecologica 22: 219–227. [Google Scholar]
- Gurnell J. 1993. Tree seed production and food conditions for rodents in an oak wood in southern England. Forestry 66: 291–315. [Google Scholar]
- Hanley ME, Sykes RJ. 2009. Impacts of seedling herbivory on plant competition and implications for species coexistence. Annals of Botany 103: 1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biometrical Journal. Biometrische Zeitschrift 50: 346–363. [DOI] [PubMed] [Google Scholar]
- Hunter PJ. 1978. Slugs – a study in applied ecology. In: Fretter V, Peake J, eds. Pulmonates: systematics, evolution and ecology. London: Academic Press, 271–286. [Google Scholar]
- Iida S. 2006. Dispersal patterns of Quercus serrata acorns by wood mice in and around canopy gaps in a temperate forest. Forest Ecology and Management 227: 71–78 [Google Scholar]
- Janzen DH. 1970. Herbivores and the number of tree species in tropical forests. The American Naturalist 104: 501–528. [Google Scholar]
- Jennings TJ. 1976. Seed detection by the wood mouse Apodemus sylvaticus. Oikos 27: 174–177. [Google Scholar]
- Jennings TJ, Barkham JP. 1975. Food of slugs in mixed deciduous woodland. Oikos 26: 211–221. [DOI] [PubMed] [Google Scholar]
- Jones EW. 1959. Biological flora of the British Isles. Quercus L. Journal of Ecology 47: 169–222. [Google Scholar]
- Koenig WD, Knops JMH. 2000. Patterns of annual seed production by Northern Hemisphere trees: a global perspective. The American Naturalist 155: 59–69. [DOI] [PubMed] [Google Scholar]
- Kotler BP, Brown JS. 2003. Environmental heterogeneity and the coexistence of desert rodents. Annual Review of Ecology and Systematics 19: 281–307. [Google Scholar]
- Lankau RA. 2007. Specialist and generalist herbivores exert opposing selection on a chemical defense. New Phytologist 175: 176–184. [DOI] [PubMed] [Google Scholar]
- Legendre P, Dale MRT, Fortin MJ, Casgrain P, Gurevitch J. 2004. Effects of spatial structures on the results of field experiments. Ecology 85: 3202–3214. [Google Scholar]
- Liu X, Liang M, Etienne RS, Wang Y, Staehelin C, Yu S. 2012. Experimental evidence for a phylogenetic Janzen–Connell effect in a subtropical forest. Ecology Letters 15: 111–118. [DOI] [PubMed] [Google Scholar]
- Mitchell CE. 2003. Trophic control of grassland production and biomass by pathogens. Ecology Letters 6: 147–155. [Google Scholar]
- Moreira X, Abdala-Roberts L, Bruun HH, et al. . 2020. Latitudinal variation in seed predation correlates with latitudinal variation in seed defensive and nutritional traits in a widespread oak species. Annals of Botany 125: 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopp-Mayr U, Kempter I, Muralt G, Gratzer G. 2012. Seed survival on experimental dishes in a central European old-growth mixed-species forest – effects of predator guilds, tree masting and small mammal population dynamics. Oikos 121: 337–346. [Google Scholar]
- den Ouden J, Jansen PA, Smit R. 2005. Jays, mice and oaks: predation and dispersal of Quercus robur and Q. petraea in North-western Europe. In: Forget PM, Lambert JE, Hulme PE, Vander Wall SB, eds. Seed fate: predation, dispersal and seedling establishment. Wallingford, UK: CABI Publishing, 223–239. [Google Scholar]
- Ostfeld RS, Canham CD. 1993. Effects of meadow vole population density on tree seedling survival in oil fields. Ecology 74: 1792–1801. [Google Scholar]
- Perea R, López D, San Miguel A, Gil L. 2012. Incorporating insect infestation into rodent seed dispersal: better if the larva is still inside. Oecologia 170: 723–733. [DOI] [PubMed] [Google Scholar]
- Piechowicz B, Stawarczyk K, Stawarczyk M. 2012. Insecticide and food consumption of Spanish slug (Arion lusitanicus Mabille 1868). Chemistry Didactics Ecology Metrology 17: 113–120. [Google Scholar]
- Pigot AL, Leather SR. 2008. Invertebrate predators drive distance-dependent patterns of seedling mortality in a temperate tree Acer pseudoplatanus. Oikos 117: 521–530. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team . 2020. nlme: linear and nonlinear mixed effects models. R package version 3.1-145. https://CRAN.R-project.org/package=nlme.
- R Core Team . 2019. R: a language and microenvironment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Reinhart KO, Clay K. 2009. Spatial variation in soil-borne disease dynamics of a temperate tree, Prunus serotina. Ecology 90: 2984–2993. [DOI] [PubMed] [Google Scholar]
- Ribeiro JF, Vieira EM. 2016. Microhabitat selection for caching and use of potential landmarks for seed recovery by a neotropical rodent. Journal of Zoology 300: 274–280. [Google Scholar]
- Rich PM. . 1990. Characterizing plant canopies with hemispherical photographs. Remote Sensing Reviews 5: 13–29. [Google Scholar]
- Root RB. 1996. Herbivore pressure on goldenrods (Solidago altissima): its variation and cumulative effects. Ecology 77: 1074–1087. [Google Scholar]
- Schnurr JL, Canham CD, Ostfeld RS, Inouye RS. 2004. Neighborhood analyses of small mammal dynamics: impacts on seed predation and seedling establishment. Ecology 85: 741–755. [Google Scholar]
- Schupp EW. 1988. Seed and early seedling predation in the forest understory and in treefall gaps. Oikos 51: 71–78. [Google Scholar]
- Silvertown JW. 1980. The evolutionary ecology of mast seeding in trees. Biological Journal of the Linnean Society 14: 235–250. [Google Scholar]
- Steele, MA, Carlson, JE, McEuen AB, Contrerrras, TA, Terzagh WB. 2007. Linking seed and seedling shadows: a case study in the oaks (Quercus). In: Dennis AJ, Schupp EW, Green RJ, Westcott DA. eds. Seed dispersal: theory and its application in a changing world. Wallingford, UK: CABI Publishing, 322–339. [Google Scholar]
- Taylor K, Rowland AP, Jones HE. 2001. Molinia caerulea (L.) Moench. Journal of Ecology 89: 126–144. [Google Scholar]
- Terborgh J. 2012. Enemies maintain hyperdiverse tropical forests. The American Naturalist 179: 303–314. [DOI] [PubMed] [Google Scholar]
- Vialatte A, Bailey RI, Vasseur C, et al. . 2010. Phylogenetic isolation of host trees affects assembly of local Heteroptera communities. Proceedings of the Royal Society B: Biological Sciences 277: 2227–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warton DI, Hui FK. 2011. The arcsine is asinine: the analysis of proportions in ecology. Ecology 92: 3–10. [DOI] [PubMed] [Google Scholar]
- Watts CHS. 1968. The foods eaten by wood mice (Apodemus sylvaticus) and Bank voles (Clethrionomys glareolus) in Wytham woods, Berkshire. Journal of Animal Ecology 37: 25–41. [Google Scholar]
- Webb CO, Gilbert GS, Donoghue MJ. 2006. Phylodiversity-dependent seedling mortality, size structure, and disease in a Bornean rain forest. Ecology 87: 123–131. [DOI] [PubMed] [Google Scholar]
- Yguel B, Bailey R, Tosh ND, et al. . 2011. Phytophagy on phylogenetically isolated trees: why hosts should escape their relatives. Ecology Letters 14: 1117–1124. [DOI] [PubMed] [Google Scholar]
- Yguel B, Courty PE, Jactel H, et al. . 2014. a. Mycorrhizae support oaks growing in a phylogenetically distant neighborhood. Soil Biology and Biochemistry 78: 204–212. [Google Scholar]
- Yguel B, Bailey R, Villemant C, Brault A, Jactel H, Prinzing A. 2014b. Enemy release of insect herbivores on phylogenetically isolated trees: why phytophages should follow plants escaping their relatives? Oecologia 176: 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieger MV, Vakoliuk IA, Tuchina OP, Zhukov VV, Meyer-Rochow VB. 2009. Eyes and vision in Arion rufus and Deroceras agreste (Mollusca; Gastropoda; Pulmonata): what role does photoreception play in the orientation of these terrestrial slugs? Acta Zoologica 90: 189–204. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.