Abstract
Purpose
30-day mortality (30-DM) is a parameter with widespread use as an indicator of avoidance of harm used in medicine. Our objective is to determine the 30-DM followed by palliative radiation therapy (RT) in our department and to identify potential prognosis factors.
Material/Methods
We conducted a retrospective cohort study including patients treated with palliative RT in our center during 2018 and 2019. Data related to clinical and treatment characteristics were collected.
Results
We treated 708 patients to whom 992 palliative irradiations were delivered. The most frequent primary tumor sites were lung (31%), breast (14.8%), and gastrointestinal (14.8%). Bone was the predominant location of the treatment (56%), and the use of single doses was the preferred treatment schedule (34.4%). The 30-DM was 17.5%. For those who died in the first month the median survival was 17 days. Factors with a significant impact on 30-DM were: male gender (p < 0.0001); Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) of 2–3 (p = 0.0001); visceral metastases (p = 0.0353); lung, gastrointestinal or urinary tract primary tumors (p = 0.016); and single dose RT (p = <0.0001). In the multivariate analysis, male gender, ECOG PS 2–3, gastrointestinal and lung cancer were found to be independent factors related to 30-DM.
Conclusion
Our 30-DM is similar to previous studies. We have found four clinical factors related to 30-DM of which ECOG was the most strongly associated. This data may help to identify terminally ill patients with poor prognosis in order to avoid unnecessary treatments.
Keywords: 30-day mortality, palliative radiation, end-of-life, prognosis, clinical indicator
Introduction
Radiation therapy (RT) has a well-established role in the palliative approach of patients with cancer. When using palliative RT, symptom relief is usually obtained in a wide range of time which varies depending on the primary tumor, the location of the treatment or the patient’s health. However, when survival is too short, these patients may die before they benefit from RT.
The use of chemotherapy in dying patients has been previously reported as an aggressive and poor tailored end-of-life care indicator (1). In the last years the use of RT at the end of life has also been a matter of concern (2–4). When proposing a palliative treatment with radiation, the presumed survival is an essential factor taken into account and may condition fractionation regimen. Therefore, the use of a larger number of fractions in terminally ill patients is likely to require spending a significant amount of their final days visiting a radiation therapy suite (2, 3). This has been suggested to be a consequence of an overoptimistism at survival prediction of dying patients (5).
The National Health Service of the United Kingdom proposed the 30-day mortality (30-DM) parameter as an indicator of aggressive management at the end of the life. Thus, when the estimated survival is less than one month, palliative RT is unlikely to be beneficial. The Royal College of Radiologist agreed that less than 20% of patients receiving palliative RT should die within 30 days of treatment (6). Therefore, it’s important to identify these patients with shortened survival and to carefully consider if the treatment should be avoided or not.
The use of palliative RT in the last 30 days of life varies substantially between centers and ranges between 0.7 and 33% (7). The purpose of this study was to determine 30-DM in patients who have received palliative RT in our center and to identify potential prognostic factors for 30-DM.
Material and Methods
We performed a retrospective study including adult patients treated with palliative RT in our center from January 2018 to December 2019. Exclusion criteria were: patients under the age of 18, hematologic tumors, non-melanomatous skin cancer, treatment with stereotactic body radiation therapy or radiosurgery, and when survival status at 30 days was unavailable. All RT treatments were identified using Aria® (Varian Medical Systems) which is a specific electronic record for patients referred for RT. Clinical data was recorded from the hospital electronic medical record. The study was approved by the institutional ethics committee of our center.
Demographic data, radiation treatment parameters, and disease characteristics were collected for each patient. The type of primary tumor was classified into eight groups according to the most frequent tumors: lung, breast, prostate, gastrointestinal, urinary tract, gynecological, head and neck, and others. Episodes were identified when the treatment intent was registered as palliative by a radiation oncologist, and radiotherapy was delivered in less than 15 fractions. Site of the treatment was allocated by primary tumor, bone, brain, lymph nodes, and soft tissue. For patients who were treated more than once, we took into account the last treatment to avoid data duplication. All patients were followed for at least one month and until 6 months.
The primary endpoint of our study was to determine 30-DM. The secondary endpoint was to identify potential prognostic factors in our cohort. 30-DM was assessed from the start of treatment to the moment of death. Patients were grouped according to their vital status within 30 days from the start of treatment: group “better survival” (BS) for survivors and group “lower survival” (LS) for non-survivors at 30 days. All patients were followed up during that period and none was lost. A descriptive analysis was carried using Chi-squared or Exact Fisher as adequate. Potential clinical and dosimetric variables related to mortality were checked fitting a univariate and multivariate logistic regression. Variables that improve the likelihood (p < 0.1) were included in the final mode. Covariates considered were the following: age, sex, ECOG PS, primary tumor, presence of visceral disease, treatment location, number of fractions, and reirradiation. A multivariable analysis was performed to identify independent prognostic factors. A Kaplan–Meier survival curve with six months of follow-up has been also estimated. All analyses were carried out with Stata 15.1.
Results
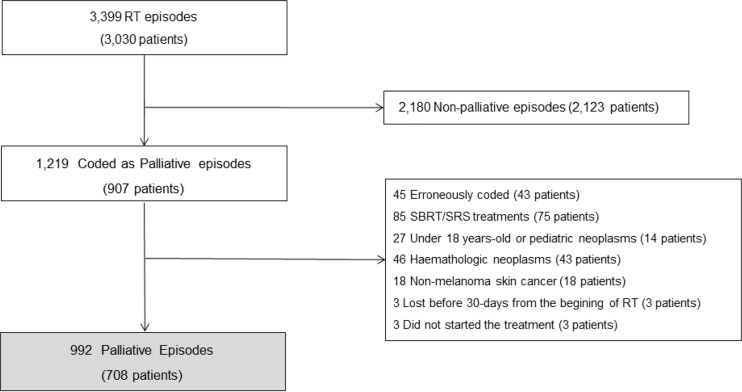
A total of 708 patients were analyzed. Figure 1 shows the consort flow diagram. The median age of the entire population at treatment was 66 years, male gender was predominant (58.2%), and the majority had a good performance status with an ECOG PS of 0–1 (59%). The most prevalent tumors were lung, breast, and gastrointestinal (31, 14.8, and 14.8% respectively). Bone was the most frequent site of radiation (56%), and the preferred schedule was single doses (34.4%) followed closely by 10–15 fractions (34%). The completion rate of the treatment was 94.8%. No differences were found according to age, location of the treatment, and reirradiation between groups. Of the 37 patients who did not end the treatment, 28 belonged to the LS group. Patient’s characteristics are shown in Table 1 .
Figure 1.
Consort diagram demonstrating exclusions from the study population.
Table 1.
Sample characteristics and descriptive analysis according to the state at 30-days.
| All patients(n = 708) | BS Group(n = 584) | LS Group(n = 124) | p value | |
|---|---|---|---|---|
| Age | 0.9865 | |||
| 18–64 years | 327 (46.2%) | 267 (45.7%) | 60 (48.4%) | |
| ≥ 65 years | 381 (53.8%) | 317 (54.3%) | 64 (51.6%) | |
| Sex | 0.0001 | |||
| Male | 412 (58.2%) | 320 (54.8%) | 92 (74.2%) | |
| Female | 296 (41.8%) | 264 (45.2%) | 32 (25.8%) | |
| ECOG PS | <0.0001 | |||
| ECOG PS 0-1 | 418 (59%) | 380 (65.1%) | 38 (30.6%) | |
| ECOG PS 2-3 | 290 (41%) | 204 (34.9%) | 86 (69.4%) | |
| Primary tumor | 0.0016 | |||
| Breast | 105 (14.8%) | 100 (17.1%) | 5 (4%) | |
| Gastrointestinal | 105 (14.8%) | 79 (13.5%) | 26 (21%) | |
| Lung | 219 (31%) | 169 (28.9%) | 50 (40.3%) | |
| Prostate | 82 (11.6%) | 70 (12%) | 12 (9.7%) | |
| Urinary tract | 72 (10.2%) | 59 (10.1%) | 13 (10.5%) | |
| Gynecological | 40 (5.6%) | 37 (6.3%) | 3 (2.4%) | |
| Head and neck | 34 (4.8%) | 28 (4.8%) | 6 (4.8%) | |
| Other | 51 (7.2%) | 42 (7.2%) | 9 (7.3%) | |
| Visceral metastases | 0.0353 | |||
| Present | 414 (58.5%) | 331 (56.7%) | 83 (66.9%) | |
| Absent | 294 (41.5%) | 253 (43.3%) | 41 (33.1%) | |
| Location of the treatment | 0.6162 | |||
| Bone | 397 (56%) | 324 (55.5%) | 73 (58.9%) | |
| Brain | 181 (25.6%) | 153 (26.2%) | 28 (22.6%) | |
| Pimary tumour | 70 (9.9%) | 56 (9.6%) | 14 (11.3%) | |
| Soft tissue | 43 (6.1%) | 35 (6%) | 8 (6.5%) | |
| Lymph nodes | 17 (2.4%) | 16 (2.7%) | 1 (0.8%) | |
| Number of fractions | <0.0001 | |||
| Single dose | 243 (34.4%) | 187 (32%) | 56 (45.2%) | |
| 2-9 fractions | 224 (31.6%) | 171 (29.3%) | 53 (42.7%) | |
| 10-15 fractions | 241 (34%) | 226 (38.7%) | 15 (12.1%) | |
| Reirradiation | 0.6242 | |||
| Yes | 66 (9.3%) | 53 (9.1%) | 13 (10.5%) | |
| No | 642 (90.7%) | 531 (90.9%) | 111 (89.5%) | |
| End of the treatment | 0.6242 | |||
| Yes | 671 (94.8%) | 575 (98.5%) | 96 (77.4%) | |
| No | 37 (5.2%) | 9 (1.5%) | 28 (22.6%) |
Bold values: statistically significant.
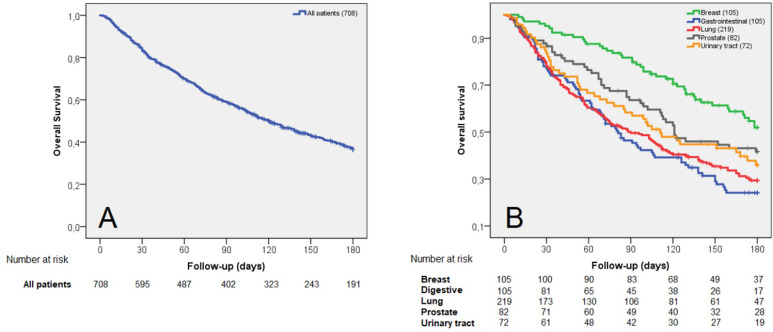
Overall, 124 out of 708 patients died at 30 days (17.5%). The median survival was 17 days for the LS group. For the entire cohort, the median survival was 120 days ( Figure 2A ). Descriptive analysis according to the state at 30-days showed a higher prevalence in the LS group of ECOG 2–3 (p = 0.0001), male gender (p < 0.0001), visceral metastasis (p = 0.0353), and use of single doses (p < 0.0001). Primary tumor distribution between groups was different (p = 0.016) with a higher prevalence of lung, gastrointestinal, urinary tract, and other tumors in the LS group. Survival according to primary tumor is shown in Figure 2B .
Figure 2.
Survival curves including: all patients (A) and the five more prevalent primary tumors (B).
The multifactorial analysis shows that male patients were 58% more likely to die within the first month after RT in comparison to female patients (OR 2.37, 95% CI: 1.5; 3.66). ECOG PS was the parameter with the highest impact in 30-DM with an increased risk of 77% of dying for those with an ECOG PS 2–3 (OR 4.22, 95% CI: 2.78; 6.40). According to primary tumor, lung and gastrointestinal neoplasms were also related to 30-DM (OR 1.66, 95% CI: 1.11; 2.48 and OR 1.7, 95% CI: 1.04; 2.78). In patients in whom visceral metastases were present, an increased risk of dying in the first month of 36% was assessed (OR 1.55, 95% CI: 1.03; 2.33). Age, treatment site, and reirradiation did not show any impact in 30-DM ( Table 2 ). After adjusting for other characteristics, the multivariate analysis found that male sex, ECOG PS 2–3, gastrointestinal and lung tumors were found to be independent related factors to 30-DM. Although visceral metastases confidence interval includes 1, a trend toward a higher mortality was observed (OR 1.53, 95% CI: 0.98; 2.40) ( Table 3 ).
Table 2.
Univariate analysis investigating potential risk factors of 30-DM.
| OR | CI 95% | p value | |
|---|---|---|---|
| Age | 0.8460 | ||
| 18–64 years | 1 | 1 | |
| ≥ 65 years | 1.001 | (0.987; 1.016) | |
| Sex | 0.0001 | ||
| Female | 1 | ||
| Male | 2.37 | (1.5.; 3.66) | |
| ECOG PS | <0.0001 | ||
| ECOG PS 0–1 | 1 | ||
| ECOG PS 2–3 | 4.22 | (2.78; 6.40) | |
| Primary tumor | |||
| Breast | 1 | ||
| Lung | 1.66 | (1.11; 2.48) | 0.0133 |
| Prostate | 0.79 | (0.41; 1.50) | 0.4665 |
| Gastrointestinal | 1.7 | (1.04; 2.78) | 0.0358 |
| Urinary tract | 1.04 | (0.55; 1.97) | 0.8985 |
| Gynecologic | 0,367 | (0,111; 1,208) | 0,0990 |
| Head and neck | 1.01 | (0.41; 2.49) | 0.9833 |
| Other | 1.01 | (0.48; 2.13) | 0.9793 |
| Visceral Metastases | |||
| Absent | 1 | ||
| Present | 1.55 | (1.03; 2.33) | 0.0362 |
| Location of the treatment | 0.6517 | ||
| Primary tumor | 1 | ||
| Bone | 0.90 | (0.44; 1.71) | |
| Brain | 0.73 | (0.36; 1.49) | |
| Lymph nodes | 0.25 | (0.003; 2.05) | |
| Soft tissue | 0.91 | (0.35; 2.40) |
Bold values: statistically significant.
Table 3.
Multivariate analysis investigating potential risk factors of 30-DM.
| OR | CI 95% | p value | |
|---|---|---|---|
| Sex | |||
| Female | 1 | ||
| Male | 2.38 | (1.538; 3.703) | 0.0001 |
| ECOG PS | |||
| ECOG PS 0-1 | 1 | ||
| ECOG PS 2-3 | 4.38 | (2.84; 6.74) | <0.0001 |
| Primary tumor | |||
| Lung | 1.66 | (1.11; 2.48) | 0.0133 |
| Gastrointestinal | 2.38 | (1.32; 4.27) | 0.0038 |
| Visceral metastases | |||
| Absent | 1 | ||
| Present | 1.53 | (0.98; 2.40) | 0.0606 |
Bold values: statistically significant.
Discussion
30-DM after palliative RT observed in our center was 17.5% of the palliative treatments. For those who died in the first month, the median survival was 17 days. A recent systematic review, showed an overall use of palliative RT rates in the last 30 days of life of 9–15.3% (7). Our results are slightly higher than previous studies (2–4, 7–12), but they are still adjusted to The Royal College of Radiologist recommendation of 30-DM to be inferior to 20%. Therefore, we consider that the selection of our patients for palliative treatment is adequate.
Park and et al. (7) conducted a systematic review and found that major predictors for 30-DM among single institution studies were ECOG PS, lung cancer primary, bladder cancer primary, multiple metastases, and evidence of progressive disease. In our analysis, we have also found the presence of a gastrointestinal tumor to be associated with 30-DM. Our center receives many patients with multi-treated digestive tumors for phase I trials. We believe this fact may partly explain this data. We have not found the presence of visceral metastases to have a statistically significant impact on survival in the multivariate analysis, but there is a clear trend we cannot ignore.
Studies analyzing patients with bone metastases treated with RT show a rather wide range of 30-DM. Ellsworth et al. (13), reported a 30-DM of 26%. The most frequent scheme consisted of 6–10 fractions (56%), while the use of single doses was 8%. On the other hand, a large Canadian population cohort study including 8,301 patients with bone metastases, showed a 30-DM of 14.5%, and a single dose was used in 64.2% of the patients in the last month of life (14). This imbalance is thought to be multifactorial and partially related to historical practice patterns and financing of the treatments. When considering patients treated for bone metastases in our series, 30-DM was 18.3%. The use of single doses was by far the most used (91%). These results are adjusted to international recommendations of a use of single fractions in patients with advanced cancer who have uncomplicated bone metastases (15, 16). Although single fractions schemes seem to be advantageous in terminally ill patients, published data show that the use multiple fractions are preferred among institutions (7). This overuse of fractionated regimens may be related to unrealistic concerns about late radiation damage and can expose dying patients to who are not expected to require a re-treatment. On the contrary, the choice of prescribing single doses has the potential to reduce cost and unnecessary visits to the hospital of terminally ill patients. Our high use of single doses for bone metastases in those with shortened survival, indirectly suggests that the fractionation approach was adapted to the end-of-life.
Since the life expectancy of these patients is sometimes too short, when palliative RT is indicated, its impact on quality of life might doubtful. Symptom relief is usually obtained in a wide range of time. When treating painful bone metastases mostly it is achieved at 3–4 weeks (17), while this benefit can be delayed up to months in brain metastases related symptoms (18). Gripp found that half of the patients treated with palliative RT spend most of their remaining time on therapy, of which a large part did not complete the treatment. Out of the patients who died in one month from the first visit, only 16% of survival estimations were correct (2). Despite the fact that the vast majority of our patients completed the treatment, 37 patients did not, of whom 28 died within the first month from the start of the treatment. This means that this small group of patients probably did not benefit from treatment and their life expectancy was expected to be longer.
Predicting survival in terminally patients evaluated for palliative RT is a difficult task since several factors are involved. The clinical predictors’ factor does not seem to be accurate enough to estimate the patient’s real-life expectancy (5, 19). Hemoglobin levels or life-threatening related symptoms, such as dyspnea or cachexia, are also relevant in advanced disease. Hence, it is important to develop survival prediction tools to achieve tailored-end-of-life strategies. In our study, we were able to construct a calculator of 30-DM using the variables with impact on 30-DM in the multivariate analysis. Of them, the ECOG PS is the one with the greatest impact on 30-DM. So that, when a male patient referred for palliative RT presents with an ECOG PS 0–1 and lung cancer, the probability of dying within the first month would be 12.3%, but if the same patient presents with an ECOG PS 2–3, this percentage would increase to 21.4%. Nonetheless, this data is not yet validated. Nowadays, there are several prognostic scores for patients with advanced cancer (9, 19–21), although most of them have not been validated in a prospective cohort of patients treated with palliative RT. Angelo et al. developed a six-parameter decision tree that was able to predict the use of palliative RT in the last 30 days of life. However, it was only applicable to patients with primary lung or bladder cancer (9). A recent study performed by Kain et al. applied the TEACHH model retrospectively to 1,744 consecutive patients. This score consists of six easy-collectable variables specifically addressed to patients referred for palliative RT. They were able to separate patients into three different and clinically relevant survival groups (12). There are few prospective studies using prognostic scores in the palliative RT set. PROGRAD stands out as a prospective study which applies two validated prognostic systems in the initial assessment of patients referred for palliative RT. Using the Palliative Prognostic Index (PPI) and the Number of Risk Factors (NRF) score, they were able to stratify the patients into three groups with different prognoses. PPI score seemed to be the one that best discriminated those patients with the worst prognosis (22).
The relevance of this data is that it can discriminate clinically relevant groups based on scales that are simple to apply and include variables easily collectable in the patient’s first visit. From our part, we have found the presence of factors related to 30-DM that may help develop a more tailored to life expectancy strategy. However, our study is inherently biased by its retrospective design and reflects the clinical practice of a single center, so its interpretation and generalization must be made cautiously. In the current study we only included patients who started the treatment, but a few patients who were planned for palliative RT and died before initiation are not taken into account. For a better understanding of the decision-making, it would be valuable to include for the analysis the patients who were considered unfit for palliative RT. Other variables with demonstrated impact on 30-DM, such as white blood count, dyspnea, or cachexia, could not be collected in a retrospective setting.
RT can provide the necessary relief of symptoms in patients with advanced cancer. However, the use of palliative RT in the last days of life may not be useful. It is therefore important to select appropriately which patients can benefit from palliative RT. While clinical prediction alone seems to be an inaccurate method for decision-making in these patients, 30-DM is objective and can set a clinically relevant time endpoint for symptom relief in patients with short survival. The reliability of survival prediction might be improved with the implementation of objective prognostic systems including variables related to early mortality. Our study provides useful and comparable results with previous, which may be useful to decide whether palliative RT should be indicated or not. In addition, it also may contribute to a better understanding of the patterns of usual clinical practice. There is now a growing body of evidence supporting the implementation of predicting tools in the palliative RT approach. The challenge is to identify those patients who will not benefit from palliative RT in order to provide a better care near the end of life.
Nomenclature
30-DM, 30-Day Mortality; ECOG, Cooperative Oncology; PS, Performance Status; RT, Radiation Therapy; BS, Better Survival; LS, Lower Survival; PPI, Palliative Prognostic Index; NRF, Number of Risk Factors.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Comitè Ètic d’Investigació Clínica (CEIC) of Vall d’Hebron University Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Concept and design: MV, MA, and JG. Collection and assembly of data: MV, DM, and AG. Data analysis and interpretation: SP-H and MV. Manuscript writing: MV and JG. Final approval of the manuscript: All authors. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The statistical analysis has been carried out in the Statistics and Bioinformatics Unit (UEB) of the Research Institute of the Vall d’Hebron Hospital (VHIR).
References
- 1. Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol (2004) 22(2):315–21. [DOI] [PubMed] [Google Scholar]
- 2. Gripp S, Mjartan S, Boelke E, Willers R. Palliative radiotherapy tailored to life expectancy in end-stage cancer patients: Reality or myth? Cancer (2010) 116(13):3251–6. [DOI] [PubMed] [Google Scholar]
- 3. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buchholz TA. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol (2013) 31(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murphy JD, Nelson LM, Chang DT, Mell LK, Quynh-Thu L. Patterns of care in palliative radiotherapy: A populationbased study. J Oncol Pract (2013) 9(5). [DOI] [PubMed] [Google Scholar]
- 5. Hartsell WF, Desilvio M, Bruner DW, Scarantino C, Ivker R, Roach M, 3rd, et al. Can physicians accurately predict survival time in patients with metastatic cancer? Analysis of RTOG 97-14. J Palliat Med (2008) 11(5):723–8. [DOI] [PubMed] [Google Scholar]
- 6. Department of Health . Improving Outcomes : A Strategy for Cancer - 4th Annual Report. United Kingdom: Department of Health and Public Health; (2014). pp. 1–101. Available at: www.dh.gov.uk/publications. [Google Scholar]
- 7. Park KR, Lee CG, Tseng YD, liao JJ, Reddy S, Bruera E, et al. Palliative radiation therapy in the last 30 days of life: A systematic review. Radiother Oncol (2017) 125(2):193–9. [DOI] [PubMed] [Google Scholar]
- 8. Spencer K, Morris E, Dugdale E, Newsham A, Sebag-Montefiore D, Turner R, et al. 30 Day mortality in adult palliative radiotherapy - A retrospective population based study of 14,972 treatment episodes. Radiother Oncol (2015) 115(2):264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Angelo K, Norum J, Dalhaug A, Pawinski A, Aadahl G, Haukland E, et al. Development and validation of a model predicting short survival (death within 30 days) after palliative radiotherapy. Anticancer Res (2014) 34(2):877–86. [PubMed] [Google Scholar]
- 10. Lopez AN, Bingham B, Strickler S, Dvorak T, Morris CG, Yeung AR. 30- and 90-Day Mortality Rates in Patients Treated with Curative or Palliative Radiation Therapy. Int J Radiat Oncol Abstract (2017) 99(2):E558. [Google Scholar]
- 11. Wu SY, Singer L, Boreta L, Garcia MA, Fogh SE, Braunstein SE. Palliative radiotherapy near the end of life. BMC Palliat Care (2019) 18(1):4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kain M, Bennett H, Yi M, Robinson B, James M. 30-Day Mortality Following Palliative Radiotherapy. J Med Imaging Radiat Oncol (2020) 64(4):570–9. [DOI] [PubMed] [Google Scholar]
- 13. Ellsworth S, Alcorn S, Hales R, McNutt TR, DeWeese TL, Smith TJ. Patterns of Care Among Patients Receiving Radiation Therapy for Bone Metastases at a Large Academic Institution. Int J Radiat Oncol (2014) 89(5):1100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tiwana MS, Barnes M, Kiraly A, Olson RA. Utilization of palliative radiotherapy for bone metastases near end of life in a population-based cohort Cancer palliative care. BMC Palliat Care (2016) 15(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chow E, van der Linden Y, Roos D, Hartsell WF, Hoskin P, Wu JSY, et al. Single versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncoly (2014) 15(2):164–71. [DOI] [PubMed] [Google Scholar]
- 16. Lutz S, Balboni T, Jones J, Lo S, Petit J, Rich SE, et al. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol (2017) 7(1):4–12. [DOI] [PubMed] [Google Scholar]
- 17. McDonald R, Ding K, Brundage M, Meyer RM, Nabid A, Chabot P, et al. Effect of radiotherapy on painful bone metastases: A secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol (2017) 3(7):953–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin NU, Lee EQ, Aoyama H, Barani IJ, Baumert BG, Brown PD, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 1: patient population, response, and progression. A report from the RANO group. Lancet Oncol (2013) 14(10):e396–406. [DOI] [PubMed] [Google Scholar]
- 19. Krishnan MS, Epstein-Peterson Z, Chen YH, Tseng YD, Wright AA, Temel JS, et al. Predicting life expectancy in patients with metastatic cancer receiving palliative radiotherapy: The TEACHH model. Cancer (2014) 120(1):134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baba M, Maeda I, Morita T, Inoue S, Ikenaga M, Matsumoto Y, et al. Survival prediction for advanced cancer patients in the real world: A comparison of the Palliative Prognostic Score, Delirium-Palliative Prognostic Score, Palliative Prognostic Index and modified Prognosis in Palliative Care Study predictor model. Eur J Cancer (2015) 51(12):1618–29. [DOI] [PubMed] [Google Scholar]
- 21. Zhang WY, Li HF, Su M, Lin RF, Chen XX, Zhang P, et al. A simple scoring system predicting the survival time of patients with bone metastases after RT. PloS One (2016) 11(7):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen ATC, Mauro GP, Gabrielli F, Gonçalves C, Castro I, Moutinho K, et al. PROGRAD – An observational study of the prognosis of inpatients evaluated for palliative radiotherapy. Radiother Oncol (2018) 127(2):299–303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.