Abstract
Background:
We investigated the effects of spirulina under high-intensity interval training (HIIT) on levels of nesfatin-1, omentin-1, and lipid profiles in overweight and obese females.
Materials and Methods:
This is a randomized, quasi-experimental controlled, single-blind with a pre- and post-test design, in which twenty overweight and obese women (body mass index = 29.32 ± 3.01 kg/m2, age = 21.55 ± 1.76 years), were randomly divided into the following groups: HIIT + spirulina (n = 10) and HIIT + placebo (n = 10). Running anaerobic sprint test was used for HIIT protocol consisting of six intervals at 35 m maximal speed runs, with a rest of 10 s in each trial (3 times/week, 4 weeks). HIIT + spirulina group received 500 mg of the spirulina pills twice daily for 4 weeks and the second group took placebo.
Results:
There was a significant increase in serum levels of nesfatin-1 in HIIT + spirulina (P < 0.0001) but not in HIIT + placebo (P = 0.61) group. Furthermore, results indicated a significant difference between two groups with respect to serum levels of nesfatin-1 (P = 0.04). Serum levels of omentin-1 significantly increased in both groups (P < 0.05). However, there was no significant difference between two groups (P = 0.49). In addition, results showed no significant inter- and intra-group differences in total cholesterol levels, triglycerides, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol between groups (P > 0.05).
Conclusion:
The spirulina under HIIT increased levels of nesfatin-1 and omentin-1 with no effects on the levels of lipid profiles in overweight and obese females.
Keywords: High-intensity interval training, lipid profiles, nesfatin-1, obese, omentin-1, spirulina
INTRODUCTION
In the 21st century, increasing prevalence of obesity has become a medical global challenge. A wealth of clinical and epidemiological evidence has linked obesity to cardiovascular diseases (CVD) and cerebrovascular disease.[1] It was indicated that physical activity reduced risk factors of CVD such as total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), body fat percentage (BFP), and increased high-density lipoprotein cholesterol (HDL-C) which is a protective factor of CVD.[1]
Adipose tissue is an endocrine organ that produces hormones as adipokines including nesfatin-1 and omentin-1.[2] Nesfatin-1 contains some proteins which play important role in food intake and weight loss.[3] Omentin is produced in visceral adipose tissue and increases insulin sensitivity in muscle, liver, and adipose tissue.[4] Levels of omentin-1 and nefastin-1 are decreased in obese individuals, Type 2 diabetes, and insulin resistance.[5] There is a negative correlation between serum levels of nesfatin-1 and omentin-1 with body mass index (BMI), waist circumference, and a positive correlation with HDL-C levels.[3,5]
Various factors such as exercise can alter adipokines secretion involved in energy regulation. Exercise is known as an effective strategy for weight management and can improve obesity risk factors in normal and obese individuals.[6] High-intensity interval training (HIIT) involves short bouts of vigorous exercise with intensity greater than lactate threshold and near VO2max followed by a return to the initial low-intensity state, which results in various metabolic adaptations.[1] Although the duration of HIIT is very short, it has a beneficial effect on acceleration of fat loss and improves aerobic and anaerobic endurance.[7] HIIT is a time-efficient way to combine vigorous physical activity into an exercise program.[7] Furthermore, it is less intimidating and more acceptable to overweight and obese individuals.[7] In addition to exercise, diet and supplements have also been recognized as a way of weight loss and obesity-related risk factors in recent years. Spirulina is a species of microalgae[8,9] and a rich source of protein and vitamins, particularly, vitamin B12, mineral, carotenoid, and phycocyanin[10] which can prevent fatty liver, cardiovascular disease, and reduce serum lipid levels.[10]
Few studies in regarding to the effect of exercise on omentin-1 and nesfatin-1 have been carried out,[11,12] however, based on the author's knowledge, the effects of spirulina under HIIT on levels of nesfatin-1, omentin-1, and lipid profiles in overweight and obese females have not yet been examined. Therefore, we investigated the effects of spirulina under (HIIT) on levels of nesfatin-1, omentin-1, and lipid profiles in overweight and obese females and compared it with HIIT effects.
MATERIALS AND METHODS
Participants and study design
This was a randomized, quasi-experimental, controlled, single-blind study with a pre- and post-test design. A total of twenty overweight and obese young women (BMI = 29.32 ± 3.01 kg/m2, age = 21.55 ± 1.76 years) were recruited to the present study. The study population included overweight and obese students of the University of Birjand, which is located in the southern Khorasan province, Iran. According to CONSORT statement, Figure 1 shows the flowchart of this study. The inclusion criteria included; 18–25 years, overweigh (25 ≥BMI ≥29.99 kg/m2) and obese (30 ≥BMI ≥34.99 kg/m2), lack of professional training for last 6 months, lack of physical ills, and stable weight for at least 3 months. They were free of chronic disease and no other medical conditions that would impact their ability to engage in intense exercise. Written informed consent was obtained from all subjects. Prior baseline measurements, all subjects became familiar with all testing and procedure. The Ethics Committee of Birjand University of Medical Science (BUMS) approved this project (Code number; IR-BUMS.REC.1397.182).
Figure 1.
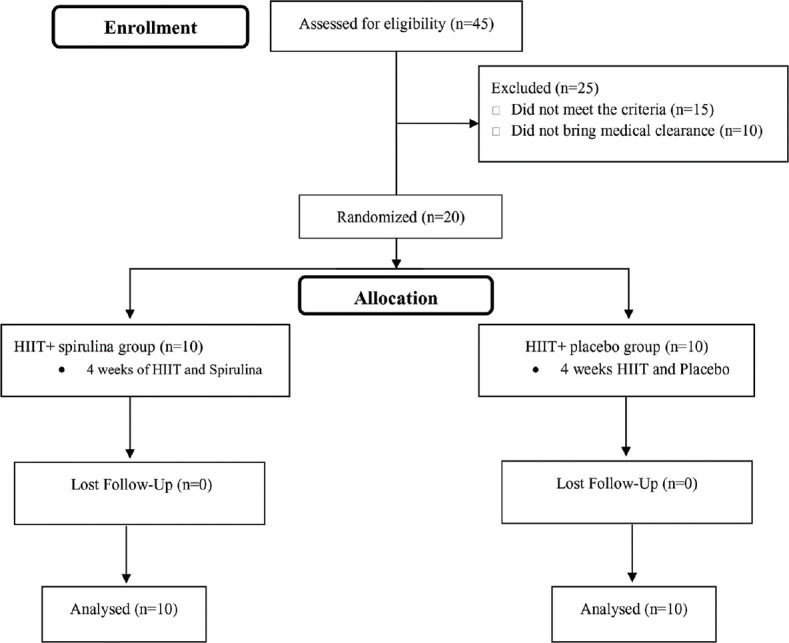
Flowchart of the study
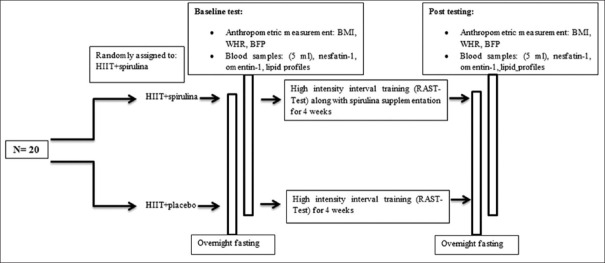
Subjects were randomly divided into two groups including: HIIT + spirulina (n = 10) and HIIT + placebo (n = 10) groups. A schematic of the of study design is shown in Figure 2. They were excluded if they had any nutritional supplements that may alter the results. All subjects were asked to adhere to their normal and similar dietary patterns and daily activity throughout the study. Furthermore, Food Frequency Questionnaires were used to measure dietary habits of subjects. Measurements were collected at two-time points, baseline and after 4 weeks, during the same time of day (±1 h) and under the same environmental conditions (~20°C and ~55% humidity) in the morning following an overnight fast and abstinence from caffeinated drinks, alcohol, and approximately 48 h after the last training session. Information about the physical activity level and health condition of all the subjects was collected through questionnaire. Randomization was performed by used a digital tool available at www.randomizer.org. Furthermore, the calculation of the sample size was carried out using G*Powers software (Heinrich-Heine-Universität, Düsseldorf, Germany).
Figure 2.
Schematic of the study design. Both baseline and post-testing were conducted after a 12-hour overnight fast and avoidance of exercise. Abbreviations: HIIT, high intensity interval training; BMI, body mass index; BFP, body fat percentage; WHR: waist-hip ratio
Physical composition measurements
Physical composition measurements of the subjects were assessed at the baseline (48 h before) and end (48 h after) of 4 weeks of intervention protocols. To determine the physical composition measurements, all subjects were wearing usual indoor clothing without shoes (weight, height, BMI, BFP, and waist-to-hip ratio [WHR]). Body weight was measured using a digital TCM scale (to the nearest 0.1) and standing height was measured using wall-mounted made in Yagami Japan (to the nearest 0.5 cm). BMI was obtained for each subject by dividing the weight in kilograms into the height in m2. Assessment of waist–hip ratio was calculated as the waist circumference in centimeters (mid between the lowest ribs and the iliac crest) divided by the hip circumference in centimeters (in the gluteus Maximus). BFP was predicted from the skinfold thickness measurement using caliper (Harpenden Skinfold Fat Caliper Made in England). A three-site skinfold (the triceps, the suprailiac, and the front thigh) protocol was used to estimate BFP.
Spirulina supplementation
The subjects of HIIT + spirulina groups received the spirulina pills 500 mg,) (Far East Microalgae Ind. Co. LTD, the exclusive distributor in Iran: QSM Co.) twice-daily, an hour before meals for 4 weeks. The HIIT + placebo received (twice-daily, an hour before each meals) pills which contained 500 g starch in similar shape to spirulina pill. Compliance with spirulina and any complication about, it was checked through weekly telephone interviews.[13]
High-intensity interval training protocol
Subjects performed HIIT by 100% maximum speed. The intensity of HIIT was measured by Carvonen by the following method:
Maximum heart rate = 220 – age
Heart rate reserve = HR max – HR rest
(Resting heart rate)
%60 Target heart rate = (0.60 × HRR) + HR rest
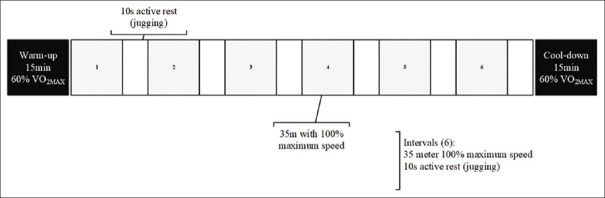
Subjects completed the running anaerobic sprint test (RAST)-test for HIIT protocol. The test included six intervals at a distance of 35 m maximal speed runs with a 10-s active rest between each trial (4 weeks, three sessions per week). HIIT program was during the same time of day and under the same environmental conditions (~20°C and ~55% humidity) in the afternoon. The protocol consisted of two sets of RAST-test with 3 min of rest between each set in the 1st week, 3 sets in the 2nd week, 4 sets in the 3rd week, and 3 sets in the fourth.[14] At the beginning and end of RAST test, subjects performed 15 min warm-up and 15 min cool down [Figure 3]. To measure the intensity of training, the heart rate of participants was randomly measured by Polar heart rate watch in every session.
Figure 3.
High intensity interval training program
Blood collection and processing
In the present study, following 12 h of fasting to measure the biochemical variables, 5 ml of blood were taken from all the subjects. Blood sampling was performed 48 h before and 48 h after the last exercise training session, from antecubital vein of the subjects by a technician in laboratory medicine. Serum was separated by centrifugation (Hettich, MIKRO 120, Germany) at 3000 rpm for 5 min and stored at − 70°C until analyses were performed. Pars test kit (Germany) was used to measure levels of lipid profiles by the Auto Analyzer System. Serum nesfatin-1 and omentin-1 were evaluated using sandwich ELISA using human kit (ZellBio GmbH [Germany]) with a sensitivity of 4.8 ng/ml and 1 ng/ml, respectively.
Statistical analysis
All data were analyzed using Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) software version 22.0 and were expressed as means ± standard deviation. The determination of normality of data distribution was performed by Shapiro–Wilk test. Leven's test and Covariance Matrix were used to examine the homogeneity of variance in groups. To confound such pretest values as the covariate, we used analysis of covariance (ANCOVA) tests was applied to examine. Paired-samples t-test and ANCOVA were applied to examine the intra- and inter-group differences, respectively. P < 0.05 was considered statistically significant. It should be noted that the present study did not have any missing data and the analyses were done on original assigned groups (HIIT + spirulina group and HIIT + placebo group).
RESULTS
The interactive effects of HIIT and spirulina supplementation on all variables are presented in Table 1. Figure 1 shows that there were not any losses and exclusion in each group. The results of the present study showed serum levels of omentin-1 increased significantly in two groups as compared to pretest (HIIT + spirulina = 34.14 mg/dl [95% confidence interval [CI] =19.25–49.03], [P < 0.001] and HIIT + placebo = 22.17 mg/dl [CI = 9.5–34.84], [P = 0.03]). There were no significant differences on serum levels of omentin-1 between two groups (P = 0.49). Furthermore, Nesfatin-1 increased significantly in HIIT + spirulina group (0.99 mg/dL [CI = 0.07–1.92], [P < 0.001]), but not in HIIT + placebo (0.12 mg/dl [CI = 0.33–0.58], [P > 0.05]). In addition, serum levels of nesfatin-1 were greater in HIIT + spirulina group than HIIT + placebo group (P = 0.04). Significant reduction of BMI was observed in HIIT + placebo group (−0.78 kg/m2, [CI=−1.34 to − 0.21], [P = 0.01]) than HIIT + spirulina group (−0.41 kg/m2, [CI = −0.92 to − 0.09], [P = 0.10]). No significant reduction was found in levels of TC (HIIT + spirulina = −4.10 mg/dl, [CI = −27.04 to − 18.83], [P = 0.69] and HIIT + placebo = −11.67 mg/dl, [CI = −28.16 to − 4.81], [P = 0.96]), TG (HIIT + spirulina = −0.26 mg/dl, [CI = −21.14 to − 20.60], [P = 0.22] and HIIT + placebo = −11.81 mg/dl, [CI = −32.14 to − 8.46], [P = 0.14]), HDL-C (HIIT + spirulina = −3.38 mg/dl, [CI = −8.37 to − 1.59], [P = 0.15] and HIIT + placebo = −0.87 mg/dl, [CI = −5.11 to − 3.36], [P = 0.65]), LDL-C (HIIT + spirulina = −3.38 mg/dl, [CI = −17.69 to − 12.96], [P = 0.37] and HIIT + placebo = −6.31 mg/dl, [CI = −18.79 to − 6.16], [P = 0.21]), and WHR (HIIT + spirulina = −0.007 cm, [CI = −0.02 to − 0.04], [P = 0.59] and HIIT + placebo = −0.03 cm, [CI = −0.02 to − 0.08), [P = 0.25]). The results found significant decrease in BFP in (HIIT + spirulina = −3.57%, [CI = −5.79 to − 1.36], [P < 0.001] and HIIT + placebo = −2.21%, [CI = −3.79 to − 0.63], [P = 0.01]). Results indicted no significant inter- and intra-group differences in TC, TG, HDL-C, LDL-C, BMI, BFP, and WHR (P > 0.05). No unintended or any important harms was reported into both groups.
Table 1.
Main descriptive characteristics of the population (age, cognitive status, body composition, lipid profiles, and biochemical factors) at baseline after 4 weeks of intervention are presented
| Variable | HIIT-SPIRO | HIIT-PLA | P | ||||
|---|---|---|---|---|---|---|---|
| Pre | Post | P | Pre | Post | P | ||
| Body composition | |||||||
| Weight (kg) | 73.88±14.57 | 73.22±14.58 | 0.16 | 72.70±2.35 | 70.80±7.81 | 0.01* | 0.06 |
| BMI (kg/m2) | 29.54±3.97 | 29.28±4.07 | 0.10 | 29.13±2.02 | 28.35±2.00 | 0.01* | 0.13 |
| WHR (cm) | 0.81±0.08 | 0.80±0.08 | 0.59 | 0.83±0.06 | 0.80±0.09 | 0.25 | 0.58 |
| BFP (%) | 30.58±3.05 | 27.00±3.60 | <0.001* | 26.95±3.70 | 24.74±2.00 | 0.01* | 0.83 |
| Lipid profiles | |||||||
| TG (mg/dl) | 94.09±61.44 | 93.82±55.29 | 0.97 | 86.57±33.26 | 74.76±18.41 | 0.22 | 0.22 |
| TC (mg/dl) | 167.35±39.48 | 163.24±38.85 | 0.69 | 170.81±26.62 | 159.13±23.85 | 0.14 | 0.82 |
| LDL-C (mg/dl) | 132.43±19.80 | 125.32±30.59 | 0.37 | 130.93±17.23 | 123.04±10.90 | 0.21 | 0.88 |
| HDLC (mg/dl) | 54.23±6.97 | 57.62±5.18 | 0.15 | 59.49±3.85 | 60.37±5.39 | 0.65 | 0.63 |
| Biochemical factors | |||||||
| Nesfatin-1 (mg/dl) | 0.47±0.55 | 1.35±1.05 | <0.001* | 0.54±0.58 | 0.66±0.91 | 0.61 | 0.04* |
| Omentin-1 (mg/dl) | 29.91±10.40 | 62.06±27.55 | <0.001* | 21.33±6.86 | 43.51±20.47 | 0.03* | 0.49 |
Data are presented as mean±SD. *P<0.05. HIIT-SPIRO=High intensity interval training-spirulina; HIIT-PLA=High intensity interval training-placebo; BMI=Body mass index; WHR=Waist-hip ratio; BFP=Body fat percent; TG=Triglyceride; TC=Total cholesterol; LDL-C=Low density lipoprotein-chain; HDL-C=High density lipoprotein-chain; SD=Standard deviation
DISCUSSION
We investigated the effects of spirulina under HIIT on levels of nesfatin-1, omentin-1, and lipid profiles in overweight and obese females. The present study was a quasi-experimental model of spirulina and HIIT which was the first study and resulted in significant increase on serum levels of nesfatin-1, omentin-1, and improving body composition. In addition, we observed no significant improvement in lipid profiles after HIIT and spirulina intervention.
The present investigation demonstrated no significant effects of HIIT in serum levels of nesfatin-1. Although lack of physical activity contributes to the prevalence of obesity-associated diseases and exercise can manage body composition in normal and obese individuals.[15] The results of the present study were similar to those seen by Ghanbari-Niak et al., who found that two different anaerobic exercise sessions had no significant effects on levels of nesfatin-1.[11] Findings of the present study are contrary to Ahmadizad et al., who reported that levels of nesfatin-1 were significantly increased after 6 weeks of HIIT.[16] In other study, Mogharnasi et al. observed that 10 weeks of resistance training increased significantly levels of nesfatin-1 in women with Type 2 diabetes mellitus.[17] Furthermore, Jafari and Mogharnasi found that levels of nesfatin-1 were increased in response to 8 weeks of endurance and strength training in overweight and obese women.[18] The contradiction reasons for the present study with other studies can be due to differences in duration and exercise intensity and diversity in the type of exercise.[19] Low number of subjects of the present study may be considereds a limitation responsible for no intergroup differences. The response of nesfatin-1 to HIIT is ambiguous. However, the increasing in the serum levels of nesfatin-1 following HIIT may be due to attribute to the greater production of lactate in HIIT. It has been demonstrated that the peripheral and central administration of lactate increases hypothalamic levels of lactate, which in turn, suppresses food intake through the hypothalamic AMP kinase/malonyl-CoA signaling system. Furthermore, lactate increases significantly the expression of proopiomelanocortin mRNA, which encodes α-MSH (anorectic molecule), that in turn, affects food intake in a similar manner to nesfatin-1.[16,20] In addition, studies suggested that nesfatin-1 is affected by some factors. For example, serum levels of nesfatin-1 decreased 18% in fasting and it returns to normal levels after 12 h of re-feeding.[21] Tsuchiya et al. demonstrated that may be there is a negative correlation between BMI and levels of nesfatin-1.[22] In the present study, despite significant decrease of BMI, there were no significant changes on levels of nesfatin-1. However, effect of exercise on nesfatin-1 has not widely been investigated and there is no available information about the increase of levels of nesfatin-1 after spirulina consumption. Obesity is known a low-grade inflammation state which is related to the development of obesity-related disorders, anti-inflammatory nutritional interventions have identified as strategy to manage inflammatory status.[23] The omega-3 fatty acid, polyphenols, PC, and β-carotene are the main components of spirulina which are necessary in anti-inflammatory dietary patterns.[24] It seems that PC and β-carotene are the most effective antioxidative and anti-inflammatory components of spirulina.[24] This effect of spirulina on nesfatin-1 might be due to the anti-inflammatory properties of spirulina in HIIT + spirulina group.
Other results of the present study demonstrated that HIIT protocol with and without spirulina supplementation can significantly increase serum levels of omentin-1 in overweight and obese women. The results are similar to those seen by Ouerghi et al., in which an 8-week HIIT increase significantly levels of omentin-1 in overweigh/obese youth.[12] Conversely, Nikseresht et al. found 12 weeks of moderate interval training had no change in omentin-1 in middle-age obese men.[25] Changing in levels of inflammation is an important factor in regulating the omentin-1 expression.[26] Physical activity is another effective strategy in regulating endocrine and metabolic process of body. It can increase basal metabolism by altering body composition and increasing muscle mass. The mechanism of the effect of HIIT on omentin-1 has not yet been well characterized. HIIT is appropriate training protocol which can decrease BFP and BMI and improve cardiovascular factors. Evidences showed that any alternation in levels of omentin-1 is almost due to reduction of weight, BFP, and BMI.[5] Since the adipose tissue is the main source of omentin-1 and increased of adipose tissue leads to the secretion of inflammatory adipokines and decrease in anti-inflammatory adipokines.[27] On other hand, it results in size of fat cells and thus in its secretion.[28] Hence, size of fat cells may be an effective factor that alters omentin-1 concentration which confirms the results of the present study. HIIT induced a reduction of visceral fat in overweight and obese people because it results in weight loss and calorie intake. Improvement of metabolic syndrome indexes following the reduction of weight may lead to increase in levels of omentin-1. Because it was indicated that hypoglycemia is an inhibitory factor in production of omentin-1.[29]
The training and interaction effect of training and spirulina supplementation did not have a significant influence on LDL-C, TG, TC, and HDL levels after 4 weeks of HIIT. Whyte et al. studied the effect of 2 weeks of sprit interval training on lipid profiles of sedentary overweight/obese men.[30] The results of this study indicated that there were no significant changes on lipid profiles. Tjønna et al. observed that 3 months of aerobic interval training improved significantly HDL-C, BFP, and BMI in overweight adolescents.[31] Maybe low sample size was cause of results of the present study. Based on results of previous studies, it was indicated that changes in levels of lipoproteins are likely affected by changes in weight.[32] In addition, may be initial levels of factors at the beginning of the intervention caused results of the present study. In other words, lower levels of HDL-C and higher levels of TG and LDL-C of subjects are most affected by training. Hence, the higher initial levels of blood lipids induced to more tangible changed in lipid profile. However, results of the present study showed despite significant change of BMI and BFP, there was no significant change in lipid profile. In addition to low initial HDL-C levels, high volume of training caused to greater increase HDL-C levels.[1] Other studies reported that HDL-C and LDL-C are difficultly affected by HIIT, especially HDL-C, and they are most influenced by intensity and volume of exercise.[30] Kessler et al. in a review demonstrated that at least 8-week interval aerobic or anaerobic exercise are essential for significant increase of HDL-C, and it was observed no significant differences on lipid profiles in intervention lasting <8 weeks.[33] Maybe the type of training and short duration (4 weeks) of HIIT program is the main cause of the results of the present study. Thus, it seems normal initial lipid levels and short duration of training has led to no significant change in lipid profiles of this study. Spirulina has a hypolipidemic activity and decreases levels of liver profiles. The main component of spirulina that acts to decrease concentration of TG, TC, and LDL-C is PC. PC prevents the intestinal absorption of cholesterol and increases concentration of HDL-C.[34] In a study of Szulinska et al., it was found that 12-week of spirulina consumption decreased significantly levels of LDL-C in obese patients.[34] Moura et al. demonstrated that aerobic exercise and spirulina supplementation leaded to lower plasma concentrations of LDL-C in diabetic Wistar rats.[35] Previous studies have been demonstrated that there were no significant differences on lipid profiles in intervention <3 months of spirulina supplementation in obese hypertensive patients receiving standard hypotensive therapy.[34,36] In the present study, interactive effects of HIIT and spirulina consumption could not improve TC, TG, LDL-C, and HDL-C that maybe due to insufficient dose and short duration of intervention. Also, the present study was probability the first one to examine interactive effect of HIIT and spirulina consumption on lipid profiles, it is required to be careful in interpreting the findings and need more further research. The short duration of the training and the use of spirulina supplementation encouraged all of the subjects to fully participate throughout this study. The results of this study can widely use for overweight and obese women. We did not observe any missing data in the present study. Therefore, these results are highly generalizable.
The present study has some limitation. The small number of samples and control group is lacking. Other limitation of the preset study was lack of controlled subjects' diet. However, there is no evidence to suggest that subjects kept exactly the same normal dietary habits. Calories consumption and nutrition can alter circulation of nesfatin-1, omention-1, and body composition, levels of nesfatin-1 influences food intake. Finally, estimation of body fat based on skin thickness method was not as fully accurate as DEXA or MRI scans. Further studies are necessitated with higher sample size and dose of spirulina, and study duration and exercise duration. We expect dose of training program (e.g., duration, intensity, etc.) and supplementation will consider for design for; however, given the uncertain influences of spirulina on the results, more investigation is needed in future works to make more effective prescription in this overweight and obese population.
CONCLUSION
In summary, we concluded that effect of 4 weeks of spirulina supplementation under HIIT were efficient in alternating anti-inflammatory adipokines in overweight and obese females, with the most important contribution being the increase in levels of nesfatin-1 and omentin-1.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank all of the participants for their time and effort in completing this study. All authors conceived the present study and its design and coordination. FG, MM, SHAE, and MEF were participated in data collection, data analysis and interpretation, and manuscript preparation. Finally, all authors approved the final version of the paper. The present study was approved by the Research Ethics Committee (number: IR.BUMS.REC.1397.182) of Birjand university of medical sciences.
REFERENCES
- 1.Saghebjoo M, Farrokhi-Fard M, Hedayati M, Sadeghi-Tabas S. The effect of high-intensity interval training and L-arginine supplementation on the serum levels of adiponectin and lipid profile in overweight and obese young men. Obes Med. 2019;16:100139. [Google Scholar]
- 2.Mirzaei K, Hossein-nezhad A, Keshavarz SA, Koohdani F, Eshraghian MR, Saboor-Yaraghi AA, et al. Association of nesfatin-1 level with body composition, dietary intake and resting metabolic rate in obese and morbid obese subjects. Diabetes Metab Syndr. 2015;9:292–8. doi: 10.1016/j.dsx.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Hofmann T, Weibert E, Ahnis A, Obbarius A, Elbelt U, Rose M, et al. Alterations of circulating NUCB2/nesfatin-1 during short term therapeutic improvement of anxiety in obese inpatients. Psychoneuroendocrinology. 2017;79:107–15. doi: 10.1016/j.psyneuen.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Babaei P, Pourrahim Ghouroghchi A, Damirchi A, Soltani Tehrani B. The interactive effect of aerobic-resistance training and estrogen therapy on metabolic syndrome indices and omentin-1. Physiol Pharmacol. 2015;19:200–7. [Google Scholar]
- 5.Shang FJ, Wang JP, Liu XT, Zheng QS, Xue YS, Wang B, et al. Serum omentin-1 levels are inversely associated with the presence and severity of coronary artery disease in patients with metabolic syndrome. Biomarkers. 2011;16:657–62. doi: 10.3109/1354750X.2011.622789. [DOI] [PubMed] [Google Scholar]
- 6.Algul S, Ozdenk C, Ozcelik O. Variations in leptin, nesfatin-1 and irisin levels induced by aerobic exercise in young trained and untrained male subjects. Biol Sport. 2017;34:339–44. doi: 10.5114/biolsport.2017.69821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadeghi-Tabas S, Saghebjoo M, Sarir H, Hedayati M. Effects of work/rest interval manipulation of high-intensity interval training and detraining on telomerase activity and p53 levels in cardiac muscle. Sci Sports. 2020;35:170–e1. [Google Scholar]
- 8.Heo MG, Choung SY. Anti-obesity effects of Spirulina maxima in high fat diet induced obese rats via the activation of AMPK pathway and SIRT1. Food Funct. 2018;9:4906–15. doi: 10.1039/c8fo00986d. [DOI] [PubMed] [Google Scholar]
- 9.Dehghani K, Mogharnasi M, Saghebjoo M, Sarir H, Malekaneh M. The effect of eight weeks of circuit resistance training and spirulina supplementation on plasma levels of irisin and some body composition in overweight and obese men. Armaghane Danesh. 2020;25:332–45. [Google Scholar]
- 10.Aladaileh SH, Khafaga AF, Abd El-Hack ME, Al-Gabri NA, Abukhalil MH, Alfwuaires MA, et al. Spirulina platensis ameliorates the sub chronic toxicities of lead in rabbits via anti-oxidative, anti-inflammatory, and immune stimulatory properties. Sci Total Environ. 2020;701:134879. doi: 10.1016/j.scitotenv.2019.134879. [DOI] [PubMed] [Google Scholar]
- 11.Ghanbari-Niaki A, Kraemer RR, Soltani R. Plasma nesfatin-1 and glucoregulatory hormone responses to two different anaerobic exercise sessions. Eur J Appl Physiol. 2010;110:863–8. doi: 10.1007/s00421-010-1531-6. [DOI] [PubMed] [Google Scholar]
- 12.Ouerghi N, Ben Fradj MK, Bezrati I, Feki M, Kaabachi N, Bouassida A. Effect of high-intensity interval training on plasma omentin-1 concentration in overweight/obese and normal-weight youth. Obes Facts. 2017;10:323–31. doi: 10.1159/000471882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeinalian R, Farhangi MA, Shariat A, Saghafi-Asl M. The effects of Spirulina Platensis on anthropometric indices, appetite, lipid profile and serum vascular endothelial growth factor (VEGF) in obese individuals: A randomized double blinded placebo controlled trial. BMC Complement Altern Med. 2017;17:225. doi: 10.1186/s12906-017-1670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosseini A, Valipour DV, Azizi M, Khanjari M. Effect of high-intensity interval training (HIT) for 4 weeks with and without L-Arginine supplementation on the performance of women's futsal players. 2015;21:113–9. [Google Scholar]
- 15.Mogharnasi M, TaheriChadorneshin H, Papoli-Baravati SA, Teymuri A. Effects of upper-body resistance exercise training on serum nesfatin-1 level, insulin resistance, and body composition in obese paraplegic men. Disabil Health J. 2019;12:29–34. doi: 10.1016/j.dhjo.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Ahmadizad S, Avansar AS, Ebrahim K, Avandi M, Ghasemikaram M. The effects of short-term high-intensity interval training vs.moderate-intensity continuous training on plasma levels of nesfatin-1 and inflammatory markers. Horm Mol Biol Clin Investig. 2015;21:165–73. doi: 10.1515/hmbci-2014-0038. [DOI] [PubMed] [Google Scholar]
- 17.Mogharnasi M, TajiTabas A, Tashakorizadeh M, Nayebifar SH. The Effects of resistance and endurance training on levels of nesfatin-1, HSP70, insulin resistance and body composition in women with type 2 diabetes mellitus. Sci Sports. 2019;34:e15–23. [Google Scholar]
- 18.Jafari M, Mogharnasi M. The protective effect of different methods of exercise training on plasma levels of nesfatin-1, cardiorespiratory endurance and body composition in overweight and obese females. Mod Care J. 2015;12:16–27. [Google Scholar]
- 19.Tofighi A, Mehrabani J, Khadivi SM. The effect of 8 weeks aerobic exercise on Nesfatin-1 and acylated Ghrelin in young obese men. Med J Mashhad Univ Med Sci. 2014;57:562–70. [Google Scholar]
- 20.Cha SH, Lane MD. Central lactate metabolism suppresses food intake via the hypothalamic AMP kinase/malonyl-CoA signaling pathway. Biochem Biophys Res Commun. 2009;386:212–6. doi: 10.1016/j.bbrc.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Stengel A, Goebel M, Yakubov I, Wang L, Witcher D, Coskun T, et al. Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology. 2009;150:232–8. doi: 10.1210/en.2008-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuchiya T, Shimizu H, Yamada M, Osaki A, Oh-I S, Ariyama Y, et al. Fasting concentrations of nesfatin-1 are negatively correlated with body mass index in non-obese males. Clin Endocrinol. 2010;73:484–90. doi: 10.1111/j.1365-2265.2010.03835.x. [DOI] [PubMed] [Google Scholar]
- 23.Yousefi R, Mottaghi A, Saidpour A. Spirulina platensis effectively ameliorates anthropometric measurements and obesity-related metabolic disorders in obese or overweight healthy individuals: A randomized controlled trial. Complement Ther Med. 2018;40:106–12. doi: 10.1016/j.ctim.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Serban MC, Sahebkar A, Dragan S, Stoichescu-Hogea G, Ursoniu S, Andrica F, et al. A systematic review and meta-analysis of the impact of Spirulina supplementation on plasma lipid concentrations. Clin Nutr. 2016;35:842–51. doi: 10.1016/j.clnu.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Nikseresht M, Hafezi Ahmadi MR, Hedayati M. Detraining-induced alterations in adipokines and cardiometabolic risk factors after nonlinear periodized resistance and aerobic interval training in obese men. Appl Physiol Nutr Metab. 2016;41:1018–25. doi: 10.1139/apnm-2015-0693. [DOI] [PubMed] [Google Scholar]
- 26.Kazama K, Usui T, Okada M, Hara Y, Yamawaki H. Omentin plays an anti-inflammatory role through inhibition of TNF-α-induced superoxide production in vascular smooth muscle cells. Eur J Pharmacol. 2012;686:116–23. doi: 10.1016/j.ejphar.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 27.Bremer AA, Jialal I. Adipose tissue dysfunction in nascent metabolic syndrome. J Obes. 2013;2013:393192. doi: 10.1155/2013/393192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–33. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 29.de Souza Batista CM, Yang RZ, Lee MJ, Glynn NM, Yu DZ, Pray J, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–61. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 30.Whyte LJ, Gill JM, Cathcart AJ. Effect of 2 weeks of sprint interval training on health-related outcomes in sedentary overweight/obese men. Metabolism. 2010;59:1421–8. doi: 10.1016/j.metabol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Tjønna AE, Stølen TO, Bye A, Volden M, Slørdahl SA, Odegård R, et al. Aerobic interval training reduces cardiovascular risk factors more than a multitreatment approach in overweight adolescents. Clin Sci (Lond) 2009;116:317–26. doi: 10.1042/CS20080249. [DOI] [PubMed] [Google Scholar]
- 32.Ramezankhany A, Nazar Ali P, Hedayati M. Comparing effects of aerobics, Pilates exercises and low calorie diet on leptin levels and lipid profiles in sedentary women. Iran J Basic Med Sci. 2011;14:256–63. [Google Scholar]
- 33.Kessler HS, Sisson SB, Short KR. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med. 2012;42:489–509. doi: 10.2165/11630910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Szulinska M, Gibas-Dorna M, Miller-Kasprzak E, Suliburska J, Miczke A, Walczak-Gałezewska M, et al. Spirulina maxima improves insulin sensitivity, lipid profile, and total antioxidant status in obese patients with well-treated hypertension: A randomized double-blind placebo-controlled study. Eur Rev Med Pharmacol Sci. 2017;21:2473–81. [PubMed] [Google Scholar]
- 35.Moura LP, Puga GM, Beck WR, Teixeira IP, Ghezzi AC, Silva GA, et al. Exercise and spirulina control non-alcoholic hepatic steatosis and lipid profile in diabetic Wistar rats. Lipids Health Dis. 2011;10:77. doi: 10.1186/1476-511X-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira-Hermosillo A, Torres-Duran PV, Juarez-Oropeza MA. Hepatoprotective effects of Spirulina maxima in patients with non-alcoholic fatty liver disease: A case series. J Med Case Rep. 2010;4:103. doi: 10.1186/1752-1947-4-103. [DOI] [PMC free article] [PubMed] [Google Scholar]