Abstract
Background
Previous studies suggest that the vaginal microbiome is associated with polycystic ovary syndrome (PCOS). However, the clinical manifestations of PCOS are heterogeneous. Whether the vaginal microbiome is related with different clinical symptoms was unknown.
Materials and Methods
In this cross-sectional study, 89 female patients with PCOS admitted to Zhongda Hospital (Nanjing, China) were included. Basic demographic information, health-related behaviors, clinical manifestations and sex hormone levels were comprehensively recorded for all patients. Vaginal swabs were acquired for microbiota sequencing of the V3–V4 region of the 16S rRNA gene.
Results
The prevalence of bacterial vaginitis and vulvovaginal candidiasis was 15.7% and 13.5%, respectively, within the PCOS patients, which were the most important factors affecting the vaginal microbiome (permutational multivariate analysis of variance test, R2 = 0.108, P = 0.001). The vaginal microbiome was associated with specific clinical manifestations of PCOS, including acanthosis nigricans, intermenstrual bleeding, pregnancy history, testosterone level and anti-müllerian hormone level, with P values < 0.05. The abundance of Lactobacillus crispatus was higher (P = 0.010) while that of Lactobacillus iners was lower (P = 0.036) among PCOS patients with elevated testosterone levels. Other potential bacterial biomarkers were not statistically significant after adjusting for confounding factors. No evidence of associations of other common manifestations of PCOS, such as obesity and acne, with the vaginal microbiome was obtained.
Conclusion
Vaginal bacterial species among PCOS patients with variable clinical manifestations, especially differences in testosterone levels, are distinct. Further studies are essential to investigate the microbiota and molecular mechanisms underpinning this disease.
Keywords: polycystic ovary syndrome, vaginal microbiome, clinical manifestations, testosterone, Lactobacillus
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common causes of infertility with ~10% global prevalence (1). Due to the unclear etiology of PCOS, it is necessary to explore the potential influencing factors to develop effective therapeutic strategies (2). The most common clinical symptoms include hyperandrogenism, oligomenorrhea, and polycystic ovarian morphology, often accompanied by obesity and acne (3). Clinically, different treatments are recommended for various symptoms. Therefore, efforts to develop precise prevention and intervention approaches should take into account the complexity of PCOS mechanisms.
Based on improved next-generation sequencing (NGS) technologies, our group initially identified associations between the vaginal microbiome and PCOS. The relative abundance of Lactobacillus crispatus (L. crispatus) in the PCOS patient group was significantly lower while that of Mycoplasma and Prevotella was higher than that in the control group (4). In the same year, comparable findings were reported by other research groups (5). However, these earlier studies were a case-control design, with heterogeneous manifestations among patients in the case group. For instance, a fraction of patients were positive for hyperandrogenism while the rest were negative (5). Owing to the limitations of small sample sizes, evidence on potential associations between the vaginal microbiome and different clinical manifestations of PCOS is still lacking.
A study by Wang et al. (6) using a letrozole-induced rat PCOS model supports the existence of a sex steroid hormone-microbiota-inflammation axis and critical roles of the vaginal and gut microbiomes in PCOS development. Population-based studies to date have focused on the hormonal changes during menopause and vaginal microbiota structure changes (7) but not in PCOS patients. The success of vaginal microflora transplants in some cases (8) has facilitated significant interest in analysis of the vaginal microbiome, with the aim of establishing effective clinical interventions (9). In the current study, we comprehensively explored the potential associations between common clinical manifestations of PCOS and the vaginal microbiome, and identified vaginal bacterial biomarkers for PCOS with variable symptoms.
Subjects and Methods
Study Population
From March 2019 to May 2020, 89 PCOS patients undergoing medical interventions at the reproductive clinic of Zhongda Hospital (Nanjing, China) who met the specific criteria described below were included for study. PCOS diagnosis followed the current Chinese Guidelines for the Diagnosis of PCOS (10) based on improved Rotterdam criteria (11). In brief, patients (1) developing at least two out of three symptoms, including oligo- and/or anovulation (fewer than eight cycles per year or more than three months without menstruation), clinical and/or biochemical signs of hyperandrogenism (hirsutism with a modified Ferriman-Gallwey score of ≥ 5 (10) or total testosterone level > 1.77 nmol/L), and polycystic ovaries (12 or more follicles in each ovary measuring 2–9 mm in diameter and/or increased ovarian volume (>10 mL) determined from ultrasound examination), (2) aged between 20–40 years, (3) reporting no use of oral estrogen for medical problems for at least six months, and (4) with the last menstrual period at least two months before the study were included. To avoid interference with the vaginal microbiome, the following exclusion criteria were used: (a) usage of antibiotics in the previous three days (None); (b) patients who were sexually active or complained of vaginal irrigation in the previous three days (3 women); (c) menstruating patients (None); (d) those diagnosed with Neisseria gonorrhoeae or Trichomonad (1 woman); and (e) those unwilling to participate (5 women). All participants signed an informed consent form and the study was approved by the Ethics Committee of Zhongda Hospital (2018ZDSYLL072-P01). In order to further illustrate the rules between vaginal microbiome and clinical manifestations of PCOS, we also included the original sequencing data of healthy women from our previous study (4).
Clinical Manifestations
A standard questionnaire was completed by all participants. The basic information collected included age, marital status and education level. Health-related behaviors were assessed, including tobacco exposure (active smoker or exposed to second-hand smoke every day), alcohol intake (more than once a week for any type of liquor), underwear replacement frequency (once in 1–2 days or once more than two days), pad usage habit when not menstruating (Yes/No), and condom usage habit during sexual behavior in the preceding six months. Self-reported pregnancy history and menstruation conditions, including intermenstrual bleeding and oligomenorrhea, were additionally recorded. Intermenstrual bleeding was defined as spontaneous bleeding occurring between menstrual periods, either cyclical or random, and oligomenorrhea as a period of more than 35 days without menstruation (12). Physical examination was conducted by a gynecologist. The items included height, weight, waistline and hipline measurements, in addition to records of body hair, acne and acanthosis nigricans. Body mass index was calculated as weight in kilograms divided by square of height in meters. According to Chinese standard guidelines (13), patients were classified into normal (< 23.9 kg/m2) and overweight/obesity (≥ 24.0 kg/m2) groups. In terms of waist-to-hip ratio (WHR), WHR values ≥0.8 were regarded as abnormal. Total body hair status was evaluated based on modified Ferriman-Gallwey criteria and hirsutism defined as mFG scores ≥ 5 (14).
Biochemical Parameters
Serum samples were collected in the early follicular phase (days 2-5) and hormonal and biochemical parameters measured the morning after overnight fasting, including follicle-stimulating hormone (FSH), luteinizing hormone (LH), total testosterone, sex hormone binding globulin, prolactin, anti-müllerian hormone (AMH), fasting glucose and fasting insulin. Biochemical analyses were performed in the hospital laboratory. The chemiluminescent immunoassay method was applied to measure indices using the DXI800 Immune Analyzer of Beckman Coulter Inc (CA. USA). According to laboratory standards, indices were divided into two groups, specifically, normal and elevated ( Supplementary Table S1 ).
Vaginal Swab Collection and Microenvironment Assessment
Two vaginal swabs were collected from each patient by gynecologists using standard operating procedures. Patients were placed in a lithotomy position, following which sterile swabs were used to scrape secretions at the posterior fornix with the aid of a speculum. Swabs were rotated three times to uniformly scrape any discharge. Vaginal pH was assessed using pH indicator paper. Smear microscopy examinations were completed by a professional member of staff to evaluate vaginal cleanliness grading according to the Chinese standards (details in Supplementary Table S2 ). Grades I and II were regarded as normal and grades III and IV as disordered. Meanwhile, bacterial vaginitis (BV), vulvovaginal candidiasis (VVC) and non-vaginitis were distinguished based on smear microscopy examinations according to Chinese standards ( Supplementary Table S3 ). The second swab was stored in a dry tube with a unique identification number and immediately placed in a 4°C collection box. Swabs were stored at −80°C within 8 hours for subsequent nucleic acid extraction.
Microbiome DNA Extraction and 16S rRNA Gene Sequencing
The detailed procedures for DNA extraction and 16S rRNA gene sequencing have been described previously (4). In brief, PBS buffer was used for elution of microorganisms in the swabs and the TIANamp Bacterial DNA Kit (Tiangen Biochemical Technology, Beijing, China) employed to extract and purify nucleic acids. The V3-V4 region of the 16S rRNA gene was amplified via polymerase chain reaction (PCR) with universal primers (338F: 5’-ACTCCTACGGGAGGCAGCA-3’, 806R: 5’-GGACTACHVGGGTWTCTAAT-3’). A library was constructed and amplified products sequenced on an Illumina HiSeq 2500 platform (Beijing Biomarker Technologies Co. Ltd. Beijing, China). According to sample collection times, we sequenced two batches at different times (39 samples on October 2019 and 50 samples on May 2020).
Sequencing data were processed using a standard procedure. Paired-end reads were merged with FLASH (v1.2.7, http://ccb.jhu.edu/software/FLASH/) and Trimmomatic software (v0.33) was used to remove tags of low quality (with more than six mismatches compared to the primers, average quality score < 20 in a 50 bp sliding window or shorter than 350 bp). Denoised sequences were clustered using USEARCH (version 10.0), and tags with ≥ 97% similarity regarded as an operational taxonomic unit (OTU). For each representative sequence, the NCBI dataset was used to annotate taxonomic information using QIIME. The numbers of reads for each sample were normalized corresponding to the sample with the least sequence. The relative abundance of OTU was comparable among different samples. All bioinformatics analyses were completed on the Biomarker BioCloud platform (www.biocloud.org)
Statistical Analysis
Based on OTU data, Shannon α-diversity index was calculated using mothur software (version 1.30). Higher Shannon index is associated with a more diverse and rich vaginal microbiome (15). Due to abnormal distribution, Shannon indices were presented as median and interquartile range for different groups. The non-parametric Kruskal-Wallis method was used to examine differences between groups. The binary Jaccard distance, one of the β-diversity indices that focuses on differences in taxonomic abundance profiles from multiple samples, was assessed with QIIME software. The β-diversity index comparisons between groups were directly presented using Principal Coordinate Analyses (PCoA) plots. Next, the permutational multivariate analysis of variance (PERMANOVA) method was used to adjust for potential confounding factors with the R package vegan. Linear discriminant analysis effect sizes (LEfSe) were evaluated to determine potential biomarkers between groups (LDA value > 4.0). Tests for differential abundance of genera were performed using covariate-adjusted zero-inflated negative binomial regression using the R package pscl. In cases where zero-inflated models failed to converge, the standard negative binomial was implemented as a secondary modeling strategy using the R package MASS (16). The relative abundance of species was logarithmically transformed and presented in scatter diagrams for visualization of differences. A two-sided P value ≤ 0.05 was considered statistically significant.
Results
Basic Demographic Characteristics and Vaginal Microbiomes
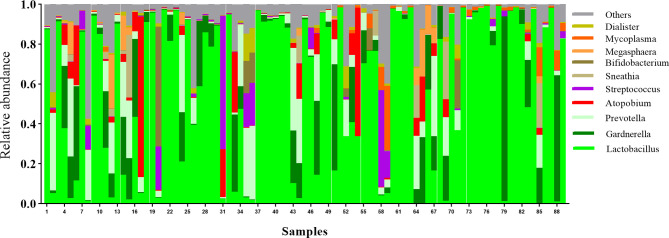
In total, 89 PCOS patients with an average age of 26.75 years were included for analysis. The majority of subjects were married (55/89, 61.8%), with graduate or postgraduate degrees (50/89, 56.2%) and no exposure to tobacco (56/89, 62.9%) or alcohol intake (71/89, 79.8%). Overall, 15 (17.4%) women changed their underwear once over more than two days and 23 (26.7%) had a habit of using sanitary pads when not menstruating. Twenty-three participants (25.8%) reported no sexual activity in the six-month period preceding the study. Among the remaining subjects, 48 (53.9%) women did not have a condom usage habit. The results of the vaginal microbiome sequencing are shown in the Figure 1 , the most abundant genus include Lactobacillus (average relative abundance: 58.52%), Gardnerella (10.40%), Prevotella (7.94%), Atopobium (4.36%), Streptococcus (2.76%), Sneathia (1.57%), Bifidobacterium (1.55%), Megasphaera (1.54%) and Mycoplasma (1.25%). Other than condom usage, no significant associations between these factors and the vaginal microbiome were observed based on α- and β-diversity (P > 0.05). Participants that used condoms had lower vaginal microbiome diversity than those with no condom usage habit or sexual activity (Shannon index: 0.56 vs 1.25 vs 1.78, P = 0.017). The details are presented in Supplementary Table S4 .
Figure 1.
Histogram plot for relative abundance of different genera among all samples.
The prevalence of BV and VVC was 15.7% and 13.5%, respectively ( Table 1 ). These factors were the most important determinants of the 10.8% variation in the vaginal microbiota structure (PERMANOVA test, R2 = 0.108, P = 0.001; PCoA plots are shown in Supplementary Figure S1A ). The Shannon index was highest among women with BV (P = 0.001). Accordingly, adjustment of vaginitis status was considered for further multivariate analysis. Women with vaginal pH > 4.5 showed higher vaginal microbiota diversity than those with pH ≤ 4.5 (Shannon index: 1.37 vs 0.81, P = 0.006). The vaginal cleanliness grade was positively correlated with Shannon index (P = 0.040), signifying that bacterial diversity increases with higher grades. Our results indicate that the vaginal microenvironment could partly, but not wholly, account for the sequencing results.
Table 1.
Vaginal microenvironment of PCOS patients and their associations with Shannon index and PERMANOVA results.
| Frequency | % | Shannon index* | PERMANOVA&R2 (P value) | ||
|---|---|---|---|---|---|
| Vaginitis | None | 63 | 70.8 | 0.99 (0.95) | 0.108 (0.001) |
| Bacterial vaginosis | 14 | 15.7 | 2.12 (0.16) | ||
| Vulvovaginal candidiasis | 12 | 13.5 | 1.22 (1.11) | ||
| P<0.001 | |||||
| PH | ≤ 4.5 | 18 | 20.2 | 0.81 (0.53) | 0.049 (0.002) |
| >4.5 | 71 | 79.8 | 1.37 (1.36) | ||
| P=0.006 | |||||
| Vaginal cleaness grading | I | 6 | 6.7 | 0.80 (1.15) | 0.066 (0.008) |
| II | 32 | 36.0 | 0.83 (0.74) | ||
| III | 40 | 44.9 | 1.56 (1.30) | ||
| IV | 11 | 12.4 | 1.57 (1.20) | ||
| P=0.040 |
*Data are presented as median (interquartile range). The Kruskal-Wallis method was used to examine differences between the groups.
&PERMANOVA test performed based on binary Jaccard distance among different groups. R2 signifies the proportion of variation in distances that can be explained by the groups tested. P values were adjusted for sequencing batch.
Clinical Manifestations and the Vaginal Microbiome
The main clinical features of PCOS are shown in Table 2 . No evidence of association of the vaginal microbiome with overweight/obesity, higher WHR, hirsutism and acne was obtained (α- and β-diversity comparisons, P > 0.05). The prevalence of acanthosis nigricans was 32.6%, which was associated with the vaginal microbiome based on binary Jaccard distance (PERMANOVA test, R2 = 0.070, P = 0.001). In total, 28.1% participants reported intermenstrual bleeding, which was associated with richer vaginal microbiota diversity (Shannon index: 1.71 vs 1.06), although this difference was not statistically significant. Thirty-two participants had a history of pregnancy, which was also significantly associated with the vaginal microbiome (PERMANOVA test, R2 = 0.025, P = 0.006).
Table 2.
Clinical features of PCOS and associations with vaginal microbiota.
| Clinical features | Frequency | % | Shannon index* | R2 (P value)& | |
|---|---|---|---|---|---|
| Overweight/Obesity | No | 54 | 60.7 | 1.21 (1.25) | 0.012 (0.094) |
| Yes | 35 | 39.3 | 1.18 (1.52) | ||
| P=0.675 | |||||
| WHR | <0.8 | 34 | 38.2 | 1.15 (1.21) | 0.019 (0.211) |
| ≥0.8 | 54 | 60.7 | 1.19 (1.31) | ||
| Missing | 1 | P=0.644 | |||
| Body hair | Normal | 43 | 48.3 | 1.22 (1.08) | 0.011 (0.139) |
| Hirsutism | 46 | 51.7 | 1.19 (1.54) | ||
| P=0.736 | |||||
| Acne | No | 8 | 9.0 | 0.83 (1.70) | 0.010 (0.199) |
| Yes | 81 | 91.0 | 1.22 (1.27) | ||
| P=0.315 | |||||
| Acanthosis nigricans | No | 60 | 67.4 | 1.15 (1.43) | 0.070 (0.001) |
| Yes | 29 | 32.6 | 1.22 (1.26) | ||
| P=0.834 | |||||
| Intermenstrual bleeding | No | 64 | 71.9 | 1.06 (1.10) | 0.024 (0.010) |
| Yes | 25 | 28.1 | 1.71 (1.36) | ||
| P=0.093 | |||||
| Pregnancy history | No | 57 | 64.0 | 1.20 (1.27) | 0.025 (0.006) |
| Yes | 32 | 36.0 | 1.23 (1.50) | ||
| P=0.578 |
*Data are presented as median (interquartile range). The Kruskal-Wallis method was used to examine differences between groups.
&PERMANOVA was performed based on binary Jaccard distance among different groups. R2 signifies the proportion of variation in distances that can be explained by the groups tested. P values were adjusted for sequencing batch.
WHR, Waist-hip ratio. Bold value means it is statistically significant (P < 0.05).
As shown in Table 3 , we observed no significant correlations of LH/FSH, prolactin and FBG with the vaginal microbiome (P > 0.05). Patients with elevated testosterone or insulin levels were likely to have richer vaginal microbiota diversity but P values were not significant (> 0.05). From the binary Jaccard distance comparisons, elevated testosterone, AMH and insulin were identified as contributory factors to microbiome variations (4.5%, 6.2% and 3.1%, respectively).
Table 3.
Biochemical parameters of PCOS patients and associations with vaginal microbiota.
| Frequency | % | Shannon index* | R2 (P value)& | ||
|---|---|---|---|---|---|
| LH/FSH | < 2 | 57 | 64.0 | 1.10 (1.49) | 0.006 (0.527) |
| ≥2 | 32 | 36.0 | 1.30 (0.99) | ||
| P=0.791 | |||||
| Testosterone | Normal | 22 | 24.7 | 0.93 (1.64) | 0.045 (0.008) |
| Elevated | 64 | 71.9 | 1.23 (1.22) | ||
| missing | 2 | P=0.874 | |||
| Prolactin | Normal | 78 | 87.6 | 1.20 (1.30) | 0.025 (0.071) |
| Elevated | 7 | 7.9 | 1.20 (0.72) | ||
| missing | 4 | P=0.512 | |||
| AMH | Normal | 60 | 67.4 | 1.19 (1.24) | 0.062 (0.001) |
| Elevated | 11 | 12.4 | 1.03 (1.15) | ||
| missing | 18 | P=0.645 | |||
| FBG | Normal | 45 | 50.6 | 1.10 (1.28) | 0.018 (0.214) |
| Elevated | 14 | 15.7 | 1.22 (1.34) | ||
| missing | 30 | P=0.521 | |||
| Insulin | Normal | 66 | 74.2 | 1.19 (1.40) | 0.031 (0.028) |
| Elevated | 12 | 13.5 | 1.44 (1.25) | ||
| missing | 11 | P=0.489 |
*Data are presented as median (interquartile range). The Kruskal-Wallis method was applied to examine differences between groups.
&The PERMANOVA test was performed based on binary Jaccard distance among different groups. R2 signifies the proportion of variation in distances that can be explained by the groups tested. P values were adjusted for sequencing batch.
AMH, Anti-müllerian hormone; FBG, Fasting blood glucose; FSH, Follicle-stimulating hormone; LH, Luteinizing hormone. Bold value means it is statistically significant (P < 0.05).
Specific Genera Associated With Clinical Manifestations
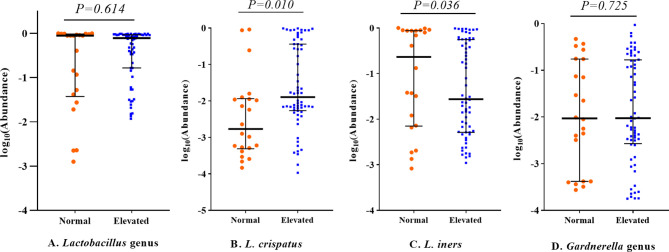
LEfSe analysis was further conducted for identification of potential biomarkers. The results are shown in Supplementary Figure S2 . For confirmation of these associations, we applied zero-inflated negative or standard negative binomial models to adjust for potential confounding factors. L. crispatus was positively correlated with the testosterone level (β = 0.98, P = 0.025; Table 4 ). Other associations, including Lactobacillus gasseri (L. gasseri) and acanthosis nigricans (β = 1.10, P = 0.065), Streptococcus and intermenstrual bleeding (β = 0.29, P = 0.085), Streptococcus and pregnancy history (β = −0.99, P = 0.087) and Mycoplasma and AMH (β = 0.73, P = 0.194) were not statistically significant, although all LDA scores were > 4.0 in LEfSe analysis. At the genus level, abundance of Lactobacillus and Gardnerella was similar between different testosterone groups (P > 0.05 for all). However, at the species level, abundance of L. crispatus of the testosterone-elevated group was higher than that of the normal group (P= 0.01), while that of Lactobacillus iners (L. iners) was lower (P=0.036, see Figure 2 ). We further compared these genera abundance with the healthy women, who were reported in previous study ( Supplementary Figure S3 ) (4). The abundance of Gardnerella among healthy women was lower than PCOS women, regardless of testosterone level (all P values < 0.05). Meanwhile, the abundance of L. crispatus of healthy group was higher than that of PCOS women, regardless of testosterone level (all P values < 0.05).
Table 4.
Potential biomarker constituents of the vaginal microbiota for clinical features and hormone levels of PCOS patients*.
| Genera/Species | Crude β (SE) | P | aβ (SE)& | P | |
|---|---|---|---|---|---|
| Acanthosis nigricans | L.gasseri | 0.97 (0.51) | 0.058 | 1.10 (0.59) | 0.065 |
| Intermenstrual bleeding | Streptococcus | 0.35 (0.19) | 0.061 | 0.29 (0.17) | 0.085 |
| Megasphaera | 0.37 (0.22) | 0.083 | 0.24 (0.20) | 0.224 | |
| Pregnancy history | Streptococcus | -1.58 (0.47) | <0.001 | -0.99 (0.58) | 0.087 |
| Testosterone | L. crispatus | 1.25 (0.47) | 0.008 | 0.98 (0.44) | 0.025 |
| AMH | Mycoplasma | 0.50 (0.59) | 0.401 | 0.73 (0.56) | 0.194 |
*All potential biomarkers were selected using LEfSe analysis with LDA scores > 4.0 ( Supplementary Figures 1A–E ). Estimates are coefficients (β) from zero-inflated negative or standard negative binomial models. The findings are interpreted as change in log genera/species sequencing read counts for patients with corresponding clinical features.
&Models were adjusted for batch of sequencing, vaginitis status and condom usage during sexual activity.
AMH, Anti-müllerian hormone; SE, standard error. Bold value means it is statistically significant (P < 0.05).
Figure 2.
Relative abundance of Lactobacillus and Gardnerella genera among PCOS patients with different testosterone status. All P values were adjusted for sequencing batch, vaginitis status and condom usage during sexual activity using linear regression models. The middle lines represent the median, and the error bars represent the range interquartile. (A) Lactobacillus genus; (B) Lactobacillus crispatus; (C) Lactobacillus iners; (D) Gardnerella genus.
Discussion
In this study, we showed that the vaginal microbiome is associated with a number of clinical manifestations of PCOS, including acanthosis nigricans, intermenstrual bleeding, pregnancy history, testosterone level and AMH level. The abundance of L. crispatus was higher among PCOS cases with elevated testosterone levels while that of L. iners was lower. To our knowledge, this is the first study to provide population-based evidence that the vaginal microbiome is associated with hormone levels in PCOS patients.
In terms of bacterial populations, the vaginal microbiome is a low-diversity, Lactobacilli- dominated community where bacteria function to maintain vaginal homoeostasis (17). Previous investigations have generally focused on the role of microbiota in vaginitis (18). With the development of detection technology, involvement of the vaginal microbiome in progression of diseases, such as preterm birth (19), infertility (20) and endometriosis (21), has been demonstrated. However, findings on the reported functions of different Lactobacilli species in the vaginal microbiome are inconsistent (17) and require further investigation. Notably, we observed distinct β-diversity of vaginal microbiota between PCOS patient groups with high and normal testosterone levels. At the micro-level, no differences in BV-related bacteria, such as Gardnerella and Atopobium, were evident but differences in specific Lactobacilli species were detected among the two groups. L. crispatus are often considered ‘beneficial bacteria’ (22). However, L. iners bacteria contain features of probiotic lactobacilli as well as vaginal pathogens (17) and an inverse association exists between L. gasseri and L. iners (23). Although the difference for their associations with hormone levels, including androgen and estrogen, remain unclear, previous studies support potential correlations. Muhleisen et al. (7) demonstrated a decrease in the Lactobacilli population in line with menopause-related hormonal and vaginal epithelial changes but no convincing conclusions were reached. Pregnancy is a typical process involving changes in hormone levels. A number of studies have reported alterations in the vaginal microbiome in association with pregnancy (24). While we observed an association of higher testosterone levels with elevated L. crispatus, this may not be beneficial, since abundance of Lactobacillus was lower among PCOS patients regardless of the testosterone level (5). Our findings just suggest that L. crispatus and L. iners populations are sensitive to the testosterone level among PCOS women. However, the associated mechanisms remain to be established.
Three potential pathways have been proposed to explain the association between the vaginal microbiome and female hormone levels. Initially, sex hormones affect mucosal immunity to determine the vaginal microbiome (25). Antigen presentation, cytokine production, immunoglobulin production and transport are influenced by variations in sex hormones levels (26) and vaginal mucosal immunity directly impacts the stability of the vaginal microbiome. Meanwhile, estrogen stimulates accumulation of glycogen in the vaginal epithelium, which is thought to play a major role in maintaining protective Lactobacillus-dominated microbiota (25). Secondly, disorder of the vaginal microbiome potentially leads to an increase in local inflammatory factors, such as interleukin-8 and tumor necrosis factor -α (27), which, along with a number of metabolites, trigger chronic systemic inflammation through the blood and lymph system, and in turn, affect the hypothalamic-pituitary-ovarian axis (28). Finally, the association between sex steroid hormones and human gut microbiome has been widely investigated. Women in the high testosterone group clearly harbor more diverse gut microbial communities (29). Interestingly, the vaginal microbiome is reported to be affected by the gut microbiome (21). Sánchez et al. (30) showed that the presence of PCOS is associated with longer anogenital distance in adults, which is a biomarker of prenatal androgen exposure. PCOS patients with elevated testosterone level might due to the prenatal androgen milieu, too (31). So it is a reasonable assumption that the longer anogenital distance might reduce the interaction between vaginal and rectum microbiome, which could partly explain the relative high L. crispatus level among PCOS patients with elevated testosterone. This is worthy of further verification.
Our results also suggest that the abundance of Streptococcus is potentially associated with intermenstrual bleeding and no history of pregnancy. Komiya et al. (32) demonstrated that Streptococcus in the gut microbiome was more abundant among infertile women. Since PCOS is a common cause of infertility (1), the issue of whether Streptococcus could serve as the key biomarker for PCOS patients with infertility warrants further research. Intermenstrual bleeding is a common symptom of PCOS and closely related to hormone level disorders (33). Continuous menstrual blood flow along the vaginal wall would significantly affect the microbiome, but the underlying mechanisms need further investigation. AMH is a glycoprotein hormone structurally related to inhibin and activin from the transforming growth factor beta superfamily whose key roles are growth differentiation and folliculogenesis (34). Women with PCOS often display elevated AMH levels and its potential association with vaginal Mycoplasma colonization was first reported in this study. The most common Mycoplasma species in the vagina is the opportunistic pathogen M. hominis, with a 20%–50% prevalence (35). Previous studies by our group support the utility of Mycoplasma as a potential biomarker in PCOS screening (4). Experiments with mouse models have disclosed significant effects of female sex hormones on vaginal colonization of M. hominis (36), but the issue of whether AMH induces Mycoplasma colonization remains unknown. Further studies are required to clarify this association and the detailed mechanisms.
The strengths of this study include comprehensive collection of data on clinical features, use of advanced microbiome analysis technology and statistical approaches. Multi-dimensional information on PCOS, including basic demographic characteristics, unhealthy behavior and common clinical manifestations, could aid in clarifying the associations between the vaginal microbiome and PCOS. For example, while obesity is thought to be linked with the human microbiome (37), our results did not support its association with the vaginal microbiome among PCOS subjects. Meanwhile, condom usage was identified as an important contributory factor to changes in the vaginal microbiome. In terms of the association between PCOS and infertility, a large proportion of women did not use condoms during sexual activity. This information should be collected and further assessed in future related studies. Enrichment of data facilitated adjustment for confounding factors during our exploration of the true associations using statistical approaches. While a number of significant differences were observed in LEfSe analysis, the multiple adjustment model did not support these conclusions, which highlights directions for future research.
A number of limitations of this study were inevitable. Since our study is cross-sectional in design, causal associations could not be inferred. PCOS is a progressive disease, and therefore, collection of samples before its occurrence is difficult. Moreover, the vaginal ecosystem is ecologically dynamic (38) and a single sample is not representative. Future studies should therefore involve successive multiple sample collections and cohort designs. Secondly, our sample size was limited, especially since many confounding factors were simultaneously considered. Several differences were observed, but not to a statistically significant extent, which could be attributable to the relatively small sample size. Our preliminary results provide a platform for future investigation of potential research queries. Thirdly, vaginal microenvironments were evaluated according to routine Chinese protocols not including the Nugent score, which limits comparability of our data with relevant literature. Fourthly, we just excluded the women who used antibiotics in the previous three days, which seemed a short interval, because of the impact of antibiotics on the human microbiome would be longer (39, 40). We should extend this interval appropriately in future studies. Lastly, this study mainly focused on the PCOS women, without a matched healthy control. Although we had supplemented some analysis through including healthy women data from our previous research, it still attenuated the persuasion of this study. Further works should be done to explore whether the association between vaginal microbiome and testosterone levels is consistent among healthy women.
In conclusion, significant variations in vaginal bacterial populations were recorded among PCOS patients with distinct clinical manifestations, in particular, different testosterone levels. Further research is essential to establish the microbiotal factors underpinning this disease and provide new insights that could facilitate improvement of PCOS prevention, screening and treatment strategies.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI PRJNA699990.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Zhongda Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
BW, DP, and XH contributed to conception and design of the study. XH and PQ organized the database. XH and JY performed the statistical analysis. XH wrote the first draft of the manuscript. YS, YX, ZC, XZ, and HY wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
WB is supported by the National Natural Science Foundation of China (No. 81872634). HX is supported by the Scientific Research Foundation of Graduate School of Southeast University (grant No. YBPY1983).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to staff at Zhongda Hospital for their assistance with data collection. We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.662725/full#supplementary-material
References
- 1. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic Ovary Syndrome: Etiology, Pathogenesis and Diagnosis. Nat Rev Endocrinol (2011) 7:219–31. 10.1038/nrendo.2010.217 [DOI] [PubMed] [Google Scholar]
- 2. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, Prevalence, and Phenotypes of Polycystic Ovary Syndrome. Fertil Steril (2016) 106:6–15. 10.1016/j.fertnstert.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 3. McCartney CR, Marshall JC, PRACTICE CLINICAL. Polycystic Ovary Syndrome. New Engl J Med (2016) 375:54–64. 10.1056/NEJMcp1514916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hong X, Qin P, Huang K, Ding X, Ma J, Xuan Y, et al. Association Between Polycystic Ovary Syndrome and the Vaginal Microbiome: A Case-Control Study. Clin Endocrinol (2020) 93:52–60. 10.1111/cen.14198 [DOI] [PubMed] [Google Scholar]
- 5. Tu Y, Zheng G, Ding G, Wu Y, Xi J, Ge Y, et al. Comparative Analysis of Lower Genital Tract Microbiome Between PCOS and Healthy Women. Front Physiol (2020) 11:1108. 10.3389/fphys.2020.01108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang T, Sha L, Li Y, Zhu L, Wang Z, Li K, et al. Dietary α-Linolenic Acid-Rich Flaxseed Oil Exerts Beneficial Effects on Polycystic Ovary Syndrome Through Sex Steroid Hormones-Microbiota-Inflammation Axis in Rats. Front Endocrinol (2020) 11:284. 10.3389/fendo.2020.00284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Muhleisen AL, Herbst-Kralovetz MM. Menopause and the Vaginal Microbiome. Maturitas (2016) 91:42–50. 10.1016/j.maturitas.2016.05.015 [DOI] [PubMed] [Google Scholar]
- 8. Lev-Sagie A, Goldman-Wohl D, Cohen Y, Dori-Bachash M, Leshem A, Mor U, et al. Vaginal Microbiome Transplantation in Women With Intractable Bacterial Vaginosis. Nat Med (2019) 25:1500–4. 10.1038/s41591-019-0600-6 [DOI] [PubMed] [Google Scholar]
- 9. Quaranta G, Sanguinetti M, Masucci L. Fecal Microbiota Transplantation: A Potential Tool for Treatment of Human Female Reproductive Tract Diseases. Front Immunol (2019) 10:2653. 10.3389/fimmu.2019.02653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Endocrinology Group and Guideline Expert Group of Obstetrics and Gynecology Branch of Chinese Medical Association . Gynecology, Chinese Guidelines for the Diagnosis and Treatment of Polycystic Ovary Syndrome. Chin J Obstetr Gynecol (2018) 53:2–6. 10.3760/cma.j.issn.0529-567x.2018.01.002 [DOI] [Google Scholar]
- 11. E.A.-S.P.c.w.g . Rotterdam, Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome (PCOS). Hum Reprod (2004) 19:41–7. 10.1093/humrep/deh098 [DOI] [PubMed] [Google Scholar]
- 12. Munro MG, Critchley HOD, Fraser IS. The Two FIGO Systems for Normal and Abnormal Uterine Bleeding Symptoms and Classification of Causes of Abnormal Uterine Bleeding in the Reproductive Years: 2018 Revisions. Int J Gynaecol Obstetr: Off Organ Int Fed Gynaecol Obstetr (2018) 143:393–408. 10.1002/ijgo.12666 [DOI] [PubMed] [Google Scholar]
- 13. Mi YJ, Zhang B, Wang HJ, Yan J, Han W, Zhao J, et al. Prevalence and Secular Trends in Obesity Among Chinese Adults, 1991-2011. Am J Prev Med (2015) 49:661–9. 10.1016/j.amepre.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao X, Ni R, Li L, Mo Y, Huang J, Huang M, et al. Defining Hirsutism in Chinese Women: A Cross-Sectional Study. Fertil Steril (2011) 96:792–6. 10.1016/j.fertnstert.2011.06.040 [DOI] [PubMed] [Google Scholar]
- 15. Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Sci (N Y NY) (2009) 324:1190–2. 10.1126/science.1171700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sitarik AR, Arora M, Austin C, Bielak LF, Eggers S, Johnson CC, et al. Fetal and Early Postnatal Lead Exposure Measured in Teeth Associates With Infant Gut Microbiota. Environ Int (2020) 144:106062. 10.1016/j.envint.2020.106062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petrova MI, Reid G, Vaneechoutte M, Lebeer S. Lactobacillus Iners: Friend or Foe? Trends Microbiol (2017) 25:182–91. 10.1016/j.tim.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 18. Onderdonk AB, Delaney ML, Fichorova RN. The Human Microbiome During Bacterial Vaginosis. Clin Microbiol Rev (2016) 29:223–38. 10.1128/CMR.00075-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fettweis JM, Serrano MG, Brooks JP, Edwards DJ, Girerd PH, Parikh HI, et al. The Vaginal Microbiome and Preterm Birth. Nat Med (2019) 25:1012–21. 10.1038/s41591-019-0450-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. García-Velasco JA, Menabrito M, Catalán IB. What Fertility Specialists Should Know About the Vaginal Microbiome: A Review. Reprod Biomed Online (2017) 35:103–12. 10.1016/j.rbmo.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 21. García-Peñarrubia P, Ruiz-Alcaraz AJ, Martínez-Esparza M, Marín P, Machado-Linde F. Hypothetical Roadmap Towards Endometriosis: Prenatal Endocrine-Disrupting Chemical Pollutant Exposure, Anogenital Distance, Gut-Genital Microbiota and Subclinical Infections. Hum Reprod Update (2020) 26:214–46. 10.1093/humupd/dmz044 [DOI] [PubMed] [Google Scholar]
- 22. Petrova MI, Lievens E, Malik S, Imholz N, Lebeer S. Lactobacillus Species as Biomarkers and Agents That can Promote Various Aspects of Vaginal Health. Front Physiol (2015) 6:81. 10.3389/fphys.2015.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Backer E, Verhelst R, Verstraelen H, Alqumber MA, Burton JP, Tagg JR, et al. Quantitative Determination by Real-Time PCR of Four Vaginal Lactobacillus Species, Gardnerella Vaginalis and Atopobium Vaginae Indicates an Inverse Relationship Between L. Gasseri and L. Iners. BMC Microbiol (2007) 7:115. 10.1186/1471-2180-7-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. MacIntyre DA, Chandiramani M, Lee YS, Kindinger L, Smith A, Angelopoulos N, et al. The Vaginal Microbiome During Pregnancy and the Postpartum Period in a European Population. Sci Rep (2015) 5:8988. 10.1038/srep08988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brotman RM, Ravel J, Bavoil PM, Gravitt PE, Ghanem KG. Microbiome, Sex Hormones, and Immune Responses in the Reproductive Tract: Challenges for Vaccine Development Against Sexually Transmitted Infections. Vaccine (2014) 32:1543–52. 10.1016/j.vaccine.2013.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wira CR, Patel MV, Ghosh M, Mukura L, Fahey JV. Innate Immunity in the Human Female Reproductive Tract: Endocrine Regulation of Endogenous Antimicrobial Protection Against HIV and Other Sexually Transmitted Infections. Am J Reprod Immunol (N Y NY: 1989) (2011) 65:196–211. 10.1111/j.1600-0897.2011.00970.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sabo MC, Lehman DA, Wang B, Richardson BA, Srinivasan S, Osborn L, et al. Associations Between Vaginal Bacteria Implicated in HIV Acquisition Risk and Proinflammatory Cytokines and Chemokines. Sexually Transmitted Infect (2020) 96:3–9. 10.1136/sextrans-2018-053949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldsammler M, Merhi Z, Buyuk E. Role of Hormonal and Inflammatory Alterations in Obesity-Related Reproductive Dysfunction At the Level of the Hypothalamic-Pituitary-Ovarian Axis. Reprod Biol Endocrinol: RB&E (2018) 16:45. 10.1186/s12958-018-0366-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shin JH, Park YH, Sim M, Kim SA, Joung H, Shin DM. Serum Level of Sex Steroid Hormone is Associated With Diversity and Profiles of Human Gut Microbiome. Res Microbiol (2019) 170:192–201. 10.1016/j.resmic.2019.03.003 [DOI] [PubMed] [Google Scholar]
- 30. Sánchez-Ferrer ML, Mendiola J, Hernández-Peñalver AI, Corbalán-Biyang S, Carmona-Barnosi A, Prieto-Sánchez MT, et al. Presence of Polycystic Ovary Syndrome is Associated With Longer Anogenital Distance in Adult Mediterranean Women. Hum Reprod (2017) 32:2315–23. 10.1093/humrep/dex274 [DOI] [PubMed] [Google Scholar]
- 31. Hernández-Peñalver AI, Sánchez-Ferrer ML, Mendiola J, Adoamnei E, Prieto-Sánchez MT, Corbalán-Biyang S, et al. Assessment of Anogenital Distance as a Diagnostic Tool in Polycystic Ovary Syndrome. Reprod Biomed Online (2018) 37:741–9. 10.1016/j.rbmo.2018.08.020 [DOI] [PubMed] [Google Scholar]
- 32. Komiya S, Naito Y, Okada H, Matsuo Y, Hirota K, Takagi T, et al. Characterizing the Gut Microbiota in Females With Infertility and Preliminary Results of a Water-Soluble Dietary Fiber Intervention Study. J Clin Biochem Nutr (2020) 67:105–11. 10.3164/jcbn.20-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crawford NM, Pritchard DA, Herring AH, Steiner AZ. Prospective Evaluation of the Impact of Intermenstrual Bleeding On Natural Fertility. Fertil Steril (2016) 105:1294–300. 10.1016/j.fertnstert.2016.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rzeszowska M, Leszcz A, Putowski L, Hałabiś M, Tkaczuk-Włach J, Kotarski J, et al. Anti-Müllerian Hormone: Structure, Properties and Appliance. Ginekol Polska (2016) 87:669–74. 10.5603/GP.2016.0064 [DOI] [PubMed] [Google Scholar]
- 35. Taylor-Robinson D. Mollicutes in Vaginal Microbiology: Mycoplasma Hominis, Ureaplasma Urealyticum, Ureaplasma Parvum and Mycoplasma Genitalium. Res Microbiol (2017) 168:875–81. 10.1016/j.resmic.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 36. Furr PM, Taylor-Robinson D. Oestradiol-Induced Infection of the Genital Tract of Female Mice by Mycoplasma Hominis. J Gen Microbiol (1989) 135:2743–9. 10.1099/00221287-135-10-2743 [DOI] [PubMed] [Google Scholar]
- 37. Maruvada P, Leone V, Kaplan LM, Chang EB. The Human Microbiome and Obesity: Moving Beyond Associations. Cell Host Microbe (2017) 22:589–99. 10.1016/j.chom.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 38. Ma B, Forney LJ, Ravel J. Vaginal Microbiome: Rethinking Health and Disease. Annu Rev Microbiol (2012) 66:371–89. 10.1146/annurev-micro-092611-150157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dethlefsen L, Relman DA. Incomplete Recovery and Individualized Responses of the Human Distal Gut Microbiota to Repeated Antibiotic Perturbation. Proc Natl Acad Sci United States America (2011) 108(Suppl 1):4554–61. 10.1073/pnas.1000087107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ferrer M, Méndez-García C, Rojo D, Barbas C, Moya A. Antibiotic Use and Microbiome Function. Biochem Pharmacol (2017) 134:114–26. 10.1016/j.bcp.2016.09.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI PRJNA699990.