Abstract
Background and Aims
The epidermis constitutes the outermost tissue of the plant body. Although it plays major structural, physiological and ecological roles in embryophytes, the molecular mechanisms controlling epidermal cell fate, differentiation and trichome development have been scarcely studied across angiosperms, and remain almost unexplored in floral organs.
Methods
In this study, we assess the spatio-temporal expression patterns of GL2, GL3, TTG1, TRY, MYB5, MYB6, HDG2, MYB106-like, WIN1 and RAV1-like homologues in the magnoliid Aristolochia fimbriata (Aristolochiaceae) by using comparative RNA-sequencing and in situ hybridization assays.
Key Results
Genes involved in Aristolochia fimbriata trichome development vary depending on the organ where they are formed. Stem, leaf and pedicel trichomes recruit most of the transcription factors (TFs) described above. Conversely, floral trichomes only use a small subset of genes including AfimGL2, AfimRAV1-like, AfimWIN1, AfimMYB106-like and AfimHDG2. The remaining TFs, AfimTTG1, AfimGL3, AfimTRY, AfimMYB5 and AfimMYB6, are restricted to the abaxial (outer) and the adaxial (inner) pavement epidermal cells.
Conclusions
We re-evaluate the core genetic network shaping trichome fate in flowers of an early-divergent angiosperm lineage and show a morphologically diverse output with a simpler genetic mechanism in place when compared to the models Arabidopsis thaliana and Cucumis sativus. In turn, our results strongly suggest that the canonical trichome gene expression appears to be more conserved in vegetative than in floral tissues across angiosperms.
Keywords: Aristolochia, epidermis development, multicellular trichomes, perianth, petaloid sepals, Piperales
INTRODUCTION
The epidermis is the continuous surface tissue in embryophytes, and it is fundamental for plant integrity. Specialized epidermal cells such as stomatal guard cells and trichomes are essential for the structural integrity and physiology of plants, and for their interplay with the environment (Javelle et al., 2011). Trichomes are highly differentiated epidermal cells present mainly on the exposed aerial plant organs, thus acting as micromorphological structures often correlated to environmental conditions (Pattanaik et al., 2014; Ioannidi et al., 2016). The morphology, function, shape, size, location and density of trichomes are species-specific, thus being exceedingly diverse and specialized (Netolitzky, 1932; Wagner et al., 2004).
Trichome functions depend primarily on the organs from which they develop. For instance, trichomes in leaves can regulate temperature, reduce water loss, increase light reflectance, prevent abrasion, and protect inner tissues from UV radiation as well as from insect and pathogen attack (Levin, 1973; Wagner, 1991; Karabourniotis et al., 1992; Werker, 2000; Wagner et al., 2004; Pattanaik et al., 2014). Trichomes in flowers might play structural roles during flower synorganization (El Ottra et al., 2013; Tan et al., 2016), promote toxin synthesis, and act as active mechano-sensory switches and wave detectors during herbivory (Zhou et al., 2016; Liu et al., 2017). Floral trichomes also play key mechanical and biochemical functions in various pollination syndromes (Young et al., 1984; Cropper and Calder, 1990; Cocucci, 1991; Hu et al., 2008; Martins et al., 2013; Boff et al., 2015; Płachno et al., 2018, 2019; Stpiczyńska et al., 2018). For instance, trichomes inside the Aristolochia (Aristolochiaceae) perianth are specialized either as mechanical structures to temporarily trap insects, or as secretory trichomes to feed them (Dafni, 1984; Oelschlägel et al., 2009; Pabón-Mora et al., 2015; Erbar et al., 2016; Suárez-Baron et al., 2019).
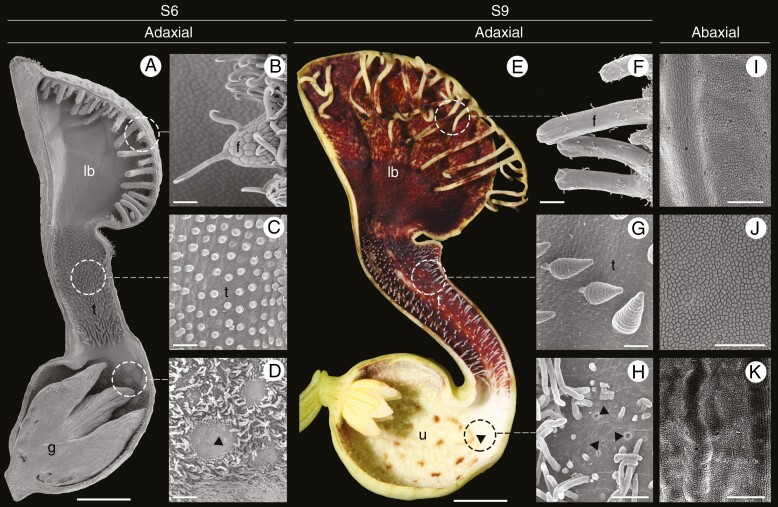
The flowers of Aristolochia are considered ‘trap-flowers’ with a complex architecture and pollination mechanism (Oelschlägel et al., 2009; Pabón-Mora et al., 2015; Erbar et al. 2016). The perianth is monosymmetric and formed by three fused sepals that variously curve forming a basal, inflated portion called the utricle, a narrow portion called the tube, and a distal, expanded, laminar coloured portion called the limb, which serves as the platform for visiting insects (Fig. 1) (González and Stevenson, 2000). Their pollinators – mainly flies – land on the limb, probably attracted by colour and scent, then pass through the tube and reach the utricle. The perianth tube is covered by downward-pointing multicellular trichomes that trap the flies in the utricle for a few days. Here flies release pollen grains in the mature stigmas. After ovule fertilization, stamens mature and deposit new pollen on the flies, which after perianth withering and loss of trichome turgor, escape loaded with fresh pollen (Oelschlägel et al., 2009; Pabón-Mora et al., 2015). For adequate functioning of such a floral trap, the adaxial (inner) epidermis has different specialized trichomes in each of the floral portions. The adaxial epidermis of the utricle is carpeted with multicellular, filamentous, secretory trichomes interspersed by osmophores. Conversely, the adaxial epidermis of the limb and the tube is covered by multicellular hooked and conical trichomes, respectively, both performing mechanical roles (Fig. 1).
Fig. 1.
Aristolochia fimbriata, flower morphology and epidermal differentiation of the perianth. (A and E) Sagittal section through flowers at S6 (A) and S9 (E) stages. (B and F) Detail of fimbriae at S6 (B) and S9 (F); note hooked trichomes. (C and G) Secretory multicellular, conical trichomes on the adaxial epidermis of the tube at S6 (C) and S9 (G). (D and H) Detail of the utricle adaxial epidermis with osmophores surrounded by secretory, filamentous multicellular trichomes. (I–K) Detail of the abaxial epidermis of the limb (I), tube (J) and utricle (K), with scattered stomata. Arrowheads: osmophores; f, fimbriae; g, gynostemium; lb, limb; t, tube; u, utricle. Scale bars: 1 mm (A); 100 µm (B–D, I, K); 5 mm (E); 50 µm (F–H, J).
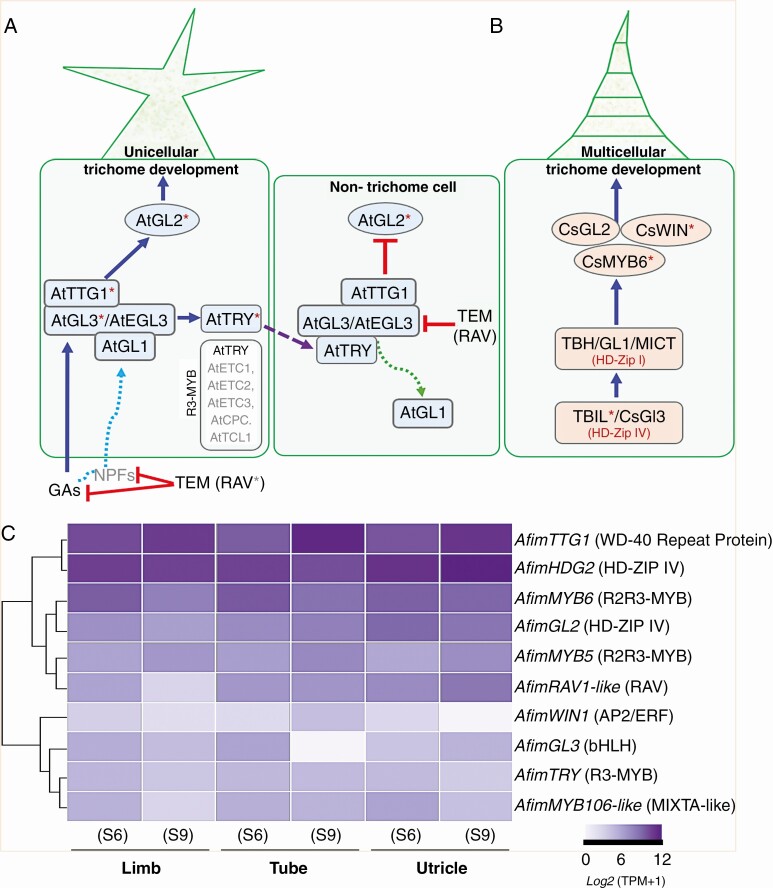
Trichome differentiation responds to both external signals and endogenous developmental programmes; thus, it is the result of a tightly regulated interplay between positional cues, intercellular communication and morphogenesis that results in cell fate specification (Szymanski et al., 2000; Hulskamp, 2004). The core genetic mechanisms underlying trichome identity and development have been identified in model flowering plants (Larkin et al., 1996, 1997; Glover et al., 1998; Glover, 2000; Schwab et al., 2000; Szymanski et al., 2000; Kärkkäinen and Ågren, 2002; Schnittger and Hülskamp, 2002, 2005; Machado et al., 2009; Zhao et al., 2015b). In Arabidopsis, the development of unicellular trichomes is the result of an activator–inhibitor complex. Mutant plants of positive regulators for trichome development have fewer or no trichomes, compared to the wild type. (Digiuni et al., 2008). Trichome activators include the R2R3 MYB transcription factor (TF), GLABRA1 (GL1), the redundant basic helix–loop–helix (bHLHs) factors GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3), and the WD40-repeat protein TRANSPARENT TESTA GLABRA1 (TTG1) (Oppenheimer et al., 1991; Galway et al., 1994; Walker et al., 1999; Payne et al., 2000; Zhang et al., 2003; Bernhardt et al., 2003, 2005; Kirik et al., 2005; Simon et al., 2013). Yeast two-hybrid protein interaction assays indicate that GL3 physically interacts with GL1 and TTG1; in turn this MYB–bHLH–WD40 (MBW) activation complex directly controls trichome formation (Rerie et al., 1994; Payne et al., 2000; Schiefelbein, 2003; Pesch and Hülskamp, 2004; Serna and Martin, 2006). The downstream target gene of the MBW complex is the homeodomain-leucine zipper GLABRA2 (GL2), which initiates trichome differentiation (Rerie et al., 1994; Cristina et al., 1996; Masucci et al., 1996; Hülskamp, 2004; Pesch and Hülskamp, 2009). This complex is inhibited when the R2R3 MYB protein is replaced by an R3 MYB protein (Payne et al., 2000). Thus, negative regulators for trichome development include several small single-repeat R3 MYB TFs, such as TRIPTYCHON (TRY), CAPRICE (CPC), and ENHANCER OF TRY AND CPC1 and 2 (ETC1 and ETC2) (Hülskamp et al., 1994; Wada et al., 1997, 2002; Szymanski and Marks, 1998; Schnittger et al., 1999; Schellmann et al., 2002; Esch et al., 2004; Kirik et al., 2004a, b) (Fig. 2A). Furthermore, additional regulatory genes have been reported such as MYB5, having a redundant role with the R2R3 MYB gene TRANSPARENT TESTA GLABRA2 (TTG2) in the control of trichome development, branching and tannin production (González et al., 2009; Li et al., 2009; Marks et al., 2009). Also, MYB106 controls trichome branching in Arabidopsis (Folkers et al., 1997; Stracke et al., 2001; Jakoby et al., 2008), epidermal conical cell development in flowers of Antirrhinum majus and Nicotiana tabacum (Noda et al., 1994; Baumann et al., 2007; Jaffé et al., 2007), and multicellular trichomes in Cucumis sativus (Zhao et al., 2015b; Yang et al., 2018). In addition, the HOMEODOMAIN GLABROUS 2 (HDG2) has been reported as a key epidermal component promoting stomatal differentiation and trichome cell wall development in Arabidopsis (Marks et al., 2009; Peterson et al., 2013). Finally, RAV genes have been associated with the regulation of trichome initiation in Arabidopsis (Matías-Hernández et al., 2016) and Cucumis sativus (Zhao et al., 2015a).
Fig. 2.
Canonical signalling pathways for unicellular and multicellular trichome development and comparative expression in Aristolochia fimbriata. (A) Schematic model of Arabidopsis trichome differentiation and surrounding pavement cell fate specification (modified from Zhao et al., 2008; Matías-Hernández et al., 2016). The activation complex is formed by GL1, TTG1 and GL3/EGL3 proteins. This complex activates transcription of GL2 to promote trichome formation while an R3-MYB repressor protein (TRY) moves to the neighbouring cells to repress trichome formation. R3-MYB members including TRY, CPC, ETC1, 2, 3 and TCL1 repress trichome initiation. RAV genes repress trichome initiation via gibberellins. (B) Proposed model for multicellular trichome patterning in Cucumis sativus (modified from Liu et al., 2016). Asterisks indicate the homologues evaluated in this study. (C) Heatmap displaying the expression of the ten candidate genes selected from the two models above. The relative abundance of transcript per million (TPM) values is shown for AfimGL2, AfimGL3, AfimHDG2, AfimMYB5, AfimMYB6, AfimMYB106-like, AfimRAV1-like, AfimTRY, AfimTTG1 and AfimWIN1 in the three perianth portions at S6 and S9.
Unicellular trichomes have been, by far, more studied than multicellular trichomes. However, scattered data on the genetic bases for the latter have been gathered in a few core eudicot model taxa, suggesting that the same MBW complex is in place (Yang et al., 2015). In Nicotiana tabacum, homologues of MIXTA (also a MYB homologue) are able to induce multicellular trichome formation (Payne et al., 1999; Perez-Rodriguez et al., 2005). In Cucumis sativus, CsGL2, CsMYB6 and CsWIN1 are directly involved in multicellular trichome development (Chen et al., 2014; Li et al., 2015; Pan et al., 2015; Zhao et al., 2015a, b; Cui et al., 2016; Liu et al., 2016; Wang et al., 2016; Yang et al., 2018).
The genetic mechanisms underlying the formation of floral trichomes in non-model species outside rosids and asterids are poorly understood. Here we aim to assess the spatio-temporal expression patterns of the candidate trichome-development homologues (GL2, GL3, TTG1, TRY, MYB5, MYB6, HDG2, MYB106-like, WIN1 and RAV1-like) in Aristolochia fimbriata, by combining different state-of-the-art methodologies, including comparative transcriptomics [RNA-sequencing (RNA-seq)] and in situ hybridization assays. We present a detailed expression profile of key regulatory genes that participate in the epidermal differentiation of the A. fimbriata perianth. Thus, our goal is to test whether candidate genes for trichome development and epidermal elaboration identified in model species are involved in the initiation and development of specialized, multicellular trichomes in vegetative and floral organs of Aristolochia.
MATERIALS AND METHODS
Transcriptome analysis
De novo transcriptomes from Aristolochia fimbriata were generated. Each transcriptome was obtained from three independent biological replicates of the three dissected portions of the sepal-derived perianth (utricle, tube and limb), at two floral developmental stages, namely S6 and S9 (stages follow Pabón-Mora et al., 2015; Fig. 1). Total RNA from all dissected perianth portions at both stages (Fig. 1) was extracted using TRIZOL reagent (Invitrogen). The RNA-seq experiment was conducted using the Truseq stranded mRNA library construction kit (Illumina) and sequenced on a NovaSeq 6000 system reading 100-bp, paired-end reads. Read cleaning was performed with a homebrew program with a quality threshold of Q30 at both ends and only keeping those longer that 70 bases after trimming. Contig assembly was computed using the Trinity package following default settings. Transcriptome assembly was performed for each perianth portion, in both developmental stages (Supplementary Data Table S1). In addition, a combined global transcriptome from all experiments was assembled as a reference with the following metrics: total assembled bases: 85 608 833 bp; total number of contigs (> 101 bp): 118 941; average contig length: 719 bp; contig N50: 14 432 sequences ≥ 1823 bp; contig N75: 31 828 sequences ≥ 746 bp; contig GC%: 42.71%.
Gene homologue searches and phylogenetic analyses
The sequences of all candidate genes involved in epidermis and trichome development were retrieved from the global Aristolochia fimbriata transcriptome using tblastx. The queries included the canonical target genes of Arabidopsis GL2, GL3, TTG1, TRY, MYB5, MYB6, HDG2, WIN1, RAV1-like and MYB106/NOK, as well as the MIXTA gene from Antirrhinum majus. All resulting contigs were confirmed by performing reciprocal searches in GenBank (https://blast.ncbi.nlm.nih.gov/Blast.cgi), and manually curated to identify the open reading frame, coding DNA sequence and untranslated regions (UTRs). Isolated sequences in this study correspond to GenBank accession numbers: MT365930–MT365939.
To assess homology for all genes isolated, we performed comprehensive phylogenetic analyses for all gene lineages. These analyses were based on an expanded sampling of the GL2, GL3, TTG1, TRY, MYB5, MYB6, HDG2, MYB106-like, WIN1 and RAV1-like homologues obtained from eudicots, monocots and magnoliids, using the following repositories: the genome database Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html), GenBank (NCBI) nucleotide database (https://www.ncbi.nlm.nih.gov/nucleotide/) and the transcriptome database OneKP (https://db.cngb.org/blast/). Sequences were compiled using BioEdit (http://www.mbio.ncsu.edu/bioedit/bioedit.html). Nucleotide sequences were then aligned using the online version of MAFFT (https://mafft.cbrc.jp/alignment/server/) (Katoh et al., 2002), with a gap open penalty of 3.0, offset value of 1.0 and all other settings as default. Then, alignments were refined by hand using BioEdit considering the representative conserved domains for each of the targeted gene families. Maximum likelihood (ML) phylogenetic analyses using the full nucleotide sequences for each gene were performed with RaxML-HPC2 BlackBox (https://www.phylo.org/) (Stamatakis et al., 2008), through the CIPRES Science Gateway (Miller et al., 2010). Bootstrapping was performed according to the default criteria in RaxML where the bootstrapping stopped after 200–600 replicates when the criteria were met. Trees were observed and edited using FigTree v.1.4.4. (http://tree.bio.ed.ac.uk/software/figtree/) (Rambaut, 2014). Homologues from Amborella trichopoda were used as the outgroups in all phylogenetic analyses (Supplementary Data Table S2).
Mapping, gene annotation and expression by RNA-seq
To estimate the relative abundance of the assembled contigs, cleaned reads were mapped against the de novo assembled dataset implementing the algorithm Kallisto v.0.46.0 with default settings (https://pachterlab.github.io/kallisto/). Kallisto quantifies transcript expression normalizing the relative abundance of each contig/transcript using transcript per million (TPM) metrics (Bray et al., 2016) (Supplementary Data Table S3). The relative abundance of GL2, GL3, TTG1, TRY, MYB5, MYB6, HDG2, MYB106-like, WIN1 and RAV1-like transcripts was used to identify their expression level in each portion of the Aristolochia fimbriata perianth. This expression was calculated for the six generated transcriptomes corresponding to the utricle, the tube, and the limb at two different developmental stages (S6 and S9). Expression data from each sample were used to construct the heatmaps using the R package pheatmap (https://cran.r-project.org/web/packages/pheatmap/index.html).
Material collection and expression analyses by in situ hybridization assays
Flowers of Aristolochia fimbriata at different developmental stages were collected from plants growing in the Nolen glasshouses at the New York Botanical Garden (NYBG) or at the Universidad de Antioquia (UdeA), and fixed under vacuum in freshly prepared, cold formaldehyde–acetic acid–ethanol (FAA solution: 50% ethanol, 37% formaldehyde and 5% glacial acetic acid). After a 4-h incubation, samples were dehydrated in an ethanol series and then transferred to fresh Paraplast X-tra tissue embedding medium (Fisher, Waltham, MA, USA) in a Leica TP1020 automatic tissue processor and stored at 4 °C until use. Samples were prepared and sectioned at 10 µm on a Microm HM3555 rotary microtome. DNA templates for RNA probe synthesis were obtained by PCR amplification of 300–550-bp fragments. To ensure specificity, the probe templates were designed to amplify the 3′ UTR sequences from each evaluated gene (Supplementary Data Table S4). Fragments were cleaned using the QIAquick PCR purification Kit (Qiagen, Valencia, CA, USA). Digoxigenin-labelled RNA probes were prepared using T7 polymerase (Roche, Switzerland), murine RNAse inhibitor (New England Biolabs, Ipswich, MA, USA), and RNA labelling mix (Roche) according to each manufacturer’s protocols. RNA in situ hybridization was performed according to Ambrose et al. (2000) and Ferrándiz et al. (2000), optimized to hybridize overnight at 55 °C. Probe concentration was identical for all the experiments, including the sense control hybridizations. In situ hybridized sections were subsequently dehydrated and permanently mounted in Permount (Fisher). All sections were digitally photographed using a Zeiss Axioplan microscope equipped with a Nikon DXM1200C digital camera.
RESULTS
Identification of epidermis- and trichome-related genes in Aristolochia fimbriata
Targeted blast searches on the global transcriptome of Aristolochia fimbriata followed by phylogenetic analyses (Supplementary Data Figs S1–S10) allowed us to identify single-copy gene homologues for GL2 (named AfimGL2), GL3 (AfimGL3), HDG2 (AfimHDG2), MYB5 (AfimMYB5), MYB6 (AfimMYB6), MYB106-like (AfimMYB106-like), RAV1-like (AfimRAV1-like), TRY (AfimTRY), TTG1 (AfimTTG1) and WIN1 (AfimWIN1). We did not find homologues of GL1 or EGL3 genes.
Comparative gene expression from RNA-seq analyses
Comparative expression levels of the ten candidate genes were tested in the utricle, tube and limb at developmental stages S6 and S9 (Fig. 1). The analyses revealed active expression of a subset of the targeted genes, namely the TFs AfimTTG1, AfimHDG2, AfimMYB6, AfimGL2, AfimMYB5 and AfimRAV1-like (Fig. 2C). Most genes that were differentially expressed in the tube and the utricle tended to increase in expression from S6 to S9. This was the case for AfimTTG1 and AfimMYB5 in the limb, tube and utricle. Those with the opposite trend (reduced levels at S9 when compared to S6) include AfimRAV1-like in the limb, and AfimMYB6 in the limb and tube. Conversely, AfimWIN1, AfimGL3, AfimTRY and AfimMYB106-like had lower expression levels in all floral portions during the two developmental stages. From the core Arabidopsis trichome-activating complex (GL1–GL3/EGL3–TTG1), only AfimTTG1 had significant expression levels in the limb, tube and utricle. In contrast, AfimWIN1 had a homogeneous low expression across developmental stages in the limb, tube and utricle, with increased expression at S6. AfimGL2, which is the putative downstream target of this complex, was expressed in all perianth portions, with significant expression levels in both the tube and the utricle. The single-repeat MYB AfimTRY, which in Arabidopsis negatively regulates the trichome-activating complex, presented a homogeneous lower expression. Similar patterns were found for AfimMYB106-like which is a positive regulator of trichome development in Cucumis sativus (Fig. 2C).
Detailed expression by in situ hybridization
With the aim of identifying putative trichome patterning candidate genes, we investigated the spatial and temporal expression patterns of ten TFs. We used RNA in situ hybridization assays on transverse and longitudinal sections of the Aristolochia fimbriata flower throughout developmental stages S1–S6 (stages following Pabón-Mora et al., 2015) and the leaves. These experiments provided information on the mRNA spatio-temporal expression patterns of the candidate genes. mRNA localization is probably correlated with, but are not necessarily equivalent to, the protein, as the latter can be narrowed by degradation or broadened by translocation between cells. Descriptions hereafter focus on expression changes between the adaxial (inner) and the abaxial (outer) perianth epidermis in flowers at S5–S6. Particular attention was given to expression in the floral trichomes.
Most highly expressed TFs in both the adaxial and the abaxial floral epidermis include AfimGL2, AfimRAV1-like, AfimWIN1 and AfimMYB106-like homologues
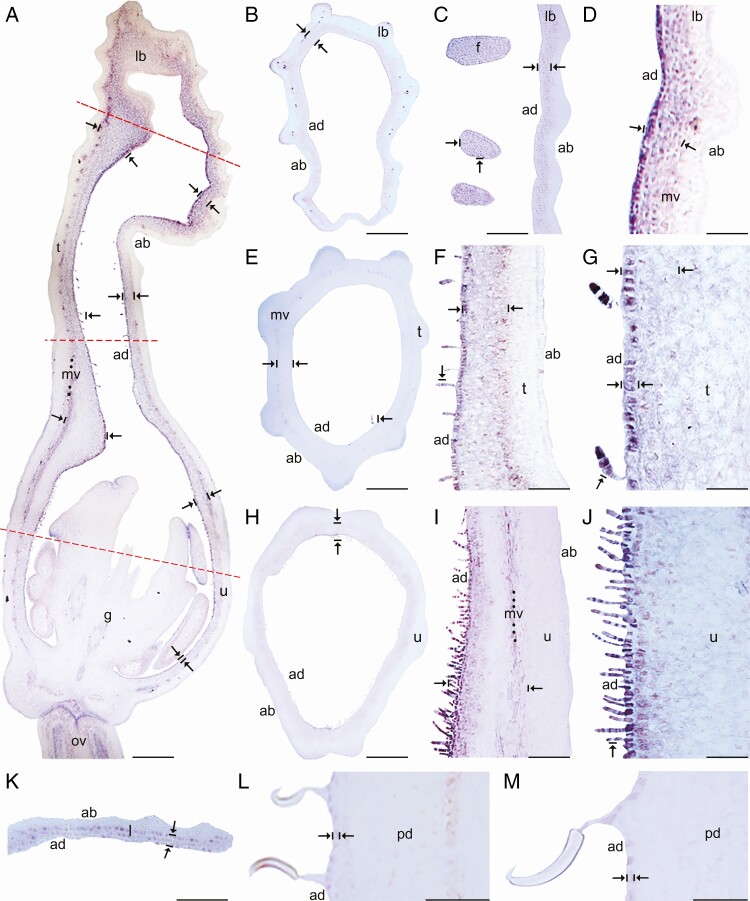
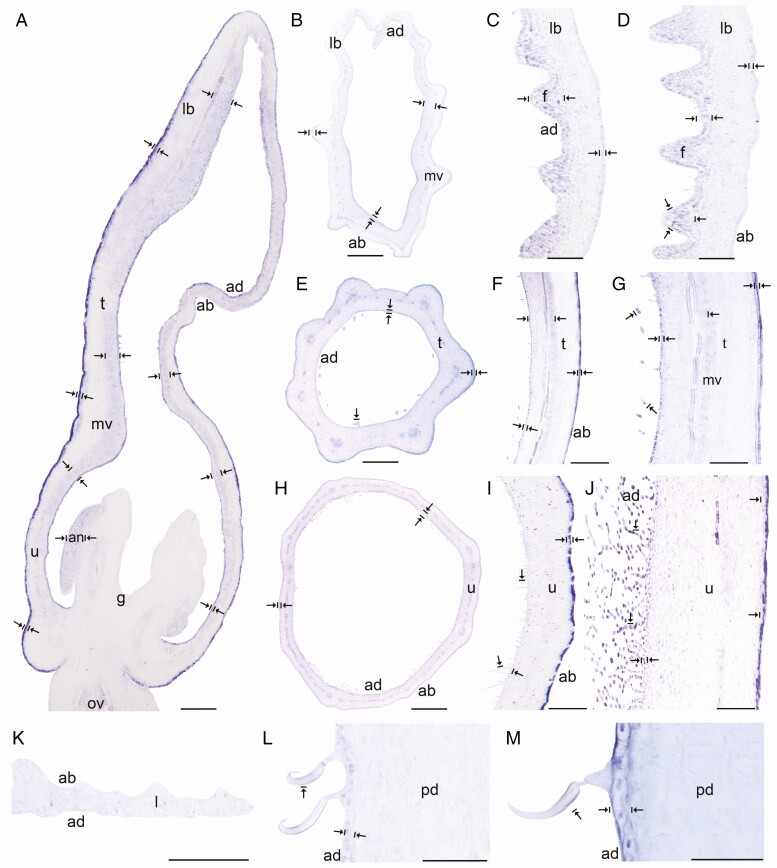
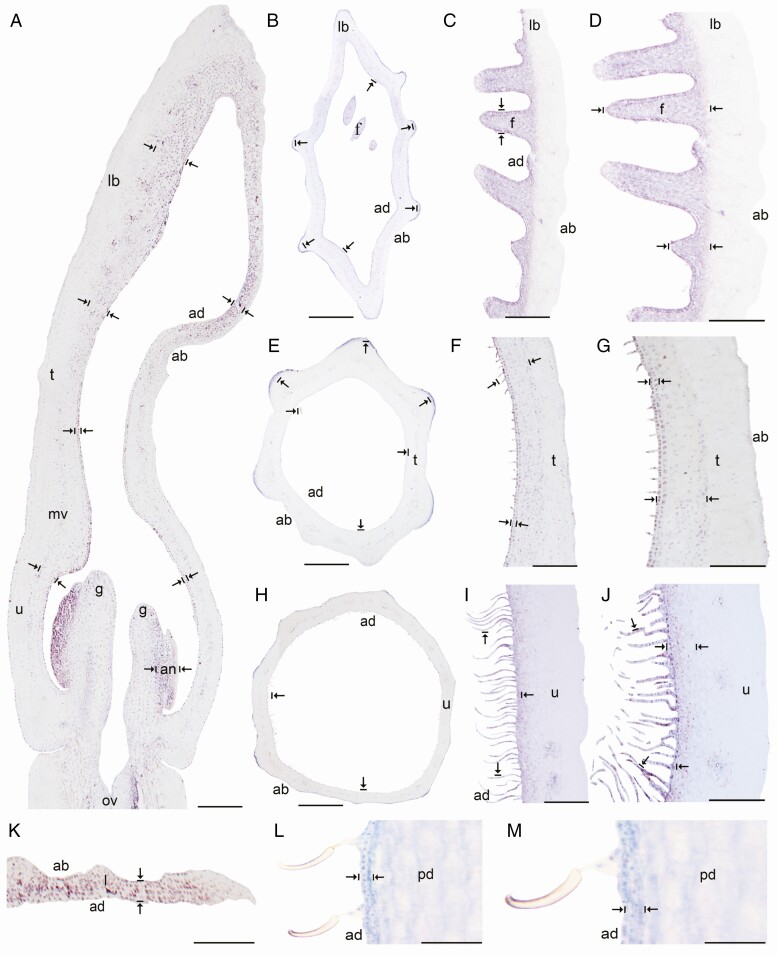
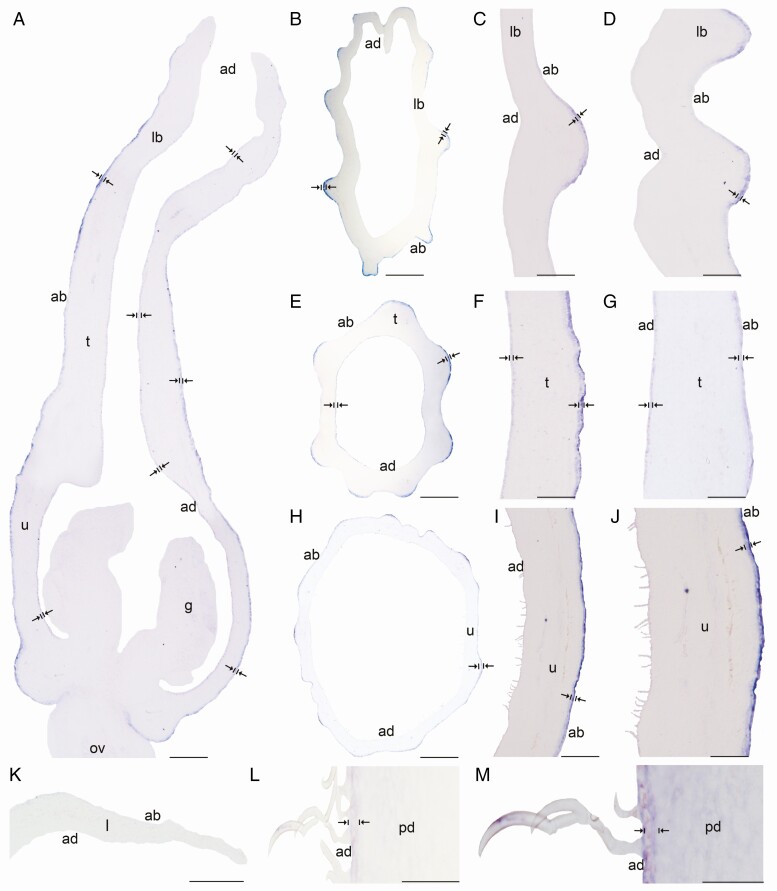
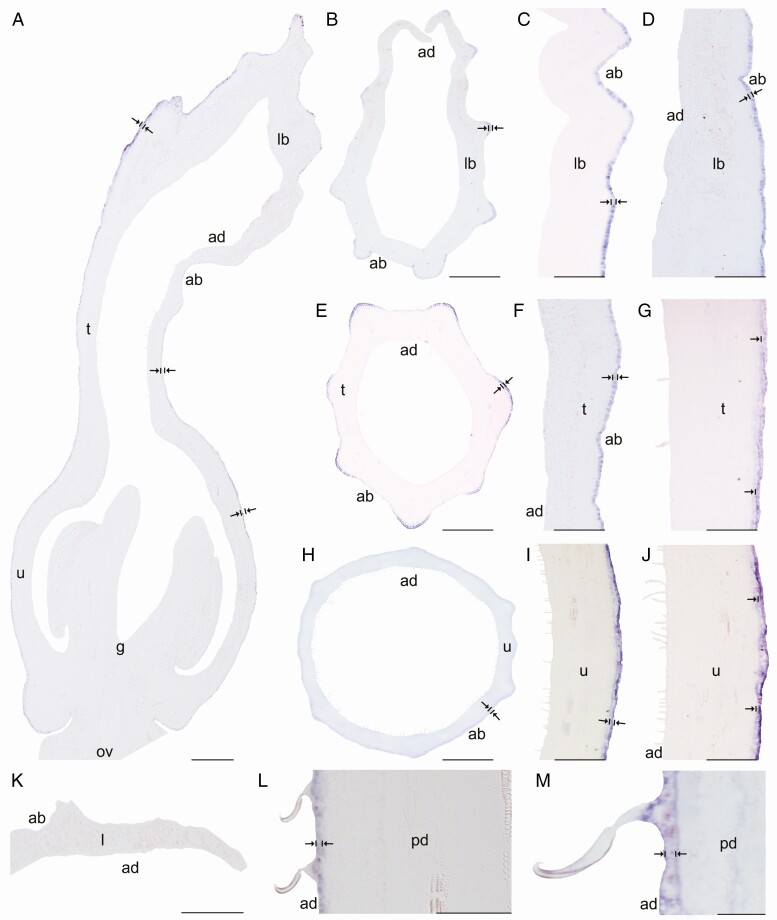
Expression of AfimGL2, AfimWIN1 and AfimRAV1-like genes was detected in the shoot apical meristem, as well as in the young leaves and in the perianth at S1–S2 (Supplementary Data Fig. S11), while expression of AfimMYB106-like at these early stages was weak (Fig. S11). Expression of these four TFs during S3–S4 (perianth and ovary differentiation) was found throughout the perianth, in the developing gynostemium and in the ovary (Fig. S11). During S6 all four TFs were expressed between the midvein and the adaxial epidermis of the perianth (Figs 3A–6A), where more subepidermal cell layers showed expression in the limb and tube (~12) compared to the utricle (~4–6). Expression of these four TFs was also evident in the fimbriae, which are vascularized limb extensions with distal osmophores probably involved in attracting pollinators (González and Pabón-Mora, 2015) (Fig. 1B, F). Furthermore, AfimMYB106 was expressed in the marginal portion of the limb and AfimWIN1 and AfimRAV1-like were also detected in the hooked trichomes scattered in the limb (Figs 3C, D, 4C, D, 5C, D and 6C, D). AfimGL2, AfimMYB106-like, AfimRAV1-like and AfimWIN1 were also detected in the multicellular, conical trichomes formed on the adaxial epidermis of the tube (Figs 3F, G, 4F, G, 5F, G and 6F, G), and the secretory, multicellular, filamentous trichomes inside the utricle (Figs 3I, J, 4I, J, 5I, J and 6I, J). Also, flowers at S6 showed expression of AfimMYB106-like and AfimWIN1 in the abaxial epidermis of the utricle, tube and limb (Figs 4A–J and 5A–J). Conversely, AfimGL2 was weakly expressed and AfimRAV1-like was undetected in the abaxial perianth epidermis (Figs 2A–J and 6A–J). Interestingly, only AfimMYB106-like and AfimWIN1 were clearly expressed in the perianth vascular bundles (Figs 4A–J and 5A–J). AfimGL2, AfimMYB106-like, AfimWIN1 and AfimRAV1-like also presented similar expression patterns in the floral pedicel, specifically in the epidermis and the hooked trichomes (Figs 3L, M, 4L, M, 5L, M and 6L, M). Expression in leaves was detected only for AfimGL2 and AfimRAV1-like (Figs 3K and 6K), but not for AfimWIN1 or AfimMYB106-like (Figs 4K and 5K). Control sense probes for AfimGL2, AfimRAV1-like, AfimWIN1 and AfimMYB106-like genes showed no signal (Fig. S12).
Fig. 3.
In situ hybridization of AfimGL2. (A) Sagittal section of the Aristolochia fimbriata flower at S6; note low expression in the abaxial epidermis with respect to the adaxial epidermis. (B–D) Transverse (B) and longitudinal (C, D) sections of the limb at low (B), mid- (C) and high (D) magnification. (E–G) Transverse (E) and longitudinal (F, G) sections of the tube at low (E), mid- (F) and high (G) magnification. (H–J) Transverse (H) and longitudinal (I, J) sections of the utricle at low (H), mid- (I) and high (J) magnification. (K) Transverse section of the leaf. (L–M) Longitudinal section of the floral pedicel and detail of its hooked trichomes (M) adaxial (ad) of the pedicel here and in Figs 4–8 is given in reference to the shoot axis. Arrow/bar signs point to tissue with positive expression; ab, abaxial epidermis; ad, adaxial epidermis; fi, fimbriae; g, gynostemium; l, leaf; lb, limb; mv, midvein; ov, ovary; pd, pedicel; t, tube; u, utricle. Scale bars: 100 µm (A, B, E, H); 150 µm (C, F, I); 200 µm (D, G, J); 60 µm (K–M).
Fig. 4.
In situ hybridization of AfimMYB106-like gene. (A) Sagittal section of the Aristolochia fimbriata flower at S6; note strong expression in the adaxial limb mesophyll and the abaxial epidermis. (B–D) Transverse (B) and longitudinal (C, D) sections of the limb at low (B), mid- (C) and high (D) magnification. (E–G) Transverse (E) and longitudinal (F, G) sections of the tube at low (E), mid- (F) and high (G) magnification. (H–J) Transverse (H) and longitudinal (I–J) sections of the utricle at low (H), mid- (I) and high (J) magnification. (K) Transverse section of the leaf. (L–M) Longitudinal section of the floral pedicel and detail of its hooked trichomes (M). Arrow/bar signs point to tissue with positive expression; ab, abaxial epidermis; ad, adaxial epidermis; an, anther; fi, fimbriae; g, gynostemium; l, leaf; lb, limb; mv, midvein; ov, ovary; pd, pedicel; t, tube; u, utricle. Scale bars: 100 µm (A, B, E, H); 150 µm (C, F, I); 200 µm (D, G, J); 60 µm (K–M).
Fig. 5.
In situ hybridization of AfimWIN1. (A) Sagittal section of the Aristolochia fimbriata flower at S6; note higher expression in the adaxial perianth mesophyll, the fimbriae and the abaxial epidermis of the perianth. (B–D) Transverse (B) and longitudinal (C, D) sections of the limb at low (B), mid- (C) and high (D) magnification. (E–G) Transverse (E) and longitudinal (F, G) sections of the tube at low (E), mid- (F) and high (G) magnification. (H–J) Transverse (H) and longitudinal (I–J) sections of the utricle at low (H), mid- (I) and high (J) magnification. (K) Transverse section of the leaf. (L–M) Longitudinal section of the floral pedicel (L) and detail of its hooked trichomes (M). Arrow/bar signs point to tissue with positive expression; ab, abaxial epidermis; ad, adaxial epidermis; an, anther; fi, fimbriae; g, gynostemium; l, leaf; lb, limb; mv, midvein; ov, ovary; pd, pedicel; t, tube; u, utricle. Scale bars: 100 µm (A, B, E, H); 150 µm (C, F, I); 200 µm (D, G, J); 60 µm (K–M).
Fig. 6.
In situ hybridization of AfimRAV1-like. (A) Sagittal section of the Aristolochia fimbriata flower at S6; note expression restricted to the adaxial mesophyll of the perianth. (B–D) Transverse (B) and longitudinal (C, D) sections of the limb at low (B), mid- (C) and high (D) magnification. (E–G) Transverse (E) and longitudinal (F, G) sections of the tube at low (E), mid- (F) and high (G) magnification. (H–J) Transverse (H) and longitudinal (I–J) sections of the utricle at low (H), mid- (I) and high (J) magnification. (K) Transverse section of the leaf. (L–M) Longitudinal section of the floral pedicel (L) and detail of its hooked trichomes (M). Arrow/bar signs point to detailed expression patterns; ab, abaxial epidermis; ad, adaxial epidermis; an, anther; fi, fimbriae; g, gynostemium; l, leaf; lb, limb; mv, midvein; ov, ovary; pd, pedicel; t, tube; u, utricle. Scale bars: 100 µm (A, B, E, H); 150 µm (C, F, I); 200 µm (D, G, J); 60 µm (K–M).
TFs preferentially restricted to the abaxial floral epidermis include AfimHDG2, AfimTTG1, AfimGL3, AfimTRY, AfimMYB5 and AfimMYB6
AfimHDG2 was weakly detected in the perianth at S4 (Supplementary Data Fig. S11). During S6 (Fig. 7A), expression of AfimHDG2 was detected in both the abaxial and the adaxial perianth epidermis. However, expression was always higher in the abaxial epidermis, when compared to the adaxial counterpart. Similar expression levels were detected in the limb (Fig. 7B, C), tube (Fig. 7F, G) and utricle (Fig. 7I, J). Expression of AfimHDG2 beyond the perianth was found in the hooked trichomes and the epidermis of the pedicel (Fig. 7L, M). Finally, no expression was detected in the young leaves (Fig. 7K). Control sense probes for AfimHDG2 resulted in no signal (Fig. S12).
Fig. 7.
In situ hybridization of AfimHDG2. (A) Sagittal section of the Aristolochia fimbriata flower at S6; note expression in the abaxial epidermis of the perianth, and weak expression in its adaxial counterpart. (B–D) Transverse (B) and longitudinal (C, D) sections of the limb at low (B), mid- (C) and high (D) magnification. (E–G) Transverse (E) and longitudinal (F, G) sections of the tube at low (E), mid- (F) and high (G) magnification. (H–J) Transverse (H) and longitudinal (I–J) sections of the utricle at low (H), mid- (I) and high (J) magnification. (K) Transverse section of the leaf. (L–M) Longitudinal section of the floral pedicel (L) and detail of its the hooked trichomes (M). Arrow/bar signs point to detailed expression patterns; ab, abaxial epidermis; ad, adaxial epidermis; fi, fimbriae; g, gynostemium; l, leaf; lb, limb; mv, midvein; ov, ovary; pd, pedicel; t, tube; u, utricle. Scale bars: 100 µm (A, B, E, H); 150 µm (C, F, I); 200 µm (D, G, J); 60 µm (K–M).
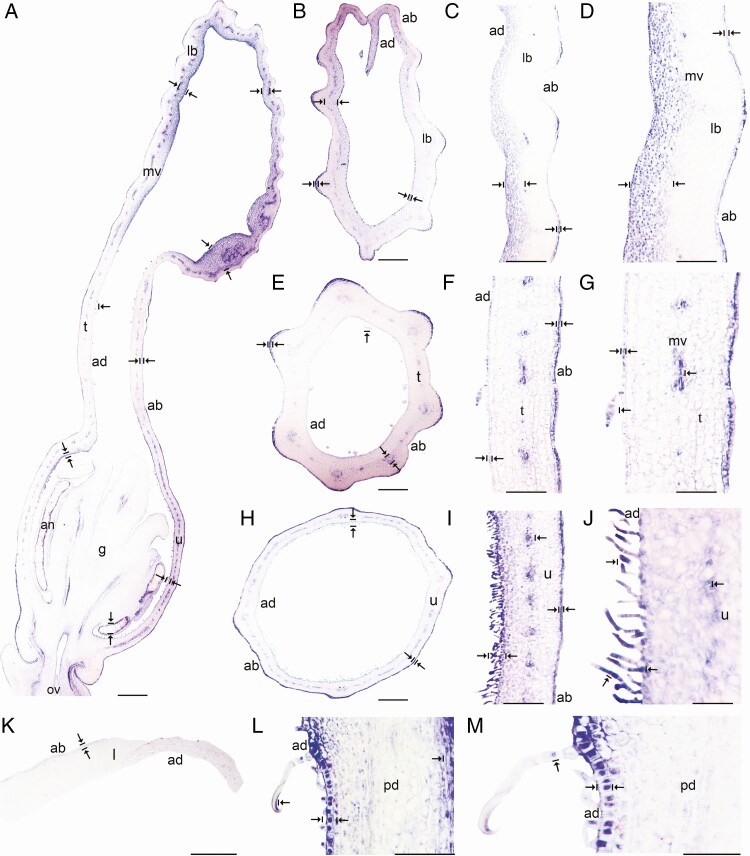
The TFs AfimGL3, AfimMYB5, AfimMYB6, AfimTRY and AfimTTG1 were weakly expressed at S1–S4 in the perianth, anther primordia and ovary (Supplementary Data Fig. S11). During S6, AfimGL3, AfimMYB5, AfimMYB6, AfimTRY and AfimTTG1 homologues presented a similar expression pattern restricted to the abaxial epidermis of the perianth (Fig. 8; Figs S13–S16). Expression in the abaxial epidermis was detected in the limb (Fig. 8A, C–D; Figs S13–S16A–B, D), tube (Fig. 8A, E-G; Figs S13–S16A, E–G) and utricle (Fig. 8A, H–J; Figs S13–S16A, H–J). At S6, none of these five TFs was detected in the multicellular, conical trichomes inside the tube or the secretory, multicellular, filamentous trichomes inside the utricle (Fig. 8F, G, I, J; Figs S13–S16F, G, I, J) or in the vascular bundles of the perianth (Fig. 8; Figs S13–S16). However, expression of AfimTTG1, AfimGL3, AfimTRY, AfimMYB5 and AfimMYB6 was detected in the epidermis and the subepidermal layer of the floral pedicel, and in the three-celled hooked trichomes (Fig. 8L–M; Figs S13–S16L, M). Control sense probes for AfimGL3, AfimMYB5, AfimMYB6, AfimTRY and AfimTTG1 showed no signal (Fig. S12).
Fig. 8.
In situ hybridization of AfimTTG1. (A) Sagittal section of the Aristolochia fimbriata flower at S6; note expression restricted to the abaxial epidermis of the perianth. (B–D) Transverse (B) and longitudinal (C, D) sections of the limb at low (B), mid- (C) and high (D) magnification. (E–G) Transverse (E) and longitudinal (F, G) sections of the tube at low (E), mid- (F) and high (G) magnification. (H–J) Transverse (H) and longitudinal (I, J) sections of the utricle at low (H), mid- (I) and high (J) magnification. (K) Transverse section of the leaf. (L and M) Longitudinal section of the floral pedicel (L) and detail of its hooked trichomes (M). Arrow/bar signs point to detailed expression patterns; ab, abaxial epidermis; ad, adaxial epidermis; fi, fimbriae; g, gynostemium; l, leaf; lb, limb; mv, midvein; ov, ovary; pd, pedicel; t, tube; u, utricle. Scale bars: 100 µm (A, B, E, H); 150 µm (C, F, I); 200 µm (D, G, J); 60 µm (K–M).
DISCUSSION
We provide a comprehensive analysis of trichome-related gene expression patterns in flowers of an early diverging angiosperm with a highly elaborate, trichome-dependent pollination system. Based on our expression data in Aristolochia fimbriata, floral trichomes use subset of core trichome development genes that appear to be deeply conserved, not only in sequence but also in expression patterns, across angiosperms. Altogether, the core trichome-related genes may control hooked trichome development in extra-floral organs such as the pedicel and the leaves of Aristolochia fimbriata. Thus, trichome formation inside the perianth may only require a group of genes including AfimGL2, AfimHDG2, AfimMYB106-like, AfimRAV1-like and AfimWIN. Expression of the remaining genes included in this study (AfimGL3, AfimMYB5, AfimMYB6, AfimTRY and AfimTTG1) show unexpected localization patterns, restricted to the abaxial and the adaxial perianth epidermis. Our results re-evaluate the core genetic network shaping trichome fate in flowers of early diverging angiosperms, resulting in a morphologically diverse output with a simpler genetic mechanism in place when compared to the model Arabidopsis thaliana and Cucumis sativus. In turn, it is likely that only the leaf trichome identity genetic regulatory network (GRN) is conserved across angiosperms.
Few genes are common hubs for multicellular trichome development across distantly related angiosperm lineages
Aristolochia fimbriata, as a member of the magnoliid family Aristolochiaceae, whose ancestor pre-dates the diversification of monocots and eudicots, is particularly well positioned to assess floral trichome development genes recruited during early angiosperm evolution. Based on quantitative and qualitative expression patterns we showed that AfimGL2, AfimMYB106-like, AfimRAV1-like and AfimWIN1 probably control the development of multicellular trichomes in the perianth of Aristolochia fimbriata. In addition, AfimHDG2 may be involved in the differentiation of the inner and outer floral epidermis. Data available from other angiosperms confirm that these five TFs are common hubs for trichome development in stems, leaves and perianth. We discuss these TFs in order to identify the contribution of each gene lineage to trichome development in various angiosperms.
In Aristolochia fimbriata, the pattern of AfimGL2 expression is maintained in the fimbriae and all trichome types distributed in the adaxial perianth epidermis (Fig. 3). These results suggest a fundamental role of AfimGL2, in both the initiation and the differentiation of multicellular trichomes. Similar processes have been attributed to GL2 homologues in model eudicots with both unicellular and multicellular trichomes. In Arabidopsis, GLABRA2 (GL2) controls unicellular trichome initiation and branching as well as cell expansion and cell wall maturation (Koornneef et al., 1982; Rerie et al., 1994; Marks and Esch, 1994; Cristina et al., 1996; Ohashi et al., 2002). In Cucumis sativus CsGL2 also controls multicellular trichome morphogenesis (Zhao et al., 2015b; Liu et al., 2016). The csgl2 mutant exhibits few trichomes on the tendrils, sepals, ovaries and fruits, and glabrous stems and leaves (Yang et al., 2015). In turn, GL2 homologues seem to be a key hub for trichome development across angiosperms, regardless of whether they are uni- or multicellular.
The MYB106 homologues, best known as MIXTA-like R2R3 MYB genes, are pivotal conserved TFs in trichome development and epidermal specializations across angiosperms. Our study shows that AfimMYB106-like is expressed in the adaxial and the abaxial epidermis of the Aristolochia fimbriata perianth, including the secretory multicellular trichomes inside the tube and utricle (Fig. 4F, G, I, J). These expression patterns suggest that AfimMYB106-like plays a role in epidermal patterning and in multicellular development of both structural and secretory types of trichomes, similarly to homologues of other species. The MYB106 homologue in Arabidopsis (NOECK) controls trichome branching (Folkers et al., 1997; Stracke et al., 2001; Jakoby et al., 2008). MIXTA-like genes regulate the growth of epidermal conical cells in the Antirrhinum majus and Nicotiana tabacum petals (Noda et al., 1994; Baumann et al., 2007; Jaffé et al., 2007). The MYB106 homologue in cotton is GhMYB25 and is expressed during the development of the long unicellular seed coat trichomes as well as trichomes in petals, stems and leaves (Wu et al., 2006; Walford et al., 2011; Machado et al., 2009). Likewise, homologues of AtMYB106 have been reported in Cucumis sativus as potential candidates for epidermal cell differentiation and trichome morphogenesis (Li et al., 2012; Zhao et al., 2015b; Yang et al., 2018). Furthermore, in the non-core eudicot genus Thalictrum (Ranunculaceae), the orthologue of MIXTA-like2 controls epidermal cell shape in flowers (Di Stilio et al., 2009).
In comparison to the two TFs discussed before, WIN1 and RAV-like homologues have been less studied. In turn, their association with trichome development across diverse angiosperm species is unclear. In Aristolochia fimbriata, AfimWIN1 is continuously expressed in the adaxial and abaxial epidermis of the perianth and in all floral trichomes (Fig. 5). The WIN1 homologue in Arabidopsis regulates the production of epicuticular waxes (Kannangara et al., 2007). However, in Cucumis sativus, CsWIN1 works as an essential regulator of multicellular trichome development, together with CsGL2 and CsMYB6 (Zhao et al., 2015a; Liu et al., 2016). Thus, it is possible that WIN1 homologues were recruited early on during plant diversification in multicellular trichome development and unicellular trichomes do not actively need them.
It is also uncertain whether RAV1-like homologues are commonly recruited in trichome development across angiosperms. In Aristolochia fimbriata, AfimRAV1-like is continuously expressed in the adaxial pavement epidermal cells as well as the trichomes of the limb, tube, and utricle, which may suggest its positive role in multicellular trichome development (Fig. 6F-G, I-J). However, members of the Arabidopsis RAV family, such as TEM1 and TEM2, repress trichome development by directing gibberellin accumulation and distribution in the leaf mesophyll (Matías-Hernández et al., 2016), and regulating GL1 and GL2. Conversely, a RAV1-like homolog has been suggested as a candidate gene in the development of multicellular trichomes in Cucumis sativus (Zhao et al., 2015a). In turn, more information is needed across angiosperms to assess whether RAV1-like genes positively or negatively control uni- or multicellular trichome development.
Overall, our results regarding the expression patterns of AfimGL2, AfimMYB106-like, AfimWIN1 and AfimRAV1-like genes in the perianth of Aristolochia fimbriata are consistent with those described above for model species such as Cucumis sativus, suggesting that these genes are players in the multicellular trichome genetic regulatory network. Our evidence indicates that the network in Aristolochia fimbriata is more similar to the GRN reported in cucumber. More comparative studies in non-model species will allow the identification of a common GRN for epidermal differentiation across angiosperms and the specific shifts in uni- versus multicellular trichome development.
Different GRNs control floral versus non-floral trichomes in Aristolochia fimbriata
Several genes have been identified as key TFs for unicellular and multicellular trichome development in Arabidopsis thaliana and Cucumis sativus, respectively. In Arabidopsis various trichome-related mutants have allowed the identification of trichome-positive and trichome-negative regulators (Fig. 2A). The positive regulators include GLABROUS1 (GL1), which encodes an R2R3 MYB transcriptional regulator (Oppenheimer et al., 1991) and a WD40 repeat-containing protein TRANSPARENT TESTA GLABRA1 (TTG1) (Bernhard et al., 2005). Two additional positive regulators redundantly control trichome development; these are GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3), both bHLH-like TFs (Payne et al., 2000; Balkunde et al., 2011). These positive regulators stimulate trichome cell fate by activating the expression of GLABRA2 (GL2), which encodes a homeodomain leucine-zipper (HD-Zip IV) (Rerie et al., 1994). The trichome-negative regulators are represented by a set of single-repeat MYB proteins encoded by six redundant genes, namely TRIPTYCHON (TRY), CAPRICE (CPC), ENHANCER OF TRY AND CPC1 (ETC1), ETC2, ETC3 and TRICHOMELESS1 (TCL1) (Schnittger et al., 1999; Kirik et al., 2004a, b; S. Wang et al., 2007; Tominaga et al., 2008; Y.L. Wang et al., 2008; Yang and Ye, 2013). In addition to these negative regulators, MYB5 has been reported to have a redundant role with the R2R3 MYB gene TRANSPARENT TESTA GLABRA2 (TTG2), in the control of trichome development and tannin production (González et al., 2009; Marks et al., 2009). A slightly different positive regulatory network for multicellular trichome development has been proposed in Cucumis sativus. Positive trichome regulators include TRIL/CsGL3, which encodes a homeodomain leucine-zipper (HD-Zip) and its protein partner, an HD-Zip I protein. The complex putatively activates downstream genes triggering trichome development, including CsMYB6, CsWIN1 and CsGL2 (Chen et al., 2014; Zhao et al., 2015a, b; Liu et al., 2016).
Using a candidate gene approach, we have identified and tested the expression patterns of GL2, GL3, HDG2, MYB5, MYB6, MYB106-like, RAV1-like, TTG1, TRY and WIN1 homologues in Aristolochia fimbriata. All these genes might be controlling three-celled hooked trichome development in the floral pedicel, stem and leaves in this species (Figs 3–8K–M; Supplementary Data Figs S13–S16). In the hooked trichomes of the floral pedicel the contribution of AfimMYB5 and AfimMYB6 stands out, as the two TFs are highly expressed in the apical, hooked-shaped cell (Figs S15, S16K–M). In Arabidopsis, MYB5 has a redundant role regulating pleiotropic control of trichome development (González et al., 2009; Marks et al., 2009). Likewise, MYB6 has been reported as a candidate gene involved directly in multicellular trichome development (Zhao et al., 2015a, b). The MYB6 homologue CsMYB6 in Cucumis sativus positively regulates multicellular trichome development in stems, tendrils, leaves and flowers (Liu et al., 2016), while it negatively regulates fruit trichome formation as part of the CsMYB6-CsTRY complex (Yang et al., 2018). In Aristolochia fimbriata MYB5 and MYB6 are strongly expressed in the abaxial epidermis of the perianth and the pedicel epidermis. In turn, expression of the TFs GL2, GL3, HDG2, MYB5, MYB6, MYB106-like, TRY, TTG1, RAV1-like and WIN1 in extra-floral trichomes suggests that they were already active in magnoliids, and their role later diverged in the core eudicots Arabidopsis thaliana and Cucumis sativus. Conversely, floral trichome fate in Aristolochia fimbriata relies on a smaller subset of genes that comprises AfimGL2, AfimHDG2, AfimMYB106-like, AfimRAV1-like and AfimWIN1 (Figs 3–8).
Vascularized limb extensions (fimbriae) retain expression of AfimGL2, AfimMYB106-like, AfimRAV1-like and AfimWIN1
Our results show that genes controlling floral trichomes, such as AfimGL2, AfimMYB106-like, AfimWIN1 and AfimRAV1-like, are also expressed in the marginal limb extensions, known as the fimbriae (Figs 1B, F, 3, 5 and 6C, D). The fimbriae are multicellular, vascularized outgrowths with epidermal osmophores and hooked trichomes (Pabón-Mora et al., 2015). The fimbriae probably act as the first visual and olfactory signals to pollinators in Aristolochia fimbriata (Aliscioni et al., 2017). Expression of AfimGL2, AfimMYB106-like, AfimRAV1-like and AfimWIN1 is detected throughout the fimbria epidermis and vascular tissue, and also in the three to six subepidermal cell layers (Figs 3, 4C, D, 5 and 6C, D). Different homeobox-leucine zipper genes that encode proteins such as GL2 and ANTHOCYANINLESS2 (ANL2) have been reported in transcriptional networks involved in cell differentiation and anthocyanin pigmentation, not only in the epidermis but also in the leaf subepidermal tissue layers (Kubo et al., 1999; Qing and Aoyama, 2012). In addition, members of the RAV family such as the TEMPRANILLO (TEM1 and TEM2) genes, also play an essential role in controlling trichome initiation at the epidermal and subepidermal levels (Matías-Hernández et al., 2016). Together, these results point to common GRNs for the limb fimbriae, the adaxial epidermis of the tube and the utricle, and floral trichome initiation and differentiation in Aristolochia fimbriata.
The abaxial perianth epidermis retains expression of a subset of TFs
In Aristolochia fimbriata, AfimTTG1, AfimGL3 and AfimTRY showed expression patterns restricted to the abaxial glabrous epidermis of the perianth in the limb (Fig. 8A–D; Supplementary Data Figs S13 and S14A–D), tube (Fig. 8E–G; Figs S13 and S14E, G) and utricle (Fig. 8H–J; Figs S13 and S14H–J). Thus, our results suggest that although these TFs are signalling in the epidermal layers, they do not contribute directly to the formation of floral trichomes in Aristolochia fimbriata. The TFs AfimGL3, AfimTRY and AfimTTG1 are homologues to those of the Arabidopsis genetic regulatory network that drive unicellular trichome development. TTG1 is the common denominator of overlapping regulatory complexes that control cell fate and trichome differentiation (Galway et al., 1994; Walker et al., 1999). GL3 encodes an activator of trichome development (Hülskamp et al., 1994; Koornneef et al., 1982). Finally, TRY was the first negative regulator of trichome development identified (Hülskamp et al., 1994; Balkunde et al., 2011). The try mutants have trichomes occasionally arranged in clusters of two or three, suggesting that TRY represses trichome initiation in the neighbouring pavement epidermal cells (Schellmann et al., 2002). In particular, expression of AfimTRY in the abaxial epidermis might be associated with the lack of trichomes in this layer of the perianth, probably functioning as a repressor of the genetic pathway. In turn, our results show that specialization and elaboration between the adaxial and the abaxial perianth epidermis relies on different sets of TFs, where repressive TFs become restricted to the non-specialized and highly homogeneous abaxial perianth surface. Thus, the Aristolochia perianth becomes paradoxical as the extremely elaborate adaxial epidermis and its structural and secretory trichomes require fewer trichome-related genes than its abaxial counterpart.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1. ML phylogenetic analysis of the GL2 gene lineage in flowering plants. Figure S2. ML phylogenetic analysis of the GL3 gene lineage in flowering plants. Figure S3. ML phylogenetic analysis of the TTG1 gene lineage in flowering plants. Figure S4. ML phylogenetic analysis of the TRY gene lineage in flowering plants. Figure S5. ML phylogenetic analysis of the RAV1-like gene lineage in flowering plants. Figure S6. ML phylogenetic analysis of the MYB5 gene lineage in flowering plants. Figure S7. ML phylogenetic analysis of the MYB6 gene lineage in flowering plants. Figure S8. ML phylogenetic analysis of the MYB106-like gene lineage in flowering plants. Figure S9. ML phylogenetic analysis of the WIN1 gene lineage in flowering plants. Figure S10. ML phylogenetic analysis of the HDG2 gene lineage in flowering plants. Figure S11. In situ hybridization of AfimGL2, AfimGL3, AfimTTG1, AfimTRY, AfimRAV1-like, AfimMYB5, AfimMYB6, AfimHDG2-like, AfimMYB106-like and AfimWIN1 at the flowering shoot apex. Figure S12. In situ sense probes used as controls for the Aristolochia fimbriata epidermal flower candidate genes. Figure S13. In situ hybridization of AfimGL3. Figure S14. In situ hybridization of AfimTRY. Figure S15. In situ hybridization of AfimMYB5. Figure S16. In situ hybridization of AfimMYB6. Table S1. De novo assembly statistics of the floral transcriptomes of Aristolochia fimbriata at two different developmental stages. Table S2. Accession numbers of GL2, GL3, HDG2-like, MIXTA-like, MYB5, MYB6, RAV1-like, TTG1, TRY and WIN1 homologue sequences used in this study. Table S3. Expression of the candidate genes included in this study. Table S4. Specific primers used in this study for the in situ hybridization experiments, including AfimGL2, AfimGL3, AfimHDG2, AfimMYB5, AfimMYB6, AfimMYB106-like, AfimRAV1-like, AfimTRY, AfimTTG1 and AfimWIN1 orthologues in Aristolochia fimbriata.
ACKNOWLEDGEMENTS
We especially thank the Arnold Arboretum at Harvard University and the New York Botanical Garden (NYBG) for their support during this research. We also thank Cecilia Zumajo-Cardona (NYBG) for assistance during lab work. N.P.-M., F.G., S.P., B.A. and H.S.-B. conceived and designed the study; N.P.-M. and H.S.-B. performed the experiments, and acquired, analysed and interpreted the data; J.F.A., N.P.-M. and H.S.-B. executed the bioinformatics analysis and analysed data; H.S.-B., F.G. and N.P.-M. wrote the manuscript, and all authors revised and approved the final manuscript.
FUNDING
This work was supported by COLCIENCIAS/COLFUTURO (Conv. Doctorados Nacionales No. 727 de 2015), the Deland Award, 2018 to H.S.-B. (the Arnold Arboretum at Harvard University), and Estrategia de Sostenibilidad (2018–2019) awarded to the Grupo Evo-Devo en Plantas by the Universidad de Antioquia, Medellín, Colombia.
LITERATURE CITED
- Aliscioni SS, Achler AP, Torretta JP. 2017. Floral anatomy, micromorphology and visitor insects in three species of Aristolochia L. (Aristolochiaceae). New Zealand Journal of Botany 55: 496–513. [Google Scholar]
- Ambrose BA, Lerner DR, Ciceri P, Padilla CM, Yanofsky MF, Schmidt RJ. 2000. Molecular and genetic analyses of the Silky1 gene reveal conservation in floral organ specification between eudicots and monocots. Molecular Cell 5: 569–579. [DOI] [PubMed] [Google Scholar]
- Balkunde R, Pesch M, Hülskamp M. 2011. Trichome patterning in Arabidopsis thaliana: from genetic to molecular models. Current Topics in Developmental Biology 91: 299–321. [DOI] [PubMed] [Google Scholar]
- Baumann K, Perez-Rodriguez M, Bradley D, et al. . 2007. Control of cell and petal morphogenesis by R2R3 MYB transcription factors. Development (Cambridge, England) 134: 1691–1701. [DOI] [PubMed] [Google Scholar]
- Bernhardt C, Lee MM, Gonzalez A, Zhang F, Lloyd A, Schiefelbein J. 2003. The bHLH genes GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3) specify epidermal cell fate in the Arabidopsis root. Development (Cambridge, England) 130: 6431–6439. [DOI] [PubMed] [Google Scholar]
- Bernhardt C, Zhao M, Gonzalez A, Lloyd A, Schiefelbein J. 2005. The bHLH genes GL3 and EGL3 participate in an intercellular regulatory circuit that controls cell patterning in the Arabidopsis root epidermis. Development (Cambridge, England) 132: 291–298. [DOI] [PubMed] [Google Scholar]
- Boff S, Demarco D, Marchi P, Alves-Dos-Santos I. 2015. Perfume production in flowers of Angelonia salicariifolia attracts males of Euglossa annectans which do not promote pollination. Apidologie 46: 84–91. [Google Scholar]
- Bray NL, Pimentel H, Melsted P, Pachter L. 2016. Near-optimal probabilistic RNA-seq quantification. Nature Biotechnology 34: 525–527. [DOI] [PubMed] [Google Scholar]
- Chen C, Liu M, Jiang L, et al. . 2014. Transcriptome profiling reveals roles of meristem regulators and polarity genes during fruit trichome development in cucumber (Cucumis sativus L.). Journal of Experimental Botany 65: 4943–4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci AA. 1991. Pollination biology of Nierembergia (Solanaceae). Plant Systematics and Evolution 174: 17–35. [Google Scholar]
- Cropper SC, Calder DM. 1990. The floral biology of Thelymitra epipactoides (Orchidaceae), and the implications of pollination by deceit on the survival of this rare orchid. Plant Systematics and Evolution 170: 11–27. [Google Scholar]
- Cui JY, Miao H, Ding LH, et al. . 2016. A new glabrous gene (csgl3) identified in trichome development in cucumber (Cucumis sativus L.). PLoS One 11: e0148422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafni A. 1984. Mimicry and deception in pollination. Annual Review of Ecology, Evolution, and Systematics 15: 259–278. [Google Scholar]
- Di Cristina M, Sessa G, Dolan L, et al. . 1996. The Arabidopsis Athb-10 (GLABRA2) is an HD-Zip protein required for regulation of root hair development. The Plant Journal: for Cell and Molecular Biology 10: 393–402. [DOI] [PubMed] [Google Scholar]
- Di Stilio VS, Martin C, Schulfer AF, Connelly CF. 2009. An ortholog of MIXTA-like2 controls epidermal cell shape in flowers of Thalictrum. The New Phytologist 183: 718–728. [DOI] [PubMed] [Google Scholar]
- Digiuni S, Schellmann S, Geier F, et al. . 2008. A competitive complex formation mechanism underlies trichome patterning on Arabidopsis leaves. Molecular Systems Biology 4: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ottra JH, Pirani JR, Endress PK. 2013. Fusion within and between whorls of floral organs in Galipeinae (Rutaceae): structural features and evolutionary implications. Annals of Botany 111: 821–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbar C, Heiler A, Leins P. 2016. Nectaries in fly-deceptive pitcher-trap blossoms of Aristolochia. Flora 232: 128–141. [Google Scholar]
- Esch JJ, Chen MA, Hillestad M, Marks MD. 2004. Comparison of TRY and the closely related At1g01380 gene in controlling Arabidopsis trichome patterning. The Plant Journal: for Cell and Molecular Biology 40: 860–869. [DOI] [PubMed] [Google Scholar]
- Ferrándiz C, Gu Q, Martienssen R, Yanofsky MF. 2000. Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWER. Development (Cambridge, England) 127: 725–734. [DOI] [PubMed] [Google Scholar]
- Folkers U, Berger J, Hülskamp M. 1997. Cell morphogenesis of trichomes in Arabidopsis: differential control of primary and secondary branching by branch initiation regulators and cell growth. Development (Cambridge, England) 124: 3779–3786. [DOI] [PubMed] [Google Scholar]
- Galway ME, Masucci JD, Lloyd AM, Walbot V, Davis RW, Schiefelbein JW. 1994. The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Developmental Biology 166: 740–754. [DOI] [PubMed] [Google Scholar]
- Glover BJ. 2000. Differentiation in plant epidermal cells. Journal of Experimental Botany 51: 497–505. [DOI] [PubMed] [Google Scholar]
- Glover BJ, Perez-Rodriguez M, Martin C. 1998. Development of several epidermal cell types can be specified by the same MYB-related plant transcription factor. Development (Cambridge, England) 125: 3497–3508. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Mendenhall J, Huo Y, Lloyd A. 2009. TTG1 complex MYBs, MYB5 and TT2, control outer seed coat differentiation. Developmental Biology 325: 412–421. [DOI] [PubMed] [Google Scholar]
- González F, Pabón-Mora N. 2015. Trickery flowers: the extraordinary chemical mimicry of Aristolochia to accomplish deception to its pollinators. New Phytologist 206: 10–13. [DOI] [PubMed] [Google Scholar]
- González F, Stevenson DW. 2000. Perianth development and systematics of Aristolochia. Flora 195: 370–391. [Google Scholar]
- Hu S, Dilcher DL, Jarzen DM, Taylor DW. 2008. Early steps of angiosperm–pollinator coevolution. Proceedings of the National Academy of Sciences USA 105: 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hülskamp M. 2004. Plant trichomes: a model for cell differentiation. Nature Reviews Molecular Cell Biology 5: 471–480. [DOI] [PubMed] [Google Scholar]
- Hülskamp M, Miséra S, Jürgens G. 1994. Genetic dissection of trichome cell development in Arabidopsis. Cell 76: 555–566. [DOI] [PubMed] [Google Scholar]
- Ioannidi E, Rigas S, Tsitsekian D, et al. . 2016. Trichome patterning control involves TTG1 interaction with SPL transcription factors. Plant Molecular Biology 92: 675–687. [DOI] [PubMed] [Google Scholar]
- Jaffé FW, Tattersall A, Glover BJ. 2007. A truncated MYB transcription factor from Antirrhinum majus regulates epidermal cell outgrowth. Journal of Experimental Botany 58: 1515–1524. [DOI] [PubMed] [Google Scholar]
- Jakoby MJ, Falkenhan D, Mader MT, et al. . 2008. Transcriptional profiling of mature Arabidopsis trichomes reveals that NOECK encodes the MIXTA-like transcriptional regulator MYB106. Plant Physiology 148: 1583–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javelle M, Vernoud V, Rogowsky PM, Ingram GC. 2011. Epidermis: the formation and functions of a fundamental plant tissue. The New Phytologist 189: 17–39. [DOI] [PubMed] [Google Scholar]
- Kannangara R, Branigan C, Liu Y, et al. . 2007. The transcription factor WIN1/SHN1 regulates cutin biosynthesis in Arabidopsis thaliana. The Plant Cell 19: 1278–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabourniotis G, Papadopoulos K, Papamarkou M, Manetas Y. 1992. Ultraviolet-B radiation absorbing capacity of leaf hairs. Physiologia Plantarum 86: 414–418. [Google Scholar]
- Kärkkäinen K, Agren J. 2002. Genetic basis of trichome production in Arabidopsis lyrata. Hereditas 136: 219–226. [DOI] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik V, Lee MM, Wester K, et al. . 2005. Functional diversification of MYB23 and GL1 genes in trichome morphogenesis and initiation. Development (Cambridge, England) 132: 1477–1485. [DOI] [PubMed] [Google Scholar]
- Kirik V, Simon M, Huelskamp M, Schiefelbein J. 2004a. The ENHANCER OF TRY AND CPC1 gene acts redundantly with TRIPTYCHON and CAPRICE in trichome and root hair cell patterning in Arabidopsis. Developmental Biology 268: 506–513. [DOI] [PubMed] [Google Scholar]
- Kirik V, Simon M, Wester K, Schiefelbein J, Hulskamp M. 2004b. ENHANCER of TRY and CPC2 (ETC2) reveals redundancy in the region-specific control of trichome development of Arabidopsis. Plant Molecular Biology 55: 389–398. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Dellaert LW, van der Veen JH. 1982. EMS- and radiation-induced mutation frequencies at individual loci in Arabidopsis thaliana (L.) Heynh. Mutation Research 93: 109–123. [DOI] [PubMed] [Google Scholar]
- Kubo H, Peeters AJ, Aarts MG, Pereira A, Koornneef M. 1999. ANTHOCYANINLESS2, a homeobox gene affecting anthocyanin distribution and root development in Arabidopsis. The Plant Cell 11: 1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JC, Marks MD, Nadeau J, Sack F. 1997. Epidermal cell fate and patterning in leaves. The Plant Cell 9: 1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JC, Young N, Prigge M, Marks MD. 1996. The control of trichome spacing and number in Arabidopsis. Development (Cambridge, England) 122: 997–1005. [DOI] [PubMed] [Google Scholar]
- Levin DA. 1973. The role of trichomes in plant defense. The Quarterly Review of Biology 48: 3–15. [Google Scholar]
- Li Q, Cao C, Zhang C, et al. . 2015. The identification of Cucumis sativus Glabrous1 (CsGL1) required for the formation of trichomes uncovers a novel function for the homeodomain-leucine zipper I gene. Journal of Experimental Botany 66: 2515–2526. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhang C, Li J, Wang L, Ren Z. 2012. Genome-wide identification and characterization of R2R3MYB family in Cucumis sativus. PLoS One 7: e47576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SF, Milliken ON, Pham H, et al. . 2009. The Arabidopsis MYB5 transcription factor regulates mucilage synthesis, seed coat development, and trichome morphogenesis. The Plant Cell 21: 72–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Jiao J, Lu TJ, Xu F, Pickard BG, Genin GM. 2017. Arabidopsis leaf trichomes as acoustic antennae. Biophysical Journal 113: 2068–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Bartholomew E, Cai Y, Ren H. 2016. Trichome-related mutants provide a new perspective on multicellular trichome initiation and development in cucumber (Cucumis sativus L). Frontiers in Plant Science 7: 1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A, Wu Y, Yang Y, Llewellyn DJ, Dennis ES. 2009. The MYB transcription factor GhMYB25 regulates early fibre and trichome development. The Plant Journal: for Cell and Molecular Biology 59: 52–62. [DOI] [PubMed] [Google Scholar]
- Marks MD, Esch JJ. 1994. Morphology and development of mutant and wild type trichomes on the leaves of Arabidopsis thaliana. In: Bowman J, ed. Arabidopis: An atlas of morphology and development. New York: Springer, 56–73. [Google Scholar]
- Marks MD, Wenger JP, Gilding E, Jilk R, Dixon RA. 2009. Transcriptome analysis of Arabidopsis wild-type and gl3-sst sim trichomes identifies four additional genes required for trichome development. Molecular Plant 2: 803–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins AC, Aguiar AJC, Alves-Dos-Santos I. 2013. Interaction between oil-collecting bees and seven species of Plantaginaceae. Flora 208: 401–411. [Google Scholar]
- Masucci JD, Rerie WG, Foreman DR, et al. . 1996. The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development (Cambridge, England) 122: 1253–1260. [DOI] [PubMed] [Google Scholar]
- Matías-Hernández L, Aguilar-Jaramillo AE, Osnato M, et al. . 2016. TEMPRANILLO reveals the mesophyll as crucial for epidermal trichome Formation. Plant Physiology 170: 1624–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES science gateway for inference of large phylogenetic trees. Gateway Computing Environments Workshop (GCE) 1-8. [Google Scholar]
- Netolitzky F. 1932. Die Pflanzenhaare. Handbuch der Pflanzenanatomie. Berlin: Gebrüder Borntraeger. [Google Scholar]
- Noda K, Glover BJ, Linstead P, Martin C. 1994. Flower colour intensity depends on specialized cell shape controlled by a Myb-related transcription factor. Nature 369: 661–664. [DOI] [PubMed] [Google Scholar]
- Oelschlägel B, Gorb S, Wanke S, Neinhuis C. 2009. Structure and biomechanics of trapping flower trichomes and their role in the pollination biology of Aristolochia plants (Aristolochiaceae). The New Phytologist 184: 988–1002. [DOI] [PubMed] [Google Scholar]
- Ohashi Y, Oka A, Ruberti I, Morelli G, Aoyama T. 2002. Entopically additive expression of GLABRA2 alters the frequency and spacing of trichome initiation. The Plant Journal 21: 5036–5046. [DOI] [PubMed] [Google Scholar]
- Oppenheimer DG, Herman PL, Sivakumaran S, Esch J, Marks MD. 1991. A myb gene required for leaf trichome differentiation in Arabidopsis is expressed in stipules. Cell 67: 483–493. [DOI] [PubMed] [Google Scholar]
- Pabón-Mora N, Suárez-Baron H, Ambrose BA, González F. 2015. Flower development and perianth identity candidate genes in the basal angiosperm Aristolochia fimbriata (Piperales: Aristolochiaceae). Frontiers in Plant Science 6: 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Bo K, Cheng Z, Weng Y. 2015. The loss-of-function GLABROUS3 mutation in cucumber is due to LTR-retrotransposon insertion in a class IV HD-ZIP transcription factor gene CsGL3 that is epistatic over CsGL1. BMC Plant Biology 15: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanaik S, Patra B, Singh SK, Yuan L. 2014. An overview of the gene regulatory network controlling trichome development in the model plant, Arabidopsis. Frontiers in Plant Science 5: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne CT, Zhang F, Lloyd AM. 2000. GL3 encodes a bHLH protein that regulates trichome development in arabidopsis through interaction with GL1 and TTG1. Genetics 156: 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne T, Clement J, Arnold D, Lloyd A. 1999. Heterologous myb genes distinct from GL1 enhance trichome production when overexpressed in Nicotiana tabacum. Development (Cambridge, England) 126: 671–682. [DOI] [PubMed] [Google Scholar]
- Pérez-Rodríguez M, Jaffé FW, Butelli E, Glover BJ, Martin C. 2005. Development of three different cell types is associated with the activity of a specific MYB transcription factor in the ventral petal of Antirrhinum majus flowers. Development 132: 359–370. [DOI] [PubMed] [Google Scholar]
- Pesch M, Hülskamp M. 2004. Creating a two-dimensional pattern de novo during Arabidopsis trichome and root hair initiation. Current Opinion in Genetics & Development 14: 422–427. [DOI] [PubMed] [Google Scholar]
- Pesch M, Hülskamp M. 2009. One, two, three.models for trichome patterning in Arabidopsis? Current Opinion in Plant Biology 12: 587–592. [DOI] [PubMed] [Google Scholar]
- Peterson KM, Shyu C, Burr CA, et al. . 2013. Arabidopsis homeodomain-leucine zipper IV proteins promote stomatal development and ectopically induce stomata beyond the epidermis. Development (Cambridge, England) 140: 1924–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Płachno BJ, Stpiczńska M, Adamec L, Oliveira-Miranda VF, Światek P. 2018. Nectar trichome structure of aquatic bladderworts from the section Utricularia (Lentibulariaceae) with observation of flower visitor and pollinators. Protoplasma 255: 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Płachno BJ, Stpiczńska M, Światek P, et al. . 2019. Floral micromorphology and nectar composition of the early evolutionary lineage Utricularia (subgenus Polypompholyx, Lentibulariaceae). Protoplasma 256: 1531–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing L, Aoyama T. 2012. Pathways for epidermal cell differentiation via the homeobox gene GLABRA2: update on the roles of the classic regulator. Journal of Integrative Plant Biology 54: 729–737. [DOI] [PubMed] [Google Scholar]
- Rambaut A. 2014. FigTree: Tree Figure Drawing Tool, version 1.4.4. Institute of Evolutionary Biology, University of Edinburgh. http://tree.bio.ed.ac.uk/software/figtree/ (1 December 2019). [Google Scholar]
- Rerie WG, Feldmann KA, Marks MD. 1994. The GLABRA2 gene encodes a homeodomain protein required for normal trichome development in Arabidopsis. Genes and Development 8: 1388–1399. [DOI] [PubMed] [Google Scholar]
- Schellmann S, Schnittger A, Kirik V, et al. . 2002. TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. The EMBO Journal 21: 5036–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein J. 2003. Cell-fate specification in the epidermis: a common patterning mechanism in the root and shoot. Current Opinion in Plant Biology 6: 74–78. [DOI] [PubMed] [Google Scholar]
- Schnittger A, Folkers U, Schwab B, Jürgens G, Hülskamp M. 1999. Generation of a spacing pattern: the role of TRIPTYCHON in trichome patterning in Arabidopsis. The Plant Cell 11: 1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittger A, Hülskamp M. 2002. Trichome morphogenesis: a cell-cycle perspective. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 357: 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab B, Folkers U, Ilgenfritz H, Hülskamp M. 2000. Trichome morphogenesis in Arabidopsis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 355: 879–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serna L, Martin C. 2006. Trichome: different regulatory network lead to convergent structures. Trends in Plant Science 11: 1360–1385. [DOI] [PubMed] [Google Scholar]
- Simon M, Bruex A, Kainkaryam RM, et al. . 2013. Tissue-specific profiling reveals transcriptome alterations in Arabidopsis mutants lacking morphological phenotypes. The Plant Cell 25: 3175–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML Web servers. Systematic Biology 57: 758–771. [DOI] [PubMed] [Google Scholar]
- Stpiczyńska M, Kamińska M, Davies KL, Pansarin ER. 2018. Nectar-secreting and nectarless Epidendrum: structure of the inner floral spur. Frontiers in Plant Science 9: 840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Werber M, Weisshaar B. 2001. The R2R3-MYB gene family in Arabidopsis thaliana. Current Opinion in Plant Biology 4: 447–456. [DOI] [PubMed] [Google Scholar]
- Suárez-Baron H, Alzate JF, González F, Ambrose BA, Pabón-Mora N. 2019. Genetic mechanisms underlying perianth epidermal elaboration of Aristolochia ringens Vahl (Aristolochiaceae). Flora 253: 56–66. [Google Scholar]
- Szymanski DB, Lloyd AM, Marks MD. 2000. Progress in the molecular genetic analysis of trichome initiation and morphogenesis in Arabidopsis. Trends in Plant Science 5: 214–219. [DOI] [PubMed] [Google Scholar]
- Szymanski DB, Marks MD. 1998. GLABROUS1 overexpression and TRIPTYCHON alter the cell cycle and trichome cell fate in Arabidopsis. The Plant Cell 10: 2047–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Walford SA, Dennis ES, Llewellyn D. 2016. Trichomes control flower bud shape by linking together young petals. Nature Plants 2: 16093. [DOI] [PubMed] [Google Scholar]
- Tominaga R, Iwata M, Sano R, Inoue K, Okada K, Wada T. 2008. Arabidopsis CAPRICE-LIKE MYB 3 (CPL3) controls endoreduplication and flowering development in addition to trichome and root hair formation. Development (Cambridge, England) 135: 1335–1345. [DOI] [PubMed] [Google Scholar]
- Wada T, Kurata T, Tominaga R, et al. . 2002. Role of a positive regulator of root hair development, CAPRICE, in Arabidopsis root epidermal cell differentiation. Development (Cambridge, England) 129: 5409–5419. [DOI] [PubMed] [Google Scholar]
- Wada T, Tachibana T, Shimura Y, Okada K. 1997. Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science (New York, N.Y.) 277: 1113–1116. [DOI] [PubMed] [Google Scholar]
- Wagner GJ. 1991. Secreting glandular trichomes: more than just hairs. Plant Physiology 96: 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GJ, Wang E, Shepherd RW. 2004. New approaches for studying and exploiting an old protuberance, the plant trichome. Annals of Botany 93: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walford SA, Wu Y, Llewellyn DJ, Dennis ES. 2011. GhMYB25-like: a key factor in early cotton fibre development. The Plant Journal: for Cell and Molecular Biology 65: 785–797. [DOI] [PubMed] [Google Scholar]
- Walker AR, Davison PA, Bolognesi-Winfield AC, et al. . 1999. The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. The Plant Cell 11: 1337–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Hubbard L, Chang Y, Guo J, Schiefelbein J, Chen JG. 2008. Comprehensive analysis of single-repeat R3 MYB proteins in epidermal cell patterning and their transcriptional regulation in Arabidopsis. BMC Plant Biology 8: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Kwak SH, Zeng Q, et al. . 2007. TRICHOMELESS1 regulates trichome patterning by suppressing GLABRA1 in Arabidopsis. Development (Cambridge, England) 134: 3873–3882. [DOI] [PubMed] [Google Scholar]
- Wang YL, Nie JT, Chen HM, et al. . 2016. Identification and mapping of Tril, a homeodomain-leucine zipper gene involved in multicellular trichome initiation in Cucumis sativus. TAG. Theoretical and Applied Genetics. Theoretische und Angewandte Genetik 129: 305–316. [DOI] [PubMed] [Google Scholar]
- Werker E. 2000. Trichome diversity and development. Advances in Botanical Research 31: 1–35. [Google Scholar]
- Wu Y, Machado AC, White RG, Llewellyn DJ, Dennis ES. 2006. Expression profiling identifies genes expressed early during lint fibre initiation in cotton. Plant & Cell Physiology 47: 107–127. [DOI] [PubMed] [Google Scholar]
- Yang C, Gao Y, Gao S, et al. . 2015. Transcriptome profile analysis of cell proliferation molecular processes during multicellular trichome formation induced by tomato Wov gene in tobacco. BMC Genomics 16: 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Ye Z. 2013. Trichomes as models for studying plant cell differentiation. Cellular and Molecular Life Sciences: CMLS 70: 1937–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Cai Y, Liu X, et al. . 2018. A CsMYB6-CsTRY module regulates fruit trichome initiation in cucumber. Journal of Experimental Botany 69: 1887–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AM, Schaller M, Strand M. 1984. Floral nectaries and trichomes in relation to pollination in some species of Theobroma and Herrania (Sterculiaceae). American Journal of Botany 71: 466–480. [Google Scholar]
- Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd A. 2003. A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development (Cambridge, England) 130: 4859–4869. [DOI] [PubMed] [Google Scholar]
- Zhao JL, Pan JS, Guan Y, et al. . 2015. a. Transcriptome analysis in Cucumis sativus identifies genes involved in multicellular trichome development. Genomics 105: 296–303. [DOI] [PubMed] [Google Scholar]
- Zhao JL, Pan JS, Guan Y, et al. . 2015. b. Micro-trichome as a class I homeodomain-leucine zipper gene regulates multicellular trichome development in Cucumis sativus. Journal of Integrative Plant Biology 57: 925–935. [DOI] [PubMed] [Google Scholar]
- Zhao M, Morohashi K, Hatlestad G, Grotewold E, Lloyd A. 2008. The TTG1-bHLH-MYB complex controls trichome cell fate and patterning through direct targeting of regulatory loci. Development (Cambridge, England) 135: 1991–1999. [DOI] [PubMed] [Google Scholar]
- Zhou LH, Liu SB, Wang PF, et al. . 2016. The Arabidopsis trichome is an active mechanosensory switch. Plant, Cell & Environment 40: 611–621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.