Abstract
Objectives
To evaluate the feasibility of trialling taxonomy for the rehabilitation of knee conditions—ACL (TRAK-ACL), a digital health intervention that provides health information, personalised exercise plans and remote clinical support combined with treatment as usual (TAU), for people following ACL reconstruction.
Methods
The study design was a two-arm parallel randomised controlled trial (RCT). Eligible participants were English-speaking adults who had undergone ACL reconstruction within the last 12 weeks, had access to the internet and could provide informed consent. Recruitment took place at three sites in the UK. TRAK-ACL intervention was an interactive website informed by behaviour change technique combined with TAU. The comparator was TAU. Outcomes were: recruitment and retention; completeness of outcome measures at follow-up; fidelity of intervention delivery and engagement with the intervention. Individuals were randomised using a computer-generated random number sequence. Blinded assessors allocated groups and collected outcome measures.
Results
Fifty-nine people were assessed for eligibility at two of the participating sites, and 51 were randomised; 26 were allocated to TRAK-ACL and 25 to TAU. Follow-up data were collected on 44 and 40 participants at 3 and 6 months, respectively. All outcome measures were completed fully at 6 months except the Client Service Receipt Inventory. Two patients in each arm did not receive the treatment they were randomised to. Engagement with TRAK-ACL intervention was a median of 5 logins (IQR 3–13 logins), over 18 weeks (SD 12.2 weeks).
Conclusion
TRAK-ACL would be suitable for evaluation of effectiveness in a fully powered RCT.
Keywords: ACL, anterior cruciate ligament, physiotherapy, exercise rehabilitation
Key messages.
What is already known
Rehabilitation following Anterior Cruciate Ligament Reconstruction can be lengthy and challenging.
Digital health interventions may provide an opportunity to improve access to care and support self-management for people following Anterior Cruciate Ligament reconstruction.
What are the new findings
Digital health tools such as the TRAK-ACL website, may provide an opportunity to teach and reinforce the key lessons and exercises at each stage of rehabilitation, as well as engage patients through behaviour change functions.
DHI may be crucial for patients who do not have access to physiotherapy throughout rehabilitation.
TRAK-ACL is a digital health intervention would be suitable for evaluation of effectiveness in a full powered RCT.
Background
ACL injury is common in the active population and can require lengthy and challenging rehabilitation.1–3 Not all patients may have access to the physiotherapy care, information, education, exercise and knowledge needed at each stage of rehabilitation due to lack of time, experienced physiotherapists or specialist resources.4 5 It is reported that only 55% of individuals return to competitive sport and better outcomes are associated with individuals that complete at least 6 months supervised rehabilitation.4 6 7 However, only 30% of individuals with musculoskeletal conditions complete any rehabilitation beyond 6 months.8 It has been argued that digital health interventions (DHIs), such as websites and apps, may provide an opportunity to improve access to care and support self-management in areas where ACL rehabilitation may be inadequately resourced.9
There is a lack of evidence to support the use of DHIs for patient’s post-ACL reconstruction. One DHI which may be suitable for supporting patient’s post-ACL reconstruction is taxonomy for the rehabilitation of knee conditions—ACL (TRAK-ACL). TRAK-ACL stems from TRAK, an interactive for self-management support website,10 which is based on an ontology that describes standard care for the rehabilitation of knee conditions.11 TRAK-ACL focuses specifically on stage-by-stage rehabilitation after ACL reconstruction with the corresponding information presented using animations, videos, text and infographics. It includes a stage-by-stage exercise library and self-assessment criteria for progression. It was developed in line with the principles of the Behaviour Change Wheel, a framework for designing interventions and includes tools for self-monitoring and prompting engagement.12 Previous studies have indicated the acceptability of TRAK-ACL to both patients and clinicians as an adjunct to care.13 14
Given this preliminary evidence suggesting that TRAK-ACL may be acceptable to patients and physiotherapists, and could be integrated into routine National Health Service (NHS) care, it is appropriate to determine whether the intervention is an effective and cost-effective use of NHS resources. The Medical Research Council framework for developing and evaluating complex interventions highlights the importance of feasibility studies for testing procedures, estimating recruitment and retention and determining the sample size of a future randomised controlled trial (RCT).15 The process provides an opportunity to test the acceptability of recruitment pathways, outcome measures and uptake of the intervention to ultimately determine if a full-scale trial can be completed successfully.16 This paper details a randomised feasibility trial of TRAK for patients following ACL reconstruction which aimed to determine the feasibility of an RCT comparing TRAK-ACL plus treatment as usual to treatment as usual (TAU). Specific objectives were to: (1) assess the feasibility of recruiting and retaining participants to the RCT; (2) assess the feasibility of gathering costings data and patient reported outcomes; (3) assess implementation and fidelity issues such as participants’ and physiotherapists’ engagement with the website; (4) assess engagement with the mechanisms of behaviour change and (5) inform the protocol for a fully powered RCT to determine the clinical and cost-effectiveness of TRAK-ACL compared with TAU.
Methods
Design—a randomised controlled feasibility trial
This study was a parallel arm, individually randomised, feasibility RCT comparing postoperative ACL rehabilitation TAU with TAU plus TRAK-ACL. It is reported in line with the Consolidated Standards of Reporting Trials (CONSORT) reporting standards extension for pilot and feasibility trials.17 The trial was supported by the PRIMENT clinical trials unit, a registered UK Clinical Trials Collaboration and a trial steering committee was established to oversee the trial processes.
Patient and public involvement (PPI)
This study won an award for patient involvement. TRAK-ACL was developed with a PPI group in a London NHS hospital.13 18 The group participated in choosing and developing content for the website, ensuring the website met an acceptable standard of diversity and inclusion and the design of the feasibility study. One member of the PPI group sat on the trial steering committee. The opinions of NHS Physiotherapists contributed to the development of the TRAK-ACL website and design of the feasibility study which was informed by the findings of two previous usability and acceptability studies carried out by this team of researchers.13 14
Recruitment
The study took place at three NHS sites; a large University Hospital Foundation Trust, in an ethnically and socio-economically diverse English metropolitan area (site 1), a large South of England Trust covering a mixed urban and rural population (site 2) and large University Health Board in Wales, also mixed urban and rural population (site 3). Recruitment opened in July 2018 at sites 1 and 2. Site 2 was unable to recruit patients and was closed. Site 3 was added in November 2018 and recruitment closed in March 2019. Sites 1 and 3 are reported hereafter.
Sample size
Feasibility study sample sizes are usually 50–70 participants.19–21 However, we looked to the ACL rehabilitation clinical trial literature for estimates of retention and compliance in similar studies.22 The literature suggested that we could expect between 20% and 25% loss to follow-up.23 24 We therefore estimated 75% retention. We estimated that 25 in each arm would give a 95% CI of 0.60 to 0.85, suggesting that we could be 95% certain that at least 60% of the target population would remain in the trial for at least 6 months.
Randomisation
The trial statistician used computer-generated random number sequences to draw up a spread sheet where trial participant numbers could be added sequentially. Participants were allocated to the intervention or control arm by a member of the research team. Randomisation was performed after informed consent and baseline data were collected by the blinded assessor.
Intervention
Participants randomised to the intervention arm received treatment as usual (TAU) plus TRAK-ACL website. The intervention was delivered by qualified chartered physiotherapists working in the musculoskeletal out-patient setting.
TRAK-ACL website
TRAK-ACL is a DHI whose content was drawn from the literature on ACL rehabilitation. It was designed to support patients after ACL reconstruction by reinforcing teaching and exercise prescriptions given by a physiotherapist in face-to-face care. It includes an extensive exercise library and information from the ACL literature and physiotherapists and orthopaedic surgeons experienced in managing patients with ACL reconstruction. The exercises and information were provided phase by phase (early, middle, advanced and return to sport) as videos, animations, infographs and in written text format. Interactive features such as personal goal setting, progress logs and dashboards of progress were informed by the Behaviour Change Wheel framework for intervention design and are known to promote engagement with rehabilitation behaviours.12 The TRAK website can be accessed at: https://spas.cs.cf.ac.uk/trakacl/.
A training package was provided for physiotherapists using TRAK-ACL, including how to induct patients to TRAK-ACL. The TRAK-ACL intervention and the training programme are described in online supplemental file 1 and reported according to the Template for Intervention Description and Replication (TIDieR) guidelines.25 Each patient was given a TRAK-ACL alphanumeric login and they were invited to seek extra support with using TRAK-ACL if needed. Tablet computers were provided to participating sites and Wi-Fi availability was ensured before the study began.
bmjsem-2020-001002supp001.pdf (2.9MB, pdf)
The treating physiotherapist inducted the patient on how to use the TRAK-ACL website and give the participant a login during their first face-to-face consultation. The patient was shown a playlist of exercises which were individualised to their needs by the physiotherapist. Goals were set in discussion with the patient. Both goals and exercise playlists were progressed according to the patient reaching the rehabilitation milestones. All the rehabilitation exercises were on the TRAK-ACL website.
Control group
Physiotherapy TAU varied across the included sites and is described in detail in online supplemental file 1. Common features included face-to-face time with physiotherapists, monitoring of the recovery from surgery and achievement of early rehabilitation goals. The duration of care was not restricted at either site and was expected to last for 6–12 months according to patient needs. A progression through phases of care was evident. The main difference between sites was the mode of delivery: at sites 1 and 2 care was delivered in an ACL group and at site 3 care was delivered though individual one-to-one appointments, with the option of attending a generalised lower limb class. The timing of appointments was different at each site. At sites 1 and 2 appointments were on a weekly basis dropping to fortnightly. At site 3 the scheduling was based on patient need and service capacity.
Outcome measures
The primary outcomes were feasibility outcomes (recruitment and retention) plus measures to inform the parameters of a future trial.26 Study outcomes were taken at baseline, 3 and 6 months.
Primary study outcomes
Feasibility of recruitment, measured by the number of people recruited to the trial (goal of four participants per month per site).
Feasibility of retention, measured by the number of people still in the trial at the end of the study (goal of <30% drops-outs).
Feasibility of collecting outcome measures, measured by the number of complete outcomes that were taken for each time point (goal of >80% at 6 months).
Feasibility of collecting participants’ intervention usage data (goal of three or more logins per week per participant).
Frequency of adverse events (goal <5%).
Secondary study outcomes
Knee Injury & Osteoarthritis Outcome Score27 (KOOS): primary outcome of a future trial. This has five patient-rated scales to assess pain, symptoms, sport, activities of daily living and knee-related quality of life.
Stanford Self-Efficacy Questionnaire; a patient-rated six-item questionnaire to evaluate self-efficacy of condition management in people with long-term conditions.28
EQ-5D-5L, a patient-rated questionnaire to evaluate health-related quality of life.29
The Client Services Receipt Inventory (CSRI) health economic tool, a questionnaire to evaluate health resource use and total costs incurred by patients, their employers, families and local healthcare services.
Strength of quadriceps, which was measured using a standard gym leg press (kg).
Calculation of sample size for a full trial.
The KOOS was chosen as the primary outcome of a future trial on patients with ACL reconstruction using a DHI because of its ability to measure both the impact of knee injury and longer term health outcomes such as osteoarthritis.30
Data collection
Outcomes were collected at physiotherapy appointments at baseline, 3 and 6 months, except quadriceps strength, which was collected at site 1 at 3 and 6 months only. Outcome assessors and the lead researcher were blinded to treatment allocation but neither patients nor treating physiotherapists could be blinded as they needed to use the DHI.
Data analysis
All primary outcome measures were summarised separately by study arm. Differences in secondary outcomes between arms were estimated using linear and logistic regression. Quantitative data were analysed as follows: binary and other categorical measures such as recruitment, retention and adverse events were summarised using frequencies and percentages. Continuous measures were summarised using means and SDs (or medians and IQRs for skewed distributions). The precision of estimates was assessed using 95% CIs. Power analyses were conducted to calculate the sample size necessary to detect an effect of the intervention in a future RCT with the KOOS as the primary outcome. Intention to treat principles were applied to data for all recruited patients.31 Progression of a full-scale trial would be considered if all of the feasibility criteria based on the primary outcomes were met.
Results
Recruitment
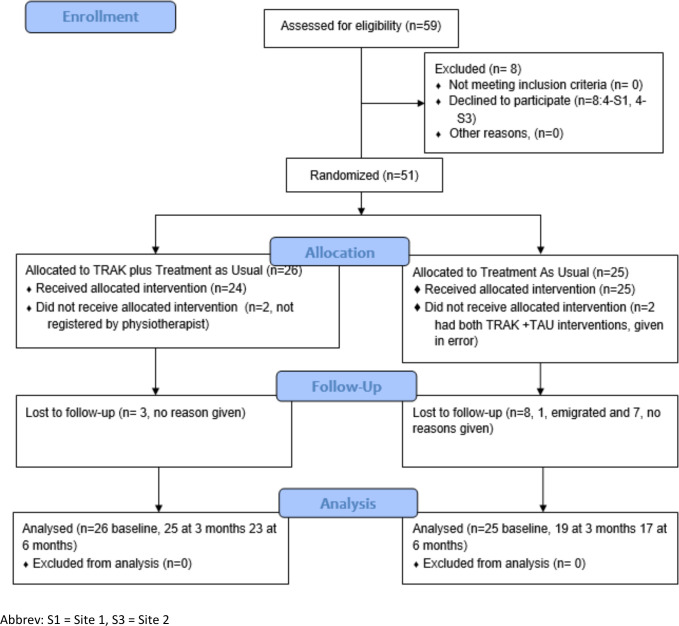
Flow of participants through the trial is illustrated using a CONSORT flow diagram shown in figure 1. Fifty-nine people were assessed for eligibility across two sites, of whom eight people declined to participate and 51 were randomised. Of these, 26 were allocated to TRAK-ACL and 25 to TAU.
Figure 1.
CONSORT flow diagram. TAU, treatment as usual; TRAK, taxonomy for the rehabilitation of knee conditions.
Characteristics at baseline
There were 51 study participants overall. The TAU arm and the TRAK-ACL arm were well-matched for baseline characteristics, presented in table 1.
Table 1.
Baseline characteristics of participants
| Patient characteristics | TAU | TRAK-ACL | Site 3 | Site 1 |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 28.4 (8.2) | 30.8 (11.4) | 29.0 (9.0) | 29.82 (10.2) |
| Site | n25 | n26 | n10 | n41 |
| Freq (%) | Freq (%) | Freq (%) | Freq (%) | |
| Gender | ||||
| Female | 12 (48.0) | 11 (42.3) | 3 (30.0) | 20 (48.8) |
| Male | 13 (52.0) | 15 (57.7) | 7 (70.0) | 21 (51.2) |
| Ethnicity | ||||
| Asian | 1 (4.0) | 0 (0.0) | 0 (0.0) | 1 (2.4) |
| Asian other | 0 (0.0) | 1 (3.9) | 0 (0.0) | 1 (2.4) |
| Black | 3 (12.0) | 2 (7.7) | 0 (0.0) | 5 (12.2) |
| Mixed black and white | 2 (8.0) | 1 (3.9) | 0 (0.0) | 3 (7.3) |
| Mixed other | 1 (4.0) | 0 (0.0) | 0 (0.0) | 1 (2.4) |
| Mixed white and Asian | 0 (0.0) | 1 (3.9) | 0 (0.0) | 1 (2.4) |
| South Asian | 0 (0.0) | 2 (7.7) | 0 (0.0) | 2 (4.9) |
| White | 18 (72.0) | 19 (73.1) | 10 (100) | 27 (65.9) |
| Education level | ||||
| A level or equivalent* | 3 (12.0) | 5 (19.2) | 1 (10.0) | 7 (17.1) |
| Degree/higher degree | 18 (72.0) | 13 (50.0) | 6 (60.0) | 25 (61.0) |
| Diploma higher education | 2 (2.0) | 4 (15.4) | 3 (30.0) | 3 (7.3) |
| GCSE or equivalent† | 2 (2.0) | 4 (15.4) | 0 (0.0) | 6 (14.6) |
| Employment status | ||||
| Currently employed | 20 (80.0) | 19 (73.1) | 7 (70.0) | 32 (78.0) |
| Student | 5 (20.0) | 7 (26.9) | 3 (30.0) | 9 (22.0) |
*A level: subject specific qualification for 16–18 years old.
†GCSE: subject specific qualification taken by 14–16 years old.
CONSORT, Consolidated Standard for Reporting Trials; GCSE, general certificate of seconday education; TAU, treatment as usual; TRAK-ACL, taxonomy for the rehabilitation of knee conditions—ACL.
Randomisation integrity
In TAU, two people were given the intervention by the treating physiotherapist by mistake. However, this was not known until the end of the feasibility study. Not all patients that were allocated to TRAK-ACL received the intervention. At site 3, two patients were allocated to the intervention but were never signed up to TRAK-ACL by the treating physiotherapist (table 2), although they continued to provide outcomes. At site 1, two patients were signed up to TRAK-ACL but never logged in. Hence usage data were only available for 22 participants from the TRAK-ACL group.
Table 2.
Allocation to TRAK-ACL
| TRAK-ACL usage | Total | Site 1 | Site 3 |
| Freq (%) | Freq (%) | Freq (%) | |
| Allocated to TRAK-ACL | 26 (100) | 21 (81.0) | 5 (19.0) |
| Did not receive the intervention Reason |
4 (15.0) | 2 (8) Technical problems prevented login and unknown |
2 (7.5) Not signed up by PT |
| Total for analysis | 22 (85.0) | 19 (73.0) | 3 (11.5) |
| Received the intervention by error Excluded from analysis |
n=2 | n=2 | n=0 |
PT, Physiotherapist; TRAK-ACL, taxonomy for the rehabilitation of knee conditions—ACL.
Retention: numbers analysed
Retention was measured by the proportion of participants providing outcome data at 3 and 6 months. In total, 44 patients were retained to the study at 3 months and 40 at 6 months. Over the course of the study, three people were lost to follow-up in the TRAK-ACL arm and eight were lost to follow-up in TAU (table 3).
Table 3.
Retention per treatment group and across sites
| Retention | Total | TAU | TRAK-ACL |
| Freq (%) | Freq (%) | Freq (%) | |
| Retention | |||
| Baseline | 51 (100) | 25 (100) | 26 (100) |
| 3 months | 44 (86) | 19 (76) | 25 (96) |
| 6 months | 40 (78) | 17 (68) | 23 (88) |
| Retention by site | Site 3 | Site 1 | |
| Freq (%) | Freq (%) | ||
| Retention | |||
| Baseline | 10 (100) | 41 (100) | |
| 3 months | 7 (70) | 37 (90) | |
| 6 months | 7 (70) | 33 (80) | |
TAU, treatment as usual; TRAK-ACL, taxonomy for the rehabilitation of knee conditions—ACL.
Completeness of outcome data
The completeness of outcome data suggested that the number, complexity and time taken to complete items were all acceptable to participants, indicating that data collection for a fully powered RCT will be feasible. All but one outcome measure had 100% item completion for each outcome (table 4). The CSRI was the exception in that it was only completed by 54% of participants at baseline, and then 69% and 89% at 3 and 6 months.
Table 4.
Outcome and data completeness
| Outcome completeness | Total | TAU | TRAK-ACL |
| Freq (%) | Freq (%) | Freq (%) | |
| KOOS | |||
| 0 | 51 (100) | 25 (100) | 26 (100) |
| 3 | 44 (86.27) | 19 (76) | 25 (96.15) |
| 6 | 40 (83.96) | 17 (68) | 23 (88.46) |
| No of items | 42 | 42 | 42 |
| Complete items | 42 (100) | 42 (100) | 42 (100) |
| Self-efficacy | |||
| 0 | 51 (100) | 25 (100) | 26 (100) |
| 3 | 44 (86.27) | 19 (76) | 25 (96.15) |
| 6 | 40 (78.43) | 17 (68) | 23 (88.46) |
| No of items | 6 | 6 | 6 |
| Complete items | 6 (100) | 6 (100) | 6 (100) |
| CSRI | |||
| 0 | 51 (100) | 25 (100) | 26 (100) |
| 3 | 44 (86.27) | 19 (76) | 25 (96.15) |
| 6 | 40 (78.43) | 17 (68) | 23 (88.46) |
| No of items | 114 | 114 | 114 |
| Complete items | 64 (54.38) | 79 (69.29) | 102 (89.47) |
| WPAI | |||
| 0 | 51 (100) | 25 (100) | 26 (100) |
| 3 | 44 (86.27) | 19 (76) | 25 (96.15) |
| 6 | 40 (78.43) | 17 (68) | 23 (31.08) |
| No of items | 6 | 6 | 6 |
| Complete items | 6 (100) | 6 (100) | 6 (100) |
| EQ-5D-5L | |||
| 0 | 51 (100) | 25 (100) | 26 (100) |
| 3 | 44 (86.27) | 19 (76) | 25 (96.15) |
| 6 | 40 (78.43) | 17 (68) | 23 (31.08) |
| No of items | 5 | 5 | 5 |
| Complete items | 5 (100) | 5 (100) | 5 (100) |
| Strength outcome | 3 months | 6 months | |
| Leg extension | |||
| Total in study at site 1 | 37 | 33 | |
| Yes | 33 (89.18) | 30 (90.90) | |
| No | 4 (10.81) | 3 (9.09) | |
| Reason | Pain or DNA | Pain or DNA |
| Physiotherapy appointments | n=51, mean (SD) | ||
| Total physiotherapy appointments per person | 16.01 (5.04) |
EQ-5D-5L, an instrument for measuring generic health related quality of life
CSRI, Client Services Receipt Inventory; KOOS, Knee Injury and Osteoarthritis Outcome Score; TAU, treatment as usual; TRAK—ACL, taxonomy for the rehabilitation of knee conditions—ACL; WPAI, work productivity and impairment.
TRAK-ACL usage data
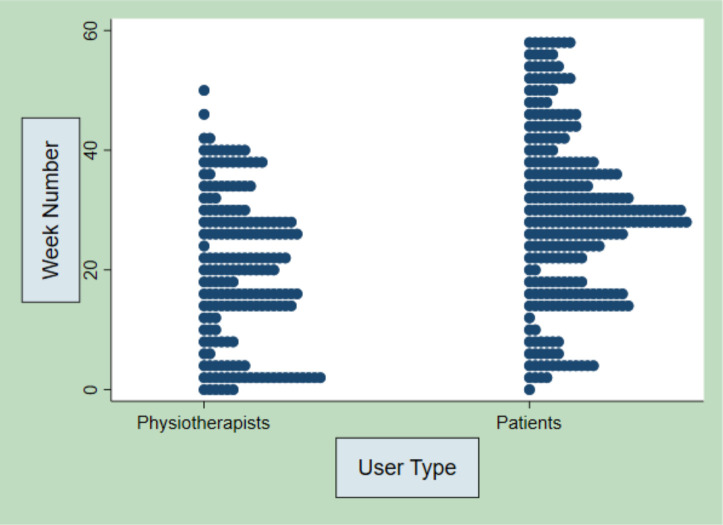
Usage of the TRAK-ACL intervention was measured by the number of logins to the website, videos watched and behaviour change functions used, for example, exercise log, goal setting or weekly progress. These findings are displayed in full detail in online supplemental information 2—TRAK-ACL usage data. In summary, the median number of logins per patient participant was 5 with IQR of 3–13. The median (IQR) patient logins per week was 4 (2–7). The time between patients’ first and last login was a mean of 18 weeks (12.2SD), which suggests that users continue to engage over time and across phases of care. The median (range) physiotherapist logins per week was 2 (0–5). The median (range) logins per physiotherapist over the duration of the trial was 11.5 (6–18.5). The logins of physiotherapists and patients over 60 weeks, given in figure 2, showed consistency of patient usage as physiotherapist usage dwindles and disappears over the last 20 weeks.
Figure 2.
Physiotherapist and patient logins over 60-week duration of the feasibility trial.
bmjsem-2020-001002supp002.pdf (310.7KB, pdf)
There were no reported adverse events for participants enrolled on this feasibility trial.
Analysis of secondary outcomes
The analysis of secondary outcomes is discussed in online supplemental information 3. The results show that the primary outcome of a future substantive RCT would be the KOOS at 6 months follow-up. A power calculation assuming a SD of 17.2671 on the KOOS estimates that a sample size of 172 participants (86 per study arm) will be required to detect a nine point difference on the KOOS between intervention and control groups with 90% power and 5% alpha. After inflation for 20% loss to follow-up, this figure increases to 108 participants per arm, 216 in total.
bmjsem-2020-001002supp003.pdf (630.6KB, pdf)
Discussion
Principal findings
The aim of the study was to determine the feasibility of an RCT comparing TRAK-ACL plus TAU to TAU in the management of postoperative patients with ACL. The findings suggest a future RCT to determine effectiveness, as measured by the KOOS, would be feasible, as patients were successfully recruited and retained and provided adequate outcome data, with the exception of the CSRI. Further work may be needed to improve data collection for an assessment of cost-effectiveness. The findings on usage suggest that patients and physiotherapists engaged with the TRAK-ACL intervention, in terms of number of log-ins, uptake of material and duration of engagement.
Recruitment and retention
Eighty-six per cent of those approached about the study were recruited. The recruitment target of four patients per month was exceeded at site 1 where there was a dedicated researcher to facilitate recruitment. Site 2 recruitment was affected by changes to orthopaedic team leading to a significant drop in patients with ACL reconstruction. This could have been anticipated by better engagement with orthopaedics through the planning stage. It is known that recruitment challenges can be a key reason why randomised trials fail32 so a robust recruitment outcome was important.33 Recruitment method was highlighted as a key barrier to participation in previous DHI studies where in one example less than half of the physiotherapists allocated to delivering DHI actually recruited patients34 and in another only 30% of eligible patients were recruited.35 Strategies such as personalising the intervention (personal exercise plans) and dedicated staff support were implemented in the current study which may have contributed to recruitment success.36
At 6-month follow-up 78% of participants were retained, which exceed the criteria set for this study of less than 30% drop-outs. Eysenbach et al described two types of attrition; non-usage attrition (non-use of the DHI) and drop out attrition (lost to follow-up in the trial).37 Some patient loss can be explained by typical drop out from physiotherapy over the duration of ACL rehabilitation,38 and dropout rates can exceed 30% in trials for DHI and exercise.14 39 This study suggests that patients with ACL reconstruction will engage with the DHI and trial process over time and that the role of the research assistant seems key to facilitating outcome collection.
Usage of TRAK-ACL
Usage data from TRAK-ACL show that there was consistent engagement by some users over time, indicating improved access to care. For the current study, a progression criterion of three logins per week per patient was set based on current evidence.4 Use of the behaviour change mechanisms such as logs, goal setting and educational and motivational content was accessed by up to 50% of patient TRAK-ACL users which may have improved these patients adherence to the target behaviour.
The usage data shown in this trial are similar to reported use data for health websites and apps used alongside treatment as usual.35 40–42 Additionally, levels of usage found in this study are similar to those found for other physiotherapy digital intervention feasibility studies14 43 and may indicate a growing acceptability of DHIs. A future trial would benefit from addressing physiotherapist engagement which may be affected by factors such as time and willingness to adopt new technology, understanding of new technology, the extent to which new technology meets user needs and the workplace support to maximise the potential of new technology.34 44
Outcomes
The gathering of clinical outcomes such as KOOS, the self-efficacy score, EQ-5D-5L and work productivity and impairment was also shown to be feasible in this trial. There was 100% completion by all patients who were retained to the trial at 6 months in four out of five outcomes, which exceeded the feasibility progression criterion of 80% completion set for this study. The exception to good outcome collection was the CSRI. It is considered a valuable tool of health economic analysis and should be included where possible.45 46 In a future RCT, a digital rather than paper version could facilitate cleaner more usable data. Likewise, strength testing would be a key outcome of a future trial.3 47 The method of collecting strength testing across sites was not standardised and in a future trial isokinetic muscle strength testing of quadriceps and hamstrings at 180°/s should be considered. Feasibility of strength testing was successful however, clinical trial standard strength testing requires standardised conditions and calibration of machines.48
Strengths and limitations
The strength of this study was the success of recruitment pathways, outcome measures and intervention toward determining feasibility. Progression to a full RCT would be recommended as the feasibility progression criteria for recruitment, retention, completeness of outcome measures at 6 months and adverse outcomes have all been met. The total sample size calculated for a full RCT was 216 participants (allowing for drop-outs). This would be achievable based on the recruitment to this study but would require 12 months’ recruitment over a minimum of five sites.
Not all patient participants engaged with all aspects of the behaviour change mechanisms built into TRAK-ACL and physiotherapists did not appear to sustain engagement with TRAK-ACL through to the return to sport phase of rehabilitation. Exploring this in a qualitative study would have strengthened this study and design of a future RCT. Measuring the number of face-to-face physiotherapist contacts would help evaluate if TRAK-ACL resulted in reduced physiotherapist contacts. The study was limited by the specificity of usage measurement that TRAK-ACL was capable of when considered against the AMUsED framework, a standard for reporting usage in digital trials.49 In a future trial, TRAK-ACL would need more specific usage measurement capabilities.
Conclusions
The results of this study suggest that TRAK-ACL is suitable to go forward to a fully powered RCT investigating whether patients using a DHI as well as treatment as usual have better outcomes than patients receiving just treatment as usual. It is essential that sufficient support for trial delivery is built into a future RCT. Future research should aim to ensure that the DHI is stable and capable of measuring all relevant aspects of engagement before going to trial.
New findings
Patient engagement with educational resources and exercises on the DHI occurs across multiple phases of the rehabilitation.
Digital health tools such as the TRAK-ACL website may provide an opportunity to teach and reinforce the key lessons and exercises at each stage of rehabilitation, as well as engage patients through behaviour change functions.
DHI may be crucial for patients who do not have access to physiotherapy throughout rehabilitation.
Footnotes
Twitter: @k8button
Contributors: ED wrote this manuscript with contributions from all authors. IS, KB and ED co-designed the TRAK-ACL website. JW, KB and IS helped with the acquisition of data. ED, KB, FH and EM contributed to the interpretation of the statistical data and involved in discussing the clinical implications of the findings. All authors read and approved the final manuscript.
Funding: This study/project is funded by the National Institute for Health Research (NIHR) Clinical Doctoral Research Fellow programme (ICA-CDRF-2016-02-027). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Deidentified participant data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Ethical approval was obtained (18/LO/0403). The trial was registered with the ISRCTN (ID ISRCTN55635910) and the NIHR Clinical Research Network Portfolio: CPMS ID 37879.
References
- 1.Hewett TE, Ford KR, Hoogenboom BJ. Understanding and preventing ACL injuries: current biomechanical and epidemiologic considerations. 5. North American journal of sports physical therapy : NAJSPT, 2010: 234–51. [PMC free article] [PubMed] [Google Scholar]
- 2.Bollen S. Epidemiology of knee injuries: diagnosis and triage. Br J Sports Med 2000;34:227–8 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1763268/pdf/v034p00227a.pdf 10.1136/bjsm.34.3.227-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grindem H, Snyder-Mackler L, Moksnes H, et al. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Br J Sports Med 2016;50:804–8. 10.1136/bjsports-2016-096031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunphy E, Hamilton FL, Button K, et al. A scoping review of the resources needed to deliver anterior cruciate ligament physiotherapy rehabilitation in randomised controlled trials. Phys Ther Rev [Internet] 2020;25:81–95 https://www.tandfonline.com/doi/full/ [Google Scholar]
- 5.A H, Dixon J. The NHS long term plan. Br Med J Publ Gr 2019;364:l84. 10.1136/bmj.l84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker A, Hing W, Lorimer A. The influence, barriers to and facilitators of anterior cruciate ligament rehabilitation adherence and participation: a scoping review. Sports Med Open 2020;6:32. 10.1186/s40798-020-00258-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards PK, Ebert JR, Joss B, et al. Patient characteristics and predictors of return to sport at 12 months after anterior cruciate ligament reconstruction: the importance of patient age and postoperative rehabilitation. Orthop J Sports Med 2018;6:2325967118797575. 10.1177/2325967118797575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey DL, Holden MA, Foster NE, et al. Defining adherence to therapeutic exercise for musculoskeletal pain: a systematic review. Br J Sports Med 2020;54:326–31. 10.1136/bjsports-2017-098742 [DOI] [PubMed] [Google Scholar]
- 9.NHS Digital. Digital inclusion for health and social care, 2019. [Google Scholar]
- 10.Spasić I, Button K, Divoli A, et al. TRAK APP suite: a web-based intervention for delivering standard care for the rehabilitation of knee conditions. JMIR Res Protoc 2015;4:e122 http://www.researchprotocols.org/2015/4/e122/ 10.2196/resprot.4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Button K, van Deursen RW, Soldatova L, et al. TRAK ontology: defining standard care for the rehabilitation of knee conditions. J Biomed Inform 2013;46:615-25. 10.1016/j.jbi.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 12.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6:42. 10.1186/1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunphy E, Hamilton FL, Spasić I, et al. Acceptability of a digital health intervention alongside physiotherapy to support patients following anterior cruciate ligament reconstruction. BMC Musculoskelet Disord 2017;18:471. 10.1186/s12891-017-1846-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Button K, Nicholas K, Busse M, et al. Integrating self-management support for knee injuries into routine clinical practice: TRAK intervention design and delivery. Musculoskelet Sci Pract 2018;33:53-60. 10.1016/j.msksp.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 15.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new medical Research Council guidance. BMJ 2008;337:a1655 http://www.bmj.com/content/337/bmj.a1655 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Research NI for H . Definition of feasibility versus pilot studies, 2019. [Google Scholar]
- 17.Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355:i5239. 10.1136/bmj.i5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunphy E, Hamilton FL, Button K. Taxonomy for the rehabilitation of knee conditions (TRAK), a digital intervention to support the self-care components of anterior cruciate ligament rehabilitation: protocol of a feasibility study. JMIR Res Protoc 2016;5:e234 http://www.researchprotocols.org/2016/4/e234/ 10.2196/resprot.6402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 2005;4:287–91. 10.1002/pst.185 [DOI] [Google Scholar]
- 20.Billingham SAM, Whitehead AL, Julious SA. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom clinical research network database. BMC Med Res Methodol 2013;13:104. 10.1186/1471-2288-13-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sim J, Lewis M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J Clin Epidemiol 2012;65:301–8. 10.1016/j.jclinepi.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 22.Whitehead AL, Sully BGO, Campbell MJ. Pilot and feasibility studies: is there a difference from each other and from a randomised controlled trial? Contemp Clin Trials 2014;38:130–3. 10.1016/j.cct.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 23.Frobell RB, Roos EM, Roos HP, et al. A randomized trial of treatment for acute anterior cruciate ligament tears. N Engl J Med 2010;363:331–42. 10.1056/NEJMoa0907797 [DOI] [PubMed] [Google Scholar]
- 24.Risberg MA, Holm I, Myklebust G, et al. Neuromuscular training versus strength training during first 6 months after anterior cruciate ligament reconstruction: a randomized clinical trial. Phys Ther 2007;87:737–50. 10.2522/ptj.20060041 [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 26.Blatch-Jones AJ, Pek W, Kirkpatrick E, et al. Role of feasibility and pilot studies in randomised controlled trials: a cross-sectional study. BMJ Open 2018;8:e022233. 10.1136/bmjopen-2018-022233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roos EM, Roos HP, Lohmander LS, et al. Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. J Orthop Sports Phys Ther 1998;28:88–96. 10.2519/jospt.1998.28.2.88 [DOI] [PubMed] [Google Scholar]
- 28.Lorig KR, Sobel DS, Ritter PL, et al. Effect of a self-management program on patients with chronic disease. Eff Clin Pract 2001;4:256–62 http://www.ncbi.nlm.nih.gov/pubmed/11769298 [PubMed] [Google Scholar]
- 29.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Quality of Life Research 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roos EM, Lohmander LS. The knee injury and osteoarthritis outcome score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes 2003;1:64. 10.1186/1477-7525-1-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollis S, Campbell F. What is meant by intention to treat analysis? survey of published randomised controlled trials. BMJ 1999;319:670–4. 10.1136/bmj.319.7211.670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Briel M, Olu KK, von Elm E, et al. A systematic review of discontinued trials suggested that most reasons for recruitment failure were preventable. J Clin Epidemiol 2016;80:8–15. 10.1016/j.jclinepi.2016.07.016 [DOI] [PubMed] [Google Scholar]
- 33.Bower P, Brueton V, Gamble C, et al. Interventions to improve recruitment and retention in clinical trials: a survey and workshop to assess current practice and future priorities. Trials 2014;15:399. 10.1186/1745-6215-15-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kloek CJJ, Bossen D, de Vries HJ, et al. Physiotherapists' experiences with a blended osteoarthritis intervention: a mixed methods study. Physiother Theory Pract 2020;36:572–9. 10.1080/09593985.2018.1489926 [DOI] [PubMed] [Google Scholar]
- 35.Goode K. Evaluation of a digital health resource providing physiotherapy information for postnatal women in a tertiary public hospital in Australia. 4. mHealth, 2018: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Connor S, Hanlon P, O'Donnell CA, et al. Understanding factors affecting patient and public engagement and recruitment to digital health interventions: a systematic review of qualitative studies. BMC Med Inform Decis Mak 2016;16:120. 10.1186/s12911-016-0359-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eysenbach G. The law of attrition. J Med Internet Res 2005;7:e11. 10.2196/jmir.7.1.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartigan EH, Axe MJ, Snyder-Mackler L. Time line for noncopers to pass return-to-sports criteria after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 2010;40:141–54. 10.2519/jospt.2010.3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levinger P, Dunn J, Bifera N, et al. High-speed resistance training and balance training for people with knee osteoarthritis to reduce falls risk: study protocol for a pilot randomized controlled trial. Trials 2017;18:384 http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med14&NEWS=N&AN=28821271 10.1186/s13063-017-2129-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kristjánsdóttir OB, Fors EA, Eide E, et al. A smartphone-based intervention with diaries and therapist-feedback to reduce catastrophizing and increase functioning in women with chronic widespread pain: randomized controlled trial. J Med Internet Res 2013;15:e5. 10.2196/jmir.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthews L, Pugmire J, Moore L, et al. Study protocol for the 'HelpMeDoIt!' randomised controlled feasibility trial: an app, web and social support-based weight loss intervention for adults with obesity. BMJ Open 2017;7:e017159. 10.1136/bmjopen-2017-017159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray E, Sweeting M, Dack C, et al. Web-based self-management support for people with type 2 diabetes (HeLP-Diabetes): randomised controlled trial in English primary care. BMJ Open 2017;7:e016009. 10.1136/bmjopen-2017-016009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowe CJM, Kelly P, Milton K, et al. WALK30X5”: a feasibility study of a physiotherapy walking programme for people with mild to moderate musculoskeletal conditions. Physiother 2020;107:275–85. [DOI] [PubMed] [Google Scholar]
- 44.Topol E. The Topol review. preparing the healthcare workforce to deliver the digital future, 2019. [Google Scholar]
- 45.Jessep SA, Walsh NE, Ratcliffe J, et al. Long-term clinical benefits and costs of an integrated rehabilitation programme compared with outpatient physiotherapy for chronic knee pain. Physiotherapy 2009;95)::94–102. Jun;. 10.1016/j.physio.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 46.Knapp M, Chisholm D. Client socio-demographic and service receipt inventory–European version, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Lynch AD, Logerstedt DS, Grindem H, et al. Consensus criteria for defining 'successful outcome' after ACL injury and reconstruction: a Delaware-Oslo ACL cohort investigation. Br J Sports Med 2015;49:335–42. 10.1136/bjsports-2013-092299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winter EM, Jones AM, Davison RR, et al. Sport and exercise physiology testing guidelines: volume I–Sport testing: the British association of sport and exercise sciences guide. Routledge, 2006. [Google Scholar]
- 49.Miller S, Ainsworth B, Yardley L, et al. A framework for analyzing and measuring usage and engagement data (AMUsED) in digital interventions: viewpoint. J Med Internet Res 2019;21:e10966 http://www.jmir.org/2019/2/e10966/ 10.2196/10966 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjsem-2020-001002supp001.pdf (2.9MB, pdf)
bmjsem-2020-001002supp002.pdf (310.7KB, pdf)
bmjsem-2020-001002supp003.pdf (630.6KB, pdf)
Data Availability Statement
Deidentified participant data are available upon reasonable request.