Abstract
Background and Aims
Internal seed morphological traits such as embryo characteristics and nutritive tissue can vary considerably within a plant lineage. These traits play a prominent role in germination processes and the success of seedling establishment, and are therefore under high selective pressure, especially in environments hostile to seedlings, such as arid, saline or highly dynamic habitats. We investigated the relationships of seed internal morphology and germination characteristics of 84 species of Amaranthaceae s.l., a family with numerous lineages that have adapted to stressful growing conditions.
Methods
We used seed cross-sections to assess embryo type and the ratios of embryo to seed surface and radicle to cotyledon length. Furthermore, seed mass, mean time to germination, habitat preferences and further plant traits such as C3 or C4 photosynthesis and life form were compiled for each species. Data were analysed using phylogenetic comparative methods.
Key results
We found embryo type (λ = 1), log seed mass (λ = 0.86) and the ratio of embryo to seed size (λ = 0.78) to be evolutionarily stable, with an annular embryo as ancestral in the family. Linked to shifts to the three derived embryos types (spiral, horseshoe-shaped and curved) is an increase in the ratio of root to cotyledon length and a reduction of nutritive tissue. We observed stabilizing selection towards seeds with relatively large embryos with longer radicles and less nutritive tissue that are able to germinate faster, especially in lineages with C4 photosynthesis and/or salt tolerance.
Conclusions
We conclude that the evolutionary shift of nutrient storage from perisperm to embryo provides an ecological advantage in extreme environments, because it enables faster germination and seedling establishment. Furthermore, the evolutionary shift towards a higher ratio of root to cotyledon length especially in small-seeded Amaranthaceae growing in saline habitats can provide an ecological advantage for fast seedling establishment.
Keywords: Amaranthaceae, Chenopodiaceae, ecology of seed mass, embryo type, fast seed germination, perisperm, radicle, seedling establishment, salinity stress, drought stress
INTRODUCTION
The seed and seedling establishment stages are crucial in a plant’s life cycle as they determine where and when an individual plant will establish. Due to a strong selection pressure on germination timing (Donohue et al., 2005), various seed morphological and physiological traits are adapted to a plants’ life cycle, ecology and habitat preferences, while being sometimes restricted by phylogenetic constraints. Most research in seed functional ecology has focused on determining how germination and dormancy lead to optimization of germination timing (Baskin and Baskin, 2014) and on the ecology and evolution of seed mass (e.g. Moles et al., 2005; Vandelook et al., 2018), while less attention has been paid to the role of seed internal morphological traits that may have evolved to optimize chances for seedling establishment.
The Amaranthaceae are a peculiar plant family composed of the former Amaranthaceae s.s., which are mostly (sub-)tropical ruderals, and the Chenopodiaceae, which comprises many stress-tolerant species such as halophytes and desert species (Walker et al., 2018; Morales-Briones et al., 2020). The C4 photosynthesis mechanism has evolved multiple times in the Amaranthaceae and is an adaptation to dry and saline environmental conditions (Kadereit et al., 2012). Numerous case studies have shown that seeds of Amaranthaceae are also adapted to these conditions (e.g. Bhatt and Santo, 2016; Muñoz-Rodíguez et al., 2017). A study by Kadereit et al. (2017) revealed that germination speed co-evolved with the photosynthesis mechanism (C3 or C4) in Amaranthaceae, suggesting a strong link between germination speed and habitat conditions.
Flowers of Amaranthaceae mostly develop only a single, basal, campylotropous ovule per flower. The ovules are bitegmic and crassinucellar, with the micropyle formed by the inner integument (endostome). The fruits are usually nuts, achenes, capsules that open with a lid or by bursting irregularly, and rarely berries (Corner, 1976). The fruit wall might consist of the pericarp alone or of the persistent perianth plus the pericarp, and might be woody, spongy, membranous or fleshy. The seeds are usually relatively small (i.e. <5 mg) and kidney- or lentil-shaped. The testa can be thick to thin, black to light brown in colour and smooth to variously sculptured (Kühn, 1993; Townsend, 1993; Shepherd et al., 2005; Sukhorukov and Zhang, 2013). Most Amaranthaceae studied show only traces of endosperm at the tip of the radicle, the remainder having been digested by the developing embryo (e.g. Veselova and Timonin, 2009; Veselova et al., 2016). As substitute nutritive tissue the seeds often contain copious perisperm (Martin, 1946), but there are also a number of species with seeds that either lack nutritive tissue (e.g. many Salsoleae) or show copious endosperm (e.g. Salicornia, including Sarcocornia; Shepherd et al., 2005). The embryo orientation, shape and size differ considerably in Amaranthaceae. Members of Salsoleae, Caroxyloneae and Suaedoideae, for example, show long, spirally coiled embryos, which was the reason why these tribes were formerly considered as a natural group and grouped into one subfamily (Salsoloideae in Kühn et al., 1993). Other embryo shapes are straight, bent, horseshoe-shaped and annular. If nutritive tissue is present, the embryo always lies peripherally, with the outer edge of the embryo aligned against the inner surface of the bitegmic seed coat (e.g. Shepherd et al., 2005). Sometimes the embryo is chlorophyllous, and schizocotyly, a deep division of the cotyledons, has also been observed (Kühn et al., 1993).
Two review studies showed that amongst species capable of germinating within 1 d after imbibition, more than half of them were Amaranthaceae (Parsons, 2012; Parsons et al., 2014). Very fast germination is considered an adaptation to very short periods of suitable conditions for germination and seedling establishment, such as occurs after a rainfall event, and particularly in saline environments (Kadereit et al., 2017; Duncan et al., 2019). An extreme example of a rapid seedling establishment strategy can be found in mangrove species with viviparous seeds (Elmqvist and Cox, 1996), in which the seed germination stage after dispersal is completely absent. To realize such very rapid seedling establishment, it can be expected that seeds have evolved morphological adaptions. First of all, morphological features associated with a delay of germination, such as an underdeveloped embryo or a water-impermeable seed coat, must be absent. For Apiaceae it has been shown that embryo size relative to seed size is positively related to germination speed (Vandelook et al., 2012). Shepherd et al. (2005) also mentioned that a larger embryo, for example in Salicornia (including Sarcocornia), could facilitate rapid germination and establishment. Storage of nutrient reserves outside the embryo in nutritive tissue such as endosperm or perisperm, in the case of Amaranthaceae, means that a transfer of reserves from the nutritive tissue to the embryo has to occur after dispersal, which may slow down germination and establishment (Stebbins, 1974). The relationship between germination speed and seed mass is less equivocal (Milberg et al., 1996) as both negative (e.g. Wang et al., 2012) and positive (e.g. Parker et al., 2006) associations have been found, as well as no association at all (e.g. Kos and Poschlod, 2010; Kadereit et al., 2017).
Much more evident than the relationship between seed mass and germination speed is the ecological role of the quantity of food reserves in the seeds, often expressed as seed mass, in seedling establishment. Large seeds are thought to be particularly advantageous in shaded (Salisbury, 1974; Mazer, 1989) or drought-prone (Baker, 1972; but see Leishman et al., 2000) habitats. Both Salisbury (1942) and Baker (1972) argued that larger food reserves might allow seedlings to develop a larger root system, although it has been noted that the water-acquiring capacity of roots should be considered in relation to evaporation (Jurado and Westoby, 1992a). It has also been shown that in arid environments large-seeded species might be able to shift root/shoot allocation further in favour of roots than small-seeded species during seedling establishment (Lloret et al., 1999; but see Jurado and Westoby, 1992a). More generally, a more extensive rooting system providing access to moist soil horizons in dry environments has been shown to improve survival (Padilla and Pugnaire, 2007). As far as we know, all studies dealing with seedling establishment in dry environments have focused on seed mass or root/shoot allocation after germination, but it can be expected that adaptations of the seedling to their environment are already realized in the embryo prior to germination. We may expect, for example, that the root to cotyledon length ratio of the embryo inside the seed of Amaranthaceae is higher for species growing in water-limited dry or saline habitats. We may also expect that in favour of germination speed the embryo is relatively large in dry or saline habitat species and there is less nutritive tissue than in mesic or shady habitats.
Seed morphological traits are known to be valuable characteristics for species delimitation and taxonomical analyses (e.g. Shepherd et al., 2005; Sukhorukov and Zhang, 2013). This suggests that many seed morphological traits are evolutionarily stable traits and that there has been a certain inertia in their evolution. Also, seed functional traits, such as seed size and relative embryo size, consistently show a high phylogenetic signal (e.g. Moles et al., 2005; Vandelook et al., 2012). Since seed morphological traits are tightly linked to species ecology, as has been shown by the extensive literature on the ecology and evolution of seed size (Moles et al., 2005), it has become indispensable to take into account phylogenetic information in order to get a thorough understanding of the role of evolutionary history in current-day patterns of variation in seed ecological traits.
To study how seed morphological and physiological traits of Amaranthaceae are adapted to the environment, we applied comparative methods to analyse more specifically (1) the distribution and evolution of embryo types across Amaranthaceae, (2) how embryo and seed size are related to species ecology, habitat and climate characteristics, (3) the relationship between seed internal morphology and germination speed, and (4) whether the ratio of root to cotyledon length of mature ungerminated seeds is potentially adapted to the environment, taking into account seed size variation.
MATERIALS AND METHODS
A total of 84 species of Amaranthaceae were included in this study (Supplementary Data Table S1). Seed samples were obtained from the Millennium Seed Bank, Kew, UK. Representatives of all major clades of the Amaranthaceae s.l. subfamilies were included in the analyses and the species were representative of the different habitat types, climatic regions and ecological niches occupied by the different species in this family.
Microscopic sections
Preparation of seeds for sectioning was strongly dependent on their morphology and could include the (partial) removal of persistent perianth or pericarp, rehydration in a steam bath or thinning of a thick testa by gently rubbing the seeds on sandpaper. To inhibit activation of germination, the embryo was killed by immersing the seeds in methanol for 7 d. Two methods of fixation were employed. In the first method, seeds were rinsed twice with FAA solution (85:20:10:1 EtOH:formalin:acetic acid:aerosol; Ruzin, 1999; W. Stuppy, Royal Botanic Gardens, Kew, UK, pers. comm.) to remove methanol and transferred into FAA for 6 weeks. The solution was changed twice on the first day and once again after 8 d. In the second method, the seeds were transferred into Karnosky fixative (Ruzin, 1999) at 4 °C for 6 weeks. The fixative was changed once on the first or second day. After fixation with either method, the material was washed with cold buffer and transferred to an ascending ethanol series using 70, 80, 90 and 100 % ethanol. The first three steps were applied for 24 h and the last step for 6 d.
After dehydration, the seeds were embedded in either Technovit 7100 (Kulzer Technik, Germany) or LR White (Electron Microscopy Sciences, USA). For embedding with Technovit 7100, solution preparation and the general process followed the manufacturer’s protocol, with a few additional steps. Two steps of pre-infiltration were used with 1:3 and 1:1 ratios of Technovit 7100 liquid and 100 % ethanol. The durations were 1 h for the first and 15 min for the second step, both under vacuum. Subsequently, the infiltration solution was prepared and applied as described in the manufacturer’s protocol. The material was stored at 4 °C until embedding. For embedding, the seeds were individually transferred into moulds (8 × 8 × 6 mm) filled with polymerization mix (750 µL infiltration solution + 50 µL hardener II) and oriented as desired. Embedding was done at room temperature. The curing process was finished after ~24 h at 60 °C. For embedding with LR White, to slowly replace ethanol with LR White we used an ascending graded resin series, starting with a 1:3 mixture of LR White and absolute ethanol. The series was conducted in open glass vials under a 440-mmHg vacuum. For curing, the seeds were transferred into individual gelatine capsules (recommended by Luft, 1961), which were filled with resin and remained open to allow pressure equilibration under vacuum. Polymerization took place at 60 °C and a 440-mmHg vacuum for 19–23 h.
Sectioning, dying and documentation were the same for both types of embedding medium. Sections were cut at a thickness of 5–10 µm using a D-knife and a rotary microtome (Leitz, Germany). The cut surface was carefully covered with egg white glycerine for Technovit blocks or distilled water for LR White blocks between individual sections to increase quality and stability. The sections were stretched directly on the slide and dried at 40 °C for at least 1 h. Dried sections were dyed with a freshly made dye mix (1 mL Eosin Y/1 mL Azure II/0.77 mL methylene blue/1 mL aqua bidest) for 1 min, rinsed briefly with 70 % ethanol and immediately washed with distilled water several times to completely remove the ethanol. After drying, coverslips were applied using Eukitt (O. Kindler, Germany). Sections were imaged with a Diaplan microscope (Leitz, Germany) using a Leica DFC420 C digital camera (Leica, Germany) and the Leica Application Suite version 3.8.0 (build 878).
Plant functional traits and environmental data
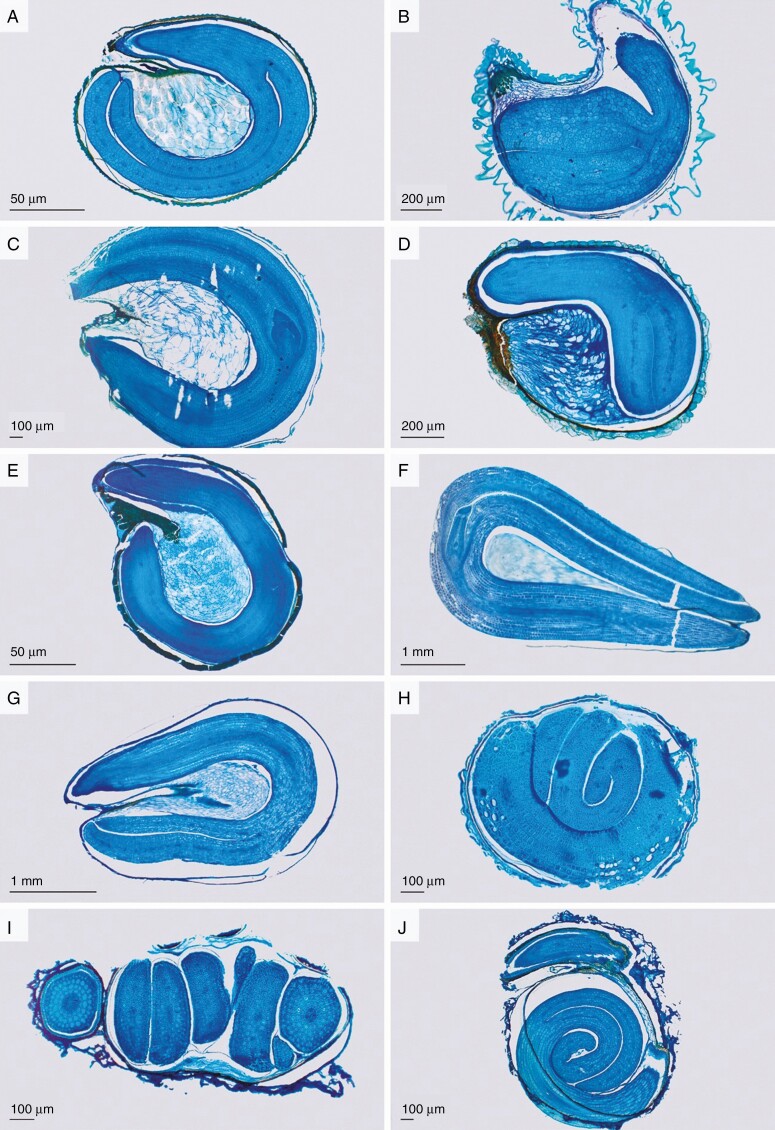
The surface areas of the perisperm and embryo were measured using ImageJ (version 1.47) and used to determine the embryo to seed size ratio as embryo area/(embryo + perisperm area). Four distinct types of embryo shape were discerned according to the variation found in all species studied: annular (Fig. 1A, C, E), curved (including folded and bent embryos; Fig. 1B, D), horseshoe-shaped (Fig. 1F, G) and spirally coiled embryos (Fig. 1H–J). The ratio of root to cotyledon length was determined quantitatively for 47 species. Hence, all analyses including the ratio of root to cotyledon length were performed on the subset of these 47 species.
Fig. 1.
Diversity of embryo shapes and ratio of embryo to nutritive tissue in Amaranthaceae. (A) Ptilotus helipteriodes: annular embryo and central perisperm. (B) Salicornia blackiana: bent embryo and little endosperm. (C) Bassia scoparia: annular embryo and central perisperm. (D) Arthroceras subterminale: curved embryo. (E) Atriplex halimus: annular embryo and central perisperm. (F) Ceratocarpus arenarius: horseshoe-shaped embryo, little central perisperm. (G) Krascheninnikovia lanata: horseshoe-shaped embryo, little central perisperm. (H) Salsola gaetula: spiral embryo, nutritive tissue lacking. (I, J) Suaeda corniculata: spiral embryo, nutritive tissue lacking.
Germination tests were generally performed with two replicates of ~50 intact seeds (Supplementary Data Table S1). When the number of seeds was limited, at least 20 seeds divided over two replicate dishes were tested. Viability was at least 79 % for all nine species with <40 seeds tested for germination. In some species, the number of seeds sown was increased to account for limited viability. Seeds were sown on 1 % agar in 9-cm Petri dishes and placed in temperature-controlled incubators (LMS Cooled Incubator A 280) at 20 and 25 °C, illuminated laterally by 30 W cool white light (photosynthetically active radiation = 36 µmol m−2 s−1) with a 12-h photoperiod. The number of germinated seeds was recorded daily during the first week and twice weekly thereafter until no further germination occurred over a 4-week period. Germinated seeds were removed after counting. Seeds that had not germinated were dissected and their viability was assessed. Empty or infested seeds or seeds without an embryo were excluded from the total number of seeds sown, as they would never have been able to germinate. Firm seeds with an intact embryo were considered dormant while soft or discoloured seeds were considered non-viable. Mean time to germination (MTG = Σniti/Σni, where ti is time from the start of the experiment and ni the number of germinated seeds at the ith time) and final germination percentage were calculated for both temperature conditions. Since seeds of different species were stored for variable periods of time (Supplementary Data Table S1), we cannot exclude the possibility that dormancy was broken during storage, which may have affected germination speed. No significant relation was observed between germination percentage at 20 °C and time of storage (R2 = 0.01; P = 0.14), between time of storage and seed viability (R2 = 0.04, P = 0.081) and between time of storage and germination speed (R2 = 0.01; P = 0.525). Similar results were obtained for seeds incubated at 25 °C. Storage effects were therefore considered to be of minor importance.
The seed mass of each species was determined by weighing at least 50 air-dried dispersal units (referred to as seeds). Data on maximum plant height, C3 or C4 photosynthesis, saline habitat (yes, no), ruderal (yes, no) and adult longevity (annual, short-lived perennial, long-lived perennial) were gathered from the literature and local floras (main sources: Tutin et al., 1964; Kadereit et al., 2003; Sage et al., 2007; efloras, 2008). The geographical coordinates of the sampling sites were used to obtain data on altitude and 19 climate variables through WorldClim version 1.3 (Hijmans et al., 2005), accessed through DIVA-GIS version 7.1.7 (Hijmans et al., 2001). These 19 climate variables were reduced to two non-correlated climate variables (MDS1 and MDS2) using non-metric multidimensional scaling (NMDS) in the vegan package (Oksanen et al., 2015) in R version 3.6.3 (R Development Core Team, 2020). Low values for MDS1 coincide with hot and dry climate conditions, as opposed to the cold and wet conditions associated with high values for MDS1. Low values for MDS2 are associated with little variation in temperature throughout the year and high minimum temperatures, i.e. tropical climates as opposed to the high values for MDS2 that are associated with temperate climates (Kadereit et al., 2017).
Statistical analyses
The phylogenetic tree used in this study was derived from that published by Kadereit et al. (2017). Species that were present in the original phylogenetic tree but not in this study were removed from the phylogenetic tree using the drop.tip function in the APE package (Paradis, 2006) in R.
An ancestral state reconstruction of the embryo type was made based on a maximum likelihood estimation (Pagel, 1994), using the ace function in the APE package (Paradis, 2006) in R. Phylogenetic signal λ was calculated for all variables included in the analyses (Pagel, 1999). The λ statistic proposed by Pagel (1997, 1999) measures how well a Brownian motion (BM) model fits the data, i.e. measures the phylogenetic signal (Lynch, 1991). When λ equals zero, related taxa are not more similar than expected by chance and the trait is evolving as a star-like phylogeny (Pagel, 1999). In such a scenario, phylogenetic correction becomes meaningless. Significant phylogenetic signal or clumping of trait states on the phylogenetic tree occurs when λ is significantly larger than zero, meaning taxa are more similar than expected by chance. When λ = 1, the trait is evolving following a BM model. If 1 > λ > 0, traits are less similar among species than expected from their phylogenetic relationships, but more similar than expected by chance. Phylogenetic signal was calculated using the Geiger package (Harmon et al., 2009).
We tested the adaptive nature of the embryo to seed size ratio using several models reflecting hypothetical selective regimes on the relative embryo size. The simplest model is a BM model of trait evolution, which assumes that embryo length evolved following a pure drift process. The second model, an Ornstein–Uhlenbeck (OU) model, is a simple linear model that allows the quantification of the effects of both natural selection and inertia (Hansen, 1997, 2008; Butler and King, 2004). In its simplest form, the model assumes that the relative embryo size evolves towards a single hypothetical optimum θ. The model also includes a parameter measuring the rate of adaptation towards the optimum and a stochasticity component (r), which is a measure of the intensity of the random fluctuations in the evolutionary process. We also calculated a more intuitive measure of the phylogenetic signal in this OU model: phylogenetic half-life, t1⁄2 = ln(2)/α. This half-life indicates how long it takes before adaptation to a new selective regime is expected to be more influential than the constraints from the ancestral state (Hansen, 1997). As the evolutionary optimum is expected to differ according to habitat conditions and other discrete plant characteristics, we further refined this model so that it included different evolutionary optima based on different hypotheses regarding evolutionary adaptation, such as whether or not species grow in saline habitats (yes, no), they have a ruderal ecology (yes, no), C3 or C4 photosynthesis, embryo type (annular, curved, horseshoe-shaped, spirally coiled) and adult longevity (annual, short-lived perennial, long-lived perennial). Models of evolutionary adaptation to habitat conditions and evolution of longevity were based on a maximum likelihood analysis of the trait states of each of these variables performed using the ace function in the APE package. We also analysed whether the embryo to seed size ratio had evolved in relation to other continuous traits (seed mass and plant height) and climate variables (MDS1, hot/dry versus cold/wet; and MDS2, tropical versus temperate), which were assumed to have changed along the phylogeny according to a BM model of evolution (Hansen et al., 2008). Multiple regression models were tested, including both categorical and continuous variables, but only those are mentioned with corrected Akaike information criterion (AICc) values lower than the univariate regression models (Akaike, 1973). All analyses were performed using the SLOUCH package (Köpperud et al., 2019) in R. The performance of the models was tested by means of AICc. Percentile confidence intervals for the model parameters were computed using a parametric bootstrap with 10 000 replicates.
The relationship between germination speed and the ratios of embryo to seed size and root to cotyledon length was analysed using ordinary least squares regression, since phylogenetically informed models performed consistently worse than non-phylogenetic models based on the AICc (Supplementary Data Tables S2 and S3). For the same reason, ordinary least squares regression was used to analyse the relationship between the ratio of root to cotyledon length and plant functional traits, habitat and climate. Post hoc Tukey tests were performed to test for significant differences between groups. Seed mass, plant height and the ratio of root to cotyledon length were log10-transformed prior to analyses to meet assumptions of normality and equality of variance.
RESULTS
Phylogenetic signal of seed traits
Highly significant phylogenetic signals were found for seed morphological traits such as embryo type (λ = 1; P < 0.001), log seed mass (λ = 0.86; P < 0.001) and embryo to seed size ratio (λ = 0.78; P < 0.001), while no significant phylogenetic signal was observed for seed physiological characteristics such as germination percentage and log MTG at 20 and 25 °C (λ < 0.12; P > 0.30 for all traits) and for one morphological trait, root to cotyledon length ratio (λ = 0.11; P = 0.39).
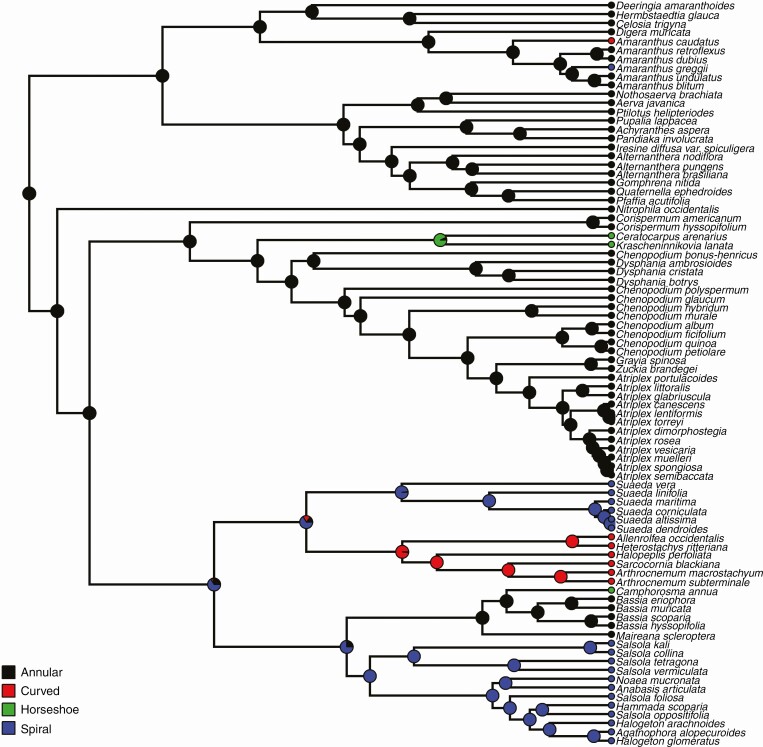
The high phylogenetic signal for embryo type is reflected in the distribution of embryo types across the phylogenetic tree (Fig. 2). The annular embryo type was by far the most commonly observed type and most likely is the ancestral character state in Amaranthaceae. The spirally coiled embryo type was observed in all members of the Suaedeae, Salsoleae and Caroxyloneae subfamilies and in one representative of the Amaranthoideae, Amaranthus greggii. All members of the Salicornieae had a curved embryo type, as well as two Amaranthoideae, Pandiaka involucrata and Amaranthus caudatus. The horseshoe type of embryo was much less common and was only found in Camphorosma annua, Krascheninnikovia lanata and Ceratocarpus arenarius.
Fig. 2.
Ancestral state reconstruction and contemporary states of embryo type across the Amaranthaceae studied.
Evolution of embryo to seed size ratio and seed mass
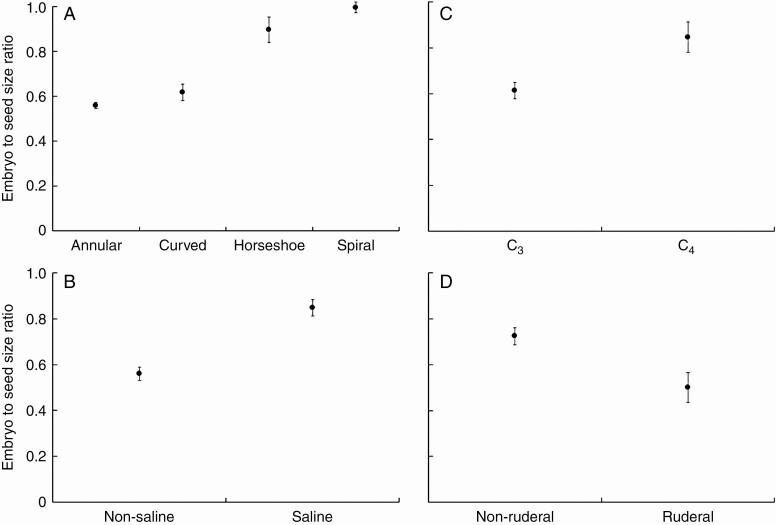
Evolution of embryo to seed size ratio was very closely related to embryo type (Table 1 and Supplementary Data Table S4). Very little or no perisperm at all was observed in seeds with horseshoe-shaped and spiral embryos, while species with annular and curved embryos had an evolutionarily optimal embryo to seed size ratio of ~0.6 (Fig. 3A). The OU models with embryo to seed size ratio evolving in association with habitat salinity, photosynthesis type and ruderality performed much better compared with models including other habitats or plant traits (Table 1). The evolutionarily optimal embryo to seed size ratio was considerably higher in species growing in saline habitats (Fig. 3B), species with a C4 photosynthesis mechanism (Fig. 3C) and non-ruderal species (Fig. 3D).
Table 1.
Fit of OU regression models of embryo to seed size ratio in relation to plant functional traits and habitat and climate characteristics
| AICc | R 2 | |
|---|---|---|
| Embryo type (annular, curved, horseshoe, spiral) | −139.0 | 0.7690 |
| Saline (no, yes) | −83.0 | 0.3100 |
| Photosynthesis type (C3, C4) | −67.9 | 0.0984 |
| Ruderal (no, yes) | −67.6 | 0.0925 |
| Adult longevity (annual, long-lived perennial, short-lived perennial) | −62.0 | 0.0469 |
| Log height | −63.9 | 0.0335 |
| MDS1 (hot/dry versus cold/wet) | −62.8 | 0.0261 |
| Log mass | −61.0 | 0.0025 |
| MDS2 (tropical versus temperate) | −60.7 | 0.0001 |
| Saline + photosynthesis type (no C3, yes C3, no C4, yes C4) | −88.3 | 0.4220 |
Fig. 3.
Evolutionarily optimal ratio of embryo to seed size as a function of (A) embryo type, (B) habitat salinity, (C) photosynthesis mechanism and (D) (non-)ruderal ecology. Error bars represent ±1 s.e.
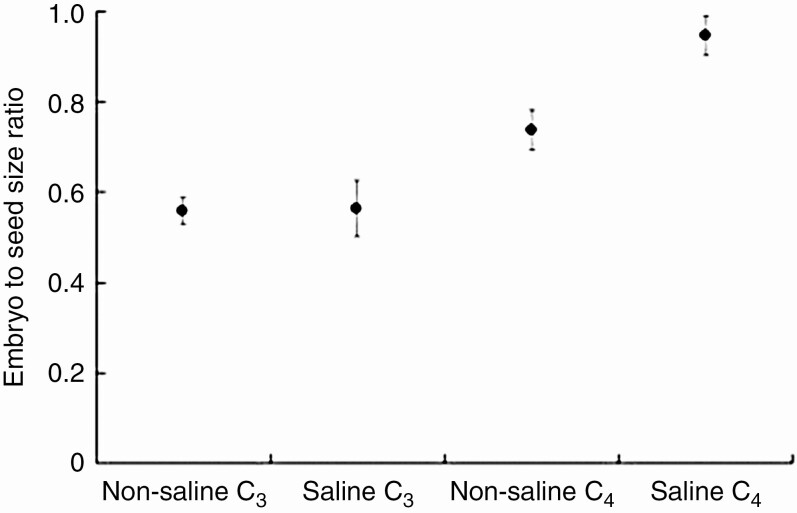
An OU model of embryo to seed size ratio evolution as a function of both habitat salinity and photosynthesis type was the best-performing model after the model including embryo type (Table 1). The embryo to seed size ratio of C4 species growing in a saline habitat was significantly higher (0.94 ± 0.04; mean ± s.e.) than that of non-saline C4 and all C3 species (Fig. 4).
Fig. 4.
Evolutionarily optimal ratio of embryo to seed size as a function of habitat salinity preference and photosynthesis mechanism. Error bars represent ± 1 s.e.
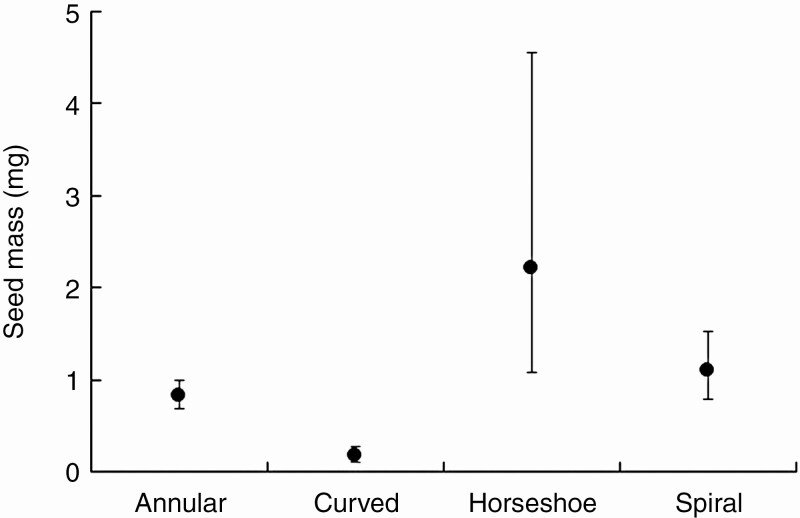
Seed mass evolution was only related to embryo type, as OU models including other variables to explain seed mass evolution performed less well (Table 2 and Supplementary Data Table S5). Seeds with curved embryos typically had a lower seed mass compared with species with other embryo types, while species with horseshoe-shaped embryos possessed the heaviest seeds, albeit with large variation among species (Fig. 5).
Table 2.
Fit of OU regression models of seed mass in relation to plant functional traits and habitat and climate characteristics
| AICc | R 2 | |
|---|---|---|
| Embryo type (annular, curved, horseshoe, spiral) | 118.0 | 0.1290 |
| Adult longevity (annual, long-lived perennial, short-lived perennial) | 122.0 | 0.0434 |
| MDS2 (tropical versus temperate) | 122.0 | 0.0227 |
| Ruderal (no, yes) | 122.0 | 0.0155 |
| Photosynthesis type (C3, C4) | 123.0 | 0.0071 |
| Log height | 123.0 | 0.0024 |
| MDS1 (hot/dry versus cold/wet) | 124.0 | 0.0003 |
| Saline (no, yes) | 124.0 | 0.0001 |
Fig. 5.
Evolutionarily optimal seed mass as a function of embryo type. Error bars represent ± 1 s.e.
Relationship between germination speed and embryo to seed size ratio
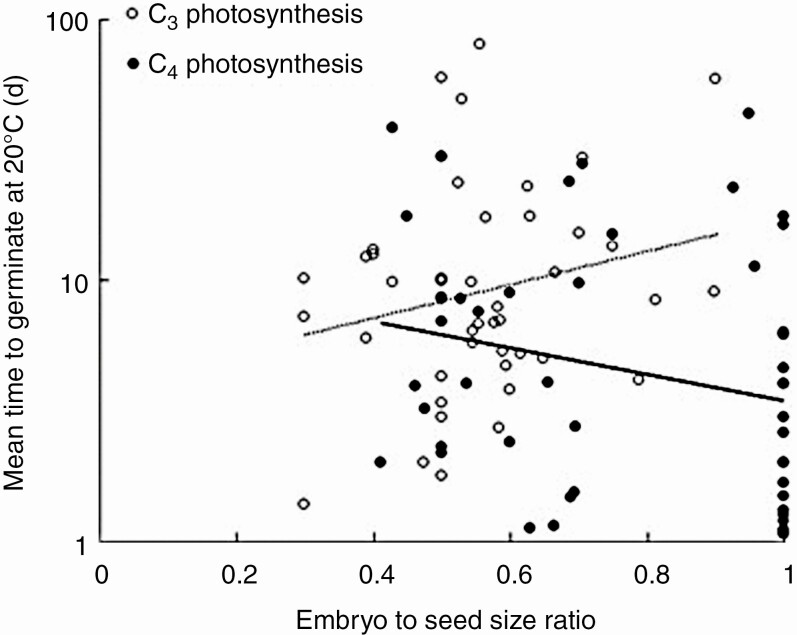
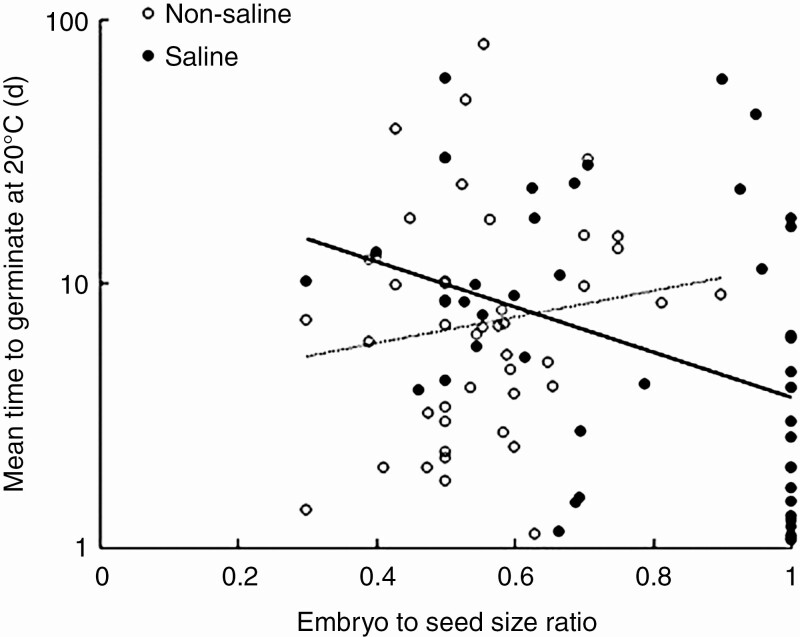
A significant negative relationship was observed between embryo to seed size ratio and MTG at 20 °C (F1,82 = 7.411; P = 0.008), as well as MTG at 25 °C (F1,82 = 5.876; P = 0.018). Significant interaction terms for germination speed at 20 °C were observed between embryo to seed size ratio and saline habitat (F1,82 = 6.256; P = 0.014) and embryo to seed size ratio and photosynthesis type (F1,80 = 7.910; P = 0.006). Models including these interaction terms also performed better based on the AICc (Supplementary Data Table S2). Seeds from C4 plants, adapted to growing in hot and dry environments, germinated faster with decreasing amounts of perisperm, while a weak opposite pattern was observed for C3 plants (Fig. 6). Similarly, for plants growing in saline habitats, seeds germinated faster when the proportion of the embryo was higher, while the opposite relationship was observed in plants from non-saline habitats (Fig. 7). Seeds with spiral embryos germinated significantly faster compared with seeds with annular embryos both at 20 °C (post hoc Tukey test, P = 0.009) and at 25 °C (post hoc Tukey test, P = 0.008). No significant relationship was observed between root to cotyledon length ratio and MTG at 20 °C (F1,45 = 0.107, P = 0.745) or at 25 °C (F1,45 = 0.067, P = 0.797).
Fig. 6.
Relationship between mean time to germinate and embryo to seed size ratio was significantly different for C4 and C3 plants (P = 0.01).
Fig. 7.
Relationship between mean time to germinate and embryo to seed size ratio was significantly different for plants from saline and non-saline habitats (P = 0.03).
Functional ecology of root to cotyledon length ratio
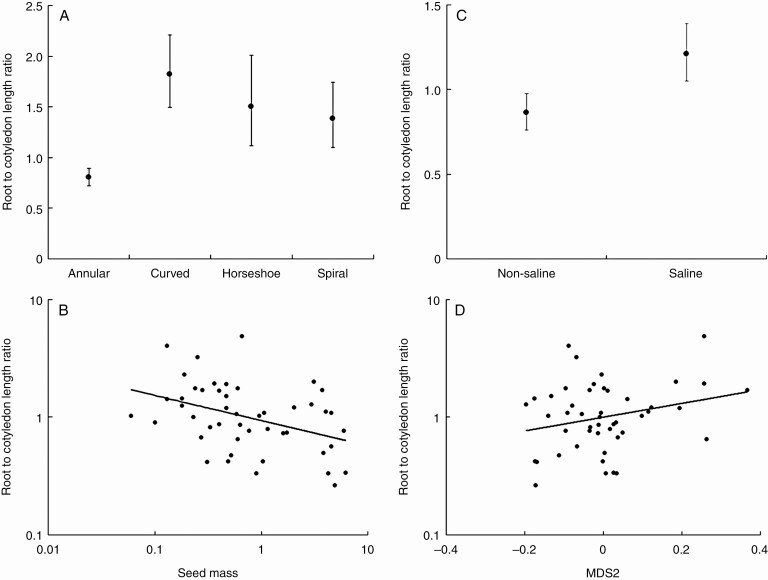
Variation in root to cotyledon length ratio was best explained by embryo type, species with annular embryos having a significantly (post hoc Tukey test, P < 0.05) lower root to cotyledon length ratio compared with other embryo types (Fig. 8A). The root to cotyledon length ratio also significantly decreased with increasing seed mass (R2 = 0.158, F1,45 = 3.185, P = 0.006; Fig. 8B). There was a marginally significant (F1,45 = 3.223, P = 0.079) increase in root to cotyledon ratio in saline species compared with species growing in non-saline habitats (Fig. 8C). However, a significant effect of habitat salinity was observed when seed mass was included as a covariable (Table 3). No significant interaction between habitat salinity and seed mass was recorded. A positive but marginally significant (F1,45 = 3.185, P = 0.081) relationship was observed between root to cotyledon length ratio and MDS2 (i.e. higher root to cotyledon length ratio in temperate climates; Fig. 8D), which was also significant when seed mass was included in the multiple regression model (Table 4). In both cases, models including seed mass also had lower AICc values (salinity AICc 17.6 versus salinity + log mass AICc 10.5; MDS2 AICc 17.6 versus MDS2 + log mass AICc 10.4). No significant relationship was observed with other variables tested.
Fig. 8.
Mean ± s.e. ratio of root to cotyledon length as a function of (A) embryo type, (B) seed mass, (C) habitat salinity preference and (D) MDS2 (tropical versus temperate).
Table 3.
Covariance analysis with log-transformed root to cotyledon length ratio as the dependent variable, habitat salinity as the predictor variable and log mass as a covariable
| d.f. | SS | MS | F | P-value | |
|---|---|---|---|---|---|
| Intercept | 1 | 0.018 | 0.018 | 0.281 | 0.599 |
| Log (mass) | 1 | 0.611 | 0.611 | 9.354 | 0.004 |
| Saline (no, yes) | 1 | 0.269 | 0.269 | 4.119 | 0.048 |
| Error | 44 | 2.872 | 0.065 | ||
| Total | 46 | 3.732 |
SS, sum of squares; MS, mean square.
Table 4.
Multiple regression model with log-transformed root to cotyledon ratio as the dependent variable and log mass and MDS2 as predictor variables
| Coefficient | s.e. | t | P-value | |
|---|---|---|---|---|
| Intercept | −0.03 | 0.039 | −0.775 | 0.442 |
| Log (mass) | −0.219 | 0.071 | −3.082 | 0.004 |
| MDS2 (tropical versus temperate) | 0.617 | 0.301 | 2.052 | 0.046 |
DISCUSSION
The annular embryo type was by far the most commonly observed type and most likely it is the ancestral character state in Amaranthaceae, as was suggested in previous studies (Kühn, 1993; Pratt, 2003). The ancestry of annular embryos might even go back to deeper nodes within the lineage of Caryophyllales to which Amaranthaceae belong. The species-poor sister family of Amaranthaceae, Achatocarpaceae, exhibits peripheral, annular embryos around a mealy perisperm (Heimerl, 1934), and the diverse Caryophyllaceae, which are sister to Amaranthaceae plus Achatocarpaceae, also have predominantly curved embryos peripherally surrounding the perisperm (Martin, 1946). Interestingly, some Caryophyllaceae also have straight or spiral embryos and therefore seem to show diversity of embryo types similar to that in Amaranthaceae (Bittrich, 1993). Connected with a deviation from the ancestral embryo type in Amaranthaceae is a shift towards higher root to cotyledon length ratio in all derived embryo types. While the cotyledons are slightly to distinctly longer than the radicle in annular embryos, the opposite occurs in curved, horseshoe-shaped and spiral embryos (Figs 1 and 8A). Furthermore, a reduction of nutritive tissue is found in derived embryo types, most prominently in species with spiral embryos (Figs 1 and 3A). The spirally coiled embryo type probably evolved three times, once in the ancestor of the Suaedeae plus Bienertieae, once in the ancestor of Salsoleae plus Caroxyloneae, and in one representative of the Amaranthaceae s.s., A. greggii. In contrast to Salsoleae and Caroxyloneae, in which the embryo is mostly conical-spiral (i.e. showing a cone-like 3-D outline when rolled up), Bienertieae and Suaedeae show a plano-spiral embryo (Fig. 1I, J; Kühn et al., 1993). All members of the Salicornieae had a curved embryo type, as well as two Amaranthoideae, P. involucrata and A. caudatus. The horseshoe type of embryo was much less common and was only found in C. annua, K. lanata and C. arenarius.
Although seed mass is widely regarded as one of the most important plant functional traits (Diaz et al., 2016), its ecological relevance seems much lower in Amaranthaceae and deviates from that of other angiosperms. Typical seed mass relations, such as increasing seed mass in larger plants or smaller seeds in annual species (Moles et al., 2005), are absent in Amaranthaceae. Also, relations between seed mass and habitat, such as larger seeds in saline habitat species, as observed for the Central European flora (Vandelook et al., 2018), were absent. The absence of such correlations may partly be explained by the fact that in the Amaranthaceae there are no shade-tolerant species, which typically widen the seed mass spectrum (Salisbury, 1974). Also, many Amaranthaceae grow in rather stressful open environments, where establishment under drought stress, rather than competing for light, is important (Ungar, 1991; Kadereit et al., 2017). Evolution of seed mass in Amaranthaceae was related to embryo type, as the evolutionarily optimal seed mass was much lower in Betoideae, Chenopodioideae (including Corispermoideae), Polycnemoideae and Amaranthoideae, all clades with predominantly annular embryos. Both embryo type and seed mass show high phylogenetic signal, suggesting that an early transition to smaller seeds with larger embryos, most notably in Salicornioideae (including former Suaedoideae and Salsoloideae; Morales-Briones et al., 2020), may be an adaptation to the extremely stressful habitats of these lineages that was independently realized and retained in Salicornieae, Salsoleae/Caroxyloneae and Suaedeae. The transition to smaller seeds with larger embryos was likely initially triggered by the onset of drier climatic conditions during the Early Oligocene, when these three lineages started to diversify (Piirainen et al., 2017; Morales-Briones et al., 2020).
The two other plant traits we measured, embryo to seed size ratio and root to cotyledon length ratio, seem to be more evolutionarily labile as they change quickly among closely related lineages. The traits are probably highly ecologically relevant and might shift rather quickly under selective pressure. Within the Amaranthaceae, evolution towards spirally coiled embryos was associated with a loss of perisperm and storage of nutrient reserves inside the embryo, hence an increased embryo to seed size ratio. Reduction of perisperm has also been observed in species with horseshoe-shaped embryos, e.g. C. annua and K. lanata, or bent embryos, e.g. Salicornia and Sarcocornia species (Shepherd et al., 2005). Our results showed that stabilizing selection towards relatively large embryos has occurred in Amaranthaceae growing in saline environments and in plants with C4 photosynthesis. We argue that storage of nutrients in the embryo provides an ecological advantage, because it enables faster germination in extreme environments, such as saline habitats and deserts, where rapid germination and establishment are crucial after short rainfall events (Gutterman, 2012; Gul et al., 2013; Liu et al., 2013). Positive associations between relative embryo size and germination speed were recorded for Apiaceae (Vandelook et al., 2012) and Mediterranean plants (Vivrette, 1995), but not when analysed across all angiosperms (Verdú, 2006). The positive relationship between germination speed and relative embryo size is also confirmed by our data, as seeds of Amaranthaceae with larger embryos in general, and spiral embryos in particular, germinated faster. Embryo morphology itself may also facilitate faster germination, since one can easily imagine that the puncture force of a spiral embryo squeezed inside the seed may increase rapidly upon imbibition. Moreover, we observed that the relationship between relative embryo size and germination speed was habitat-dependent. Faster germination in seeds with relatively large embryos was only evident in species with C4 photosynthesis or in species growing in saline habitats, suggesting that selection for faster germination and an adapted seed morphology was stronger in dry, saline or hot habitats. Seeds of many saline habitat species germinate best in fresh water (Khan and Ungar, 1984), while both germination and early root elongation are slowed by increased salt concentrations (Katembe et al., 1998). Such seed morphological characteristics adapted to very dry and saline environments are not consistently found in halophytes from other plant families. Plumbaginaceae and Zygophyllaceae, which represent quite a few halophytes, for example usually have well-developed straight or curved embryos (Corner, 1976). In Brassicaceae, however, some species are known to germinate very fast as well (Parsons, 2012), while spirally coiled embryos are known to occur in some ephemeral species (Iltis et al., 2011). Nonetheless, more studies are required to assess seed morphology and ecology relationships in other plant families in general and in halophytes specifically. A strong selection pressure thus exists on very rapid germination and seedling establishment during the short period with sufficiently low soil osmotic potential in saline habitats or water availability in desert habitats (Ungar, 1978; Jurado and Westoby, 1992b; Duncan et al., 2019). It should be noted that several species had very low germination percentages (Supplementary Data Table S1), which indicates that germination speed in these species is only represented by a few non-dormant seeds. Further studies are required to determine whether germination speed after a dormancy-breaking treatment is the same as that for seeds without primary dormancy. Hydrothermal time models accounting for dormancy break (Meyer et al., 2000; Arène et al., 2017), although very laborious, may be a good way forward to obtain a more accurate assessment of germination speed.
The absence of phylogenetic signal in root to cotyledon length ratio implies that the role of phylogenetic descent is less important in explaining patterns of variation in root to cotyledon length ratio. Also, the absence of a relationship between the root to cotyledon length ratio and mean time to germination confirms that this ratio is not important during the germination process, but only during early seedling establishment, as we will discuss now. Taschereau (1972) showed that in Atriplex prostrata the first 6 mm (± 8 mm) of root elongation after germination was achieved by cell expansion through imbibition. Therefore, high soil salinity will also affect early root elongation, although other species, like Halocnemum strobilaceum, can cope very well with high salinity levels during germination and early seedling establishment, which can be viewed as an alternative strategy to salt avoidance through rapid elongation of the root (Qu et al., 2008). Also, Mediterranean species with deep-rooting seedlings, such as Salsola, have been shown to survive drought better compared with species with shallow roots (Padilla and Pugnaire, 2007). We therefore expected a higher root to cotyledon length ratio in species occupying saline or dry environments, which was, to a certain extent, confirmed by our analyses. We did find that Amaranthaceae growing in saline habitats have a higher root to cotyledon length ratio, albeit only when seed mass was taken into account. The root to cotyledon length ratio was higher in smaller seeds, which may indicate that it is more important for small seeds to have an embryo with relatively large roots. The negative relationship between seed mass and root to cotyledon length ratio was much more pronounced for saline habitat species, although no significant interaction term was recorded (results not shown). It has been shown that in arid environments large-seeded species might be able to shift root/shoot allocation more in favour of roots than small-seeded species can during seedling establishment (Lloret et al., 1999; but see Jurado and Westoby, 1992a).
The Amaranthaceae is quite a unique plant family, because many species have evolved adaptations to grow in stressful environments such as those characterized by high soil salinity and/or extreme drought. Besides the well-known adaptations, such as succulence and a C4 photosynthesis mechanism, it is clear from this and from previous studies (e.g. Parsons, 2012; Kadereit et al., 2017) that the seeds have also evolved morphological and physiological adaptations to ensure successful establishment in these stressful environments. To our knowledge, this is the first study to examine the functional significance of cotyledon relative to radicle size, a characteristic that was, at least for Amaranthaceae, more relevant than seed mass in explaining the potential importance in seedling establishment.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: species of Amaranthaceae s.l. sampled and information. Table S2: AICc values for regression models of mean time to germinate at 20 and 25 °C in relation to embryo to seed size ratio in interaction with plant functional traits and habitat and climate characteristics. Table S3: AICc values for regression models of root to cotyledon length as a function of plant functional traits and habitat and climate characteristics. Table S4: OU model parameters for embryo to seed size ratio. Table S5: OU model parameters for seed mass.
ACKNOWLEDGEMENTS
We thank Beatrice Dewenter, Lara Hennig and Ursula Martiné (Mainz) for their help with generating seed sections, and Ann Van de Vyver and Sarah Le Pajolec (Meise Botanic Garden) for help with the germination tests. Special thanks to Dr Robert Parsons (La Trobe University) for providing valuable input at the early stages of the study. We thank the Kunming Institute of Botany, Bulgarian Academy of Sciences and National Botanical Garden of Georgia for permission to use seed collections.
FUNDING
This work was supported by the German Science Foundation (DFG) (grant KA1816/8-1) to G.K. and F.V. The Royal Botanic Gardens, Kew, is partially funded by a grant-in-aid from Defra.
LITERATURE CITED
- Akaike H. 1973. Maximum likelihood identification of Gaussian autoregressive moving average models. Biometrika 60: 255–265. [Google Scholar]
- Arène F, Affre L, Doxa A, Saatkamp A. 2017. Temperature but not moisture response of germination shows phylogenetic constraints while both interact with seed mass and lifespan. Seed Science Research 27: 110–120. [Google Scholar]
- Baker HG. 1972. Seed weight in relation to environmental conditions in California. Ecology 53: 997–1010. [Google Scholar]
- Baskin CC, Baskin JM. 2014. Seeds: ecology, biogeography, and evolution of dormancy and germination, 2nd edn. Amsterdam: Elsevier. [Google Scholar]
- Bhatt A, Santo A. 2016. Germination and recovery of heteromorphic seeds of Atriplex canescens (Amaranthaceae) under increasing salinity. Plant Ecology 217: 1069–1079. [Google Scholar]
- Bittrich V. 1993. Caryophyllaceae. In: Kubitzki K, Rohwer JG, Bittrich V, eds. The families and genera of vascular plants, Vol. II. Heidelberg: Springer, 206–236. [Google Scholar]
- Butler MA, King AA. 2004. Phylogenetic comparative analysis: a modeling approach for adaptive evolution. American Naturalist 164: 683–695. [DOI] [PubMed] [Google Scholar]
- Corner EJH. 1976. The seeds of dicotyledons, Vol. 1. Cambridge: Cambridge University Press. [Google Scholar]
- Díaz S, Kattge J, Cornelissen JH, et al. . 2016. The global spectrum of plant form and function. Nature 529: 167–171. [DOI] [PubMed] [Google Scholar]
- Donohue K, Dorn L, Griffith C, et al. . 2005. The evolutionary ecology of seed germination of Arabidopsis thaliana: variable natural selection on germination timing. Evolution 59: 758–770. [PubMed] [Google Scholar]
- Duncan C, Schultz N, Lewandrowski W, Good MK, Cook S. 2019. Lower dormancy with rapid germination is an important strategy for seeds in an arid zone with unpredictable rainfall. PLoS ONE 14: e0218421. eFloras. http://www.efloras.org (August 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist T, Cox P. 1996. The evolution of vivipary in flowering plants. Oikos 77: 3–9. [Google Scholar]
- Gul B, Ansari R, Flowers TJ, Khan MA. 2013. Germination strategies of halophyte seeds under salinity. Environmental and Experimental Botany 92: 4–18. [Google Scholar]
- Gutterman Y. 2012. Survival strategies of annual desert plants. Berlin: Springer Science and Business Media. [Google Scholar]
- Hansen TF. 1997. Stabilizing selection and the comparative analysis of adaptation. Evolution 51: 1341–1351. [DOI] [PubMed] [Google Scholar]
- Hansen TF, Pienaar J, Orzack SH. 2008. A comparative method for studying adaptation to a randomly evolving environment. Evolution 62: 1965–1977. [DOI] [PubMed] [Google Scholar]
- Harmon L, Weir J, Brock C, Glor R, Challenger W, Gene H. 2009. Analysis of evolutionary diversification. R package version 1.3-1. https://cran.r-project.org/web/packages/geiger/index.html 1 June 2020.
- Heimerl A. 1934. Achatocarpaceae. In: Engler A, Prantl K, eds. Die Natürlichen Pflanzenfamilien, 2nd edn. Leipzig: Engelmann, 174–178. [Google Scholar]
- Hijmans RJ, Guarino L, Cruz M, Rojas E. 2001. Computer tools for spatial analysis of plant genetic resources data: 1. DIVA-GIS. Plant Genetic Resources Newsletter 127: 15–19. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high-resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- Iltis HH, Hall JC, Cochrane TS, Sytsma KJ. 2011. Studies in the Cleomaceae I. On the separate recognition of Capparaceae, Cleomaceae, and Brassicaceae. Annals of the Missouri Botanical Garden 98: 28–36. [Google Scholar]
- Jurado E, Westoby M. 1992a. Seedling growth in relation to seed size among species of arid Australia. Journal of Ecology 80: 407–416. [Google Scholar]
- Jurado E, Westoby M. 1992b. Germination biology of selected central Australian plants. Australian Journal of Ecology 17: 341–348. [Google Scholar]
- Kadereit G, Borsch T, Weising K, Freitag H. 2003. Phylogeny of Amaranthaceae and Chenopodiaceae and the evolution of C4 photosynthesis. International Journal of Plant Sciences 164: 959–986. [Google Scholar]
- Kadereit G, Ackerly D, Pirie MD. 2012. A broader model for C4 photosynthesis evolution in plants inferred from the goosefoot family (Chenopodiaceae s.s.). Proceedings of the Royal Society B: Biological Sciences 279: 3304–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadereit G, Newton RJ, Vandelook F. 2017. Evolutionary ecology of fast seed germination—a case study in Amaranthaceae/Chenopodiaceae. Perspectives in Plant Ecology, Evolution and Systematics 29: 1–11. [Google Scholar]
- Katembe WJ, Ungar IA, Mitchell JP. 1998. Effect of salinity on germination and seedling growth of two Atriplex species (Chenopodiaceae). Annals of Botany 82: 167–175. [Google Scholar]
- Khan MA, Ungar IA. 1984. The effect of salinity and temperature on germination of polymorphic seeds and growth of Atriplex triangularis. American Journal of Botany 71: 481–489. [Google Scholar]
- Köpperud BT, Pienaar J, Voje KJ, Orzack SH, Hansen TF, Grabowski M. 2019. Stochastic linear Ornstein-Uhlenbeck comparative hypotheses. R package version 2.1.2. https://cran.r-project.org/web/packages/slouch/index.html 1 June 2020.
- Kos M, Poschlod P. 2010. Why wait? Trait and habitat correlates of variation in germination speed among Kalahari annuals. Oecologia 162: 549–559. [DOI] [PubMed] [Google Scholar]
- Kühn U, Bittrich V, Carolin R, et al. . 1993. Chenopodiaceae. In: Kubitzki K, ed. Flowering plants. Dicotyledons. Heidelberg: Springer, 253–281. [Google Scholar]
- Leishman MR, Wright IJ, Moles AT, Westoby M. 2000. The evolutionary ecology of seed size. In: Fenner M, ed. Seeds: the ecology of regeneration in plant communities. Wallingford: CABI, 31–57. [Google Scholar]
- Liu K, Baskin JM, Baskin CC, Du G. 2013. Very fast-germinating seeds of desert species are cryptoviparous-like. Seed Science Research 23: 163–167. [Google Scholar]
- Lloret F, Casanovas C, Penuelas J. 1999. Seedling survival of Mediterranean shrubland species in relation to root:shoot ratio, seed size and water and nitrogen use. Functional Ecology 13: 210–216. [Google Scholar]
- Luft JH. 1961. Improvements in epoxy resin embedding methods. Journal of Biophysical and Biochemical Cytology 9: 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M. 1991. Methods for the analysis of comparative data in evolutionary biology. Evolution 45: 1065–1080. [DOI] [PubMed] [Google Scholar]
- Martin AC. 1946. The comparative internal morphology of seeds. American Midland Naturalist 36: 513–660. [Google Scholar]
- Mazer SJ. 1989. Ecological, taxonomic, and life history correlates of seed mass among Indiana dune angiosperms. Ecological Monographs 59: 153–175. [Google Scholar]
- Meyer SE, Debaene-Gill SB, Allen PS. 2000. Using hydrothermal time concepts to model seed germination response to temperature, dormancy loss, and priming effects in Elymus elymoides. Seed Science Research 10: 213–223. [Google Scholar]
- Milberg P, Andersson L, Elfverson C, Regnér S. 1996. Germination characteristics of seeds differing in mass. Seed Science Research 6: 191–198. [Google Scholar]
- Moles AT, Ackerly DD, Webb CO, et al. . 2005. Factors that shape seed mass evolution. Proceedings of the National Academy of Sciences of the USA 102: 10540–10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Briones DF, Kadereit K, Tefarikis DT, et al. . 2020. Disentangling sources of gene tree discordance in phylogenomic data sets: testing ancient hybridizations in Amaranthaceae s.l. Systematic Biology 70: 219–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Rodríguez AF, Sanjosé I, Márquez-García B, et al. . 2017. Germination syndromes in response to salinity of Chenopodiaceae halophytes along the intertidal gradient. Aquatic Botany 139: 48–56. [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, et al. . 2015. Vegan: community ecology package. R package vegan, version 2.2-1. https://cran.r-project.org/web/packages/vegan/index.html 14 May 2020.
- Padilla FM, Pugnaire FI. 2007. Rooting depth and soil moisture control Mediterranean woody seedling survival during drought. Functional Ecology 21: 489–495. [Google Scholar]
- Pagel M. 1994. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proceedings of the Royal Society B. Biological Sciences 255: 37–45. [Google Scholar]
- Pagel M. 1997. Inferring evolutionary processes from phylogenies. Zoologica Scripta 26: 331–348. [Google Scholar]
- Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401: 877–884. [DOI] [PubMed] [Google Scholar]
- Paradis E. 2006. Analysis of phylogenetics and evolution with R. New York: Springer. [Google Scholar]
- Parker WC, Noland TL, Morneault AE. 2006. The effects of seed mass on germination, seedling emergence, and early seedling growth of eastern white pine (Pinus strobus L.). New Forests 32: 33–49. [Google Scholar]
- Parsons RF. 2012. Incidence and ecology of very fast germination. Seed Science Research 22: 161–167. [Google Scholar]
- Parsons RF, Vandelook F, Janssens SB. 2014. Very fast germination: additional records and relationship to embryo size and phylogeny. Seed Science Research 24: 159–163. [Google Scholar]
- Piirainen M, Liebisch O, Kadereit G. 2017. Phylogeny, systematics and taxonomy of Salicornioideae (Amaranthaceae/Chenopodiaceae) – a cosmopolitan, highly specialized hygrohalophyte lineage dating back to the Oligocene. Taxon 66: 109–132. [Google Scholar]
- Pratt DB. 2003. Phylogeny and morphological evolution of the Chenopodiaceae-Amaranthaceae alliance. PhD Thesis, Iowa State University, USA. [Google Scholar]
- R Development Core Team . 2020. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Ruzin SE. 1999. Plant microtechnique and microscopy, Vol. 198. New York: Oxford University Press. [Google Scholar]
- Qu XX, Huang ZY, Baskin JM, Baskin CC. 2008. Effect of temperature, light and salinity on seed germination and radicle growth of the geographically widespread halophyte shrub Halocnemum strobilaceum. Annals of Botany 101: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Pearcy RW, Borsch T. 2007. The taxonomic distribution of C4 photosynthesis in Amaranthaceae sensu stricto. American Journal of Botany 94: 1992–2003. [DOI] [PubMed] [Google Scholar]
- Salisbury EJ. 1942. The reproductive capacity of plants. London: Bell. [Google Scholar]
- Salisbury EJ. 1974. Seed size and mass in relation to environment. Proceedings of the Royal Society B. Biological Sciences 186: 83–88. [Google Scholar]
- Shepherd KA, Macfarlane TD, Colmer TD. 2005. Morphology, anatomy and histochemistry of Salicornioideae (Chenopodiaceae) fruits and seeds. Annals of Botany 95: 917–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins GL. 1974. Flowering plants. Evolution above the species level. Cambridge: Belknap Press of Harvard University Press. [Google Scholar]
- Sukhorukov AP, Zhang M. 2013. Fruit and seed anatomy of Chenopodium and related genera (Chenopodioideae, Chenopodiaceae/Amaranthaceae): implications for evolution and taxonomy. PLoS ONE 8: e0061906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschereau PM. 1972. Taxonomy and distribution of Atriplex species in Nova Scotia. Canadian Journal of Botany 50: 1571–1594. [Google Scholar]
- Townsend CC. 1993. Amaranthaceae. In: Kubitzki K, ed. Families and genera of vascular plants, Vol. 2. Berlin: Springer, 70–91. [Google Scholar]
- Tutin TG, Heywood VH, Burges NA, Valentine DH, Walters SM, Webb DA. 1964. Flora Europaea, Vol. 1. Cambridge: Cambridge University Press. [Google Scholar]
- Ungar IA. 1978. Halophyte seed germination. Botanical Review 44: 233–263. [Google Scholar]
- Ungar IA. 1991. Ecophysiology of vascular halophytes. Boca Raton: CRC Press. [Google Scholar]
- Vandelook F, Janssens SB, Probert RJ. 2012. Relative embryo length as an adaptation to habitat and life cycle in Apiaceae. New Phytologist 195: 479–487. [DOI] [PubMed] [Google Scholar]
- Vandelook F, Janssens SB, Matthies D. 2018. Ecological niche and phylogeny explain distribution of seed mass in the central European flora. Oikos 127: 1410–1421. [Google Scholar]
- Verdú M. 2006. Tempo, mode and phylogenetic associations of relative embryo size evolution in angiosperms. Journal of Evolutionary Biology 19: 625–634. [DOI] [PubMed] [Google Scholar]
- Veselova TD, Timonin AC. 2009. Pleuropetalum Hook. F. is still an anomalous member of Amaranthaceae Juss. An embryological evidence. Wulfenia 16: 99–116. [Google Scholar]
- Veselova TD, Dzhalilova KK, Timonin AC. 2016. Embryology of Polycnemum arvense L. (lower core Caryophyllales). Wulfenia 23: 221–240. [Google Scholar]
- Vivrette NJ. 1995. Distribution and ecological significance of seed-embryo types in Mediterranean climates in California, Chile, and Australia. In: Arroyo MKT, Zedler PH, Fox MD, eds. Ecology and biogeography of Mediterranean ecosystems in Chile, California and Australia. New York: Springer, 274–288. [Google Scholar]
- Walker JF, Yang Y, Feng T, et al. . 2018. From cacti to carnivores: improved phylotranscriptomic sampling and hierarchical homology inference provide further insight into the evolution of Caryophyllales. American Journal of Botany 105: 446–462. [DOI] [PubMed] [Google Scholar]
- Wang J, Chen W, Baskin CC, et al. . 2012. Variation in seed germination of 86 subalpine forest species from the eastern Tibetan Plateau: phylogeny and life-history correlates. Ecological Research 27: 453–465. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.