Abstract
Aims:
This study was aimed at investigating the prognostic impact of pretreatment thrombocytosis in epithelial ovarian cancer (EOC) patients in Lagos, Nigeria.
Methods:
This was a retrospective cohort study involving the review of the clinical record of 72 patients with histologically confirmed EOC who were managed at the Lagos University Teaching Hospital, Lagos, Nigeria over a 7-year period from January 2010 to December 2016. Information on the sociodemographic data and platelet counts at diagnosis of EOC were retrieved from the patients’ medical records. Descriptive statistics were then computed for all baseline patients’ characteristics. Survival analyses were carried out using the Kaplan-Meier estimates. Multivariate analysis of these data was performed with the Cox proportional hazards model.
Results:
This study revealed that the prevalence of pretreatment thrombocytosis was 41.7% among the women with EOC. Fifty-three (73.6%) of the women had the advanced-stage disease (FIGO stage III-IV) while 52 (72.2%) had high-grade disease (II-III). The majority (66.7%) of the women had a serous histological type of EOC while 76.4% had documented recurrence. Pretreatment thrombocytosis was significantly associated with the women’s parity (P = 0.009), serum carbohydrate antigen 125 levels (P = 0.018), median progression-free survival (PFS) (P < 0.001), 3-year median overall survival (OS) (P < 0.001), type of primary treatment (P = 0.002), extent of cytoreduction (P < 0.001), presence of ascites (P = 0.002), International Federation of Gynecology and Obstetrics (FIGO) stage (P = 0.008), and histological type (P = 0.011). Pretreatment thrombocytosis was negatively associated with PFS (hazard ratio [HR] = 0.25; 95% CI 0.83, 0.75; P = 0.014) and 3-year OS (HR = 0.03; 95% CI 0.03, 0.27; P = 0.002).
Conclusions:
The study suggests that pretreatment thrombocytosis may be a useful predictor of survivals in EOC patients.
Keywords: Nigeria, overall survival, platelets, progression-free survival, thrombocytosis
Introduction
Ovarian cancer is the sixth most common cancer among women worldwide.[1] There are almost 300,000 new cases of ovarian cancer diagnosed with approximately 180,000 deaths per year.[1] Several studies from Africa and Nigeria have shown that ovarian cancer is the second most common gynecological cancer[2–5] constituting about 7–8.2% of all gynecological malignancies[6,7] and accounting for the second commonest cause of deaths among women admitted on the gynecological ward of a teaching hospital in Lagos.[6] It is the leading cause of deaths in women with gynecological malignancies worldwide.[8] In Nigeria, over 70% of ovarian cancer patients were diagnosed at an advanced stage.[7,9,10]
Lack of early predictive biomarkers is responsible for the high mortality rate. Until now, the most widely used biomarker for monitoring of ovarian cancer is carbohydrate antigen 125 (CA-125)[11] with approximately 83% of the patients at advanced stage having CA-125 levels >35 U/mL.[12] However, CA-125 is also elevated in a small proportion of people with endometriosis, pelvic inflammatory disease, pregnancy, hepatic cirrhosis, acute heart failure, tuberculosis, pancreatic cancer, lung cancer, liver cancer, and so on.[13,14] Therefore, it has a relatively low positive predictive value and is usually not considered as an independent predictor.[15] Thus, the effort to find other reliable and cheaper biomarkers that could be used alone (in resource-limited settings) or in combination with CA-125 for the effective evaluation and prognostic prediction of ovarian cancer is long-expected. The eminent role of platelet, a ubiquitously available parameter in regions with limited economic resources, has previously been discussed in the diagnosis of pelvic mass[16] and, therefore, its impact on prognosis also needs to be examined.
Platelets are highly reactive cellular orchestrators of primary hemostasis, immunity, and inflammation[17] and it is widely believed that platelets may play important roles in cancer growth and metastasis. The finding of thrombocytosis in patients with solid tumors was first made over a century ago.[18] Incidentally, almost 40% of persons found to have thrombocytosis in the absence of iron deficiency and benign inflammatory conditions have occult malignant neoplasia including ovarian cancer.[19] Experimental evidence had suggested that platelets actively promote cancer progression through different mechanisms and these may include shielding of cancer cells from immune attacks and stimulation of angiogenesis.[20] A recent systematic review and meta-analysis conducted by Ye et al. reported that pretreatment thrombocytosis was a significant negative predictor of both survival in women with epithelial ovarian cancer (EOC).[21] However, it is still not clearly understood whether prognosis in ovarian cancer patients is directly affected by the presence of a high level of platelets or if thrombocytosis is a surrogate for other prognostic indicators, such as advanced-stage disease, suboptimal cytoreduction, or even medical comorbidity. On this basis, the current study, therefore, investigated the impact of pretreatment thrombocytosis on progression-free survival (PFS) and overall survival (OS) in black Nigerian women with EOC.
Subjects and Methods
Study design and setting
This was a retrospective cohort study involving the review of case records of all histologically confirmed EOC patients managed at the Lagos University Teaching Hospital (LUTH), Lagos, Nigeria over a 7-year period from January 2010 to December 2016. LUTH is the largest tertiary institution that provides services to patients in Lagos and from the neighboring Southwestern states. The hospital is located in the central Lagos metropolis and offers mainly clinical services among which include gynecological oncology services.
Eligibility criteria
Eligible participants were women with histologically confirmed EOC. Further eligibility criteria included those who commenced treatment within 6 weeks of diagnosis. Patients with non-EOC; those who failed to complete treatment or yet to complete the treatment at least in the last 3 years of the review period were excluded from the study.
Data collection
The registration numbers of all women with EOC who were managed during the period under review were obtained from the gynecological oncology ward registers and the patients’ case notes were subsequently retrieved from the medical records department. Relevant information such as sociodemographic characteristics, menopausal status, body mass index (BMI), presence of major comorbidity (hypertension/diabetes mellitus/kidney disease), pretreatment platelets counts and serum CA-125 levels, type of primary treatment (primary debulking or neoadjuvant chemotherapy), extent of cytoreduction (optimal or suboptimal), presence of ascites (≥500 mL), FIGO stage of disease, histological subtype of tumor, and tumor grade were extracted using a standardized pro forma.
Exposure and confirmatory outcome variables
Platelet counts at diagnosis of EOC were evaluated in all patients treated in the hospital and for whom there were complete follow-up data on PFS and 3-year OS. PFS was defined as those women without any clinical, biochemical, or radiological evidence of the disease within the first 36 months of completion of treatment. Evidence of the disease was defined clinically as presence of a pelvic tumor on bimanual pelvic examination; and/or biochemically as abnormal (>35 IU/mL) or increasing serum CA-125 level; and/or radiologically as the presence of a new pelvic tumor on abdominopelvic USS or CT scan. The 3-year OS was defined as those women who were still 36-month alive after completion of treatment. The endpoints for statistical analysis were 3-year OS and PFS while the exposure variable is elevated pretreatment platelet counts (thrombocytosis).
Statistical analysis
All relevant data were analyzed using SPSS version 23.0 statistical package for Windows manufactured by IBM Corp., Armonk, NY, United States. Descriptive statistics were then computed for all baseline patients’ characteristics. Characteristics of patients were described by mean and SD (if normally distributed) or median and percentiles (if not-normally distributed) for continuous variables and by frequencies and percentages for categorical variables. PFS and OS of patients with normal platelet counts (150–450 × 109/L) were compared with that of patients with thrombocytosis (>450 × 109/L) using the Mann-Whitney U test. Survival analyses were carried out using the Kaplan-Meier estimates and statistical significance was determined by the log-rank test. Patients who were alive at last follow-up or those without recurrence were censored. Multivariate analysis of these data was performed with the Cox proportional hazards model. All testing was two-sided, and a P value of less than 0.05 was considered to indicate statistical significance.
Ethical approval
Ethical approval for the study was obtained from the Health Research and Ethics committee of the LUTH (Approval number—ADM/DCST/HREC/1912) prior to the review of case records and data collection. Ethical principles according to the Helsinki declaration were considered during the course of the study.
Results
On review of the case records of the 81 EOC cases managed in the hospital during the study period, only 72 cases had their complete clinical data available or eligible for final analyses. Excluded from the final analyses were two women who did not have documented pretreatment platelet counts, four women who failed to complete their primary treatment, and three women who were lost to follow-ups. The baseline characteristics of the study cohorts are shown in Table 1. The majority of the patients were postmenopausal (54.2%) and more than 50 years of age (66.7%).
Table 1:
Baseline characteristics of study cohorts (n=72)
| Variable | Frequency | Percentage |
|---|---|---|
| Age, in years | ||
| <50 | 24 | 33.3 |
| ≥50 | 48 | 66.7 |
| Mean age±SD=54.6±10.7 years | ||
| Menopausal status | ||
| Premenopausal | 33 | 45.8 |
| Postmenopausal | 39 | 54.2 |
| Parity | ||
| Low parity (≤1) | 23 | 31.9 |
| High parity (≥2) | 49 | 68.1 |
| Median parity (interquartile range [IQR]) = 2.0 (1.0, 4.0) | ||
| Body mass index (BMI), in kg/m2 | ||
| ≤25.0 | 37 | 51.4 |
| >25.0 | 35 | 48.6 |
| Median BMI (IQR) = 24.7 (22.1, 28.9) kg/m2 | ||
| Co-morbidity | ||
| No | 55 | 76.4 |
| Yes | 17 | 23.6 |
| Pretreatment CA-125 levels, in U/mL | ||
| <250 | 25 | 34.7 |
| ≥250 | 47 | 65.3 |
| Median CA125 (IQR) = 437.0 (144.0, 924.5) U/mL | ||
| Type of cytoreductive surgery | ||
| Optimal | 37 | 51.4 |
| Suboptimal | 35 | 48.6 |
| Presence of significant ascites | ||
| No | 37 | 51.4 |
| Yes | 35 | 48.6 |
| FIGO stage | ||
| Early (I-II) | 19 | 26.4 |
| Advanced (III-IV) | 53 | 73.6 |
| Tumour grade | ||
| Low grade (grade I) | 20 | 27.8 |
| High grade (grade II-III) | 52 | 72.2 |
| Histological type | ||
| Serous | 48 | 66.7 |
| Non-serous | 24 | 33.3 |
| Type of primary treatment | ||
| Primary debulking | 39 | 54.2 |
| Neoadjuvant chemotherapy | 33 | 45.8 |
| Thrombocytosis | ||
| No | 42 | 58.3 |
| Yes | 30 | 41.7 |
| Median platelet counts (IQR) = 428 (301, 503) × 109/L | ||
| Recurrence within 36 months | ||
| Yes | 55 | 76.4 |
| No | 17 | 23.6 |
| Median PFS (IQR) = 15.0 (8.0, 29.5) months | ||
| Vital status at 36-months | ||
| Dead | 27 | 37.5 |
| Alive | 45 | 62.5 |
Median OS (IQR) 36.0 (23.0, 36.0) months. SD: standard deviation; IQR: interquartile range; PFS: progression-free survival; OS: Overall survival; FIGO: International Federation of Obstetrics and Gynecology
The median (interquartile range [IQR]) platelet count was 428 (301–503) × 109/L and 30 (41.7%) women had pretreatment thrombocytosis. Fifty-three (73.6%) women had the advanced-stage disease (FIGO stage III-IV) while 52 (72.2%) had high-grade disease (II-III). The majority of the cases of EOC were of serous histological type (66.7%). Fifty-five (76.4%) of the women had documented recurrence and 27 (37.5%) deaths were documented. The median PFS and 3-year OS were 15.0 (IQR: 8.0–29.5) and 36.0 (IQR: 23.0–36.0) months, respectively.
Table 2 shows comparison of the clinicopathological characteristics of patients with and without pretreatment thrombocytosis. Pretreatment thrombocytosis was significantly associated with the patients’ parity (P = 0.009), serum CA125 levels (P = 0.018), median PFS (P < 0.001), 3-year median OS (P < 0.001), type of primary treatment (P = 0.002), extent of cytoreduction (P < 0.001), presence of ascites (P = 0.002), FIGO stage (P = 0.008), and histological type (P = 0.011). There were no statistically significant association with age (P = 0.782), BMI (P = 0.052), menopausal status (P = 0.341), comorbidity (P = 0.963), and disease grade (P = 0.075).
Table 2:
Clinicopathological characteristics of study cohorts and thrombocytosis (n=72)
| Characteristics | Platelet counts | P | |
|---|---|---|---|
| Normal counts (%) | Thrombocytosis (%) | ||
| Age in year, mean±SD | 54.3±10.1 | 55.0±11.6 | 0.782a |
| Parity, median (IQR) | 3.0 (1.0, 4.3) | 2.0 (1.0, 2.3) | 0.009b |
| BMI in kg/m2, median (IQR) | 23.8 (19.9, 28.9) | 27.3 (22.8, 29.7) | 0.052b |
| CA-125 in U/mL, median (IQR) | 268.0 (67.0, 541.5) | 451.0 (362.4, 1150.8) | 0.018b |
| Median PFS (IQR) months | 24.0 (13.0, 35.3) | 7.5 (6.0, 13.0) | <0.001*b |
| Median OS (IQR) months | 36.0 (36.0, 36.0) | 21.0 (17.0, 24.0) | <0.001*b |
| Menopausal status | 0.341 | ||
| Premenopausal | 17 (51.5) | 16 (48.5) | |
| Postmenopausal | 25 (64.1) | 14 (35.9) | |
| Comorbidity | 0.963 | ||
| No | 32 (58.2) | 23 (41.8) | |
| Yes | 10 (58.8) | 7 (41.2) | |
| Type of primary treatment | 0.002 | ||
| Primary debulking | 26 (78.8) | 7 (21.2) | |
| Neoadjuvant chemotherapy | 16 (41.0) | 23 (58.0) | |
| Extent of cytoreductive surgery | <0.001* | ||
| Optimal | 29 (78.4) | 8 (21.6) | |
| Suboptimal | 13 (37.1) | 22 (62.9) | |
| Ascites | 0.002 | ||
| No | 28 (75.7) | 9 (24.3) | |
| Yes | 14 (40.0) | 21 (60.0) | |
| FIGO stage | 0.008 | ||
| Early | 16 (84.2) | 3 (15.8) | |
| Advanced | 26 (49.1) | 27 (50.9) | |
| Disease grade | 0.075 | ||
| Low grade | 15 (75.0) | 5 (25.0) | |
| High grade | 27 (61.9) | 25 (48.1) | |
| Histological type | 0.011 | ||
| Serous | 23 (47.9) | 25 (52.1) | |
| Non-serous (n=10) | 19 (79.2) | 5 (20.8) | |
SD: standard deviation; IQR: interquartile range; PFS: progression-free survival; OS: overall survival; BMI: body mass index.
Independent sample T-test;
Mann-Whitney U test;
P: Significant values at 95% confidence interval
Figure 1 states the PFS while Figure 2 states the 3-year OS. Pretreatment thrombocytosis was associated with a shorter PFS (P < 0.001) and 3-year OS (P < 0.001). Multivariate analysis using Cox-regression statistics revealed that pretreatment thrombocytosis was an independent predictor of PFS (hazard ratio [HR] = 0.25; 95% CI 0.83, 0.75; P = 0.014) [Table 3] and 3-year OS (HR = 0.03; 95%CI 0.03, 0.27; P = 0.002) [Table 4].
Figure 1:
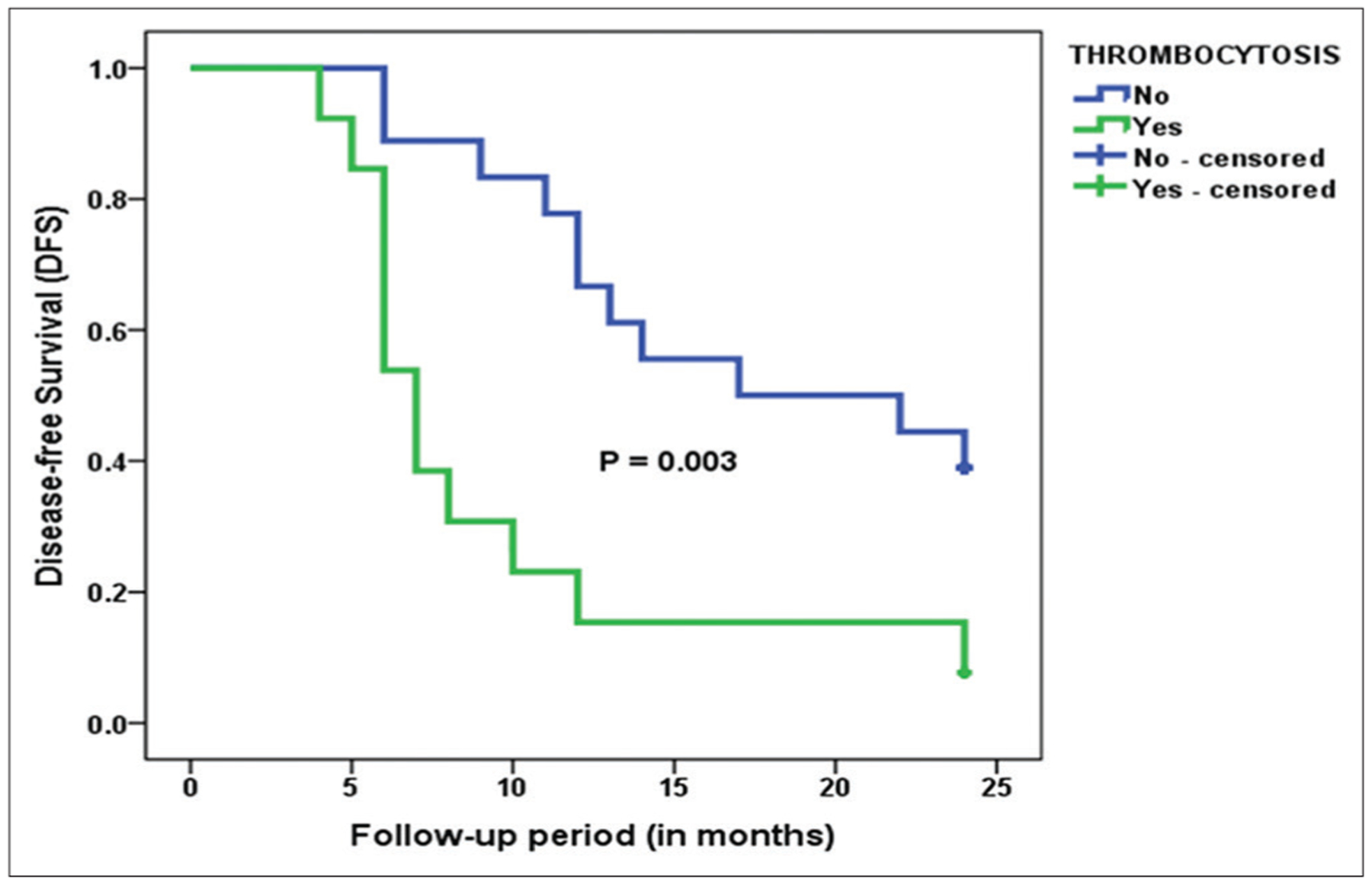
Kaplan-Meier curve of progression-free survival (PFS) stratified by thrombocytosis—Pretreatment thrombocytosis was associated with a shorter PFS (P = 0.003)
Figure 2:
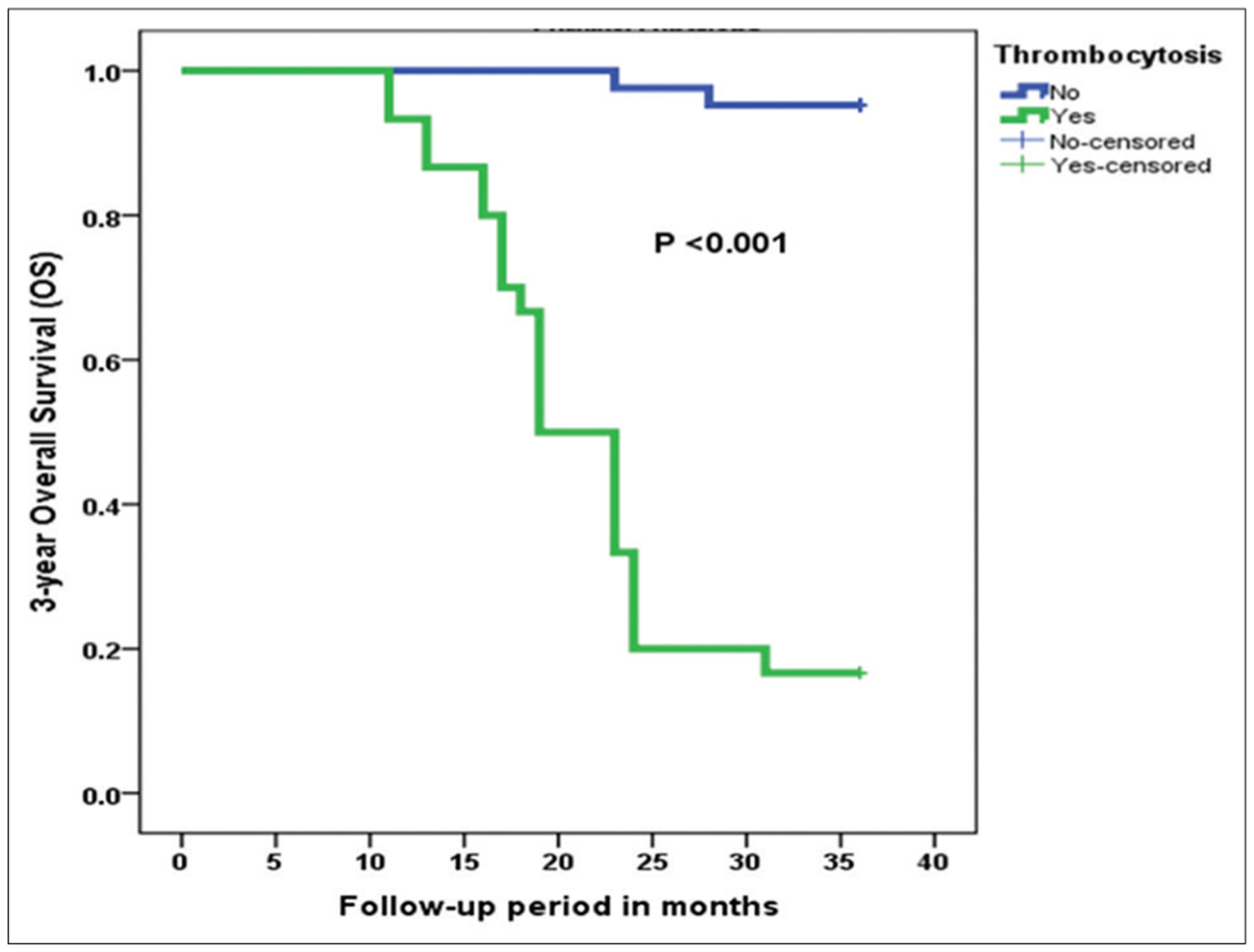
Kaplan-Meier curve of 3-year overall survival (OS) stratified by thrombocytosis—Pretreatment thrombocytosis was associated with a shorter OS (P < 0.001)
Table 3:
Cox Regression analysis of the clinicopathological factors associated with PFS
| Characteristics | PFS | P | |
|---|---|---|---|
| Hazard ratio (HR) | 95% CI | ||
| Age | 0.67 | 0.23–1.96 | 0.467 |
| Menopausal status | 3.05 | 0.89–10.52 | 0.077 |
| Parity | 1.13 | 0.56–2.28 | 0.738 |
| BMI | 0.69 | 0.30–1.58 | 0.374 |
| Serum CA-125 levels | 0.14 | 0.03–0.60 | 0.008 |
| Comorbidity | 3.50 | 0.83–14.87 | 0.089 |
| Cytoreduction | 1.35 | 0.39–4.71 | 0.634 |
| Primary treatment | 1.28 | 0.43–3.75 | 0.658 |
| FIGO stage | 152.19 | 16.55–1399.43 | <0.001* |
| Tumor grade | 3.94 | 0.45–34.50 | 0.216 |
| Histological type | 0.46 | 0.12–1.79 | 0.262 |
| Ascites | 0.99 | 0.40–2.41 | 0.974 |
| Thrombocytosis | 0.25 | 0.83–0.75 | 0.014 |
CI: confidence interval; BMI: body mass index; FIGO: International Federation of Obstetrics and Gynecology.
P: Significant values at 95% confidence interval
Table 4:
Cox Regression analyses of clinicopathological factors associated with 3-year OS
| Characteristics | OS | P | |
|---|---|---|---|
| Hazard Ratio (HR) | 95% CI | ||
| Age | 0.62 | 0.10–3.84 | 0.737 |
| Menopausal status | 2.34 | 0.32–17.10 | 0.404 |
| Parity | 0.83 | 0.29–2.41 | 0.737 |
| BMI | 1.45 | 0.59–3.56 | 0.419 |
| Serum CA125 levels | 0.50 | 0.04–5.84 | 0.579 |
| Comorbidity | 0.38 | 0.06–2.47 | 0.310 |
| Cytoreduction | 0.10 | 0.01–3.07 | 0.189 |
| Primary treatment | 0.89 | 0.16–4.81 | 0.891 |
| FIGO stage | 1.74 | 0.43–74.64 | 0.761 |
| Tumor grade | 3.17 | 0.043–235.40 | 0.599 |
| Histological type | 0.24 | 0.01–6.01 | 0.387 |
| Ascites | 1.27 | 0.31–5.26 | 0.737 |
| Thrombocytosis | 0.03 | 0.01–0.27 | 0.002 |
Abbreviations: CI, confidence interval; BMI, body mass index; FIGO, International Federation of Obstetrics and Gynecology.
P: values at 95% confidence interval
Discussion
This study is investigating the prognostic impact of pretreatment thrombocytosis in black Nigerian women with EOC. The majority of the patients were postmenopausal (54.2%) and this was higher than the proportion (40.0%) reported in a study conducted by Odukogbe et al. in Ibadan[9] and our previous study conducted in the same setting in Lagos.[7] This is, however, not unexpected as this current study only reported cases with histological diagnosis of EOC, which is commoner among older women, unlike our previous study that involved all cases of ovarian cancer in its final analysis.[7]
We reported that about 41.7% of our study cohorts in this study had pretreatment thrombocytosis and this is slightly higher than the 31.0% and 22.3% reported by Stone et al.[22] and Allensworth et al.,[23] respectively in the United States. This may be because of the racial differences in platelet counts as reported by Saxena et al.[24] in their study of platelet counts in three racial groups where they indicated that black women had significantly higher platelet counts than their Caucasian counterparts. However, a much lower proportion of thrombocytosis (13.8%) was reported by Feng et al.[25] in their study in China, and this apart from the reason adduced above may also be attributed to this study involving only women with high-grade serous ovarian cancer. The mechanisms of thrombocytosis in ovarian cancer and the role that this plays in abetting cancer growth are, however, still unclear.[22]
We demonstrated in this study that pretreatment thrombocytosis was associated with some clinicopathological characteristics and poorer survival in the Nigerian female population with EOC. We found that thrombocytosis was associated with the stage and histological type of the tumor, type of primary treatment, the extent of cytoreductive surgery and the presence of ascites at the surgery. These findings are consistent with several published studies involving patients with EOCs regardless of histological type[22,23,26–28] and this indicates that platelet counts tend to increase concurrently with tumor progression and metastasis. Stone et al.[22] reported that patients with thrombocytosis were significantly more likely to have advanced-stage disease and higher pretreatment levels of serum CA-125 than those with normal platelet counts. Ma et al.[26] also found that EOC patients with thrombocytosis have a greater likelihood of having suboptimal cytoreduction.
Previous studies outside the African continent have suggested that platelet count and fibrinogen levels can serve as potential prognostic indicators in ovarian cancer patients.[22,23,25–29] It was postulated that once the coagulation system is activated, it can directly or indirectly promote disease progression and metastasis with a resultant poorer prognostic outcome.[30,31] Consequently, we were able to confirm in the current study that pretreatment thrombocytosis is an independent negative predictor of PFS and OS in Nigerian women with EOC. These findings were also confirmed in a recent systematic review and meta-analysis of 11 pooled studies conducted by Ye et al.[21] reported that pretreatment thrombocytosis was a significant negative predictor of both PFS and OS in women with EOC for all stages and degrees of differentiation. This is at variance to the work of Allensworth et al.[23] reported that thrombocytosis is not independently correlated with survival even after stratifying according to the FIGO stage. Such stratification was, however, not carried out in our current study.
The major limitation of this study was its retrospective design that depended on effective documentation of patients’ history with the potential for missed data. The poor record-keeping in our center also resulted in the unacceptably high number of EOC cases with incomplete clinical data which limited the follow-up period in the study to 3-years instead of the standard 5-years for assessment of survivors and subsequently the small sample size used. Additionally, our center is a foremost tertiary referral hospital in Nigeria and thus the majority of the patients seen with ovarian cancer have advanced disease and this may not be a fair representation of the general population. However, this study is a pilot research effort among women with ovarian cancer in Nigeria and therefore the preliminary data generated will form the basis for a future robust longitudinal study.
Conclusions
This study revealed a high proportion of thrombocytosis among black Nigerian women with EOC. It further suggested that preoperative thrombocytosis reflects tumor burden and may also be a predictive determinant of survival in EOC patients. We, therefore, suggest that preoperative platelet levels may have value in counseling patients with ovarian cancer about their prognosis and the need for a more intense follow-up monitoring, prior to their primary treatment.
Acknowledgments
We thank the staff of the medical record department attached to the oncology and pathology studies (OPS) unit of LUTH for their assistance in ensuring the retrieval of the patients’ case records. This work was supported in part by the Fogarty International Center of the National Institutes of Health (Award numbers D43TW010134 and D43TW010543) through the Building Research and Innovation in Nigeria’s Science (BRAINS) and the Harvard, Boston, Northwestern and University of New Mexico (HBNU) Training Fellowship programs. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Iyoke CA, Ugwu GO. Burden of gynaecological cancers in developing countries. World Obstet Gynaecol. 2013;2:1–7. [Google Scholar]
- 3.Nkyekyer K Pattern of gynaecological cancers in Ghana. East Afr Med J 2000;77:534–8. [DOI] [PubMed] [Google Scholar]
- 4.Ugwu EO, Iferikigwe ES, Okeke TC, Ugwu AO, Okezie OA, Agu PU. Pattern of gynaecological cancers in University of Nigeria Teaching Hospital, Enugu, South Eastern Nigeria. Niger J Med 2011;20:266–9. [PubMed] [Google Scholar]
- 5.Buhari MO, Ojo BA, Ijaiya MA, Aboyeji PA. Ovarian cancers in Ilorin, Nigeria-A review of over 80 cases. Nig Q J Hosp Med 2005;15:127–30. [Google Scholar]
- 6.Onyiaorah IV, Anunobi CC, Banjo AA, Fatima AA, Nwankwo KC. Histopathological patterns of ovarian tumours seen in Lagos University Teaching Hospital: A ten-year retrospective study. Nig Q J Hosp Med. 2011;21:114–8. [PubMed] [Google Scholar]
- 7.Okunade KS, Okunola H, Okunowo AA, Anorlu RI. A five-year review of ovarian cancer at a tertiary institution in Lagos, South-West, Nigeria. Niger J Gen Pract 2016;14:23–7. [Google Scholar]
- 8.Zhang WW, Liu KJ, Hu GL, Liang WJ. Preoperative platelet/lymphocyte ratio is a superior prognostic factor compared to other systemic inflammatory response markers in ovarian cancer patients. Tumour Biol 2015;36:8831–7. [DOI] [PubMed] [Google Scholar]
- 9.Odukogbe AA, Adebamowo CA, Ola B, Olayemi O, Oladokun A, Adewole IF, et al. Ovarian cancer in Ibadan: Characteristics and management. J Obstet Gynaecol 2004;24:294–7. [DOI] [PubMed] [Google Scholar]
- 10.Gharoro EP, Eirewele O. Cancer of the ovary at the University of Benin Teaching Hospital: A 10-year review, 1992–2001. Afr J Med Sci 2006;35:143–7. [PubMed] [Google Scholar]
- 11.Wei SU, Li H, Zhang B. The diagnostic value of serum HE4 and CA-125 and ROMA index in ovarian cancer. Biomed Rep 2016;5:41–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogata Y, Heppelmann CJ, Charlesworth MC, Madden BJ, Miller MN, Kalli KR, et al. Elevated levels of phosphorylated fibrinogen-alpha-isoforms and differential expression of other post-translationally modified proteins in the plasma of ovarian cancer patients. J Proteome Res 2006;5:3318–25. [DOI] [PubMed] [Google Scholar]
- 13.Miralles C, Orea M, Espana P, Provencio M, Sánchez A, Cantos B, et al. Cancer antigen 125 associated with multiple benign and malignant pathologies. Ann Surg Oncol 2003;10:150–4. [DOI] [PubMed] [Google Scholar]
- 14.Shiau CS, Chang MY, Chiang CH, Hsieh CC, Hsieh TT. Ovarian endometrioma associated with very high serum CA-125 levels. Chang Gung Med J 2003;26:695–9. [PubMed] [Google Scholar]
- 15.Tiwari RK, Saha K, Mukhopadhyay D, Datta C, Chatterjee U, Ghosh TK. Evaluation of preoperative serum levels of CA 125 and expression of p53 in ovarian neoplasms: A prospective clinicopathological study in a tertiary care hospital. J Obstet Gynaecol India 2016;66:107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watrowski R, Heinze G, Jäger C, Forster J, Zeillinger R. Usefulness of the preoperative platelet count in the diagnosis of adnexal tumors. Tumour Biol 2016;37:12079–87. [DOI] [PubMed] [Google Scholar]
- 17.Leslie M Cell biology: Beyond clotting: The powers of platelets. Science 2010;328:562–4. [DOI] [PubMed] [Google Scholar]
- 18.Riess L Zur pathologischen anatomie des blutes. Arch Anat Physiol Wissensch Med 1872;39:237–49. [Google Scholar]
- 19.Levin J, Conley CL. Thrombocytosis associated with malignant disease. Arch Intern Med. 1964;114:497–500. [DOI] [PubMed] [Google Scholar]
- 20.Borsig L The role of platelet activation in tumor metastasis. Expert Rev Anticancer Ther 2008;8:1247–55. [DOI] [PubMed] [Google Scholar]
- 21.Ye Q, Cheng J, Ye M, Liu D, Zhang Y. Association of pretreatment thrombocytosis with prognosis in ovarian cancer: A systematic review and meta-analysis. J Gynecol Oncol 2019;30:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med 2012;366:610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allensworth SK, Langstraat CL, Martin JR, Lemens MA, McGree ME, Weaver AL, et al. Evaluating the prognostic significance of preoperative thrombocytosis in epithelial ovarian cancer. Gynecol Oncol 2013;130:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saxena S, Cramer AD, Weiner JM, Carmel R. Platelet counts in three racial groups. Am J Clin Pathol 1987;88:106–9. [DOI] [PubMed] [Google Scholar]
- 25.Feng Z, Wen H, Bi R, Duan Y, Yang W, Wu X. Thrombocytosis and hyperfibrinogenemia are predictive factors of clinical outcomes in high-grade serous ovarian cancer patients. BMC Cancer 2016;16:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma X, Wang Y, Sheng H, Tian W, Qi Z, Teng F, et al. Prognostic significance of thrombocytosis, platelet parameters and aggregation rates in epithelial ovarian cancer. J Obstet Gynaecol Res 2014;40:178–83. [DOI] [PubMed] [Google Scholar]
- 27.Qiu J, Yu Y, Fu Y, Ye F, Xie X, Lu W. Preoperative plasma fibrinogen, platelet count and prognosis in epithelial ovarian cancer. J Obstet Gynaecol Res 2012;38:651–7. [DOI] [PubMed] [Google Scholar]
- 28.Polterauer S, Grimm C, Seebacher V, Concin N, Marth C, Tomovski C, et al. Plasma fibrinogen levels and prognosis in patients with ovarian cancer: A multicenter study. Oncologist 2009;14:979–85. [DOI] [PubMed] [Google Scholar]
- 29.Man YN, Wang YN, Hao J, Liu X, Liu C, Zhu C, et al. Pretreatment plasma D-dimer, fibrinogen, and platelet levels significantly impact prognosis in patients with epithelial ovarian cancer independently of venous thromboembolism. Int J Gynecol Cancer 2015;25:24–32. [DOI] [PubMed] [Google Scholar]
- 30.Goubran HA, Burnouf T, Radosevic M, El-Ekiaby M. The platelet-cancer loop. Eur J Intern Med 2013;24:393–400. [DOI] [PubMed] [Google Scholar]
- 31.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer 2011;11:123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


