Abstract
Objective
Metformin has been linked to anti-proliferative and anti-inflammatory mechanisms. In this study, we aimed to examine the long-term impact of metformin on mortality and organ damage in patients with autoimmune diseases and type 2 diabetes mellitus (T2DM).
Methods
We conducted a cohort study using the National Health Insurance Research Database in Taiwan between 1997 and 2013. Based on metformin and other anti-diabetic agent prescriptions, we categorized all patients with autoimmune diseases into either the metformin group (metformin administration for at least 28 days) or the non-metformin group. The primary outcomes were all-cause mortality and annual admission rate, while the secondary outcome was target organ damage. We followed patients from the index date to the date on which the event of interest occurred, death, or the end of this study.
Results
Our cohort study included 3,359 subjects for analysis. During a mean follow up of 5.2 ± 3.8 years, the event rate of all-cause mortality was 228 (33.6%) in the metformin group and 125 (36.9%) in the non-metformin group. The risk of both all-cause mortality and annual number of admissions for autoimmune diseases was significantly lower in the metformin group than in the non-metformin group [hazard ratio (HR) 0.77; 95% CI 0.62–0.96 and risk ratio (RR) 0.81; 95% CI 0.73–0.90, respectively].
Conclusion
Metformin may add benefits beyond T2DM control with regard to reducing all-cause mortality and admission rate, as well as minimizing end-organ injury in lungs and kidneys among patients with autoimmune diseases.
Keywords: autoimmune diseases, inflammatory disorders, hospital admissions, metformin, mortality
Introduction
Immunosuppressive agents and corticosteroids have long been a cornerstone of treatment for various autoimmune diseases (AD). However, infectious complications resulting from immunosuppression may result in a high burden of morbidity in patients with AD. A recent clinical trial has demonstrated that the use of metformin in patients with systemic lupus erythematosus (SLE) resulted in decreased disease activity (1). Data regarding whether metformin can be used as a disease modifying treatment in other ADs are scarce (2).
Type 2 diabetes mellitus (T2DM), combined with a number of risk factors of metabolic origin, can result in an increased risk for early mortality (3). Considerable evidence has demonstrated that chronic low-grade inflammation caused by activation of the innate immune system is vital in the pathogenesis of T2DM and major complications, as well as that the antidiabetic drug metformin exhibits various anti-proliferative and anti-inflammatory mechanisms (3). Furthermore, increasing studies have indicated that metformin can reduce cardiovascular mortality (4), cancer mortality (5), all-cause mortality (6), and intensive care unit mortality (7). However, previous studies have rarely investigated the role of metformin in patients with AD and T2DM with regard to mortality (8).
According to the international treatment guidelines for T2DM, metformin is the first-line therapy, followed by dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide 1 (9). Therefore, using a nationwide database, we conducted an observational pharmacoepidemiological study to examine the impact of metformin on mortality and AD-related complications among patients with both AD and T2DM.
Material and Methods
Data Source
We obtained real-world data from Taiwan’s National Health Insurance Research Database (NHIRD). NHIRD is an administrative database from Taiwan’s National Health Insurance (NHI) program, which is a compulsory single-payer national health insurance system. The NHI program covers almost all outpatient visits, emergency room services, admission services, medical care services, and prescription drugs. This study was approved by the Institutional Review Board of National Cheng Kung University Hospital (A-EX-109-017) and Chang Gung Medical Foundation (IRB No.: 201600763B1).
Study Design and Participants
Between 1st January 1997 and 31st December 2013, patients with AD, including systemic lupus erythematosus, rheumatoid arthritis, primary Sjogren’s syndrome, systemic sclerosis, polymyositis, dermatomyositis, anti-neutrophil cytoplasmic antibodies-associated vasculitis, Buerger’s disease, Takayasu arteritis, Kawasaki disease, Behçet’s syndrome, pemphigus, Crohn’s disease, ulcerative colitis, hypersensitivity angiitis, and seronegative spondylopathy, were identified by the corresponding international classification of diseases, ninth revision, clinical modification (ICD-9-CM), and the list of ICD-9 coding is presented in the supplementary table. Most AD diagnoses were confirmed by catastrophic illness certification (CIC) according to NHI program regulations (10, 11). The application of CIC for major AD required a strict review process by two rheumatologists: one application rheumatologist and one anonymous senior rheumatologist as an adjudicator.
T2DM was identified using a physician’s diagnosis with a disease code (ICD-9 CM code: 250) combined with the prescription of glucose-lowering drugs. The accuracy of T2DM diagnosis has been validated in previous studies (12). Only patients that had been diagnosed with AD prior to the first diagnosis of T2DM were included in this study. Patients who had been diagnosed with T2DM prior to the first diagnosis of AD were excluded from this study since metformin may affect the occurrence of AD and become a confounding factor. We further excluded patients with any history of respiratory failure or end-stage renal disease. Study participants were categorized into the metformin group if they had received metformin treatment for at least 28 days. The date of the first metformin prescription was assigned as the index date in the metformin group, while the index date of the non-metformin group was the date of the first prescription of other oral antihyperglycemic agents such as sulfonylureas, α-glucosidase, thiazolidinediones, meglitinides, DPP-4 inhibitor, and insulin.
Identification of Covariates and Outcomes
Since the NHIRD does not include laboratory test results (i.e., glycohemoglobin, C-reactive protein), we selected several clinical indicators to represent surrogate markers of disease severity, including history of plasmapheresis or plasma exchange, prior admission due to AD, diabetic ketoacidosis (DKA) or hyperosmolar hyperglycemic state (HHS), DM disease duration, and autoimmune disease duration. Such baseline comorbidities as hypertension, dyslipidemia, gout, chronic kidney disease, coronary artery disease, interstitial lung disease, malignancy, stroke, and hepatitis B virus (HBV)/hepatitis C virus (HCV) infections were identified in the period of 365 days prior to the index date by using corresponding ICD-9-CM diagnosis codes in the ambulatory record at least twice or in the inpatient record at least once. We also recorded exposure to medications, including aspirin, clopidogrel, statins, fibrates, anticoagulants, systemic non-steroidal anti-inflammatory drugs (NSAIDs), immunosuppressants (hydroxychloroquine, steroids, cyclosporine, cyclophosphamide, mycophenolate, tacrolimus, azathioprine, methotrexate, sulfasalazine), and antihyperglycemic agents (sulfonylureas, α-glucosidase, thiazolidinediones, meglitinides, DPP-4 inhibitors, insulin), within three months of the index date. Patients were designated as users of certain drugs if they refilled the prescription for the 80% of the required dosage or more.
The primary outcomes included mortality rate and the number of hospitalizations due to AD. The number of AD admissions was calculated after the index date. The secondary outcomes included sepsis, cerebrovascular or cardiovascular event, respiratory failure, initiation of long-term dialysis, admission related to viral infection (viral upper respiratory tract infection, viral pneumonia, viral meningitis, viral gastroenteritis), DKA or HHS, acute hepatitis, or newly-onset malignancy. The diagnosis of respiratory failure, long-term dialysis, and malignancy had to be verified by the presence of CIC. The occurrence of a cerebral or cardiovascular event, DKA or HHS, or acute hepatitis was determined through an emergency room’s principal diagnosis or hospitalization. Each patient was followed until the date of event occurrence, death, or 31 December 2013, whichever came first.
Statistical Analysis
We performed propensity score matching in order to compare the metformin and non-metformin groups. The propensity score was calculated by logistic regression model, using metformin as the dependent variable. The independent variables used to construct the logistic regression model are listed in Table 1 , including demographics (gender and age), types of autoimmune diseases, surrogate variables of disease severity, comorbidities, glucose-lowering medications, immunomodulatory medications, and other medications that may influence the occurrence of outcome, and the index date. We matched a patient in the non-metformin group with two corresponding patients in the metformin group (13). We carried out the matching using the greedy nearest neighbor algorithm with a caliper width of 0.2 times the pooled standard deviation of the propensity score.
Table 1.
Characteristics of the study patients before and after propensity score matching.
| Variable | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| Metformin (n = 3,015) | Non-metformin (n = 344) | P-value | Metformin (n = 678) | Non-metformin (n = 339) | P-value | |
| Characteristic | ||||||
| Age (years) | 59.8 ± 11.6 | 63.7 ± 12.2 | <0.001 | 63.2 ± 11.1 | 63.4 ± 12.2 | 0.733 |
| Age ≥ 65 years | 1024 (34.0) | 169 (49.1) | <0.001 | 309 (45.6) | 164 (48.4) | 0.398 |
| Female gender | 2,332 (77.3) | 274 (79.7) | 0.332 | 541 (79.8) | 270 (79.6) | 0.956 |
| Existing autoimmune diseases in enrollment | 0.244 | 0.990 | ||||
| Rheumatoid arthritis | 1,882 (62.4) | 205 (59.6) | 415 (61.2) | 202 (59.6) | ||
| Sjogren’s syndrome | 232 (7.7) | 26 (7.6) | 53 (7.8) | 26 (7.7) | ||
| Systemic lupus erythematosus | 435 (14.4) | 61 (17.7) | 118 (17.4) | 61 (18.0) | ||
| Polymyositis + dermatomyositis | 84 (2.8) | 5 (1.5) | 10 (1.5) | 5 (1.5) | ||
| Crohn’s disease + ulcerative colitis | 59 (2.0) | 4 (1.2) | 9 (1.3) | 4 (1.2) | ||
| Other | 323 (10.7) | 43 (12.5) | 73 (10.8) | 41 (12.1) | ||
| Severity | ||||||
| Plasmapheresis or plasma exchange | 187 (6.2) | 38 (11.0) | 0.001 | 77 (11.4) | 36 (10.6) | 0.724 |
| Admission due to autoimmune disease | 1,381 (45.8) | 157 (45.6) | 0.954 | 320 (47.2) | 153 (45.1) | 0.534 |
| DKA or HHS | 49 (1.6) | 9 (2.6) | 0.181 | 16 (2.4) | 9 (2.7) | 0.775 |
| Autoimmune diseases duration (year) | 7.0 ± 4.5 | 6.9 ± 4.9 | 0.949 | 6.6 ± 4.6 | 6.9 ± 4.9 | 0.299 |
| Comorbidity | ||||||
| Hypertension | 1,457 (48.3) | 197 (57.3) | 0.002 | 380 (56.0) | 192 (56.6) | 0.858 |
| Dyslipidemia | 628 (20.8) | 62 (18.0) | 0.222 | 121 (17.8) | 61 (18.0) | 0.954 |
| Gout | 196 (6.5) | 28 (8.1) | 0.248 | 47 (6.9) | 28 (8.3) | 0.445 |
| Coronary artery disease | 364 (12.1) | 48 (14.0) | 0.314 | 83 (12.2) | 45 (13.3) | 0.640 |
| Chronic kidney disease | 278 (9.2) | 55 (16.0) | <0.001 | 100 (14.7) | 53 (15.6) | 0.710 |
| Interstitial lung disease | 55 (1.8) | 6 (1.7) | 0.916 | 14 (2.1) | 6 (1.8) | 0.749 |
| Malignancy | 165 (5.5) | 12 (3.5) | 0.119 | 22 (3.2) | 12 (3.5) | 0.805 |
| Hepatitis B virus infection | 42 (1.4) | 7 (2.0) | 0.347 | 15 (2.2) | 7 (2.1) | 0.879 |
| Hepatitis C virus infection | 105 (3.5) | 8 (2.3) | 0.259 | 11 (1.6) | 8 (2.4) | 0.413 |
| Stroke | 135 (4.5) | 24 (7.0) | 0.039 | 42 (6.2) | 23 (6.8) | 0.717 |
| Medication | ||||||
| Hypoglycemic drugs | ||||||
| Glinide | 176 (5.8) | 48 (14.0) | <0.001 | 83 (12.2) | 43 (12.7) | 0.840 |
| Alpha glucosidase inhibitors | 183 (6.1) | 55 (16.0) | <0.001 | 84 (12.4) | 50 (14.7) | 0.294 |
| Sulfonylurea | 1,705 (56.6) | 248 (72.1) | <0.001 | 503 (74.2) | 246 (72.6) | 0.580 |
| DPP-4 inhibitor | 123 (4.1) | 18 (5.2) | 0.312 | 31 (4.6) | 17 (5.0) | 0.754 |
| Thiazolidinediones | 107 (3.5) | 15 (4.4) | 0.446 | 33 (4.9) | 15 (4.4) | 0.754 |
| Insulin | 287 (9.5) | 38 (11.0) | 0.364 | 73 (10.8) | 37 (10.9) | 0.943 |
| Autoimmune drugs | ||||||
| Hydroxychloroquine | 997 (33.1) | 121 (35.2) | 0.432 | 246 (36.3) | 118 (34.8) | 0.644 |
| Steroid | 1,887 (62.6) | 241 (70.1) | 0.006 | 485 (71.5) | 236 (69.6) | 0.526 |
| Cyclosporine | 102 (3.4) | 10 (2.9) | 0.641 | 18 (2.7) | 10 (2.9) | 0.786 |
| Cyclophosphamide | 66 (2.2) | 9 (2.6) | 0.611 | 21 (3.1) | 9 (2.7) | 0.694 |
| Azathioprine | 211 (7.0) | 26 (7.6) | 0.701 | 56 (8.3) | 26 (7.7) | 0.745 |
| Methotrexate | 655 (21.7) | 73 (21.2) | 0.830 | 156 (23.0) | 73 (21.5) | 0.596 |
| Sulfasalazine | 628 (20.8) | 76 (22.1) | 0.585 | 155 (22.9) | 75 (22.1) | 0.791 |
| Other drugs | ||||||
| Antiplatelet (Aspirin or Clopidogrel) | 611 (20.3) | 71 (20.6) | 0.870 | 141 (20.8) | 70 (20.6) | 0.956 |
| Statin | 761 (25.2) | 69 (20.1) | 0.035 | 136 (20.1) | 69 (20.4) | 0.912 |
| NSAID | 2,390 (79.3) | 269 (78.2) | 0.643 | 545 (80.4) | 266 (78.5) | 0.473 |
| Cox-2 | 1,293 (42.9) | 151 (43.9) | 0.720 | 309 (45.6) | 149 (44.0) | 0.624 |
DKA, diabetic ketoacidosis; HHS, hyperglycemic hyperosmolar state; DM, diabetes mellitus; DPP4, dipeptidyl peptidase 4; NSAID, non-steroidal anti-inflammatory drug.
After propensity score matching, the characteristics of the metformin and non-metformin groups were compared using the t-test for continuous variables and the chi-square test for categorical variables. Mortality was reported as the proportion of events and incidence density (number of events per 100 person-years). We compared the risk of mortality between the two groups with a Cox proportional hazard model and adopted a Poisson regression model to compare the annual number of admissions between the metformin and non-metformin groups. We compared each secondary outcome between the two groups using a sub-distribution hazard model that considered death a competing risk (14). We considered a p-value < 0.05 to be statistically significant. Data analysis was conducted using Stata 13 software (StataCorp LLC, College Station, TX, US) and SAS software version 9.4 (SAS Institute, Cary, NC).
Results
Eligible Patients
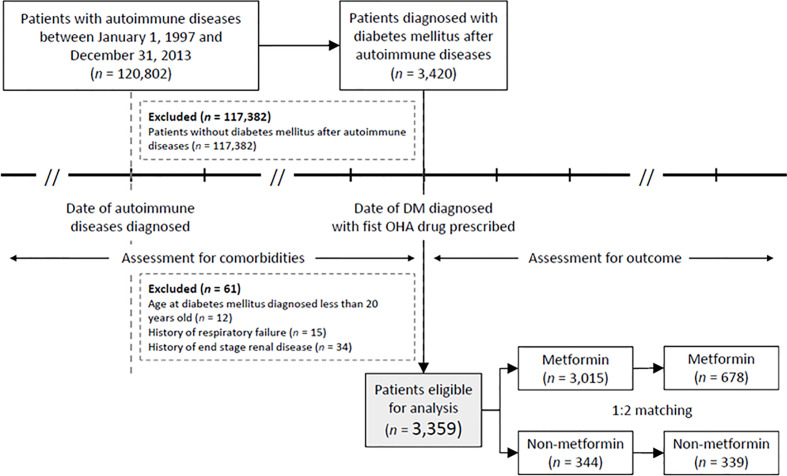
We found a total of 120,802 patients with AD between January 1, 1997 and December 31, 2013. Of those, 117,382 patients were excluded because they did not have a type 2 diabetes mellitus (T2DM) diagnosis after their AD diagnosis; only 3,420 AD patients were diagnosed with T2DM (2.83%). We further excluded patients under the age of 20 years old (n = 12), with a history of respiratory failure (n = 15), or with end-stage renal disease (ESRD, n = 34). Ultimately, 3,359 patients (2.78%) were eligible for analysis. After reviewing their medication history, we observed that 3,015 patients (89.75%) were found to be prescribed metformin, while only 344 patients (10.24%) were prescribed other anti-diabetic agents ( Figure 1 ).
Figure 1.
Study design and patient inclusion criteria.
Baseline Characteristics for Study Participants
Table 1 lists patient characteristics before and after propensity score matching. Prior to matching, the metformin group had 3,015 patients, and the non-metformin group had 344 patients. Patients in the metformin group had a younger age (59.8 ± 11.6 vs. 63.7 ± 12.2, P < 0.001) and a similar prevalence rate of underlying autoimmune disease (P = 0.244). However, the AD disease activity differed, with patients in the metformin group having fewer episodes of plasmapheresis or plasma exchange and an increased interval between the autoimmune disease and the T2DM diagnosis. Furthermore, patients in the metformin group had a lower prevalence of comorbidities (e.g., hypertension, chronic kidney disease, and stroke), were less likely to be prescribed such medications as glinides, alpha glucosidase inhibitors or sulfonylurea, and steroids, and were more likely to be prescribed a statin (P < 0.05).
After propensity score matching, all the parameters shown in Table 1 were balanced between the metformin and non-metformin groups (all P > 0.2). In the end, 678 and 339 patients remained in the metformin group and non-metformin group, respectively, for further analysis.
Primary Outcomes
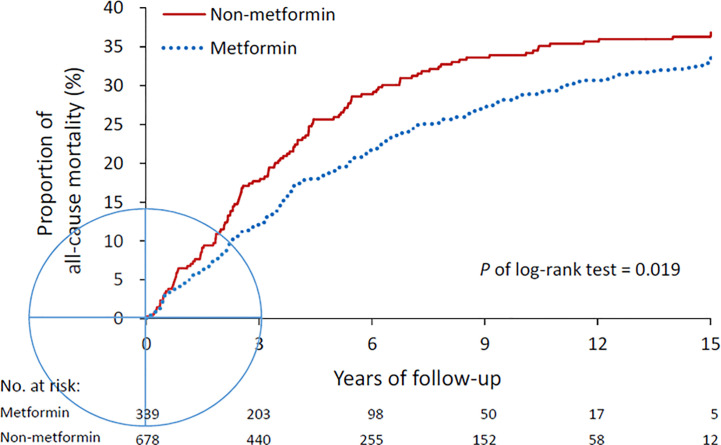
With a mean of 5.2 years (SD = 3.8 years) of follow-up duration, the event rate of all-cause mortality was 228 (33.6%) in the metformin group and 125 (36.9%) in the non-metformin group. The risk of all-cause mortality was significantly lower in the metformin group (Hazard ratio [CI], 0.77; 95% confidence interval [CI], 0.62–0.96). The cumulative incidence of mortality during the follow-up period was also significantly lower in metformin users compared to non-metformin users (P of log-rank test = 0.019, Figure 2 ). The annual number of admissions for AD was significantly lower in the metformin group (0.35 ± 0.81) than the non-metformin group (0.43 ± 1.08) with a rate ratio of 0.81 (95% CI, 0.73–0.90) ( Table 2 ).
Figure 2.
All-cause mortality in the metformin and non-metformin groups. Mortality was compared using log-rank test.
Table 2.
Event numbers and incidence density of the primary outcomes between the study cohorts.
| Variable | Metformin(n = 678) | Non-metformin(n = 339) | P-value |
|---|---|---|---|
| All-cause mortality | |||
| Follow-up (years), mean ± SD | 5.5 ± 4.1 | 4.7 ± 3.7 | |
| Event number, n (%) | 228 (33.6) | 125 (36.9) | |
| Incidence density (95% CI)§ | 6.15 (5.35–6.95) | 7.79 (6.42–9.16) | |
| Hazard ratio (95% CI) | 0.77 (0.62, 0.96) | Reference | 0.020 |
| Annual number of admissions for autoimmune disease | |||
| Mean ± SD | 0.35 ± 0.81 | 0.43 ± 1.08 | |
| Rate ratio (95% CI) | 0.81 (0.73, 0.90) | Reference | <0.001 |
SD, standard deviation; CI, confidence interval.
§Incidence density (ID), event numbers per 100 person-years.
Secondary Outcomes
We observed that metformin had a neutral effect on secondary outcomes, except for chronic respiratory failure and long-term dialysis ( Table 3 ). The risk of chronic respiratory failure was significantly reduced in the metformin group (HR, 0.56; 95% CI, 0.31–0.99), as was the risk of long-term dialysis (HR, 0.30; 95% CI, 0.14–0.65) ( Table 3 ).
Table 3.
Secondary outcome between metformin and non-metformin groups.
| Outcome | Metformin (n = 678) | Non-metformin (n = 339) | Metformin vs. Non-metformin§ | |
|---|---|---|---|---|
| HR (95% CI) | P-value | |||
| Organ damage | ||||
| Vascular outcomes# | 106 (15.6) | 41 (12.1) | 1.26 (0.88, 1.80) | 0.216 |
| Respiratory failure | 25 (3.7) | 21 (6.2) | 0.56 (0.31, 0.99) | 0.048* |
| ESRD on dialysis | 11 (1.6) | 16 (4.7) | 0.30 (0.14, 0.65) | 0.002* |
| Acute hepatitis | 6 (0.9) | 1 (0.3) | 2.91 (0.35, 24.11) | 0.323 |
| Infectious diseases | ||||
| Sepsis | 78 (11.5) | 42 (12.4) | 0.86 (0.60, 1.26) | 0.442 |
| Virus infection¶ | 23 (3.4) | 13 (3.8) | 0.86 (0.44, 1.69) | 0.661 |
| Diabetes-related outcome | ||||
| DKA or HHS | 28 (4.1) | 6 (1.8) | 2.28 (0.95, 5.51) | 0.066 |
| Newly-onset malignancy | 52 (7.7) | 30 (8.8) | 0.81 (0.52, 1.26) | 0.350 |
HR, hazard ratio; CI, confidence interval; DKA, diabetic ketoacidosis; HHS, hyperglycemic hyperosmolar state; ESRD, end-stage renal disease.
§estimated using Fine and Gray (1999) sub-distribution hazard model that considered all-cause mortality as a competing risk.
#Vascular outcomes include stroke or acute coronary syndrome.
¶Virus infection includes viral meningitis, gastroenteritis, flu and herpes infections.
*indicates p < 0.05.
Subgroup Analysis
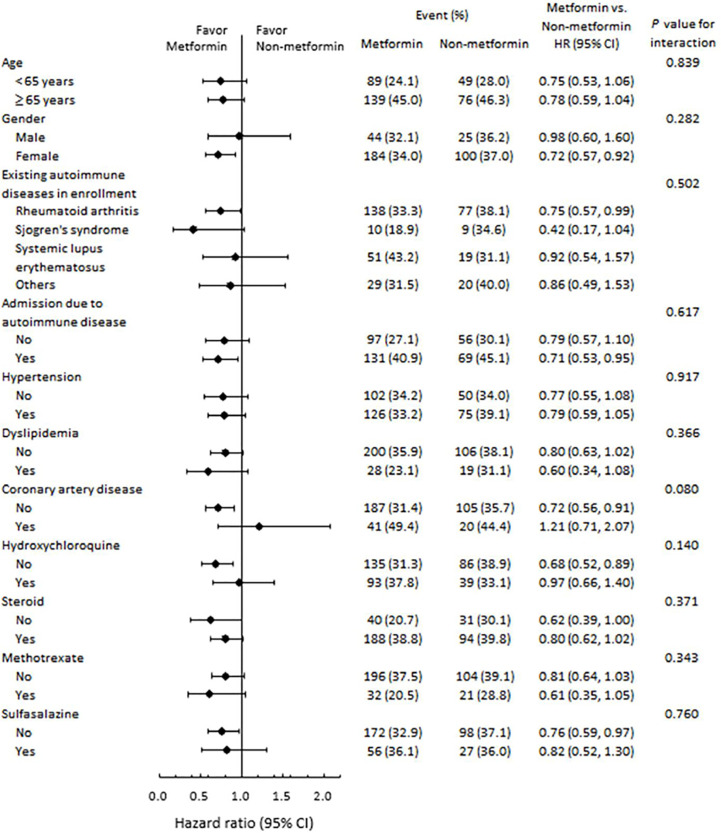
Figure 3 presents the subgroup analysis for all-cause mortality stratified by age, gender, immune diseases, and various medication. The beneficial effects of metformin on mortality were similar across all strata (P for interaction > 0.05).
Figure 3.
Subgroup analysis of all-cause mortality. The beneficial effects of metformin on risk of mortality were similar across various strata.
Discussion
This large AD cohort study demonstrates that patients prescribed metformin have been associated with a significantly decreased rate of all-cause mortality and annual admissions compared to those who do not take metformin but still have a similar incidence rate of sepsis death, vascular event, viral infection, diabetic ketoacidosis, acute hepatitis, or new-onset malignancy. Whether exposure to metformin has a deteriorating or neutral effect on kidney function is still a question of debate (15), but in this study, we prove that metformin may have benefits related to reducing end-stage renal disease and respiratory failure in diabetic AD patients. While some studies have been performed on metformin and reducing mortality in cardiovascular events, hepatoma, heart failure, and atherothrombosis (16), no specific research dealing with the metformin effect on AD has yet been published.
In the current study, the clinical vascular outcome comparison between metformin users and non-metformin users seems to be neutral, which is not in line with previous studies on the protective effect of metformin with regard to the mortality of cardiovascular events, heart failure, and atherothrombosis (16). This discrepancy may be attributed to the underlying more pronounced inflammation of AD patients, which may offset the potential beneficial effect of metformin in this cohort (17, 18). Therefore, the detailed mechanism of metformin regarding cardiovascular events in AD patients warrants additional study.
Chronic renal disease patients account for 12% to 14% of all AD patients, including those with lupus glomerulonephritis (19, 20), vasculitis (21), thromboembolism (22), scleroderma renal crisis (23), interstitial nephritis (24), and drug toxicity (25). Most AD-related chronic kidney diseases are associated with inflammation and oxidative stress (26). Previous studies have shown that diabetic nephropathy, with an incidence rate of around 4%~6% in Asian populations, may be related to oxidative stress (27). Our study results demonstrated that the risk of ESRD is significantly lower in the metformin group. This observation may be explained by the anti-inflammatory effects of metformin.
Likewise, respiratory failure can be lethal in AD (28). The relationship between respiratory failure and T2DM has been discussed before (29, 30). Previous studies have revealed that patients with cystic fibrosis and T2DM had worse outcomes compared to those without T2DM (31), in which oxidative stress was a potential deteriorating marker (32, 33). Our large cohort study indicated that metformin may significantly reduce the respiratory failure rate in AD patients with T2DM, which further shows its extra benefit in immune modulation.
Our study has some limitations that should be mentioned at this point. First, the exact percentage of AD in our cohort was unknown since we could only establish target AD patients through a mixture of different ICD-9-CM codes representing different AD, which could potentially result in selection bias. For example, antiphospholipid syndrome is an autoimmune disorder that may manifest vascular events and often appears in AD patients, but it has no specific ICD-9-CM code. To overcome that problem, we collected some of the medications that may be used to treat antiphospholipid syndrome, such as aspirin and warfarin, and matched them when possible. Second, the enrolled patients were mostly Taiwanese, so the external validity of these results may be questionable. Additional studies for other ethnic groups are required to verify our results. However, our large-scale study may provide an insight for long-term treatment of T2DM in patients with AD.
Conclusion
In actual usage, metformin seems to have benefits beyond T2DM control with regard to reducing real-world all-cause mortality and admission rates, as well as minimizing end-organ injury in lungs and kidneys among autoimmune disease patients.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by National Cheng Kung University Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
All authors contributed to the acquisition of research data. C-YL and Y-JS conducted the literature review, data analysis, and drafted the manuscript. C-YL and Y-JS are the guarantors of this work, have full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by research grants from the National Cheng Kung University Hospital (NCKUH-10903023 and NCKUH-10801002), the Ministry of Science and Technology (MOST 108-2314-B-006-007-MY2), and Chang Gung Memorial Hospital [grant number CORPG8K0131, NMRPG8G6183].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.641635/full#supplementary-material
References
- 1. Wang H, Li T, Chen S, Gu Y, Ye S. Neutrophil Extracellular Trap Mitochondrial DNA and Its Autoantibody in Systemic Lupus Erythematosus and a Proof-of-Concept Trial of Metformin. Arthritis Rheumatol (2015) 67(12):3190–200. 10.1002/art.39296 [DOI] [PubMed] [Google Scholar]
- 2. Ahlqvist E, Storm P, Karajamaki A, Martinell M, Dorkhan M, Carlsson A, et al. Novel Subgroups of Adult-Onset Diabetes and Their Association With Outcomes: A Data-Driven Cluster Analysis of Six Variables. Lancet Diabetes Endocrinol (2018) 6(5):361–9. 10.1016/S2213-8587(18)30051-2 [DOI] [PubMed] [Google Scholar]
- 3. Su YJ, Lin HY, Weng SW, Lu CH, Lin CY, Chiu WC, et al. Metformin Represses Interferonopathy Through Suppression of Melanoma Differentiation-Associated Protein 5 and Mitochondrial Antiviral Signaling Protein Activation: Comment on the Article by Wang Et al. Arthritis Rheumatol (2016) 68(12):3042–3. 10.1002/art.39935 [DOI] [PubMed] [Google Scholar]
- 4. Johnson JA, Simpson SH, Toth EL, Majumdar SR. Reduced Cardiovascular Morbidity and Mortality Associated With Metformin Use in Subjects With Type 2 Diabetes. Diabetes Med (2005) 22(4):497–502. 10.1111/j.1464-5491.2005.01448.x [DOI] [PubMed] [Google Scholar]
- 5. Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin Associated With Lower Cancer Mortality in Type 2 Diabetes: ZODIAC-16. Diabetes Care (2010) 33(2):322–6. 10.2337/dc09-1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pantalone KM, Kattan MW, Yu C, Wells BJ, Arrigain S, Jain A, et al. Increase in Overall Mortality Risk in Patients With Type 2 Diabetes Receiving Glipizide, Glyburide or Glimepiride Monotherapy Versus Metformin: A Retrospective Analysis. Diabetes Obes Metab (2012) 14(9):803–9. 10.1111/j.1463-1326.2012.01604.x [DOI] [PubMed] [Google Scholar]
- 7. Christiansen C, Johansen M, Christensen S, O’Brien JM, Tonnesen E, Sorensen H. Preadmission Metformin Use and Mortality Among Intensive Care Patients With Diabetes: A Cohort Study. Crit Care (2013) 17(5):R192. 10.1186/cc12886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun Y, Tian T, Gao J, Liu X, Hou H, Cao R, et al. Metformin Ameliorates the Development of Experimental Autoimmune Encephalomyelitis by Regulating T Helper 17 and Regulatory T Cells in Mice. J Neuroimmunol (2016) 292:58–67. 10.1016/j.jneuroim.2016.01.014 [DOI] [PubMed] [Google Scholar]
- 9. Qaseem A, Barry MJ, Humphrey LL, Forciea MA. Clinical Guidelines Committee of the American College of P. Oral Pharmacologic Treatment of Type 2 Diabetes Mellitus: A Clinical Practice Guideline Update From the American College of Physicians. Ann Intern Med (2017) 166(4):279–90. 10.7326/M16-1860 [DOI] [PubMed] [Google Scholar]
- 10. Lin CC, Lai MS, Syu CY, Chang SC, Tseng FY. Accuracy of Diabetes Diagnosis in Health Insurance Claims Data in Taiwan. J Formos Med Assoc (2005) 104(3):157–63. [PubMed] [Google Scholar]
- 11. Sung SF, Hsieh CY, Lin HJ, Chen YW, Chen CH, Kao Yang YH, et al. Validity of a Stroke Severity Index for Administrative Claims Data Research: A Retrospective Cohort Study. BMC Health Serv Res (2016) 16(1):509. 10.1186/s12913-016-1769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu CS, Lai MS, Gau SS, Wang SC, Tsai HJ. Concordance Between Patient Self-Reports and Claims Data on Clinical Diagnoses, Medication Use, and Health System Utilization in Taiwan. PloS One (2014) 9(12):e112257. 10.1371/journal.pone.0112257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Austin PC. Statistical Criteria for Selecting the Optimal Number of Untreated Subjects Matched to Each Treated Subject When Using Many-to-One Matching on the Propensity Score. Am J Epidemiol (2010) 172(9):1092–7. 10.1093/aje/kwq224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jaeger C, Hatziagelaki E, Stroedter A, Becker F, Scherer S, Petzoldt R, et al. The Giessen-Bad Oeynhausen Family Study: Improved Prediction of Type I Diabetes in a Low Incidence Population of Relatives Using Combinations of Islet Autoantibodies in a Dual Step Model. Exp Clin Endocrinol Diabetes (1999) 107(8):496–505. 10.1055/s-0029-1232558 [DOI] [PubMed] [Google Scholar]
- 15. Hussain MI, Hall BM, Depczynski B, Connor SJ. Acute Renal Failure and Metformin-Associated Lactic Acidosis Following Colonoscopy. Diabetes Res Clin Pract (2014) 105(1):e6–8. 10.1016/j.diabres.2013.12.055 [DOI] [PubMed] [Google Scholar]
- 16. Roussel R, Travert F, Pasquet B, Wilson PW, Smith SC, Jr., Goto S, et al. Metformin Use and Mortality Among Patients With Diabetes and Atherothrombosis. Arch Intern Med (2010) 170(21):1892–9. 10.1001/archinternmed.2010.409 [DOI] [PubMed] [Google Scholar]
- 17. Bessant R, Duncan R, Ambler G, Swanton J, Isenberg DA, Gordon C, et al. Prevalence of Conventional and Lupus-Specific Risk Factors for Cardiovascular Disease in Patients With Systemic Lupus Erythematosus: A Case-Control Study. Arthritis Rheum (2006) 55(6):892–9. 10.1002/art.22343 [DOI] [PubMed] [Google Scholar]
- 18. Yang L, Tao J, Tang X, Wang Y, He X, Xu G, et al. Prevalence and Correlation of Conventional and Lupus-Specific Risk Factors for Cardiovascular Disease in Chinese Systemic Lupus Erythematosus Patients. J Eur Acad Dermatol Venereol (2012) 26(1):95–101. 10.1111/j.1468-3083.2011.04211.x [DOI] [PubMed] [Google Scholar]
- 19. Yu F, Haas M, Glassock R, Zhao MH. Redefining Lupus Nephritis: Clinical Implications of Pathophysiologic Subtypes. Nat Rev Nephrol (2017) 13(8):483–95. 10.1038/nrneph.2017.85 [DOI] [PubMed] [Google Scholar]
- 20. Yung S, Yap DY, Chan TM. Recent Advances in the Understanding of Renal Inflammation and Fibrosis in Lupus Nephritis. F1000Res (2017) 6:874. 10.12688/f1000research.10445.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang WJ, Wu HS, Chu TS. Anti-Neutrophil Cytoplasmic Antibody-Associated Pauci-immune Crescentic Glomerulonephritis Complicating Sjogren’s Syndrome. J Formos Med Assoc (2011) 110(7):473–7. 10.1016/S0929-6646(11)60070-3 [DOI] [PubMed] [Google Scholar]
- 22. Gigante A, Gasperini ML, Cianci R, Barbano B, Giannakakis K, Di Donato D, et al. Antiphospholipid Antibodies and Renal Involvement. Am J Nephrol (2009) 30(5):405–12. 10.1159/000235941 [DOI] [PubMed] [Google Scholar]
- 23. Al Aranji G, White D, Solanki K. Scleroderma Renal Crisis Following Silicone Breast Implant Rupture: A Case Report and Review of the Literature. Clin Exp Rheumatol (2014) 32(2):262–6. [PubMed] [Google Scholar]
- 24. Yung S, Chan TM. Molecular and Immunological Basis of Tubulo-Interstitial Injury in Lupus Nephritis: A Comprehensive Review. Clin Rev Allergy Immunol (2017) 52(2):149–63. 10.1007/s12016-016-8533-z [DOI] [PubMed] [Google Scholar]
- 25. Launay-Vacher V, Izzedine H, Deray G. Statins’ Dosage in Patients With Renal Failure and Cyclosporine Drug-Drug Interactions in Transplant Recipient Patients. Int J Cardiol (2005) 101(1):9–17. 10.1016/j.ijcard.2004.04.005 [DOI] [PubMed] [Google Scholar]
- 26. Rodriguez-Iturbe B, Franco M, Tapia E, Quiroz Y, Johnson RJ. Renal Inflammation, Autoimmunity and Salt-Sensitive Hypertension. Clin Exp Pharmacol Physiol (2012) 39(1):96–103. 10.1111/j.1440-1681.2011.05482.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Atkins RC, Briganti EM, Lewis JB, Hunsicker LG, Braden G, Champion de Crespigny PJ, et al. Proteinuria Reduction and Progression to Renal Failure in Patients With Type 2 Diabetes Mellitus and Overt Nephropathy. Am J Kidney Dis (2005) 45(2):281–7. 10.1053/j.ajkd.2004.10.019 [DOI] [PubMed] [Google Scholar]
- 28. Holdsworth S, Boyce N, Thomson NM, Atkins RC. The Clinical Spectrum of Acute Glomerulonephritis and Lung Haemorrhage (Goodpasture’s Syndrome). Q J Med (1985) 55(216):75–86. [PubMed] [Google Scholar]
- 29. Block MB. Respiratory Failure and Diabetic Control. Ann Intern Med (1982) 96(4):538. 10.7326/0003-4819-96-4-538_1 [DOI] [PubMed] [Google Scholar]
- 30. Umeki S. Glucose Intolerance in Chronic Respiratory Failure. Angiology (1994) 45(11):937–42. 10.1177/000331979404501105 [DOI] [PubMed] [Google Scholar]
- 31. Stecenko AA, Moran A. Update on Cystic Fibrosis-Related Diabetes. Curr Opin Pulm Med (2010) 16(6):611–5. 10.1097/MCP.0b013e32833e8700 [DOI] [PubMed] [Google Scholar]
- 32. Dickerhof N, Pearson JF, Hoskin TS, Berry LJ, Turner R, Sly PD, et al. Oxidative Stress in Early Cystic Fibrosis Lung Disease is Exacerbated by Airway Glutathione Deficiency. Free Radic Biol Med (2017) 113:236–43. 10.1016/j.freeradbiomed.2017.09.028 [DOI] [PubMed] [Google Scholar]
- 33. Skov M, Pressler T, Lykkesfeldt J, Poulsen HE, Jensen PO, Johansen HK, et al. The Effect of Short-Term, High-Dose Oral N-acetylcysteine Treatment on Oxidative Stress Markers in Cystic Fibrosis Patients With Chronic P. Aeruginosa Infection – a Pilot Study. J Cyst Fibros (2015) 14(2):211–8. 10.1016/j.jcf.2014.09.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.