Abstract
Peptic ulcer refers to the inflammatory response and necrotic lesions of the mucosa under the action of various pathogenic factors, which goes deeply into the mucosal muscle layer and often occurs to the gastrointestinal mucosa related to gastric acid secretion, among which the stomach and duodenum are the most common. The clinical manifestations include slow onset, prolonged course and weekly upper abdominal pain. Nitric oxide (NO) is an intracellular and intercellular signaling molecule that plays an important role in many physiological and pathological processes. Studies have found that a small amount of NO produced in vivo plays a role in many physiological homeostasis, such as regulating blood pressure, platelet aggregation, nitrogenization of hemoglobin, and regulating proliferation and differentiation of stem cells. However, under the action of some cytokines and oxidative stress, intracellular NO synthase will catalyze the synthesis of large amounts of NO and participate in the inflammatory response, causing beneficial or harmful effect on the body. Numerous basic studies have focused on the relationship between NO and peptic ulcer. The purpose of this review is to summarize the role of NO in peptic ulcer and its possible mechanism.
Keywords: inflammation, nitric oxide synthase, nitric oxide, peptic ulcer, pH, prostaglandin, vascular smooth muscle, vessel endothelium
INTRODUCTION
Peptic ulcer disease refers a mucosal break deeper than 3–5 mm in the duodenum or stomach.1 It is mainly due to the imbalance of protective and injury factors between gastric mucosa and duodenum. Gastric ulcer and duodenal ulcer are similar in appearance, often accompanied by upper or posterior sternum pain, early satiety, nausea, bloating, or post-meal pain. These symptoms are nonspecific and may be clinically difficult to distinguish from functional dyspepsia. Studies have shown a low correlation between symptoms and endoscopic results.2 Instead, patients may not develop symptoms until complications appear, or an ulcer may be accidentally discovered during an endoscopy for other reasons. Peptic ulcer can lead to serious complications such as bleeding or perforation, which can lead to a high death rate.3 In recent years, peptic ulcer is still one of the most common diseases of the digestive system and the incidence of peptic ulcer disease is 1–2 per 1000 person-years,4,5,6 although its occurrence rates have decreased slightly.3,7 But many studies have shown that peptic ulcer complications have not decreased and are increasing year by year.8 The pathogenesis of peptic ulcer is mainly related to the injury of gastric and duodenal mucosa, it is reported that 70% of gastric ulcers and 90% of duodenal ulcers are related to Helicobacter pylori and because of its serious harm, it is urgent to curb its spread.9,10,11,12 Non-steroidal anti-inflammatory drugs (NSAIDs) and other medications such as aspirin are important factors which cause peptic ulcer. Noteworthily, NSAID seems to be more likely to cause gastric ulcers, the mechanism of which is unknown.13,14 In addition, there are many causes of peptic ulcer, such as selective serotonin reuptake inhibitors,15 Zollinger-Ellison syndrome, physiological stress associated with serious trauma and critical illness,16 cigarette smoking.17 With the exploration of more pathogenic factors of peptic ulcer, it is beneficial to us to understand its pathogenesis and put forward relevant prevention, diagnosis and treatment strategies.
Nitric oxide (NO) was once considered to be a kind of harmful gas, which can be converted into nitrogen dioxide in air and damage to the respiratory tract, even can cause pulmonary fibrosis, the methemoglobin levels can lead to disease. In recent years, it is generally believed that NO, as a biological signal molecule, plays an important role in human signal transduction. One of the most important functions is to act on the smooth muscle cells of the blood vessels to relax the blood vessels.18,19 Due to the extensive research on NO, some studies have shown that the role of NO includes regulating vasodilation and contraction of blood vessels, participating in inflammatory response, and coagulation process, and regulating cell cycle. In peptic ulcer, NO takes an important role in keeping the gastric mucosa20 health. NO is mainly synthesized by NO synthase (NOS), and three NOS subtypes have been shown to exist in the gastrointestinal tract, neuronal NOS, endothelial NOS and inducible NOS. NOS composed to neuronal NOS or endothelial NOS is involved in maintaining gastrointestinal mucosal integrity by regulating gastric mucosal blood flow, mucous secretion and defense barrier. However, NO synthesized by inducible NOS is involved in tissue damage in the inflammatory response. Several pieces of evidence showed that NO can be used as a protective agent for gastric ulcer by promoting mucosal blood flow, stimulating gastric mucus secretion and reducing leukocyte infiltration (Table 1).21,22,23
Table 1.
The role of nitric oxide in peptic ulcer
| Disease | Injury type | Animal | Result |
|---|---|---|---|
| Gastric ulcer | Hypertonic NaCl solution | Rat | NO is involved in the mechanism of the gastric alkaline response which occurs in the stomach after exposing to hypertonic NaCl. |
| Gastric ulcer | Non-steroidal anti-inflammatory drugs | Rat | NO could reduce the severity of gastric and intestinal damage. |
| Gastric ulcer | Ethanol | Rat | NO may have therapeutic effect on gastric ulcer mucosal lesions. This effect may be realized by the anti-inflammatory, angiogenesis and antiapoptosis effects of NO. |
| Gastric lesion | HCl | Rat | NO significantly inhibits the HCl-induced gastric lesion formation in rats. |
| Duodenal ulcer | Helicobacter pylori infection | Mouse | NO binds to superoxide to produce peroxynitrite that is involved in epithelial damage associated with H. pylori infection. |
Note: NO: Nitric oxide.
THE CRITICAL ROLE OF NITRIC OXIDE INCREASES THE PH IN THE STOMACH
Existing studies have shown that stimulation can improve the pH of gastric environment.24,25 A common phenomenon after gastric injury called gastric alkaline reaction, which is characterized as increased gastric pH after injury, has been shown to be mediated by endogenous peptidoglycans (PGs) pathway.26 Further researches also show that the generation and release of endogenous NO could increase the pH somehow.27 After exposure of the stomach to hypertonic sodium chloride solution, acid secretion stopped. We found that when NOS inhibitor nitro-L-arginine methyl ester was added, gastric pH stopped rising, which is suggesting that NO may be related to this gastric alkaline reaction. Besides, slight stimulation to gastric mucosa could also activate NOS to increase the local release of NO, promote gastric alkaline reaction and adaptive cell protection. This condition can also be supported by the fact that the adding D-arginine in the same experimental conditions was completely ineffective in antagonizing the inhibitory action of nitro-L-arginine methyl ester on gastric alkaline response. We believe that mild mucosal stimulation may activate NOS, increase the local release of NO, and promote gastric alkaline response and adaptive cell protection. Previous studies indicated that NO could promote cyclooxygenase activity through a kind of mechanism independent of cyclic guanosine-3′,5′-monophosphate.28 They also noted that when NO synthase and cyclooxygenase systems are existed at the same time, there is a no-mediated increase in PGs production, which may lead to multiple pro-inflammatory effects. Therefore, it is reasonable for us to deduce that NO may control the release and biosynthesis of endogenous PGs after gastric injury, while NO strengthens the gastric alkaline response by inhibiting gastric acid secretion in directly or indirectly way through endogenous PGs mediated. In summary, the results indicate that the gastric alkaline reaction observed after hypertonic sodium chloride solution treatment is mediated by endogenous NO and PGs pathways.25,29 It is believed that inflammatory cells such as macrophages, granulocytes in the mucosa and vascular endothelial cells may be involved in the release of NO by hypertonic sodium chloride.
THE CRITICAL ROLE OF NITRIC OXIDE IN VASCULAR SYSTEM
Peptic ulcer is usually thought to be caused by an imbalance between aggressive factors and antagonistic defense mechanisms. The most aggressive factor is the use of NSAIDs.30 Decreased mucosal blood flow is considered to be the main mechanism of gastric injury caused by NSAID.31 Mucosal defense mechanisms are mechanisms by which the mucosa remains stable despite frequent exposure to various stimuli, such as changes in temperature, pH and osmotic pressure, toxic substances, bacterial products, and inflammatory infiltration.32 Microcirculation perfusion of gastric mucosa provides essential substances for epithelial cells, and plays a key role in removing metabolic substances and foreign bodies, maintaining normal secretion of mucus, and maintaining pH in the stomach.33 In conclusion, the mucosa becomes more fragile when blood flow decreases.34 NO synthesized by endothelial NOS acts on vascular smooth muscle to make it relaxed, thereby enhancing local blood flow. Meanwhile, it also increases blood flow by stimulating mucosal angiogenesis in the wound.35 The mechanism of NO causing vascular smooth muscle relaxation is NO binds to Fe2+-heme of soluble guanylyl-cyclase effectively activates soluble guanylyl-cyclase and promote the production of cyclic guanosine-3′,5′-monophosphate. The second messenger (cyclic guanosine-3′,5′-monophosphate) immediately activates protein kinase G and then launches the signal cascades, such as K+ channels. Activation of K+ channels leads to hyperpolarization of cell membranes, and Ca2+ channels are blocked, leading to relaxation of blood vessels (Figure 1).36 Histamine and bradykinin induced vasodilation is considered to be a powerful vasodilator, and its mechanism is to stimulate endothelial cells to release NO.37 On the other side, numerous studies have shown that because of the insufficient release of NO, vascular endothelial cells are damaged and vasodilation is restricted.38 In addition, vascular endothelial growth factor can stimulate the expression of endothelial NOS in endothelial cells to produce active NO to stimulate angiogenesis and repair damaged endothelial cells.39
Figure 1.
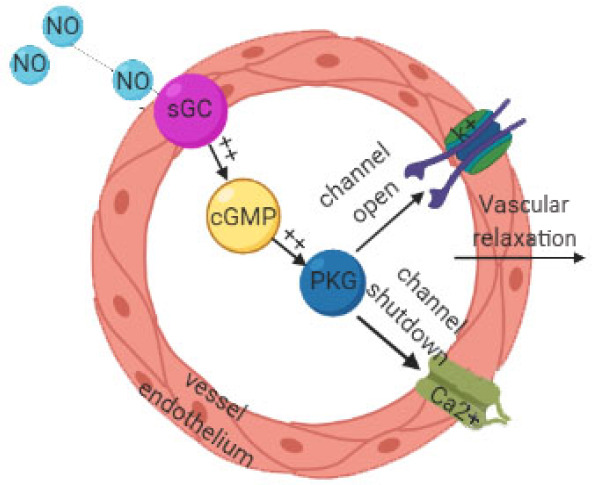
The mechanism of nitric oxide (NO) causing vascular smooth muscle relaxation is NO binds to soluble guanylyl-cyclase (sGC) effectively activates sGC and promote the production of cyclic guanosine-3′,5′-monophosphate (cGMP).
Note: The second messenger (cGMP) immediately activates protein kinase G (PKG) and then launches the signal cascades, such as K+ channels. Activation of K+ channels lead to hyperpolarization of cell membranes, and Ca2+ channels are blocked, leading to relaxation of blood vessels.
THE CRITICAL ROLE OF NITRIC OXIDE IN INFLAMMATION
The immune system in the lining of the gastrointestinal tract ensures that the gastrointestinal tract remains stable against various microorganisms, microbial products, and other antigens. Mutual adhesion between white blood cells and endothelial cells is considered a rate-control step in inflammatory response, so factors that regulate these cell-cell interactions have received considerable attention. Antibodies against white blood cells and endothelial cell adhesion molecules can reduce the degree of injury. NO is an important regulator of leukocyte adhesion to vascular endothelium under various acute inflammatory conditions. Increased NO can lead to decreased myeloperoxidase activity (an indicator of the number of neutrophils) and tissue damage. Existing studies have shown that fewer neutrophils infiltrate the gastric mucosa after administration of nitroprusside or acetylcholine.40 Other studies have shown that NO inhibits the production of superoxide from neutrophils and directly inhibits NADPH oxidase.41 Thus, in addition to removing superoxide, NO also prevents the synthesis of superoxide and related oxidants, including hydrogen peroxide. Both superoxide and hydrogen peroxide are involved in mucosal damage associated with ischemia reperfusion and NSAIDs.42,43 Kubes et al.44 provided the first clear evidence that inhibition of the NOS enzyme leads to inflammation. Further studies found that NOS inhibition enhanced leukocyte - endothelial interaction in rats.45 Platelets are also thought to play a role in inflammatory processes, and NO is a very effective inhibitor of platelet aggregation and adhesion,46 which may play an anti-inflammatory role by inhibiting platelet activation. Studies have suggested that the role of NO in anti-aggregation is regulated by increasing the level of cyclic guanosine-3′,5′-monophosphate in platelets.47 In the gastrointestinal tract, the most significant immune regulatory role of NO is to inhibit the ability of mast cells to release mediators. Mast cells can produce NO, and NO produced by mast cells can automatically regulate the release of other inflammatory mediators.48 On the other hand, H. pylori can induce the expression of inducible NOS in the pathogenesis of peptic ulcer. The increase of inducible NOS leads to duodenal epithelial injury, which can be inhibited by NOS inhibitors and superoxide dismutase inhibitors. These findings indicate that NO binds to superoxide to produce peroxynitrite that is involved in epithelial damage associated with H. pylori infection.49
CONCLUSION
In recent years, the study of NO as a signaling molecule emerges in endlessly. We should correctly recognize and profoundly understand the different effects of NO in peptic ulcer, which leads to a great awareness of the pathological process, and provides a better solution for future clinical diagnosis and treatment.
Footnotes
Conflicts of interest
The authors declare that they have no competing interests.
Financial support
None.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
REFERENCES
- 1.Sverdén E, Agréus L, Dunn JM, Lagergren J. Peptic ulcer disease. BMJ. 2019;367:l5495. doi: 10.1136/bmj.l5495. [DOI] [PubMed] [Google Scholar]
- 2.Werdmuller BF, van der Putten AB, Loffeld RJ. The clinical presentation of peptic ulcer disease. Neth J Med. 1997;50:115–119. doi: 10.1016/s0300-2977(96)00075-7. [DOI] [PubMed] [Google Scholar]
- 3.Sung JJY, Kuipers EJ, El-Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther. 2009;29:938–946. doi: 10.1111/j.1365-2036.2009.03960.x. [DOI] [PubMed] [Google Scholar]
- 4.Cai S, García Rodríguez LA, Massó-González EL, Hernández-Díaz S. Uncomplicated peptic ulcer in the UK: trends from 1997 to 2005. Aliment Pharmacol Ther. 2009;30:1039–1048. doi: 10.1111/j.1365-2036.2009.04131.x. [DOI] [PubMed] [Google Scholar]
- 5.Bardhan KD, Royston C. Time, change and peptic ulcer disease in Rotherham, UK. Dig Liver Dis. 2008;40:540–546. doi: 10.1016/j.dld.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Aro P, Storskrubb T, Ronkainen J, et al. Peptic ulcer disease in a general adult population: the Kalixanda study: a random population-based study. Am J Epidemiol. 2006;163:1025–1034. doi: 10.1093/aje/kwj129. [DOI] [PubMed] [Google Scholar]
- 7.Agréus L, Hellström PM, Talley NJ, et al. Towards a healthy stomach? Helicobacter pylori prevalence has dramatically decreased over 23 years in adults in a Swedish community. United European Gastroenterol J. 2016;4:686–696. doi: 10.1177/2050640615623369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leow AHR, Lim YY, Liew WC, Goh KL. Time trends in upper gastrointestinal diseases and Helicobacter pylori infection in a multiracial Asian population--a 20-year experience over three time periods. Aliment Pharmacol Ther. 2016;43:831–837. doi: 10.1111/apt.13550. [DOI] [PubMed] [Google Scholar]
- 9.Marshall BJ, McGechie DB, Rogers PA, Glancy RJ. Pyloric Campylobacter infection and gastroduodenal disease. Med J Aust. 1985;142:439–444. doi: 10.5694/j.1326-5377.1985.tb113444.x. [DOI] [PubMed] [Google Scholar]
- 10.Graham DY, Klein PD, Opekun AR, Boutton TW. Effect of age on the frequency of active Campylobacter pylori infection diagnosed by the [13C]urea breath test in normal subjects and patients with peptic ulcer disease. J Infect Dis. 1988;157:777–780. doi: 10.1093/infdis/157.4.777. [DOI] [PubMed] [Google Scholar]
- 11.Hansson LE, Nyrén O, Hsing AW, et al. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N Engl J Med. 1996;335:242–249. doi: 10.1056/NEJM199607253350404. [DOI] [PubMed] [Google Scholar]
- 12.Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. doi: 10.1136/bmj.g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gisbert JP, Calvet X. Review article: Helicobacter pylori-negative duodenal ulcer disease. Aliment Pharmacol Ther. 2009;30:791–815. doi: 10.1111/j.1365-2036.2009.04105.x. [DOI] [PubMed] [Google Scholar]
- 14.Konturek SJ, Bielański W, Płonka M, et al. Helicobacter pylori, non-steroidal anti-inflammatory drugs and smoking in risk pattern of gastroduodenal ulcers. Scand J Gastroenterol. 2003;38:923–930. doi: 10.1080/00365520310004696. [DOI] [PubMed] [Google Scholar]
- 15.Dall M, Schaffalitzky de Muckadell OB, Lassen AT, Hallas J. There is an association between selective serotonin reuptake inhibitor use and uncomplicated peptic ulcers: a population-based case-control study. Aliment Pharmacol Ther. 2010;32:1383–1391. doi: 10.1111/j.1365-2036.2010.04472.x. [DOI] [PubMed] [Google Scholar]
- 16.Krag M, Perner A, Wetterslev J, et al. Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive Care Med. 2015;41:833–845. doi: 10.1007/s00134-015-3725-1. [DOI] [PubMed] [Google Scholar]
- 17.Kurata JH, Nogawa AN. Meta-analysis of risk factors for peptic ulcer. Nonsteroidal antiinflammatory drugs, Helicobacter pylori, and smoking. J Clin Gastroenterol. 1997;24:2–17. doi: 10.1097/00004836-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 19.Cacanyiova S, Dovinova I, Kristek F. The role of oxidative stress in acetylcholine-induced relaxation of endothelium-denuded arteries. J Physiol Pharmacol. 2013;64:241–247. [PubMed] [Google Scholar]
- 20.Tepperman BL, Soper BD. Nitric oxide synthase induction and cytoprotection of rat gastric mucosa from injury by ethanol. Can J Physiol Pharmacol. 1994;72:1308–1312. doi: 10.1139/y94-188. [DOI] [PubMed] [Google Scholar]
- 21.Bandarage UK, Janero DR. Nitric oxide-releasing nonsteroidal anti-inflammatory drugs: novel gastrointestinal-sparing drugs. Mini Rev Med Chem. 2001;1:57–70. doi: 10.2174/1389557013407160. [DOI] [PubMed] [Google Scholar]
- 22.McCarty MF. Dietary nitrate and reductive polyphenols may potentiate the vascular benefit and alleviate the ulcerative risk of low-dose aspirin. Med Hypotheses. 2013;80:186–190. doi: 10.1016/j.mehy.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Ohta Y, Nishida K. L-arginine protects against stress-induced gastric mucosal lesions by preserving gastric mucus. Clin Exp Pharmacol Physiol. 2002;29:32–38. doi: 10.1046/j.1440-1681.2002.03607.x. [DOI] [PubMed] [Google Scholar]
- 24.Swierczek JS, Konturek SJ. Gastric alkaline response to mucosa-damaging agents: effect of 16,16-dimethyl prostaglandin E2. Am J Physiol. 1981;241:G509–G515. doi: 10.1152/ajpgi.1981.241.6.G509. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi K, Ueki S, Tanaka H. Endogenous prostaglandins in gastric alkaline response in the rat stomach after damage. Am J Physiol. 1986;250:G842–G849. doi: 10.1152/ajpgi.1986.250.6.G842. [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi K, Ohno T, Okabe S. Irritative and protective activity of mild irritants in rat stomach. Dig Dis Sci. 1987;32:889–896. doi: 10.1007/BF01296714. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi K, Okabe S. Mechanism of gastric alkaline response in the stomach after damage. Roles of nitric oxide and prostaglandins. Dig Dis Sci. 1995;40:865–871. doi: 10.1007/BF02064993. [DOI] [PubMed] [Google Scholar]
- 28.Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nobuhara Y, Takeuchi K. Possible role of endogenous prostaglandins in alkaline response in rat gastric mucosa damaged by hypertonic NaCl. Dig Dis Sci. 1984;29:1142–1147. doi: 10.1007/BF01317090. [DOI] [PubMed] [Google Scholar]
- 30.Sivri B. Trends in peptic ulcer pharmacotherapy. Fundam Clin Pharmacol. 2004;18:23–31. doi: 10.1111/j.1472-8206.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 31.Santos CL, Souza MHLP, Gomes AS, et al. Sildenafil prevents indomethacin-induced gastropathy in rats: role of leukocyte adherence and gastric blood flow. Br J Pharmacol. 2005;146:481–486. doi: 10.1038/sj.bjp.0706361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallace JL, Granger DN. The cellular and molecular basis of gastric mucosal defense. FASEB J. 1996;10:731–740. doi: 10.1096/fasebj.10.7.8635690. [DOI] [PubMed] [Google Scholar]
- 33.Petersson J, Phillipson M, Jansson EA, Patzak A, Lundberg JO, Holm L. Dietary nitrate increases gastric mucosal blood flow and mucosal defense. Am J Physiol Gastrointest Liver Physiol. 2007;292:G718–G724. doi: 10.1152/ajpgi.00435.2006. [DOI] [PubMed] [Google Scholar]
- 34.El-Demerdash E, El-Mesallamy HO, Abu-Zaid NM, Gad MZ. The potential therapeutic effect of nitric oxide modulators in experimentally-induced gastric ulcers. Drug Discov Ther. 2010;4:276–284. [PubMed] [Google Scholar]
- 35.Tian JS, Peng GJ, Gao XX, et al. Dynamic analysis of the endogenous metabolites in depressed patients treated with TCM formula Xiaoyaosan using urinary (1)H NMR-based metabolomics. J Ethnopharmacol. 2014;158(Pt A):1–10. doi: 10.1016/j.jep.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Silva BR, Paula TD, Paulo M, Bendhack LM. Nitric oxide signaling and the cross talk with prostanoids pathways in vascular system. Med Chem. 2016 doi:10.2174/1573406412666161228115627. [PubMed] [Google Scholar]
- 37.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 38.Mollnau H, Wendt M, Szöcs K, et al. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circul Res. 2002;90:E58–E65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira de Almeida L, Della Coletta Francescato H, Antunes-Rodrigues J, et al. Imbalance of pro- and anti-angiogenic factors due to maternal vitamin D deficiency causes renal microvasculature alterations affecting the adult kidney function. Nutrients. 2019;11:1929. doi: 10.3390/nu11081929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrews FJ, Malcontenti-Wilson C, O’Brien PE. Protection against gastric ischemia-reperfusion injury by nitric oxide generators. Dig Dis Sci. 1994;39:366–373. doi: 10.1007/BF02090210. [DOI] [PubMed] [Google Scholar]
- 41.Clancy RM, Leszczynska-Piziak J, Abramson SB. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest. 1992;90:1116–1121. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Granger DN, Höllwarth ME, Parks DA. Ischemia-reperfusion injury: role of oxygen-derived free radicals. Acta Physiol Scand Suppl. 1986;548:47–63. [PubMed] [Google Scholar]
- 43.Vaananen PM, Meddings JB, Wallace JL. Role of oxygen-derived free radicals in indomethacin-induced gastric injury. Am J Physiol. 1991;261:G470–G475. doi: 10.1152/ajpgi.1991.261.3.G470. [DOI] [PubMed] [Google Scholar]
- 44.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davenpeck KL, Gauthier TW, Lefer AM. Inhibition of endothelial-derived nitric oxide promotes P-selectin expression and actions in the rat microcirculation. Gastroenterology. 1994;107:1050–1058. doi: 10.1016/0016-5085(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 46.Radomski MW, Palmer RM, Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci U S A. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 48.Hogaboam CM, Befus AD, Wallace JL. Modulation of rat mast cell reactivity by IL-1 beta. Divergent effects on nitric oxide and platelet-activating factor release. J Immunol. 1993;151:3767–3774. [PubMed] [Google Scholar]
- 49.Wilson KT, Ramanujam KS, Mobley HL, Musselman RF, James SP, Meltzer SJ. Helicobacter pylori stimulates inducible nitric oxide synthase expression and activity in a murine macrophage cell line. Gastroenterology. 1996;111:1524–1533. doi: 10.1016/s0016-5085(96)70014-8. [DOI] [PubMed] [Google Scholar]


