Abstract
Epidemiological studies demonstrated an association between heavy metal exposure and the incidence of obesity and metabolic syndrome. However, the particular effects of metal toxicity on adipose tissue functioning are unclear. Therefore, recent findings of direct influence of heavy metals (mercury, cadmium, and lead) and metalloid (arsenic) on adipose tissue physiology are discussed while considering existing gaps and contradictions. Here, we provide a literature review addressing adipose tissue as a potential target of heavy metal toxicity. Experimental in vivo studies demonstrated a significant influence of mercury, cadmium, lead, and arsenic exposure on body adiposity. In turn, in vitro experiments revealed both up- and downregulation of adipogenesis associated with aberrant expression of key adipogenic pathways, namely CCAAT/enhancer-binding protein (C/EBP) and peroxisome proliferator-activated receptor gamma (PPARγ). Comparison of the existing studies on the basis of dose and route of exposure demonstrated that the effects of heavy metal exposure on adipose tissue may be dose-dependent, varying from increased adipogenesis at low-dose exposure to inhibition of adipose tissue differentiation at higher doses. However, direct dose-response data are available in a single study only for arsenic. Nonetheless, both types of these effects, irrespective of their directionality, contribute significantly to metabolic disturbances due to dysregulated adipogenesis. Particularly, inhibition of adipocyte differentiation is known to reduce lipid-storage capacity of adipose tissue, leading to ectopic lipid accumulation. In contrast, metal-associated stimulation of adipogenesis may result in increased adipose tissue accumulation and obesity. However, further studies are required to reveal the particular dose- and species-dependent effects of heavy metal exposure on adipogenesis and adipose tissue functioning.
Keywords: mercury, cadmium, lead, adipogenesis, adipocyte
Introduction
Adipose tissue is a metabolically active tissue that evolutionarily developed as a specialized lipid depot also capable of secreting a wide range of signaling molecules (adipokines)1. Perturbations in adipose tissue physiology are known to be implicated in a wide range of metabolic disturbances, and obesity is the most widespread2. Findings in the last decades demonstrated that exposure to environmental pollutants may play a significant role in the development of obesity because of their role as endocrine disruptors, raising the concept of “obesogens”3. Known chemical obesogens include phenols, polycyclic aromatic hydrocarbons, amides, metallic compounds, esters, halogenated compounds, air pollutants, and flavoring agents, to name a few4.
Specifically, both epidemiological and experimental studies demonstrated an association between persistent organic pollutant exposure and pathogenesis of obesity and this was due mainly to interference with adipogenesis through modulation of peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT/enhancer-binding protein (C/EBP) expression. Certain obesogens—organotin compounds, in particular—are considered agonists of nuclear receptors (PPARγ and RXR). Transgenerational effects of obesogens may also be mediated through epigenetic mechanisms, including altered DNA and histone methylation, as well as impaired chromatin structure4.
Recent findings demonstrated the association between heavy metal exposure and prevalence of obesity. Exposure to markers of mercury5, cadmium6, lead7, and arsenic8 as well as metal mixture9 were found to be correlated with anthropometric and metabolic parameters in obesity and metabolic syndrome. However, the only metal considered a classic obesogen is tin (Sn), particularly its organic compounds, organotins10, which, being both PPARγ and RXR agonists, were shown to interfere with adipogenesis11. Data on the impact of other metals on adipogenesis and adipocyte functions are extremely insufficient. Specifically, a crude PubMed-Medline search using the terms “adipocyte” and “cadmium” or “mercury” revealed 21 and 13 papers, compared with 52, (48) 193, and 77 for “organotin”, “(polychlorinated) biphenyl”, and “dioxin”, respectively, indicating a scant number of studies on the adipocyte-targeted effects of heavy metals. Therefore, we aimed to discuss recent findings on the direct influence of heavy metals (Hg, Cd, and Pb) and metalloid (As) on adipose tissue physiology in view of the existing gaps and contradictions.
A brief introduction to the role of adipose tissue in the regulation of body adiposity
The cardinal morphological feature of obesity is increased white adipose tissue (WAT) mass (adiposity) that results from the imbalance between energy intake and energy expenditure. At positive caloric balance, excessive energy is stored as lipids in adipocyte lipid droplets as a result of lipogenesis, whereas increased energy expenditure activates mobilization of the stored lipids through lipolysis, which is regulated by neuroendocrine signals12. In parallel to the changes in adipocyte size, which are dependent on the balance between lipogenic and lipolytic enzymes, adipose tissue remodeling is also associated with modulation of adipogenesis13. Adipogenesis is the process of proliferation and subsequent maturation of adipocytes from adipocyte progenitors regulated by C/EBPs and PPARγ to meet the increasing lipid-storing requirements. In turn, adipogenesis dysregulation was shown to be associated with altered metabolic profile in obesity14. In addition, owing to the role of adipose tissue as an endocrine organ, adipocyte dysfunction results in impaired adipokine secretion15. Leptin, the main endocrine product of adipose tissue, is known to play a significant role in the central control of energy balance by influencing hypothalamic centers of appetite and satiety, also underlining the role of adipose tissue in the regulation of energy intake. Therefore, altered leptin signaling is known to be associated with impaired feeding behavior and obesity16. It is also notable that under certain signals WAT may modulate energy expenditure through modulation of beige adipogenesis and white-to-beige adipocyte transformation and subsequent beige thermogenesis. In turn, beige-to-white adipocyte transformation may further aggravate obesity17. Therefore, the role of adipose tissue is mediated not only by its role as lipid reservoir but also through modulation of adipogenesis, adipokine secretion, involvement in the central regulation of appetite and satiety as well as the capability to regulate thermogenesis via beige (“brite”) adipocyte formation18.
Adipotropic effects of heavy metals
Mercury (Hg)
High total blood Hg levels were found to be associated with significantly increased visceral adipose tissue mass in Korean adults19. Experimental studies also demonstrated the impact of Hg exposure on adipose tissue accumulation. Specifically, periconceptional maternal exposure to methylmercury (MeHg) and cadmium chloride (CdCl2) (both 2 mg/kg) resulted in increased adipose tissue mass and body weight in offspring with transgenerational effect that persisted to the F4 generation20.
Rizzetti et al. (2019) demonstrated that mercury chloride (HgCl2), when injected intramuscularly (4.6 μg/kg as the first dose with subsequent 0.07 μg/kg per day exposure for 60 days) to male Wistar rats, may be considered a “powerful environmental WAT disruptor” that is capable of reducing adipocyte size21. The latter was shown to be accompanied by increased adiponectin, leptin, PPARα, and PPARγ mRNA expression, indicative of impaired adipogenesis and adipokine secretion. The revealed increase in adipose tissue GRP78, CHOP, and CD11 mRNA expression indicated the potential role of endoplasmic reticulum stress and pro-inflammatory signaling in Hg-induced alteration of adipose tissue physiology21. These findings only partially corroborate the pioneering observation by Kawakami et al. (2012)22, who also demonstrated Hg-induced decrease in adipose tissue mass and adipocyte size when high-fat diet (HFD)-fed male Slc:ICR mice were subcutaneously injected with 1.0 mg/kg body weight HgCl2. However, inorganic mercury exposure in HFD-fed animals significantly reduced WAT-specific leptin, PPARα, and PPARγ mRNA expression that may be at least partially mediated by Hg-induced AMPK upregulation22. Certain inconsistencies between the adipotropic effects of Hg reported in these two studies may be explained by the difference in the dose of metal exposure; higher dose22 results in adipogenic response inhibition due to the potential toxic (including pro-oxidant) effects. In addition to the studies in mice demonstrating a significant impact of Hg exposure on PPARγ, our recent findings from a Caenorhabditis elegans model revealed a significant impact of 10 to 20 µM MeHg on lipid metabolism regulatory genes, including pro-adipogenic worm orthologs to human SREBP and C/EBPs23, due to MeHg being another regulator of adipogenesis. In contrast to the previously mentioned studies, one study demonstrated that in C57BL/6J mice orally exposed to 0.5 or 5 ppm MeHg (concentrations that failed to induce adipogenic gene expression in visceral adipose tissue), a reduction of adipose tissue cumulation was associated with MeHg-induced increase in hypothalamic pro-opiomelanocortin (POMC) expression24. Given the role of POMC as an anorexigenic peptide that reduces food intake25, the obtained data are indicative of the role of central effects of Hg in the modulation of body adiposity24.
The role of Hg as a potential factor affecting adipogenesis was also demonstrated in in vitro studies. The most recent study demonstrated that MeHg exposure in vitro (0, 0.3, 1.7, or 3.8 mM) for 6 days increased lipid accumulation in 3T3-L1 preadipocytes isolated from perivisceral adipose tissue. Although these changes were accompanied by an increase in fatty acid synthase and perilipin expression, certain other adipogenic markers, namely fatty acid transport protein 1, glycerol-3-phosphate dehydrogenase, and C/EBPδ, were downregulated26. It is also notable that Hg is capable of regulating adipogenesis-related genes, including C/EBPβ, DDIT3 (both upregulation), LPIN1, and SREBF1 (both downregulation), in BEAS-2B cells when administered together with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)27.
The observed effects of Hg on adipogenesis also corroborated in vitro data on its impact on adipokine secretion. Specifically, treatment of 3T3-L1 adipocytes to 200 ng/mL MeHg resulted in the formation of a lower number of adipocytes and clumped lipid droplets as well as activation of apoptosis through induction of oxidative stress as evaluated by increased culture 4-hydroxynonenal (4-HNE) levels. These changes were accompanied by aberrant adipokine expression patterns characterized predominantly by elevated adiponectin and resistin production28. In addition, MeHg exposure (100–200 ng/mL) resulted in a significant increase in vascular endothelial growth factor (VEGF) production, which is known to play a certain role in the pathogenesis of metabolic syndrome29, in mature 3T3-L1 adipocytes30.
Lead (Pb)
Pb effectively accumulates in human adipose tissue31, although direct effects of Pb exposure on adipocyte physiology have yet to be discerned. Lifetime Pb exposure (200–500 ppm) in C57Bl/6 mice resulted in a significant increase in bone marrow adiposity characterized by increased adipocyte size and number through upregulation of PPARγ gene expression32, indicative of Pb-induced adipogenesis. The results of in vitro studies have also demonstrated a significant effect of Pb on adipogenesis through modulation of key regulators (PPARγ and C/EBPβ). Particularly, a study in 3T3-L1 fibroblasts demonstrated that Pb exposure (0–10 µM) increased cytosolic lipid accumulation and perilipin expression occurring during adipogenesis. These effects were mediated by upregulation of C/EBPβ and ERK expression with subsequent activation of PPARγ33. These findings corroborate earlier observations on the interactive effects of Pb exposure (2 µM) and high-fat medium on the expression of proadipogenic PPARγ and adipogenic marker fatty acid-binding protein 4 (FABP4) in MC3T3-E1 cells34. However, higher concentrations (>10 µM) of Pb were shown to inhibit proliferation of 3T3-L1 fibroblasts35 that may be associated with toxic effects of increasing metal concentrations.
It is also notable that Pb exposure may affect central adipokine signaling through downregulating adiponectin receptor 1b and especially leptin receptor gene expression in zebrafish brain36. Given the role of altered leptin receptor expression in leptin resistance and obesity37, these findings may be indicative of the central role of Pb in alterations of energy homeostasis and excessive adiposity.
Arsenic (As)
As was considered a potential obesogen affecting normal adipose tissue physiology, although direct data on the impact of As species on adipogenesis and adipocyte functions are inconsistent38. Experimental in vivo studies demonstrated that As exposure is capable of affecting WAT mass, although the observed effects of As on adipogenesis seem to be dose-dependent. Specifically, exposure to As-containing drinking water (300 μg/L sodium arsenite [NaAsO2]) for 9 weeks in male C57BL/6J mice resulted in a significant increase in WAT mass, accompanied by impaired mitochondrial biogenesis and thermogenesis due to modulation of PPARα and PPARγ-specific genes, including Slc27a2, Fabp3, Ucp1, Acsl5, Scd2, and Cpt1β39. However, exposure to a higher dose (50 mg/L for 16 weeks) significantly reduced adipose tissue mass in HFD-fed male C57BL/6J mice without alteration of energy expenditure as assessed by indirect calorimetry40. The authors propose that the observed “antiobesogenic” effect of As could be attributed to its insulin-sensitizing activity40. Of interest, an inverse association between As dose and adipogenic response was reported by Shearer et al. (2017)41. Particularly, oral exposure to 300 ppb inorganic As in male C57BL/6J mice resulted in a significant decrease in the expression of adipocyte-specific genes in isolated adipose-derived mesenchymal stem/stromal cells (ASCs), whereas ASCs obtained from mice exposed to a higher dose (1000 ppb) were characterized by a significant increase in adiponectin, leptin, and FABP4 expression, indicative of a biphasic response of adipogenesis to inorganic As exposure41.
High-dose As exposure was also shown to alter the production of adiponectin. Drinking NaAsO2-containing water (5 and 50 ppm in drinking water) for 18 weeks resulted in a significant decrease in serum adiponectin levels in male C57BL/6 mice42. However, the impact of As on adiponectin production seems to be diet-dependent. Particularly, As exposure in male mice fed a low- and high-fat diet resulted in a significant decrease and increase of circulating adiponectin levels, respectively43. Reduced levels of adiponectin in response to As toxicity may also indirectly indicate inhibitory effects of the metalloid on adipogenesis and production of adipocyte-specific proteins, although this relationship may be mediated by factors such as insulin resistance, inflammation, atherogenic dyslipidemia, and total body adiposity44.
The existing in vitro studies further unraveled the mechanisms underlying the modulatory effect of As on adipogenesis. Generally, previously discussed in vivo studies corroborate earlier data on the inhibitory effect of As (0.2–4 μM) on adipogenic differentiation of mesenchymal stem cells through downregulation of PPARγ and C/EBP expression45. The results of a recent study performed in stromal vascular fraction cells isolated from WATs of Nrf1(f)-knockout mice allow investigators to propose that As-induced inhibition of PPARγ signaling and adipogenesis may be NRF1-dependent46. Inhibition of PPARγ signaling was shown to occur because of As (5–10 μM)-induced endoplasmic reticulum stress in 3T3-L1 preadipocytes with subsequent induction of C/EBP homologous protein (CHOP10) that is known to reduce C/EBPβ DNA-binding activity47. Modulation of SIRT3-FOXO3a, endothelin-1, Ras-MAP-AP-1, and PI3K-Akt pathways by As exposure may also significantly contribute to disturbed adipocyte functioning48. As exposure (0 or 100 μg/L in drinking water or 1 μM in culture medium) was also shown to inhibit adipogenesis by increasing miR-29b in human mesenchymal stem cells and murine adipose tissue and sustained cyclin D expression, preventing cell cycle exit and providing a shift from differentiation to proliferation49.
Brown adipose tissue (BAT) was also shown to be the potential target of As toxicity mediating the effects of the metalloid on body adiposity. Specifically, oral administration of NaAsO2 (5–10 mg/kg) to male C57BL/6J mice was accompanied by a more pronounced As accumulation in BAT as compared with WAT and also resulted in inhibition of brown adipocyte differentiation and decreased expression of PPARγ and other brown adipocyte-specific markers (UCP1 and PGC1). Moreover, As-induced inhibition of autophagy was proposed to play a role in BAT dysfunction50. Correspondingly, prolonged exposure of adult C57BL/6J female mice to inorganic As (5–20 ppm in drinking water for 17 weeks) was shown to impair energy homeostasis, resulting in metabolic disturbances attributed to BAT whitening and impaired thermogenesis51. These findings corroborate data on As-induced alteration of mitochondrial biogenesis and beige adipocyte formation, indicative of the significant role of reduced thermogenesis in the observed increase in adiposity40.
Cadmium (Cd)
Although the role of Cd exposure in the development of diabetes mellitus type 2 was demonstrated in epidemiological and laboratory studies, the mechanisms of particular involvement of Cd in obesity pathogenesis are unclear6. Owing to high abundance in the human body, adipose tissue was considered a significant Cd depot in spite of a rather low Cd level (42 μg/kg)52. Correspondingly, Cd levels in adipose tissue were found to be directly associated with smoking53, which was the most significant source of non-occupational metal exposure. These findings indicate that adipose tissue may be considered a potential target for Cd toxicity. However, data on the particular effects of Cd in adipose tissue or adipocyte cultures are inconsistent6.
Cd levels in prenatal blood were found to be significantly associated with the risk of pediatric obesity in children (4 to 5 years old) living in North Carolina and this is in agreement with the effects of prenatal Cd exposure on juvenile lipid accumulation in zebrafish54. Early-life low-dose Cd exposure (100 nM CdCl2 with drinking water) was shown to increase body mass, adiposity, and elevated leptin levels in male C57BL/6J mice55. It is also notable that periconceptional exposure to Cd and Hg (2 mg/kg each) in CD1 mice caused transgenerational metabolic effects characterized by abdominal obesity and glucose intolerance up to the F4 generation, although the particular impact of combined metal exposure on adipogenesis was not specified20. The observed effects of Cd on body adiposity may be mediated by its impact on adipogenesis as demonstrated in vitro. Particularly, chronic exposure to 0.5 to 2 µmol/L CdCl2 was shown to increase the abundance of bone marrow adipocytes through upregulation of mesenchymal stem cell adipogenic differentiation with concomitant upregulation of PPARγ expression56.
However, certain in vivo and in vitro studies reported opposite effects of Cd exposure on adipose tissue cellularity and function and were also supported by epidemiological data. Particularly, a study originating from Mexico City demonstrated an inverse association between maternal urinary Cd levels and abdominal and peripheric adiposity57. An experimental in vivo study demonstrated that administration of 100 ppm CdCl2 in drinking water to ICR mice for 8 weeks resulted in a significant decrease in adipocyte size but that Cd chelation using dimercaptosuccinic acid (DMSA) ameliorated this effect58. This effect may be at least partially mediated by the earlier demonstrated Cd-induced (3 μM) inhibition of adipogenesis and a dose-dependent decrease in C/EBPα and PPARγ protein expression in 3T3-L1 preadipocytes59.
The existing experimental studies revealed significant contradictions in the effects of Cd on adiposity and adipogenesis in particular. Hypothetically, “low”-dose exposure in vivo (100 nM CdCl2 with drinking water, C57BL/6J mice55) and in vitro (0.5–2 µmol/L CdCl2, bone marrow adipocytes56) may promote body adiposity through upregulation of adipogenesis, whereas “high” doses studied in ICR mice (100 ppm CdCl2 in drinking water58) and 3T3-L1 preadipocytes (3 μM59) may reduce adipose tissue levels at least partially through inhibition of adipogenesis.
Conclusion and perspectives
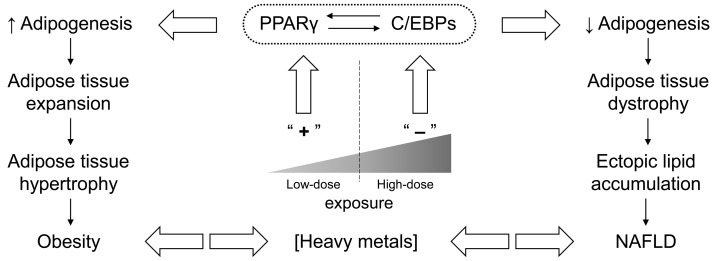
Generally, the existing in vivo and in vitro studies demonstrate that heavy metal(loid)s affect adipose tissue mass and function through modulation of adipogenesis (via C/EBPα and PPARγ), indicating the “adipotropic” effects of heavy metals. Despite some existing contradictions, the experimental data provide insight into dose-dependent effects of heavy metals on adipogenesis. It is proposed that the effects of heavy metal exposure on adipose tissue is biphasic (Figure 1), varying from increased adipogenesis at low-dose exposure to inhibition of adipose tissue differentiation at higher doses, as demonstrated for Hg21,22, Cd55,56,58,59, and As41. However, direct dose-response analysis was performed only for the latter41. Similar patterns of biological action were shown to underlie the hermetic effect of chemical substances, including heavy metals and metal nanoparticles60.
Figure 1. A schematic representation of biphasic adipogenic response to heavy metal exposure.
Briefly, low-dose exposure (left) may upregulate key adipogenic factors C/EBPs and PPARγ, thus promoting excessive adipogenesis and contributing to obesity and diabetes mellitus. In turn, “high-dose” metal exposure (right) may inhibit adipogenesis through downregulation of C/EBPs and PPARγ that may be associated with toxic effects of the metals because of pro-inflammatory and pro-oxidant activity. Under positive caloric balance, reduced adipogenic capacity results in increased ectopic lipid accumulation and lipotoxicity, including that in non-alcoholic fatty liver disease (NAFLD). However, in vivo and in vitro dose-response studies are required to clarify the association between toxic metal exposure and adipogenesis. C/EBP, CCAAT/enhancer-binding protein; PPARγ, peroxisome proliferator-activated receptor gamma.
Both stimulation and inhibition of adipogenesis in response to heavy metal exposure might contribute significantly to metabolic disturbances. Particularly, inhibition of adipocyte differentiation is known to reduce lipid-storage capacity of adipose tissue, leading to ectopic lipid accumulation61. This assumption is indirectly confirmed by the observed association between heavy metal exposure and non-alcoholic fatty liver disease62–64. On the other hand, metal-associated stimulation of adipogenesis may result in increased adipose tissue accumulation and obesity. At the same time, in view of pro-inflammatory and pro-oxidant65,66 effects of heavy metals as well as their contribution to insulin resistance67, expanded adipose tissue seems to be dysfunctional, also contributing to aggravated metabolic risk.
Further studies are required to reveal the particular dose- and species-dependent effects of heavy metal exposure on adipogenesis and adipose tissue functioning. Moreover, it is unclear whether other toxic metals, including aluminum, nickel, and beryllium, may also target adipose tissue. These findings could help to unravel the particular role of heavy metal exposure in obesity and metabolic syndrome and the subsequent development of protective strategies.
The peer reviewers who approve this article are:
Jen-Chywan Wang, Superfund Research Program, Department of Nutritional Sciences & Toxicology, University of California, Berkeley, California, USA
Marion Ehrich, Department of Biomedical Sciences and Pathobiology, Virginia Tech, Blacksburg, VA, USA
Funding Statement
The current investigation is supported by the Russian Foundation for Basic Research within project 20-515-S52003. MA was supported in part by National Institutes of Health grants NIEHS R01 10563 and R01ES07331.
References
- 1. Zwick RK, Guerrero-Juarez CF, Horsley V, et al. : Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metab. 2018; 27(1): 68–83. 10.1016/j.cmet.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Trim W, Thompson D, Turner JE: Adipose Tissue Dysfunction. In Encyclopedia of Behavioral Medicine. Living edition. Edited by Gellman M. Springer. 2018; 1–5. 10.1007/978-1-4614-6439-6_101903-1 [DOI] [Google Scholar]
- 3. Trasande L, Blumberg B: Endocrine disruptors as obesogens. In Pediatric Obesity. Contemporary Endocrinology. Edited by Freemark MS. Humana Press, Cham. 2018; 243–253. 10.1007/978-3-319-68192-4_14 [DOI] [Google Scholar]
- 4. Egusquiza RJ, Blumberg B: Environmental Obesogens and Their Impact on Susceptibility to Obesity: New Mechanisms and Chemicals. Endocrinology. 2020; 161(3): bqaa024. 10.1210/endocr/bqaa024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee K: Blood mercury concentration in relation to metabolic and weight phenotypes using the KNHANES 2011-2013 data. Int Arch Occup Environ Health. 2018; 91(12): 185–93. 10.1007/s00420-017-1269-0 [DOI] [PubMed] [Google Scholar]
- 6. Tinkov AA, Filippini T, Ajsuvakova OP, et al. : The role of cadmium in obesity and diabetes. Sci Total Environ. 2017; 601–602: 741–55. 10.1016/j.scitotenv.2017.05.224 [DOI] [PubMed] [Google Scholar]
- 7. Wang G, DiBari J, Bind E, et al. : Association Between Maternal Exposure to Lead, Maternal Folate Status, and Intergenerational Risk of Childhood Overweight and Obesity. JAMA Netw Open. 2019; 2: e1912343. 10.1001/jamanetworkopen.2019.12343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stahr S, Su J: Positive association between salivary arsenic concentration and obesity in a pilot study of women living in rural communities in the United States. Environmental Epidemiology. 2019; 3: 381. 10.1097/01.EE9.0000610240.85951.68 [DOI] [Google Scholar]
- 9. Wang X, Mukherjee B, Park SK: Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003-2014. Environ Int. 2018; 121(Pt 1): 683–94. 10.1016/j.envint.2018.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chamorro-Garcia R, Blumberg B: Current Research Approaches and Challenges in the Obesogen Field. Front Endocrinol (Lausanne). 2019; 10: 167. 10.3389/fendo.2019.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tinkov AA, Ajsuvakova OP, Skalnaya MG, et al. : Organotins in obesity and associated metabolic disturbances. J Inorg Biochem. 2019; 191: 49–59. 10.1016/j.jinorgbio.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 12. Braun K, Oeckl J, Westermeier J, et al. : Non-adrenergic control of lipolysis and thermogenesis in adipose tissues. J Exp Biol. 2018; 221(Pt Suppl 1): jeb165381. 10.1242/jeb.165381 [DOI] [PubMed] [Google Scholar]
- 13. Choe SS, Huh JY, Hwang IJ, et al. : Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front Endocrinol (Lausanne). 2016; 7: 30. 10.3389/fendo.2016.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vishvanath L, Gupta RK: Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J Clin Invest. 2019; 129(10): 4022–4031. 10.1172/JCI129191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Longo M, Zatterale F, Naderi J, et al. : Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int J Mol Sci. 2019; 20(9): 2358. 10.3390/ijms20092358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pandit R, Beerens S, Adan RAH: Role of leptin in energy expenditure: The hypothalamic perspective. Am J Physiol Regul Integr Comp Physiol. 2017; 312(6): R938–R947. 10.1152/ajpregu.00045.2016 [DOI] [PubMed] [Google Scholar]
- 17. McQueen AE, Koliwad SK, Wang J-C: Fighting obesity by targeting factors regulating beige adipocytes. Curr Opin Clin Nutr Metab Care. 2018; 21(6): 437–443. 10.1097/MCO.0000000000000509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu Q, Glazier BJ, Hinkel BC, et al. : Neuroendocrine Regulation of Energy Metabolism Involving Different Types of Adipose Tissues. Int J Mol Sci. 2019; 20(11): 2707. 10.3390/ijms20112707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park JS, Ha KH, He K, et al. : Association between Blood Mercury Level and Visceral Adiposity in Adults. Diabetes Metab J. 2017; 41(2): 113–20. 10.4093/dmj.2017.41.2.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Camsari C, Folger JK, Rajput SK, et al. : Transgenerational Effects of Periconception Heavy Metal Administration on Adipose Weight and Glucose Homeostasis in Mice at Maturity. Toxicol Sci. 2019; 168(2): 610–9. 10.1093/toxsci/kfz008 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 21. Rizzetti DA, Corrales P, Piagette JT, et al. : Chronic mercury at low doses impairs white adipose tissue plasticity. Toxicology. 2019; 418: 41–50. 10.1016/j.tox.2019.02.013 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 22. Kawakami T, Hanao N, Nishiyama K, et al. : Differential effects of cobalt and mercury on lipid metabolism in the white adipose tissue of high-fat diet-induced obesity mice. Toxicol Appl Pharmacol. 2012; 258(1): 32–42. 10.1016/j.taap.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 23. Caito SW, Newell-Caito J, Martell M, et al. : Methylmercury Induces Metabolic Alterations in Caenorhabditis elegans: Role for C/EBP Transcription Factor. Toxicol Sci. 2020; 174(1): 112–123. 10.1093/toxsci/kfz244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferrer B, Peres TV, Dos Santos AA, et al. : Methylmercury Affects the Expression of Hypothalamic Neuropeptides That Control Body Weight in C57BL/6J Mice. Toxicol Sci. 2018; 163(2): 557–68. 10.1093/toxsci/kfy052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mountjoy KG: Pro-Opiomelanocortin (POMC) Neurones, POMC-Derived Peptides, Melanocortin Receptors and Obesity: How Understanding of this System has Changed Over the Last Decade. J Neuroendocrinol. 2015; 27(6): 406–18. 10.1111/jne.12285 [DOI] [PubMed] [Google Scholar]
- 26. Tinant G, Neefs I, Rees J-F, et al. : Is methylmercury a new candidate obesogen? Proc Nutr Soc. 2020; 79. 10.1017/S0029665120002281 [DOI] [Google Scholar]
- 27. Jagannathan L, Jose CC, Tanwar VS, et al. : Identification of a unique gene expression signature in mercury and 2,3,7,8-tetrachlorodibenzo-p-dioxin co-exposed cells. Toxicol Res (Camb). 2017; 6(3): 312–23. 10.1039/C6TX00432F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chauhan S, Dunlap K, Duffy LK: Effects of Methylmercury and Theaflavin Digallate on Adipokines in Mature 3T3-L1 Adipocytes. Int J Mol Sci. 2019; 20(11): 2755. 10.3390/ijms20112755 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 29. Mazidi M, Rezaie P, Kengne AP, et al. : VEGF, the underlying factor for metabolic syndrome; fact or fiction? Diabetes Metab Syndr. 2017; 11 Suppl 1: S61–S64. 10.1016/j.dsx.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 30. Vertigan T, Dunlap K, Reynolds A, et al. : Effects of Methylmercury exposure in 3T3-L1 Adipocytes. AIMS Environ Sci. 2017; 4(1): 94–111. 10.3390/ijms20112755 [DOI] [Google Scholar]
- 31. Freire C, Vrhovnik P, Fiket Ž, et al. : Adipose tissue concentrations of arsenic, nickel, lead, tin, and titanium in adults from GraMo cohort in Southern Spain: An exploratory study. Sci Total Environ. 2020; 719: 137458. 10.1016/j.scitotenv.2020.137458 [DOI] [PubMed] [Google Scholar]
- 32. Beier EE, Holz JD, Sheu TJ, et al. : Elevated Lifetime Lead Exposure Impedes Osteoclast Activity and Produces an Increase in Bone Mass in Adolescent Mice. Toxicol Sci. 2016; 149(2): 277–88. 10.1093/toxsci/kfv234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martini CN, Gabrielli M, Bonifacino G, et al. : Lead enhancement of 3T3-L1 fibroblasts differentiation to adipocytes involves ERK, C/EBPβ and PPARγ activation. Mol Cell Biochem. 2018; 437(1–2): 37–44. 10.1007/s11010-017-3093-y [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 34. Beier EE, Inzana JA, Sheu TJ, et al. : Effects of Combined Exposure to Lead and High-Fat Diet on Bone Quality in Juvenile Male Mice. Environ Health Perspect. 2015; 123(10): 935–43. 10.1289/ehp.1408581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martini CN, Sosa FN, Fuchs J, et al. : Effect of lead on proliferation, oxidative stress and genotoxic damage of 3T3-L1 fibroblasts. Toxicol Res (Camb). 2020; 9(3): 158–63. 10.1093/toxres/tfaa018 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 36. Meyer DN, Crofts EJ, Akemann C, et al. : Developmental exposure to Pb 2+ induces transgenerational changes to zebrafish brain transcriptome. Chemosphere. 2020; 244: 125527. 10.1016/j.chemosphere.2019.125527 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 37. Engin A: Diet-induced obesity and the mechanism of leptin resistance. Adv Exp Med Biol. In: Obesity and Lipotoxicity, Springer, Cham, 2017; 960: 381–397. 10.1007/978-3-319-48382-5_16 [DOI] [PubMed] [Google Scholar]
- 38. Ceja-Galicia ZA, Daniel A, Salazar AM, et al. : Effects of arsenic on adipocyte metabolism: Is arsenic an obesogen? Mol Cell Endocrinol. 2017; 452: 25–32. 10.1016/j.mce.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 39. Castriota F, Zushin PJH, Sanchez SS, et al. : Chronic arsenic exposure impairs adaptive thermogenesis in male C57BL/6J mice. Am J Physiol Endocrinol Metab. 2020; 318(5): E667–E677. 10.1152/ajpendo.00282.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 40. Carmean CM, Kirkley AG, Landeche M, et al. : Arsenic Exposure Decreases Adiposity During High-Fat Feeding. Obesity (Silver Spring). 2020; 28(5): 932–41. 10.1002/oby.22770 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 41. Shearer JJ, Neto MF, Umbaugh CS, et al. : In Vivo Exposure to Inorganic Arsenic Alters Differentiation-Specific Gene Expression of Adipose-Derived Mesenchymal Stem/Stromal Cells in C57BL/6J Mouse Model. Toxicol Sci. 2017; 157(1): 172–82. 10.1093/toxsci/kfx026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song X, Li Y, Liu J, et al. : Changes in Serum Adiponectin in Mice Chronically Exposed to Inorganic Arsenic in Drinking Water. Biol Trace Elem Res. 2017; 179(1): 140–7. 10.1007/s12011-017-0950-1 [DOI] [PubMed] [Google Scholar]
- 43. Ahangarpour A, Alboghobeish S, Oroojan AA, et al. : Effects of Combined Exposure to Chronic High-Fat Diet and Arsenic on Thyroid Function and Lipid Profile in Male Mouse. Biol Trace Elem Res. 2018; 182(1): 37–48. 10.1007/s12011-017-1068-1 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 44. Katsiki N, Mantzoros C, Mikhailidis DP: Adiponectin, lipids and atherosclerosis. Curr Opin Lipidol. 2017; 28(4): 347–54. 10.1097/MOL.0000000000000431 [DOI] [PubMed] [Google Scholar]
- 45. Yadav S, Anbalagan M, Shi Y, et al. : Arsenic inhibits the adipogenic differentiation of mesenchymal stem cells by down-regulating peroxisome proliferator-activated receptor gamma and CCAAT enhancer-binding proteins. Toxicol In Vitro. 2013; 27(1): 211–9. 10.1016/j.tiv.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 46. Xue P, Hou Y, Zuo Z, et al. : Long isoforms of NRF1 negatively regulate adipogenesis via suppression of PPARγ expression. Redox Biol. 2020; 30: 101414. 10.1016/j.redox.2019.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 47. Hou Y, Xue P, Woods CG, et al. : Association between arsenic suppression of adipogenesis and induction of CHOP10 via the endoplasmic reticulum stress response. Environ Health Perspect. 2013; 121(2): 237–43. 10.1289/ehp.1205731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Renu K, Madhyastha H, Madhyastha R, et al. : Role of arsenic exposure in adipose tissue dysfunction and its possible implication in diabetes pathophysiology. Toxicol Lett. 2018; 284: 86–95. 10.1016/j.toxlet.2017.11.032 [DOI] [PubMed] [Google Scholar]
- 49. Beezhold K, Klei LR, Barchowsky A: Regulation of cyclin D1 by arsenic and microRNA inhibits adipogenesis. Toxicol Lett. 2017; 265: 147–55. 10.1016/j.toxlet.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bae J, Jang Y, Kim H, et al. : Arsenite exposure suppresses adipogenesis, mitochondrial biogenesis and thermogenesis via autophagy inhibition in brown adipose tissue. Sci Rep. 2019; 9(1): 14464. 10.1038/s41598-019-50965-9 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 51. Zuo Z, Liu Z, Gao T, et al. : Prolonged inorganic arsenic exposure via drinking water impairs brown adipose tissue function in mice. Sci Total Environ. 2019; 668: 310–7. 10.1016/j.scitotenv.2019.03.008 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 52. Egger AE, Grabmann G, Gollmann-Tepeköylü C, et al. : Chemical imaging and assessment of cadmium distribution in the human body. Metallomics. 2019; 11(12): 2010–9. 10.1039/c9mt00178f [DOI] [PubMed] [Google Scholar]
- 53. Echeverría R, Vrhovnik P, Salcedo-Bellido I, et al. : Levels and determinants of adipose tissue cadmium concentrations in an adult cohort from Southern Spain. Sci Total Environ. 2019; 670: 1028–36. 10.1016/j.scitotenv.2019.03.114 [DOI] [PubMed] [Google Scholar]
- 54. Green AJ, Hoyo C, Mattingly CJ, et al. : Cadmium exposure increases the risk of juvenile obesity: A human and zebrafish comparative study. Int J Obes (Lond). 2018; 42(7): 1285–95. 10.1038/s41366-018-0036-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ba Q, Li M, Chen P, et al. : Sex-Dependent Effects of Cadmium Exposure in Early Life on Gut Microbiota and Fat Accumulation in Mice. Environ Health Perspect. 2017; 125(3): 437–46. 10.1289/EHP360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Knani L, Bartolini D, Kechiche S, et al. : Melatonin prevents cadmium-induced bone damage: First evidence on an improved osteogenic/adipogenic differentiation balance of mesenchymal stem cells as underlying mechanism. J Pineal Res. 2019; 67(3): e12597. 10.1111/jpi.12597 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 57. Moynihan M, Telléz-Rojo MM, Colacino J, et al. : Prenatal Cadmium Exposure Is Negatively Associated With Adiposity in Girls Not Boys During Adolescence. Front Public Health. 2019; 7: 61. 10.3389/fpubh.2019.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 58. Prabhu R, Ribeiro M, Kajdacsy-Balla A: The Toxic Effect of Environmental Cadmium on Visceral Adipose Tissue. The FASEB Journal. 2020; 34(S1): 1. 10.1096/fasebj.2020.34.s1.06577 [DOI] [Google Scholar]
- 59. Lee EJ, Moon JY, Yoo BS: Cadmium inhibits the differentiation of 3T3-L1 preadipocyte through the C/EBPα and PPARγ pathways. Drug Chem Toxicol. 2012; 35(2): 225–31. 10.3109/01480545.2011.591401 [DOI] [PubMed] [Google Scholar]
- 60. Calabrese EJ: The Emergence of the Dose-Response Concept in Biology and Medicine. Int J Mol Sci. 2016; 17(12): 2034. 10.3390/ijms17122034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bosy-Westphal A, Braun W, Albrecht V, et al. : Determinants of ectopic liver fat in metabolic disease. Eur J Clin Nutr. 2019; 73(2): 209–14. 10.1038/s41430-018-0323-7 [DOI] [PubMed] [Google Scholar]
- 62. Zhai H, Chen C, Wang N, et al. : Blood lead level is associated with non-alcoholic fatty liver disease in the Yangtze River Delta region of China in the context of rapid urbanization. Environ Health. 2017; 16(1): 93. 10.1186/s12940-017-0304-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen R, Xu Y, Xu C, et al. : Associations between mercury exposure and the risk of nonalcoholic fatty liver disease (NAFLD) in US adolescents. Environ Sci Pollut Res Int. 2019; 26(30): 31384–91. 10.1007/s11356-019-06224-5 [DOI] [PubMed] [Google Scholar]
- 64. Frediani JK, Naioti EA, Vos MB, et al. : Arsenic exposure and risk of nonalcoholic fatty liver disease (NAFLD) among U.S. adolescents and adults: An association modified by race/ethnicity, NHANES 2005-2014. Environ Health. 2018; 17(1): 6. 10.1186/s12940-017-0350-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen P, Bornhorst J, Neely MD, et al. : Mechanisms and Disease Pathogenesis Underlying Metal-Induced Oxidative Stress. Oxid Med Cell Longev. 2018; 2018: 7612172. 10.1155/2018/7612172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bondy SC: Metal toxicity, inflammation and oxidative stress. In: Inflammation, Aging, and Oxidative Stress. Springer, Cham. 2016; 3–16. 10.1007/978-3-319-33486-8_1 [DOI] [Google Scholar]
- 67. Planchart A, Green A, Hoyo C, et al. : Heavy Metal Exposure and Metabolic Syndrome: Evidence from Human and Model System Studies. Curr Environ Health Rep. 2018; 5(1): 110–24. 10.1007/s40572-018-0182-3 [DOI] [PMC free article] [PubMed] [Google Scholar]