Abstract
Background
In patients with ST-segment elevation myocardial infarction (STEMI), it is unknown how patient delay modulates the beneficial effects of timely reperfusion.
Aims
To assess the prognostic significance of a contact-to-balloon time of less than 90 min on in-hospital mortality in different categories of symptom-onset-to-first-medical-contact (S2C) times.
Methods
A total of 20 005 consecutive patients from the Feedback Intervention and Treatment Times in ST-segment Elevation Myocardial Infarction (FITT-STEMI) programme treated with primary percutaneous coronary intervention (PCI) were included.
Results
There were 1554 deaths (7.8%) with a J-shaped relationship between mortality and S2C time. Mortality was 10.0% in patients presenting within 1 hour, and 4.9%, 6.0% and 7.3% in patient groups with longer S2C intervals of 1–2 hours, 2–6 hours and 6–24 hours, respectively. Patients with a short S2C interval of less than 1 hour (S2C<60 min) had the highest survival benefit from timely reperfusion with PCI within 90 min (OR 0.27, 95% CI 0.23 to 0.31, p<0.0001) as compared with the three groups with longer S2C intervals of 1 hour<S2C≤2 hours (OR 0.44, 95% CI 0.33 to 0.59, p<0.0001), 2 hours<S2C≤6 hours (OR 0.49, 95% CI 0.38 to 0.64, p<0.0001) and 6 hours<S2C≤24 hours (OR 0.42, 95% CI 0.30 to 0.58, p<0.0001).
Conclusions
Timely reperfusion with a contact-to-balloon time of less than 90 min is most effective in patients presenting with short S2C intervals of less than 1 hour, but has also beneficial effects in patients with S2C intervals of up to 24 hours.
Trial registration number
Keywords: acute coronary syndrome, chest pain, myocardial infarction
Key questions.
What is already known about this subject?
In ST-segment elevation myocardial infarction (STEMI) patients, the relationship between in-hospital mortality and total ischaemic time defined as the interval from symptom onset to balloon inflation is J-shaped. However, how patient delay modulates the effects of timely reperfusion remains unknown.
What does this study add?
Timely reperfusion within 90 min of the first medical contact is most effective in STEMI patients presenting within a short interval from symptom onset to first medical contact of less than 1 hour. However, it also has beneficial effects in patients with much longer intervals with respect to better survival.
How might this impact on clinical practice?
Our data suggest for the first time that measures for timely reperfusion should be applied to all patients irrespective of patient delay.
Introduction
Early reversal of abnormal epicardial flow by primary percutaneous coronary intervention (PCI) is the primary strategy in acute ST-segment elevation myocardial infarction (STEMI) management to reduce mortality and improve clinical outcome.1–5 Numerous clinical studies have demonstrated that, in STEMI patients, longer ischaemic time following occlusion of an epicardial coronary artery is associated with higher mortality.6–16 In comparison to prolonged occlusion, shorter ischaemic time resulted in decreased infarct size and reduced myocardial damage.17–19 However, how various categories with respect to the duration from symptom onset to first medical contact affect mortality and, in particular, how patient delay modulates the beneficial effects of timely reperfusion remains unknown.
Therefore, in this post hoc analysis, we tested whether early PCI treatment within 90 min of the first medical contact, which is an established standard process metric in the guideline-directed management of STEMI patients,4 20 had beneficial effects on hospital mortality in different patient groups as stratified by the time interval from of the onset of symptoms to the emergency medical service (EMS) arrival at the scene.
Methods
Participating PCI hospitals
The Feedback Intervention and Treatment Times in STEMI (FITT-STEMI) study is an ongoing multicentre trial currently including a network of 47 PCI hospitals throughout Germany. The study aims to assess the prognostic impact of a standardised feedback-driven quality management initiative for timely reperfusion therapy on treatment times and mortality in local STEMI treatment networks. The current analysis included data from the period from January 2006 to December 2017. Details of the protocol of the FITT-STEMI study including the predefined outcome measures have been published elsewhere.5 21 22 The PCI hospitals are obligated to prospectively enrol, without exception, all consecutive STEMI patients who presented within less than 24 hours after onset of infarct symptoms. The population analysed in the present paper comprised all STEMI patients who arrived at the primary PCI hospital via EMS with treatment times of less than 6 hours from arrival at the scene to balloon inflation.
Assessment of symptom onset
Patients were asked to provide information about their precise time of symptom onset related to the current episode of myocardial infarction. Symptoms of angina pectoris were defined as any complaint of chest pain or compression due to myocardial ischaemia, which did not subside spontaneously and occurred both at rest or during exercise. When related to the current episode, pain radiating to left arm, neck or jaw was taken into account. Symptoms indicative of preinfarction unstable angina pectoris were documented. Symptom-to-contact time intervals were categorised in groups with ‘very early’ (S2C≤1 hour), ‘early’ (1 hour<S2C≤2 hours), ‘intermediate’ (2 hours<S2C≤6 hours) and ‘late’ (6 hours<S2C≤24 hours) presentation. Total ischaemic time was the time elapsed from symptom onset, when chest pain became more intense and sustained, to the balloon inflation in the catheterisation laboratory following wire crossing of the culprit lesion.
Clinical data collection
Besides clinical information on symptom onset, data on pre-hospital and in-hospital treatment times were collected for each consecutive STEMI patient on a case-report form. Key treatment times included the time of arrival on the scene by EMS which in Germany is usually staffed both with a trained physician and experienced paramedics. The duration of the transport to the PCI centre, the intra-hospital transfer of the patient to the catheterisation laboratory as well as the time points of puncture and first balloon inflation of the culprit lesion were documented. Furthermore, data were obtained on comorbid diagnoses, medical history, prior medication, blood pressure, heart rate, Killip classification and the thrombolysis in myocardial infarction (TIMI) risk score for STEMI, which is a well-validated prognostication scheme categorising the patient’s risk of death. Results from coronary angiography and the assessment of Killip class were recorded. The FITT-STEMI study was conducted in accordance with the Declaration of Helsinki.
Data analysis and feedback interventions
Site-specific data assessment was completed for each year, and annual feedback interventions were performed. At these meetings, outcome data for each local PCI clinic were discussed in interactive sessions with members of the interdisciplinary STEMI treatment teams, including staff from the local EMS, all physicians and nurses working in the emergency department and the emergency responding system as well as staff members in the cardiac catheterisation laboratory and interventional cardiologists. Based on the site-specific descriptive data, particular attention was given to identify components in the treatment chain which require further improvement in order to shorten transportation times and prevent procedural delays during the time from the first medical contact to direct handoff in the cardiac catheterisation laboratory.
Statistical analysis
For each STEMI patient, relevant treatment time intervals from first medical contact at the scene to balloon inflation were determined from raw data. Study patients were grouped according to the following four S2C intervals: S2C≤1 hour (group 1), 1 hour<S2C≤2 hours (group 2), 2 hours<S2C≤6 hours (group 3) and 6 hours<S2C≤24 hour (group 4). Continuous data were reported as means and SD and compared using analysis of variance, whereas χ2 tests were used to compare categorical variables among the four groups. In addition, the study population was dichotomised along the guideline-recommended cut-off level for contact-to-balloon time of equal to or less than 90 min. Logistic regression analyses were performed to examine the impact of duration of symptom onset to EMS arrival at the field on mortality and how this relationship was affected by the treatment time to reperfusion. In a basic model, contact-to-balloon time of ≤90 min and its interaction term with symptoms duration as classified by the four groups were used as independent variables. The group with the shortest symptom onset (S2C≤1 hour) was set as the reference category. To identify significant predictors of in-hospital mortality, a logistic regression model was computed using a stepwise backward-selection method. Likewise, the model used dichotomised data for contact-to-balloon time along the 90 min cut-off level as the independent variable and was adjusted for the following potential confounders: age, gender, arterial hypertension, diabetes mellitus, hyperlipoproteinaemia, family history of myocardial infarction, smoker status, previous stroke, renal failure, number of coronary arteries narrowed, electrocardiographic localisation and recanalisation of the culprit coronary artery, chronic vessel occlusion in a non-infarct-related coronary artery, and TIMI flow grades before and after PCI (score ≤2 vs 3). The results from the regression analyses are presented as OR with their 95% CIs. Supporting analyses were carried out taking only patients without out-of-hospital cardiac arrest (OHCA) into consideration; furthermore, the thresholds 60 min and 120 min rather than 90 min for the contact-to-balloon time were additionally used. The reported p values are all two sided, and p values of <0.05 were considered statistically significant. No formal adjustment for multiple testing was carried out. Statistical analyses were performed on a personal computer using the software program SAS V.9.4 (SAS Institute).
Results
Baseline characteristics and treatment times in patients with different symptom-to-first medical contact intervals
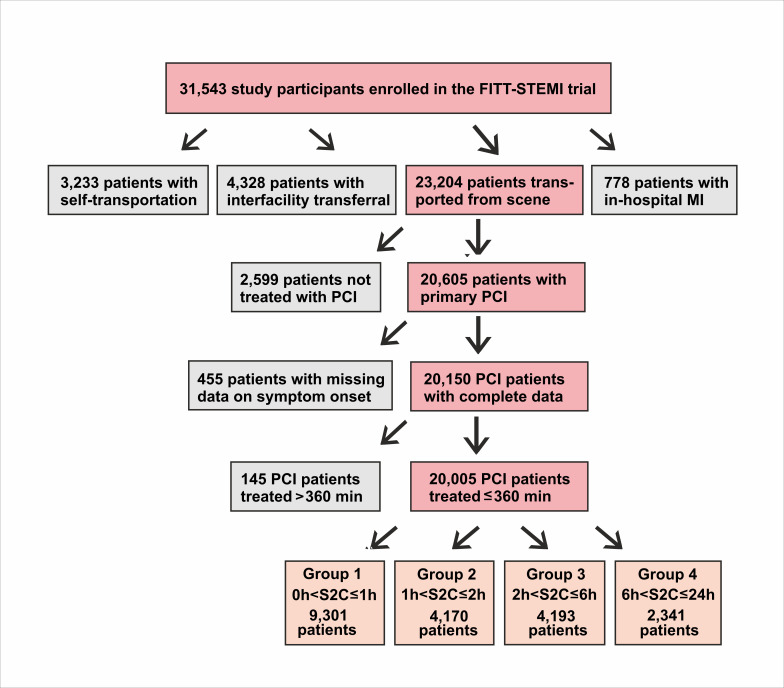
The total study population comprised 20 005 PCI-treated STEMI patients with complete survival data who were transported by EMS and had treatment times of less than 360 min from arrival at the scene to reperfusion (figure 1). The mean age of the study population was 63.8±12.8 years and the majority were men (14 721; 74%). Epidemiological and clinical data including comorbidities of the total study population are presented in table 1. The proportions of females and older subjects increased in patient groups with longer S2C intervals (both p<0.0001). Pre-hospital and intra-hospital times to reperfusion differed with respect to duration of chest pain. Patients with an S2C time lasting longer than 6 hours had the shortest mean contact-to-door time (37.0±15.9 min, p<0.0001) (table 1). In contrast, this group also had the longest documented door-to-balloon time (60.8±44.0 min, p<0.0001) and contact-to-balloon time (97.7±47.3 min, p<0.0001), indicating significant delays in the intra-hospital treatment chain. These delays during intra-hospital transport did not result from a higher proportion of patients transported via the emergency department, since the ratio of patients bypassing the emergency department in this group (54%) was in the same range as in patients with shorter symptom duration (p=0.2640).
Figure 1.
Flow diagram of the PCI-treated STEMI patients from the FITT-STEMI study population as stratified by four groups of symptom-onset-to-first-medical-contact times. FITT-STEMI, Feedback Intervention and Treatment Times in ST-segment Elevation Myocardial Infarction; PCI, percutaneous coronary intervention.
Table 1.
Demographic, clinical and angiographic characteristics of STEMI patients
| Total cohort | Group 1 | Group 2 | Group 3 | Group 4 | P value | |
| 0 hour<S2C≤24 hours | S2C≤1 hour | 1 hour<S2C≤2 hours | 2 hours<S2C≤6 hours | 6 hours<S2C≤24 hours | ||
| (n=20 005) | (n=9301) | (n=4170) | (n=4193) | (n=2341) | ||
| Demographic data | ||||||
| Male gender | 14 721 (74%) | 7180 (77%) | 3007 (72%) | 2943 (70%) | 1591 (68%) | <0.0001 |
| Age±SD (years) | 63.8±12.8 | 62.5±12.7 | 64.1±12.9 | 65.3±12.9 | 65.5±12.5 | <0.0001 |
| Age >80 years | 2171 (11%) | 866 (9%) | 478 (11%) | 544 (13%) | 283 (12%) | <0.0001 |
| Clinical data | ||||||
| Body mass index (kg/m²) | 27.5±4.6 | 27.5±4.4 | 27.6±4.7 | 27.6±4.6 | 27.6±4.8 | 0.142 |
| Hypertension | 11 897 (59%) | 5332 (57%) | 2522 (60%) | 2595 (62%) | 1448 (62%) | <0.0001 |
| Diabetes mellitus | 3486 (17%) | 1420 (15%) | 764 (18%) | 811 (19%) | 491 (21%) | <0.0001 |
| Prior angina pectoris | 2432 (12%) | 966 (10%) | 529 (13%) | 560 (13%) | 377 (16%) | <0.0001 |
| Hyperlipidaemia | 5825 (29%) | 2671 (29%) | 1.212 (29%) | 1281 (31%) | 661 (28%) | 0.1226 |
| Family history | 3799 (19%) | 1753 (19%) | 814 (19%) | 791 (19%) | 441 (18%) | 0.8195 |
| Current smoker | 8433 (42%) | 4082 (44%) | 1792 (43%) | 1665 (40%) | 894 (38%) | <0.0001 |
| Previous MI | 2216 (11%) | 1136 (12%) | 487 (12%) | 389 (9%) | 204 (9%) | <0.0001 |
| Previous stroke | 823 (4%) | 391 (4%) | 184 (4%) | 166 (4%) | 82 (4%) | 0.3108 |
| Previous angioplasty | 2325 (12%) | 1191 (13%) | 522 (13%) | 411 (10%) | 201 (9%) | <0.0001 |
| Previous CABG | 422 (2%) | 175 (2%) | 97 (2%) | 97 (2%) | 53 (2%) | 0.2218 |
| Renal failure | 956 (5%) | 434 (5%) | 175 (4%) | 213 (5%) | 134 (6%) | 0.0336 |
| ECG (STEMI site) | 0.0003 | |||||
| Anterior | 8835 (44%) | 4256 (46%) | 1773 (42%) | 1743 (42%) | 1063 (45%) | |
| Inferior | 9930 (50%) | 4468 (48%) | 2155 (52%) | 2181 (52%) | 1126 (48%) | |
| Lateral | 1051 (5%) | 485 (5%) | 210 (5%) | 228 (5%) | 128 (5%) | |
| LBBB | 189 (1%) | 89 (1%) | 35 (1%) | 41 (1%) | 24 (1%) | |
| TIMI risk score | ||||||
| 0–2 | 7.011 (35%) | 3525 (38%) | 1640 (39%) | 1313 (31%) | 533 (23%) | <0.0001 |
| 3–4 | 5782 (29%) | 2622 (28%) | 1272 (30%) | 1233 (29%) | 655 (28%) | |
| 5–8 | 6255 (31%) | 2714 (29%) | 1126 (27%) | 1442 (34%) | 973 (42%) | |
| >8 | 965 (5%) | 443 (5%) | 136 (3%) | 206 (5%) | 180 (8%) | |
| Cardiogenic shock | 2423 (12%) | 1646 (18%) | 309 (7%) | 299 (7%) | 169 (7%) | <0.0001 |
| OHCA | 1881 (9%) | 1474 (16%) | 171 (4%) | 156 (4%) | 80 (3%) | <0.0001 |
| Procedural data | ||||||
| Time of EMS at scene (min) | 23.4±11.8 | 24.1±12.7 | 21.3±10.5 | 21.1±10.8 | 20.2±10.7 | <0.0001 |
| Transportation time (min) | 17.0±10.7 | 17.0±10.6 | 17.2±10.7 | 17.1±10.3 | 16.7±11.6 | 0.222 |
| Contact-to-door time (min) | 39.5±16.2 | 41.1±16.8 | 38.5±15.4 | 38.2±15.3 | 37.0±15.9 | <0.0001 |
| Door-to-cath. lab. time (min) | 24.1±33.1 | 23.4±31.0 | 23.0±31.5 | 24.8±35.1 | 27.3±39.2 | <0.0001 |
| Cath. lab.-to-puncture (min) | 12.0±7.1 | 12.2±7.3 | 11.9±6.7 | 11.7±6.8 | 11.9±7.1 | 0.0002 |
| Puncture-to-balloon (min) | 20.4±12.6 | 20.1±12.7 | 20.2±12.2 | 20.5±12.0 | 21.6±14.2 | <0.0001 |
| Door-to-balloon time (min) | 56.5±37.6 | 55.7±35.6 | 55.1±35.9 | 57.0±39.4 | 60.8±44.0 | <0.0001 |
| Contact-to-balloon (min) | 95.9±41.3 | 96.8±39.9 | 93.6±39.6 | 95.3±42.2 | 97.7±47.3 | <0.0001 |
| Direct transfer to cath. lab. (%) | 11 022 (55%) | 5076 (55%) | 2332 (56%) | 2345 (56%) | 1269 (54%) | 0.264 |
| Angiographic data | ||||||
| No of coronary arteries narrowed | ||||||
| 0 | 60 (0.3%) | 26 (0.3%) | 11 (0.3%) | 17 (0.4%) | 6 (0.3%) | <0.0001 |
| 1 | 7761 (39%) | 3788 (41%) | 1612 (39%) | 1531 (37%) | 830 (35%) | |
| 2 | 6230 (31%) | 2800 (30%) | 1336 (32%) | 1337 (32%) | 757 (32%) | |
| 3 | 5831 (29%) | 2617 (28%) | 1191 (29%) | 1287 (31%) | 736 (31%) | |
| LMCA | 130 (0.7%) | 72 (0.8%) | 124 (0.6%) | 22 (0.5%) | 12 (0.5%) | |
| CTO in NIRA | 2293 (12%) | 1027 (12%) | 462 (12%) | 491 (13%) | 313 (15%) | 0.0078 |
| STEMI recanalisation vessel | 0.0004 | |||||
| LAD | 8787 (44%) | 4209 (45%) | 1794 (43%) | 1742 (42%) | 1042 (45%) | |
| RCA | 8265 (41%) | 3743 (40%) | 1782 (43%) | 1800 (43%) | 940 (40%) | |
| LCX | 2575 (13%) | 1168 (13%) | 530 (13%) | 568 (14%) | 309 (13%) | |
| LMCA | 207 (1%) | 114 (1%) | 29 (1%) | 43 (6%) | 21 (3%) | |
| Graft | 177 (1%) | 68 (1%) | 39 (1%) | 41 (1%) | 29 (1%) | |
| TIMI angiographic flow grade before PCI | ||||||
| Score 0–2 | 18 488 (92%) | 8549 (92%) | 3839 (92%) | 3893 (93%) | 2207 (94%) | |
| Score 3 | 1523 (8%) | 753 (8%) | 335 (8%) | 301 (7%) | 134 (6%) | 0.0007 |
| TIMI angiographic flow grade after PCI | ||||||
| Score 0–2 | 1306 (7%) | 533 (6%) | 251 (6%) | 3894 (7%) | 222 (9%) | |
| Score 3 | 18 705 (93%) | 8769 (94%) | 3923 (94%) | 569 (93%) | 2119 (91%) | <0.0001 |
| Treatment and outcome | ||||||
| IABP | 278 (1.4%) | 178 (1.9%) | 34 (0.8%) | 43 (1.0%) | 23 (1.0%) | <0.0001 |
| In-hospital mortality | 1554 (7.8%) | 928 (10.0%) | 205 (4.9%) | 251 (6.0%) | 170 (7.3%) | <0.0001 |
Data are presented as means and SD or percentages.
*P values refer to the comparisons between the four groups.
CABG, coronary artery bypass grafting; CTO, chronic total occlusion; ECMO, extracorporeal membrane oxygenation; EMS, emergency medical service; IABP, intra-aortic balloon pump; cath. lab, catheterisation laboratory; LBBB, left bundle branch block; LCA, left coronary artery; LCX, left circumflex artery; LMCA, left main coronary artery; MI, myocardial infarction; NIRA, non-infarct-related arteries; OHCA, out-of-hospital cardiac arrest; RCA, right coronary artery; TIMI, Thrombolysis In Myocardial Infarction score.
Hospital mortality depending on patient delay
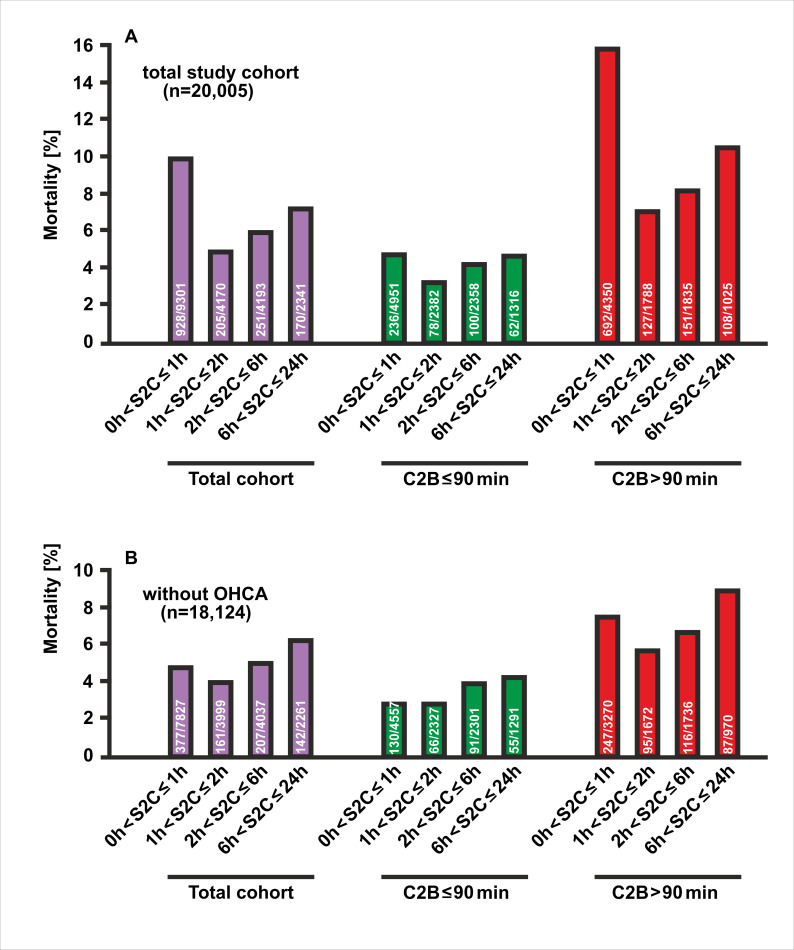
There were 1554 (7.8%) deaths in the total study population. Hospital mortality differed significantly according to the time from symptom onset to first medical contact (p<0.0001). The cohort of 455 patients with unknown times of symptom onset, who were excluded from the present analysis (see figure 1), had the highest mortality rate (79 deaths, 17.4%). Mortality was also high in patients reporting a short S2C time, as 928 out of 9301 study participants in group 1 (10%) died during the hospital treatment (figure 2A). The poor outcome in this group most probably resulted from a more than twofold higher prevalence of cardiogenic shock (18% vs 7%, p<0.0001) and a fourfold higher occurrence of OHCA (16% vs 4%, p<0.0001, table 1) compared with the remaining groups with an interval from symptom onset to EMS arrival of more than one hour. Mortality was lowest in STEMI patients with a moderate S2C time of one to two hours (205 deaths out of 4170 patients, 4.9%). Prolonged chest pain in the two groups with symptom durations ranging from 2 to 6 hours (group 3) and 6 to 24 hours (group 4), respectively, resulted in an increase in mortality (6.0% and 7.3%), which however did not reach the high mortality in STEMI patients with the shortest symptom duration of ≤1 hour (group 1). Excluding patients with OHCA, mortality was considerably reduced in the group with very early presentation (S2C≤1 hour: 377 deaths out of 7827 patients, 4.8%), and decreased moderately in the second group with early presentation (161 deaths out of 3999 patients, 4,0%), and again increased in the other two groups with intermediate (207 deaths out of 4037 patients, 5.1%) and late presentation (142 deaths out of 2261patients, 6.3%) (figure 2B).
Figure 2.
In-hospital mortality of PCI-treated STEMI patients by different categories of time intervals from the symptom onset to the arrival of the EMS at the scene. (A) Depicted are the percentages of deaths among the four groups in the total study population (purple), in patients with a contact-to-balloon time of equal to or less than 90 min (green) or longer (red). (B) Mortality data as in (A) for the subgroup of STEMI patients who had not experienced out-of-hospital cardiac arrest (OHCA). EMI, emergency medical service; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Better survival after timely reperfusion irrespective of symptom duration
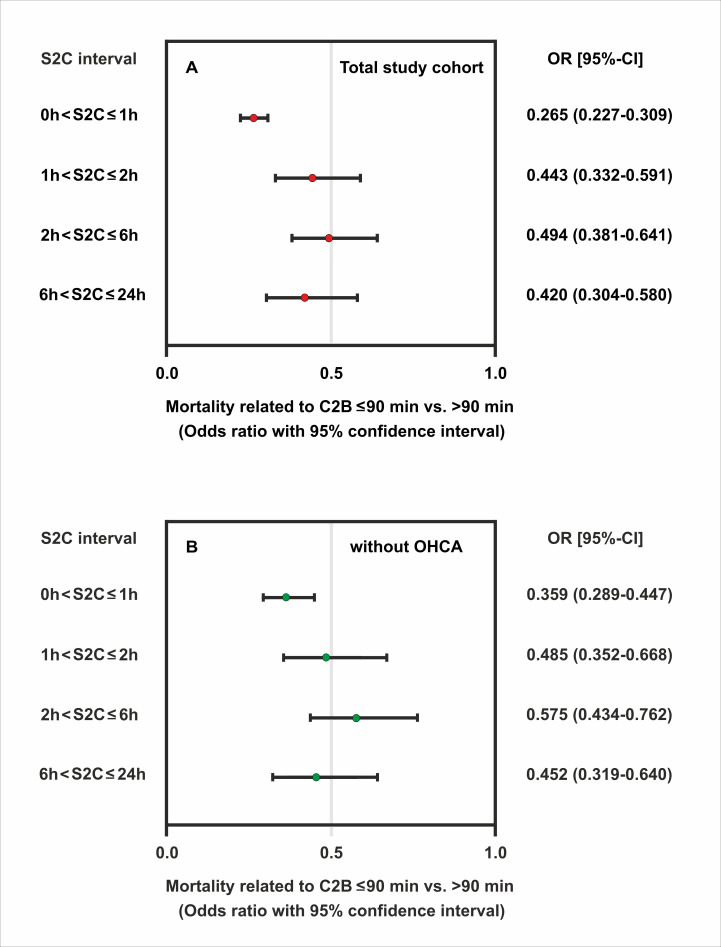
Reperfusion within 90 min of the first medical contact was a significant predictor of better survival, as for all S2C groups there were fewer deaths when time to reperfusion after EMS arrival did not exceed 90 min. Again, there were two peaks of high mortality, irrespective of whether or not the aim of a 90 min contact-to-balloon time was achieved. Mortality was highest in patients with an extremely short symptom duration of not more than 1 hour (4.8% and 15.9%, respectively), followed by a second peak at prolonged symptom duration (4.7% and 10.5%) (figure 2A). In an unadjusted model, patients with a reported symptom onset of ≤1 hour benefited the most from timely reperfusion as determined by a contact-to-balloon time of ≤90 min compared with their counterparts with a time to PCI treatment of >90 min (OR 0.27, 95% CI 0.23 to 0.31, p<0.0001) (figure 3A). Similar results were observed for the remaining three groups with longer symptom onset, as in-hospital mortality was always significantly lower when PCI was performed within 90 min after first medical contact.
Figure 3.
Odds ratios of mortality related to timely reperfusion as determined by a contact-to-balloon time of ≤90 min compared with >90 min and their corresponding 95% CI by categories of symptom-onset-to-first-medical contact time. Data are shown for the total study sample (A) and in the subgroup of patients without OHCA (B). OHCA, out-of-hospital cardiac arrest.
Notably, in the subgroup of STEMI patients without OHCA (n=18 124), timely treatment within the guideline-recommended 90 min interval after first medical contact significantly improved survival in each of the four symptom-to-contact groups (figures 2B and 3B). Beneficial effects of timely PCI treatment were also observed for all symptom-to-contact groups using different cut-offs for contact-to-balloon times. Similar mortality profiles were found for the two contact-to-balloon times at 60 min and 120 min, respectively (online supplemental figure 1 and online supplemental table 1).
openhrt-2021-001650supp001.pdf (548.7KB, pdf)
Finally, we assessed the impacts of S2C time on timely PCI treatment and their relation to mortality using a logistic regression model adjusted for baseline characteristics (table 2). This model used the four groups of symptom onset and the dichotomised data for contact-to-balloon time along the 90 min cut-off level and was adjusted to a variety of clinically important variables. Again, patients with an onset of symptoms within 60 min of the arrival of the EMS at the scene benefited the most from timely reperfusion with respect to survival (OR 0.56; 95% CI 0.46 to 0.68). Although data indicated better survival with a contact-to-balloon time of ≤90 min across the entire patient population, this did not reach statistical significance for the second to the fourth group.
Table 2.
Multivariable logistic regression model with in-hospital mortality as dependent variable and the guideline-recommended cut-off level of contact-to-balloon time (≤90 min) for four groups of symptom onset as independent variable adjusted to the indicated confounders
| Variable | OR | 95% CI | P value |
| Symptom-onset-to-first-medical-contact time (S2C) groups | |||
| Group 1 (S2C≤1 hour) | 0.555 | 0.456 to 0.675 | <0.0001 |
| Group 2 (1 hour<S2C≤2 hours) | 0.803 | 0.560 to 1.151 | |
| Group 3 (2 hours<S2C≤6 hours) | 0.769 | 0.558 to 1.060 | |
| Group 4 (6 hours<S2C≤24 hours) | 0.762 | 0.509 to 1.142 | |
| Age (year) | 1.054 | 1.047 to 1.062 | <0.0001 |
| Female gender | 1.395 | 1.200 to 1.622 | <0.0001 |
| Hypertension | 0.785 | 0.676 to 0.911 | 0.0014 |
| Diabetes mellitus | 1.384 | 1.170 to 1.636 | 0.0001 |
| Family history | 0.636 | 0.498 to 0.812 | 0.0003 |
| Smoker | 0.758 | 0.641 to 0.897 | 0.0012 |
| Previous stroke | 1.442 | 1.101 to 1.890 | 0.0079 |
| Renal failure | 1.552 | 1.231 to 1.956 | 0.0002 |
| No of coronary arteries narrowed | |||
| 0 versus LMCA | 0.171 | 0.030 to 0.971 | 0.0462 |
| one versus LMCA | 0.491 | 0.276 to 0.902 | 0.0219 |
| two versus LMCA | 0.551 | 0.301 to 1.007 | 0.0528 |
| three versus LMCA | 0.604 | 0.332 to 1.100 | 0.0992 |
| ECG (STEMI site) | |||
| Inferior versus anterior | 0.642 | 0.488 to 0.844 | 0.0015 |
| LBBB versus anterior | 0.747 | 0.460 to 1.213 | 0.2383 |
| Lateral versus anterior | 0.534 | 0.365 to 0.783 | 0.0013 |
| Chronic total occlusion in NIRA | |||
| RCA | 1.682 | 1.289 to 2.194 | 0.0001 |
| RCX | 1.722 | 1.286 to 2.306 | 0.0003 |
| LAD | 1.468 | 1.106 to 1.949 | 0.0079 |
| Recanalisation | |||
| Graft versus LAD | 1.313 | 0.726 to 2.375 | 0.3674 |
| LMCA versus LAD | 2.031 | 1.266 to 3.260 | 0.0033 |
| RCA versus LAD | 1.043 | 0.780 to 1.395 | 0.775 |
| LCX versus LAD | 1.245 | 0.932 to 1.663 | 0.1374 |
| TIMI angiographic flow grade after PCI (Score ≤2 vs 3) | 4.053 | 3.361 to 4.887 | <0.0001 |
Data are presented as ORs and their 95% CI.
CABG, coronary artery bypass grafting; cath. lab, catheterisation laboratory; CTO, chronic total occlusion; ECG, electrocardiogram; ECMO, extracorporeal membrane oxygenation; EMS, emergency medical service; IABP, intra-aortic balloon pump; LAD, left anterior descending artery; LBBB, left bundle branch block; LCA, left coronary artery; LCX, left circumflex artery; LMCA, left main coronary artery; MI, myocardial infarction; NIRA, non-infarct-related arteries; OHCA, out-of-hospital cardiac arrest; RCA, right coronary artery; TIMI, Thrombolysis In Myocardial Infarction score.
Discussion
Analysing the prognostic role of different S2C intervals in STEMI patients, we noted two important findings: first, our data show a J-shaped relationship between the time duration from symptom onset to first medical contact and in-hospital mortality. Second and most importantly, we found that timely reperfusion within 90 min of the first medical contact is most effective in STEMI patients presenting within a short S2C interval of less than 1 hour, but also has beneficial effects in patients with much longer S2C intervals.
While some authors reported that the total ischaemic time (ie, symptom-onset-to-balloon time) was inversely linked to survival,6 10 23 one study demonstrated that the relationship between total ischaemic time and mortality appeared to be rather complex not with a single, but two peaks separated by a trough, indicating lower mortality at 5 hours after symptom onset.24 The investigators in this prospective, observational Japan Acute Myocardial Infarction registry suggested that the link between ischaemic time and mortality was determined by the influence of two independent components: One was the outcome during the initial 3 hours for high-risk STEMI patients, while the other was the outcome of patients who survived the initial high risk period and were then exposed to the haemodynamic consequences of a long-lasting vessel occlusion.24
We observed the highest mortality rates in PCI-treated STEMI patients presenting within 1 hour of onset of symptoms (10%). Patients with S2C times between 1 and 2 hours had the lowest rate of hospital deaths (4.9%). A second mortality peak was seen in the group of patients with prolonged symptom duration lasting for longer than 6 hours before EMS arrival (7.3%).
In our analysis comparing different intervals from symptom onset to first medical contact, we found that patients from the high-risk group with a short symptom onset of within one hour were the youngest and had the lowest proportion of diabetes mellitus. Nevertheless, in this group, the prevalence of cardiogenic shock as a consequence of myocardial infarction was more than twice as high as in any of the other remaining groups (18% vs 7%). Similarly, we observed a high prevalence for OHCA with need of instant cardiopulmonary resuscitation in the patient group with a short interval from symptom onset to arrival of EMS, which was more than four times higher as compared with STEMI patients with a prolonged symptom duration exceeding one hour (15.8% vs 3.8%). The high mortality observed in STEMI patients presenting with an S2C time of ≤1 hour may have resulted from adverse haemodynamic complications, reflecting the severity of the ischaemic myocardial damage in this high-risk group. Excluding patients with OHCA resulted in considerably reduced mortality in the group with very early presentation (S2C≤1 hour). Timely treatment from first medical contact to balloon inflation of less than 90 min resulted in an improved survival in this group and in the other groups with early, intermediate and late presentation.
Terkelsen et al divided the total ischaemic time in ‘patient delay’ and ‘system delay’,2 whereby the latter but not the former can be influenced by the healthcare provider. From the perspective of the healthcare provider, the question is whether the magnitude of the benefit of timely PCI depends on the extent of patient delay (ie, the time from symptom onset to first medical contact). In our analysis, it is striking that shortening system delay (ie, the time from first medical contact to PCI) still has a beneficial effect even with extended patient delays and long S2C time intervals. These beneficial effects of timely reperfusion even in STEMI patients with prolonged duration of symptoms could be due to a variety of mechanisms such as incomplete or intermittent occlusion of the infarct-related artery, residual blood flow by recruitment of collaterals and ischaemic preconditioning, thereby preventing complete necrosis and preserving viable myocardium.25–27
Several issues merit consideration in the interpretation of our findings. First of all, the time point of symptom onset is critical to our analysis but hard to define. Since our study is observational in nature, the collection of patients’ data might be susceptible to unmeasured confounding and selection bias, which were not been systematically evaluated, such as objective criteria for the onset of ischaemia (eg, increase in serum markers such as troponin and imaging). Thus, any conclusion about causality between the time from symptom onset to first medical contact and outcome is of limited validity from our observational study, which of course limits the generalisability of our results in other clinical settings. Owing to the limited follow-up, only in-hospital mortality was included in our analysis, while data on longitudinal outcomes at longer follow-up periods were not available. In addition, our findings from the FITT-STEMI study conducted in Germany may not be directly applicable to STEMI management care systems in other countries, in which physicians experienced in emergency medicine are not part of the EMS teams. Moreover, it cannot be excluded that the attendance of both physicians and paramedics in EMS transportation might have accounted for the beneficial effects of shortened treatment times on survival seen in our different patient groups. Another limitation relates to the fact that it is unknown how the exclusion of formalised data analysis and interactive feedback sessions would affect the results from similar investigations without this intervention, since quality management improvement is an integral and essential component of the FITT-STEMI study design.
In summary, our findings demonstrate that timely revascularisation by means of PCI therapy within the guideline-recommended 90 min from first medical contact is a critical component of improvement of outcomes in myocardial infarction, irrespective of the time from symptom onset. In order to improve outcome, minimising system delay should be followed in all patients across a wide range of S2C time intervals up to 24 hours.
Acknowledgments
We thank Hans-Joachim Radtke and Michael Jur (UniPro Software corporation, Halberstadt, Germany) providing web-based data acquisition and delivery of raw data, as well as Phillip Scholz and Nikos Tirilomis (Universitätsklinikum Göttingen) preparing regular feedback analyses for each single participating hospital. Hospitals participating in the FITT STEMI consortium and list of contributors: St. Bernward-Krankenhaus Hildesheim (Karl Heinrich Scholz; Barbara Bartels; Christina Planke); Universitätsklinikum Würzburg (Björn Lengenfelder; Verena Grünewald; Sebastian K.G. Maier); Klinikum Worms (Jens Jung; Birgit Nicklas); Klinikum Darmstadt (Hiller Moehlis; Gerald S. Werner); Klinikum Wolfsburg (Claus Fleischmann; Rüdiger Becker; Rolf Engberding); Universitätsklinikum Göttingen (Tim Seidler; Claudius Jacobshagen; Kristina Schröder; Svetlana Hartmann; Nadine Heydrich; Lars S. Maier); Asklepios Klinikum Langen (Marcus Mittag; Melanie Hauff; Ralf Lehmann; Hans G. Olbrich; Kerstin Eck); Klinikum Oldenburg (Susanne Grafmüller; Albrecht Elsässer; Annette Schütz); Städtisches Klinikum München Neuperlach (Anamaria Stote; Michael Danner; Stefan Sack; Martin Hug; Harald Mudra); Helios Klinikum Krefeld (Rainer Ott; Ursula Jansen; Heinrich G. Klues; Alexander Bufe); Krankenhaus Rothenburg ob der Tauber (Christian Wacker); Marienkrankenhaus Soest (Roland Bürger; Markus Flesch); Kreiskrankenhaus Eschwege (Peter Schott; Marco Lubitz; Tobias-Richard Meinel); Krankenhaus Landshut-Achdorf (Ute Zrenner; Bernhard Zrenner; Josef Haimerl); Klinik am Eichert Göppingen (Stephen Schröder; Marion Steindl; Josef Steindl; Sophia Atseles); Klinikum Ingolstadt (Silke Gläser; Katrin Thiele; Karlheinz Seidl); Universitätsklinikum Jena (Corinna Schneider; P. Christian Schulze; Daniel Kretzschmar); Klinikverbund Kempten-Oberallgäu (Carsten Bauer; Wulf Ito); Robert-Bosch-Krankenhaus Stuttgart (Stephan Hill; Andrea Bullinger; Udo Sechtem); Klinikum Lüneburg (Jan Priesack; Christian Weiss; Claus H. Müller); Krankenhaus Buchholz (Werner Raut; Klaus Hertting; Liana Hertting); Regio Klinikum Pinneberg (Konrad Gorski; Thomas Hofmann); Klinikum Lippe-Detmold (Melanie Kriete; Dirk Haertel; Ulrich Tebbe); Klinikum Region Hannover-Siloah (Andreas Franke; Jan Fürste; Rania Jebrini; Fred Aumüller); Klinikum Deggendorf (Martina Silova; Rainer Burckhard; Martin Giesler); Klinikum Leer (Christian Vahlhaus; Ralf Pretzsch); Asklepios Harzklinik Goslar (Gaby Lehnert; Thomas Wittlinger; Tobias Steffen; Arnd B. Buchwald; Christoph Engelhardt); Kliniken Ostallgäu-Kaufbeuren Füssen (Simon Delladio; Martin Hinterseer; Myriam Parvanov); Klinikum St. Elisabeth Straubing (Sebastian K.G. Maier; Elke Grassl; Florian Brattinger; Stilla Jacobs); Klinikum Neumarkt (Christian Schmidt; Melina Dykczak; Veronika Lingg); Universitätsklinikum Augsburg (Georg Waidhauser; Christian Thilo; Wolfgang von Scheidt); Klinikum Ludwigsburg (Stefan Stefanow; Ralf Berroth; Joachim Geiger; Friederike Wunsch; Christian Wolpert); Sana Kliniken Lübeck (Hans-Martin Grusnick; Joachim Weil); Allgemeines Krankenhaus Viersen (Nicolas von Beckerath; Barbara Vogel; Birger Horn); Medizinische Hochschule Hannover (Jörn Tongers; Cicek Yarcu; Ulrike Flierl; Benedikta Ritter; Johann Bauersachs); Klinikum REGIOMED-Kliniken Coburg (Steffen Schnupp; Andrea Scharmentke; Karoline Kleinecke; Andrea Linss; Kerstin Truthan; Hans-Joachim Goller; Johannes Brachmann); Kreiskrankenhaus Dormagen (Benjamin Orth; Georg Haltern); Hermann-Josef-Krankenhaus Erkelenz (Vera Schiwietz; Christina Ridt; Klaus Dieter Winter); Klinikum Landkreis Erding (Lorenz Bott-Flügel); Krankenhaus Henriettenstift Hannover (Thomas Weiss; Lisa Landsmann; Thorsten Grundmann); SLK Kliniken Heilbronn (Marcus Hennersdorf; Jens Martin Maier; Eva Schropp); Kliniken Maria Hilf Mönchengladbach (Jürgen vom Dahl; Gregor Nothofer; Dierk Rulands); Vinzenzkrankenhaus Hannover (Beate Bugdoll; Arno Lutz; Christian Zellerhoff; Petra Wucherpfennig; Jan-Bernd Schüttert); Universitätsklinikum Regensburg (Stefan Neef; Bailey-Marie Johnson; Christina Strack; Dierk Endemann; Lars S. Maier); SLK Kliniken Am Plattenwald Bad Friedrichshall (Thomas Dengler; Christine Lindner); Evangelisches Krankenhaus Bethesda Mönchengladbach (Thomas Lickfeld); Krankenhaus Bethanien Moers (Alexander Donath; Stefan Möhlenkamp); DRK-Krankenhaus Clementinenhaus Hannover (Heinz-Peter Remmlinger; Constanze Behne); Johanna-Etienne-Krankenhaus Neuss (Till Falke); Rems-Murr Kliniken Winnenden (Andreas Jeron; Michaela Schorer); Universitätsklinikum Aachen (Jörg Schröder; Gabriele Heuer; Stefan Beckers); St. Antonius Hospital Eschweiler (Andrea Wings; Andreas Niedeggen; Johannes Nußbaum; Uwe Janssens); Städtische Kliniken Mönchengladbach (Dierk Rulands; Katharina Grün-Himmelmann); Universitätsklinikum Münster (Lena-Maria Makowski; Marion Pohl; Christian Pogoda; Georg Haltern); Krankenhaus Reinbek St. Adolf Stift (Claudia Zeiler-Reich; Ali Aydin)
Footnotes
Contributors: KHS was responsible for conception, design of the study and obtaining funding. KHS, BL, CV, JT, SS, RB, NvB, HMG, AJ, KDW, SM, MD, JvD, SN and SS contributed to subject recruitment and data acquisition. KHS, TM and TF analysed the clinical data. TF performed the statistical analysis. All authors made substantial contributions to analysis and interpretation of data. KHS, TM and TF drafted the manuscript. All authors critically revised and reviewed the manuscript. All authors read and approved the final manuscript. KHS, TM and TF agreed to be responsible for the overall content as guarantors.
Funding: This study was supported by a grant from the Deutsche Herzstiftung e.V. and the Arbeitsgemeinschaft Leitender Kardiologischer Krankenhausärzte to KHS.
Competing interests: TF reports personal fees for consultancies (including data monitoring committees) from Novartis, Bayer, Biogen, AstraZeneca, Janssen, Grünenthal, Pharmalog, SGS, Boehringer Ingelheim, Daiichi-Sankyo, Mediconomics and Roche, all outside the submitted work. Furthermore, he has received research funding by the European Commission for statistical analyses on the EUTrigTreat (NCT01209494) and EU-CERT-ICD (NCT02064192) clinical studies. All relationships declared are modest.
Provenance and peer review: Not commissioned; externally and internally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article and are available on reasonable request from the last author (TF).
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by the ethics committee of the Medical Faculty at the University of Göttingen and the local ethics committees of all participating PCI centres (GAU1/10//07).
References
- 1.McNamara RL, Wang Y, Herrin J, et al. Effect of door-to-balloon time on mortality in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2006;47:2180–6. 10.1016/j.jacc.2005.12.072 [DOI] [PubMed] [Google Scholar]
- 2.Terkelsen CJ, Sørensen JT, Maeng M, et al. System delay and mortality among patients with STEMI treated with primary percutaneous coronary intervention. JAMA 2010;304:763–71. 10.1001/jama.2010.1139 [DOI] [PubMed] [Google Scholar]
- 3.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: Executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task force on Practice Quidelines. Circulation 2013;127:e529–55. 10.1161/CIR.0b013e3182742c84 [DOI] [PubMed] [Google Scholar]
- 4.Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–77. 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 5.Scholz KH, Maier SKG, Maier LS, et al. Impact of treatment delay on mortality in ST-segment elevation myocardial infarction (STEMI) patients presenting with and without haemodynamic instability: results from the German prospective, multicentre FITT-STEMI trial. Eur Heart J 2018;39:1065–74. 10.1093/eurheartj/ehy004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson CM, Murphy SA, Kirtane AJ, et al. Association of duration of symptoms at presentation with angiographic and clinical outcomes after fibrinolytic therapy in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2004;44:980–7. 10.1016/j.jacc.2004.05.059 [DOI] [PubMed] [Google Scholar]
- 7.De Luca G, Suryapranata H, van 't Hof AWJ, et al. Prognostic assessment of patients with acute myocardial infarction treated with primary angioplasty: implications for early discharge. Circulation 2004;109:2737–43. 10.1161/01.CIR.0000131765.73959.87 [DOI] [PubMed] [Google Scholar]
- 8.Sejersten M, Birnbaum Y, Ripa RS, et al. Influences of electrocardiographic ischaemia grades and symptom duration on outcomes in patients with acute myocardial infarction treated with thrombolysis versus primary percutaneous coronary intervention: results from the DANAMI-2 trial. Heart 2006;92:1577–82. 10.1136/hrt.2005.085639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasoul S, Ottervanger JP, de Boer M-J, et al. Predictors of 30-day and 1-year mortality after primary percutaneous coronary intervention for ST-elevation myocardial infarction. Coron Artery Dis 2009;20:415–21. 10.1097/MCA.0b013e32832e5c4c [DOI] [PubMed] [Google Scholar]
- 10.Fokkema ML, Wieringa WG, van der Horst IC, et al. Quantitative analysis of the impact of total ischemic time on myocardial perfusion and clinical outcome in patients with ST-elevation myocardial infarction. Am J Cardiol 2011;108:1536–41. 10.1016/j.amjcard.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 11.Mohanan PP, Mathew R, Harikrishnan S, et al. Presentation, management, and outcomes of 25 748 acute coronary syndrome admissions in Kerala, India: results from the Kerala ACS registry. Eur Heart J 2013;34:121–9. 10.1093/eurheartj/ehs219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song F, Yu M, Yang J, et al. Symptom-onset-to-balloon time, ST-segment resolution and in-hospital mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention in China: from China Acute Myocardial Infarction registry. Am J Cardiol 2016;118:1334–9. 10.1016/j.amjcard.2016.07.058 [DOI] [PubMed] [Google Scholar]
- 13.Kim HK, Jeong MH, Ahn Y, et al. Relationship between time to treatment and mortality among patients undergoing primary percutaneous coronary intervention according to Korea Acute Myocardial Infarction Registry. J Cardiol 2017;69:377–82. 10.1016/j.jjcc.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 14.Wah W, Pek PP, Ho AFW, et al. Symptom-to-door delay among patients with ST-segment elevation myocardial infarction in Singapore. Emerg Med Australas 2017;29:24–32. 10.1111/1742-6723.12689 [DOI] [PubMed] [Google Scholar]
- 15.Kawecki D, Morawiec B, Gąsior M, et al. Annual trends in total ischemic time and one-year fatalities: the paradox of STEMI network performance assessment. J Clin Med 2019;8:78. 10.3390/jcm8010078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park J, Choi KH, Lee JM, et al. Prognostic implications of door-to-balloon time and onset-to-door time on mortality in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Heart Assoc 2019;8:e012188. 10.1161/JAHA.119.012188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeng M, Nielsen PH, Busk M, et al. Time to treatment and three-year mortality after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction-a DANish trial in Acute Myocardial Infarction-2 (DANAMI-2) substudy. Am J Cardiol 2010;105:1528–34. 10.1016/j.amjcard.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 18.Denktas AE, Anderson HV, McCarthy J, et al. Total ischemic time: the correct focus of attention for optimal ST-segment elevation myocardial infarction care. JACC Cardiovasc Interv 2011;4:599–604. 10.1016/j.jcin.2011.02.012 [DOI] [PubMed] [Google Scholar]
- 19.Solhpour A, Chang KW, Arain SA, et al. Ischemic time is a better predictor than door-to-balloon time for mortality and infarct size in ST-elevation myocardial infarction. Catheter Cardiovasc Interv 2016;87:1194–200. 10.1002/ccd.26230 [DOI] [PubMed] [Google Scholar]
- 20.Wijns W, Naber CK. Reperfusion delay in patients with high-risk ST-segment elevation myocardial infarction: every minute counts, much more than suspected. Eur Heart J 2018;39:1075–7. 10.1093/eurheartj/ehy069 [DOI] [PubMed] [Google Scholar]
- 21.Scholz KH, Hilgers R, Ahlersmann D, et al. Contact-to-balloon time and door-to-balloon time after initiation of a formalized data feedback in patients with acute ST-elevation myocardial infarction. Am J Cardiol 2008;101:46–52. 10.1016/j.amjcard.2007.07.078 [DOI] [PubMed] [Google Scholar]
- 22.Scholz KH, Maier SKG, Jung J, et al. Reduction in treatment times through formalized data feedback: results from a prospective multicenter study of ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2012;5:848–57. 10.1016/j.jcin.2012.04.012 [DOI] [PubMed] [Google Scholar]
- 23.Shiomi H, Nakagawa Y, Morimoto T, et al. Association of onset to balloon and door to balloon time with long term clinical outcome in patients with ST elevation acute myocardial infarction having primary percutaneous coronary intervention: observational study. BMJ 2012;344:e3257. 10.1136/bmj.e3257 [DOI] [PubMed] [Google Scholar]
- 24.Nakamura M, Yamagishi M, Ueno T, et al. Current treatment of ST elevation acute myocardial infarction in Japan: door-to-balloon time and total ischemic time from the J-AMI registry. Cardiovasc Interv Ther 2013;28:30–6. Erratum in: Cardiovasc Interv Ther 2013;28(4):427. 10.1007/s12928-012-0128-x [DOI] [PubMed] [Google Scholar]
- 25.Yusuf S, Lopez R, Maddison A, et al. Variability of electrocardiographic and enzyme evolution of myocardial infarction in man. Br Heart J 1981;45:271–80. 10.1136/hrt.45.3.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kloner RA, Shook T, Antman EM, et al. Prospective temporal analysis of the onset of preinfarction angina versus outcome: an ancillary study in TIMI-9B. Circulation 1998;97:1042–5. 10.1161/01.cir.97.11.1042 [DOI] [PubMed] [Google Scholar]
- 27.Schömig A, Mehilli J, Antoniucci D, et al. Mechanical reperfusion in patients with acute myocardial infarction presenting more than 12 hours from symptom onset: a randomized controlled trial. JAMA 2005;293:2865–72. 10.1001/jama.293.23.2865 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2021-001650supp001.pdf (548.7KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article and are available on reasonable request from the last author (TF).