Abstract
Background
The ATP-binding cassette transporter A1 (ABCA1) is likely associated with the risk of type 2 diabetes mellitus (T2DM) via β cell function modification, but the evidence on the association remains unclear. This study aimed to investigate the relationship between the ABCA1 69C>T polymorphism and the risk of T2DM through a systematic review and meta-analysis.
Materials and Methods
The PubMed, Web of Science, and Embase databases were searched for qualified studies published until August 2020. Studies that included the association between the ABCA1 69C>T polymorphism and the risk of T2DM were reviewed. The odds ratios (ORs) and 95% confidence intervals (CIs) were evaluated.
Results
We analyzed data from a total of 10 studies involving 17,742 patients. We found that the CC or CT genotype was associated with increased risk of T2DM than the TT genotype (OR, 1.41; 95% CI, 1.02-1.93). In the Asian population, the C allele carriers had a higher risk of T2DM than those with the TT genotype; the ORs of the CC and CT genotypes were 1.80 (95% CI, 1.21-2.68) and 1.61 (95% CI, and 1.29-2.01), respectively.
Conclusions
This meta-analysis confirmed that the ABCA1 69C>T genotype showed a decrease risk of T2DM compared to the CC or CT genotypes.
Keywords: ABCA1 69 C>T, meta-analysis, systematic review, polymorphism, type 2 diabetes mellitus
Introduction
Diabetes is a major global health issue estimated to have affected approximately 463 million people in 2019, with this number predicted to reach 700 million by 2045 according to the International Diabetes Federation (1). In addition, the annual cost of diabetes care is USD 760 billion (2). Diabetes is a serious, chronic endocrine disease that occurs when the blood glucose level is elevated due to insufficient insulin secretion and low sensitivity of target organs or cells to insulin (3–5). Diabetes is also associated with several comorbidities such as neuropathy and angiopathy, which have become leading causes of mortality and morbidity worldwide (1, 6).
Diabetes is classified into 2 types, and type 2 diabetes mellitus (T2DM) is the most prevalent. The development of T2DM is complex and involves a combination of several genetic and environmental factors (7–9). Several genes contribute to the overall susceptibility to T2DM by influencing the baseline glucose tolerance level (10). Genetic linkage analysis and association studies have identified several candidate genes contributing to T2DM.
The ATP-binding cassette transporter A1 (ABCA1) is considered an important gene that can modify β cell function, although its primary function is associated with cholesterol metabolism. ABCA1 contributes to the reverse transportation of cholesterol from peripheral tissues to the liver via high-density lipoprotein-cholesterol (HDL-C). Polymorphism in the ABCA1 gene is reportedly related to HDL-C deficiency, which leads to coronary heart disease or coronary artery disease (11–13). ABCA1 may also be crucial to maintaining β cell cholesterol homeostasis and function (14, 15). Because cholesterol is an important factor for membrane organization and survival of β cells, cholesterol accumulation in β cells impairs glucose metabolism and reduces insulin secretion, resulting in the development of T2DM (16). A study showed that β cell–specific Abca1 knockout mice had significantly higher fasting blood glucose levels than their littermate controls because of a defect in the first-phase glucose-stimulated insulin release (17). Studies have shown that several common variants of ABCA1 gene are also associated with the development of T2DM in humans (18). However, evidence regarding the association between ABCA1 gene polymorphisms and the risk of T2DM remains unclear. Therefore, we aimed to investigate the relationship between the extensively studied ABCA1 69C>T polymorphism and the risk of T2DM through a systematic review and meta-analysis.
Methods and Materials
Literature Search Strategy
Two researchers independently searched three databases (PubMed, Web of Science, and Embase) in August 2020 for studies on the association between ABCA1 69C>T and T2DM. The following search terms were used: [(ABCA1 OR ATP-binding cassette transporter 1 OR ATP-binding cassette transporter A1 OR adenosine triphosphate-binding cassette transporter A1 OR ATP Binding Cassette Sub Family A Member 1 OR ATP Binding Cassette Transporter, Subfamily A) AND (polymorph* OR variant* OR mutation* OR genotyp* OR allele* OR SNP*) AND (diabetes mellitus OR diabet* OR NIDDM OR T2D* OR T2DM)]. The search was not restricted by publication date. Duplicates and irrelevant studies were removed through the initial screening of titles and abstracts according to the eligibility criteria. This meta-analysis was conducted according to the checklist outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (19).
Study Inclusion and Exclusion Criteria
The following criteria were used to identify eligible studies: (i) evaluating the association between the ABCA1 polymorphisms and the risk of T2DM; (ii) using prospective or retrospective cohort study or case-control study design; (iii) providing sufficient information to calculate odds ratios (ORs) and 95% confidence intervals (CIs); and (iv) being published in English. Exclusion criteria included: (i) conference or meeting abstracts, summaries, reviews, comments, letters, news, and editorials; (ii) in vitro or animal studies; or (iii) unable to extract the data. In case of overlapping data, only the most recent and comprehensive data were included in the meta-analysis.
Data Extraction and Quality Assessment
All data were extracted independently by two researchers, and discrepancies were resolved by consensus. The following information was extracted from each study: name of the first author, publication year, study design, country, the number of participants, percentage of T2DM and females, age, body mass index (BMI), genotyping method, and the Newcastle–Ottawa scale (NOS) score. Two researchers independently assessed the selected studies based on the NOS for cohort studies and case-control studies (20). NOS has three categories: selection of study sample, comparability between the case and control groups, and outcome or exposure assessment. Each study can be assessed with a total score of 0-9. In this review, we rated 1 point for each item of comparability, if age and other known risk factors (such as BMI) were matched or adjusted for in the analysis.
Statistical Analysis
Review Manager (version 5.3; The Cochrane Collaboration, Copenhagen, Denmark) was used for data review. ORs and 95% CIs were calculated using the Z test to estimate the strength of the association between the ABCA1 69C>T polymorphism and the risk of T2DM. A p value <0.05 was considered statistically significant.
The heterogeneity across studies was estimated using a chi-square test, and an I² statistic. I² >50% was considered to indicate significant heterogeneity. In the absence of any statistical evidence of heterogeneity, the fixed-effects model was used; otherwise, the random-effects model was used to calculate pooled estimates (21, 22). Subgroup analysis was performed according to ethnic groups. Both the Begg test and the Egger regression test of the funnel plot were conducted using R Studio software (version 3.6.0; R Foundation for Statistical Computing, Vienna, Austria) to identify publication bias (23, 24).
Results
Literature Search and Characteristics of Included Studies
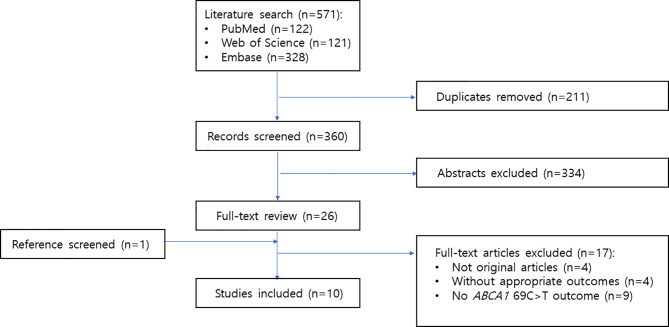
A detailed flow chart of the study selection process is presented in Figure 1 . A total of 571 studies were retrieved through the electronic databases. After duplicate removal, 360 records were initially identified, and the titles and abstracts were screened for inclusion in the study. From this initial review, the full texts of 26 studies were assessed for eligibility. Of these studies, 17 were excluded for the following reasons: not original articles (n = 4), not having appropriate outcomes (n = 4), and not containing ABCA1 69C>T outcomes (n = 9). One study was added through manual search. Thus, 10 articles were identified for this meta-analysis. All the 10 articles were written in English.
Figure 1.
Flow diagram of included studies.
The characteristics of the studies included are summarized in Table 1 . The studies were published between 2012 and 2020, most of them were case-control studies, and they mainly included Asian populations. Quality scores evaluated using the NOS for all included studies ranged from 4 to 8 ( Table 1 ).
Table 1.
Characteristics of studies included in the systematic review.
| First author, year, ref number | Study design | Country | Participants | Age (years) T2DM/Control(mean ± SD) | BMIT2DM/Control (kg/m2± SD) | Genotyping | NOS | |
|---|---|---|---|---|---|---|---|---|
| Total (T2DM %) | Female %T2DM/Control | |||||||
| Alharbi, 2013 (25) | Case-control study | Saudi Arabia | 756 (49.7) | 40.2/46.8 | 50.6 ± 10.4/46.0 ± 7.7 | 29.5 ± 5.9/29.2 ± 5.5 | PCR-RFLP | 6 |
| Du, 2020 (26) | Case-control study | China | 1998 (49.9) | 50.2/49.6 | 60.2 ± 8.6/59.7 ± 8.9 | 26.4 ± 3.2/25.1 ± 3.6 | SNaPshot | 7 |
| Ergen, 2012 (27) | Case-control study | Turkey | 157 (68.2) | 66.4/34.0 | 56 (25-85)/49 (29-85)1 | 27.5 ± 5.0/25.2 ± 3.8 | PCR-RFLP | 6 |
| Ghafar, 2020 (28) | Case-control study | Egypt | 197 (52.8) | 62.5/51.6 | 49.7 ± 9.0/48.0 ± 9.7 | 29.2 ± 4.1/22.6 ± 2.9 | TaqMan real-time PCR | 7 |
| Haghvirdizadeh, 2015 (29) | Case-control study | Malaysia | 329 (49.9) | 37.2/47.3 | 62.1 ± 9.6/55.0 ± 11.8 | 27.9 ± 5.1/27.1 ± 6.2 | PCR-HRM | 5 |
| Hasan, 2019 (30) | Case-control study | Bangladeshi | 200 (51.0) | 70.6/69.4 | 40.4 ± 1.3/39.0 ± 1.7 | 20.3 ± 0.4/20.9 ± 0.2 | PCR-RFLP | 5 |
| Li, 2018 (31) | Case-control study | China | 1122 (45.3) | 40.9/42.8 | 55.3 ± 13.3/55.2 ± 10.2 | 26.1 ± 4.7/25.4 ± 4.6 | matrix-assisted laser desorption/lionization time-of-flight mass spectrometry | 8 |
| Schou, 2012 (32) | Prospective cohort study | Denmark | 10185 (7.8) | 45.2/56.6 | 64 (57-71)/58 (43-69)2 | 28.9 (26.1-32.2)/24.6 (22.3-27.5)2 | The ABI PRISM 7900HT Sequence Detection System | 7 |
| Singh, 2015 (33) | Case-control study | India | 590 (47.5) | 35.7/35.5 | 48.5 ± 14.5/49.0 ± 16.2 | 27.2 ± 3.3 (F),25.1 ± 4.9 (M) /25.9 ± 4.1 (F), 24.8 ± 5.5 (M) |
PCR-RFLP | 4 |
| Yan 2020 (18) | Case-control study | China | 2208(49.2) | 51.7/49.1 | 58.8 ± 9.7/59.2 ± 9.9 | 26.8 ± 3.5/25.9 ± 3.8 | SNaPshot | 7 |
SD, standard deviation; BMI, body mass index; NOS, Newcastle–Ottawa scale; F, female; M, male; PCR, polymerase chain reaction; RFLP, restriction fragment length polymorphism; HRM, high resolution melting.
1 median (minimum-maximum).
2 median (interquartile range).
Associations of the ABCA1 69C>T With T2DM
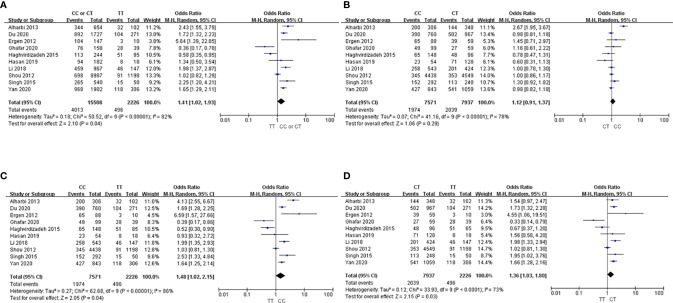
Ten studies with a total of 17,742 participants were evaluated for the association between ABCA1 variants and the risk of T2DM (18, 25–33) ( Figure 2 ). Because significant heterogeneity by the chi-square and I² tests (I² > 50%) was found, the analysis was conducted using the random-effects model to calculate the pooled ORs. The CC or CT genotype was associated with increased risk of T2DM than the TT genotype (OR, 1.41; 95% CI, 1.02-1.93; Figure 2A ). Neither the Begg test nor the Egger test showed significant publication bias (Begg test, p = 0.655; Egger test, p = 0.958, Supplementary Figure 1 ). Sensitivity analysis was performed by sequentially excluding each study; the estimates showed a similar trend, with ORs ranging from 1.31 to 1.56.
Figure 2.
Forest plots showing the association between the ABCA1 69C>T genotype and T2DM risk. (A) CC or CT vs. TT, (B) CC vs. CT, (C) CC vs. TT, (D) CT vs. TT.
In respective comparisons of the three genotypes, the CC genotype was associated with 1.1-fold (95% CI, 0.91-1.37) and 1.5-fold (95% CI 1.02-2.15) higher risk of T2DM compared to the CT and TT genotypes, respectively ( Figures 2B, C ), although statistical significance was only obtained in the comparison between the CC and TT genotypes. Regarding the CT and TT genotypes, the CT genotype had 1.4-fold (95% CI, 1.03-1.80) higher risk of T2DM compared to the TT genotype ( Figure 2D ).
Subgroup Analysis in Asians
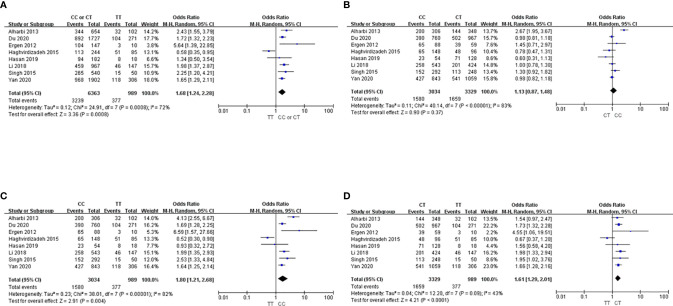
In the subgroup analysis for the Asian population, we found that the C allele carriers had significantly higher risk of T2DM than those with the TT genotype (OR 1.68; 95% CI, 1.24-2.28; Figure 3A ). Although the CC genotype was associated with no significant risk of T2DM compared to the CT genotype among Asians (OR, 1.13; 95% CI, 0.87-1.48; Figure 3B ), the CC and CT genotypes were associated with 1.8-fold (95% CI, 1.21-2.68; Figure 3C ) and 1.6-fold (95% CI, 1.29-2.01; Figure 3D ) higher risks than the TT genotype, respectively.
Figure 3.
Forest plots showing the association between the ABCA1 69C>T genotype and T2DM risk in Asians. (A) CC or CT vs. TT, (B) CC vs. CT, (C) CC vs. TT, (D) CT vs. TT.
Discussion
The inconsistency in the results regarding the association between the ABCA1 69C>T polymorphism and the risk of T2DM underlines the need for a meta-analysis on this topic. Therefore, we performed a meta-analysis including 10 studies and found that ABCA1 69TT is associated with a decreased risk of T2DM; this tendency was more pronounced in the Asian population.
ABCA1 is an efficient transporter of cholesterol from the cell to the liver and is highly expressed in β cells (17, 34). The absence of ABCA1 in β cell results in accumulation of cellular cholesterol, reduction in insulin secretion, and progressive impairment in glucose tolerance (17). In this regard, several studies investigating the association between the ABCA1 polymorphisms and the risk of T2DM have been conducted. A meta-analysis investigating the association between ABCA1 219R>K polymorphism and the risk of T2DM revealed that patients with a variant allele had a lower risk of T2DM (35). Another meta-analysis on the association between the ABCA1 230R>C and ABCA1 69C>T polymorphisms and T2DM showed that these single nucleotide polymorphisms were not associated with increased susceptibility to T2DM (36). However, the aforementioned meta-analysis included data only from three studies; hence, further meta-analyses including recently published studies are warranted.
Cholesterol accumulation in β cells impairs glucose metabolism and reduces insulin secretion (16). Several clinical studies have shown that ABCA1C69T is associated with lipoprotein metabolism. Patients with the ABCA1 69CC genotype had higher plasma triacylglycerol and very-low-density lipoprotein cholesterol levels than patients with the CT genotype (37). In addition, a study that included 391 Han Chinese adults showed that patients with the ABCA1 69CT or TT genotype had 0.68-fold lower risk of non-alcoholic fatty liver disease than those with the CC genotype (38). In line with these studies, our results indicate that the ABCA1 69T allele is associated with decreased risk of T2DM.
Because eight of the 10 included studies were conducted in Asian populations, we performed a subgroup analysis for the Asian population. Similar to the overall result, the ABCA1 69CC or CT genotype was significantly associated with higher risk of T2DM than the TT genotype in the Asian population; the association size (OR value) was greater than that for the entire study.
Our study has some limitations. First, it was not possible to perform the subgroup analysis for the non-Asian population because only two studies were available. Second, T2DM is a complex and multifactorial disease; therefore, potential gene-gene and gene-environment interactions should be considered. However, insufficient information, including nutrition, lifestyle, and demographic details, precluded further adjustments in the analysis. Third, our meta-analysis had substantial heterogeneity, possibly because of the small number of studies included.
In conclusion, our findings indicate a significant association between the ABCA1 69C>T polymorphism and T2DM risk. Large-scale population-based association studies should be conducted to validate the risk indicated by our meta-analysis and investigate potential gene-gene and gene-environment interactions on T2DM risk.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author Contributions
All the authors have made substantial contributions to the conception of the study. HY, ML, and HG contributed to designing the study. HY and ML contributed to acquisition and analysis of data. JY, GS, and HG contributed to interpretation of data. HY and ML contributed to drafting of the manuscript. HG contributed to critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.639524/full#supplementary-material
References
- 1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results From the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res Clin Pract (2019) 157:107843. 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 2. Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besançon S, et al. Global and Regional Estimates and Projections of Diabetes-Related Health Expenditure: Results From the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res Clin Pract (2020) 162:108072. 10.1016/j.diabres.2020.108072 [DOI] [PubMed] [Google Scholar]
- 3. Bansal V, Gassenhuber J, Phillips T, Oliveira G, Harbaugh R, Villarasa N, et al. Spectrum of Mutations in Monogenic Diabetes Genes Identified From High-Throughput DNA Sequencing of 6888 Individuals. BMC Med (2017) 15:213. 10.1186/s12916-018-1012-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nawaz MS, Shah KU, Khan TM, Rehman AU, Rashid HU, Mahmood S, et al. Evaluation of Current Trends and Recent Development in Insulin Therapy for Management of Diabetes Mellitus. Diabetes Metab Syndr (2007) Suppl 2:S833–9. 10.1016/j.dsx.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization and International Diabetes Federation . Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia. Report of a WHO/IDF Consultation. Geneva: World Health Organization: (2016). Available at: https://apps.who.int/iris/bitstream/handle/10665/43588/9241594934_eng.pdf. [Google Scholar]
- 6. Forbes JM, Cooper ME. Mechanisms of Diabetic Complications. Physiol Rev (2013) 93:137–88. 10.1152/physrev.00045.2011 [DOI] [PubMed] [Google Scholar]
- 7. Li Z, Ye CY, Zhao TY, Yang L. Model of Genetic and Environmental Factors Associated With Type 2 Diabetes Mellitus in a Chinese Han Population. BMC Public Health (2020) 20:1024. 10.1186/s12889-020-09130-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lara-Riegos JC, Ortiz-López MG, Peña-Espinoza BI, Montúfar-Robles I, Peña-Rico MA, Sánchez-Pozos K, et al. Diabetes Susceptibility in Mayas: Evidence for the Involvement of Polymorphisms in HHEX, Hnf4α, KCNJ11, Pparγ, CDKN2A/2B, Slc30a8, CDC123/CAMK1D, Tcf7l2, ABCA1 and SLC16A11 Genes. Gene (2015) 565:68–75. 10.1016/j.gene.2015.03.065 [DOI] [PubMed] [Google Scholar]
- 9. Miranda-Lora AL, Cruz M, Molina-Díaz M, Gutiérrez J, Flores-Huerta S, Klünder-Klünder M. Associations of Common Variants in the SLC16A11, TCF7L2, and ABCA1 Genes With Pediatric-Onset Type 2 Diabetes and Related Glycemic Traits in Families: A Case-Control and Case-Parent Trio Study. Pediatr Diabetes (2017) 18:824–31. 10.1111/pedi.12497 [DOI] [PubMed] [Google Scholar]
- 10. de Lorenzo C, Greco A, Fiorentino TV, Mannino GC, Hribal ML. Variants of Insulin-Signaling Inhibitor Genes in Type 2 Diabetes and Related Metabolic Abnormalities. Int J Genomics (2013) 2013:376454. 10.1155/2013/376454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oram JF. Tangier Disease and ABCA1. Biochim Biophys Acta (2000) 1529:321–30. 10.1016/S1388-1981(00)00157-8 [DOI] [PubMed] [Google Scholar]
- 12. Evans D, Beil FU. The Association of the R219K Polymorphism in the ATP-binding Cassette Transporter 1 (ABCA1) Gene With Coronary Heart Disease and Hyperlipidaemia. J Mol Med (Berl) (2003) 81:264–70. 10.1007/s00109-003-0426-y [DOI] [PubMed] [Google Scholar]
- 13. Zargar S, Wakil S, Mobeirek AF, Al-Jafari AA. Involvement of ATP-binding Cassette, Subfamily a Polymorphism With Susceptibility to Coronary Artery Disease. BioMed Rep (2013) 1:883–8. 10.3892/br.2013.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vergeer M, Brunham LR, Koetsveld J, Kruit JK, Verchere CB, Kastelein JJ, et al. Carriers of Loss-of-Function Mutations in ABCA1 Display Pancreatic Beta-Cell Dysfunction. Diabetes Care (2010) 33:869–74. 10.2337/dc09-1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wijesekara N, Kaur A, Westwell-Roper C, Nackiewicz D, Soukhatcheva G, Hayden MR, et al. ABCA1 Deficiency and Cellular Cholesterol Accumulation Increases Islet Amyloidogenesis in Mice. Diabetologia (2016) 59:1242–6. 10.1007/s00125-016-3907-6 [DOI] [PubMed] [Google Scholar]
- 16. Perego C, Da Dalt L, Pirillo A, Galli A, Catapano AL, Norata GD. Cholesterol Metabolism, Pancreatic β-Cell Function and Diabetes. Biochim Biophys Acta Mol Basis Dis (2019) 1865:2149–56. 10.1016/j.bbadis.2019.04.012 [DOI] [PubMed] [Google Scholar]
- 17. Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasanji Z, et al. Beta-Cell ABCA1 Influences Insulin Secretion, Glucose Homeostasis and Response to Thiazolidinedione Treatment. Nat Med (2007) 13:340–7. 10.1038/nm1546 [DOI] [PubMed] [Google Scholar]
- 18. Yan R, Luo J, He X, Li S. Association Between ABC Family Variants rs1800977, rs4149313, and rs1128503 and Susceptibility to Type 2 Diabetes in a Chinese Han Population. J Int Med Res (2020) 48:300060520941347. 10.1177/0300060520941347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med (2009) 6:264–9. 10.1371/journal.pmed.1000097 [DOI] [PubMed] [Google Scholar]
- 20. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (Accessed 25 April 2020).
- 21. Mantel N, Haenszel W. Statistical Aspects of the Analysis of Data From Retrospective Studies of Disease. J Natl Cancer Inst (1959) 22:719–48. 10.1093/jnci/22.4.719 [DOI] [PubMed] [Google Scholar]
- 22. DerSimonian R, Laird N. Meta-Analysis in Clinical Trials. Control. Clin Trials (1986) 7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 23. Begg CB, Mazumdar M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics (1994) 50:1088–99. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ (1997) 315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alharbi KK, Khan IA, Al-Daghri NM, Munshi A, Sharma V, Mohammed AK, et al. Abca1 C69T Gene Polymorphism and Risk of Type 2 Diabetes Mellitus in a Saudi Population. J Biosci (2013) 38:893–7. 10.1007/s12038-013-9384-x [DOI] [PubMed] [Google Scholar]
- 26. Du W, Hu Z, Wang L, Li M, Zhao D, Li H, et al. Abca1 Variants rs1800977 (C69T) and rs9282541 (R230c) Are Associated With Susceptibility to Type 2 Diabetes. Public Health Genomics (2020) 23:20–5. 10.1159/000505344 [DOI] [PubMed] [Google Scholar]
- 27. Ergen HA, Zeybek U, Gök O, Karaali ZE. Investigation of ABCA1 C69T Polymorphism in Patients With Type 2 Diabetes Mellitus. Biochem Med (Zagreb) (2012) 22:114–20. 10.11613/bm.2012.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghafar MTA, Shalaby KH, Okda HI, Rizk FH. Association of ABCA1 (C69T) Gene Polymorphism With Dyslipidemia and Type 2 Diabetes Among the Egyptian Population. Meta Gene (2020) 25:100714. 10.1016/j.mgene.2020.100714 [DOI] [Google Scholar]
- 29. Haghvirdizadeh P, Ramachandran V, Etemad A, Heidari F, Ghodsian N, Bin Ismail N, et al. Association of ATP-Binding Cassette Transporter A1 Gene Polymorphisms in Type 2 Diabetes Mellitus Among Malaysians. J Diabetes Res (2015) 2015:289846. 10.1155/2015/289846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hasan MM, Hosen MB, Rahman MM, Howlader MZH, Kabir Y. Association of ATP Binding Cassette Transporter 1 (ABCA 1) Gene Polymorphism With Type 2 Diabetes Mellitus (T2DM) in Bangladeshi Population. Gene (2019) 688:151–4. 10.1016/j.gene.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 31. Li C, Fan D. Association Between the ABCA1 rs1800977 Polymorphism and Susceptibility to Type 2 Diabetes Mellitus in a Chinese Han Population. Biosci Rep (2018) 38:BSR20171632. 10.1042/BSR20171632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schou J, Tybjærg-Hansen A, Møller HJ, Nordestgaard BG, Frikke-Schmidt R. ABC Transporter Genes and Risk of Type 2 Diabetes: A Study of 40,000 Individuals From the General Population. Diabetes Care (2012) 35:2600–6. 10.2337/dc12-0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh U, Tripathi A, Tripathi J, Parihar S, Saket R, Chauhan U, et al. ABCA1 (C69T) Gene Polymorphism and Serum TSH Level in Susceptibility to Diabetes Type 2: A Study on Central Indian Polulation. J Int Res Med Pharm Sci (2015) 5:152–60. [Google Scholar]
- 34. Dean M, Hamon Y, Chimini G. The Human ATP-binding Cassette (ABC) Transporter Superfamily. (2018) 50:308–16. 10.1055/a-0583-0201 [DOI] [PubMed] [Google Scholar]
- 35. Jung D, Cao S, Liu M, Park S. A Meta-Analysis of the Associations Between the ATP-Binding Cassette Transporter ABCA1 R219K (rs2230806) Polymorphism and the Risk of Type 2 Diabetes in Asians. Horm Metab Res (2018) 50(4):308–16. 10.1055/a-0583-0201 [DOI] [PubMed] [Google Scholar]
- 36. Haerian BS, Haerian MS, Roohi A, Mehrad-Majd H. ABCA1 Genetic Polymorphisms and Type 2 Diabetes Mellitus and its Complications. Meta Gene (2017) 13:104–14. 10.1016/j.mgene.2017.05.007 [DOI] [Google Scholar]
- 37. Ergen A, Isbir S, Tekeli A, Isbir T. Investigation of ABCA1 C69T and G-191C Polymorphisms in Coronary Artery Disease. In Vivo (2008) 22:187–90. [PubMed] [Google Scholar]
- 38. Wang C, Liu S, Lu L, Liao S, Yue H, Dong Q, et al. Association Between Four ABCA1 Gene Polymorphisms and Risk of non-Alcoholic Fatty Liver Disease in a Chinese Han Population. Hepatitis Monthly (2018) 18:e66149. 10.5812/hepatmon.66149 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.